Abstract
Background:
We investigated differences in gonococcal antimicrobial susceptibility by anatomic site among cisgender men who have sex with men (MSM) using specimens collected through the Centers for Disease Control and Prevention’s enhanced Gonococcal Isolate Surveillance Project and Strengthening the US Response to Resistant Gonorrhea.
Methods:
During the period January 1, 2018–December 31, 2019, 12 enhanced Gonococcal Isolate Surveillance Project and 8 Strengthening the US Response to Resistant Gonorrhea sites collected urogenital, pharyngeal, and rectal isolates from cisgender MSM in sexually transmitted disease clinics. Gonococcal isolates were sent to regional laboratories for antimicrobial susceptibility testing by agar dilution. To account for correlated observations, linear mixed-effects models were used to calculate geometric mean minimum inhibitory concentrations (MICs), and mixed-effects logistic regression models were used to calculate the proportion of isolates with elevated or resistant MICs; comparisons were made across anatomic sites.
Results:
Participating clinics collected 3974 urethral, 1553 rectal, and 1049 pharyngeal isolates from 5456 unique cisgender MSM. There were no significant differences in the geometric mean MICs for azithromycin, ciprofloxacin, penicillin, and tetracycline by anatomic site. For cefixime and ceftriaxone, geometric mean MICs for pharyngeal isolates were higher compared with anogenital isolates (P < 0.05). The proportion of isolates with elevated ceftriaxone MICs (≥0.125 μg/mL) at the pharynx (0.67%) was higher than at rectal (0.13%) and urethral (0.18%) sites (P < 0.05).
Conclusions:
Based on data collected from multijurisdictional sentinel surveillance projects, antimicrobial susceptibility patterns of Neisseria gonorrhoeae isolates may differ among MSM at extragenital sites, particularly at the pharynx. Continued investigation into gonococcal susceptibility patterns by anatomic site may be an important strategy to monitor and detect the emergence of antimicrobial resistant gonorrhea over time.
Neisseria gonorrhoeae has been designated as an urgent antibiotic resistance threat-level pathogen in the United States since 2013.1,2 The organism’s ability to acquire antimicrobial resistance has complicated the treatment and control of N. gonorrhoeae.3,4 In the United States, the Gonococcal Isolate Surveillance Project (GISP) functions as the national surveillance system to monitor susceptibility trends in N. gonorrhoeae strains,5 providing critical data to inform the Centers for Disease Control and Prevention sexually transmitted infections treatment guidelines.6
Data from GISP are based on isolates collected from men presenting with gonococcal urethritis in selected public STD clinics, with the assumption that the antimicrobial susceptibility patterns among male urethral isolates are representative of all circulating strains in the United States.7 Although this sampling strategy was practical given the high culture yield of urethral gonorrhea, gonococcal infections at extragenital anatomic sites (i.e., rectum and pharynx) may have different susceptibility patterns. It is important to note that extragenital infections may facilitate the acquisition or development of resistance mutations.8–14 Notably, the pharynx may serve as an incubator of resistance owing to genetic reassortment and plasmid uptake of resistance genes between N. gonorrhoeae and other Neisseria species that colonize the pharynx.11–15 Furthermore, gonococcal infections at the pharynx are predominantly asymptomatic and, compared with urogenital and rectal infections, are more difficult to eradicate because of poor bioavailability at the pharynx, such that they may provide an optimal setting for selection of resistance mutations.16–19
Rates of gonorrhea have been increasing among men in the United States, including gay, bisexual, and other men who have sex with men (MSM), and high rates of resistance and elevated minimum inhibitory concentrations (MICs) have been observed historically in GISP and continue today among gonococcal isolates from MSM.5,20–24 Extragenital gonococcal infections are common among MSM; however, data comparing antimicrobial susceptibilities of urogenital and extragenital N. gonorrhoeae isolates are limited.25–30,31s Characterizing antimicrobial susceptibility patterns of gonococcal isolates among MSM is critical to ensure the development of adequate treatment approaches and ultimately to reduce the spread of antimicrobial-resistant N. gonorrhoeae. The CDC’s enhanced GISP (eGISP) and Strengthening the US Response to Resistant Gonorrhea (SURRG) expand antimicrobial-resistant N. gonorrhoeae surveillance by collecting specimens from multiple anatomic sites from persons attending STD clinics. In this analysis, we describe the antimicrobial susceptibility profiles of urogenital and extragenital isolates collected from cisgender MSM who attended participating STD clinics in 2018 to 2019 and investigate differences in susceptibility across anatomic sites.
METHODS
Study Design and Population
We conducted a cross-sectional analysis of cisgender MSM who attended participating STD clinics in 12 eGISP and 8 SURRG jurisdictions in 2018 to 2019 (Supplemental Text, http://links.lww.com/OLQ/A748). We included MSM with a positive gonococcal culture obtained from any anatomic site (urogenital, rectal, pharyngeal) and antimicrobial susceptibility testing (AST) performed by agar dilution. We restricted isolate collection to consecutive MSM who had related clinical and epidemiologic data submitted to CDC and whose records contained a unique patient ID. For the purpose of this analysis, MSM were defined as a patient who self-identified as male gender and reported having any male gender sex partners within the past 3 months; consequently, patients with self-reported female gender, transgender persons, and patients who reported only female, nonbinary, or unknown gender sex partners were excluded.
