Abstract
Motorized spiral enteroscopy (MSE) is the latest advance in device-assisted enteroscopy. Adverse events related to MSE were discussed in a recent large systematic review and meta-analysis and were directly compared with those of balloon enteroscopy in a case-matched study and a randomized controlled trial. Following the real-life application of MSE, an unexpected safety issue emerged regarding esophageal injury and the technique has been withdrawn from the global market, despite encouraging results in terms of diagnostic and therapeutic yield. We conducted an Italian multicenter real-life prospective study, which was prematurely terminated after the withdrawal of MSE from the market. The primary goals were the evaluation of MSE performance (both diagnostic and therapeutic) and its safety in routine endoscopic practice, particularly in the early phase of introduction in the endoscopic unit. A subanalysis, which involved patients who underwent MSE after unsuccessful balloon enteroscopy, demonstrated, for the first time, the promising performance of MSE as a rescue procedure. Given its remarkable performance in clinical practice and its potential role as a backup technique following a previously failed enteroscopy, it may be more appropriate to refine and enhance MSE in the future rather than completely abandoning it.
Keywords: Motorized spiral enteroscopy, Rescue enteroscopy, Diagnostic yield, Adverse events, AGREE classification
Core Tip: In July 2023, motorized spiral enteroscopy (MSE) was withdrawn from the global market, accompanied by an urgent safety notification. Our multicenter real-life prospective study, although prematurely interrupted, demonstrated the promising performance of MSE as a rescue procedure after a previously failed balloon enteroscopy. After a literature review about the safety of MSE, we propose that the technique should not be completely discarded but instead refined due to its very high diagnostic and therapeutic yield and its potential role as a backup enteroscopy.
INTRODUCTION
Exploration of the small bowel has challenged endoscopists for many years because the anatomical mobility and length hamper standard intubation with an endoscope.
Device-assisted enteroscopy (DAE) has overcome some of the limitations of push enteroscopy[1,2].
Balloon enteroscopy requires an overtube with an inflatable end (double bowel enteroscopy (DBE); single balloon enteroscopy (SBE) or a balloon catheter through the working channel of the endoscope for anchoring (balloon-guided or on-demand enteroscopy)[3]. Double and single balloon techniques have long been considered the gold standard for enteroscopy, providing good diagnostic yield in expert hands[4-6]. However, the back-and-forth movements needed for anchoring and advancing make balloon enteroscopy a time-consuming procedure, and the learning curve is often discouraging[7]. Furthermore, the maximum depth of insertion varies widely, depending on each patient’s small bowel looping.
Spiral enteroscopy, which was introduced in 2007, offered a new approach to examining the small bowel since the manual rotation of the spiral-shaped overtube resulted in the pleating of the jejunum and ileum instead of advancing the scope through them[8]. This procedure provided a shorter procedure time, albeit with comparable diagnostic yield and depth of insertion[3,9]. However, this technique never took hold because the manual rotation of the overtube handles was cumbersome, and two operators were needed.
The spiral technique came back to prominence in 2015, when the first clinical case of motorized spiral enteroscopy (MSE) was performed (PowerSpiral Enteroscopy, Olympus Medical Systems Corporation, Tokyo, Japan)[10]. MSE is a single-operator technique based on a self-propelled spiral segment incorporated into the scope shaft and controlled by a foot pedal. This technique was the real game-changer in enteroscopy, with the highest rate of total enteroscopies (60.6%-70.0% overall and 16.6%-31.1% with the antegrade approach alone) and the shortest procedure time in the first clinical feasibility trials[11,12]. The 3.2-mm working channel, the separated irrigation channel, and the controlled movements provided by the motor unit, granted excellent therapeutic capabilities[3,9,13].
In July 2023, MSE was withdrawn from the global market due to an urgent safety notification regarding an unpublished fatality following the surgical removal of a lodged device (probably in the esophagus)[14]. The manufacturer conducted a post-marketing analysis of adverse events (AEs) and complaints, concluding that the motor stop of the spiral segment, during both insertion and withdrawal, could result in the need for surgical removal and injury to the patient.
