Abstract
One of the leading causes of cancer-related death is gastrointestinal cancer, which has a significant morbidity and mortality rate. Although preoperative risk assessment is essential for directing patient care, its biological behavior cannot be accurately predicted by conventional imaging investigations. Potential pathophysiological information in anatomical imaging that cannot be visually identified can now be converted into high-dimensional quantitative image features thanks to the developing discipline of molecular imaging. In order to enable molecular tissue profile in vivo, molecular imaging has most recently been utilized to phenotype the expression of single receptors and targets of biological therapy. It is expected that molecular imaging will become increasingly important in the near future, driven by the expanding range of biological therapies for cancer. With this live molecular fingerprinting, molecular imaging can be utilized to drive expression-tailored customized therapy. The technical aspects of molecular imaging are first briefly discussed in this review, followed by an examination of the most recent research on the diagnosis, prognosis, and potential future clinical methods of molecular imaging for GI tract malignancies.
Keywords: Molecular imaging, Personalized medicine, Gastrointestinal cancers, Positron emission tomography-computed tomography
Core Tip: In this study, we explored the important role of molecular imaging methods including single-photon emission computed tomography, positron emission tomography, magnetic resonance spectroscopy, photoacoustic imaging, magnetic resonance imaging in gastrointestinal cancers (GIC). These techniques require a forward-looking attitude and clear objectives, which will ultimately guarantee the efficient utilization of these promising tools for identifying GIC characteristics, assessing treatment effectiveness, and providing guidance for therapy and monitoring. By incorporating these sophisticated imaging and molecular technologies into the therapeutic process, medical professionals can customize treatment approaches for each patient, track the effectiveness of treatment, and make well-informed choices on ongoing care.
INTRODUCTION
The World Health Organization reported that gastrointestinal (GI) malignancies account for 26.3% of diagnosed cancers and 35.4% cancer-related deaths globally. Colorectal, stomach, liver, esophagus, and pancreatic neoplasms account for 10.2%, 5.7%, 4.7%, 3.2%, and 2.5% of new cancer cases, respectively. In terms of mortality, GI malignancies account for one-third of all cancer fatalities. Colorectal, stomach, liver, esophageal, and pancreatic cancers (PC) account for 9.2%, 8.2%, 8.2%, 5.3%, and 4.5% of cancer mortality, respectively[1].
"Molecular Imaging" refers to a non-invasive medical imaging technique that allows for the characterization, visualization, and assessment of biological activities in malignancies at both molecular and cellular scales[2-6]. Molecular imaging, unlike anatomic imaging techniques, focuses on the expression status or physiological activity of certain molecules inside of an organ or tissue[7]. Single-photon emission computed tomography (SPECT)[8,9], positron emission tomography (PET)[10,11], magnetic resonance spectroscopy[12], photoacoustic imaging[13,14], magnetic resonance imaging (MRI)[15], optical imaging (optical bioluminescence, optical fluorescence)[16,17], chemical exchange saturation transfer[18], near-infrared fluorescence[19,20], and multimodal imaging[21] including PET-magnetic resonance and also PET-computed tomography (CT)[22,23], are some of the techniques used for molecular imaging.
With the advancement of molecular imaging in recent years, molecular imaging has become a crucial technique for diagnosing, assessing, and treating GI cancer cases. It can identify lesions and define the type of early lesions more precisely than conventional anatomic imaging since cancerous cells have a high metabolic rate, cell surface markers, cell cycle regulators, growth factors, or DNA-binding transcription factors[24,25]. For instance, PET-MRI has higher accuracy compared with CT and endoscopic ultrasound for staging and detecting occult metastases of gastric cancer (GC)[26,27]. Also, PET-CT has a better accuracy than contrast CT for evaluating the stage of liver cancer[28]. Accordingly, the Centers for Medicare and Medicaid Services in the United States reimburse most oncological indications of PET. These indications include staging or restaging gastric, colorectal, esophageal, and other GI malignancies[29].
Personalized medicine (PM) is a concept that attempts to provide the proper management to the appropriate patient at the ideal moment[30]. PM might be closely tied to molecular imaging, considering molecular imaging has the potential to help in determining therapeutic targets and choosing patients to identify individuals likely to benefit from targeted treatment[31]. It can also be used in assessing drug pharmacokinetics and directing doses to guide medication dosing and minimize harmful effects by evaluating drug transport, delivery, and clearance in cancerous or normal cells[32]. During the last few years, molecular classifications have progressed from histology-based to genomic, epigenomic, and transcriptome data, revealing novel prognostic indicators and assisting in therapy selection[33-35]. Understanding the complicated molecular alterations associated with abdominal malignancies, particularly colon and pancreatic tumors, is progressing. Molecular imaging agents can detect changes in cancer induced by genetic mutations[36].
Regardless of the number of papers or meta-analyses published on this topic, a complete review in narrative form addresses the significance of molecular imaging and personalized treatment in GI tumors. This article tries to illuminate the ever-changing landscape of GI cancer research by digging into the nuances of classifications, advances in genomics, and the correlation between agents and genetic variables. Finally, the study aims to bridge the gap between imaging, individualized care, and the complexity involved with cancer. It provides insight into accuracy, therapy techniques, and their possible impact on patient outcomes.
IMPACT OF MOLECULAR IMAGING ON PROGNOSIS AND DIAGNOSIS OF GI CANCER AND THE ROLE IN PM
PM is a new branch of medicine that attempts to improve diagnosis accuracy and treatment efficacy. Molecular imaging, a visualization of the metabolic activity and localization of the lesions that are not evident at the structural imaging level, is widely employed in numerous illnesses, notably cancer treatment. Imaging is often divided into three categories: functional, molecular, and anatomical imaging. Tumors are visualized and measured using anatomical imaging methods including ultrasound, CT, and MRI. In contrast, such procedures do not offer information and can be difficult when tumors share physical properties with adjacent tissues[37]. Functional imaging measures processes such as blood flow changes, allows us to greater comprehend disease dynamics in angiogenic cancer therapy[38-41]. On the one hand, molecular imaging enables us to visualize processes at the cellular and molecular levels. It gives information regarding activities such as enzyme processes and receptor numbers, allowing for a more in-depth understanding of cancer development. With advances in genomes, proteomics, and targeted drug discovery, molecular imaging has emerged as a tool for cancer detection, staging, therapy response prediction, and effectiveness monitoring. Despite laboratory approaches that provide snapshots of cancer biology, molecular imaging offers a dynamic understanding of how tumors progress in laboratory settings[42].
The utilization of imaging modalities, particularly integrating anatomical and molecular or functional data, has considerably increased diagnostic accuracy, specificity, and sensitivity. PET-CT, SPECT-CT, PET-MRI, and MRI-optical imaging are some examples of this method[43-47]. A retrospective investigation showed that PET-CT performed well in tumor staging, with an 84% accuracy rate. In comparison, PET-CT obtained 76% accuracy, whereas CT alone achieved 63% and PET alone achieved 64%[44]. Although anatomic imaging has limitations, functional imaging can shed light on them, and molecular imaging could enhance cancer diagnosis and treatment monitoring. The use of modal imaging is a significant advancement since it provides a more comprehensive and accurate view of malignancies[48].
The development of imaging techniques, plays an important role in improving diagnostic skills. It emphasizes the importance of biomarkers and advanced detection methods while distinguishing between two types of probes: Exogenous probes and genetically encoded probes. Exogenous probes for PET imaging, such as 18 F labeled fluorodeoxyglucose (FDG), are designed to target cancer biomarkers. When there is inflammation, the specificity may be reduced. Various types of molecules, including antibodies and peptides, are being studied for their potential as imaging agents in techniques such as ultrasound, CT, MRI, SPECT, and PET methods[49-53].
Molecular imaging has enormous potential in clinical oncology, going beyond laboratory applications to play a crucial part in early cancer detection, and prognosis. It has the greatest impact on tumors that can be treated using non-invasive or minimally invasive approaches, boosting traditional imaging methods by highlighting minor abnormalities and detecting molecular alterations in tissues. This increased sensitivity enables more precise staging, prognostic predictions, and tailored treatment methods. As a cornerstone in clinical oncology, molecular imaging considerably improves 5-year survival rates by allowing for early detection and diagnosis of tumors, particularly in cases such as colorectal cancer (CRC), where screening programs have been implemented to reduce mortality rates. The changing landscape of genetic markers linked to tumor biology and medication responsiveness highlights molecular imaging's expanding role in influencing the future of cancer care[48].
Significantly, malignancies that can be diagnosed without surgery, such as GI cancers, benefit greatly from the use of imaging. This innovative approach gives information about the biology and progression of the lesions. It is a prognostic tool that highlights anomalies and aids in the differentiation of normal or inflammatory tissues from malignant tissue based on early molecular changes rather than evident morphological distinctions. The great precision of biomarkers is critical for collecting data from repeated investigations and comparing before and after therapy. Furthermore, molecular imaging approaches adds information to colonoscopy, while increases sensitivity and diagnostic capacities[48].
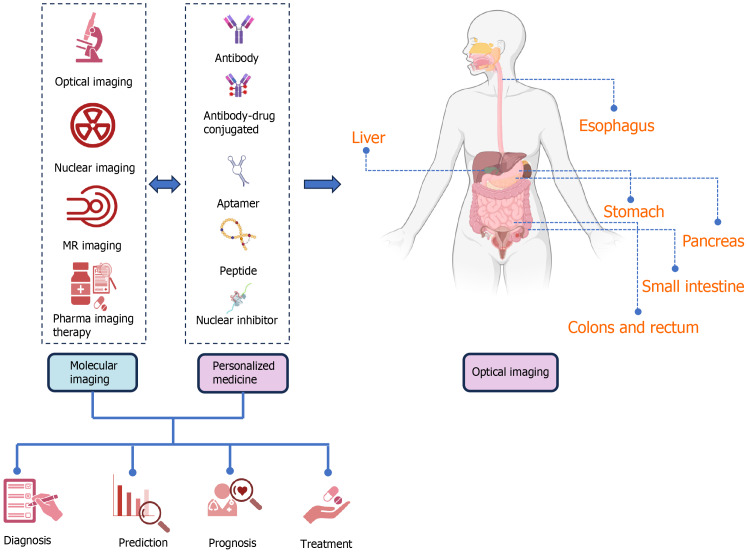
As sequencing and proteomic data, tumor vessels, and tumor stroma assessments become available, new targets may emerge for both imaging and targeted therapies. For example, fibroblasts present in tumors are potential targets worth exploring. This expanding knowledge enhances our understanding of subsets within diseases like colon and PC, which opens up possibilities for more precise targeted therapies. Changes in proteins found in tumors and the surrounding tumor environment are used as points for therapies that target molecular processes, immunotherapies, and other precision treatments. Finally, PM seeks to improve the accuracy of diagnosis while decreasing treatment failures. Molecular imaging plays a significant role in PM. Despite many parts of molecular imaging in their early stages, the ultimate aim is to use these approaches to reach superior diagnoses and treatment decisions and determine patient outcomes. Molecular imaging technologies are expected to improve much more technologically over the next few years, substantially influencing PM[54] (Figure 1, Table 1).
