Key Points
Question
What is the association between maternal obesity and risk of sudden unexpected infant death (SUID)?
Findings
In this cohort study of 18 857 694 live births with 16 545 postperinatal SUID cases in the US from 2015 through 2019, maternal obesity showed a dose-dependent, monotonically increasing association with SUID risk. Approximately 5.4% of SUID cases were attributable to maternal obesity.
Meaning
Maternal obesity should be added to the list of known risk factors for SUID.
Abstract
Importance
Rates of maternal obesity are increasing in the US. Although obesity is a well-documented risk factor for numerous poor pregnancy outcomes, it is not currently a recognized risk factor for sudden unexpected infant death (SUID).
Objective
To determine whether maternal obesity is a risk factor for SUID and the proportion of SUID cases attributable to maternal obesity.
Design, Setting, and Participants
This was a US nationwide cohort study using Centers for Disease Control and Prevention National Center for Health Statistics linked birth–infant death records for birth cohorts in 2015 through 2019. All US live births for the study years occurring at 28 weeks’ gestation or later from complete reporting areas were eligible; SUID cases were deaths occurring at 7 to 364 days after birth with International Statistical Classification of Diseases, Tenth Revision cause of death code R95 (sudden infant death syndrome), R99 (ill-defined and unknown causes), or W75 (accidental suffocation and strangulation in bed). Data were analyzed from October 1 through November 15, 2023.
Exposure
Maternal prepregnancy body mass index (BMI; calculated as weight in kilograms divided by height in meters squared).
Main Outcome and Measure
SUID.
Results
Of 18 857 694 live births eligible for analysis (median [IQR] age: maternal, 29 [9] years; paternal, 31 [9] years; gestational, 39 [2] weeks), 16 545 died of SUID (SUID rate, 0.88/1000 live births). After confounder adjustment, compared with mothers with normal BMI (BMI 18.5-24.9), infants born to mothers with obesity had a higher SUID risk that increased with increasing obesity severity. Infants of mothers with class I obesity (BMI 30.0-34.9) were at increased SUID risk (adjusted odds ratio [aOR], 1.10; 95% CI, 1.05-1.16); with class II obesity (BMI 35.0-39.9), a higher risk (aOR, 1.20; 95% CI, 1.13-1.27); and class III obesity (BMI ≥40.0), an even higher risk (aOR, 1.39; 95% CI, 1.31-1.47). A generalized additive model showed that increased BMI was monotonically associated with increased SUID risk, with an acceleration of risk for BMIs greater than approximately 25 to 30. Approximately 5.4% of SUID cases were attributable to maternal obesity.
Conclusions and Relevance
The findings suggest that infants born to mothers with obesity are at increased risk of SUID, with a dose-dependent association between increasing maternal BMI and SUID risk. Maternal obesity should be added to the list of known risk factors for SUID. With maternal obesity rates increasing, research should identify potential causal mechanisms for this association.
This cohort study of US live births investigates whether maternal obesity is a risk factor for sudden unexpected infant death.
Introduction
Maternal obesity is associated with a number of adverse outcomes, including stillbirth, preterm birth, congenital anomalies, and neonatal intensive care unit admission.1,2,3 High maternal body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) is also associated with all-cause infant mortality, defined as death before the first birthday, with a clear dose-response association.4,5,6,7 The obesity link has been documented most strongly for deaths caused by congenital anomalies, birth asphyxia, and other neonatal morbidities,6 and the overall dose-response association has been taken to indicate a biological link between infant death and maternal adiposity.7
The association between maternal prepregnancy obesity and unexpected infant death is unclear. Sudden unexpected infant death (SUID) is a significant cause of infant mortality, with approximately 3500 SUID cases reported each year in the US. SUID includes 3 causes of death classified by the International Statistical Classification of Diseases, 10th Revision (ICD-10)8: sudden infant death syndrome (SIDS; code R95), ill-defined or unknown causes (code R99), and accidental suffocation and strangulation in bed (code W75). Throughout the 1990s, SUID rates in the US decreased dramatically in large part due to education campaigns about safe sleep position for infants, although rates have plateaued since the late 1990s despite decreases in the prevalence of risk factors, such as maternal smoking.9,10
In contrast to smoking, maternal obesity rates are increasing.11 Thus far, maternal obesity has not been identified as a risk factor for SUID in expert reviews and public education campaigns, nor has it been included in the American Academy of Pediatrics evidence or recommendations to prevent sudden infant death.12,13,14,15,16,17 Nonetheless, some have suggested that maternal obesity could increase SUID risk in the context of bed sharing,18,19 although these suggestions were based on a small number of case reports of overlaying deaths involving a mother with obesity and were not based on cohort or case-control studies.20,21 One case-control study found that, although bed sharing was associated with increased SIDS risk, maternal obesity was not a statistically significant independent risk factor, nor was the risk associated with bed sharing statistically significantly higher among mothers who were overweight or obese.22 In contrast, 2 studies on maternal obesity and all-cause infant mortality reported associations between high maternal BMI and SIDS risk in subanalyses, although there were inconsistent results with no evident dose-response association.5,6 However, each of these studies had a small number of cases, so associations may not have been detectable.
The aim of this study was to identify whether an association between maternal obesity and SUID risk exists by using whole-population data from the US, thus providing a substantially larger sample.
