Abstract
Background
The selection of an appropriate chemical solution in wound care reduces the severity of wounds and accelerates the healing process. Povidone-iodine (PV-I), a chemical solution popularly known as an antiseptic, is frequently used in studies of wound care to prevent wound infection and accelerate woud the process of wound healing.
Objective
To identify the latest evidence on the benefits of PV-I in wound infection prevention and healing in all areas.
Methods
This review is a scoping review by Arskey and O’Malley and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) is used for reporting. The literature search used databases including PubMed, CINAHL, and search engines such as Scopus. This study was conducted using thematic analysis.
Results
This review yielded 19 out of 2109 studies that were identified in the initial search. Four outcomes comprised 20 sub-outcomes have been identified. Although strong evidence is lacking, PV-I in the form of foam is effective in improving wound healing, shortening healing time, and producing fewer adverse events than hydrocelluers. Moreover, saline and PV-I are effective as skin disinfectants when compared to saline alone in reducing surgical site infection (SSI), and they are also more effective than hypochlorous acid in improving wound healing. Nevertheless, there was no difference between PV-I and hypochlorous acid in the prevention of SSI and bacterial growth. In terms of healing time, silver foam and hyaluronic acid were more favorable than PV-I. Moreover, the use of chlorhexidine to improve SSI and silver dressing to improve hospital stay is more favorable than that of PV-I.
Conclusion
There is limited evidence regarding the effectiveness of wound care outcomes, and the efficacy of PV-I as a surface disinfectant for wound infection prevention remains controversial. Consequently, PV-I is not highly recommended for wound care, and further investigation into the effectiveness of PV-I as a surface disinfectant is required for various types of surgeries.
Keywords: disinfectant, povidone-iodine, surgical site infection, wound healing
Background
The biological process of wound healing is intricate and leads to recovery of tissue integrity. This process can be divided physiologically into four main stages: hemostasis, inflammation, proliferation, and tissue remodeling.1,2 Hemostasis involves cellular and molecular systems that act synergistically to repair ruptured blood vessels and prevent severe bleeding.3 To prevent bacterial contamination and clear the cellular debris of the wound, biochemical agents are released during the inflammatory response.4 Although the inflammatory phase of wound healing removes excess germs and debris, prolonged inflammation may damage tissues, hinder cell growth, and lead to chronic wounds.1 Once inflammation is controlled and the wound is clean, the proliferative stage begins with wound repair through angiogenesis, granulation, collagen deposition, epithelialization, and retraction. The final stage involves scar tissue maturation and epithelium formation, potentially lasting up to two years.1
Achieving successful wound healing requires a conducive environment for the wound. It has been widely reported that A variety of topical therapies, antimicrobial agents, and dressings have been widely reported to cover wounds, maintain optimal moisture levels, and absorb excessive exudate to support the healing process.1,4 The effectiveness of povidone-iodine (PV-I) as a treatment agent for facilitating wound healing has been evaluated in several studies. It has been argued that PV-I offers a wider range of antibacterial actions, strong antibiofilm efficacy, no cross-resistance or acquired bacterial resistance, minimal cytotoxicity, beneficial tolerability, and the capacity to accelerate wound healing. Within 1–5 min, PV-I exhibits bactericidal, fungicidal, and virucidal activity against encapsulated viruses.5,6 As such, the PV-I solution has been used as a disinfectant for the treatment of minor skin and mucous membrane wounds.5 However, PV-I has not been suggested for use in chronic wounds because of its cytotoxicity and lack of synergy with silver dressings.7
Moreover, PV-I is used in surgical applications to prevent surgical site infections (SSI). SSI represents a prevalent type of hospital-acquired infection, comprising 14–16% of infections among hospitalized individuals and 38% of infections among patients who have undergone surgery.8 The approach to minimize SSI encompasses pre-operative, perioperative, and post-operative outcomes. According to the Surgical Site Infection Prevention Guidelines by the Centers for Disease Control and Prevention (CDC), PV-I and chlorhexidine (CHX) are deemed appropriate for cleaning the skin preoperatively to lower the risk of SSI without giving preference to either disinfectant.9 While some studies suggest that CHX may be favorable to PV-I in reducing the incidence of SSI,10,11 other studies of PV-I have observed significant effectiveness.12,13 This finding remains inconsistent with the efficacy of povidone-iodine in SSI prevention. Therefore, this study aimed to identify the use of PV-I to prevent wound infection and improve wound healing outcomes in all areas using the latest evidence. This study is necessitated by the growing body of conflicting evidence, aiming to map out the diverse findings and synthesize the latest data to clarify PV-I’s current standing in medical practice. This study aimed to provide healthcare professionals with a comprehensive overview of safer and more effective clinical decisions.
Methods
Study Design
The Arskey and O’Malley methodology framework was used to conduct this scoping review. The framework consisted of five steps: (1) identifying the research questions, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collating, summarizing, and reporting the results. Preferred reporting items for systematic reviews and the meta-analysis extension for coping reviews (PRISMA-ScR) were used in this study.14
Eligibility Criteria
The inclusion of this study including (1) patient with any wound (acute, chronic, or post-surgery) (1) focused on the use of PV-I (3) comparation in this study was any modality in wound care (4) the outcome focused on wound infection prevention and wound healing (5) were published in English between 2019 and 2023. Moreover, studies were excluded if they met the following criteria: (1) study protocols, (2) lacked full text, (3) used in vitro or in vivo samples, (4) case reports and/or case studies (5) were performed for device development, and (6) PV-I was not applied to the individuals’ skin or any other tissues.
Electronic databases, such as PubMed, CINAHL, and Scopus, were used to search for relevant studies. Search terms include (“wound care” or “wound healing” or “wound management” or “wound treatment”) and (“povidone iodine” or “povidone-iodine” or “polyvinylpyrrolidone iodine” or “polyvinylpyrrolidone-iodine” or “polyvidone iodine” or “polyvidone-iodine” or “iodopovidone”) were used using Boolean operator system.
