Abstract
Background
Consistent patterns of reduced cortical thickness have been identified in early Alzheimer’s disease (AD). However, the pathological factors that influence rates of cortical thinning within these AD signature regions remain unclear.
Methods
Participants were from the Insight 46 substudy of the MRC National Survey of Health and Development (NSHD; 1946 British birth cohort), a prospective longitudinal cohort study. Linear regression was used to examine associations of baseline cerebral β-amyloid (Aβ) deposition, measured using florbetapir positron emission tomography, and baseline white matter hyperintensity volume (WMHV) on MRI, a marker of cerebral small vessel disease, with subsequent longitudinal changes in AD signature cortical thickness quantified from baseline and repeat MRI (mean [SD] interval 2.4 [0.2] years).
Results
In a population-based sample of 337 cognitively normal older white adults (mean [SD] age at baseline 70.5 [0.6] years; 48.1% female), higher global WMHV at baseline related to faster subsequent rates of cortical thinning in both AD signature regions (~0.15%/year faster per 10 mL additional WMHV), whereas baseline Aβ status did not. Among Aβ positive participants (n=56), there was some evidence that greater global Aβ standardised uptake value ratio at baseline related to faster cortical thinning in the AD signature Mayo region, but this did not reach statistical significance (p=0.08).
Conclusions
Cortical thinning within AD signature regions may develop via cerebrovascular pathways. Perhaps reflecting the age of the cohort and relatively low prevalence of Aβ-positivity, robust Aβ-related differences were not detected. Longitudinal follow-up incorporating additional biomarkers will allow assessment of how these relationships evolve closer to expected dementia onset.
Keywords: CEREBROVASCULAR DISEASE, AMYLOID, ALZHEIMER'S DISEASE
Introduction
Previous MRI studies have identified consistent patterns of decreased cortical thickness in early Alzheimer’s disease (AD)—termed AD signatures—which predict cognitive decline and AD dementia in cognitively normal (CN) older adults.1–4 Similar findings have also been detected longitudinally in presymptomatic autosomal dominant AD.5
The relationship between β-amyloid (Aβ) deposition, one of the neuropathological hallmarks of AD and cortical thickness is unclear. Findings from MRI studies have been mixed, with some studies reporting Aβ-related reductions in cortical thickness,3 6 while a previous cross-sectional analysis from our group did not detect significant differences,7 and some researchers have observed Aβ-associated increases in cortical thickness.8 This has led to suggestions that the relationship may be non-linear or perhaps mediated via or interactive with tau pathology or other disease processes.9 Alternatively, discrepancies between studies might relate to difficulties accounting for heterogeneity between individuals, either reflecting premorbid differences in brain structure—an issue in cross-sectional studies—or the effects of age and other pathologies which often coexist in later life.
To investigate this further, this study examines associations of baseline cerebral Aβ deposition, measured using florbetapir positron emission tomography (PET), and baseline white matter hyperintensity volume (WMHV) on MRI, a marker of cerebral small vessel disease (CSVD), with subsequent longitudinal changes in AD signature cortical thickness quantified from MRI in CN older adults of almost identical age.
Methods
Participants were scanned on a single Biograph mMR 3T PET/MRI (Siemens Healthcare) at two time points as part of the Insight 46 substudy of the MRC National Survey of Health and Development (NSHD; the 1946 British birth cohort).10
Baseline Aβ PET data obtained postinjection of 370 MBq 18F florbetapir were processed with pseudo-CT attenuation correction.11 Global standardised uptake value ratios (SUVRs) were generated using a cortical region of interest, based on a previously defined composite,12 and an eroded subcortical white matter reference region. A gaussian mixture model was applied to global SUVRs and the 99th percentile of the lower gaussian was taken as the cut-point for Aβ positivity (0.6104).