SAFETY OF MSE
Definitions
Unfortunately, premature withdrawal of MSE from the market blocked any further evaluation of its safety, both in large prospective studies and in a real-life setting. The long clinical practice with balloon enteroscopy, spanning over 20 years, has facilitated systematic reviews comprising > 10 000 procedures. In contrast, available data on MSE is based on < 1000 procedures[15,16]. The AE rate for DBE is 5.2%-14% for minor events and 0.56%-0.90% for major events, with the most frequent being perforation and pancreatitis[17,18].
DAE benchmarking performance measures established by the European Society of Gastrointestinal (GI) Endoscopy indicated that the serious AE (SAE) rate should be < 1% for diagnostic procedures and < 5% for therapeutic procedures[19]. However, it is important to note that this guideline was solely derived from data obtained from balloon enteroscopy. Expert authors defined a more realistic threshold of 8% for MSE, since a certain degree of wall trauma is intrinsic to the procedure and is associated with the engagement of the mucosal folds during spiral rotation, as seen in manual spiral enteroscopy[16,20,21].
Another issue is the different approaches to reporting MSE AEs in the literature. These are based on arbitrary distinctions into minor and major AEs[13,22], according to the MedDRA (Medical Dictionary for Regulatory Activities; www.meddra.org)[20], or the Clavien–Dindo classification for surgical AEs[23,24]. Yamamoto et al[4] are to be commended for reclassifying AEs from previous publications according to the recently introduced AGREE classification (acronym for AEs in GI endoscopy) for endoscopic AEs[25].
Literature review
A systematic review and meta-analysis of nine studies of MSE[22] revealed a technical success rate of 94%-97% for the antegrade route (defined as reaching the ligament of Treitz) and 94%-100% for the retrograde route (defined as passing the ileocecal valve), with a cumulative diagnostic success rate of 72%-84% and a total enteroscopy rate ranging from 30% to 72%. This meta-analysis found a significant AE rate, with minor events varying from 11% to 20%. Major events (0%-1%) included three cases of small bowel perforations, two of acute pancreatitis, and six of significant bleeding; most of them related to therapeutic treatment and not to the spiraling technique.
The AE rate reported in this meta-analysis was mainly calculated from secondary end points of studies designed for different principal outcomes, except for two multicenter studies aimed at investigating the safety of MSE as a primary end point in a real-life setting, including patients with previous abdominal surgery or surgically altered anatomy[13,20].
The study conducted by Beyna et al[20] classified hospitals based on their different levels of MSE expertise into expert centers (> 20 MSE cases handled in the past) and new centers (< 20 cases). The allocation of the procedures consisted of the training phase (< 5 MSE cases handled), core phase 1 (excluding postsurgical patients), and core phase 2 (including postsurgical patients). The overall AE rate was 11.0% (37/337), consisting mainly of minor mucosal lacerations of the esophagus, small bowel, or mild abdominal pain. Eight SAE were reported: bradycardia, hypoventilation during deep sedation, delayed abdominal perforation, deep focal defect after argon plasma coagulation, submucosal injury of the esophagus, disassembly of the spiral segment in the esophagus during withdrawal, and two cases of rebleeding from arteriovenous malformation. With the exception of one case, all SAEs occurred in new MSE centers and were associated with the antegrade approach. Postsurgical patients, who accounted for 21.5% of the core phase group, did not experience an increased AE rate, and only one SAE was documented. The SAE rate seemed higher in the training phase (4.3%, 2/47), even though the low number of events did not allow for a subgroup analysis (2.4%, 3/124 and 1.6%, 2/127 in core phases 1 and 2, respectively).
Al-Toma et al[13] also observed that there was no difference in the AE rate between patients with surgically altered GI anatomy (34/170 patients) and those with normal anatomy. No SAEs were reported, but the total minor AE rate (15.9% in the whole cohort) included three deep mucosal tears of the upper esophageal sphincter and one submucosal hematoma of the proximal esophagus. The authors also attempted to estimate a learning curve effect. After dividing the procedures into four quartiles, they found no difference in terms of the AE rate. However, the procedure ranking may be too high for the short learning curve of MSE (first quartile < 45 procedures).
In their review, Yamamoto et al[4] reported a minor AE rate for MSE, ranging from 14.4% to 48.4% (AGREE grade I), and a major AE rate from 0.7% to 9.7% (AGREE grade ≥ II). It is worth noting that AGREE grade II included AEs conservatively treated with antibiotics or blood products during hospitalization but without the need for endoscopic, radiologic, or surgical intervention (accounted for in grade III).