Figure 1.
The function of molecular imaging along with personalized medicine in the diagnosis, screening, prognosis, treatment and also follow-up of gastrointestinal cancers, including the organs of the esophagus, stomach, intestines, colons, rectum, pancreas, and liver, is schematically shown. With the use of molecular imaging and personalized medicine in recent years, the possibility of presenting the function and role of these two concepts in the form of priority and delay is generally not proposed and can be used in parallel or earlier and later according to each organ, and finally to benefit the patients. Molecular imaging including optical imaging (submodalities, including fluorescence, bioluminescence imaging, near infrared, surface-enhanced raman scattering and chemiluminescence), nuclear imaging [positron emission tomography (PET), PET/computerized tomography (CT), and single-photon emission CT], magnetic resonance imaging, imaging and pharma imaging Therapy can be used in the stage of screening, diagnosis, prognosis, treatment, and follow-up separately or in combination with personalized medicine (PM). PM can help patients and clinicians by intervening and taking into account the differences and considerations at the molecular level of various organs of digestive cancer, including aptamer, peptide, nuclear inhibitor, and antibodies. When necessary, PM aims to treat the appropriate patient. PM may be effective in molecular imaging since it helps in the identification of therapeutic targets and persons in need of therapy. It may assess drug transport, administration, and clearance in malignant or healthy cells to reduce side effects and prescribe medication dosage. By evaluating tumor drug activity, molecular imaging can analyze medication pharmacodynamics and uncover promising therapeutic responses and early malfunction. GI: Gastrointestinal.
Table 1.
Summary of the molecular imaging in gastrointestinal cancers
|
|
Imaging modalities
|
Diagnosis
|
Staging
|
Prognosis/survival
|
Metastasis
|
Recurrence
|
RtT
|
|
| Liver | PET | 18F-FDG-PET-CT | Independent prognostic factor | |||||
| 11C-acetate vs 18F-FDG-PET | Dual-tracer PET had better sensitivity and specificity vs FDG PET alone | Dual-tracer PET-CT was superior in staging vs contrast CT | 18 F-FDG PET-CT has higher | Dual-tracer can predict recurrence after TACE | ||||
| Choline vs 18F-FDG PET-CT | ||||||||
| 11C-choline vs. 18F-FDG PET-CT | Dual-tracer had higher sensitivity compared w/each one alone | Dual-tracer had better prognostic value compared w/each one alone | Dual- tracer was a better tool for selecting patients for resection or LT | |||||
| 68Ga-FAPI PET-CT vs 18F-FDG PET-CT | 68Ga-FAPI PET-CT is more sensitive vs 18F-FDG PET-CT | |||||||
| 18F-FAPI PET-CT vs. 18F-FDG PETCT | 18F-FAPI PET-CT was superior | 18F-FAPI PET-CT was superior | 18F-FAPI PET/CT was superior in lymph node metastasis vs. distant metastasis |
18F-FAPI PET-CT was superior for local recurrence | ||||
| 68Ga-LNC1007 vs 68Ga-FAPI and 18F-FDG PET-CT | 68Ga-LNC1007 PET-CT was superior | 68Ga-LNC1007 was superior in skeletal metastases, and peritoneal metastases | ||||||
| MRI | DWI | Use in staging | Determining response after CT-guided HDR- B | |||||
| DWI vs DCE MRI | Can evaluate responses to radiotherapy | |||||||
| Colorectal | PET | 18F-FDG PET/CT | Could predict the prognosis | |||||
| Dual-time-point 18F-FDG PET/CT could liver metastasis | ||||||||
| 68Ga-FAPI PET/CT vs 18F-FDG PET/CT | 68Ga-FAPI PET/CT was superior | 68Ga-FAPI PET/CT was superior | ||||||
| Ga-DOTA-FAPI-04 PET/CT vs FDG PET/CT | Ga-DOTA-FAPI-04 PET/CT was superior | Ga-DOTA-FAPI-04 PET/CT was superior | ||||||
| MRI | Whole body DWI/MRI vs CT | WB-DWI/MRI significantly outperformed CT | ||||||
| Whole body DWI/MRI | Could improve diagnostic accuracy | |||||||
| FDG-PET/CT vs MRI vs CT | MRI had highest sensitivity in detecting liver metastasis | |||||||
| SPECT | 99mTc-MAA SPECT/CT | Could detect liver metastasis | ||||||
| Pancreas | PET | FDG-PET | Could predict RtT | |||||
| FDG-PET (CT) vs CA-19-9 | FDG-PET was univariate preoperative predictor of OS | FDG-PET was superior in predicting pathologic treatment response | ||||||
| FDG-PET/CT was superior in diagnosis | ||||||||
| FDG-PET/CT | Independent prognostic factor of PFS and OS | |||||||
| Could predict prognosis | ||||||||
| Independent prognostic values for OS | ||||||||
| FDG-PET/CT and ceCT | Equal potential in Diagnosing | FDG-PET/CT was superior in metastasis detection | ||||||
| ceCT was superior at nodal staging | FDG-PET/CT was superior | |||||||
| F-FDG PET/CT was superior | F-FDG PET/CT was superior in predicting LN metastasis | |||||||
| FDG-PET/CT was superior | ||||||||
| FDG-PET/CT and FDG-PET/MR | Equal diagnostic performance | |||||||
| 18F-FDG PET/MR was superior in TNM staging | 18F-FDG PET/MR was superior in liver metastasis | |||||||
| FDG-PET/CT vs EUS | EUS was superior in detection of locoregional and isolated locoregional recurrences | |||||||
| 68Ga-FAPI PET/CT vs ceCT | 68Ga-FAPI PET/CT was superior | |||||||
| FAPI PET/(CT) vs 18F-FDG PET/CT | Equivalent detection ability | Al18F-NOTA-FAPI-04 PET/CT was superior in TNM staging | ||||||
| 68Ga-FAPI PET was superior in diagnosis | ||||||||
| Ga-FAPI PET/CT superior | Ga-FAPI PET/CT was superior | Ga-FAPI PET/CT superior | ||||||
| FDG-PET/CT vs CA19-9 | Both potent predictors for determining the lymph node status | |||||||
| Esophagus | SPECT | C-Met targeted fluorescence molecular endoscopy | It did not improve endoscopy | |||||
| 99mTc-3PRGD2 SPEC vs [18F] FDG PET/CT | SPECT was superior | SPECT was superior | FDG-PET/CT was superior | |||||
| 99mTc-3PRGD2 SPEC vs CT | SPECT was superior | |||||||
| PET | 18F-FDG PET/CT | Showed prognostic ability | ||||||
| Could identify interval metastasis | ||||||||
| Could predict prognosis | Could predict recurrence | |||||||
| Could predict recurrence | ||||||||
| Could predict prognosis | ||||||||
| 68Ga-FAPI-04 PET/CT | Could predict lymph node metastasis | |||||||
| [68Ga] Pentixafor-PET/CT vs (FDG)-PET/CT | [68Ga] Pentixafor-PET/CT can be complementary to (FDG)-PET/CT | [68Ga] Pentixafor-PET/CT can be complementary to (FDG)-PET/CT | ||||||
| MRI | CT vs MRI vs EUS | MRI had better diagnostic performance for tumor staging | ||||||
| DW-MRI | Could predict pathologic complete response | |||||||
| DCE-MRI | Could predict response to treatment | |||||||
| FDG-PET/CT vs DWI-MRI | DWI-MRI was independent prognostic factor | DWI-MRI was superior in predicting lymph node metastasis | ||||||
| Stomach | PET | FAPI PET/CT vs FDG PET/CT | FAPI PET/CT is more effective than FDG PET/CT | |||||
TNM: Tumor-node-metastasis; PFS: Progression-free survival; FDG: Fluorodeoxyglucose; PET: Positron emission tomography; CT: Computed tomography; DWI-MRI: Diffusion-weighted imaging magnetic resonance imaging; DCE: Dynamic contrast-enhanced; EUS: Endoscopic ultrasound; HCC: Hepatocellular carcinoma; SPECT: Single photon emission computed tomography; TACE: Transarterial chemoembolization; CCRT: Concurrent chemoradiotherapy; OS: Overall survival.
LIVER NEOPLASMS
Liver cancer is one of the current global health challenges not only because of its growing incidence but also because of its prognoses[55,56]. The five-year survival of liver cancer is about 21 percent. Therefore, liver cancer is one of the most lethal GI neoplasms[57].
The most common type of liver cancer is hepatocellular carcinoma (HCC), which accounts for approximately 90 percent of liver cancer cases[58]. HCC is the second most fatal tumor, with a 20.3 percent five-year survival rate, according to the surveillance, epidemiology, and results program of the National Cancer Institute (Figure 2, Table 1).