Methods
We conducted a nationwide cohort study using data from the Centers for Disease Control and Prevention (CDC) National Center for Health Statistics for birth cohort years 2015 through 2019. Data were drawn from the cohort linked birth–infant death file for 2015 and period/cohort linked birth–infant death files for 2016 through 2019. These reflect all live births in the US for these years. Infant deaths reflect mortality within the first year of life. We analyzed births occurring at 28 weeks’ gestation or later and with CDC flags for areas with complete reporting for all variables considered in our analyses. For cases of infant death, we included only those for whom the primary cause was one of the 3 ICD-10 codes associated with SUID (code R95, R99, or W75). We additionally retained only SUID cases where the death occurred at 7 days post partum or later, as unexpected infant deaths occurring prior to the seventh day of life are likely a distinct entity from postperinatal SUID.23 After exclusions, 16 545 SUID cases and 18 841 149 controls (infants surviving ≥364 days after birth) remained for analyses (SUID rate, 0.88/1000 live births) (Table 1). As these are routinely collected health data and made publicly available for research purposes, informed consent and institutional review board approval were not needed. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Table 1. Case Flow Information.
| Step | Cases, No. | SUID rate, No./1000 births | |||||
|---|---|---|---|---|---|---|---|
| Control | Infant death | ICD-10 code | Total SUID | ||||
| R95 | R99 | W75 | |||||
| Start | 19 258 173 | 110 542 | 6884 | 6093 | 4490 | 17 467 | 0.91 |
| Remove infants born at GA <28 wk | 93 369 | 50 477 | 87 | 239 | 49 | 375 | NA |
| Remaining | 19 164 804 | 60 065 | 6797 | 5854 | 4441 | 17 092 | 0.89 |
| Remove CDC flags for incomplete reporting areas | 323 655 | 625 | 91 | 52 | 26 | 169 | NA |
| Remaining | 18 841 149 | 59 440 | 6706 | 5802 | 4415 | 16 923 | 0.90 |
| Remove all ICD-10 infant death codes except R95, R99, and W75 | NA | 42 517 | NA | NA | NA | NA | NA |
| Remaining | 18 841 149 | 16 923 | 6706 | 5802 | 4415 | 16 923 | 0.90 |
| Remove all deaths <7 d | NA | 378 | 118 | 196 | 64 | 378 | NA |
| Retained for analysis | 18 841 149 | 16 545 | 6588 | 5606 | 4351 | 16 545 | 0.88 |
Abbreviations: CDC, Centers for Disease Control and Prevention; GA, gestational age; ICD-10, International Statistical Classification of Diseases, Tenth Revision; NA, not applicable; SUID, sudden unexpected infant death.
Study Measures
The primary exposure was prepregnancy maternal BMI. For our main analysis, we used standard BMI categories: underweight (<18.5), normal weight (18.5-24.9), overweight (25.0-29.9), class I obesity (30.0-34.9), class II obesity (35.0-35.9), and class III obesity (≥40.0). In the multivariate analyses, we adjusted for the following variables: maternal age, race, Hispanic ethnicity, education, birthplace or nativity, marital status, and smoking status; paternal age and race; payment source for birth procedure; live birth order; and infant sex.5,6,17,24,25,26,27 Categorization levels and record counts or distributions for the primary exposure and all covariates are provided in Table 2. Race (American Indian or Alaska Native, Asian, Black, Native Hawaiian or Other Pacific Islander, White, ≥1 race, and unknown or not stated) and ethnicity (Central and South American, Cuban, Mexican, non-Hispanic, Puerto Rican, other or unknown Hispanic origin, and unknown or not stated) were obtained from the CDC, which aggregates and recodes these data from birth certificates.
Table 2. Counts and SUID Rates for Exposure Variable and Covariates.
| Characteristic | No. (%) | SUID rate, No./1000 births | Prepregnancy maternal BMI, mean (SD) | |
|---|---|---|---|---|
| SUID (n = 16 545) | Controls (n = 18 841 149) | |||
| Maternal BMI | ||||
| Underweight, <18.5 | 642 (3.9) | 613 535 (3.3) | 1.05 | 17.4 (0.9) |
| Normal, 18.5-24.9 | 5914 (35.7) | 7 935 244 (42.1) | 0.74 | 22.0 (1.7) |