Two recent studies have directly compared MSE with balloon enteroscopy: a case-matched study[23] with DBE and a randomized controlled trial (RCT) with SBE[26]. The case-matched study described a similar diagnostic and therapeutic performance and reported a higher AE rate for the MSE cohort (31 patients) than the DBE cohort (62 patients)[23]. In detail, one minor AE for DBE (superficial mucosal tear in the proximal esophagus), 10 minor AEs for MSE (mainly mucosal esophageal tears), and one SAE for MSE (ileal perforation) were reported. Minor AEs in the MSE group also included two deep esophageal lacerations requiring hospitalization. In both patients, endoscopists encountered resistance during withdrawal of the scope from the upper esophagus. Difficult esophageal intubation was reported in six patients despite neck overextension and tracheal tube deflation. Even in this study, postsurgical patients (35.5% and 22.6% in the DBE and MSE groups, respectively) did not experience a higher AE rate, although a subgroup analysis was not performed.
The Motor Trial RCT compared an MSE group (35 patients) with an SBE group (37 patients)[26]. A higher diagnostic yield was achieved by the MSE group (80% vs 62.1% for the SBE group, respectively; P = 0.0096). A higher total enteroscopy rate (71.4% vs 10.8%, respectively; P < 0.0001) and a shorter procedure time (mean 58 vs 114 min, respectively; P < 0.0001) were observed. In terms of AEs, only minor events were reported for MSE (8.5%) and SBE (5.4%).
Considerations
In the early phase of MSE introduction, endoscopists were primarily concerned about two main safety issues. The first was emergency withdrawal in the case of anesthesiology complications (cardiorespiratory arrest or shock). When the spiral is deeply engaged in the small bowel, an adequate amount of time is needed to slowly unscrew the pleated bowel, even adopting the emergency maneuvers suggested by the manufacturer (continuous air insufflation and scope tip movements for disengagement); otherwise, forceful withdrawal would result in intussusception or, worse, in mesentery strangulation[4]. Fortunately, no anesthesiological AEs were reported in this regard. The second issue was surgically altered anatomy. Nevertheless, despite initial reservations, subsequent literature provided reassurance to endoscopists regarding the feasibility of performing MSE in this category of patients.
However, following the real-life application of MSE, an unexpected safety issue emerged regarding potential esophageal injury, particularly in patients with a low body mass index and a small-caliber esophagus[23].
When performing MSE, endoscopists face two important differences with standard scopes: first, the large diameter of the spiral section (18.1 mm with soft fins reaching up to 31.1 mm); and second, its automated progression. The foot pedal transmits an on-off drive to the spiral, and no regulation of the force is possible, neither onward nor backward. The motor stops only when high resistance is detected during the spiral’s rotation in order to protect the mucosa from excessive force and to prevent injuries to the patient.
The intubation of a narrow section of the GI tract requires specific endoscopic expertise. The passage of the upper esophageal sphincter is particularly challenging, especially for beginners. The safety precautions involve the overextension of the patient’s head and ensuring the deflation of the tracheal tube while inserting the scope into the upper esophagus[6]. Prophylactic esophageal bougienage to 18-20 mm has also been suggested, particularly when the first cases of antegrade MSE are performed[3,11,27], but this has not been routinely accepted in many centers[13,23].
However, the majority of esophageal AEs occurred during withdrawal and were not anticipated, given that there was no particular resistance during intubation[4]. Dray et al[28] recently reported a case involving a young woman in whom the spiral segment became lodged in her upper esophagus during the withdrawal phase. The salvage withdrawal involved manually unscrewing the scope after unplugging it from the processor, similar to the maneuver needed with the previous non-motorized spiral enteroscope.
Apart from uncommon and anecdotal case reports of SAEs in high-volume referral centers, a learning curve effect may be postulated in the very early phase of training. However, it is important to exercise caution due to the above mentioned statistical limitations of the studies[4,15].
Finally, authors should strive to utilize a standardized language when reporting AEs. We recently validated the AGREE classification in a real-life 5-year setting, demonstrating a higher interobserver agreement than the ASGE classification[29,30]. We believe that the AGREE classification should be implemented in routine endoscopic practice.