Data Selection
All articles retrieved in the initial search were imported into the Zotero reference management tool. The article was evaluated based on its title and abstract, and duplicate abstracts were eliminated. Then, using the inclusion and exclusion criteria for the study, the two reviewers (HHarun and HHaroen) independently evaluated the eligibility of the articles. Discussion and consensus were used to resolve differences in the selected studies.
Data Extraction and Analysis
Data from the selected studies were gathered and analyzed by two reviewers using a data extraction tool. The extracted data contained information pertaining to the authors, year, country, study design, type of wound, sample size, materials used, and substantial findings relevant to the study objectives. After each reviewer independently extracted the data using the data charting table, they discussed whether the process was consistent with the objective of the study. Thematic analysis was used to construct an organized theme. The results were collated to provide a summary of the findings.
Results
Study Selection Result
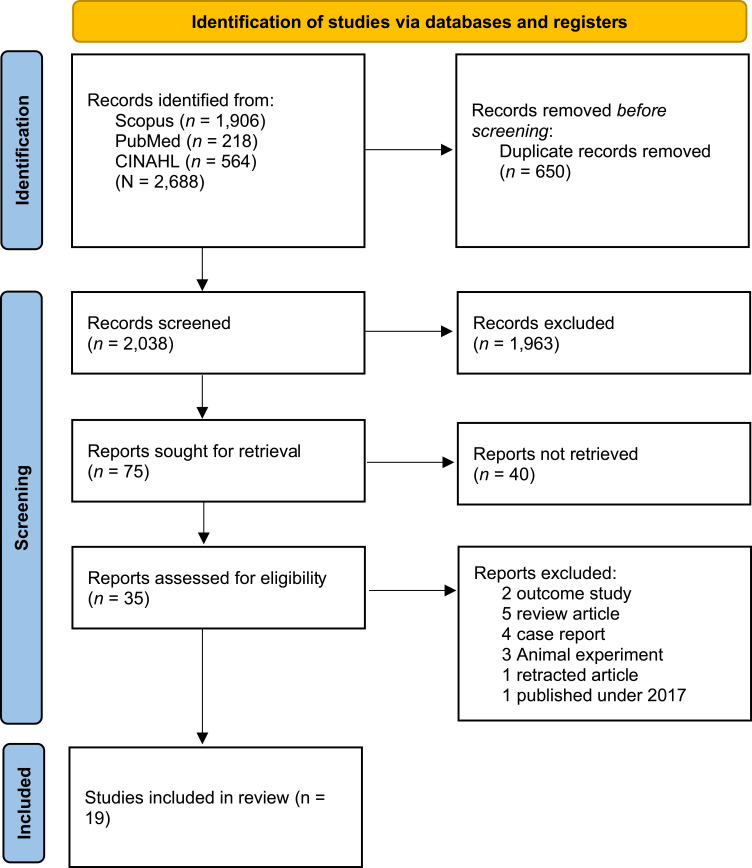
A thorough search across predetermined databases has yielded a significant number of studies. Initially, we retrieved 2327 records from the three databases. Of the retrieved articles, 281 were duplicates and were removed, resulting in 2046 articles for further screening. Then 75 articles were selected for further reading and screening, resulting in only 16 that could be fully assessed for inclusion in the review. Sixteen studies were excluded owing to several factors: two outcome studies, five review articles, four case reports, three animal experiments, one retracted article, and one article published in 2017. Ultimately, nineteen nine studies met the inclusion criteria and were included in our analysis15–33 (Figure 1).
Figure 1.
PRISMA flow diagram.
Notes: Adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.
Characteristic of Included Studies
This review found that the study was conducted across various countries, including the United States of America (USA) (n=4), South Korea (n=3), Turkey (n=3), Spain (n=1), Mali (n=1), Egypt (n=1), Argentina (n=1), Australia (n=1), Italy (n=1), China (n=1), and Germany (n=1). The included studies were randomized controlled trials (RCT) (n=13), retrospective studies (n=4), cohort studies (n=1), case-control studies (n=1), and one multicenter study conducted in the USA, Spain, and Canada. The sample included seven studies involving post-operative patients (including toenail surgery, cesarean section, fractures, spinal surgery, head and neck cancer surgery, lower limb trauma, and split-thickness skin graft), two studies on chronic wounds (specifically diabetic foot ulcers), two studies on acute wounds (pemphigus vulgaris and abscesses), six studies involving pre-operative patients administered surface disinfectants, and one study that did not specify the patient types involved. Additionally, there were 13 control modalities for PV-I treatment, including silver foam dressing, hyaluronic acid cream, topical honey, topical drugs, CHX, vancomycin powder, AF-SSD, saline, dakin (Sodium Chloride), standard treatment, hypochlorous acid, and hydrocellular foam dressing.15–33 Detailed characteristics of the studies are presented in Table 1.
Table 1.
Characteristic of Included Studies
| No | Author (Year) | Country | Study Design | Age (years) | Objectives | Type of Wound | Intervention and Sample Size | |
|---|---|---|---|---|---|---|---|---|
| PI-group | Control Group | |||||||
| 1 | Park et al (2023)22 | South Korea | A prospective, randomized non-inferiority trial | PI: 62.7±13.4 Non-PI: 65.7±15.3 |
To compare the effectiveness of PV-I foam dressing and silver foam dressing in the treatment of pressure ulcers. | Pressure ulcers | 3% PV-I dressing (n=40) | Silver foam dressing (n=40) |
| 2 | Lopezosa-Reca et al (2023)16 | Spain | A randomized clinical trial | PI: 24.19±10.15 Non-PI: 19.81±7.84 |
To evaluate the effectiveness of PV-I gel and hyaluronic acid cream in post-operative cures. | Ingrown toenail post-operative wound | PV-I gel (n=35) | 2% pure hyaluronic acid cream (n=35) |
| 3 | Bocoum et al (2023)29 | Mali | A prospective cohort study | <19 to ≥35 | To compare the effectiveness of antibiotics + PV-I and medical-grade honey on caesarean section wound healing and post-operative complication. | Caesarean section wound | Systemic antibiotics + topical PV-I (n=383) | Topical treatment with medical-grade honey (n=383) |
| 4 | Slobogean et al (2022)18 | USA, Spain, and Canada | A multiple period, cluster randomized, crossover trial | PI: 45.2±18.2 Non-PI: 44.5±17.9 |
To evaluate whether aqueous PV-I is more efficient than chlorhexidine gluconate at preventing surgical site infection. | Open extremity fracture post-operative wound | 10% aqueous PV-I (n=828) | 4% chlorhexidine gluconate (n=810) |
| 5 | Skeith et al (2021)17 | USA | A retrospective study | PI: 48.7±12.2 Non-PI: 48.1±12.2 |
To evaluate the effectiveness of pre-operative vaginal preparation with PV-I or chlorhexidine before hysterectomy in reducing the incidence of post-operative infection problems. | Hysterectomy wound | PV-I (n=2935) | Chlorhexidine (n=2935) |
| 6 | Roshdy et al (2021)30 | Egypt | A retrospective study | 45–72 | To assess the effectiveness of PV-I and hydrogen peroxide on the single-session protocol of CIED pocket infection elimination. | CIED pocket infection | 10% PV-I | 3% hydrogen peroxide |
| 7 | Roberto et al (2021)28 | USA | A retrospective study | 1.6–21 | To investigate if PV-I irrigation, intrawound vancomycin powder, or a combination of these treatments could reduce infection rates after paediatric deformity surgery. | Spinal surgery wound | 30 cc PV-I per liter irrigation (n=13) | 2 gram vancomycin powder (n=180) |
| 8 | Prezzavento et al (2021)32 | Argentina | A single-blinded randomized quantification experiment | PI: 53 Non-PI-1: 48 Non-PI-2: 45 |
To evaluate the effectiveness of an AF-SSD, PV-I, and ethanol in removing or regulating microbiological contamination from suture threads. | Postsurgical sutures | 10% PV-I (n=21) | Group 1: 96% ethanol (n=24) Group 2: AF-SSD (n=27) |
| 9 | Gwak et al (2020)31 | South Korea | A multicentre, open-labelled, randomized study | PI: 64.5±11.1 Non-PI: 60.7±12.8 | To compare the effectiveness of Betafoam, a novel PV-I foam dressing, and Medifoam, a foam dressing, in the treatment of diabetic foot ulcers. | Diabetic foot ulcers | Polyurethane + 3% PV-I foam dressing (n= 36) | Saline gauze (n= 35) |
| 10 | Dior et al (2020)15 | Australia | A double-blind randomized clinical trial | PI: 36.1±10.8 Non-PI: 35.5±10.3, |
To evaluate and contrast the impact on surgical site infection rates in patients undergoing gynaecological laparoscopies of three different skin pre-operative preparations (alcohol-based chlorhexidine, alcohol-based PV-I, and water-based povidone iodine). | Gynaecological laparoscopy wound | Water-based PV-I (n=214) | Alcohol-based chlorhexidine (n=210) |
| 11 | Unver et al (2019)24 | Turkey | A retrospective study | 27–88 | To contrast the impact of Dakin solution and PV-I solution on patient outcomes using the FGSI. | Fournier gangrene | PV-I solution (n=29) | Dakin solution (n=28) |
| 12 | Schmitz et al (2019)25 | USA | A randomized controlled trial | PI: 41.33±14.82 Non-PI: 41.76±13.57 |
To evaluate the viability of utilising a PV-I topical antiseptic solution as a therapeutic adjuvant in the treatment of superficial skin abscesses after incision and drainage. | Superficial skin abscesses | PV-I (n=52) | Standard treatment (dressing change) (n=49) |
| 13 | Lakhi et al (2019)19 | USA | A block randomized comparator-controlled, open-label trial | PI: 32.61±5.22 Non-PI: 32.49±5.56 |
To evaluate the effectiveness of PV-I and chlorhexidine gluconate in caesarean delivery vaginal cleaning. | Caesarean section wound | 10% PV-I solution (n=590) | 4% chlorhexidine gluconate solution (n=524) |
| 14 | Karuserci et al (2019)26 | Turkey | A prospective, randomized, controlled study | PI: 33.92±11.09 Non-PI: 36.26±11.33 |
To research the impact of irrigation with PV-I and subcutaneous rifampicin on incisional surgical site infection. | Gynaecologic surgery wound | Saline + 10% PV-I (n=100) | Saline (n=100) |
| 15 | Di Stadio et al (2019)27 | Italy | A case-control study | 60–75 | To determine whether Knutson’s solution, which combines PV-I and sugar, is effective at speeding up the healing of wounds in patients who had radio-chemotherapy administered after having surgery for head and neck cancer. | Wound dehiscence on head and neck cancer post-operative | PV-I + sugar (n=18) | Traditional topical drugs (n=16) |
| 16 | Chen et al (2021)33 | China | A Randomized controlled trial | ≥ 18 | To compare wet-to-dry PV-I dressings versus wet silver dressings for wound healing in pemphigus vulgaris patients | Skin erosions covering 10–70% of the body surface area. | Wet-to-dry PV-I dressings (n=30) | Wet silver dressings (n=28) |
| 17 | Ritter et al (2020)20 | Germany | A Randomized controlled trial | PI: 50.5±1.3 Non-PI: 51.1±1.6 |
To determine the clinical effectiveness of Chlorhexidine versus PV-I in reducing post-operative wound complication rates | Wound after aseptic lower limb trauma surgery | 1% PV-I and 50% 2-propanol (n=167) | 2% Chlorhexidine and 70% isopropyl alcohol (n=112) |
| 18 | Go¨zu¨ku¨¸uc¨k et al (2022)23 | Turkey | A Randomized controlled trial | PI: 15.46±10.58 Non-PI: 12.63±10.00 |
To compare PV-I and hypochlorous acid (HCOL) as surface disinfectants in neonatal circumcision | Wound after neonatal circumcision | PV-I (n=32) | Hypochlorous acid (28) |
| 19 | Pak et al (2019)21 | South Korea | Open-label randomized controlled trial with multicentre | PI: 55.5 (17.0–85.0) Non-PI: 54.0 (19.0–86.0) |
To compare the efficacy PV-I foam dressing versus hydrocellular foam dressing (Allevyn) in the management of split-thickness skin graft donor sites | Wound after split-thickness skin graft | PV-I foam dressing (n=31) | Hydrocellular foam dressing (n=33) |
Abbreviations: AF-SSD, aerosol formulation of silver sulfadiazine + vitamin A + and lidocaine; CIED, cardiovascular implantation electronic devices; FGSI, Fournier gangrene severity index; NR, not reported; PV-I, povidone-iodine; rh-EGF, recombinant human epidermal growth factor; THA, total hip arthroplasty; TKA, total knee arthroplasty; UK, United Kingdom; USA, United States of America; VAS, visual analog scale.