| Overweight, 25.0-29.9 | 3812 (23.0) | 4 831 172 (25.6) | 0.79 | 27.2 (1.4) |
| Obesity class | ||||
| I, 30.0-34.9 | 2673 (16.2) | 2 704 181 (14.4) | 0.99 | 32.1 (1.4) |
| II, 35.0-39.9 | 1551 (9.4) | 1 326 907 (7.0) | 1.17 | 37.2 (1.4) |
| III, ≥40.0 | 1387 (8.4) | 944 553 (5.0) | 1.47 | 44.9 (4.5) |
| Unknown or not stated | 566 (3.4) | 485 557 (2.6) | 1.16 | NAa |
| Maternal age, y | ||||
| <20 | 1771 (10.7) | 971 977 (5.2) | 1.82 | 25.2 (6.0) |
| 20-24 | 5522 (33.4) | 3 765 640 (20.0) | 1.46 | 26.9 (6.8) |
| 25-29 | 4954 (29.9) | 5 475 346 (29.1) | 0.90 | 27.3 (6.9) |
| 30-34 | 2870 (17.3) | 5 335 263 (28.3) | 0.54 | 26.9 (6.6) |
| 35-39 | 1184 (7.2) | 2 689 862 (14.3) | 0.44 | 27.2 (6.6) |
| ≥40 | 244 (1.5) | 603 061 (3.2) | 0.40 | 27.4 (6.5) |
| Maternal raceb | ||||
| American Indian or Alaska Native | 370 (2.2) | 182 618 (1.0) | 2.02 | 28.8 (7.0) |
| Asian | 280 (1.7) | 1 264 298 (6.7) | 0.22 | 23.8 (4.6) |
| Black | 5292 (32.0) | 2 954 743 (15.7) | 1.79 | 28.7 (7.5) |
| Native Hawaiian or Other Pacific Islander | 88 (0.5) | 59 151 (0.3) | 1.49 | 29.7 (7.2) |
| White | 9784 (59.1) | 13 897 967 (73.8) | 0.70 | 26.9 (6.5) |
| >1 Race | 731 (4.4) | 482 323 (2.6) | 1.51 | 27.4 (7.1) |
| Unknown or not stated | 0 | 49 (0.0) | 0.00 | NAa |
| Maternal ethnicityb | ||||
| Central and South American | 206 (1.2) | 711 519 (3.8) | 0.29 | 26.5 (5.5) |
| Cuban | 47 (0.3) | 111 524 (0.6) | 0.42 | 26.2 (5.8) |
| Mexican | 1349 (8.2) | 2 551 701 (13.5) | 0.53 | 27.9 (6.4) |
| Non-Hispanic | 14 081 (85.1) | 14 279 562 (75.8) | 0.99 | 26.8 (6.8) |
| Puerto Rican | 294 (1.8) | 330 147 (1.8) | 0.89 | 27.9 (7.0) |
| Other or unknown Hispanic origin | 431 (2.6) | 694 518 (3.7) | 0.62 | 27.6 (6.5) |
| Unknown or not stated | 137 (0.8) | 162 178 (0.9) | 0.84 | 26.1 (6.2) |
| Maternal education | ||||
| ≤8th Grade | 384 (2.3) | 612 339 (3.3) | 0.63 | 27.1 (5.8) |
| 9th-12th Grade with no diploma | 3570 (21.6) | 1 864 355 (9.9) | 1.91 | 27.1 (6.9) |
| High school or GED | 6402 (38.7) | 4 747 905 (25.2) | 1.35 | 27.7 (7.1) |
| Some college | 3868 (23.4) | 3 814 866 (20.2) | 1.01 | 28.0 (7.2) |
| Associate degree | 819 (5.0) | 1 546 121 (8.2) | 0.53 | 27.7 (6.9) |
| Bachelor’s degree | 888 (5.4) | 3 782 201 (20.1) | 0.23 | 25.8 (5.9) |
| ≥Master’s degree | 397 (2.4) | 2 227 840 (11.8) | 0.18 | 25.2 (5.5) |
| Unknown or not stated | 217 (1.3) | 245 522 (1.3) | 0.88 | 26.4 (6.2) |
| Maternal birthplace | ||||
| Within the 50 US states | 15 305 (92.5) | 14 536 052 (77.2) | 1.05 | 27.3 (7.0) |
| Outside the US, including possessions | 1189 (7.2) | 4 268 517 (22.7) | 0.28 | 25.8 (5.5) |
| Unknown or not stated | 51 (0.3) | 36 580 (0.2) | 1.39 | 26.3 (6.3) |
| Marital status | ||||
| Married | 4601 (27.8) | 10 486 952 (55.7) | 0.44 | 26.7 (6.5) |
| Unmarried | 11 301 (68.3) | 6 992 539 (37.1) | 1.61 | 27.5 (7.1) |
| Unknown or not stated | 643 (3.9) | 1 361 658 (7.2) | 0.47 | 26.7 (6.3) |
| Maternal smoking | ||||
| Nonsmoker | 11 357 (68.6) | 17 039 043 (90.4) | 0.67 | 26.9 (6.6) |
| Continued | 4275 (25.8) | 1 255 309 (6.7) | 3.39 | 27.2 (7.3) |
| Quit | 634 (3.8) | 408 349 (2.2) | 1.55 | 27.8 (7.2) |
| Started | 80 (0.5) | 23 187 (0.1) | 3.44 | 27.6 (7.3) |
| Unknown or not stated | 199 (1.2) | 115 261 (0.6) | 1.72 | 27.2 (6.9) |
| Paternal age, y | ||||
| <20 | 569 (3.4) | 335 801 (1.8) | 1.69 | 25.0 (5.9) |
| 20-24 | 2766 (16.7) | 2 117 102 (11.2) | 1.30 | 26.7 (6.7) |
| 25-29 | 3283 (19.8) | 4 059 514 (21.5) | 0.81 | 27.2 (6.8) |
| 30-34 | 2474 (15.0) | 4 931 982 (26.2) | 0.50 | 26.8 (6.5) |
| 35-39 | 1415 (8.6) | 3 251 276 (17.3) | 0.44 | 26.8 (6.5) |
| 40-44 | 556 (3.4) | 1 283 588 (6.8) | 0.43 | 27.2 (6.6) |
| ≥45 | 375 (2.3) | 653 591 (3.5) | 0.57 | 27.1 (6.6) |
| Unknown or not stated | 5107 (30.9) | 2 208 295 (11.7) | 2.31 | 27.8 (7.4) |
| Paternal raceb | ||||
| American Indian or Alaska Native | 210 (1.3) | 123 773 (0.7) | 1.69 | 28.6 (7.0) |
| Asian | 206 (1.2) | 1 044 188 (5.5) | 0.20 | 23.8 (4.7) |
| Black | 3369 (20.4) | 2 288 753 (12.1) | 1.47 | 28.9 (7.5) |