Isolate Collection
Per the eGISP protocol, urethral gonococcal isolates were collected from male patients presenting with symptomatic urethritis, and pharyngeal and rectal isolates from patients who reported sexual exposure at oral and/or rectal anatomic sites with a N. gonorrhoeae nucleic acid amplification test performed.7 Per the SURRG protocol, urethral, pharyngeal, and rectal isolates were collected from patients who reported sexual exposure at urogenital, pharyngeal, and/or rectal anatomic sites with a N. gonorrhoeae nucleic acid amplification test performed. For eGISP, per participating clinic, collection was limited to the first 25 isolates from male patients with symptomatic urethritis and the first 25 pharyngeal and rectal isolates from patients who reported sexual exposure at oral or rectal anatomic sites. There was no maximum collection limit for clinics participating in SURRG. There were no minimum monthly isolate contributions required by participating clinics in either project.
Laboratory Procedures
Specimens collected for N. gonorrhoeae isolation were inoculated on selective media and incubated at 36°C ± 1°C in 5% CO2 by local laboratory staff. Gonococcal isolates were subcultured on noninhibitory medium (e.g., chocolate agar) with 1% IsoVitalex to obtain a pure culture of the isolate and for subsequent species identification. For eGISP, local laboratories were only required to do presumptive identification; whereas, for SURRG, local laboratories also used matrix-assisted laser desorption-ionization time-of-flight mass spectrometry and API-NH assay for identification of N. gonorrhoeae.32s,33s After 18 to 20 hours of incubation, growth from the pure culture was suspended in trypticase soy broth containing 20% (vol/vol) glycerol and frozen to −70°C, and shipped monthly to 1 of 4 participating Antibiotic Resistance Laboratory Network (ARLN) regional laboratories. Antibiotic Resistance Laboratory Network regional laboratories performed N. gonorrhoeae AST by agar dilution. Antimicrobials tested included azithromycin (0.008–16 μg/mL), cefixime (0.002–1 μg/mL), ceftriaxone (0.001–1 μg/mL), ciprofloxacin (0.001–32 μg/mL), penicillin (0.008–64 μg/mL), and tetracycline (0.06–64 μg/mL). Isolates with a cefixime MIC of ≥1 μg/mL, ceftriaxone MIC of ≥1 μg/mL, or azithromycin MIC of ≥16 μg/mL were shipped to the CDC and tested to the end point MIC at the CDC Division of STD Prevention laboratory. All isolates were tested for β-lactamase activity using the nitrocefin test by the regional ARLN laboratories. Isolates with azithromycin MICs of ≥2 μg/mL, cefixime MICs of ≥0.25 μg/mL, or ceftriaxone MICs of ≥0.125 μg/mL were shipped from regional laboratories to the CDC for confirmatory AST. To ensure the accuracy of agar dilution AST results, control N. gonorrhoeae strains (F18/ATCC 49226, WHO L and WHO U) with known MICs were included for each susceptibility test run at both the regional ARLN laboratories and at the CDC Division of STD Prevention laboratory.34s,35s For additional quality assurance, ARLN regional laboratories participated in external quality assessment by testing a panel of 15 unidentified N. gonorrhoeae strains (provided by the CDC) twice yearly.
Interpretation of AST Results
Antimicrobial susceptibility testing results were reported as MIC for antigonococcal antimicrobials. The results were interpreted according to the criteria recommended by the Clinical and Laboratory Standards Institute for penicillin resistance (MIC ≥2 μg/mL or β-lactamase positive), ciprofloxacin resistance (MIC ≥1.0 μg/mL), and tetracycline resistance (MIC ≥2.0 μg/mL).34s The Clinical and Laboratory Standards Institute has not established the criteria for resistance to azithromycin, cefixime, and ceftriaxone. Therefore, the breakpoints used in these analyses to define elevated MIC for these antimicrobials include those previously described by the CDC GISP: azithromycin MIC ≥2.0 μg/mL, cefixime MIC ≥0.25 μg/mL, and ceftriaxone MIC ≥0.125 μg/mL.36s
Demographic, Epidemiologic, and Clinical Variables
Clinical and demographic data documented at the time of visit were abstracted from medical records by local clinic staff. Patients and each of their specimens were assigned a unique ID such that multiple specimens contributed by a single patient could be identified for a single visit and/or tracked across multiple clinic visits. In addition to anatomic site of infection, the following additional variables were included in this analysis: gender of sex partners (MSM exclusively; men who have sex with men and women), age, race/Hispanic ethnicity, and HIV status (self-reported and/or per medical chart). Given possible geographic differences in antimicrobial susceptibility patterns, we additionally categorized each clinic site by US census region (Northeast, Midwest, South, West) for stratified analyses.
Demographic, clinical, and epidemiologic data were linked to AST results at the CDC.
Data Analysis
We calculated frequencies of demographic and epidemiologic characteristics of cisgender MSM from whom N. gonorrhoeae isolates were collected. To describe antimicrobial susceptibility across anatomic sites, we calculated the MIC range, geometric mean MICs with 95% confidence intervals (CIs) for each antimicrobial by anatomic site, and the number and proportion of isolates with elevated or resistant MICs. We calculated the MIC distributions (percentage of isolates by MICs) by antimicrobial for pharyngeal, rectal, and urethral isolates. To account for correlated observations (i.e., multiple isolates provided by the same patients either at the same visit or across visits), we used linear mixed-effects models to calculate geometric mean MICs and mixed-effects logistic regression models to calculate the proportion of isolates with elevated or resistant MICs across anatomic sites. We used the likelihood ratio test to assess any MIC difference across anatomic sites (overall test); if the overall P value was less than 0.05, we performed post hoc pairwise comparisons among anatomic site pairs. We used the Holm-Bonferroni method (Holm’s sequential Bonferroni procedure) to control the family-wise error rate for the multiple comparisons. We also assessed geometric mean MICs and proportion with elevated or resistant MICs across anatomic sites of infection for each antimicrobial by geographic location using the statistical models described previously.