Outcome of Povidone-Iodine Compared to Other Various Agents
The outcomes of PV-I including four main categories and 20 subcategories. A detailed breakdown of the outcomes and sub-outcomes of PV-I use is shown in Table 2 and Figure 2. This study highlights its varied impacts on wound care. In infection prevention, PV-I is more effective in preventing surgical site and local infections than agents such as CHX and saline, with some studies suggesting comparable or inferior performance. Healing outcomes indicate that, while PV-I supports wound healing, alternative agents such as hyaluronic acid and silver dressings may enhance healing speed and effectiveness, especially in chronic wounds. Clinical effectiveness varies, with PV-I performing similarly or less effectively in reducing wound size and improving clinical indicators such as pain and bleeding compared to newer moisture-maintaining dressings. Adverse events are generally few, although PV-I occasionally leads to more skin irritation than less cytotoxic treatments. This comprehensive synthesis underscores the specific contexts in which PV-I may be most beneficial and in which alternative treatments could offer better outcomes.
Table 2.
Outcome and Sub-Outcome of Included Studies
| Outcome | Sub-outcome | Statistical value | Study | ||
|---|---|---|---|---|---|
| PI group (Even/ Mean [SD] – total sample] | Non-PI group (Even/ Mean [SD] – total sample] | p-value | |||
| Wound outcome | Wound healing | 10 (40) | 7 (40) | NA | Park et al (2023)22 |
| 16 (18)^ | 3 (16) | NA | Di Stadio et al (2019)15 | ||
| 9 (32)^ | 2 (28) | 0.036* | Gozukuuck et al (2022)23 | ||
| 26 (31)^ | 12 (33) | 0.0001* | Pak et al (2019)21 | ||
| Wound size reduction | 41.6 ± 35.3% | 49.7 ± 38.2% | 0.327 | Pak et al (2023)22 | |
| Healing time (days) | 27.0 ± 9.3 | 18.0 ± 2.8^ | 0.0073* | Pak et al (2023)22 | |
| 26.17 ± 7.75 | 22.42 ± 2.41^ | 0.007* | Lopezosa-Reca et al (2023)16 | ||
| 24.54 ± 8.168 | 19.12 ± 7.760 | NA | Bocoum et al (2023)29 | ||
| 31.0 ± 14.1 | 33.3 ± 12.6 | 0.6541 | Gwak et al (2020)31 | ||
| 55.00 ± 16.64 | 43.72 ± 11.85^ | 0.008* | Chen et al (2021)33 | ||
| 12.74 ± 3.51^ | 16.61 ± 4.45 | 0.0003* | Pak et al (2019)21 | ||
| Infection prevention | Surgical site infection | 59 (787) | 58 (784) | 0.61 | Slobogean et al (2022)18 |
| 61 (383) | 55 (383) | NA | Bocoum et al (2023)29 | ||
| 364 (18,184) | 81 (3018) | 0.07 | Skeith et al (2021)17 | ||
| 0 (13) | 5 (180) | NA | Roberto et al (2021)28 | ||
| 34 (214) | 38 (210) | 0.62 | Dior et al (2020)15 | ||
| 12 (590) | 3 (524)^ | 0.039* | Lakhi et al (2019)19 | ||
| 6 (100)^ | 12 (100) | NA | Karuserci et al (2019)26 | ||
| 9 (167) | 2 (112) | 0.073 | Ritter et al (2020)20 | ||
| 2 (32) | 3 (28) | 0.533 | Gozukuuck et al (2022)23 | ||
| Infection (non-surgery) | 3 (25) | 1 (25) | 0.302 | Chen et al (2021)33 | |
| Recurrent infection | 1 (12) | NA | NA | Rosdhy et al (2021)30 | |
| Local infection | 4 (36) | 4 (35) | NA | Gwak et al (2020)31 | |
| Superficial pus discharge | 49 (383) | 31 (383)^ | < 0.05* | Bocoum et al (2023)29 | |
| Deep pus discharge | 30 (383) | 6 (383)^ | < 0.05* | Bocoum et al (2023)29 | |
| Microbial contamination | Much higher | Much lower^ | NA | Prezzavento et al (2021)32 | |
| Bacterial growth | 12 (32) | 9 (28) | 0.755 | Gozukuuck et al (2022)23 | |
| Clinical | Perceived pain | 0.14 ± 0.49 | 0.19 ± 0.47 | 0.63 | Lopezosa-Reca et al (2023)16 |
| Bleeding | 5 (383)^ | 4 (35) | < 0.05* | Bocoum et al (2023)29 | |
| 3 (32) | 13 (383) | 0.755 | Gozukuuck et al (2022)23 | ||
| Superficial skin abscess | 8 (39) | 8 (41) | 0.96 | Schmitz et al (2019)25 | |
| Post-operative fever | 15 (590) | 14 (524) | 0.895 | Lakhi et al (2019)19 | |
| Hospital stays (median, IR) | 13 (3–34) days^ | 20 (1–41) days | NA | Unver at al (2019)24 | |
| 43.64 ± 10.54 days | 33.72 ± 9.61^ | 0.001 | Chen et al (2021)33 | ||
| Readmission | 3 (590) | 3 (524) | 0.999 | Lakhi et al (2019)19 | |
| Safety | Skin related AE at site | 1 (36) | 0 (35) | 1.000 | Gwak et al (2020)31 |
| 0 (34) | 1 (35) | NA | Pak et al (2019)22 | ||
| 6 (35) | 1 (35) | 0.1987 | Gwak et al (2020)31 | ||
| Serious AE | 31 (52) | 13 (49)^ | <0.001 | Schmitz et al (2019)25 | |
| AE | 12 (34)^ | 21 (35) | NA | Pak et al (2019)21 | |
Note: *Significant, ^favorable.
Abbreviations: AE, Adverse event; PVI, Povidone-Iodine; NA, Not available.
Figure 2.
Overview of povidone-iodine effectiveness in various wound types and clinical settings.
Infection Prevention
Several sub-outcomes were identified for infection prevention, including surgical site infection (SSI), local infection, superficial discharge, mycobacterial contamination, and bacterial growth. Only one study demonstrated that the use of PV-I and saline was more effective than saline alone in preventing SSI, although the level of significance was not reported.26 Conversely, one study reported that CHX was significantly more effective than PV-I in preventing SSI (p = 0.039).19 However, the majority of the evidence suggests that there is no significant difference between the administration of PV-I and CHX, although CHX still shows slightly favorable results.17,18,20 Additionally, there was no significant difference in the administration of PV-I compared with topical honey,15 vancomycin,28 and hypochlorous acid in preventing SSI.23
In terms of acute and chronic wounds, there was no significant difference between the administration of PV-I and silver foam dressing in acute wounds of pemphigus vulgaris,33 and there was no significant difference in the incidence of infection after the administration of PV-I solution compared with saline solution in diabetic wounds.31 Additionally, there was no significant difference in the incidence of recurrent infection after administration of PV-I compared with hydrogen peroxide.30
Moreover, in terms of the clinical signs of infection, the application of topical honey on post-operative cesarean section wounds is more effective than PV-I in reducing superficial and deep discharge.29 Additionally, the use of ethanol and Aerosol Formulation of Silver Sulfadiazine, Vitamin A, and Lidocaine (AF-SSD) compared to PV-I demonstrated fewer microbial contaminations based on microscopic observation;32 however, the administration of PV-I compared to hypochlorous acid does not show a significant difference in bacterial growth in post-operative preparation or surface disinfectant use.23
Healing Outcome
The administration of PV-I is significantly more effective in improving wound healing than the use of hypochlorous acid as a surface disinfectant,23 hydrocellular foam dressing for diabetic wounds,21 and topical drugs for post-operative wound care.27 However, silver foam dressing for diabetic foot ulcers is more effective than PV-I in enhancing wound healing.22
Regarding healing time outcomes, the administration of PV-I was significantly more effective than hydrocellular foam dressing.21 However, the application of hyaluronic acid cream to post-operative wounds and silver foam dressing for the treatment of pemphigus vulgaris is more effective than PV-I.33 There was no significant difference between the administration of PV-I and topical honey for post-operative wounds and saline solution for diabetic foot ulcers.29 In reducing wound size, the administration of silver foam dressing showed better results than PV-I, although there was no significant difference.22
Clinical Outcome
In terms of clinical outcomes, there are sub-outcomes, including bleeding, fever, pain, skin abscess, hospital stay, and readmission. The administration of PV-I is more effective than topical honey for post-operative wound care in reducing bleeding;29 however, there was no significant difference between the administration of PV-I and hypochlorous acid cream as a pre-operative surface disinfectant.23 There was no significant difference in the administration of PV-I in reducing fever compared to CHX,19 pain compared to hyaluronic acid cream,16 skin abscess,25 and readmission compared to CHX.19 However, silver foam dressing was significantly more effective than PV-I in reducing hospital stay in patients with pemphigus vulgaris.33
Adverse Event
The administration of PV-I is more favorable in reducing adverse events than the administration of hydrocellular foam dressing for post-operative wounds.21 However, the standard treatment includes instructing the patient on hand washing with soap and water, as well as wound care with regular dressing changes was significantly more effective than PV-I.25 Specifically, there was no significant difference in the occurrence of skin-related adverse events between the administration of PV-I and saline solution for diabetic ulcers31 and the administration of hydrocellular foam dressing post-operative.22
Discussion
This study had several findings. The outcome of PV-I compared with various therapeutic agents revealed significant findings across several domains. In infection prevention, PV-I shows comparable efficacy to other agents, such as CHX, topical honey, vancomycin, and hypochlorous acid, and CHX exhibits a slight advantage in preventing surgical site infections (SSI). In acute and chronic wounds, PV-I performs similarly to silver foam dressing and saline solution in managing pemphigus vulgaris and diabetic wounds, respectively. Clinical signs of infection favor the use of topical honey over PV-I for post-operative wounds, while microbial contamination rates are lower with ethanol and AF-SSD than with PV-I. Regarding healing outcomes, PV-I demonstrated superiority over hydrocellular foam dressing but lagged behind hyaluronic acid cream and silver foam dressing for certain wound types. In terms of clinical outcomes, PV-I effectively reduced bleeding but showed no significant advantage over other agents in managing fever, pain, skin abscess, or readmission rates. Moreover, silver foam dressing significantly reduced the hospital stay for patients with pemphigus vulgaris. Adverse event profiles favor PV-I over hydrocellular foam dressing, although standard treatment surpasses PV-I in terms of efficacy. Notably, PV-I exhibited comparable rates of skin-related adverse events to saline solution and hydrocellular foam dressings. Overall, although PV-I demonstrates efficacy across various outcomes, its superiority over alternative treatments varies depending on the specific clinical context.
Owing to the possibility of passing through the hemostasis, inflammation, proliferation, and remodelling phases rather quickly, surgical incisions are typically categorized as acute wounds.1 The best conditions for surgical wound healing involve a healthy wound environment and strict adherence to aseptic procedures. This study discovered six studies that examined the use of PV-I pre- or peri-operative to avoid post-operative infection. There is a lack of effectiveness of PV-I compared to CHX, and it was only more effective when compared to only applying it as a surface disinfectant pre-operative. This study is in line with a recent meta-analysis that concluded that CHX is more effective than PV-I.10,11,34 Although the difference was not statistically significant, individuals receiving rifampicin had fewer superficial incisional surgical site infections than those receiving PV-I. Nevertheless, another study in the following year demonstrated that the PV-I group had lower rates of superficial incision SII than the rifampicin or saline groups.35 In line with a previous meta-analysis, wound irrigation with antibiotics was effective in reducing the SII.36 Antibiotics, such as vancomycin, are known for their cost-effectiveness and bactericidal activity against common microorganisms implicated in SSI.37 However, some antibiotics, such as vancomycin, are not effective against most gram-negative bacteria.38
This study also evaluated the application of PV-I in post-operative wounds. In contrast to medical-grade honey, patients who received both systemic antibiotics and topical PV-I after caesarean section had fewer complications, such as superficial push discharge and wound bleeding.29 In addition, postsurgical suture threads treated with AF-SSD showed a significant reduction in the number of contaminated samples containing live microbial cells compared with those treated with PV-I.32 Confocal laser scanning microscopy demonstrated that postsurgical sutures treated with AF-SSD had much fewer adherent microbial cells than those treated with PV-I, with hardly any detectable microbial cells on the surface of the suture.32 Moreover, ingrown toenail post-operative wounds required a longer time to heal in the PV-I group than in the hyaluronic acid group.16 PV-I is not only used for operative wounds but may also be used for care in chronic wounds.
Chronic wounds require longer than expected times to complete the normal stages of wound healing.4 Vascular ulcers (such as venous and arterial ulcers), diabetic ulcers, and pressure ulcers are the three types of chronic wounds.39 Each of these wounds has some common characteristics, such as persistent infections, prolonged or severe inflammation, the development of drug-resistant microbial biofilms, and the inability of dermal and/or epidermal cells to react to reparative stimuli.4 This study found that 3% PV-I impregnated with polyurethane foam dressing (Betafoam) significantly accelerated the healing of diabetic foot ulcers over a period of eight weeks and was not shown to increase the length of time required for wound healing.31 They successfully demonstrated the role of betafoam in exudate control, antibacterial protection, and the maintenance of a moist wound environment.31 These findings were consistent with the results of a case study of older Asian patients with left sole ulcers that drained pus.40 They discovered that PV-I sugar ointment and artificial carbon dioxide foot bathing were effective treatments for both ischemic skin ulcers and bone and joint regeneration in ischemic limbs.40 Carbon dioxide foot bathing can improve the blood flow to the skin and muscles.40 PV-I sugar ointment, which is composed of up to 70% sugar and 3% PV-I, can accelerate wound healing by reducing contamination from bacteria, removing eschar quickly, likely nourishing surface cells, and filling in defects with granulation tissue and covering it with epithelium.40 However, patients who received only 3% PV-I dressing did not have a significantly reduced pressure ulcer rate that healed by more than 70% compared to the silver foam dressing group. PV-I in solution form compared to sodium hypochlorite solution also showed no significant difference in gangrene outcome.24 The findings of this study highlight that the combination of PV-I with another modality for chronic wounds has improved antibacterial, anti-biofilm, and anti-inflammatory properties.41,42
This study also found that PV-I was used in specific cases. This study found that PV-I and standard therapies were not significantly effective in patients with superficial skin abscesses.25 In addition, the proportion of patients in the standard treatment group who showed complete clinical recovery on days 7–10 and the occurrence of spread to household contact were both greater. Additionally, more new abscesses developed in the PV-I group.25
In terms of the adverse effects of PV-I use, a considerably higher rate of adverse events was observed in the group receiving PV-I than in the group receiving other modalities of care.25 Negative side effects included cold, cough, diarrhea, decreased appetite, dizziness, rash, burning, pain, pruritus, tape irritation, skin irritation around the wound, and skin discoloration.21,25,31 According to the European Guidelines, PV-I should not be administered to infants, young children, pregnant women, or breastfeeding mothers.7 Other contraindications include thyroid disorders and iodine irradiation. PV-I should not be used for longer than seven days, even if no thyroid issues have been reported.7
Implication for Practice
The implications of the study’s results are multifaceted and offer valuable insights for clinical practice. First, the comparable efficacy of PV-I to other agents underscores the slight variations in infection prevention outcomes. This suggests that PV-I remains a viable option in contexts in which CHX may not be available. Second, while PV-I performs adequately in managing acute and chronic wounds, its effectiveness falls short compared with silver foam dressing and hyaluronic acid cream in certain scenarios, highlighting the importance of tailoring treatment approaches based on wound characteristics. Moreover, the preference for topical honey over PV-I in reducing superficial and deep discharge postoperatively emphasizes the need to consider alternative therapies for specific indications. Furthermore, the observed reduction in adverse events with PV-I compared with hydrocellular foam dressing suggests its potential as a safer option for wound care. However, the superiority of standard treatments over PV-I in certain aspects underscores the importance of continued research to optimize therapeutic strategies. Overall, these findings underscore the nuanced nature of wound management and emphasize the significance of individualized treatment approaches guided by clinical evidence and patient-specific factors.
Given the complexity of wound care for chronic diseases, especially Diabetic Foot Ulcers (DFU), more advanced treatments are needed to improve the healing outcomes. Consequently, Platelet-Rich Plasma (PRP) therapy in standard care protocols for DFU management holds promise for enhancing patient outcomes and reducing the burden of this debilitating chronic condition. The current meta-analysis presents compelling evidence for the potential use of PRP as a promising modality for the treatment of chronic diseases, particularly DFU.2 PRP administration demonstrated a substantial improvement in wound healing rate, reduction in healing time, and shortened length of hospital stay.2 These findings underscore the potential of PRP as a cost-effective and efficacious treatment modality for DFU, offering improved healing outcomes, reduced hospitalization durations, and mitigated risks of amputation.
Limitation
This study provides insights into the effectiveness of PV-I in wound care; however, it is imperative to acknowledge its limitations. The included studies exhibited variability in sample sizes, study designs, and outcome measures, which may have affected the generalizability and comparability of the results. Furthermore, differences in PV-I concentrations and application methods across studies add another layer of complexity, potentially skewing the effectiveness outcomes. The methodology of this scoping review, although broad in scope, does not assess the quality of evidence and is confined to selected databases, possibly omitting significant studies due to restrictive search terms and language barriers. Such limitations highlight the need for more rigorous future studies with standardized outcome measures and expanded database searches to enhance the reliability and applicability of the findings in clinical practice.
Conclusion
A comprehensive analysis of povidone-iodine (PV-I) compared to various other agents revealed its efficacy across multiple domains of wound management. Although PV-I demonstrates comparable performance in infection prevention and wound healing, its effectiveness over alternative treatments varies depending on specific clinical contexts and outcomes. This study emphasized the importance of tailoring treatment approaches based on wound characteristics and patient factors. Moreover, the findings highlight the need for further research to evaluate the optimal use of PV-I and explore its potential synergies with other therapeutic modalities. Based on these results, clinicians should consider PV-I a valuable option for infection prevention and wound care, particularly in settings where alternatives may not be readily available or accessible. However, for specific indications such as post-operative wound care and diabetic ulcers, alternative therapies such as silver foam dressing or hyaluronic acid cream may offer better outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Singh S, Young A, McNaught CE. The physiology of wound healing. Surgery. 2017;35(9):473–477. doi: 10.1016/j.mpsur.2017.06.004 [DOI] [Google Scholar]
- 2.Platini H, Adammayanti KA, Maulana S, et al. The potential of autologous platelet-rich plasma gel for diabetic foot ulcer care among older adults: a systematic review and meta-analysis. Ther Clin Risk Manag. 2024;20(null):21–37. doi: 10.2147/TCRM.S433033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sira J, Eyre L. Physiology of haemostasis. Anaesth Intensive Care Med. 2016;17(2):79–82. doi: 10.1016/j.mpaic.2015.11.004 [DOI] [Google Scholar]
- 4.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560–582. doi: 10.1089/wound.2015.0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260–268. doi: 10.1016/j.ijsu.2017.06.073 [DOI] [PubMed] [Google Scholar]
- 6.Sauerbrei A. Bactericidal and virucidal activity of ethanol and povidone-iodine. Microbiologyopen. 2020;9(9):e1097. doi: 10.1002/mbo3.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babalska ZŁ, Korbecka-Paczkowska M, Karpiński TM. Wound antiseptics and European guidelines for antiseptic application in wound treatment. Pharmaceuticals. 2021;14(12). doi: 10.3390/ph14121253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong TS, Yee GT. Prospective multicenter surveillance study of surgical site infection after spinal surgery in Korea: a preliminary study. J Korean Neurosurg Soc. 2018;61(5):608–617. doi: 10.3340/jkns.2018.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–791. doi: 10.1001/jamasurg.2017.0904 [DOI] [PubMed] [Google Scholar]
- 10.Wang P, Wang D, Zhang L. Effectiveness of chlorhexidine versus povidone-iodine for preventing surgical site wound infection: a meta-analysis. Int Wound J. 2024;21(2):e14394. doi: 10.1111/iwj.14394 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Jalalzadeh H, Groenen H, Buis DR, et al. Efficacy of different preoperative skin antiseptics on the incidence of surgical site infections: a systematic review, GRADE assessment, and network meta-analysis. Lancet Microbe. 2022;3(10):e762–e771. doi: 10.1016/S2666-5247(22)00187-2 [DOI] [PubMed] [Google Scholar]
- 12.Shi L, Cai L, Wan F, Jiang Y, Choudhury R, Rastogi S. Does povidone-iodine application in surgical procedures help in the prevention of surgical site infections? An updated meta-analysis. Wideochir Inne Tech Maloinwazyjne. 2022;17(2):261–278. doi: 10.5114/wiitm.2021.112479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monstrey SJ, Govaers K, Lejuste P, Lepelletier D, Ribeiro de Oliveira P. Evaluation of the role of povidone-iodine in the prevention of surgical site infections. Surg Open Sci. 2023;13:9–17. doi: 10.1016/j.sopen.2023.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 15.Dior UP, Kathurusinghe S, Cheng C, et al. Effect of surgical skin antisepsis on surgical site infections in patients undergoing gynecological laparoscopic surgery: a double-blind randomized clinical trial. JAMA Surg. 2020;155(9):807–815. doi: 10.1001/jamasurg.2020.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopezosa-Reca E, Martínez-Nova A, Sánchez-Rodríguez R, et al. Effectiveness of hyaluronic acid in post-surgical cures following partial matricectomies with the phenol/alcohol technique: a randomized clinical trial. J Tissue Viability. 2023;32(1):59–62. doi: 10.1016/j.jtv.2023.01.010 [DOI] [PubMed] [Google Scholar]
- 17.Skeith AE, Morgan DM, Schmidt PC. Vaginal preparation with povidone-iodine or chlorhexidine before hysterectomy: a propensity score matched analysis. Am J Obstet Gynecol. 2021;225(5):560.e1–560.e9. doi: 10.1016/j.ajog.2021.08.035 [DOI] [PubMed] [Google Scholar]
- 18.Slobogean GP, Sprague S, Wells JL, et al. Aqueous skin antisepsis before surgical fixation of open fractures (Aqueous-PREP): a multiple-period, cluster-randomised, crossover trial. Lancet. 2022;400(10360):1334–1344. doi: 10.1016/S0140-6736(22)01652-X [DOI] [PubMed] [Google Scholar]
- 19.Lakhi NA, Tricorico G, Osipova Y, Moretti ML. Vaginal cleansing with chlorhexidine gluconate or povidone-iodine prior to cesarean delivery: a randomized comparator-controlled trial. Am J Obstet Gynecol MFM. 2019;1(1):2–9. doi: 10.1016/j.ajogmf.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 20.Ritter B, Herlyn PKE, Mittlmeier T, Herlyn A. Preoperative skin antisepsis using chlorhexidine may reduce surgical wound infections in lower limb trauma surgery when compared to povidone-iodine - a prospective randomized trial. Am J Infect Control. 2020;48(2):167–172. doi: 10.1016/j.ajic.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 21.Pak CS, Park DH, Oh TS, et al. Comparison of the efficacy and safety of povidone-iodine foam dressing (Betafoam), hydrocellular foam dressing (Allevyn), and petrolatum gauze for split-thickness skin graft donor site dressing. Int Wound J. 2019;16(2):379–386. doi: 10.1111/iwj.13043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park KH, Baek K, Kim M, Ju MJ, Jung WH, Yoon YS. A prospective, randomized, non-inferiority trial to compare the efficacy of 3% povidone-iodine foam dressing and silver foam dressing in the treatment of pressure injuries. J Wound Manag Res. 2023;19(1):13–20. doi: 10.22467/jwmr.2023.02376 [DOI] [Google Scholar]
- 23.Gözüküçük A, Çakıroğlu B. Comparison of hypochlorous acid and Povidone Iodine as a disinfectant in neonatal circumcision. J Pediatr Urol. 2022;18(3):341.e1–341.e5. doi: 10.1016/j.jpurol.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 24.Unver M, Kebapci E, Guner M, et al. Which method is better to provide wound healing in Fournier gangrene: dakin solution or conventional antiseptic dressings? A retrospective study. Int Surg. 2019;104(1–2):21–26. doi: 10.9738/INTSURG-D-16-00029.1 [DOI] [Google Scholar]
- 25.Schmitz G, Rosenblatt L, Salerno N, et al. Treatment data using a topical povidone-iodine antiseptic in patients with superficial skin abscesses. Data Brief. 2019;23:103715. doi: 10.1016/j.dib.2019.103715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karuserci ÖK, Sucu S, Özcan HÇ, et al. Topical rifampicin versus povidone-iodine for the prevention of incisional surgical site infections following benign gynecologic surgery: a prospective, randomized, controlled trial. New Microbiol. 2019;42(4):205–209. [PubMed] [Google Scholar]
- 27.Di Stadio A, Gambacorta V, Cristi MC, et al. The use of povidone‐iodine and sugar solution in surgical wound dehiscence in the head and neck following radio‐chemotherapy. Int Wound J. 2019. doi: 10.1111/iwj.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberto RF, Rowan FA, Nallur D, Durbin-Johnson B, Javidan Y, Klineberg EO. Povidone-iodine irrigation combined with vancomycin powder lowers infection rates in pediatric deformity surgery. Spine Deform. 2021;9(5):1315–1321. doi: 10.1007/s43390-021-00333-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bocoum A, Riel Van SJJM, Traoré SO, et al. Medical-grade honey enhances the healing of caesarean section wounds and is similarly effective to antibiotics combined with povidone-iodine in the prevention of infections—a prospective cohort study. Antibiotics. 2023;12(1):92. doi: 10.3390/antibiotics12010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roshdy H, Seaoud E, Elbelbesy R. Low‐budget, single‐session elimination of CIED pocket infection. Pacing Clin Electrophysiol. 2021;44(1):129–134. doi: 10.1111/pace.14133 [DOI] [PubMed] [Google Scholar]
- 31.Gwak HC, Han SH, Lee J, et al. Efficacy of a povidone‐iodine foam dressing (betafoam) on diabetic foot ulcer. Int Wound J. 2020;17(1):91–99. doi: 10.1111/iwj.13236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prezzavento GE, Mas CR, Achaval Rodríguez J, et al. Comparison of efficacy of povidone-iodine, ethanol, and an aerosol formulation of silver sulfadiazine in controlling microbial burden on sutures from clean surgeries. J Burn Care Res. 2021;42(5):975–980. doi: 10.1093/jbcr/irab022 [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Zou Q, Hamblin MR, Wen X. A preliminary clinical trial comparing wet silver dressings versus wet-to-dry povidone-iodine dressings for wound healing in pemphigus vulgaris patients. Dermatol Ther. 2021;34(3). doi: 10.1111/dth.14906 [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi N, Kamono E, Maeda K, Misumi T, Yukizawa Y, Inaba Y. Effectiveness of diluted povidone-iodine lavage for preventing periprosthetic joint infection: an updated systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1). doi: 10.1186/s13018-021-02703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karuserci ÖK, Balat Ö. Subcutaneous rifampicin versus povidone-iodine for the prevention of incisional surgical site infections following gynecologic oncology surgery - a prospective, randomized, controlled trial. Ginekol Pol. 2020;91(9):513–518. doi: 10.5603/GP.a2020.0134 [DOI] [PubMed] [Google Scholar]
- 36.Fu C, Meng L, Ma M, Li N, Zhang J. Effect of wound irrigation on the prevention of surgical site infections: a meta-analysis. Int Wound J. 2022;19(7):1878–1886. doi: 10.1111/iwj.13794 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Martin VT, Zhang Y, Wang Z, Liu QL, Yu B. A systematic review and meta-analysis comparing intrawound vancomycin powder and povidone iodine lavage in the prevention of periprosthetic joint infection of Hip and knee arthroplasties. J Orthop Sci. 2022. doi: 10.1016/j.jos.2022.11.013 [DOI] [PubMed] [Google Scholar]
- 38.Levine DP Vancomycin: a History. Available from: https://academic.oup.com/cid/article/42/Supplement_1/S5/275962. Accessed July 19, 2024.
- 39.Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech. 2014;7(11):1205–1213. doi: 10.1242/dmm.016782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hihara M, Fukui M, Mitsui T, Kakudo N, Kuro A. Osteolytic metatarsal osteomyelitis regenerated by combined treatment of artificial carbon dioxide foot bathing and povidone–iodine sugar ointment: a case report. J Med Case Rep. 2022;16(1):434. doi: 10.1186/s13256-022-03654-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alves PJ, Gryson L, Hajjar J, et al. Role of antiseptics in the prevention and treatment of infections in nursing homes. J Hosp Infect. 2023;131:58–69. doi: 10.1016/j.jhin.2022.09.021 [DOI] [PubMed] [Google Scholar]
- 42.Alves PJ, Barreto RT, Barrois BM, Gryson LG, Meaume S, Monstrey SJ. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int Wound J. 2021;18(3):342–358. doi: 10.1111/iwj.13537 [DOI] [PMC free article] [PubMed] [Google Scholar]