Baseline global WMHV was measured from distortion-corrected and bias-corrected T1 and fluid-attenuated inversion recovery MRI data using an unsupervised automated algorithm, Bayesian Model Selection, as described elsewhere.13
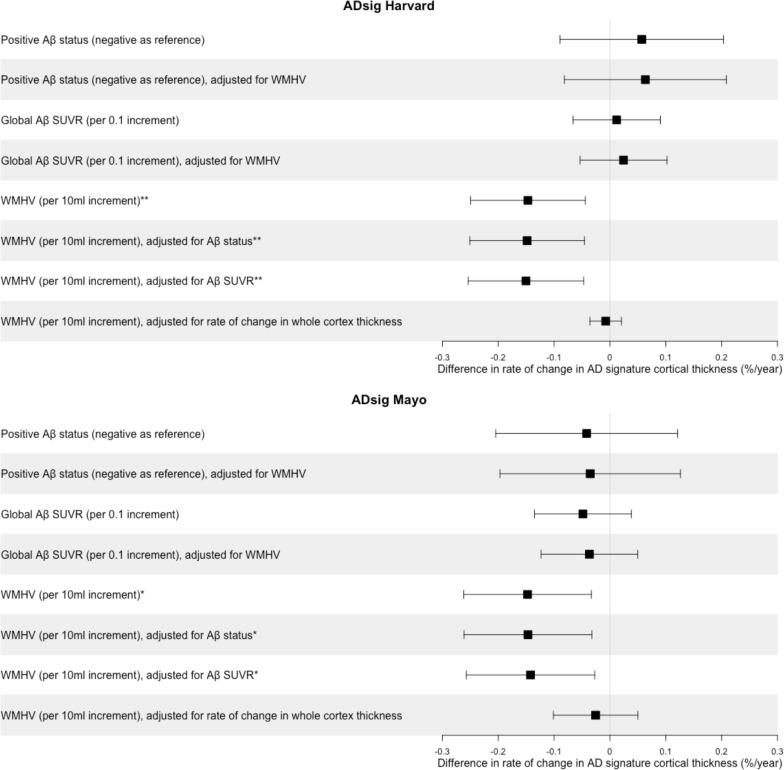
Cortical thickness was estimated at each time point using Freesurfer V.7.1.0 (https://surfer.nmr.mgh.harvard.edu/). Distortion-corrected T1 MRI underwent cross-sectional processing within Freesurfer, before being processed through the longitudinal stream.14 To form the AD signatures (ADsig Harvard and Mayo; see figure 1 for region descriptions), Desikan-Killian atlas labels were merged and single annotation files were created.15 Surface area-weighted averages of extracted left and right hemisphere AD signature cortical thickness values were then calculated.
Figure 1.
Associations of baseline Aβ deposition and baseline WMHV with subsequent rates of change in cortical thickness in Alzheimer’s disease (AD) signature regions in cognitively normal participants. Coefficients and 95% CIs are presented from linear regression models, adjusted for sex and age at baseline scan. *p≤0.05; **p≤0.01. ADsig Harvard consisted of entorhinal, inferior temporal, parahippocampal, temporal pole, precuneus, supramarginal, superior and inferior parietal, superior frontal, pars opercularis, pars triangularis and pars orbitalis areas.3 ADsig Mayo was composed of middle temporal, inferior temporal, entorhinal and fusiform areas.4 Aβ, β-amyloid; SUVR, standardised uptake value ratio; WMHV, white matter hyperintensity volume.
Statistical analyses were performed in STATA V.17. Overall, 356 of 502 participants had high-quality longitudinal MRI data, of whom those with dementia (n=2), mild cognitive impairment (n=4), other confounding brain disorders (n=4) or missing Aβ or WMHV data (n=9) at baseline were excluded.16
Differences in baseline characteristics by Aβ status were assessed using t-tests, Wilcoxon rank-sum tests or χ2 tests, as appropriate.
Associations of baseline Aβ (status or global SUVR) and baseline global WMHV with subsequent changes in AD signature cortical thickness were tested using linear regression models, similar to those previously described.16 Effects of Aβ and WMHV were assessed in separate models and then as predictors in a single model, with adjustment for sex and baseline age. WMHV was not corrected for total intracranial volume since this did not alter the results (online supplemental etable 1).
jnnp-2023-332067supp001.pdf (74.3KB, pdf)
Model assumptions were checked by examination of residual plots. Non-linear relationships were assessed by inspection of residual versus predictor plots and were formally tested by adding quadratic terms to models.
Results
A total of 337 CN participants (mean [SD] age 70.5 [0.6] years; 48.1% female) had complete imaging data (mean [SD] scan interval 2.4 [0.2] years). There were significantly more APOE ε4 carriers among Aβ positive than Aβ negative participants (60.7% vs 22.6%; p<0.001), but age, sex and other baseline characteristics did not differ significantly by Aβ status (table 1).
Table 1.
Participant characteristics
| Characteristic | All participants (n=337) |
Aβ positive (n=56) | Aβ negative (n=281) | |
| Age at baseline visit, years, mean (SD) | 70.5 (0.6) | 70.5 (0.6) | 70.5 (0.6) | |
| Sex, % female | 48.1 | 42.9 | 49.1 | |
| Childhood cognition, z-score, mean (SD) | 0.41 (0.72) | 0.34 (0.71) | 0.43 (0.73) | |
| Education level | % none | 15.4 | 17.9 | 15.0 |
| % O-level or equivalent or vocational | 32.3 | 41.1 | 30.6 | |
| % A-level or equivalent or higher | 52.2 | 41.1 | 54.5 | |
| Socioeconomic position at age 53, % manual occupation | 15.1 | 16.1 | 15.0 | |
| APOE ε4 status, % carrier* | 29.0 (n=335)† |
60.7 | 22.6 (n=279)† |
|
| Global WMHV at baseline, mL, median (IQR) | 2.7 (1.5–6.1) | 3.3 (1.8–6.2) | 2.6 (1.5–6.1) | |
| PACC at baseline, z-score, mean (SD) | 0.05 (0.67) | −0.04 (0.69) | 0.07 (0.66) | |
*Significant difference detected between Aβ positive and negative groups (p≤0.001).
†Number of participants with available data if below maximum possible.
APOE, apolipoprotein E; Aβ, β-amyloid; PACC, preclinical Alzheimer’s cognitive composite; WMHV, white matter hyperintensity volume.
There were no significant relationships between baseline Aβ (status or global SUVR) and subsequent rates of change in cortical thickness in either AD signature region, whereas higher global WMHV at baseline associated with significantly faster subsequent rates of cortical thinning in both AD signature regions: 0.15%/year faster per 10 mL additional WMHV (figure 1, online supplemental etable 1). There was no material difference in the results when the effects of Aβ and WMHV were assessed as predictors in a single model; however, effects of WMHV were attenuated and non-significant after adjustment for rate of whole cortex thickness change (figure 1, online supplemental etable 1). There were no interactions between Aβ and WMHV or between sex and Aβ or WMHV, and no non-linear relationships (p>0.1, all tests).
In post hoc analyses, effects of regional (lobar) Aβ SUVR were virtually identical to each other (online supplemental etable 2). There was some evidence that, among Aβ positive participants, higher global SUVR at baseline related to faster subsequent cortical thinning in the ADsig Mayo region, though it did not reach statistical significance (p=0.08), and a similar relationship was not detected with the ADsig Harvard region (online supplemental etable 3). In a vertex-wise analysis, rates of change in cortical thickness did not differ significantly by baseline Aβ status in any brain region after cluster-wise correction for multiple comparisons (1000 permutations; cluster-forming threshold p<0.05).
Discussion
In CN adults ~70 years old, higher baseline WMHV—a marker of CSVD—related to significantly faster subsequent rates of cortical thinning in AD signature regions, whereas baseline Aβ status did not.
The association with WMHV does not necessarily mean that CSVD has a direct role in AD pathogenesis—indeed, effects were reduced to almost zero and non-significant after adjustment for rate of whole cortex thickness change, implying that they were not disproportionate to global changes—but it may reflect that CSVD contributes to cortical thinning in later life including within regions known to be vulnerable in AD. Thus, interventions aimed at reducing development of CSVD in later life may help to slow neurodegeneration in these areas, potentially delaying or preventing progression to dementia. Moreover, studies using AD signature cortical thickness as a biomarker in AD should consider possible effects of CSVD.
Notably, rates of cortical thinning did not differ by Aβ status, either within AD signature regions or elsewhere in the brain. While there was some evidence, among Aβ positive participants, that higher global Aβ SUVR at baseline related to faster subsequent cortical thinning in the ADsig Mayo region, this was not statistically significant. Insight 46 is a relatively young cohort, and rates of Aβ-positivity (~17%) in the current sample, while broadly within those expected for age, are perhaps slightly lower than in some studies, likely reflecting that the cohort is population based.17 This might explain why some studies of CN adults—often with older age ranges or greater rates of Aβ-positivity—have detected significant Aβ-related cortical thinning,3 6 whereas Insight 46 analyses—both here and in a previous cross-sectional study7—have not observed robust differences.
Another potential issue is that there may be apparent ‘thickening’ of the cortex in early AD, perhaps related to a transient inflammatory response to Aβ. Evidence supporting this hypothesis is largely based on cross-sectional studies with small subject numbers and has not been widely replicated.8 18 However, if this were the case, effects of Aβ in opposite directions may cancel each other out when assessed at a group level, making it difficult to detect a relationship in early AD.
A further consideration is whether the absence of a significant relationship may be technique related. In the same sample, we previously reported Aβ-related differences in rates of global and hippocampal volume loss measured using the boundary shift integral (BSI).16 While comparison with this study is difficult due to the different regions assessed, this might reflect that the BSI is more precise, providing a direct rather than indirect measure of change, or that cortical thickness is computationally more difficult to quantify than volume. Indeed, whole brain volume change measured using the BSI was much less variable (SD/mean ratio ~1/3) than whole cortical thickness change measured using Freesurfer.
Strengths of this study include that participants were scanned on a single PET/MRI scanner at an almost identical age. A limitation is the absence of tau PET data. Previous studies have detected interactions between Aβ and tau, whereby Aβ-positivity associated with increased cortical thickness in tau negative individuals but reduced cortical thickness in tau positive individuals.9 18 Tau has also been suggested as a cause of WMH, perhaps via Wallerian degeneration.19 Other limitations include that there was insufficient power to assess the impact of other CSVD features (eg, lacunes or microbleeds) due to their low frequency in this sample20; and that Aβ PET may reflect both Aβ found in AD plaques and that in cerebral amyloid angiopathy,21 which may have confounded the results.
In conclusion, the findings in this study add to current understanding of the factors that might influence rates of change in AD signature cortical thickness in CN older adults, as well as highlighting important avenues for further research.
Acknowledgments
The authors are very grateful to the study participants for their contributions to Insight 46 and for their lifelong commitment to research. They are grateful to the radiographers and nuclear medicine physicians at the UCL Institute of Nuclear Medicine; the staff at the Leonard Wolfson Experimental Neurology Centre and the Bloomsbury Centre for Clinical Phenotyping at UCL; neuroradiologists Dr. Chandrashekar Hoskote and Dr. Sachit Shah for providing clinical reads for the MRI scans; the Dementia Research Centre trials team for assistance with imaging quality control; Dan Marcus and Rick Herrick for assistance with XNAT; and Dr. Philip Curran for assistance with data sharing with the MRC Unit for Lifelong Health and Ageing. They are also particularly indebted to the support of the late Chris Clark of Avid Radiopharmaceuticals who championed this study from its outset.
Footnotes
@davecash75, @dave_mri, @jmschott
Contributors: SK and JMS contributed to the study concept and design. SK, WC, DMC, JB, CAL, TP, AK, SB, AW, MS, MH, KL, S-NJ, RS, IBM, CS, DT, JD, FB, HM-S and AW contributed to data acquisition. SK, WC, DMC, JB, JMN, MR, NCF and JMS contributed to data analysis or interpretation. SK drafted the initial manuscript. All authors critically reviewed the manuscript before submission.
Funding: This study was funded by Medical Research Council (MC-UU-00019/1 PI Chaturvedi, MC-UU-00019/3 PI Richards), Alzheimer's Association (SG-666374-UK BIRTH COHORT PI Schott), Brain Research Trust (UCC14191 PI Schott), Wellcome Trust (Clinical Research Fellowship 200109/Z/15/Z Parker), Dementias Platform UK (CSUB19166 PIs Schott, Fox, Richards), Selfridges Group Foundation (22/3/18 PIs Schott, Keshavan), Alzheimer's Research UK (ARUK-PG2014-1946 PIs Schott, Fox, Richards, ARUK-PG2017-1946 PIs Schott, Fox, Richards) and the Wolfson Foundation (PR/ylr/18575 PIs Fox, Schott).
Competing interests: SK and WC report no disclosures relevant to the manuscript. DMC was supported by Alzheimer’s Research UK (ARUK-PG2017-1946), the UCL/UCLH National Institute of Health and Care Research Biomedical Research Centre and the UK Dementia Research Institute, which is funded by the UK Medical Research Council, Alzheimer’s Society, and Alzheimer’s Research UK. JB was funded by an Alzheimer’s Research UK Senior Fellowship (ARUK-SRF2016A-2). JMN reports no disclosures relevant to the manuscript. CAL is a full-time employee of Roche Products and a shareholder in F. Hoffmann-La Roche. TP has received support from a Wellcome Trust Clinical Research Fellowship and a UK National Institute of Health and Care Research academic clinical lectureship. AK was supported by a Wolfson Clinical Research Fellowship and a Weston Brain Institute and Selfridges Group Foundation award (UB170045). SB reports no disclosures relevant to the manuscript. AW is funded by a Wolfson Clinical Research Fellowship. MS, MH, KL, S-NJ, RS and IBM report no disclosures relevant to this manuscript. CS was supported by the Alzheimer’s Society Junior Fellowship (AS-JF-17–011) and serves as scientific advisor and shareholder of BrainKey. DT is funded by the Leonard Wolfson Experimental Neurology Centre and University College London Hospitals Biomedical Research Centre. JD has received payment for consultancy on the running of multicentre studies from Biogen, Bioclinica and GE Healthcare, and he is supported by the National Institute for Health Research, University College London Hospitals Biomedical Research Centre. FB is on the steering committee or is an iDMC member for Biogen, Merck, Roche, EISAI and Prothena, and is a consultant for Roche, Biogen, Merck, IXICO, Jansen and Combinostics; he has research agreements with Merck, Biogen, GE Healthcare, and Roche, is a cofounder and shareholder of Queen Square Analytics, and a board member of the journals Neurology, Radiology, MSJ, and Neuroradiology; he was Editor In Chief of Clinical Neuroradiology—the ESNR textbook (Springer), and has had projects funded by the UK MS Society, Dutch Foundation MS Research, NOW (Picture project) and IMI-EU (Amypad project). HM-S reports no disclosures relevant to this manuscript. AW is funded by the Medical Research Council (MC_UU_00,019/1, MC_UU_00,019/3). MR is funded by the Medical Research Council (MC_UU_12,019/1, MC_UU_12,019/3). NCF has served as a consultant, on advisory boards or on a data monitoring committee for Roche, Biogen and Ionis, and he acknowledges support from the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Rosetrees Trust, Alzheimer’s Research UK, and the UK Dementia Research Institute. JMS has received research funding from Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly); has consulted for Roche Pharmaceuticals, Biogen, Merck and Eli Lilly; has given educational lectures sponsored by GE Healthcare, Eli Lilly and Biogen; and is supported by University College London Hospitals Biomedical Research Centre, Engineering and Physical Sciences Research Council (EP/J020990/1), British Heart Foundation (PG/17/90/33415) and EU’s Horizon 2020 Research and Innovation Programme (666992).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Further details can be found at http://www.nshd.mrc.ac.uk/data.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by National Research Ethics Services Committee (REC reference 14/LO/1173). Participants gave informed consent to participate in the study before taking part.
References
- 1. Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology 2009;72:1048–55. 10.1212/01.wnl.0000340981.97664.2f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dickerson BC, Stoub TR, Shah RC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011;76:1395–402. 10.1212/WNL.0b013e3182166e96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 2009;19:497–510. 10.1093/cercor/bhn113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jack CR, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain 2015;138(Pt 12):3747–59. 10.1093/brain/awv283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weston PSJ, Nicholas JM, Lehmann M, et al. Presymptomatic cortical thinning in familial Alzheimer's disease. Neurology 2016;87:2050–7. 10.1212/WNL.0000000000003322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker JA, Hedden T, Carmasin J, et al. Amyloid‐Β associated cortical thinning in clinically normal elderly. Ann Neurol 2011;69:1032–42. 10.1002/ana.22333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker TD, Cash DM, Lane CA, et al. Amyloid Β influences the relationship between cortical thickness and vascular load. Alzheimers Dement (Amst) 2020;12:e12022. 10.1002/dad2.12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fortea J, Sala-Llonch R, Bartrés-Faz D, et al. Cognitively preserved subjects with transitional cerebrospinal fluid B-amyloid 1-42 values have thicker cortex in Alzheimer's disease vulnerable areas. Biol Psychiatry 2011;70:183–90. 10.1016/j.biopsych.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 9. Harrison TM, Du R, Klencklen G, et al. Distinct effects of beta‐amyloid and tau on cortical thickness in cognitively healthy older adults. Alzheimers Dement 2021;17:1085–96. 10.1002/alz.12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lane CA, Parker TD, Cash DM, et al. Study protocol: Insight 46 - a neuroscience sub-study of the MRC National Survey of Health and Development. BMC Neurol 2017;17:75. 10.1186/s12883-017-0846-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgos N, Cardoso MJ, Modat M, et al. CT synthesis in the head & neck region for PET/MR attenuation correction: an iterative multi-atlas approach. EJNMMI Phys 2015;2.(Suppl 1) 10.1186/2197-7364-2-S1-A31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landau SM, Breault C, Joshi AD, et al. Amyloid-Β imaging with Pittsburgh compound B and Florbetapir: comparing radiotracers and quantification methods. J Nucl Med 2013;54:70–7. 10.2967/jnumed.112.109009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sudre CH, Cardoso MJ, Bouvy WH, et al. Bayesian model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Trans Med Imaging 2015;34:2079–102. 10.1109/TMI.2015.2419072 [DOI] [PubMed] [Google Scholar]
- 14. Reuter M, Schmansky NJ, Rosas HD, et al. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012;61:1402–18. 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 16. Keuss SE, Coath W, Nicholas JM, et al. Associations of Β-amyloid and vascular burden with rates of neurodegeneration in cognitively normal members of the 1946 British birth cohort. Neurology 2022;99:e129–41. 10.1212/WNL.0000000000200524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jansen WJ, Janssen O, Tijms BM, et al. Prevalence estimates of amyloid abnormality across the Alzheimer's disease clinical spectrum. JAMA Neurol 2022;79:228–43. 10.1001/jamaneurol.2021.5216 [DOI] [PubMed] [Google Scholar]
- 18. Fortea J, Vilaplana E, Alcolea D, et al. Cerebrospinal fluid Β-amyloid and phospho-tau biomarker interactions affecting brain structure in preclinical Alzheimer's disease. Ann Neurol 2014;76:223–30. 10.1002/ana.24186 [DOI] [PubMed] [Google Scholar]
- 19. McAleese KE, Walker L, Graham S, et al. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol 2017;134:459–73. 10.1007/s00401-017-1738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lane CA, Barnes J, Nicholas JM, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (insight 46): an Epidemiological study. Lancet Neurol 2019;18:942–52. 10.1016/S1474-4422(19)30228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alakbarzade V, French JM, Howlett DR, et al. Cerebral amyloid angiopathy distribution in older people: a cautionary note. Alzheimers Dement (N Y) 2021;7:e12145. 10.1002/trc2.12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2023-332067supp001.pdf (74.3KB, pdf)
Data Availability Statement
Data are available on reasonable request. Further details can be found at http://www.nshd.mrc.ac.uk/data.