Abstract
Aims
To compare the diagnostic performance of 360° anterior segment optical coherence tomography assessment by applying normative percentile cut-offs versus iris trabecular contact (ITC) for detecting gonioscopic angle closure.
Methods
In this multicentre study, 394 healthy individuals were included in the normative dataset to derive the age-specific and angle location-specific normative percentiles of angle open distance (AOD500) and trabecular iris space area (TISA500) which were measured every 10° for 360°. 119 healthy participants and 170 patients with angle closure by gonioscopy were included in the test dataset to investigate the diagnostic performance of three sets of criteria for detection of gonioscopic angle closure: (1) the 10th and (2) the 5th percentiles of AOD500/TISA500, and (3) ITC (ie, AOD500/TISA500=0 mm/mm2). The number of angle locations with angle closure defined by each set of the criteria for each eye was used to generate the receiver operating characteristic (ROC) curve for the discrimination between gonioscopic angle closure and open angle.
Results
Of the three sets of diagnostic criteria examined, the area under the ROC curve was greatest for the 10th percentile of AOD500 (0.933), whereas the ITC criterion AOD500=0 mm showed the smallest area under the ROC (0.852) and the difference was statistically significant with or without adjusting for age and axial length (p<0.001). The criterion ≥90° of AOD500 below the 10th percentile attained the best sensitivity 87.6% and specificity 84.9% combination for detecting gonioscopic angle closure.
Conclusions
Applying the normative percentiles of angle measurements yielded a higher diagnostic performance than ITC for detecting angle closure on gonioscopy.
Keywords: Angle, Imaging, Glaucoma
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Anterior segment optical coherence tomography (AS-OCT) has been used for detecting iris trabecular contact (ITC) in the diagnostic assessment of angle closure, although it remains unclear how AS-OCT can be applied for detecting angle closure as determined from gonioscopy.
WHAT THIS STUDY ADDS
This study investigated the diagnostic criteria of 360° AS-OCT imaging, comparing anterior chamber angle normative cut-offs versus ITC for detecting gonioscopic angle closure. We showed that applying normative percentiles of angle measurements outperformed ITC to identify eyes with gonioscopic angle closure.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study illustrates the importance of 360° imaging and measurements of the anterior chamber angle dimensions in clinical practice for detection of gonioscopic angle closure and highlights the differences between gonioscopic angle closure and anatomical angle closure.
The diagnosis of primary angle closure disease (PACD), including primary angle closure suspect (PACS), primary angle closure (PAC) and primary angle closure glaucoma (PACG), is contingent on the assessment of angle closure by gonioscopy, which is conventionally identified when the posterior trabecular meshwork is not visible for at least 180°.1–4 Although gonioscopy is the current clinical standard to detect angle closure, gonioscopy assessment can be confounded by slit-lamp illumination and inadvertent corneal indentation. Consequently, interobserver agreement of gonioscopy grading is weak.5 6 While anterior segment optical coherence tomography (AS-OCT) can image the anterior chamber angle (ACA) in the dark and provides objective and reproducible measurements of the ACA dimensions,7–9 AS-OCT has not been routinely adopted to detect angle closure in clinical practice because there is no consensus regarding how angle closure should be defined using AS-OCT. While AS-OCT was previously shown to detect more closed ACAs than gonioscopy,10 we recently reported that only 31.8–35.3% of eyes with gonioscopic angle closure had iris trabecular contact (ITC) for ≥180° in AS-OCT.11 Our finding suggests that angle closure as determined by gonioscopy may not signify anatomical angle closure as visualised from AS-OCT.
We hypothesised applying normative percentile cut-offs of angle opening distance (AOD) or trabecular iris space area (TISA) can attain a higher diagnostic performance than ITC for the detection of gonioscopic angle closure. Determining the AS-OCT criteria for identifying gonioscopic angle closure is relevant to the management of PACD because all the treatment recommendations for PACD have been based on studies that used gonioscopy to define angle closure. In this multicentre study, we investigated the diagnostic performance of 360° AS-OCT imaging for detecting gonioscopic angle closure.
Methods
The study followed the Standards for Reporting Diagnostic Accuracy Studies12 (online supplemental appendix). This prospective study included two datasets: (1) a test dataset to investigate the specificities and sensitivities of AS-OCT to detect gonioscopic angle closure, and (2) a normative dataset to derive age-specific and angle location-specific percentiles of AOD500 and TISA500 measurements for classification of open angle versus angle closure. The test dataset has been described in a previous study.11 In brief, it comprised 289 eyes from 119 healthy individuals with gonioscopic open angles and 170 patients with gonioscopic angle closure consecutively enrolled between 1 April 2017 and 31 October 2018 (phase I) from four study sites: Hong Kong Eye Hospital (HKEH), Tokyo University (TU), University of California San Francisco and Zhongshan Ophthalmic Center (ZOC). For the normative dataset, we set out to recruit at least 300 healthy individuals, 50 subjects from each of the following age groups: 20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years and ≥70 years. They were consecutively enrolled between 1 February 2019 and 31 December 2019 (phase II) from three study sites: HKEH, TU and ZOC. Healthy individuals had open angles on dark room gonioscopy for both eyes and no evidence of ocular disease except visually insignificant cataract. Gonioscopic angle closure was defined as having posterior trabecular meshwork not visible for ≥180° on dark room gonioscopy. PACS had angle closure on gonioscopy without a history of intraocular pressure (IOP) >21 mm Hg, peripheral anterior synechiae (PAS) or glaucoma; PAC had gonioscopic angle closure with IOP >21 mm Hg in ≥2 consecutive visits or PAS; PACG had gonioscopic angle closure and glaucoma.1–4 All other forms of secondary angle closure were excluded. The inclusion criteria for healthy participants and patients with PACD were as follows: phakic eyes, best-corrected visual acuity at least 20/40, spherical equivalent greater than or equal to −6.0 D, and without any history of intraocular surgical procedure or laser treatment. If both eyes of a participant were considered eligible, one eye would be randomly selected for inclusion. All participants received comprehensive eye examinations, dark room gonioscopy and AS-OCT imaging in the same visit. There were no adverse events associated with gonioscopy or AS-OCT imaging.
bjo-2023-323860supp001.pdf (1.1MB, pdf)
Ocular examination
Clinical examination included slit-lamp biomicroscopy, Goldmann applanation tonometry and dark room gonioscopy. Best-corrected visual acuity, refractive errors and axial length (IOL Master, Carl Zeiss Meditec, Dublin, California, USA) were measured. Gonioscopy was the reference standard because it had been universally accepted for assessment of the ACA. Glaucoma specialists performed static gonioscopy using a Posner or Sussman goniolens in a darkened room using the lowest possible slit-lamp illumination. Attention was exercised to minimise illumination onto the pupil from the slit lamp. The modified Shaffer grading system was applied to grade the angle. Appositional angle closure and synechial angle closure were differentiated with dynamic gonioscopy. Twenty-six healthy subjects in the test dataset missed the measurement of the axial length.
AS-OCT imaging
The CASIA2 was selected for the study because it provided a higher scan density for circumferential imaging of the anterior segment. We applied 18 evenly spaced radial B-scans over 360° (ie, 10° resolution) for the assessment of ACA. The whole anterior segment could be imaged in less than 0.3 s, thereby minimising motion artefacts. The anterior chamber angle was imaged in a darkened room (<1.0 lux) by a blinded technician on the same day of gonioscopy and clinical examination. During AS-OCT imaging, participants fixated at an internal fixation target; the lower ACA was revealed by guiding the lower lid down towards the lower orbital rim, while the upper ACA was revealed by guiding the upper lid up towards the upper orbital rim. The scan protocol was composed of 18 radial evenly spaced B-scans, each with 800 A-scans in 16 mm (length)×14 mm (depth). Eyes with ACA obscured by eyelids for ≥1 B-scan or non-visible scleral spur for ≥5 angle locations would be excluded. For eyes with non-visible scleral spur for more than one but less than five angle locations, the scleral spurs were marked taking reference from the adjacent B-scans 10° or 20° apart. The AS-OCT scans were not available to the glaucoma specialists prior to the gonioscopy.
Analysis of ACA dimensions
The scleral spur of 24 588 angle locations from a total of 513 healthy individuals and 170 patients with PACD included in the test dataset and the normative dataset was marked by a blinded single observer (KO). The scleral spur represents an internal projection of the inner margin of the cornea and trabecular meshwork at the boundary between the ciliary muscle and the sclera.13 14 The software of CASIA2 automatically traced the corneal and the iris boundaries. AOD500/TISA500 were measured every 10° over 360°. The angle locations of the right and left eyes were annotated in left eye orientation (0° the temporal angle, 90° the superior angle, 180° the nasal angle and 270° the inferior angle). The AOD500 represented the perpendicular distance from the posterior corneoscleral junction at 500 µm anterior to the scleral spur to the anterior iris border. TISA500 represented the area enclosed by (1) the AOD500, (2) a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the opposing iris, (3) the inner corneoscleral wall and (4) the anterior iris border.
Diagnostic criteria of AS-OCT
Three sets of ACA thresholds (ie, the 5th percentile of AOD500/TISA500, the 10th percentile of AOD500/TISA500 and AOD500/TISA500=0 mm/mm2) were applied to classify whether an angle location was open or closed. The age-specific and angle location-specific ACA thresholds were derived from the normative dataset. The number of angle locations (out of the 36 angle locations) with angle closure (as per defined by a prespecified ACA threshold) for each eye was used to generate the receiver operating characteristic (ROC) curves and to identify the best sensitivity and specificity combination (ie, Youden’s index) to discriminate eyes with open angle from eyes with angle closure.
Sample size calculation
We planned to recruit at least 300 healthy subjects in the normative dataset and at least 90 healthy participants and 180 patients with PACD in the test dataset. For the normative dataset, 292 subjects were needed to establish the fifth percentile (q) with 95% confidence (ie, α=5%) that the margin of error (d) was within 2.5% based on binomial approximation: .15 For the test dataset, the number of subjects required to investigate the sensitivities and specificities for the detection of gonioscopic angle closure had been described in a previous study.11
Statistics
Statistics were analysed with Stata (StataCorp, College Station, Texas, USA, V.15.0) and R V.3.2.5 (R Foundation for Statistical Computing, Vienna, Austria). Two-tailed t-tests were applied to compare the biometric parameters between the diagnostic groups. The sensitivity and specificity for detecting gonioscopic angle closure were determined from the area under ROC curves (AUC); the AUCs were compared with ROC regression analysis, with adjustment of axial length and age.
Results
ACA measurements in the normative dataset
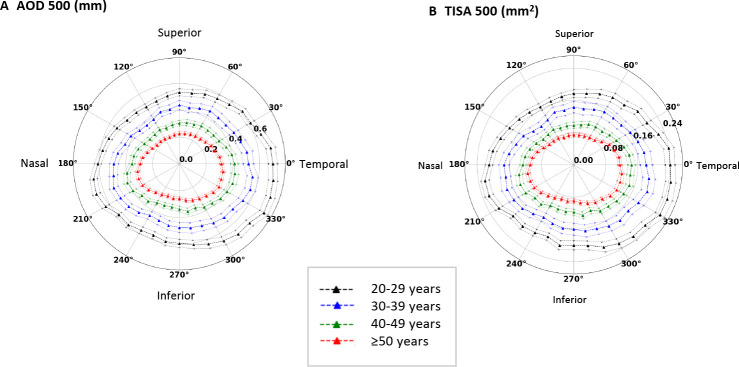
AS-OCT measurements of 394 healthy subjects were included in the normative dataset after excluding 2 subjects who had ACA obscured by eyelids and 2 subjects with scleral spur not discernible for more than five angle locations (figure 1). All participants were Asians. Increasing age was associated with decreasing mean AOD500/TISA500 (online supplemental figure 1), axial length and anterior chamber depth (online supplemental figure 2), although the associations were no longer significant beyond 50 years. The 5th percentiles and the 10th percentiles of AOD500/TISA500 were therefore derived from each of the 36 angle locations by four age groups (20–29 years (n=95), 30–39 years (n=66), 40–49 years (n=71) and ≥50 years (n=162)). There were no significant differences in AOD500/TISA500 between genders by age groups (online supplemental figure 3 and online supplemental table 1). The circumferential AOD500/TISA500 measurements of the four age groups were displayed every 10° for 360° in the polar plots (figure 2). Across the four age groups, the superior and the inferior quadrants had smaller AOD500/TISA500 than the nasal and temporal quadrants. The narrowest angles were located at the superior meridians between approximately 40° and 140°, whereas the widest angles were located at the inferotemporal meridians between approximately 300° and 350°. Online supplemental table 2 shows the demographics and the ACA dimensions over the four age groups.
Figure 1.
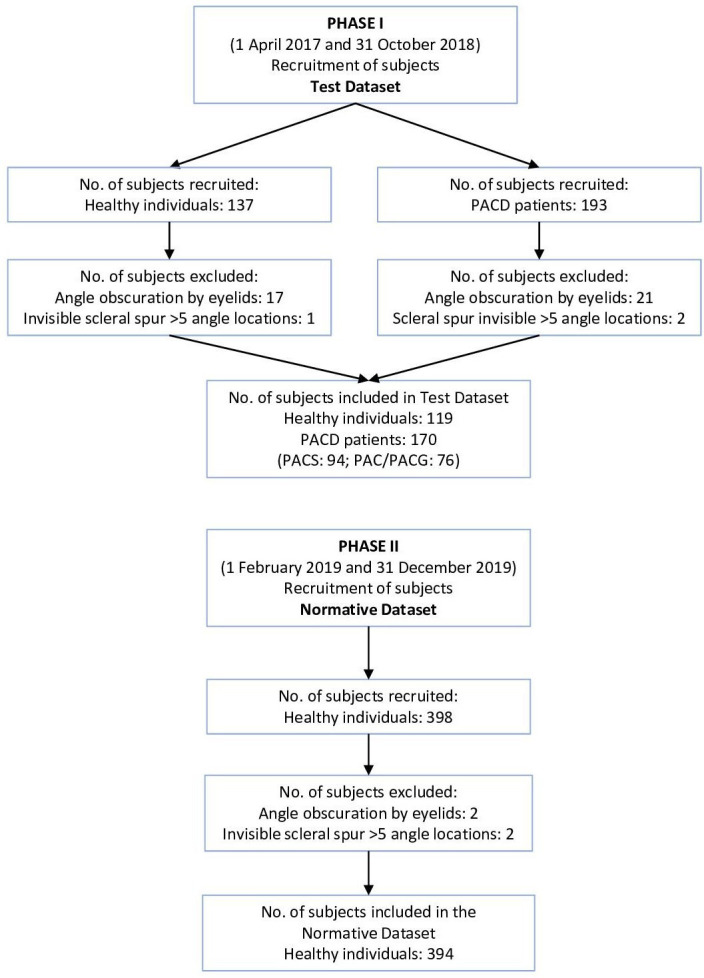
Flow chart of subject recruitment and inclusion. Recruitment and inclusion of healthy individuals and patients with primary angle closure disease (PACD) in phase I, and healthy individuals in phase II. PAC, primary angle closure; PACG, primary angle closure glaucoma; PACS, primary angle closure suspect.
Figure 2.
Circumferential analysis of the anterior chamber angle width in 394 eyes from 394 healthy individuals with gonioscopically open angle. Polar plots of angle opening distance (AOD500) (A) and trabecular space iris area (TISA500) (B) in different age groups. The angle locations of the right and left eyes were analysed in left eye orientation; 0° denotes the temporal, 90° the superior, 180° the nasal and 270° the inferior angle. Solid lines: mean; dotted lines: SEM.
Diagnostic performance of AS-OCT for detection of gonioscopic angle closure
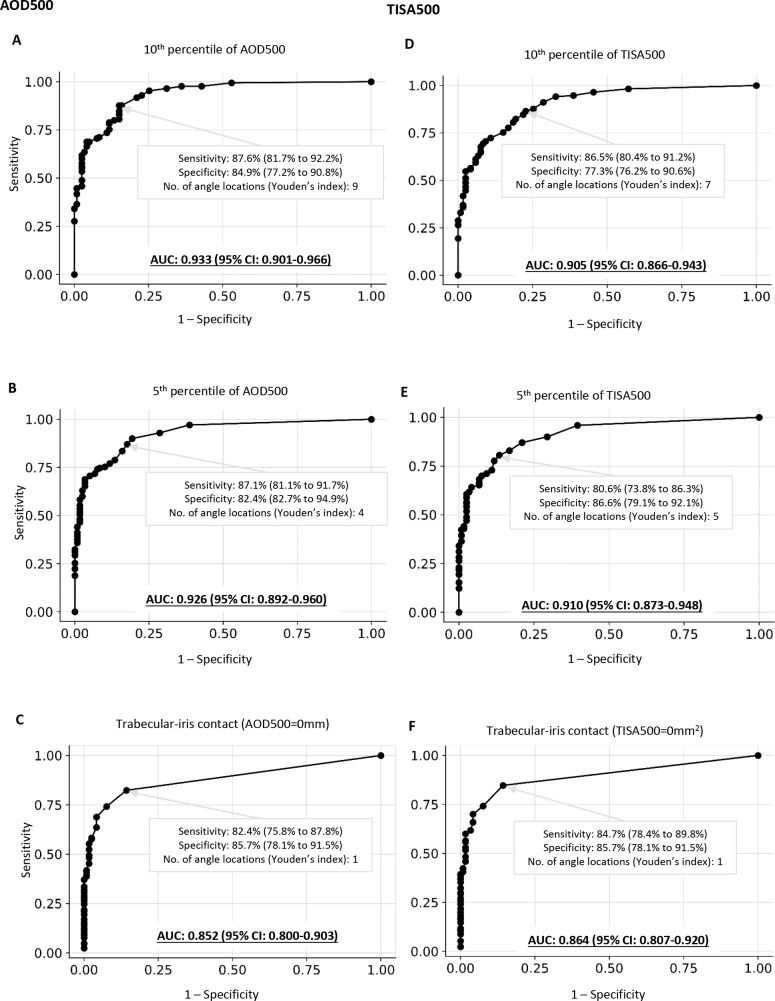
In the test dataset, we excluded 38 subjects who had ACA obscured by the lids and 3 subjects who had scleral spur not detectable for more than five angle locations. A total of 119 healthy individuals (89 Asian, 18 white, 5 Hispanic, 1 Native American, and 2 black individuals, 4 individuals of unknown ethnicity) and 170 patients with PACD (143 Asian, 14 white, 6 black, and 3 Hispanic individuals, 4 individuals of unknown ethnicity) were included, among which 94 patients had PACS and 76 patients had PAC/PACG (online supplemental table 3).11 The distribution of age and AOD500/TISA500 of the test dataset is shown in online supplemental figure 4. Among the three sets of diagnostic criteria examined, the 10th percentiles of AOD500 showed the greatest AUC (0.933, 95% CI: 0.901 to 0.966), whereas the ITC criterion AOD500=0 mm (0.852, 95% CI: 0.800 to 0.903) showed the smallest (figure 3), and the differences were statistically significant with or without adjustment of age and axial length (p<0.001). The criterion nine angle locations (ie, 90°) of AOD500 below the 10th percentiles attained the best sensitivity (87.6%) and specificity (84.9%) combination (the Youden’s index) for the detection of gonioscopic angle closure. Cross-tabulation of the AS-OCT results by the results of gonioscopy is shown in online supplemental table 4. The sensitivities and specificities in detecting gonioscopic angle closure by age groups (40–49 years vs ≥50 years) and by ethnicity (Asians vs non-Asians) are shown in online supplemental figures 5 and 6 and online supplemental figures 7 and 8, respectively. The 10th percentiles of AOD500 consistently showed a greater AUC compared with ITC.
Figure 3.
Diagnostic performance of narrow angle thresholds (the 5th and 10th percentiles of angle opening distance (AOD500) (A and B) /trabecular iris space area (TISA500) (D and E)) versus iris trabecular contact (ITC500) (C and F) for discrimination between gonioscopic open angle and gonioscopic angle closure. The area under the receiver operating characteristic (ROC) curve (AUC) was compared using ROC regression analysis with adjustment for age and axial length.
Discussion
A major benefit of AS-OCT over gonioscopy for detecting angle closure stems from its ability of objectively and reproducibly measuring the ACA dimensions in the dark. Since clinical evidence pertaining to the management of PACS, PAC and PACG has been garnered from studies with angle closure defined by gonioscopy, applying AS-OCT criteria to identify gonioscopic angle closure is useful in guiding treatment decisions for patients with PACD. Our study revealed that applying ACA threshold criteria had a higher diagnostic performance than applying ITC to detect gonioscopic angle closure (figure 3). Specifically, AOD500 below the 10th percentile for at least 90° best approximates gonioscopy assessment which yields the highest sensitivity and specificity combination for the detection of gonioscopic angle closure among the three sets of diagnostic thresholds examined in the study. In other words, gonioscopic angle closure does not necessarily signify anatomical angle closure and 360° measurements of the ACA dimension with AS-OCT are relevant to identify eyes with gonioscopic angle closure.
Diagnostic criteria for detecting gonioscopic angle closure
We have previously shown that the sensitivity of ITC for ≥180° was very low (31.8% for AOD500 and 35.3% for TISA500).11 To facilitate the comparison of diagnostic performance between normative percentile cut-offs versus ITC for detecting gonioscopic angle closure, we therefore applied prespecified percentiles to identify the number of angle locations that showed the highest sensitivity and specificity combination instead of identifying the percentiles that best detect gonioscopic angle closure for ≥180°. Likewise, we identified the number of angle locations that yield the highest sensitivity and specificity combination for ITC as shown in figure 3. We selected the fifth percentile because of the convention of adopting 5% as an arbitrary cut-off to discriminate normal from abnormal measurements in many normative data collection studies. ITC (ie, AOD500/TISA500=0 mm/0 mm2) represents anatomical angle closure. We did not select the first percentile because it approximates closely with ITC. We additionally investigated the 10th percentile to allow increased sensitivity (although at the expense of reduced specificity) to detect angle closure.
Gonioscopic angle closure versus anatomical angle closure
Prior application of AS-OCT for detection of angle closure has been essentially centred on qualitative examination of ITC in the B-scans.16–22 The ACA dimensions are rarely measured. Analysis of the ACA dimensions over 360° is important because ACA measurements varied substantially across individual angle locations. It is for this reason that we collected 360° normative ACA measurements to determine the age-specific and location-specific ACA thresholds to define angle closure. The finding that the 10th and the 5th percentiles of AOD500/TISA500 had a greater AUC than ITC (ie, AOD500/TISA500=0 mm/mm2) for the detection of gonioscopic angle closure underscores the different nature of angle closure defined by AS-OCT and gonioscopy—eyes with non-visible posterior trabecular meshwork for more than 180° on gonioscopy may have no ITC on AS-OCT.11 While the clinical implication of gonioscopic angle closure has been well established, the importance of detecting anatomical angle closure, or ITC on AS-OCT, in the management of PACD remains to be determined. A greater extent of ITC on AS-OCT has been shown to be associated with greater odds of PAC/PACG in eyes with angle closure detected by gonioscopy in a cross-sectional study.11 Prospective clinical studies and clinical trials are needed to unravel whether angle closure defined by gonioscopy or ITC as identified from AS-OCT is more predictive of disease progression and treatment outcomes in the PACD continuum.
Deep learning versus non-deep learning approaches for detecting gonioscopic angle closure
Several deep learning approaches for discrimination between open angle and angle closure have been proposed,23–28 but it is unclear whether these models work better for detecting gonioscopic angle closure compared with non-deep learning approaches. The reported deep learning models are also limited by the fact that the anatomical features that characterise gonioscopic angle closure (ie, the degree and the extent of angle narrowing) are obscured in the ‘black box’. Without knowing the optimal criteria for detecting angle closure, comparison of diagnostic performance of non-deep learning approaches versus deep learning models would be difficult. Our study was designed to investigate the optimal AS-OCT diagnostic criteria, in terms of the degree of angle narrowing and the extent of angle narrowing, for detecting gonioscopic angle closure. The finding of a higher diagnostic performance of using the normative percentiles rather than using ITC for detecting gonioscopic angle closure underscores the clinical benefits of the normative approach.
Limitations
The study has a number of weaknesses. Although more than 85% of the patients with PACG in the world reside in Asia,29 the fact that the normative ACA measurements in the study were essentially derived from Asians could limit its application in other ethnic groups. Nevertheless, the diagnostic performance of the normative dataset was found to be high even in non-Asians (online supplemental figures 6 and 7). We excluded individuals with visually significant cataract because visually significant cataract is closely associated with angle closure. For the same reason, patients with secondary angle closure were also excluded from the normative dataset. The exclusion of individuals with ocular pathology other than visually insignificant cataract can limit the study generalisability. Yet, the rationale of excluding individuals with ocular pathology stems from the fact that the study sample was not population based, and the normative percentile cut-offs generated from a clinic-based sample could potentially be biased by the specific ocular comorbidities presented in a particular cohort. Ideally, the normative dataset should be obtained from a large population-based study. To minimise potential biases introduced by over-representation or under-representation of certain ocular comorbidities in a clinic-based sample, we therefore only recruited healthy individuals without ocular pathology other than visually insignificant cataract. Since the scleral spur was marked by a single observer, the generalisability of the AOD500/TISA500 percentile cut-offs requires further validation. The inclusion of deep learning models for detecting the scleral spur can facilitate the automation of workflow for 360° measurement of ACA dimensions in the diagnostic assessment of angle closure.30 31
Footnotes
Contributors: Research design—CK-SL, SL, MA and XZ. Data acquisition and/or research execution—PYG, XZ, CL, FL, AN, RS, MY, MA, SL and CK-SL. Data analysis and/or interpretation—PYG, CL, RH, KO, MY, TA and CK-SL. CL is the gurantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: Tomey Corporation had no role in the design, execution, interpretation of the data or decision to submit results.
Competing interests: XZ, MA, SL and CK-SL have received support from Tomey in the form of instrument and speaker honorarium. SL is consultant to Aerie, Iridex, Bausch & Lomb and Allergan. TA is consultant to Allergan, Alcon, Novartis, Santen, Sun Pharma and Belkin Laser, and has received research support from Allergan and Santen. CK-SL has received research support in the form of research grant and instruments from Carl Zeiss Meditec, Topcon and Heidelberg Engineering; he has been an advisory board member for Alcon, Novartis, Santen, Aerie and Allergan. The study instruments (CASIA2, Tomey Corporation, Nagoya, Japan) were on loan from Tomey Corporation during the study period.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was conducted according to the ethical standards stated in the 2013 Declaration of Helsinki. The respective Institutional Review Boards of Hong Kong Eye Hospital, Tokyo University, University of California San Francisco and Zhongshan Ophthalmic Center approved the study (Hong Kong Hospital Authority Research Ethics Committee, approval ID: KC/KE-06-0098/ER-2; Ethics Committee of the University of Tokyo Hospital, approval ID: 11517; University of California San Francisco Committee on Human Research, approval ID: 11-07289; Ethics Committee of Zhongshan Ophthalmic Center, approval ID: 2018KYPJ074). All participants signed an informed consent form.
References
- 1. Foster PJ, Buhrmann R, Quigley HA, et al. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002;86:238–42. 10.1136/bjo.86.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European glaucoma society terminology and guidelines for glaucoma, 4TH edition-Chapter 2. classification and terminology supported by the EGS foundation: part 1: Foreword; introduction; glossary; chapter 2 classification and terminology. Br J Ophthalmol 2017;101:73–127. 10.1136/bjophthalmol-2016-EGSguideline.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Primary angle closure preferred practice pattern 2015. In: American Academy of Ophthalmology Glaucoma Preferred Practice Pattern Panel. [Google Scholar]
- 4. Asian Pacific Glaucoma Society . Asia Pacific glaucoma guidelines. Asian Pacific Glaucoma Society; 2016. Available: http://www.icoph.org/dynamic/attachments/resources/asia_pacific_glaucoma_guidelines_2016_third_edition.pdf [Google Scholar]
- 5. Phu J, Wang H, Khuu SK, et al. Anterior chamber angle evaluation using Gonioscopy: consistency and agreement between Optometrists and Ophthalmologists. Optom Vis Sci 2019;96:751–60. 10.1097/OPX.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 6. Campbell P, Redmond T, Agarwal R, et al. Repeatability and comparison of clinical techniques for anterior chamber angle assessment. Ophthalmic Physiol Opt 2015;35:170–8. 10.1111/opo.12200 [DOI] [PubMed] [Google Scholar]
- 7. Müller M, Dahmen G, Pörksen E, et al. Anterior chamber angle measurement with optical coherence tomography: Intraobserver and Interobserver variability. J Cataract Refract Surg 2006;32:1803–8. 10.1016/j.jcrs.2006.07.014 [DOI] [PubMed] [Google Scholar]
- 8. Liu S, Yu M, Ye C, et al. Anterior chamber angle imaging with swept-source optical coherence tomography: an investigation on variability of angle measurement. Invest Ophthalmol Vis Sci 2011;52:8598–603. 10.1167/iovs.11-7507 [DOI] [PubMed] [Google Scholar]
- 9. Römkens HCS, Beckers HJM, Frusch M, et al. Reproducibility of anterior chamber angle analyses with the swept-source optical coherence tomography in young, healthy Caucasians. Invest Ophthalmol Vis Sci 2014;55:3999–4004. 10.1167/iovs.13-12904 [DOI] [PubMed] [Google Scholar]
- 10. Sakata LM, Lavanya R, Friedman DS, et al. Comparison of Gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology 2008;115:769–74. 10.1016/j.ophtha.2007.06.030 [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Guo PY, Lin C, et al. Assessment of Iris trabecular contact in eyes with Gonioscopic angle-closure. Ophthalmology 2023;130:111–9. 10.1016/j.ophtha.2022.08.017 [DOI] [PubMed] [Google Scholar]
- 12. Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016;6:e012799. 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seager FE, Wang J, Arora KS, et al. The effect of Scleral spur identification methods on structural measurements by anterior segment optical coherence tomography. J Glaucoma 2014;23:e29–38. 10.1097/IJG.0b013e31829e55ae [DOI] [PubMed] [Google Scholar]
- 14. Liu S, Li H, Dorairaj S, et al. Assessment of Scleral spur visibility with anterior segment optical coherence tomography. J Glaucoma 2010;19:132–5. 10.1097/IJG.0b013e3181a98ce4 [DOI] [PubMed] [Google Scholar]
- 15. Mori S, Kitsukawa K, Hisakado M. Correlation structures of correlated binomial models and implied default distribution. J Phys Soc Jpn 2008;77. 10.1143/JPSJ.77.114802 [DOI] [Google Scholar]
- 16. Porporato N, Baskaran M, Aung T. Role of anterior segment optical coherence tomography in angle-closure disease: a review. Clin Exp Ophthalmol 2018;46:147–57. 10.1111/ceo.13120 [DOI] [PubMed] [Google Scholar]
- 17. Porporato N, Baskaran M, Tun TA, et al. Understanding diagnostic disagreement in angle closure assessment between anterior segment optical coherence tomography and Gonioscopy. Br J Ophthalmol 2020;104:795–9. 10.1136/bjophthalmol-2019-314672 [DOI] [PubMed] [Google Scholar]
- 18. Baskaran M, Ho S-W, Tun TA, et al. Assessment of Circumferential angle-closure by the Iris-trabecular contact index with swept-source optical coherence tomography. Ophthalmology 2013;120:2226–31. 10.1016/j.ophtha.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 19. Porporato N, Baskaran M, Tun TA, et al. Assessment of Circumferential angle closure with swept-source optical coherence tomography: a community based study. Am J Ophthalmol 2019;199:133–9. 10.1016/j.ajo.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 20. Porporato N, Baskaran M, Perera S, et al. Evaluation of Meridional scans for angle closure assessment with anterior segment swept-source optical coherence tomography. Br J Ophthalmol 2021;105:131–4. 10.1136/bjophthalmol-2019-315461 [DOI] [PubMed] [Google Scholar]
- 21. Lavanya R, Foster PJ, Sakata LM, et al. Screening for narrow angles in the Singapore population: evaluation of new Noncontact screening methods. Ophthalmology 2008;115:1720–7, 10.1016/j.ophtha.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 22. Leung CK, Cheung CYL, Li H, et al. Dynamic analysis of dark-light changes of the anterior chamber angle with anterior segment OCT. Invest Ophthalmol Vis Sci 2007;48:4116–22. 10.1167/iovs.07-0010 [DOI] [PubMed] [Google Scholar]
- 23. Shan J, Li Z, Ma P, et al. Deep learning classification of angle closure based on anterior segment OCT. Ophthalmol Glaucoma 2024;7:8–15. 10.1016/j.ogla.2023.06.011 [DOI] [PubMed] [Google Scholar]
- 24. Randhawa J, Chiang M, Porporato N, et al. Generalisability and performance of an OCT-based deep learning Classifier for community-based and hospital-based detection of Gonioscopic angle closure. Br J Ophthalmol 2023;107:511–7. 10.1136/bjophthalmol-2021-319470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porporato N, Tun TA, Baskaran M, et al. “Towards 'automated Gonioscopy': a deep learning algorithm for 360° angle assessment by swept-source optical coherence tomography”. Br J Ophthalmol 2022;106:1387–92. 10.1136/bjophthalmol-2020-318275 [DOI] [PubMed] [Google Scholar]
- 26. Li F, Yang Y, Sun X, et al. Digital Gonioscopy based on three-dimensional anterior-segment OCT: an international multicenter study. Ophthalmology 2022;129:45–53. 10.1016/j.ophtha.2021.09.018 [DOI] [PubMed] [Google Scholar]
- 27. Fu H, Baskaran M, Xu Y, et al. A deep learning system for automated angle-closure detection in anterior segment optical coherence tomography images. Am J Ophthalmol 2019;203:37–45. 10.1016/j.ajo.2019.02.028 [DOI] [PubMed] [Google Scholar]
- 28. Xu BY, Chiang M, Chaudhary S, et al. Deep learning classifiers for automated detection of Gonioscopic angle closure based on anterior segment OCT images. Am J Ophthalmol 2019;208:273–80. 10.1016/j.ajo.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–7. 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu P, Higashita R, Guo PY, et al. Reproducibility of deep learning based Scleral spur Localisation and anterior chamber angle measurements from anterior segment optical coherence tomography images. Br J Ophthalmol 2023;107:802–8. 10.1136/bjophthalmol-2021-319798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu BY, Chiang M, Pardeshi AA, et al. Deep neural network for Scleral spur detection in anterior segment OCT images: the Chinese American eye study. Transl Vis Sci Technol 2020;9:18. 10.1167/tvst.9.2.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjo-2023-323860supp001.pdf (1.1MB, pdf)
Data Availability Statement
Data are available upon reasonable request.