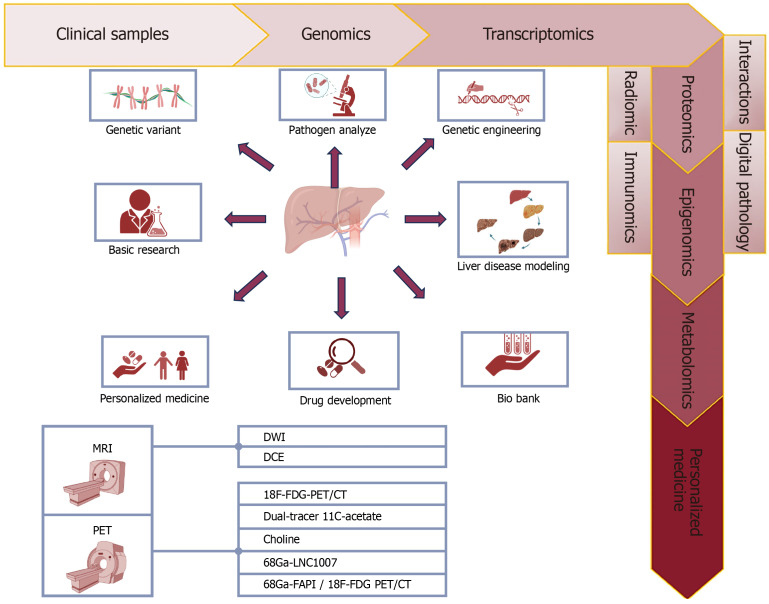
Figure 2.
Role of personalized medicine and molecular imaging in liver cancers. The main diagnostic and treatment modalities for liver cancer include positron emission tomography (PET) and magnetic resonance imaging, which are specific types of PET modalities including types bound with 18F-F-fluorodeoxyglucose, 11C-acetate, Choline, and types combined with Ga such as fibroblast activation protein inhibitor. The diagram with arrows shows the general progress and considerations made based on genetic, molecular, immunohistochemistry, microscopy, examination of pathogens at the cellular and molecular level, and background information of patients suffering from various types of liver cancer, which ultimately lead to adopting the best and most accurate and most appropriate and personalized solution available. In fact, in the pre-screening stage, collecting patient information, examining samples, and taking images to diagnose and adopt the most appropriate laboratory and imaging methods, as well as the most appropriate and personalized treatment method including medical treatment (drugs, dose, and type of drug), radiation therapy, non-intervention includes all types of surgeries on liver cancer patients according to different types of liver cancer including hepatocellular carcinoma, cholangiocarcinoma, and angiosarcoma. MRI: Magnetic resonance imaging; PET: Positron emission tomography; CT: Computed tomography; FDG: F-fluorodeoxyglucose; DCE: Dynamic contrast-enhanced imaging; DWI: Diffusion-weighted.
PET imaging using FDG
FDG-PET has a 50%-55% sensitivity for diagnosing and characterizing HCC[59-61]. A study found that 18F-FDG PET-CT has 59% accuracy, 60% specificity, and 76.5% sensitivity in identifying primary HCC and its metastases[62].
The prognostic use of FDG-PET in HCC appears to be connected to particular gene expressions. HCC cells with high expression of vinexin beta, vascular cell adhesion molecule-1, and the natural killer cell inhibitory receptor, appear to have more aggressive biological properties. A substantial association was found between the grade of FDG uptake and the pathological grade. In an experiment by Lee and colleagues on surgical samples from ten patients with HCC showed that tumors with significantly higher 18F-FDG uptake activity were more biologically malignant compared to tumors with lower 18F-FDG uptake. The presence of FDG within a diagnosed HCC might be regarded as an imaging indicator of biological aggressiveness[63].
Furthermore, in a meta-analysis, researchers indicated that an elevated tumor maximal Standardized Uptake Value (SUVmax), as well as a high ratio of lesion SUVmax to average liver SUVmax was associated with a poor outcome in HCC cases. Furthermore, the uptake of FDG in the primary lesions can differentiate the extra-hepatic HCC from intrahepatic disease with a higher uptake in extra-hepatic tumors[64].
Choline, a phosphatidyl cellular membrane component, is upregulated in tumor cells, and radiolabeled choline PET-CT detects well to moderately differentiated HCC more accurately than FDG-PET-CT. FDG-PET-CT is however superior for poorly differentiated and advanced-stage HCC. Combining choline PET-CT and FDG-PET-CT improves the detection of HCC. A systematic review and meta-analysis found that the pooled detection rate per patient and lesion-based of HCC with dual tracer PET-CT was 91% and 89%[65].
Tumor cells accumulate acetate, a precursor for fatty acid production, as a tumor activity marker. In a study by Ho et al[66] dual-tracer PET with FDG-11C-acetate showed 87.3% sensitivity in biopsy-confirmed HCC patients, while FDG had a sensitivity of 47.3%. According to a large cohort study, combining FDG PET-CT with C-11-acetate PET-CT improved sensitivity for identifying primary HCC, yet there was no improvement in detecting metastatic lesions. Additionally, they noted that FDG-PET has a 64.4% sensitivity for detecting primary HCC, whereas 11C-acetate PET has 84.4% sensitivity. A study indicates that dual-tracer PET-CT was much more percise at determining tumor-node-metastasis staging than contrast CT in liver transplantation (LT) and partial-hepatectomy patients. It also accurately assessed tumor burden regarding the size as well as the number of HCC lesions. LT individuals who have 11C-acetate-avid HCC lesions may have more prolonged survival after LT. In advanced-stage patients, 11C-acetate PET-CT might provide valuable information in addition to 18F-FDG because it is related to tumor differentiation in advanced-stages lesions; whereas 18F-FDG uptake was prevalent in those advanced-stage patients. Treatment strategies might be more personalized based on dual radiotracer PET-CT measurements of tumor metabolism features[67].
Fibroblast activation protein (FAP), a serine protease in fibroblast membranes, is excessively expressed in most of the epithelial carcinomas, such as HCC[68]. 68 Ga-FAP inhibitor (FAPI), a fibroblast activating protein inhibitor, helps diagnose various types of cancers[69], with 18F-FAPI-PET-CT being more sensitive in identifying HCC lesions[70-73]. However, 18F-FAPI benefits from a longer half-life which is equal to 68 Ga-FAPI in identifying malignant tumors[74]. A prospective study found that 18F-FAPI-PET-CT was better in detection of primary tumors and lymph node metastases in HCC compared to 18F-FDG-PET-CT[75].
Ga-68 LNC1007 is a radiotracer, created by chemically combining two ligands that target FAP and tripeptide arginine-glycine-aspartic, two key targets in cancer cell growth. A study comparing 68Ga-LNC1007 with 18F-FDG-PET-CT discovered that 68Ga-LNC1007 identified all of the HCC cases (55 primary tumors) and was superior to 2-18F-FDG for diagnosing HCC[76].
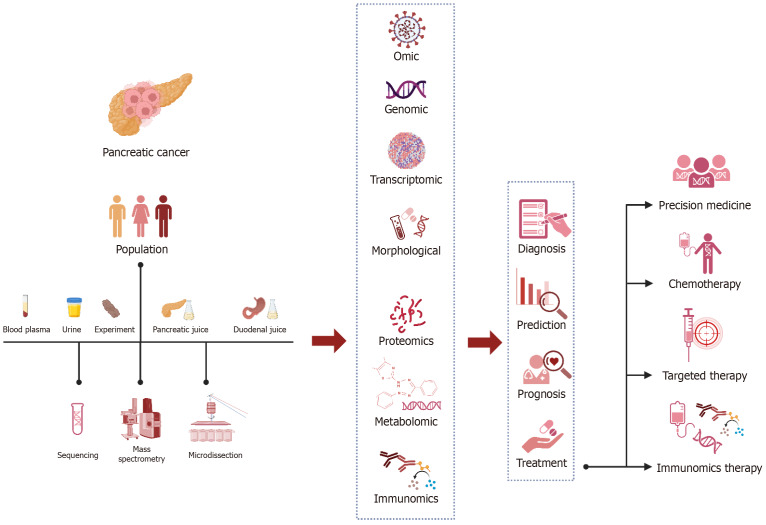
PANCREATIC NEOPLASMS
By 2025, PC is expected to become the third most common cause of cancer-related deaths, potentially overtaking breast cancer[77]. Typically, the initial phase of PC lacks noticeable symptoms which justifies the significant number of cases diagnosed at advanced stages (80%)[78,79]. Similar to other cancers, the management of PC depends on early diagnosis, suggesting the curable one-third of PC, if diagnosed at early stages[80]. Imaging techniques are necessary for PC's early detection, staging, treatment, and surgical resection procedures[81].
PET imaging using PET-CT
A meta-analysis which comprised 3567 patients with PC indicated that PET-CT for PC has 89% sensitivity, 70% specificity, and 84% diagnostic accuracy[82]. Nonetheless, PET-CT provides detailed data in volume, size, and stage[83]. The combined use with CA19-9 enhanced the indicators to 96.25%, 63.64%, and 92.31%. The SUVmax combined with CA19-9 level had a 0.94 area under the curve (AUC), substantially greater than either alone[84]. Additionally, the integrated use of PET-CT and CT exhibited even better accuracy (90.0%) and sensitivity (97.6%) than either scan alone. The investigators found that the combination of PET-CT and CT enhances the identification of recurrence. The PET-CT scan was particularly beneficial in identifying recurrences in regions that had been missed by the CT scan[85].
Moreover, PET-CT has been demonstrated to predict PC prognosis[86,87]. Low SUVmax is associated with a PC survival rate at each stage. It has been discovered that PET-CT metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were independent predictive variables of overall survival (OS) in locally progressed PC patients who received Stereotactic Body Radiation Therapy. In another study, MTV independently provided valuable information on prognosis of locally advanced PC targeted with radiation and chemotherapy. Characteristics shown via volume-based PET-CT approach may assist in identifying patients who benefit from radiation therapy[88].
Additionally, MTV and TLG can improve prediction models of prognosis of patients who are candidate for surgical management. In a study of 89 individuals with PC who experienced surgical therapy, of whom 57 treated with neoadjuvant chemotherapy, the MTV and TLG were shown to have significant potential in predicting relapse-free survival (RFS) and OS, regardless of treatment[89]. In another study, MTV and TLG predicted RFS and OS better than baseline tumor size, SUVmax, and serum CA19-9[90]. The results of a comparison study between PET-CT images obtained three months prior to and three months post-radioembolization of hepatic metastases originated from PC indicated that differences in the SUVmax and TLG could serve as predictors of progression-free survival (PFS), OS, and the time to intrahepatic progression subsequent to hepatic metastases[91].