FUTURE PERSPECTIVES
Far from being considered a total replacement for balloon enteroscopy, MSE was regarded as a complementary technique for small-bowel exploration. DBE and SBE remained the techniques of choice in the case of contraindications to MSE or when repeated motor stops during the passage of a GI segment hindered its technical success[9]. Nevertheless, the application of MSE as a backup technique after a failed balloon enteroscopy has not been investigated.
We conducted an Italian multicenter real-life prospective study (SPIRALOB, ID 2884), which was prematurely stopped after the withdrawal of MSE from the market (unpublished). We collected data from five secondary and third-level endoscopy units with prior expertise in balloon enteroscopy but lacking experience in MSE. The primary goals were the evaluation of MSE performance (both diagnostic and therapeutic) and its safety in routine endoscopic practice, particularly in the early phase of introduction in the endoscopic unit.
We collected 64 MSE procedures from 56 patients with suspected small-bowel diseases between April 2022 and May 2023. Exclusion criteria were any contraindication to standard enteroscopy (e.g., severe coagulopathy or known coagulation disorder, bowel obstruction/stenosis, suspected GI perforation) with particular regard to esophageal pathology (known gastroesophageal varices or stenosis, eosinophilic esophagitis) as judged by the endoscopist after careful individual risk assessment. Prior abdominal surgery was documented in 28/64 (43.7%) procedures, and esophageal bougienage was performed in 19/46 (41.3%) antegrade MSE procedures. The technical success rate was 60/64 (93.7%), with a total procedure time of 63 min (range 32-150 min) and a depth of insertion of 200 cm (range 25-480 cm). The diagnostic yield was 46/64 (71.9%) per procedure and 44/56 (78.6%) per patient.
In terms of safety issues, we documented 19/64 (29.7%) minor AEs. Esophageal tears were the only AEs documented in 12 cases. In four cases, esophageal tears were present along with other minor AEs (duodenal/pyloric erosions, mild acute pancreatitis, and abdominal/anal pain). We registered two cases of entrapment of the spiral during withdrawal in female patients of small stature with a probable small-caliber esophagus (Figure 1). Moderate resistance was encountered when introducing the spiral segment, and additional maneuvers were required as previously described. Repeated backward stops of the motor unit occurred during withdrawal from the esophagus, and the spiral became stuck. The spiral was disengaged with tracheal tube desufflation, neck overextension, repositioning of the spiral after controlled motor re-advancement, and gentle manual derotation. The final endoscopic inspection revealed significant mucosal lacerations of the proximal esophagus, which required hospitalization for clinical monitoring and symptomatic treatment. However, both cases were resolved conservatively with no sequelae.
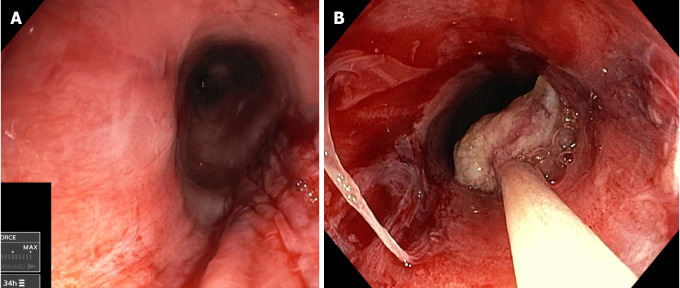
Figure 1.
The two cases of motorized spiral enteroscopy esophageal entrapment during withdrawal. A: A 49-year-old female patient (body mass index 18) with familiar adenomatous polyposis and a small tubular adenoma of the jejunum resected by en bloc polypectomy. Final inspection documented linear laceration of the cervical esophagus; B: A 29-year-old female patient (body mass index 20) with Peutz–Jeghers syndrome and a major jejunal polyp (25 mm), which was resected by piecemeal mucosal resection and retrieved with a net snare; circumferential and deep laceration of the cervical esophagus was seen during withdrawal.
The only SAE (1.6%) was esophageal perforation during insertion that precluded MSE completion and required endoscopic clip closure, fortunately with no sequelae for the patient. Therefore esophageal mucosal damages accounted for 17/46 (37%) total antegrade MSE.