| Native Hawaiian or Other Pacific Islander | 78 (0.5) | 47 672 (0.3) | 1.63 | 29.9 (7.4) |
| White | 6471 (39.1) | 11 549 341 (61.3) | 0.56 | 26.7 (6.4) |
| >1 Race | 418 (2.5) | 377 042 (2.0) | 1.11 | 26.8 (6.7) |
| Unknown or not stated | 5793 (35.0) | 3 410 380 (18.1) | 1.70 | 27.7 (7.1) |
| Payment source | ||||
| Private insurance | 3487 (21.1) | 9 249 211 (49.1) | 0.38 | 26.5 (6.4) |
| Medicaid | 11 881 (71.8) | 7 982 328 (42.4) | 1.49 | 27.7 (7.1) |
| Self-pay | 499 (3.0) | 763 262 (4.1) | 0.65 | 25.6 (5.7) |
| Other | 563 (3.4) | 725 296 (3.8) | 0.78 | 26.7 (6.2) |
| Unknown or not stated | 115 (0.7) | 121 052 (0.6) | 0.95 | 26.7 (6.5) |
| Live birth order | ||||
| 1 | 4291 (25.9) | 7 139 004 (37.9) | 0.60 | 26.2 (6.5) |
| 2 | 4886 (29.5) | 6 032 914 (32.0) | 0.81 | 27.0 (6.7) |
| 3 | 3528 (21.3) | 3 220 718 (17.1) | 1.09 | 27.6 (6.8) |
| 4 | 1923 (11.6) | 1 396 608 (7.4) | 1.38 | 28.2 (6.9) |
| 5 | 965 (5.8) | 548 977 (2.9) | 1.75 | 28.5 (6.9) |
| ≥6 | 878 (5.3) | 443 380 (2.4) | 1.98 | 28.7 (7.0) |
| Unknown or not stated | 74 (0.4) | 59 548 (0.3) | 1.24 | 26.7 (6.5) |
| Infant sex | ||||
| Female | 6888 (41.6) | 9 207 653 (48.9) | 0.75 | 27.0 (6.7) |
| Male | 9657 (58.4) | 9 633 496 (51.1) | 1.00 | 27.0 (6.7) |
| GA at birth, wk | ||||
| 28-31 | 500 (3.0) | 166 883 (0.9) | 2.99 | 28.0 (7.4) |
| 32-36 | 2936 (17.7) | 1 571 760 (8.3) | 1.86 | 27.7 (7.3) |
| ≥37 | 13 109 (79.2) | 17 102 506 (90.8) | 0.77 | 26.9 (6.6) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GA, gestational age; GED, General Educational Development test; NA, not applicable; SUID, sudden unexpected infant death.
Not estimable due to all missing values.
Data were obtained from the Centers for Disease Control and Prevention, which aggregates and recodes these data from birth certificates.
Statistical Analysis
Analyses were carried out between October 1, 2023, and November 15, 2023. We computed logistic regression models estimating the odds of SUID for each category of maternal prepregnancy BMI, where odds ratios (ORs) used normal BMI as the reference category. Crude univariate ORs used only maternal BMI category as an input; adjusted ORs (aORs) additionally included all adjustment variables described earlier. We used E-values to assess the minimum degree of residual confounding necessary to account for any observed associations with maternal obesity.28 To assess whether the associations were consistent across each of the 3 SUID cause of death classifications (ICD-10 code R95, R99, or W75), 3 logistic regression models adjusting for the covariates described earlier were fitted for each of the 3 ICD-10 codes separately. As high maternal BMI is a risk factor for preterm birth,3 which is in turn a risk factor for SUID,13 we fit an additional model adjusting for gestational age at birth (Table 2). Records with unknown or missing prepregnancy BMI values were coded as a separate level for all logistic regression analyses and are not reported in results outcome tables. To assess the shape of the association between maternal BMI and SUID risk across the entire range of BMI values and identify potential nonlinearities, we computed a generalized additive model (GAM)29,30 using maternal prepregnancy BMI as a continuous input without binning, while adjusting for the same covariates as the primary analysis described earlier. Records with missing maternal BMI values were dropped for the GAM analysis.
Finally, to assess the proportion of SUID cases attributable to maternal obesity after controlling for covariates, we computed the population attributable fraction (PAF) for each level of maternal BMI, controlling for the covariates described earlier; 95% CIs for PAFs were calculated using the stratified bootstrap method. All analyses were carried out using R version 4.0.5 statistical software (R Foundation); the GAM was fit using the mgcv package (version 1.8-40)29; and E-values were computed using the EValue package (version 4.1.3).28 For all analyses, we defined P < .05 (2-sided) as indicating statistical significance.