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.6.3. software (R Core Team, Vienna, Austria). Data were collected as sentinel surveillance activities and were exempt from CDC institutional review board review.
RESULTS
Characteristics of Population That Contributed Isolates
From January 1, 2018, to December 31, 2019, 13,363 isolates were collected from cisgender men attending participating STD clinics. Of these, 12,585 (94%) isolates were collected from men with known gender of sex partners; 6576 (49%) isolates were collected from 5456 unique cisgender MSM and are included in the analysis. Most participants contributed one isolate only (n = 4570; 83.8%). Among all participants, 13.1% (n = 714) contributed 2 isolates, 2.4% (n = 129) contributed 3 isolates, and <1% (n = 43) contributed more than 3 isolates. Most participants had only 1 clinic visit (n = 4964; 91.0%); the maximum number of clinic visits by any one MSM was 5 visits over the 2 years (n = 1).
Of the 6576 isolates, 3974 were urethral, 1553 were rectal, and 1049 were pharyngeal isolates (Table 1). There was variation in the geographic distribution of isolates across anatomic sites. Most pharyngeal and rectal isolates (62.3% and 60.4%, respectively) were contributed by participants in the West, whereas most urethral isolates were collected in the Northeast (40.4%) and West (40.0%) regions. Across anatomic sites, more than 90% of isolates were collected from MSM who only identified recent male sex partners. There was some variation in the distribution of ages and race/Hispanic ethnicity of MSM from whom isolates were collected across anatomic sites. For example, 16.9% of urethral isolates were from MSM aged 20 to 24 years, whereas 22.7% of pharyngeal isolates were from MSM aged 20 to 24 years. Overall, 17.5% of isolates were collected from MSM who had a positive HIV status documented at the time of isolate collection; this varied according to anatomic site of infection (e.g., 19.4% of urogenital isolates vs. 11.1% of pharyngeal isolates).
TABLE 1.
Descriptive Characteristics of Men Who Have Sex With Men From Whom Neisseria gonorrhoeae Isolates Were Collected by Anatomic Site of Gonococcal Infection: eGISP and SURRG, 2018 to 2019
| Total Isolates (n = 6576), n (Col %) | Urethral (n = 3974), n (Col %) | Rectal (n = 1553), n (Col %) | Pharyngeal (n = 1049), n (Col %) | |
|---|---|---|---|---|
| Region* | ||||
| Northeast | 2103 (32.0) | 1606 (40.4) | 290 (18.7) | 207 (19.7) |
| Midwest | 1003 (15.3) | 620 (15.6) | 236 (15.2) | 147 (14.0) |
| South | 281 (4.3) | 160 (4.0) | 60 (3.9) | 61 (5.8) |
| West | 3189 (48.5) | 1588 (40.0) | 967 (62.3) | 634 (60.4) |
| Gender of sex partners | ||||
| Men only | 5976 (90.9) | 3560 (89.6) | 1462 (94.1) | 954 (90.9) |
| Men and women | 600 (9.1) | 414 (10.4) | 91 (5.9) | 95 (9.1) |
| Age, y | ||||
| <20 | 306 (4.7) | 158 (4.0) | 94 (6.1) | 54 (5.2) |
| 20–24 | 1242 (18.9) | 672 (16.9) | 332 (21.4) | 238 (22.7) |
| 25–29 | 1886 (28.7) | 1132 (28.5) | 440 (28.3) | 314 (30.0) |
| 30–34 | 1301 (19.8) | 795 (20.0) | 307 (19.8) | 199 (19.0) |
| 35–39 | 668 (10.2) | 437 (11.0) | 137 (8.8) | 94 (9.0) |
| 40–44 | 395 (6.0) | 264 (6.6) | 81 (5.2) | 50 (4.8) |
| ≥45 | 778 (11.8) | 516 (13.0) | 162 (10.4) | 100 (9.5) |
| Race/Hispanic ethnicity | ||||
| Non-Hispanic Asian | 379 (5.8) | 164 (4.1) | 143 (9.2) | 72 (6.9) |
| Non-Hispanic Black | 1804 (27.4) | 1354 (34.1) | 234 (15.1) | 216 (20.6) |
| Hispanic | 1474 (22.4) | 783 (19.7) | 426 (27.4) | 265 (25.3) |
| Non-Hispanic White | 2390 (36.3) | 1349 (34.0) | 633 (40.8) | 408 (38.9) |
| Non-Hispanic other† | 340 (5.2) | 220 (5.5) | 68 (4.4) | 52 (5.0) |
| Unknown | 189 (2.9) | 104 (2.6) | 49 (3.2) | 36 (3.4) |
| HIV status at time of clinic visit for gonorrhea‡ | ||||
| Positive | 1150 (17.5) | 765 (19.4) | 270 (17.6) | 115 (11.1) |
| Negative | 4759 (72.4) | 2893 (73.3) | 1084 (70.8) | 782 (75.6) |
| Unknown | 605 (9.2) | 289 (7.3) | 178 (11.6) | 138 (13.3) |
Northeast Region: Philadelphia (PA), New York City (NY); Midwest Region: Columbus (OH), Indianapolis (IN), Chicago (IL), Milwaukee (WI), Minneapolis (MN), Pontiac (MI), Kalamazoo (MI); South Region: Miami (FL), New Orleans (LA); Greensboro (NC); West Region: Portland (OR), Las Vegas (NV), San Diego (CA), Orange County (CA), Seattle (WA), San Francisco (CA), Denver (CO), Honolulu (HI).
Other: American Indian/Alaska Native, Native Hawaiian, and other Pacific Islander, multirace, or other race indicated.
HIV status at the time of clinic visit for gonorrhea by self-report and/or per medical chart.
eGISP indicates enhanced Gonococcal Isolate Surveillance Project; SURRG, Strengthening the US Response to Resistant Gonorrhea.
Comparison of Antimicrobial Susceptibilities Across Anatomic Sites of Infection
The MIC distributions by antimicrobial for pharyngeal, rectal, and urethral isolates were unimodal across antimicrobials, with the exception of ciprofloxacin, which was bimodal (Fig. 1). There were no significant differences in the geometric mean MICs for azithromycin, ciprofloxacin, penicillin, and tetracycline across anatomic sites (Table 2). For cefixime, there were differences in the geometric mean MICs across anatomic sites (P < 0.05); the geometric mean MIC for pharyngeal isolates (geometric mean MIC, 0.0192 μg/mL) was higher compared with rectal isolates (geometric mean MIC, 0.0181 μg/mL; adjusted P < 0.05) and urethral isolates (geometric mean MIC, 0.0176 μg/mL; adjusted P < 0.05). The rectal geometric mean MIC for cefixime was not significantly different from the urethral geometric mean MIC. For ceftriaxone, there were also differences in the geometric mean MICs across anatomic sites (overall, P < 0.05); the geometric mean MIC for pharyngeal isolates (geometric mean MIC, 0.0108 μg/mL) was higher compared with rectal isolates (geometric mean MIC, 0.00987 μg/mL; adjusted P < 0.05) and urethral isolates (geometric mean MIC, 0.0098 μg/mL; adjusted P < 0.05). The rectal geometric mean MIC for ceftriaxone was not significantly different from the urethral geometric mean MIC.
Figure 1.
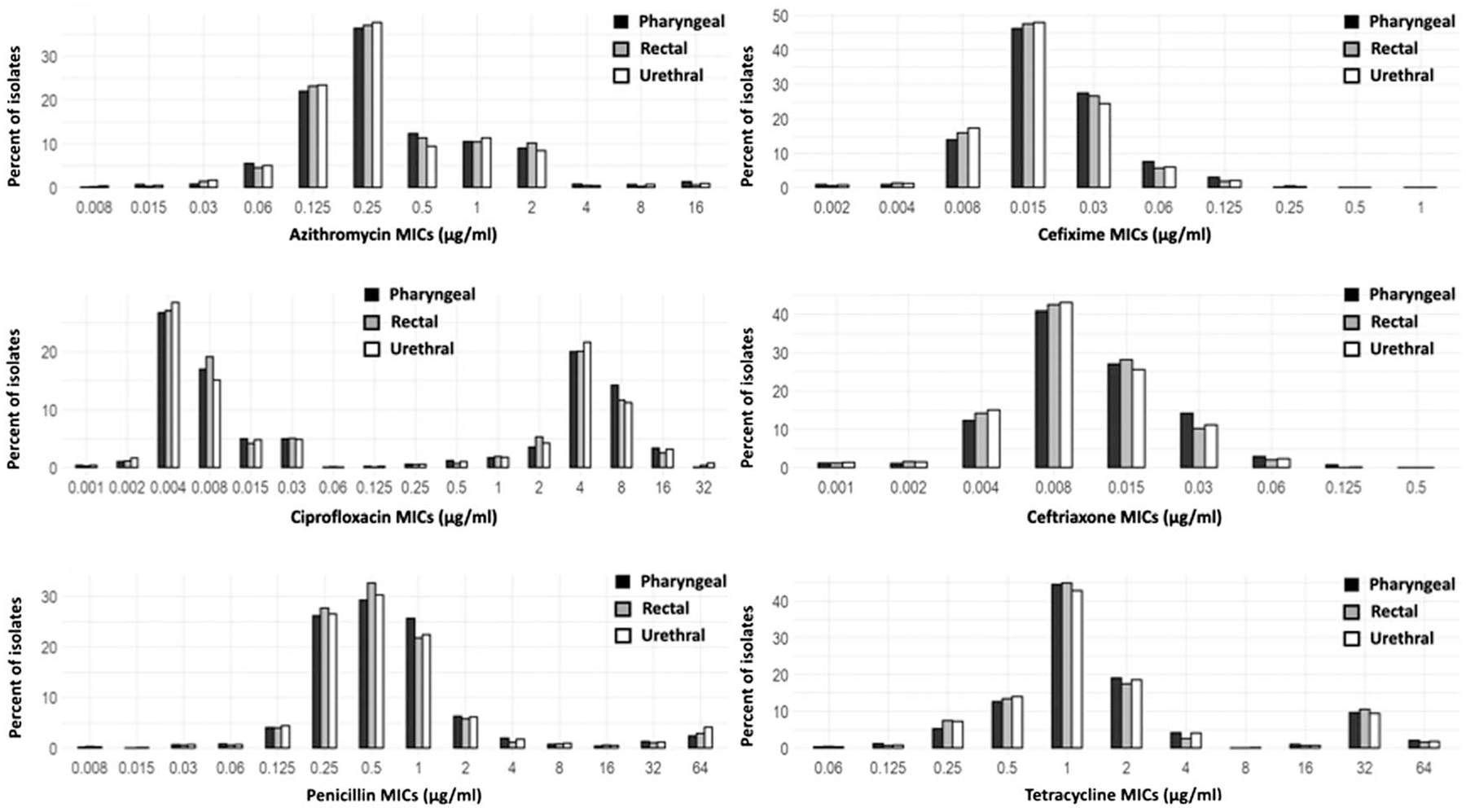
Minimum inhibitory concentration distribution by antimicrobial and anatomic site of gonococcal infection among men who have sex with men: eGISP and SURRG, 2018 to 2019. The MIC distributions (percentage of isolates by MICs) by each antimicrobial (azithromycin, cefixime, ceftriaxone, ciprofloxacin, tetracycline, and penicillin) were calculated for pharyngeal, rectal, and urethral isolates.
TABLE 2.
Comparison* of Antimicrobial Susceptibility Distribution by Antimicrobial and Anatomic Site of Gonococcal Infection Among Men Who Have Sex With Men: eGISP and SURRG, 2018 to 2019
| Antimicrobial and Measured Parameter† | Pharyngeal (n = 1049) | Rectal (n = 1553) | Urethral (n = 3974) |
|---|---|---|---|
| Azithromycin | |||
| MIC range, μg/mL | 0.008–16 | 0.008–16 | 0.008–16 |
| Geometric mean MIC (95% CI) | 0.32 (0.30–0.34) | 0.31 (0.29–0.33) | 0.30 (0.29–0.31) |
| No. (%) [95% CI] with elevated MIC | 124/1049 (11.2) [9.4–13.3] | 182/1553 (11.3) [9.8–13.1] | 420/3974 (10.2) [9.3–11.3] |
| Cefixime | |||
| MIC range, μg/mL | 0.002–0.5 | 0.002–1 | 0.002–0.25 |
| Geometric mean MIC (95% CI) | 0.0192 (0.0185–0.020) | 0.0181 (0.0175–0.0187) | 0.0176 (0.0173–0.018) |
| No. (%) [95% CI] with elevated MIC | 3/1049 (0.29) [0.09–0.88] | 8/1553 (0.52) [0.26–1.0] | 11/3974 (0.28) [0.15–0.50] |
| Ceftriaxone | |||
| MIC range, μg/mL | 0.001–0.125 | 0.001–0.5 | 0.001–0.125 |
| Geometric mean MIC (95% CI) | 0.0108 (0.0103–0.0113) | 0.00987 (0.0095–0.0102) | 0.0098 (0.0096–0.0101) |
| No. (%) [95% CI] with elevated MIC | 7/1049 (0.67) [0.32–1.4] | 2/1553 (0.13) [0.03–0.51] | 7/3974 (0.18) [0.08–0.37] |
| Ciprofloxacin | |||
| MIC range, μg/mL | 0.001–32 | 0.001–32 | 0.001–32 |
| Geometric mean MIC (95% CI) | 0.12 (0.10–0.15) | 0.11 (0.09–0.13) | 0.12 (0.10–0.13) |
| No. (%) [95% CI] with resistant MIC | 450/1049 (42.7) [39.5–46.0] | 649/1553 (41.6) [38.9–44.2] | 1701/3974 (42.6) [40.9–44.3] |
| Penicillin | |||
| MIC range, μg/mL | 0.008–64 | 0.008–64 | 0.008–64 |
| Geometric mean MIC (95% CI) | 0.63 (0.58–0.68) | 0.60 (0.57–0.65) | 0.66 (0.63–0.69) |
| No. (%) [95% CI] with resistant MIC | 140/1049 (13.0) [11.0–15.2] | 200/1553 (12.6) [11.0–14.4] | 603/3974 (14.8) [13.7–16.0] |
| Tetracycline | |||
| MIC range, μg/mL | 0.06–64 | 0.06–64 | 0.06–64 |
| Geometric mean MIC (95% CI) | 1.55 (1.44–1.68) | 1.48 (1.39–1.58) | 1.47 (1.40–1.53) |
| No. (%) [95% CI] with resistant MIC | 377/1049 (35.7) [32.7–38.8] | 512/1553 (32.9) [30.5–35.4] | 1377/3974 (34.5) [32.9–36.1] |
We fitted overall models with and without “anatomic sites” and compared the 2 models to get the overall P value; if the overall P value was statistically significant (<0.05), we conducted post hoc testing to adjust for multiple tests using the Holm adjustment. Linear mixed-effects model was used for geometric mean MICs, and mixed-effects logistic regression model was used for the proportion of isolates with elevated or resistant MICs across anatomic sites, respectively, to account for the multiple isolates provided by the same patients. All geometric mean MICs were not statistically significant (P < 0.05) except for cefixime and ceftriaxone; all proportions with elevated or resistant MICs were not statistically significant except for ceftriaxone.
The Clinical and Laboratory Standards Institute has not established the criteria for resistance to azithromycin, cefixime, and ceftriaxone; breakpoints used to define “elevated MIC” for these antimicrobials include the following: azithromycin MIC of ≥2.0 μg/mL, cefixime MIC of ≥0.25 μg/mL, and ceftriaxone MIC of ≥0.125 μg/mL. Antimicrobial susceptibility testing results were interpreted according to the criteria recommended by the Clinical and Laboratory Standards Institute for penicillin resistance (MIC ≥2 μg/mL or β-lactamase positive), ciprofloxacin resistance (MIC ≥1.0 μg/mL), and tetracycline resistance (MIC ≥2.0 μg/mL). Tested ranges for antimicrobials (μg/mL): azithromycin (0.008–16), cefixime (0.002–1), ceftriaxone (0.001–1), ciprofloxacin (0.001–32), penicillin (0.008–64), tetracycline (0.06–64).
CI, confidence interval; eGISP, enhanced Gonococcal Isolate Surveillance Project; MIC, minimum inhibitory concentration; SURRG, Strengthening the US Response to Resistant Gonorrhea.
There were no differences in the proportion of isolates with elevated or resistant MICs across anatomic sites for azithromycin, cefixime, ciprofloxacin, penicillin, and tetracycline; however, for ceftriaxone, the proportion of isolates with elevated MICs at the pharynx was higher than the proportion with elevated MICs at the rectum or urethra (P < 0.05). For ceftriaxone, 0.67% (7 of 1049; 95% CI, 0.32–1.4) of pharyngeal isolates had elevated MICs compared with 0.18% (7 of 3974; 95% CI, 0.08–0.37) of urethral isolates and 0.13% (2 of 1553; 95% CI, 0.03–0.51) of rectal isolates.
Comparison of Antimicrobial Susceptibilities Across Anatomic Sites of Infection and Geographic Region
In some geographic regions, there were differences in geometric mean MICs for azithromycin, cefixime, and ceftriaxone across anatomic sites of infection (Table S1, http://links.lww.com/OLQ/A749). For azithromycin, the geometric mean MIC differed across anatomic sites in the Northeast with higher pharyngeal geometric mean MIC (P < 0.05) compared with rectal and urethral. For cefixime and ceftriaxone, the geometric mean MICs differed across anatomic sites in the Midwest with higher pharyngeal geometric mean MICs than rectal and urethral geometric mean MICs (P < 0.05). There were no significant differences in the geometric mean MICs for ciprofloxacin, penicillin, and tetracycline according to anatomic site by geographic region.
The proportion of pharyngeal isolates with elevated or resistant MICs for ceftriaxone was higher than the other anatomic sites in each geographic region; however, there were no statistically significant differences in the proportion with elevated or resistant MICs for azithromycin, cefixime, ceftriaxone, ciprofloxacin, penicillin, and tetracycline according to anatomic site of infection by geographic region (Table S2, http://links.lww.com/OLQ/A749).
DISCUSSION
In this analysis of gonococcal isolates collected from cisgender MSM attending STD clinics participating in multijurisdiction sentinel surveillance projects, we detected differences in MICs to antigonococcal antimicrobials across anatomic sites of infection. Cefixime and ceftriaxone geometric mean MICs were higher among pharyngeal isolates compared with rectal and urethral isolates, and the proportion of isolates with elevated ceftriaxone MICs was higher at the pharynx compared with those with elevated MICs at the rectum and urethra. Furthermore, we detected differences in MICs across anatomic sites of infection for azithromycin, cefixime, and ceftriaxone in some geographic regions, reflecting geographic heterogeneity. Collectively, our findings suggest differential antimicrobial susceptibility patterns of N. gonorrhoeae at the pharynx.
This is the first combined report from 2 multijurisdiction sentinel surveillance projects; combining results was facilitated by use of similar protocols and AST conducted by the same regional laboratories. This is the largest study to date using standardized protocols to investigate differences in antimicrobial susceptibility of N. gonorrhoeae by anatomic site. Previous studies comparing antimicrobial susceptibilities of urogenital and extragenital gonococcal isolates are limited. Similar to Kidd et al.,28 which examined susceptibility patterns among MSM by anatomic site using isolates collected in 2011 to 2013 from men seeking care in 5 STD clinics, we found a difference in cefixime geometric mean MICs between pharyngeal and rectal isolates; however, we also detected a difference in geometric mean MICs between pharyngeal and urethral isolates. Given the smaller sample size included in the analysis of Kidd et al. (only 205 pharyngeal isolates included), it may not have been powered to detect small differences between anatomic sites. An earlier study by Hottes et al.30 identified a higher prevalence of elevated azithromycin, cefixime, and ceftriaxone MICs among rectal and pharyngeal isolates than among urethral isolates; however, this study did not control for sex of sex partners, and this difference may have been more reflective of the higher prevalence of gonococcal strains with elevated MICs circulating within MSM sexual networks as compared with heterosexual networks. When looking at associations between antimicrobial susceptibility and anatomical site of infection, a recent study found that resistance to cefixime and resistance combined with decreased susceptibility to ceftriaxone was associated with urogenital infections.31s However, this study used combined data from multiple counties with varying levels of reporting and differing AST methods, highlighting the need for additional studies using standardized methodology. Preliminary analyses of eGISP and SURRG data were suggestive of differences by anatomic site; however, they were not weighted to account for the geographic variation in distribution of isolates or modeled to account for clustering by patient.37s,38s
Although cefixime and ceftriaxone geometric mean MICs across anatomic sites in our analysis were very low and within the antimicrobial susceptibility range, the pattern of higher MICs at the pharynx suggests that susceptibility patterns may be different at the pharynx compared with anogenital sites and supports concerns that the pharynx may serve as an anatomic niche that fosters N. gonorrhoeae resistance. Studies suggest that N. gonorrhoeae may acquire resistance genes, such as penA, associated with cephalosporin resistance, from oropharyngeal commensal Neisseria species.11–14 Furthermore, studies of cefixime pharmacokinetics have shown variation in absorption and protein binding that contributes to poor bioavailability and negligible cefixime drug concentrations in pharyngeal fluid.18 In addition, tonsillar concentrations of ceftriaxone have also been lower than anticipated,19 suggesting lower drug penetration at the pharynx that could foster antimicrobial resistance. Rectal gonococcal infections and exposure to fecal lipids provide selective pressure for antimicrobial resistance mutations39s–42s; however, in our analysis, the rectal geometric mean MICs for cefixime and ceftriaxone were not significantly different from the urethral geometric mean MICs and were lower than the pharyngeal geometric mean MICs. This may be due to in vivo selection for transmissible antimicrobial resistance in the pharynx given the longer duration of untreated pharyngeal gonococcal infections compared with other anatomic sites.43s
Interestingly, although differences were identified in cefixime and ceftriaxone geometric mean MICs across anatomic site, differences across anatomic sites were not identified for other antimicrobials. This may be due to already circulating gonococcal strains with high levels of antimicrobial resistance to those other antimicrobial classes (e.g., penicillins, ciprofloxacin, tetracycline) at all anatomic sites. Because cephalosporin resistance is still emerging in the United States and may develop at different anatomic sites, these surveillance systems may have been able to identify small differences in antimicrobial susceptibility patterns across anatomic sites for ceftriaxone and cefixime. In addition, we may have been underpowered to detect small differences in the antimicrobial classes with a higher prevalence of resistance across anatomic sites. Furthermore, when looking at the proportion of isolates with elevated or resistant MICs, we identified a higher proportion of isolates with elevated ceftriaxone MICs at the pharynx compared with other anatomic sites, a pattern not seen for other antimicrobials. However, the proportion of isolates with elevated ceftriaxone MICs at the pharynx was small (0.67%), suggesting that ceftriaxone remains an effective treatment of pharyngeal gonococcal infections.
The GISP was established in 1986 to monitor the antimicrobial susceptibility trends in N. gonorrhoeae strains and to inform treatment recommendations with the assumption that male urethral isolates are reflective of all circulating strains. If the goal remains to monitor N. gonorrhoeae susceptibility at a population level and antimicrobial resistance is thought to emerge at the same rate at both urogenital and extragenital sites, then limiting sentinel surveillance to circulating strains at the urethra may be sufficient for monitoring national susceptibility patterns. However, if resistance emerges faster at extragenital sites, particularly the pharynx, compared with urogenital sites, routine monitoring of N. gonorrhoeae susceptibility at extragenital sites may be important for identifying new resistant strains and for monitoring resistance emergence trends over time.
In our analysis, we found differences in geometric mean MICs across anatomic sites of infection in some geographic regions, such as azithromycin in the Northeast and cefixime and ceftriaxone in the Midwest. There were no significant differences between the proportions of isolates with elevated or resistant MICs across anatomic sites by geographic region. Gonococcal antimicrobial susceptibility patterns overall are known to vary by geographic region, and our historical understanding has been that antimicrobial-resistant N. gonorrhoeae strains (such as fluoroquinolone-resistant and penicillinase-producing N. gonorrhoeae) are often imported from Asia, with early cases detected in the Pacific and West Coast, before spreading east in the United States.44s,45s The finding of differences across anatomic sites in some geographic regions suggest that resistance/elevated MICs in isolates may develop and emerge in different geographic regions.46s Furthermore, this may be reflective of diverse gonococcal strains circulating in different geographic sexual networks possibly because of sexual behavioral or antimicrobial-use patterns that may vary geographically.
Strengths of our analysis include the large, population-based sample; ability to account for clustering by individual and for heterogeneity by geographic site; and completeness of gender of sex partners data such that we could restrict the study to MSM. However, we note limitations. First, our isolates may not be representative of all circulating strains of gonococcal infections because of the population that underwent specimen collection and the known challenges with isolating N. gonorrhoeae. This may have differed by anatomic site because gonococcal culture at extragenital sites has much lower sensitivity compared with the urethral site.47s–49s In addition, collection of urethral cultures in eGISP is limited to MSM presenting with symptomatic urethritis, whereas extragenital cultures are collected from MSM who report sexual exposure at the anatomic site, regardless of presence of symptoms. Therefore, our findings would be biased if gonococcal strains associated with symptomatic infection or improved culture growth are more likely to have differing antimicrobial susceptibility patterns from those associated with asymptomatic infection or decreased ability to grow in culture. Second, our study was limited to cisgender MSM attending STD clinics participating in eGISP and SURRG, and our results may not be representative of gonorrhea antimicrobial susceptibility patterns across anatomic sites in other populations. For example, a prior study examining N. gonorrhoeae antimicrobial susceptibility patterns across women and men who have sex with women only did not find differences in MICs according to anatomic site, although this study may not have been sufficiently powered.23 Finally, although our overall sample size was large, when stratified by geographic regions, sample size was reduced, potentially limiting our ability to identify significant differences by anatomic site—particularly for antimicrobials with a low frequency of elevated MICs (e.g., cefixime and ceftriaxone).
In conclusion, we identified differences in MICs across anatomic sites of gonococcal infection with higher cefixime and ceftriaxone geometric mean MICs and a higher proportion of isolates with elevated ceftriaxone MICs among isolates from the pharynx. Continued investigation into antimicrobial susceptibility patterns of gonococcal isolates at extragenital sites may be an important strategy to detect and monitor the emergence of antimicrobial-resistant N. gonorrhoeae strains over time. Ensuring adequate treatment and eradication of pharyngeal gonococcal infections may be important to reducing the spread of antimicrobial-resistant N. gonorrhoeae in communities.50s
Supplementary Material
Acknowledgments:
This article was written on behalf of the Strengthening the US Response to Resistant Gonorrhea and the enhanced Gonococcal Isolate Surveillance Project collaborators and other public health staff who contributed to the data collection, management, and implementation of these projects. The authors thank Lindley Barbee, Robert Kirkcaldy, Jose Bazan, Aaron Hoogenboom, Rebecca Harrison, Elesi Quaye, Southern Nevada Public Health Laboratory of Southern Nevada Health District (SNHD), SNHD Sexual Health Clinic, and SNHD Office of Epidemiology and Disease Surveillance, as well as the patients and providers who contributed data to these projects.
The Strengthening the US Response to Resistant Gonorrhea and enhanced Gonococcal Isolate Surveillance Project activities described in this article were supported by Federal Antibiotic Resistance Initiative funding and administered through the US Centers for Disease Control and Prevention’s (CDC) Epidemiology and Laboratory Capacity for the Prevention and Control of Infectious Diseases Cooperative Agreement (CK19–1904).
Footnotes
Conflict of Interest and Sources of Funding: None declared.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: CDC, 2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed February 21, 2021. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Atlanta, GA: CDC, 2013. Available at: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed February 21, 2021. [Google Scholar]
- 3.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med 2012; 366:485–487. [DOI] [PubMed] [Google Scholar]
- 4.Unemo M, Golparian D, Eyre DW. Antimicrobial resistance in Neisseria gonorrhoeae and treatment of gonorrhea. Methods Mol Biol 1997; 2019:37–58. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018. Available at: https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf. Accessed February 21, 2021.
- 6.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021; 70:1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Gonococcal Isolate Surveillance Project (GISP) and Enhanced GISP, 2020. Atlanta, GA: CDC, 2020. Available at: https://www.cdc.gov/std/gisp/GISP_eGISP_Protocol_January_2020.pdf. Accessed February 21, 2021. [Google Scholar]
- 8.Shafer WM, Balthazar JT, Hagman KE, et al. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 1995; 141:907–911. [DOI] [PubMed] [Google Scholar]
- 9.Morse SA, Lysko PG, McFarland L, et al. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun 1982; 37:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland L, Mietzner TA, Knapp JS, et al. Gonococcal sensitivity to fecal lipids can be mediated by an Mtr-independent mechanism. J Clin Microbiol 1983; 18:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuya R, Onoye Y, Kanayama A, et al. Antimicrobial resistance in clinical isolates of Neisseria subflava from the oral cavities of a Japanese population. J Infect Chemother 2007; 13:302–304. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Nakayama H, Huruya K, et al. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int J Antimicrob Agents 2006; 27:20–26. [DOI] [PubMed] [Google Scholar]
- 13.Ameyama S, Onodera S, Takahata M, et al. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother 2002; 46:3744–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, Deguchi T, Mizutani KS, et al. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother 2005; 49:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp JS, Johnson SR, Zenilman JM, et al. High-level tetracycline resistance resulting from TetM in strains of Neisseria spp., Kingella denitrificans, and Eikenella corrodens. Antimicrob Agents Chemother 1988; 32:765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris SR, Klausner JD, Buchbinder SP, et al. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: The EXPLORE study. Clin Infect Dis 2006; 43: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 17.Moran JS. Treating uncomplicated Neisseria gonorrhoeae infections: Is the anatomic site of infection important? Sex Transm Dis 1995; 22:39–47. [DOI] [PubMed] [Google Scholar]
- 18.Barbee LA, Nayak SU, Blumer JL, et al. A phase 1 pharmacokinetic and safety study of extended-duration, high-dose cefixime for cephalosporin-resistant Neisseria gonorrhoeae in the pharynx. Sex Transm Dis 2018; 45:677–683. [DOI] [PubMed] [Google Scholar]
- 19.Blumer JL, Reed MD, Kaplan EL, et al. Explaining the poor bacterio-logic eradication rate of single-dose ceftriaxone in group A streptococcal tonsillopharyngitis: A reverse engineering solution using pharmacodynamic modeling. Pediatrics 2005; 116:927–932. [DOI] [PubMed] [Google Scholar]
- 20.Weston EJ, Kirkcaldy RD, Stenger M, et al. Narrative review: Assessment of Neisseria gonorrhoeae infections among men who have sex with men in national and sentinel surveillance systems in the United States. Sex Transm Dis 2018; 45:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbee LA, Soge OO, Katz DA, et al. Increases in Neisseria gonorrhoeae with reduced susceptibility to azithromycin among men who have sex with men in Seattle, King County, Washington, 2012–2016. Clin Infect Dis 2018; 66:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkcaldy RD, Zaidi A, Hook EW III, et al. Neisseria gonorrhoeae antimicrobial resistance among men who have sex with men and men who have sex exclusively with women: The Gonococcal Isolate Surveillance Project, 2005–2010. Ann Intern Med 2013; 158(5 Pt 1): 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidd S, Moore PC, Kirkcaldy RD, et al. Comparison of antimicrobial susceptibility of urogenital Neisseria gonorrhoeae isolates obtained from women and men. Sex Transm Dis 2015; 42:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC). Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men—United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep 2004; 53:335–338. [PubMed] [Google Scholar]
- 25.Chan PA, Robinette A, Montgomery M, et al. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: A review of the literature. Infect Dis Obstet Gynecol 2016; 2016:5758387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson Jones ML, Chapin-Bardales J, Bizune D, et al. , National HIV Behavioral Surveillance Sexually Transmitted Infection Study Group. Extragenital chlamydia and gonorrhea among community venue–attending men who have sex with men—Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep 2019; 68:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abara WE, Llata EL, Schumacher C, et al. Extragenital gonorrhea and chlamydia positivity and the potential for missed extragenital gonorrhea with concurrent urethral chlamydia among men who have sex with men attending sexually transmitted disease clinics—Sexually Transmitted Disease Surveillance Network, 2015–2019. Sex Transm Dis 2020; 47:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kidd S, Zaidi A, Asbel L, et al. Comparison of antimicrobial susceptibilities of pharyngeal, rectal, and urethral Neisseria gonorrhoeae isolates among men who have sex with men. Antimicrob Agents Chemother 2015; 59:2588–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagan D Comparison of Neisseria gonorrhoeae isolates from homosexual and heterosexual men. Genitourin Med 1985; 61:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hottes TS, Lester RT, Hoang LM, et al. Cephalosporin and azithromycin susceptibility in Neisseria gonorrhoeae isolates by site of infection, British Columbia, 2006 to 2011. Sex Transm Dis 2013; 40:46–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