A subanalysis including patients undergoing MSE after unsuccessful balloon enteroscopy (Table 1) demonstrated for the first time an encouraging performance of MSE as a rescue procedure (diagnostic yield 90% in 10 cases included). This indicated that MSE has brought about a significant change in patients’ clinical history, since most procedures were operative and led to a definitive diagnosis or treatment (Figure 2).
Table 1.
Subset of patients who underwent motorized spiral enteroscopy after failed balloon enteroscopy, n (%)
|
N = 10
|
|
| Age year; median (range) | 60 (20-81) |
| Gender (female) | 7 (70.0) |
| Indications for enteroscopy | |
| No findings on previous diagnostic tests | 1 (10.0) |
| Findings on videocapsule | 8 (80.0) |
| Findings on other imaging tests | 1 (10.0) |
| Previous enteroscopy | |
| SBE | 8 (80.0) |
| DBE | 1 (10.0) |
| SBE/DBE | 1 (10.0) |
| Upper enteroscopy | 7 (70.0) |
| Lower enteroscopy | 2 (20.0) |
| Both types | 1 (10.0) |
| Previous abdominal surgery | 4 (40.0) |
| Type of enteroscopy | |
| Upper enteroscopy | 5 (50.0) |
| Lower enteroscopy | 4 (40.0) |
| Both types | 1 (10.0) |
| Previous esophageal dilation | 2 (20.0) |
| Technical success rate | 10 (100.0) |
| Total procedure time, min; median (range) | 61.5 (40-150) |
| Max depth of insertion cm; median (range) | 200 (90-350) |
| Diagnostic yield | 9 (90.0) |
| Operative procedure | 9 (90.0) |
| Hemostasis | 1 (10.0) |
| Polypectomy | 3 (30.0) |
| Biopsy | 5 (50.0) |
| Adverse events | 2 (20.0) |
| Final diagnosis | |
| IBD | 3 (30.0) |
| Active bleeding | 2 (20.0) |
| Neoplasia | 2 (20.0) |
| Others | 2 (20.0) |
| Non-diagnostic | 1 (10.0) |
SBE: Single balloon enteroscopy; DBE: Double bowel enteroscopy; IBD: Inflammatory bowel disease.
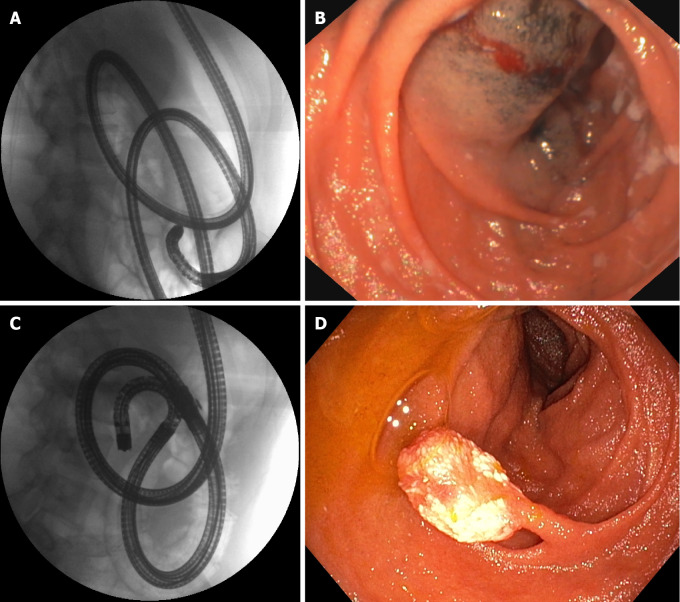
Figure 2.
A 21-year-old female patient with juvenile polyposis associated with Osler–Weber–Rendu disease (hereditary hemorrhagic telangiectasia) and a 10-mm hamartomatous polyp of the jejunum. A: Single balloon enteroscopy was used to reach the medium jejunum; B: A tattoo with submucosal ink was placed at the maximum insertion depth; C: A few months later, the same endoscopist performed motorized spiral enteroscopy, reaching the distal jejunum: D: The polyp could be detected and easily removed with standard polypectomy. Note the different looping of the scope in the fluoroscopic view.
CONCLUSION
The withdrawal of MSE from the market was justified due to major concerns following a fatality. Regrettably, our understanding of the potential of MSE has remained incomplete. However, patient safety is pivotal in our entire clinical practice, particularly when a new technique is introduced.