Results
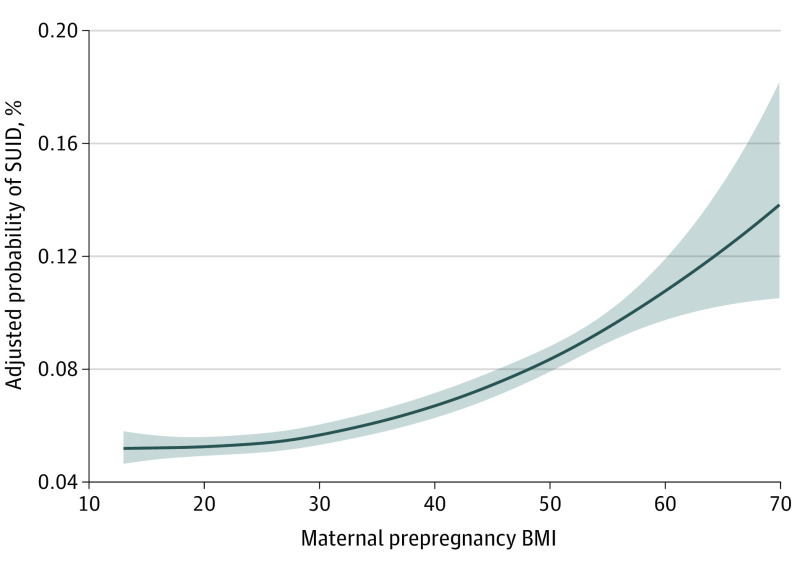
Of 18 857 694 live births (median [IQR] age: maternal, 29 [9] years; paternal, 31 [9] years; gestational, 39 [2] weeks) eligible for analysis, 16 545 died of SUID (SUID rate, 0.88/1000 live births). Results from the univariate and multivariate logistic regressions are presented in Table 3. In the univariate analysis, we found increased odds of SUID both for mothers who were underweight and for mothers who were overweight or obese, with increasing obesity classes associated with increasing odds of SUID. After adjusting for covariates, infants born to mothers with BMI values in the underweight and overweight categories were no longer found to be at statistically significantly increased risk. Infants born to mothers with prepregnancy BMIs in each of the 3 obesity class categories continued to show statistically significantly increased odds of SUID compared with those born to mothers with BMIs in the normal range, again in a dose-dependent fashion (class I obesity: aOR, 1.10; 95% CI, 1.05-1.16; class II obesity: aOR, 1.20; 95% CI, 1.13-1.27; and class III obesity: aOR, 1.39; 95% CI, 1.31-1.47). For mothers with BMIs in the class III obesity range, corresponding to approximately 5% of births, the odds of SUID were nearly 40% greater than those for mothers with normal BMIs (aOR, 1.39; 95% CI, 1.31-1.47). E-values suggest that residual confounding would need to be substantial to account for this. For example, an unmeasured confounder or set of confounders associated by an increased risk of at least 2-fold each with both obesity class III and SUID, over and above the measured confounders in our adjusted model, would erase the observed aOR of 1.39, but lesser confounding would not. Results of the GAM (Figure) showed a monotonically increasing association between maternal prepregnancy BMI and probability of SUID. Point estimates for the GAM showed a continuously increasing function across the entire BMI range; however, this increase was nonlinear. Increases were small for BMIs below approximately 25, with an acceleration of the risk increase occurring between BMIs of approximately 25 to 30.
Table 3. Risk of SUID by Maternal BMI Categorya.
| BMI category | All SUID, model estimate | By ICD-10 code | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case counts, No. | Model estimate, aOR (95% CI) | ||||||||
| Univariate OR (95% CI) | aOR (95% CI) | E-value (limit)b | R95 | R99 | W75 | R95 | R99 | W75 | |
| Underweight, <18.5 | 1.40 (1.29-1.52) | 0.99 (0.92-1.08) | 1.09 (1.00) | 261 | 218 | 163 | 1.02 (0.89-1.15) | 0.99 (0.86-1.14) | 0.96 (0.82-1.13) |
| Normal, 18.5-24.9 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 2373 | 2033 | 1508 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Overweight, 25.0-29.9 | 1.06 (1.02-1.10) | 1.00 (0.96-1.04) | 1.05 (1.00) | 1459 | 1322 | 1031 | 0.96 (0.90-1.02) | 1.00 (0.93-1.07) | 1.08 (0.99-1.17) |
| Obesity class | |||||||||
| I, 30.0-34.9 | 1.33 (1.27-1.39) | 1.10 (1.05-1.16) | 1.44 (1.29) | 1085 | 878 | 710 | 1.12 (1.04-1.21) | 1.04 (0.96-1.13) | 1.16 (1.06-1.27) |
| II, 35.0-39.9 | 1.57 (1.48-1.66) | 1.20 (1.13-1.27) | 1.69 (1.52) | 626 | 493 | 432 | 1.21 (1.11-1.32) | 1.10 (1.00-1.21) | 1.31 (1.18-1.46) |
| III, ≥40.0 | 1.97 (1.86-2.09) | 1.39 (1.31-1.47) | 2.12 (1.94) | 557 | 463 | 367 | 1.40 (1.27-1.53) | 1.34 (1.21-1.49) | 1.44 (1.28-1.61) |
Abbreviations: aOR, adjusted odds ratio; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ICD-10, International Statistical Classification of Diseases, Tenth Revision; OR, odds ratio; SUID, sudden unexpected infant death.
aORs and E-values are adjusted for maternal age, race, ethnicity, education, birthplace or nativity, marital status, and smoking status; paternal age and race; payment source for birth procedure; live birth order; and infant sex.
E-value for the 95% confidence limit closest to the null.
Figure. Generalized Additive Model Results.
Shaded area reflects the 95% CI. The model is adjusted for maternal age, race, Hispanic ethnicity, education, birthplace or nativity, marital status, and smoking status; paternal age and race; payment source for birth procedure; live birth order; and infant sex. The y-axis reflects the estimated probability of sudden unexpected infant death (SUID) at the reference level of all covariates, as a function of maternal prepregnancy body mass index (BMI; calculated as weight in kilograms divided by height in meters squared).