PET imaging using other PET tracers
Quinoline-based FAPIs have shown promising outcomes in both pre/clinical molecular imaging investigations[92,93]. The first published report of clinical research employing FAPI-PET to examine PC was released in 2018[94]. Alongside the findings of this study, pancreatic neoplasms exhibited increased 68Ga-FAPI (FAPI-02) PET-CT uptake, while its rate in normal tissues, unlike 18F-FDG, was low[95]. This low background activity enhances the image contrast and accuracy. Likewise, other observational case studies showed the superiority of 68Ga-FAPI-PET-CT to 18F-FDG-PET-CT in detecting pancreatic-related metastasis[96,97].
Using multiplex immunohistochemistry, a retrospective investigation was conducted to analyze the images of 215 treatment-naïve pancreatic ductal adenocarcinomas (PDACs). The results showed that patients with FAP-dominant and fibroblast-rich stroma had a worse prognosis compared to those with collagen-rich stroma[98]. Shi et al[99] found that there was a strong correlation between high FAP expression levels and poor outcomes in 134 individuals with PDAC. Hence, parameters obtained from FAPI-PET-CT could be potentially in correlation with the clinical prognosis of PC. Nevertheless, this necessitates additional investigations.
Antibody-mediated PET scan
Although the majority of studies using antibody mediated PET in PC s are at animal experiments, they have reported promising outcomes. For instance, Chen et al[100] recruited 89Zr-labeled Anti-Trop-2 antibody (AF650) to evaluate Trop-2 as an immunoPET target in three PC cell lines (BxPC-3, MIA PaCa-2, and AsPC-1). The use of 89Zr-DFO-AF650 showed a remarkable capacity to differentiate primary tumors in the orthotopic BxPC-3 cancer model. It suggests a strong association observed between PET imaging and great sensitivity and bio-distribution. They concluded that this method clearly demonstrates the significant promise of Trop-2-based method of non-invasive imaging in detecting PC and tracking the treatment response.
COLORECTAL NEOPLASMS
In 2020, CRC was attributed to around 150000 new cases and over 53000 fatalities[101]. Non-metastatic CRC has a good prognosis, having an approximate 5-year survival rate of around 90[101]. Regrettably, the five-year survival rate for metastatic CRC stands at a mere 14%. The main treatment options for CRC are surgical removal of the primary tumor and metastasis, systemic chemotherapy, and neoadjuvant chemoradiation[102,103].
PET imaging using FDG
Despite the limitations of FDG-PET in inflammation, infection, and some non-neoplastic conditions[104], FDG-PET-CT is invaluable due to its capacity to evaluate the abnormal metabolic activity that occurs before any visible changes in structure, as well as its capability of detecting tiny malignant tumors within structures with normal morphology[105].
Several studies have tried to improve clinical management strategies of CRC by employing imaging prognostic parameters including depth of presence of malignant lymph nodes, tumor spread, extramural vascular invasion (EMVI), and tumor deposits[106]. In this direction, Lv et al[107] designed and validated a machine learning model of predicting the prognosis of primary CRC by 18F-FDG-PET-CT radiomic and clinical-biological features. They concluded that radiomics signature, including four clinical and four PET-CT characteristics, resulted in the most efficient prognostic prediction model (C-index 0.780, 95%CI: 0.634-0.877). In addition, they reported radiomics features to be associated with tumor metabolic markers, such as SUVmax and SUVmean. This integrated model of bio-clinic and radiology depicts the potentials of 18F-FDG- PET-CT findings along other features in predicting CRC prognosis.
PET imaging using other PET tracers
Studying primary and recurrent GI malignancies, Pang et al[108] found that 68Ga-FAPI-PET-CT with a detection rate of 100% is superior in detecting primary tumors than 18F-FDG-PET-CT with a detection rate of 53%. Furthermore, the 68Ga-FAPI PET/CT scan revealed enhanced tumor delineation and increased contrast between the tumor and its surrounding background. The study revealed the significant absorption of primary CRC lesions in 18F-FDG-PET-CT and 68Ga-FAPI-PET-CT scans. The SUVmax of 68Ga-FAPI in the primary lesions was greater than that of 18F-FDG (15.9 vs 7.9). The superiority of 68Ga-FAPI-PET-CT over 18F-FDG-PET-CT was evident in the visualization and image quality of peritoneal metastases in CRC. Additionally, the average SUVmax value was significantly greater[109].
In 2022, Kömek et al[110] evaluated the patients with CRC and showed that [68 Ga]Ga-DOTA-FAPI-04 PET/CT had a sensitivity of 90% in detecting nodal involvement and a sensitivity of 100% in peritoneal seeding, while these values for 18F-FDG-PET-CT were 80% and 55%. These findings shed light on the promising efficacy of [68 Ga] Ga-DOTA-FAPI-04 PET-CT in evaluating patients with CRC, regarding the primary and secondary lesions.
Prashanth et al[111] examined 29 patients with CRC by 68Ga-FAPI-PET-CT and reported the sensitivity of FAPI in the detection of recurrence was 100%, which was higher than that of FDG-conventional imaging (88%). In another study on 68Ga-FAPI-PET-CT, moderate 68Ga-FAPI absorption in primary CRC tumors was seen (SUVmax, 8.6). In metastatic lesions, however, the SUVmax and SUVmean were 7.95 and 3.96, respectively. Simultaneously, the activity levels of the background and normal tissues were extremely low. Consequently, the tumor-to-background ratio (TBR) exceeded 3, resulting in a significant contrast between normal and tumor tissue. Therefore, the study reported that FAPI-PET-CT has promising potential for detecting metastatic CRC, particularly in cases with lymph node and liver metastasis[112].
Dynamic contrast-enhanced imaging-MRI imaging
In 2022, Chen et al[113] explored the predictive and diagnostic potentials of dynamic contrast-enhanced imaging (DCE)-MRI in EMVI in 124 patients with rectal cancer. They reported the Ktrans and Ve values of EMVI-positive patients evaluated by DCE-MRI to be 1.08 ± 0.97 and 1.03 ± 0.93, while the values for conventional MRI were 0.68 ± 0.29 and 0.65 ± 0.31, respectively. These amounts were higher significantly in EMVI-negative patients (P < 0.05). In another study, Shen et al[114] applied DCE-MRI in 40 individuals with rectal cancer and 15 controls. The time-signal intensity curve of lesions presented in MRI exhibited an outflow pattern. In addition, a moderate association between Ktrans and iAUC, and pathological differentiation was highlighted (0.3 < r < 0.8, all P < 0.05), which provides new insights to preoperative diagnosis of rectal cancer.
ESOPHAGEAL NEOPLASMS
Although there have been advancements in therapeutic approaches and an increase in survival rates for esophageal cancer (EC) patients in the last twenty years, the prognosis of EC remains unfavorable, with an overall five-year survival rate of less than 20%[115,116] (Figure 3).
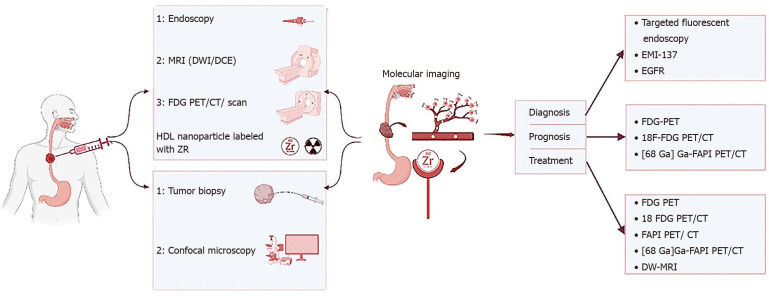
Figure 3.
Role of personalized medicine and molecular imaging in esophagus cancers. The function of molecular imaging and personalized medicine in esophageal cancers such as squamous cell carcinoma or adenocarcinoma including simultaneous or even initial and final examinations in the way of collecting temporal and personal information (specific to each person) in the diagnosis process including the collection of human samples, biopsy and blood samples and so on, all kinds of imaging modalities like endoscopy, magnetic resonance imaging (MRI) [diffusion-weighted (DWI)/dynamic contrast-enhanced imaging], [positron emission tomography (PET), PET computed tomograph (CT) and bound with F-fluorodeoxyglucose (FDG)] and also in the path of determining disease prognosis with PET types including FDG-PET, [68 Ga] Ga-FAPI PET/CT, and 18F-FDG PET/CT. Treatment with dedicated modalities FDG-PET, [68 Ga] Ga-FAPI PET/CT, 18F-FDG PET/CT, and DWI-MRI and it is tried in each stage of the currency, from the beginning of data collection to diagnosis, determining prognosis, treatment, response to treatment, assessment of relapse and follow-up, is the most appropriate and accurate and personalized solution possible based on personalized medicine in determining the dose of the substances used in the modalities imaging, choosing the most suitable imaging modality and binding element, the amount of medical treatment (drugs, amount and type of drug), and even all kinds of combined solutions with surgery and radiation therapy should be used. MRI: Magnetic resonance imaging; DW: Diffusion-weighted; PET: Positron emission tomography; CT: Computed tomography; FDG: F-fluorodeoxyglucose; EGFR: Epidermal growth factor receptor.
PET imaging using FDG
Tustumi et al[117] evaluated 113 patients with EC by 18F-FDG-PET-CT and demonstrated that TLG and MTV in the primary tumor and the SUVmax in the suspicious lymph nodes were correlated with survival after surgery (P ≤ 0.05). They concluded that pre-neoadjuvant 18F-FDG-PET-CT parameters can independently predict the prognosis (Figure 4).
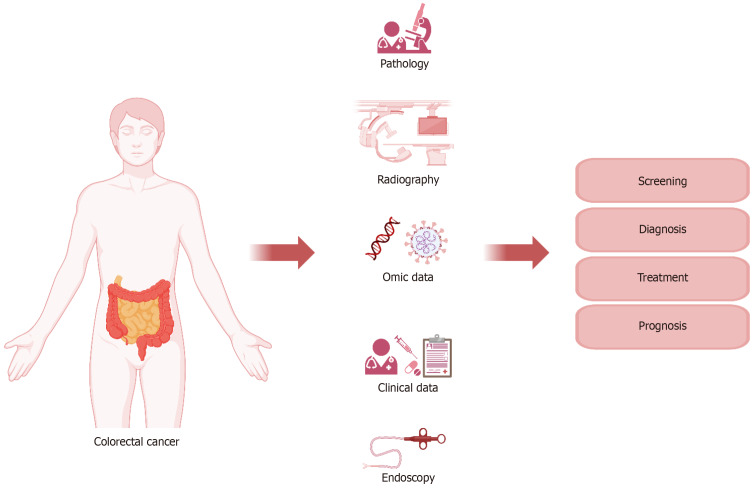
Figure 4.