One of the main issues was passage of the spiral segment through the esophagus, particularly during withdrawal. Manufacturers should develop technology that enables the safe delivery of the spiral segment to the small intestine without causing injury to the upper GI segment. Spiral retractable fins could be a viable solution to address both the issue of esophageal passage and emergency withdrawal from the small bowel in the case of anesthesiology complications.
We propose that MSE should not be completely discarded but instead refined due to its high diagnostic and therapeutic yield and its potential role as a rescue technique after previous enteroscopic failure.
Footnotes
Conflict-of-interest statement: All authors declare no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: ASGE, No. 177497; SIED, No. 21149.
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Zhou PH S-Editor: Qu XL L-Editor: Kerr C P-Editor: Yuan YY
Contributor Information
Alessandro Mussetto, Department of Gastroenterology, S Maria della Croci Hosp, Ravenna 48121, Italy.
Elettra Merola, Department of Gastroenterology Unit, G.B. Grassi Hospital (ASL Roma 3), Roma 00122, Italy.
Cesare Casadei, Department of Gastroenterology, S Maria della Croci Hosp, Ravenna 48121, Italy.
Daniele Salvi, Section of Digestive Endoscopy and Gastroenterology, Fondazione Poliambulanza, Brescia 25124, Italy.
Fabiola Fornaroli, Gastroenterology and Endoscopy Unit, Department of Medicine and Surgery, University of Parma, Parma 43125, Italy.
Silvia Cocca, Gastroenterology and Endoscopy Unit, Azienda Ospedaliero Universitaria Policlinico di Modena, Modena 41121, Italy.
Margherita Trebbi, Department of Gastroenterology, S Maria della Croci Hosp, Ravenna 48121, Italy.
Armando Gabbrielli, Center for Medical Sciences (CISMed), University of Trento, Trento 38122, Italy; Gastroenterology and Digestive Endoscopy Unit, Santa Chiara Hospital, Azienda Provinciale per i Servizi Sanitari, Trento 38122, Italy.
Cristiano Spada, Digestive Endoscopy Unit, Fondazione Policlinico Universitario A. Gemelli IRCSS, Roma 00168, Italy.
Andrea Michielan, Gastroenterology and Digestive Endoscopy Unit, Santa Chiara Hospital, Azienda Provinciale per i Servizi Sanitari, Trento 38122, Italy. andrea.michielan@apss.tn.it.
References
- 1.Yamamoto H, Ogata H, Matsumoto T, Ohmiya N, Ohtsuka K, Watanabe K, Yano T, Matsui T, Higuchi K, Nakamura T, Fujimoto K. Clinical Practice Guideline for Enteroscopy. Dig Endosc. 2017;29:519–546. doi: 10.1111/den.12883. [DOI] [PubMed] [Google Scholar]
- 2.Schneider M, Höllerich J, Beyna T. Device-assisted enteroscopy: A review of available techniques and upcoming new technologies. World J Gastroenterol. 2019;25:3538–3545. doi: 10.3748/wjg.v25.i27.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nehme F, Goyal H, Perisetti A, Tharian B, Sharma N, Tham TC, Chhabra R. The Evolution of Device-Assisted Enteroscopy: From Sonde Enteroscopy to Motorized Spiral Enteroscopy. Front Med (Lausanne) 2021;8:792668. doi: 10.3389/fmed.2021.792668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto H, Despott EJ, González-Suárez B, Pennazio M, Mönkemüller K. The evolving role of device-assisted enteroscopy: The state of the art as of August 2023. Best Pract Res Clin Gastroenterol. 2023;64-65:101858. doi: 10.1016/j.bpg.2023.101858. [DOI] [PubMed] [Google Scholar]