Results for the 3 logistic regression models for the 3 respective ICD-10 cause of death codes are also shown in Table 3. The results for each of the 3 models mimicked the primary adjusted model, with increasing classes of obesity always associated with increasing SUID odds, although the aORs for code R99 reached statistical significance only for obesity classes II and III (class II obesity: aOR, 1.10; 95% CI, 1.00-1.21; class III obesity: aOR, 1.34; 95% CI, 1.21-1.49). In the model adjusting for gestational age at birth, short gestational periods were associated with increased SUID risk, consistent with prior research. Infants born at a gestational age between 28 and 31 weeks (aOR, 2.84; 95% CI, 2.60-3.11) and between 32 and 36 weeks (aOR, 1.93; 95% CI, 1.85-2.01) had higher SUID odds than infants born at 37 weeks’ gestation or later. However, the aORs for maternal BMI categories remained relatively unchanged after adjustment for gestational age, with infants born to mothers in all 3 obesity categories continuing to show statistically significantly and dose-dependent increased odds of SUID (class I obesity: aOR, 1.12; 95% CI, 1.06-1.17; class II obesity: aOR, 1.21; 95% CI, 1.14-1.28; and class III obesity: aOR, 1.39; 95% CI, 1.31-1.47). This suggests that the obesity-associated SUID risk was not mediated through gestational age.
Finally, adjusted PAFs for each BMI category are shown in Table 4. Each of the 3 obesity categories showed statistically significant contributions to SUID burden. Approximately 5.4% of SUID cases were attributable to maternal obesity, corresponding to approximately 179 SUID cases per year in the US.
Table 4. Population Attributable Fractions (PAFs) for Sudden Unexpected Infant Death.
| BMI category | PAF (95% CI)a |
|---|---|
| Underweight, <18.5 | 0.000 (−0.004 to 0.003) |
| Normal, 18.5-24.9 | Reference |
| Overweight, 25.0-29.9 | 0.001 (−0.008 to 0.009) |
| Obesity class | |
| I, 30.0-34.9 | 0.015 (0.008 to 0.023) |
| II, 35.0-39.9 | 0.016 (0.011 to 0.020) |
| III, ≥40.0 | 0.023 (0.020 to 0.027) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared.
PAFs are adjusted for maternal age, race, ethnicity, education, birthplace or nativity, marital status, and smoking status; paternal age and race; payment source for birth procedure; live birth order; and infant sex.
Discussion
Using whole-population data from the US, we found that maternal prepregnancy obesity was associated with increased SUID risk and that the association between maternal BMI and SUID risk was dose dependent. This association was maintained after adjustment for socioeconomic, demographic, physiological, and other factors commonly adjusted for in similar prior research. The GAM analysis showed that, after confounder adjustment, SUID risk increased monotonically across the entire BMI range; although the increase was modest below BMIs in the overweight range (25.0-29.9), it accelerated thereafter. To date, maternal obesity has not been considered a risk factor for SUID in expert reviews, in public education campaigns, or by the American Academy of Pediatrics.12,13,14,15,16 Based on our results, maternal obesity should be added to the list of known risk factors.
Although we found a robust dose-response association, we were not able to elucidate specific causal mechanisms that could link high maternal BMI to SUID. Some reviews have suggested that maternal obesity increases SUID risk while bed sharing.18,19 For example, the larger breast of an obese mother could occlude the infant’s airway if she fell asleep while breastfeeding, or a bed may sag more if the mother is obese, so that the infant may roll toward the mother, obstructing the infant’s soft airways. However, the suggestion that maternal obesity is a risk specifically in the context of bed sharing was made on the basis of a small number of case reports in which descriptions of overlaying deaths noted that the mother was obese.20,21 One case-control study (1987-1990) found that, although bed sharing was an independent risk factor for SIDS, this risk was not statistically significantly higher in the presence of maternal overweight or obesity. However, that study had only 42 SIDS cases among obese mothers and only 9.7% of the controls were obese, so associations may have been difficult to detect.22 Although a strength of our study is a large sample, we lacked an explicit measure of bed sharing, so the hypothesis that obesity increases the risk of SUID via suffocation while bed sharing was not testable with our data. Future research with sufficient statistical power and a direct measure of bed sharing should address this question.
Another potential causal mechanism is that obese mothers are also more likely to have obstructive sleep apnea (OSA), which is associated with a number of adverse outcomes, including preeclampsia, postpartum hemorrhage, maternal death, preterm birth, and neonatal intensive care unit admission.31,32 OSA can result in intermittent hypoxia, which is known to cause oxidative stress, in turn having detrimental consequences on fetal growth.33,34,35,36 Fetal growth restriction can increase the postnatal risk of neurodevelopmental and cardiometabolic disorders,37 which could cause an arousal defect, thus increasing the infant’s risk of death. The consequences on fetal development caused by obesity may therefore resemble those of maternal smoking, which has also been associated with vasoconstrictions and intermittent placental hypoxia.38
We found that approximately 5.4% of SUID cases were attributable to maternal obesity after controlling for confounders, assuming causality. Although this PAF is not as high as that for some other modifiable SUID risk factors, such as bed sharing and smoking,24,39 if obesity-associated risks were eliminated, this might have resulted in approximately 179 fewer SUID cases per year, assuming a causal chain between maternal obesity and SUID. Moreover, obesity is continuing to increase in prevalence globally and is thus poised to become an even larger concern in the coming years.40 Also, unlike some other risk factors, including social determinants of health such as race, high maternal BMI is potentially modifiable. Taken in the context of a prior study in Sweden suggesting that 11% of total infant deaths might be attributable to maternal overweight and obesity,6 our work underscores the importance of understanding the nature of and casual chain between obesity as a risk factor for SUID and other causes of infant death, so that appropriate interventions to mitigate these risks can be implemented. Such mitigations could lead to substantial reductions in overall infant mortality.
