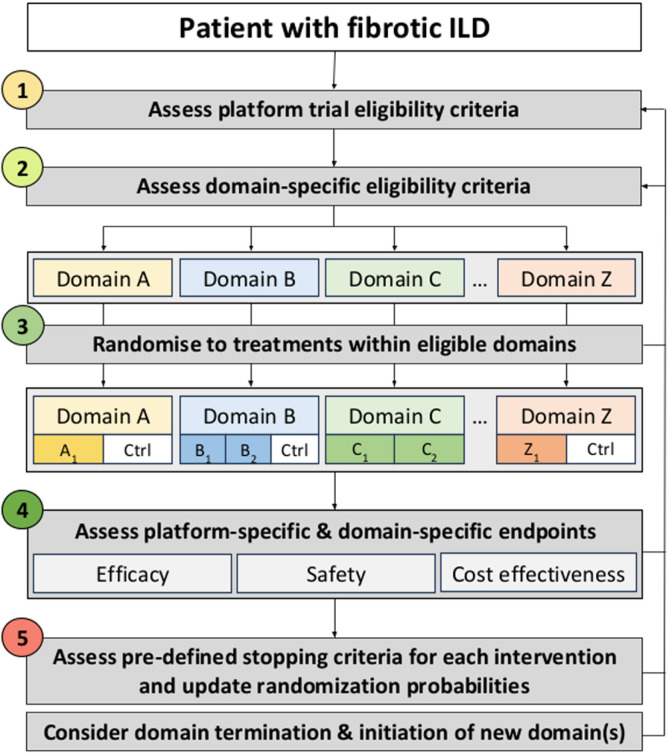
Figure 2.
Study overview. Patients with fibrotic ILD will be assessed for platform eligibility criteria (step 1), after which, screening for domain-specific eligibility criteria will be performed (step 2). Patients who meet eligibility criteria for at least one domain will be randomised to an intervention (factor) from each domain (step 3), which could include a variety of different options depending on the available interventions (indicated by subscripts). Patients would then be initiated on therapy and complete study assessments that allow analysis of efficacy, safety and cost-effectiveness outcomes (step 4). Predefined stopping criteria for each intervention will be assessed throughout the study (step 5), with termination of completed interventions (factors) and/or domains and initiation of new interventions (factors) and/or domains. Patients will be continuously assessed throughout their participation in the study for eligibility of previously existing and newly added domains. Domain refers to a group of interventions that are mutually exclusive. Interventions are called factors in the REMAP terminology. Ctrl, control arm; ILD, interstitial lung disease; REMAP, Randomised Embedded Multifactorial Adaptive Platform.