- 5.Trebbi M, Casadei C, Dari S, Buzzi A, Brancaccio ML, Feletti V, Mussetto A. Outcomes of Double Balloon-Enteroscopy in Elderly vs. Adult Patients: A Retrospective 16-Year Single-Centre Study. Diagnostics (Basel) 2023;13 doi: 10.3390/diagnostics13061112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chetcuti Zammit S, Sanders DS, Sidhu R. Device assisted enteroscopy in the elderly - A systematic review and meta-analysis. Dig Liver Dis. 2019;51:1249–1256. doi: 10.1016/j.dld.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Tee HP, How SH, Kaffes AJ. Learning curve for double-balloon enteroscopy: Findings from an analysis of 282 procedures. World J Gastrointest Endosc. 2012;4:368–372. doi: 10.4253/wjge.v4.i8.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akerman PA, Agrawal D, Chen W, Cantero D, Avila J, Pangtay J. Spiral enteroscopy: a novel method of enteroscopy by using the Endo-Ease Discovery SB overtube and a pediatric colonoscope. Gastrointest Endosc. 2009;69:327–332. doi: 10.1016/j.gie.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Neuhaus H. The Potential Role of Motorized Spiral Enteroscopy in Small Bowel Evaluation. Gastroenterol Hepatol (N Y) 2023;19:169–172. [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhaus H, Beyna T, Schneider M, Devière J. Novel motorized spiral enteroscopy: first clinical case. VideoGIE. 2016;1:32–33. doi: 10.1016/j.vgie.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyna T, Arvanitakis M, Schneider M, Gerges C, Böing D, Devière J, Neuhaus H. Motorised spiral enteroscopy: first prospective clinical feasibility study. Gut. 2021;70:261–267. doi: 10.1136/gutjnl-2019-319908. [DOI] [PubMed] [Google Scholar]
- 12.Beyna T, Arvanitakis M, Schneider M, Gerges C, Hoellerich J, Devière J, Neuhaus H. Total motorized spiral enteroscopy: first prospective clinical feasibility trial. Gastrointest Endosc. 2021;93:1362–1370. doi: 10.1016/j.gie.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Al-Toma A, Beaumont H, Koornstra JJ, van Boeckel P, Hergelink DO, van der Kraan J, Inderson A, de Ridder R, Jacobs M. The performance and safety of motorized spiral enteroscopy, including in patients with surgically altered gastrointestinal anatomy: a multicenter prospective study. Endoscopy. 2022;54:1034–1042. doi: 10.1055/a-1783-4802. [DOI] [PubMed] [Google Scholar]
- 14.Olympus Medical. Urgent field safety notice QIL FY24-EMEA-10-FY24-OMSC-05 2023. Available from: https://www.igj.nl/binaries/igj/documenten/waarschuwingen/2023/07/11/fsn-qil-fy24-emea-10-fy24-omsc-05-olympus-medical-systems-corporation-powerspiral-intestinal-videoscope-psf-1/IT2081382+FSN-QIL+FY24-EMEA-10-FY24-OMSC-05+Olympus+Medical+Systems+Corporation+PowerSpiral+Intestinal+Videoscope+PSF-1.pdf .
- 15.Pennazio M. Safety of motorized spiral enteroscopy: is what we know today enough? Endoscopy. 2022;54:1156–1157. doi: 10.1055/a-1881-4691. [DOI] [PubMed] [Google Scholar]
- 16.Despott EJ, Murino A. The return of the helix: an evaluation of motorized spiral enteroscopy in actual daily clinical practice; a few points to "torque" about and ponder upon…. Endoscopy. 2022;54:1043–1044. doi: 10.1055/a-1866-3917. [DOI] [PubMed] [Google Scholar]
- 17.Xin L, Liao Z, Jiang YP, Li ZS. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. 2011;74:563–570. doi: 10.1016/j.gie.2011.03.1239. [DOI] [PubMed] [Google Scholar]
- 18.Shiha MG, Sidhu R, Lucaciu LA, Palmer-Jones C, Ayeboa-Sallah B, Lazaridis N, Eckersley R, Hiner GE, Maxfield D, Shaheen W, Abduljabbar D, Hussain MA, O'Hare R, Phull PS, Eccles J, Caddy GR, Butt MA, Kurup A, Chattree A, Hoare J, Jennings J, Longcroft-Wheaton G, Collins P, Humphries A, Murino A, Despott EJ, Sanders DS. Device-assisted enteroscopy performance measures in the United Kingdom: DEEP-UK quality improvement project. Endoscopy. 2024;56:174–181. doi: 10.1055/a-2199-7155. [DOI] [PubMed] [Google Scholar]
- 19.Spada C, McNamara D, Despott EJ, Adler S, Cash BD, Fernández-Urién I, Ivekovic H, Keuchel M, McAlindon M, Saurin JC, Panter S, Bellisario C, Minozzi S, Senore C, Bennett C, Bretthauer M, Dinis-Ribeiro M, Domagk D, Hassan C, Kaminski MF, Rees CJ, Valori R, Bisschops R, Rutter MD. Performance measures for small-bowel endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2019;51:574–598. doi: 10.1055/a-0889-9586. [DOI] [PubMed] [Google Scholar]
- 20.Beyna T, Moreels T, Arvanitakis M, Pioche M, Saurin JC, May A, Knabe M, Agnholt JS, Bjerregaard NC, Puustinen L, Schlag C, Aabakken L, Paulsen V, Schneider M, Neurath MF, Rath T, Devière J, Neuhaus H. Motorized spiral enteroscopy: results of an international multicenter prospective observational clinical study in patients with normal and altered gastrointestinal anatomy. Endoscopy. 2022;54:1147–1155. doi: 10.1055/a-1831-6215. [DOI] [PubMed] [Google Scholar]
- 21.Rondonotti E. Device-assisted enteroscopy: take-home messages and open issues from everyday clinical practice. Endoscopy. 2024;56:182–183. doi: 10.1055/a-2234-8695. [DOI] [PubMed] [Google Scholar]
- 22.Papaefthymiou A, Ramai D, Maida M, Tziatzios G, Viesca MFY, Papanikolaou I, Paraskeva K, Triantafyllou K, Repici A, Hassan C, Binda C, Beyna T, Facciorusso A, Arvanitakis M, Gkolfakis P. Performance and safety of motorized spiral enteroscopy: a systematic review and meta-analysis. Gastrointest Endosc. 2023;97:849–858.e5. doi: 10.1016/j.gie.2023.01.048. [DOI] [PubMed] [Google Scholar]
- 23.Chan W, Wei LK, Tan T, Hsiang LG, Kong C, Salazar E, Koay D, Khor C, Asokkumar R. Motorized spiral enteroscopy versus double-balloon enteroscopy: a case-matched study. Gastrointest Endosc. 2023;97:314–324. doi: 10.1016/j.gie.2022.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 25.Nass KJ, Zwager LW, van der Vlugt M, Dekker E, Bossuyt PMM, Ravindran S, Thomas-Gibson S, Fockens P. Novel classification for adverse events in GI endoscopy: the AGREE classification. Gastrointest Endosc. 2022;95:1078–1085.e8. doi: 10.1016/j.gie.2021.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Rughwani H, Singh AP, Ramchandani M, Jagtap N, Pal P, Inavolu P, Reddy PM, Januszewicz W, Sekaran A, Nabi Z, Patel R, Lakhtakia S, Banerjee R, Memon SF, Balram P, Darishetty S, Rao GV, Reddy DN. A Randomized, Controlled Trial Comparing the Total Enteroscopy Rate and Diagnostic Efficacy of Novel Motorized Spiral Enteroscopy and Single-Balloon Enteroscopy in Patients With Small-Bowel Disorders: The Motor Trial ( NCT 05548140) Am J Gastroenterol. 2023;118:1855–1863. doi: 10.14309/ajg.0000000000002409. [DOI] [PubMed] [Google Scholar]
- 27.Ramchandani M, Rughwani H, Inavolu P, Singh AP, Tevethia HV, Jagtap N, Sekaran A, Kanakagiri H, Darishetty S, Reddy DN. Diagnostic yield and therapeutic impact of novel motorized spiral enteroscopy in small-bowel disorders: a single-center, real-world experience from a tertiary care hospital (with video) Gastrointest Endosc. 2021;93:616–626. doi: 10.1016/j.gie.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Dray X, Leenhardt R, Camus M. Stuck in a Hard Place: Salvage, Manual Withdrawal of a Motorized Spiral Overtube Out of the Esophagus and Pharynx. J Clin Gastroenterol. 2024;58:103–105. doi: 10.1097/MCG.0000000000001908. [DOI] [PubMed] [Google Scholar]
- 29.Crispino F, Merola E, Tasini E, Cammà C, di Marco V, de Pretis G, Michielan A. Adverse events in gastrointestinal endoscopy: Validation of the AGREE classification in a real-life 5-year setting. Dig Liver Dis. 2023;55:933–937. doi: 10.1016/j.dld.2023.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]