Limitations
Our study had several strengths and some limitations. This was a large, national population study with more than 16 000 SUID cases and 18 million births. Furthermore, maternal prepregnancy weight, although self-reported, was collected prior to the infant’s death and thus cannot introduce recall bias. In addition, we were able to adjust for a large number of confounders, and E-values suggest a limited role for potential residual confounding in explaining our results. Our study’s major limitation was that it did not include direct measures of bed sharing or sleep apnea; we were therefore unable to directly assess whether mechanical factors specific to bed sharing may have contributed to the obesity-SUID association, nor were we able to directly measure potential OSA-associated intermittent hypoxias. Identifying potential causal mechanisms remains an important area for future research.
Conclusions
This large, full-population study found that infants born to mothers with obesity were at increased risk of postperinatal SUID and that the association between increasing BMI and increasing risk was monotonic and dose dependent. Maternal prepregnancy obesity should be considered a risk factor for SUID. As the obesity epidemic in the US and around the world continues to accelerate, maternal obesity is likely to play an increasingly larger role in SUID prevalence over time. As our findings are observational, future work is needed to elucidate the specific causal mechanisms for the obesity-SUID association. Such research could lead to interventions that reduce or eliminate a nontrivial proportion of unexpected infant deaths.
Data Sharing Statement
References
- 1.Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Arch Dis Child Fetal Neonatal Ed. 2011;96(5):F378-F382. doi: 10.1136/adc.2009.170928 [DOI] [PubMed] [Google Scholar]
- 2.Valsamakis G, Kyriazi EL, Mouslech Z, Siristatidis C, Mastorakos G. Effect of maternal obesity on pregnancy outcomes and long-term metabolic consequences. Hormones (Athens). 2015;14(3):345-357. doi: 10.14310/horm.2002.1590 [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. doi: 10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodnar LM, Siminerio LL, Himes KP, et al. Maternal obesity and gestational weight gain are risk factors for infant death. Obesity (Silver Spring). 2016;24(2):490-498. doi: 10.1002/oby.21335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen A, Feresu SA, Fernandez C, Rogan WJ. Maternal obesity and the risk of infant death in the United States. Epidemiology. 2009;20(1):74-81. doi: 10.1097/EDE.0b013e3181878645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson S, Villamor E, Altman M, Bonamy AKE, Granath F, Cnattingius S. Maternal overweight and obesity in early pregnancy and risk of infant mortality: a population based cohort study in Sweden. BMJ. 2014;349:g6572. doi: 10.1136/bmj.g6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311(15):1536-1546. doi: 10.1001/jama.2014.2269 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . International Statistical Classification of Diseases, Tenth Revision (ICD-10). World Health Organization; 1992. [Google Scholar]
- 9.Azagba S, Manzione L, Shan L, King J. Trends in smoking during pregnancy by socioeconomic characteristics in the United States, 2010-2017. BMC Pregnancy Childbirth. 2020;20(1):52. doi: 10.1186/s12884-020-2748-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro-Mendoza CK, Parks S, Erck Lambert A, Camperlengo L, Cottengim C, Olson C. The epidemiology of sudden infant death syndrome and sudden unexpected infant deaths: diagnostic shift and other temporal changes. In: Duncan JR, Byard RW, eds. SIDS: Sudden Infant and Early Childhood Death: The Past, the Present and the Future. University of Adelaide Press; 2018:257-282. doi: 10.20851/sids-13 [DOI] [PubMed] [Google Scholar]
- 11.Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020;(392):1-8. [PubMed] [Google Scholar]
- 12.Hauck FR, Tanabe KO. Beyond “Back to Sleep”: ways to further reduce the risk of sudden infant death syndrome. Pediatr Ann. 2017;46(8):e284-e290. doi: 10.3928/19382359-20170721-01 [DOI] [PubMed] [Google Scholar]
- 13.Moon RY, Horne RSC, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370(9598):1578-1587. doi: 10.1016/S0140-6736(07)61662-6 [DOI] [PubMed] [Google Scholar]
- 14.Moon RY, Hauck FR. Risk factors and theories. In: Duncan JR, Byard RW, eds. SIDS: Sudden Infant and Early Childhood Death: The Past, the Present and the Future. University of Adelaide Press; 2018:169-186. doi: 10.20851/sids-10 [DOI] [PubMed] [Google Scholar]
- 15.Moon RY, Carlin RF, Hand I; Task Force on Sudden Infant Death Syndrome and the Committee on Fetus and Newborn . Sleep-related infant deaths: updated 2022 recommendations for reducing infant deaths in the sleep environment. Pediatrics. 2022;150(1):e2022057990. doi: 10.1542/peds.2022-057990 [DOI] [PubMed] [Google Scholar]
- 16.Eunice Kennedy Shriver National Institute of Child Health and Human Development. About safe sleep for babies: what are the known risk factors? Accessed December 8, 2023. https://safetosleep.nichd.nih.gov/about/risk-factors