Role of personalized medicine and molecular imaging in colorectal cancers. The function and role of molecular imaging and personalized medicine in charge of management of colorectal cancers is shown schematically in the picture, and the possibility of playing the role of these two concepts does not have any special priority or delay and can be simultaneous and parallel. The role of these two concepts is to adopt the most accurate, appropriate, and personalized mode for patients with colorectal cancers, including the basic steps of collecting individual demographic information of patients, collecting human samples, including blood, urine, and biopsy samples etc, and examination and analysis, screening, in the diagnostic path using common and novel imaging and anatomical laboratory methods and even at the cellular and molecular level, determining the most suitable and targeted treatment method including various medical methods (drugs, type and amount), and types of surgical methods, radiation therapy or combined methods, determining the rate of response to treatment, recurrence, prognosis and follow-up of patients according to the characteristics of each patient. Imaging modalities including positron emission tomography (PET), 18F-fluorodeoxyglucose (FDG) PET, FDG-PETPET, 68[Ga]Ga-DOTA-fibroblast activation protein inhibitor (FAPI)-04 PET/computed tomography (CT), 68Ga-FAPI PET/CT, magnetic resonance imaging (MRI), including dynamic contrast-enhanced imaging (DCE) MRI and CT perfusion imaging in determining the course diagnosis, prognosis and FDG-PET/CT, 18F-FDG PET/CT, 68Ga-FAPI PET/CT, and DCE-MRI in treatment.
PET imaging using other PET tracers
In a prospective analysis of 45 patients with locally advanced EC, the prognostic value of 68 Ga-FAPI was evaluated. Regarding the importance of T stage as a significant prognostic factor in patients with EC, groups with different T stages showed significantly different PET parameters; higher stages were associated with higher SUVmax-FAPI (P = 0.009) and GTVFAPI (P < 0.001). In addition, GTVFAPI values lower than 33.9 cm3 were correlated with better PFS. In this pilot study, Zhao et al[118] concluded that 68Ga-FAPI- PET-CT may predict the response to treatment and OS.
GASTRIC NEOPLASMS
The absolute number of cases of stomach cancer is expected to remain stable or perhaps rise in the future despite a lowering incidence, given the anticipated growth of the global population and the rise in average life expectancies in many countries. While there has been considerable progress in the clinical therapy of stomach cancer, most nations still have a 5-year survival rate of less than 30%, and reported death rates are in line with the disease's frequency. As a result, facilitating an early diagnosis are crucial.
PET imaging using FAPI
Pang et al[108] have recently demonstrated that FAPI-PET-CT is more effective than FDG-PET-CT in identifying primary and malignant lesions in a diverse group of patients, including GC. The significant detection rate observed for FAPI in primary gastric tumors with varying levels of differentiation, coupled with the recognized limitations of FDG in detecting certain subtypes of gastric carcinoma, like mucinous, poorly differentiated, and signet ring tumors[119], suggests the potential use of FAPI as a preferred radiotracer for assessing GC. The notably higher ratio of tumor uptake compared to background in primary GCs demonstrates the potential of FAPI-PET-CT for accurately identifying tumors for radiotherapy, as has been previously demonstrated in other tumor types[120] (Figure 5).
Figure 5.
Role of personalized medicine and molecular imaging in pancreatic cancers. The function and role of molecular imaging and personal medicine in the management of pancreas is shown in a semantic way, and the possibility of playing the role of these two concepts does not have any special priority or delay, and it can be both concurrent and concurrent, and it is changing day by day. The role of these two concepts is to adopt the most accurate, appropriate and personalized mode possible for pancreatic cancer patients from the initial stages of collecting individual demographic information of patients, collecting human samples including blood, urine and biopsy samples, etc. and checking and their analysis, in the path of individualization using the common methods of novel imaging and anatomical laboratory and even at the cellular and molecular level to determine the most appropriate and targeted treatment including various medical methods (drugs, type and amount) and various surgical methods, radiation Treatment or combined methods is to determine the rate of response to treatment, recurrence, prognosis and follow-up of patients according to the conditions and characteristics of each patient. Imaging modality in determining the course of diagnosis and determining prognosis includes endoscopy, computed tomography (CT), positron emission tomography (PET) and their combination with chemical agents such as 68Ga- fibroblast activation protein inhibitor (FAPI) PET/CT, 18F-fluorodeoxyglucose (FDG) PET/CT and even FAPI PET/CT alone, and Gd-enhanced magnetic resonance imaging (MRI) with diffusion-weighted along contrast-enhanced CT and adopting a treatment plan based on CT, PET, PET/CT, 18F-FDG PET/CT, FDG-PET/contrast-enhanced CT, FDG-PET/non-contrast-enhanced CT, FAPI PET/CT, single-photon emission CT/CT and dynamic contrast-enhanced-MRI are utilized to select chemotherapy, endohistochemical therapy or medical treatment based on personalized medicine.
ImmunoPET and ImmunoSPECT
Radiotracers based on antibodies are used in the imaging procedures of immunoPET and immunoSPECT. In both preclinical and clinical trials, non-invasive imaging of GC has been accomplished using immunoPET and immunoSPECT. The following examines the application of immunoPET in GC that targets the antigens CDH17, PD-1, and MG7. For PET or SPECT imaging of stomach tumors, antibodies that were either newly developed or approved by the FDA and that targeted membrane antigens were radiolabeled with gallium-68 (68 Ga), technetium-99m (99m Tc), indium-111 (111 In), copper-64 (64 Cu), zirconium-89 (89 Zr), and bromine-76 (76 Br).
The expression of MG7, an antigen specific to GC, is strongly linked with the advancement of the disease[121]. More than 90% of GC patients have MG7, which may serve as a biomarker for the disease because it is overexpressed in GC tissues as compared to benign lesions or normal mucosa[121]. Afterwards, the NOTA-MG7 compound was utilized as a probe for in vivo imaging of BGC-823 stomach xenografts after being radiolabeled with the short-lived radioisotope gallium-68. Effective labeling at room temperature is made possible by using NOTA as a bifunctional chelator to radiolabel biomolecules with gallium-68. This is crucial for maintaining the immunoreactivity of the antibody. At 60 minutes following intravenous (tail vein) injection of 68 Ga-NOTA-MG7, the 68 Ga-labeled immunoconjugate showed a tumor absorption of 2.53 ± 0.28 percent ID/g. 68 Ga-NOTA-MG7 accumulated in the liver and kidneys in addition to the tumor, most likely as a result of the probe's metabolism in these organs. Antibodies have a delayed biodistribution profile and comparatively extended biological half-life when administered systemically. Because of this, they are frequently marked with radiometals that have longer half-lives, such zirconium-89 (half-life of 3.3 days). Given that gallium-68 has a short half-life of 67.7 minutes, tagging the antibody with a radioisotope with a longer half-life could result in a larger TBR. The high expression of MG7 in Helicobacter pylori-associated stomach illnesses, which may skew patient immunoPET data, is another restriction on the use of 68 Ga-NOTA-MG7[121].
CONCLUSION
The use of cutting-edge biological technology in precision medicine allows for precise diagnosis and therapy by considering a patient's living environment in addition to their clinical data, molecular imaging methods, and bioinformatics. The inconsistent biological properties across the human genome make it challenging to pinpoint the exact clinical and biological importance for each given patient.
In terms of care, we anticipate that reducing the pool of potential patients in accordance with dosage and scheduling guidelines and choosing concurrent medications based on the molecular targeted agents' mechanisms would result in efficient therapy tailored to each patient. FDG-PET-CT and other PET Tracers are becoming a crucial component of the first diagnosis in patients with GI malignancies. Additionally, there is growing interest in studying various radiotracers, especially those that assess hypoxia and other significant characteristics of the tumor micro-environment. These techniques require a forward-looking attitude and clear objectives, which will ultimately guarantee the efficient utilization of these promising tools for identifying GI cancer characteristics, assessing treatment effectiveness, and providing guidance for therapy and monitoring. By incorporating these sophisticated imaging and molecular technologies into the therapeutic process, medical professionals can customize treatment approaches for each patient, track the effectiveness of treatment, and make well-informed choices on ongoing care. This holistic strategy has significant potential for enhancing patient outcomes in the treatment of GI cancers.
Footnotes
Conflict-of-interest statement: The authors had no conflict of interests.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medical laboratory technology
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade D
Scientific Significance: Grade C
P-Reviewer: Tang Y S-Editor: Qu XL L-Editor: A P-Editor: Zhang L
Contributor Information
Mobina Fathi, Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran 1983969411, Iran.
Hayder Jasim Taher, Department of Radiology, Hilla University College, Babylon 00964, Iraq.
Sabah Jassim Al-Rubiae, Department of Radiology, Hilla University College, Babylon 00964, Iraq.
Shirin Yaghoobpoor, Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran 1983969411, Iran.
Ashkan Bahrami, Faculty of Medicine, Kashan University of Medical Sciences, Kashan 1617768911, Iran.
Reza Eshraghi, Faculty of Medicine, Kashan University of Medical Sciences, Kashan 1617768911, Iran.
Hossein Sadri, Faculty of Medicine, Kashan University of Medical Sciences, Kashan 1617768911, Iran.
Mahsa Asadi Anar, Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran 1983969411, Iran.
Ali Gholamrezanezhad, Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, United States. gholamre@usc.edu.
References
- 1.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 3.Thakur M, Lentle BC. Report of a summit on molecular imaging. AJR Am J Roentgenol. 2006;186:297–299. doi: 10.2214/AJR.06.5020. [DOI] [PubMed] [Google Scholar]
- 4.Floyd E, McShane TM. Development and use of biomarkers in oncology drug development. Toxicol Pathol. 2004;32 Suppl 1:106–115. doi: 10.1080/01926230490425021. [DOI] [PubMed] [Google Scholar]
- 5.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 6.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 7.Bai JW, Qiu SQ, Zhang GJ. Molecular and functional imaging in cancer-targeted therapy: current applications and future directions. Signal Transduct Target Ther. 2023;8:89. doi: 10.1038/s41392-023-01366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vangestel C, Van de Wiele C, Mees G, Mertens K, Staelens S, Reutelingsperger C, Pauwels P, Van Damme N, Peeters M. Single-photon emission computed tomographic imaging of the early time course of therapy-induced cell death using technetium 99m tricarbonyl His-annexin A5 in a colorectal cancer xenograft model. Mol Imaging. 2012;11:135–147. [PubMed] [Google Scholar]
- 9.Raza Naqvi SH, Fatima M, Ali PS, Alam M, Khan MT, Qadir F. Positional syncope occurring just at the right time in a patient with a permanent pacemaker: A case report. J Pak Med Assoc. 2021:71: 2461–2463. doi: 10.47391/JPMA.06-844. [DOI] [PubMed] [Google Scholar]
- 10.Kajary K, Molnár Z. The role of 18F-FDG PET/CT before and after the treatment of multiple myeloma: Our clinical experience. Adv Mod Oncol Res. 2017;3:20. [Google Scholar]
- 11.Basu S, Hess S, Nielsen Braad PE, Olsen BB, Inglev S, Høilund-Carlsen PF. The Basic Principles of FDG-PET/CT Imaging. PET Clin. 2014;9:355–370, v. doi: 10.1016/j.cpet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 12.van der Graaf M. In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophys J. 2010;39:527–540. doi: 10.1007/s00249-009-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinberg I, Huland DM, Vermesh O, Frostig HE, Tummers WS, Gambhir SS. Photoacoustic clinical imaging. Photoacoustics. 2019;14:77–98. doi: 10.1016/j.pacs.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nat Photonics. 2009;3:503–509. doi: 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover VP, Tognarelli JM, Crossey MM, Cox IJ, Taylor-Robinson SD, McPhail MJ. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. J Clin Exp Hepatol. 2015;5:246–255. doi: 10.1016/j.jceh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadjipanayis CG, Jiang H, Roberts DW, Yang L. Current and future clinical applications for optical imaging of cancer: from intraoperative surgical guidance to cancer screening. Semin Oncol. 2011;38:109–118. doi: 10.1053/j.seminoncol.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller J, Wunder A, Licha K. Optical imaging. Recent Results Cancer Res. 2013;187:221–246. doi: 10.1007/978-3-642-10853-2_7. [DOI] [PubMed] [Google Scholar]
- 18.Wu B, Warnock G, Zaiss M, Lin C, Chen M, Zhou Z, Mu L, Nanz D, Tuura R, Delso G. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 2016;3:19. doi: 10.1186/s40658-016-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang KA, Bruley DF, Londono JM, Chance B. Highly scattering optical system identification via frequency response analysis of NIR-TRS spectra. Ann Biomed Eng. 1994;22:240–252. doi: 10.1007/BF02368231. [DOI] [PubMed] [Google Scholar]
- 20.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsoukalas C, Psimadas D, Kastis GA, Koutoulidis V, Harris AL, Paravatou-Petsotas M, Karageorgou M, Furenlid LR, Moulopoulos LA, Stamopoulos D, Bouziotis P. A Novel Metal-Based Imaging Probe for Targeted Dual-Modality SPECT/MR Imaging of Angiogenesis. Front Chem. 2018;6:224. doi: 10.3389/fchem.2018.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zasadny KR, Kison PV, Francis IR, Wahl RL. FDG-PET Determination of Metabolically Active Tumor Volume and Comparison with CT. Clin Positron Imaging. 1998;1:123–129. doi: 10.1016/s1095-0397(98)00007-7. [DOI] [PubMed] [Google Scholar]
- 23.Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20:e354–e367. doi: 10.1016/S1470-2045(19)30317-1. [DOI] [PubMed] [Google Scholar]
- 24.Pantel AR, Ackerman D, Lee SC, Mankoff DA, Gade TP. Imaging Cancer Metabolism: Underlying Biology and Emerging Strategies. J Nucl Med. 2018;59:1340–1349. doi: 10.2967/jnumed.117.199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momcilovic M, Shackelford DB. Imaging Cancer Metabolism. Biomol Ther (Seoul) 2018;26:81–92. doi: 10.4062/biomolther.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smyth E, Schöder H, Strong VE, Capanu M, Kelsen DP, Coit DG, Shah MA. A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer. 2012;118:5481–5488. doi: 10.1002/cncr.27550. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Zheng D, Liu JJ, Cui JX, Xi HQ, Zhang KC, Huang XH, Wei B, Wang XX, Xu BX, Li K, Gao YH, Liang WQ, Tian JH, Chen L. Comparing PET/MRI with PET/CT for Pretreatment Staging of Gastric Cancer. Gastroenterol Res Pract. 2019;2019:9564627. doi: 10.1155/2019/9564627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, Fung JY, Yan Chan AC, Sharr W, Yau T, Poon RT, Fan ST. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon's perspective. J Nucl Med. 2013;54:192–200. doi: 10.2967/jnumed.112.107516. [DOI] [PubMed] [Google Scholar]
- 29.Buck AK, Herrmann K, Stargardt T, Dechow T, Krause BJ, Schreyögg J. Economic evaluation of PET and PET/CT in oncology: evidence and methodologic approaches. J Nucl Med. 2010;51:401–412. doi: 10.2967/jnumed.108.059584. [DOI] [PubMed] [Google Scholar]
- 30.European Society of Radiology. Medical imaging in personalised medicine: a white paper of the research committee of the European Society of Radiology (ESR) Insights Imaging. 2011;2:621–630. doi: 10.1007/s13244-011-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farwell MD, Clark AS, Mankoff DA. How Imaging Biomarkers Can Inform Clinical Trials and Clinical Practice in the Era of Targeted Cancer Therapy. JAMA Oncol. 2015;1:421–422. doi: 10.1001/jamaoncol.2015.0667. [DOI] [PubMed] [Google Scholar]
- 32.Pantel AR, Mankoff DA. Molecular imaging to guide systemic cancer therapy: Illustrative examples of PET imaging cancer biomarkers. Cancer Lett. 2017;387:25–31. doi: 10.1016/j.canlet.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cisło M, Filip AA, Arnold Offerhaus GJ, Ciseł B, Rawicz-Pruszyński K, Skierucha M, Polkowski WP. Distinct molecular subtypes of gastric cancer: from Laurén to molecular pathology. Oncotarget. 2018;9:19427–19442. doi: 10.18632/oncotarget.24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho SWT, Tan P. Dissection of gastric cancer heterogeneity for precision oncology. Cancer Sci. 2019;110:3405–3414. doi: 10.1111/cas.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahl RL. The Interaction of Genomics, Molecular Imaging, and Therapy in Gastrointestinal Tumors. Semin Nucl Med. 2020;50:471–483. doi: 10.1053/j.semnuclmed.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Evans PM. Anatomical imaging for radiotherapy. Phys Med Biol. 2008;53:R151–R191. doi: 10.1088/0031-9155/53/12/R01. [DOI] [PubMed] [Google Scholar]
- 38.Baka S, Clamp AR, Jayson GC. A review of the latest clinical compounds to inhibit VEGF in pathological angiogenesis. Expert Opin Ther Targets. 2006;10:867–876. doi: 10.1517/14728222.10.6.867. [DOI] [PubMed] [Google Scholar]
- 39.Tang Y, Kim M, Carrasco D, Kung AL, Chin L, Weissleder R. In vivo assessment of RAS-dependent maintenance of tumor angiogenesis by real-time magnetic resonance imaging. Cancer Res. 2005;65:8324–8330. doi: 10.1158/0008-5472.CAN-05-0027. [DOI] [PubMed] [Google Scholar]
- 40.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 42.Piwnica-worms DA, Luker GD, Anderson C, Wahl RL. Molecular Imaging in Oncology. Mol Nucl Med. 2003 [Google Scholar]
- 43.Donati OF, Reiner CS, Hany TF, Fornaro J, von Schulthess GK, Marincek B, Weishaupt D. 18F-FDG-PET and MRI in patients with malignancies of the liver and pancreas. Accuracy of retrospective multimodality image registration by using the CT-component of PET/CT. Nuklearmedizin. 2010;49:106–114. doi: 10.3413/nukmed-0263. [DOI] [PubMed] [Google Scholar]
- 44.Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, Bockisch A, Debatin JF, Freudenberg LS. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol. 2004;22:4357–4368. doi: 10.1200/JCO.2004.08.120. [DOI] [PubMed] [Google Scholar]
- 45.Barwick T, Murray I, Megadmi H, Drake WM, Plowman PN, Akker SA, Chew SL, Grossman AB, Avril N. Single photon emission computed tomography (SPECT)/computed tomography using Iodine-123 in patients with differentiated thyroid cancer: additional value over whole body planar imaging and SPECT. Eur J Endocrinol. 2010;162:1131–1139. doi: 10.1530/EJE-09-1023. [DOI] [PubMed] [Google Scholar]
- 46.Mishra A, Pfeuffer J, Mishra R, Engelmann J, Mishra AK, Ugurbil K, Logothetis NK. A new class of Gd-based DO3A-ethylamine-derived targeted contrast agents for MR and optical imaging. Bioconjug Chem. 2006;17:773–780. doi: 10.1021/bc050295b. [DOI] [PubMed] [Google Scholar]
- 47.Hüber MM, Staubli AB, Kustedjo K, Gray MH, Shih J, Fraser SE, Jacobs RE, Meade TJ. Fluorescently detectable magnetic resonance imaging agents. Bioconjug Chem. 1998;9:242–249. doi: 10.1021/bc970153k. [DOI] [PubMed] [Google Scholar]
- 48.Seaman ME, Contino G, Bardeesy N, Kelly KA. Molecular imaging agents: impact on diagnosis and therapeutics in oncology. Expert Rev Mol Med. 2010;12:e20. doi: 10.1017/S1462399410001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WARBURG O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 50.Taegtmeyer H, Dilsizian V. Imaging myocardial metabolism and ischemic memory. Nat Clin Pract Cardiovasc Med. 2008;5 Suppl 2:S42–S48. doi: 10.1038/ncpcardio1186. [DOI] [PubMed] [Google Scholar]
- 51.Matsui T, Nakata N, Nagai S, Nakatani A, Takahashi M, Momose T, Ohtomo K, Koyasu S. Inflammatory cytokines and hypoxia contribute to 18F-FDG uptake by cells involved in pannus formation in rheumatoid arthritis. J Nucl Med. 2009;50:920–926. doi: 10.2967/jnumed.108.060103. [DOI] [PubMed] [Google Scholar]
- 52.Rubin DT, Surma BL, Gavzy SJ, Schnell KM, Bunnag AP, Huo D, Appelbaum DE. Positron emission tomography (PET) used to image subclinical inflammation associated with ulcerative colitis (UC) in remission. Inflamm Bowel Dis. 2009;15:750–755. doi: 10.1002/ibd.20819. [DOI] [PubMed] [Google Scholar]
- 53.Takuma K, Kamisawa T, Itoi T. Positive response to steroid therapy for autoimmune pancreatitis evaluated with fluorodeoxyglucose positron emission tomography. Clin Gastroenterol Hepatol. 2010;8:e54–e55. doi: 10.1016/j.cgh.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, White C, Lowe C, Sherba JJ, Hartmanshenn C, O'Neill KM, Balter ML, Fritz ZR, Androulakis IP, Schloss RS, Yarmush ML. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci) 2018;6:79–100. doi: 10.1142/S2339547818300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 56.Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 57.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 58.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 59.Wudel LJ Jr, Delbeke D, Morris D, Rice M, Washington MK, Shyr Y, Pinson CW, Chapman WC. The role of [18F]fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. Am Surg. 2003;69:117–24; discussion 124. [PubMed] [Google Scholar]
- 60.Kashiwagi T. FDG-PET and hepatocellular carcinoma. J Gastroenterol. 2004;39:1017–1018. doi: 10.1007/s00535-004-1458-y. [DOI] [PubMed] [Google Scholar]
- 61.Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, Zeuzem S. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314–3319. doi: 10.1111/j.1572-0241.1999.01544.x. [DOI] [PubMed] [Google Scholar]
- 62.Shaban EAIN. Can fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography detect hepatocellular carcinoma and its extrahepatic metastases? Egypt J Radiol Nucl Med. 2018;49:196–201. [Google Scholar]
- 63.Lee JD, Yun M, Lee JM, Choi Y, Choi YH, Kim JS, Kim SJ, Kim KS, Yang WI, Park YN, Han KH, Lee WJ, Yoo N, Lim SM, Park JH. Analysis of gene expression profiles of hepatocellular carcinomas with regard to 18F-fluorodeoxyglucose uptake pattern on positron emission tomography. Eur J Nucl Med Mol Imaging. 2004;31:1621–1630. doi: 10.1007/s00259-004-1602-1. [DOI] [PubMed] [Google Scholar]
- 64.Sun DW, An L, Wei F, Mu L, Shi XJ, Wang CL, Zhao ZW, Li TF, Lv GY. Prognostic significance of parameters from pretreatment (18)F-FDG PET in hepatocellular carcinoma: a meta-analysis. Abdom Radiol (NY) 2016;41:33–41. doi: 10.1007/s00261-015-0603-9. [DOI] [PubMed] [Google Scholar]
- 65.Signore G, Nicod-lalonde M, Prior JO, Bertagna F, Muoio B, Giovanella L, Furlan C, Treglia G. Detection rate of radiolabelled choline PET or PET/CT in hepatocellular carcinoma: an updated systematic review and meta-analysis. Clin Transl Imaging. 2019;7:237–253. [Google Scholar]
- 66.Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213–21. [PubMed] [Google Scholar]
- 67.Park S, Kim TS, Kang SH, Kim HB, Park JW, Kim SK. 11C-acetate and 18F-fluorodeoxyglucose positron emission tomography/computed tomography dual imaging for the prediction of response and prognosis after transarterial chemoembolization. Medicine (Baltimore) 2018;97:e12311. doi: 10.1097/MD.0000000000012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boulter L, Bullock E, Mabruk Z, Brunton VG. The fibrotic and immune microenvironments as targetable drivers of metastasis. Br J Cancer. 2021;124:27–36. doi: 10.1038/s41416-020-01172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, Adeberg S, Rathke H, Röhrich M, Winter H, Plinkert PK, Marme F, Lang M, Kauczor HU, Jäger D, Debus J, Haberkorn U, Giesel FL. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med. 2019;60:801–805. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siripongsatian D, Promteangtrong C, Kunawudhi A, Kiatkittikul P, Boonkawin N, Chinnanthachai C, Jantarato A, Chotipanich C. Comparisons of Quantitative Parameters of Ga-68-Labelled Fibroblast Activating Protein Inhibitor (FAPI) PET/CT and [(18)F]F-FDG PET/CT in Patients with Liver Malignancies. Mol Imaging Biol. 2022;24:818–829. doi: 10.1007/s11307-022-01732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo W, Pang Y, Yao L, Zhao L, Fan C, Ke J, Guo P, Hao B, Fu H, Xie C, Lin Q, Wu H, Sun L, Chen H. Imaging fibroblast activation protein in liver cancer: a single-center post hoc retrospective analysis to compare [(68)Ga]Ga-FAPI-04 PET/CT versus MRI and [(18)F]-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48:1604–1617. doi: 10.1007/s00259-020-05095-0. [DOI] [PubMed] [Google Scholar]
- 72.Shi X, Xing H, Yang X, Li F, Yao S, Congwei J, Zhao H, Hacker M, Huo L, Li X. Comparison of PET imaging of activated fibroblasts and (18)F-FDG for diagnosis of primary hepatic tumours: a prospective pilot study. Eur J Nucl Med Mol Imaging. 2021;48:1593–1603. doi: 10.1007/s00259-020-05070-9. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J, Guan Y, Jia H, Chen J, Lu L, Xie F, Qin L. (68)Ga-FAPI-04 Versus (18)F-FDG PET/CT in the Detection of Hepatocellular Carcinoma. Front Oncol. 2021;11:693640. doi: 10.3389/fonc.2021.693640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu K, Wang L, Wu H, Huang S, Tian Y, Wang Q, Xiao C, Han Y, Tang G. [(18)F]FAPI-42 PET imaging in cancer patients: optimal acquisition time, biodistribution, and comparison with [(68)Ga]Ga-FAPI-04. Eur J Nucl Med Mol Imaging. 2022;49:2833–2843. doi: 10.1007/s00259-021-05646-z. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, Jiang S, Li M, Xue H, Zhong X, Li S, Peng H, Liang J, Liu Z, Rao S, Chen H, Cao Z, Gong Y, Chen G, Zhang R, Zhang L. Head-to-head comparison of (18)F-FAPI and (18)F-FDG PET/CT in staging and therapeutic management of hepatocellular carcinoma. Cancer Imaging. 2023;23:106. doi: 10.1186/s40644-023-00626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zang J, Lin R, Wen X, Wang C, Zhao T, Jakobsson V, Yang Y, Wu X, Guo Z, Chen X, Zhang J, Miao W. A Head-to-Head Comparison of 68Ga-LNC1007 and 2-18F-FDG/68Ga-FAPI-02 PET/CT in Patients With Various Cancers. Clin Nucl Med. 2023;48:861–868. doi: 10.1097/RLU.0000000000004820. [DOI] [PubMed] [Google Scholar]
- 77.Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016;55:1158–1160. doi: 10.1080/0284186X.2016.1197419. [DOI] [PubMed] [Google Scholar]
- 78.Gits H, Tang A, Harmsen W, Bamlet W, Graham R, Mahipal A, Ashman J, Rule W, Owen D, Neben-wittich M, Ma W, Truty M, Sio T, Haddock M, Hallemeier C, Merrell K. SMAD4 as a Predictive Biomarker For Locally Aggressive Phenotype In Resected Pancreas Cancer. Int J Radiat Oncolo Biol Phys. 2020;108:e617. [Google Scholar]
- 79.Preston Hewgley W, Hester CA, Zeh HJ, Charles Yopp A, Marcelo Polanco P. Disparities in Surgical Resection for Early Stage Pancreas Cancer in the Texas-Mexico Border Population. J Am Coll Surg. 2020;231:S266–267. [Google Scholar]
- 80.Xiang M, Heestand GM, Chang DT, Pollom EL. Neoadjuvant treatment strategies for resectable pancreas cancer: A propensity-matched analysis of the National Cancer Database. Radiother Oncol. 2020;143:101–107. doi: 10.1016/j.radonc.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Horvat N, Ryan DE, LaGratta MD, Shah PM, Do RK. Imaging for pancreatic ductal adenocarcinoma. Chin Clin Oncol. 2017;6:62. doi: 10.21037/cco.2017.11.03. [DOI] [PubMed] [Google Scholar]
- 82.Toft J, Hadden WJ, Laurence JM, Lam V, Yuen L, Janssen A, Pleass H. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: A systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur J Radiol. 2017;92:17–23. doi: 10.1016/j.ejrad.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Sur YK, Kim YC, Kim JK, Lee JH, Yoo BM, Kim YB. Comparison of Ultrasound-Guided Core Needle Biopsy and Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Solid Pancreatic Lesions. J Ultrasound Med. 2015;34:2163–2169. doi: 10.7863/ultra.14.11030. [DOI] [PubMed] [Google Scholar]
- 84.Lin JL, Barthel JS, Keshishian J, Eikman EA, Klapman JB. Negative predictive value of positron emission tomography/computed tomography in patients with a clinical suspicion of pancreatic cancer. Pancreas. 2011;40:653–656. doi: 10.1097/MPA.0b013e31821b5bf7. [DOI] [PubMed] [Google Scholar]
- 85.Jung W, Jang JY, Kang MJ, Chang YR, Shin YC, Chang J, Kim SW. The clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) in follow-up of curatively resected pancreatic cancer patients. HPB (Oxford) 2016;18:57–64. doi: 10.1016/j.hpb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hyun SH, Kim HS, Choi SH, Choi DW, Lee JK, Lee KH, Park JO, Lee KH, Kim BT, Choi JY. Intratumoral heterogeneity of (18)F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2016;43:1461–1468. doi: 10.1007/s00259-016-3316-6. [DOI] [PubMed] [Google Scholar]
- 87.Parikh U, Marcus C, Sarangi R, Taghipour M, Subramaniam RM. FDG PET/CT in Pancreatic and Hepatobiliary Carcinomas: Value to Patient Management and Patient Outcomes. PET Clin. 2015;10:327–343. doi: 10.1016/j.cpet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Choi HJ, Lee JW, Kang B, Song SY, Lee JD, Lee JH. Prognostic significance of volume-based FDG PET/CT parameters in patients with locally advanced pancreatic cancer treated with chemoradiation therapy. Yonsei Med J. 2014;55:1498–1506. doi: 10.3349/ymj.2014.55.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, Lee JD. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative ¹⁸F-FDG PET/CT in Patients with Pancreatic Cancer. J Nucl Med. 2014;55:898–904. doi: 10.2967/jnumed.113.131847. [DOI] [PubMed] [Google Scholar]
- 90.Xu HX, Chen T, Wang WQ, Wu CT, Liu C, Long J, Xu J, Zhang YJ, Chen RH, Liu L, Yu XJ. Metabolic tumour burden assessed by ¹⁸F-FDG PET/CT associated with serum CA19-9 predicts pancreatic cancer outcome after resection. Eur J Nucl Med Mol Imaging. 2014;41:1093–1102. doi: 10.1007/s00259-014-2688-8. [DOI] [PubMed] [Google Scholar]
- 91.Michl M, Lehner S, Paprottka PM, Ilhan H, Bartenstein P, Heinemann V, Boeck S, Albert NL, Fendler WP. Use of PERCIST for Prediction of Progression-Free and Overall Survival After Radioembolization for Liver Metastases from Pancreatic Cancer. J Nucl Med. 2016;57:355–360. doi: 10.2967/jnumed.115.165613. [DOI] [PubMed] [Google Scholar]
- 92.Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, Debus J, Marmé F, Jäger D, Mier W, Haberkorn U. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J Nucl Med. 2019;60:1421–1429. doi: 10.2967/jnumed.118.224469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, Jäger D, Mier W, Haberkorn U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med. 2018;59:1415–1422. doi: 10.2967/jnumed.118.210443. [DOI] [PubMed] [Google Scholar]
- 94.Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, Giesel F, Kratochwil C, Barthe P, Roumestand C, Haberkorn U. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J Nucl Med. 2018;59:1423–1429. doi: 10.2967/jnumed.118.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, Wei J, Wu S, Zhao L, Luo Z, Lin X, Xie C, Sun L, Lin Q, Wu H. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–1832. doi: 10.1007/s00259-020-04769-z. [DOI] [PubMed] [Google Scholar]
- 96.Kaghazchi F, Divband G, Amini H, Adinehpour Z, Akbarian Aghdam R. 68 Ga-FAPI-46 and 18 F-FDG in Advanced Metastatic Pancreatic Cancer. Clin Nucl Med. 2022;47:e666–e669. doi: 10.1097/RLU.0000000000004316. [DOI] [PubMed] [Google Scholar]
- 97.Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y, Wu X, Guo W, Fan C, Wu J, Huang W, Lin Q, Sun L, Wu H. Usefulness of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [(18)F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. 2021;48:73–86. doi: 10.1007/s00259-020-04940-6. [DOI] [PubMed] [Google Scholar]
- 98.Ogawa Y, Masugi Y, Abe T, Yamazaki K, Ueno A, Fujii-Nishimura Y, Hori S, Yagi H, Abe Y, Kitago M, Sakamoto M. Three Distinct Stroma Types in Human Pancreatic Cancer Identified by Image Analysis of Fibroblast Subpopulations and Collagen. Clin Cancer Res. 2021;27:107–119. doi: 10.1158/1078-0432.CCR-20-2298. [DOI] [PubMed] [Google Scholar]
- 99.Shi M, Yu DH, Chen Y, Zhao CY, Zhang J, Liu QH, Ni CR, Zhu MH. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol. 2012;18:840–846. doi: 10.3748/wjg.v18.i8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen W, Li M, Younis MH, Barnhart TE, Jiang D, Sun T, Lang JM, Engle JW, Zhou M, Cai W. ImmunoPET of trophoblast cell-surface antigen 2 (Trop-2) expression in pancreatic cancer. Eur J Nucl Med Mol Imaging. 2022;49:861–870. doi: 10.1007/s00259-021-05563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 102.Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370–398. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 103.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hess S, Hansson SH, Pedersen KT, Basu S, Høilund-Carlsen PF. FDG-PET/CT in Infectious and Inflammatory Diseases. PET Clin. 2014;9:497–519, vi. doi: 10.1016/j.cpet.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 105.Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol. 2011;38:55–69. doi: 10.1053/j.seminoncol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.García-Figueiras R, Baleato-González S, Padhani AR, Luna-Alcalá A, Marhuenda A, Vilanova JC, Osorio-Vázquez I, Martínez-de-Alegría A, Gómez-Caamaño A. Advanced Imaging Techniques in Evaluation of Colorectal Cancer. Radiographics. 2018;38:740–765. doi: 10.1148/rg.2018170044. [DOI] [PubMed] [Google Scholar]
- 107.Lv L, Xin B, Hao Y, Yang Z, Xu J, Wang L, Wang X, Song S, Guo X. Radiomic analysis for predicting prognosis of colorectal cancer from preoperative (18)F-FDG PET/CT. J Transl Med. 2022;20:66. doi: 10.1186/s12967-022-03262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, Sun L, Chen H. Comparison of (68)Ga-FAPI and (18)F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology. 2021;298:393–402. doi: 10.1148/radiol.2020203275. [DOI] [PubMed] [Google Scholar]
- 109.Elboga U, Sahin E, Kus T, Cayirli YB, Aktas G, Okuyan M, Cinkir HY, Teker F, Sever ON, Aytekin A, Yılmaz L, Aytekin A, Cimen U, Mumcu V, Kilbas B, Eryilmaz K, Cakici D, Celen YZ. Comparison of (68)Ga-FAPI PET/CT and (18)FDG PET/CT Modalities in Gastrointestinal System Malignancies with Peritoneal Involvement. Mol Imaging Biol. 2022;24:789–797. doi: 10.1007/s11307-022-01729-x. [DOI] [PubMed] [Google Scholar]
- 110.Kömek H, Can C, Kaplan İ, Gündoğan C, Kepenek F, Karaoglan H, Demirkıran A, Ebinç S, Güzel Y, Gündeş E. Comparison of [(68) Ga]Ga-DOTA-FAPI-04 PET/CT and [(18)F]FDG PET/CT in colorectal cancer. Eur J Nucl Med Mol Imaging. 2022;49:3898–3909. doi: 10.1007/s00259-022-05839-0. [DOI] [PubMed] [Google Scholar]
- 111.Prashanth A, Kumar Ravichander S, Eswaran P, Kalyan S, Maheswari Babu S. Diagnostic performance of Ga-68 FAPI 04 PET/CT in colorectal malignancies. Nucl Med Commun. 2023;44:276–283. doi: 10.1097/MNM.0000000000001661. [DOI] [PubMed] [Google Scholar]
- 112.Koerber SA, Staudinger F, Kratochwil C, Adeberg S, Haefner MF, Ungerechts G, Rathke H, Winter E, Lindner T, Syed M, Bhatti IA, Herfarth K, Choyke PL, Jaeger D, Haberkorn U, Debus J, Giesel FL. The Role of (68)Ga-FAPI PET/CT for Patients with Malignancies of the Lower Gastrointestinal Tract: First Clinical Experience. J Nucl Med. 2020;61:1331–1336. doi: 10.2967/jnumed.119.237016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Z, Hu D, Ye G, Xu D. Quantitative Evaluation of Extramural Vascular Invasion of Rectal Cancer by Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Contrast Media Mol Imaging. 2022;2022:3038308. doi: 10.1155/2022/3038308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shen FU, Lu J, Chen L, Wang Z, Chen Y. Diagnostic value of dynamic contrast-enhanced magnetic resonance imaging in rectal cancer and its correlation with tumor differentiation. Mol Clin Oncol. 2016;4:500–506. doi: 10.3892/mco.2016.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang J, Liu X, Cao S, Dong X, Rao S, Cai K. Understanding Esophageal Cancer: The Challenges and Opportunities for the Next Decade. Front Oncol. 2020;10:1727. doi: 10.3389/fonc.2020.01727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li J, Xu J, Zheng Y, Gao Y, He S, Li H, Zou K, Li N, Tian J, Chen W, He J. Esophageal cancer: Epidemiology, risk factors and screening. Chin J Cancer Res. 2021;33:535–547. doi: 10.21147/j.issn.1000-9604.2021.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tustumi F, Duarte PS, Albenda DG, Takeda FR, Sallum RAA, Junior UR, Buchpiguel CA, Cecconello I. Prognostic value of 18F-fluorodeoxyglucose PET/computed tomography metabolic parameters measured in the primary tumor and suspicious lymph nodes before neoadjuvant therapy in patients with esophageal carcinoma. Nucl Med Commun. 2021;42:437–443. doi: 10.1097/MNM.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 118.Zhao L, Pang Y, Chen S, Chen J, Li Y, Yu Y, Huang C, Sun L, Wu H, Chen H, Lin Q. Prognostic value of fibroblast activation protein expressing tumor volume calculated from [(68) Ga]Ga-FAPI PET/CT in patients with esophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2023;50:593–601. doi: 10.1007/s00259-022-05989-1. [DOI] [PubMed] [Google Scholar]
- 119.Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, Honma K, Suzuki T. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–641. doi: 10.1002/1097-0142(20010801)92:3<634::aid-cncr1364>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 120.Syed M, Flechsig P, Liermann J, Windisch P, Staudinger F, Akbaba S, Koerber SA, Freudlsperger C, Plinkert PK, Debus J, Giesel F, Haberkorn U, Adeberg S. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur J Nucl Med Mol Imaging. 2020;47:2836–2845. doi: 10.1007/s00259-020-04859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo DL, Dong M, Wang L, Sun LP, Yuan Y. Expression of gastric cancer-associated MG7 antigen in gastric cancer, precancerous lesions and H. pylori -associated gastric diseases. World J Gastroenterol. 2002;8:1009–1013. doi: 10.3748/wjg.v8.i6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]