- 17.Parks SE, DeSisto CL, Kortsmit K, Bombard JM, Shapiro-Mendoza CK. Risk factors for suffocation and unexplained causes of infant deaths. Pediatrics. 2023;151(1):e2022057771. doi: 10.1542/peds.2022-057771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan JR, Byard RW. Sudden infant death syndrome: an overview. In: Duncan JR, Byard RW, eds. SIDS: Sudden Infant and Early Childhood Death: The Past, the Present and the Future. University of Adelaide Press; 2018:15-50. doi: 10.20851/sids-02 [DOI] [Google Scholar]
- 19.Byard RW. Is co-sleeping in infancy a desirable or dangerous practice? J Paediatr Child Health. 1994;30(3):198-199. doi: 10.1111/j.1440-1754.1994.tb00618.x [DOI] [PubMed] [Google Scholar]
- 20.Bass M, Kravath RE, Glass L. Death-scene investigation in sudden infant death. N Engl J Med. 1986;315(2):100-105. doi: 10.1056/NEJM198607103150206 [DOI] [PubMed] [Google Scholar]
- 21.Gilbert-Barness E, Hegstrand L, Chandra S, et al. Hazards of mattresses, beds and bedding in deaths of infants. Am J Forensic Med Pathol. 1991;12(1):27-32. doi: 10.1097/00000433-199103000-00004 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell EA, Thompson JMD. Who cosleeps? does high maternal body weight and duvet use increase the risk of sudden infant death syndrome when bed sharing? Paediatr Child Health. 2006;11(suppl A):14A-15A. 19030235 [Google Scholar]
- 23.Lavista Ferres JM, Anderson TM, Johnston R, Ramirez JM, Mitchell EA. Distinct populations of sudden unexpected infant death based on age. Pediatrics. 2020;145(1):1-8. doi: 10.1542/peds.2019-1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson TM, Lavista Ferres JM, Ren SY, et al. Maternal smoking before and during pregnancy and the risk of sudden unexpected infant death. Pediatrics. 2019;143(4):e20183325. doi: 10.1542/peds.2018-3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu T, Hackett M, Simons H. Maternal nativity and sleep-related infant injury deaths in New York City. Early Hum Dev. 2015;91(1):13-17. doi: 10.1016/j.earlhumdev.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Min KB, Jung YJ, Min JY. Disparities in infant mortality by payment source for delivery in the United States. Prev Med. 2021;145:106361. doi: 10.1016/j.ypmed.2020.106361 [DOI] [PubMed] [Google Scholar]
- 27.Johnson DL, Carlo WA, Rahman AKMF, et al. Health insurance and differences in infant mortality rates in the US. JAMA Netw Open. 2023;6(10):e2337690. doi: 10.1001/jamanetworkopen.2023.37690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 29.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol. 2011;73(1):3-36. doi: 10.1111/j.1467-9868.2010.00749.x [DOI] [Google Scholar]
- 30.Wood SN. Generalized Additive Models: An Introduction With R. 2nd ed. Chapman and Hall/CRC; 2017. doi: 10.1201/9781315370279 [DOI] [Google Scholar]
- 31.Liu L, Su G, Wang S, Zhu B. The prevalence of obstructive sleep apnea and its association with pregnancy-related health outcomes: a systematic review and meta-analysis. Sleep Breath. 2019;23(2):399-412. doi: 10.1007/s11325-018-1714-7 [DOI] [PubMed] [Google Scholar]
- 32.Passarella E, Czuzoj-Shulman N, Abenhaim HA. Maternal and fetal outcomes in pregnancies with obstructive sleep apnea. J Perinat Med. 2021;49(9):1064-1070. doi: 10.1515/jpm-2020-0551 [DOI] [PubMed] [Google Scholar]
- 33.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3(1):28. doi: 10.1186/1477-7827-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joo EH, Kim YR, Kim N, Jung JE, Han SH, Cho HY. Effect of endogenic and exogenic oxidative stress triggers on adverse pregnancy outcomes: preeclampsia, fetal growth restriction, gestational diabetes mellitus and preterm birth. Int J Mol Sci. 2021;22(18):10122. doi: 10.3390/ijms221810122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands JL. Oxidative stress in placental pathology. Placenta. 2018;69:153-161. doi: 10.1016/j.placenta.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 36.Taysi S, Tascan AS, Ugur MG, Demir M. Radicals, oxidative/nitrosative stress and preeclampsia. Mini Rev Med Chem. 2019;19(3):178-193. doi: 10.2174/1389557518666181015151350 [DOI] [PubMed] [Google Scholar]
- 37.Colson A, Sonveaux P, Debiève F, Sferruzzi-Perri AN. Adaptations of the human placenta to hypoxia: opportunities for interventions in fetal growth restriction. Hum Reprod Update. 2021;27(3):531-569. doi: 10.1093/humupd/dmaa053 [DOI] [PubMed] [Google Scholar]
- 38.Ion R, Bernal AL. Smoking and preterm birth. Reprod Sci. 2015;22(8):918-926. doi: 10.1177/1933719114556486 [DOI] [PubMed] [Google Scholar]
- 39.Mitchell EA, Thompson JM, Zuccollo J, et al. The combination of bed sharing and maternal smoking leads to a greatly increased risk of sudden unexpected death in infancy: the New Zealand SUDI nationwide case control study. N Z Med J. 2017;130(1456):52-64. [PubMed] [Google Scholar]
- 40.Afshin A, Forouzanfar MH, Reitsma MB, et al. ; GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13-27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement