Abstract
Background:
We aimed to evaluate the performance of the current algorithm the HIV diagnosis that has been performed for four years. Results of HIV Ag/Ab tests, anti-HIV 1/2 confirmatory tests, HIV-1 RNA tests and the time for official results to be approved were evaluated.
Methods:
The relationship of HIV Ag/Ab tests, anti-HIV 1/2 confirmation tests and HIV-1 RNA PCR tests, their result times and suitability to the algorithm were examined at Izmir Dokuz Eylül University between May 2017 and June 2021.
Results:
HIV Ag/Ab ELISA was reactive repetitively in 165/54628 (0.30%) serum samples. Anti-HIV 1/2 confirmation test was reactive in 54.42% (80/147) of samples. The most common pattern (18.2%) in the confirmation tests was the positivity of the antibodies against gp160 - gp41 envelope glycoproteins together. The mean reporting time of the confirmation test result was 3h 50 min after the ELISA test. The mean reporting time of the HIV-1 RNA PCR was 12.79 d (±10.22) after the ELISA test and 12.63 (± 10.12) day after the confirmation test. In ROC analysis, the estimated rate of the ELISA test for the confirmation test was highest when S/CO was >13.16 (sensitivity: 97.59 %, specificity: 97.59%).
Conclusion:
The confirmation test in the current algorithm enabled the rapid test results, early diagnosis of HIV and early antiretroviral therapy. To use the new algorithm effectively, decentralization of the validation tests would be appropriate.
Keywords: HIV infection diagnosis, Serodiagnosis, Algorithms, Testing HIV
Introduction
Human Immunodeficiency Virus (HIV) infection is an important public health problem that caused 33 million deaths worldwide. Even 39% decrease in newly diagnosed HIV infections, 1.7 million people were newly infected with HIV and 690 000 people died of HIV-related causes in 2019 (1).
According to the 2019 report of the European Centre for Disease Prevention and Control (ECDC), while the number of cases in Western Europe has decreased recently, in some Eastern European countries including Turkey, have seen an increase (2,3).
Early diagnosis and treatment are important in preventing the transmission and development of AIDS. Antiretroviral therapy can ensure a healthy and long life in infected people. Because of the high viral load during acute infection, the risk of transmission is high (4–6).
Nowadays, 4th generation “enzyme-linked immunosorbent assays” (ELISA) tests, nucleic acid amplification tests (NAAT), and immunochromatographic rapid tests that detect antigens and antibodies together are frequently used for diagnosis (7–12). The new algorithm has been published the Centers for Disease Control and Prevention (CDC): Laboratory Testing for the Diagnosis of HIV Infection Updated Recommendations 2014’’. In this algorithm, after the detection of a reactive ELISA test, the test is repeated twice using the same kit, one of the blood samples is the same as the first tested sample. If two of the three tests are detected reactive, then anti-HIV 1/2 distinctive testing and viral load monitoring should be done with NAAT (13, 14).
We assessed the HIV diagnosis algorithm used in our laboratory during the past four years. For this reason, we examined the turnaround times for the screening HIV Ag/Ab test, the anti-HIV 1/2 distinct test, and the monitoring HIV-1 RNA PCR test. Additionally, we looked at the accuracy of the HIV Ag/Ab test in predicting the findings of HIV verification and HIV-1 RNA PCR testing, as well as the relationship between the two tests.
Materials and Methods
The results of HIV Ag/Ab tests, anti-HIV 1/2 confirmation tests, and HIV-1 RNA Polymerase Chain Reaction (PCR) tests done at the Izmir Dokuz Eylül University Hospital Central Laboratory between May 2017 and June 2021 were retrospectively reviewed.
Architect® HIV Ag/Ab Combo (Abbott, ABD)
For the HIV Ag/Ab test, the Architect® HIV Ag/Ab Combo (Abbott, USA) test was used, using chemiluminescent microparticle enzyme immunological (CMIA) technology, which qualitatively detects the p24 antigen and anti-HIV 1/2 antibodies in human serum/plasma. (15). Samples with S/CO value ≥1 were included in the study.
Geenius HIV 1/2 confirmatory test (Bio-Rad Laboratories, Redmond, WA)
An Immunochromatographic based Geenius™ HIV 1/2 confirmation test (Geenius, Bio-Rad, France) was used to confirm the presence of antibodies against HIV-1/2 and to determine the virus type in the samples with recurrent reactivity with ELISA tests (16).
Artus HI Virus-1 QS-RGQ kit (QIAGEN, Germany)
The Artus HI Virus-1 QS-RGQ kit (QIAGEN, Germany), a test based on the real-time RT-qPCR principle to investigate the presence of HIV-1 RNA in plasma/serum quantitatively used. The target was a 93 bp region in the 5’UTR region of the HIV-1 genome (17). Nucleic acid extraction was performed with the EZ1 Virus Mini Kit V 2.0 (QIAGEN, Germany) on the EZ1 Advanced XL (QIAGEN, Germany) instrument (18).
Statistical analysis
The relationship between the results of the three tests and the reporting times was evaluated. ROC analysis was performed between the ELISA S/CO value, confirmatory test results and HIV-1 RNA PCR results. The relationship between ELISA and HIV-1 RNA PCR results was evaluated by Spearman correlation test analysis were performed using the SPSS 26.0 (IBM Corp., Armonk, NY, USA) package program.
Ethics approval
The research was approved by the Dokuz Eylul University Faculty of Medicine Non-Interventional Research Ethics Committee with the date of 22.09.2021 and the decision number 2021/26-01.
Results
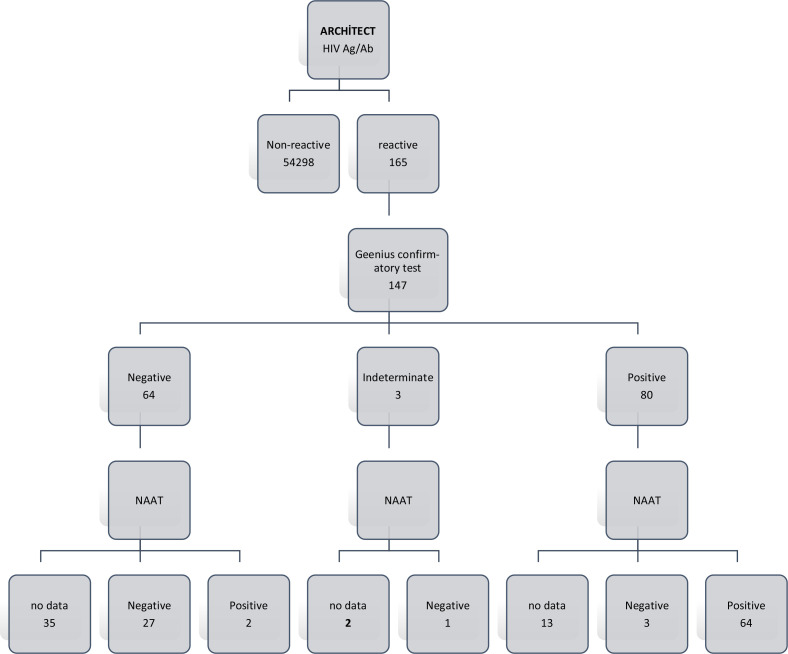
HIV Ag/Ab ELISA test in 54628 serum samples, and repetitive reactivity was detected in 165 (0.30%) samples. The reactive samples belonged to 126 (76.4%) males and 39 (23.6%) females. The mean age of the patients was 40.84 (± 16.4) yr.
While the confirmatory test was performed in 89.1% (147/165) of total reactive samples, the confirmation test could not be performed in 18 patients with confirmation results in an external center.
Anti-HIV 1/2 confirmation test was found to be reactive in 80/147 (54.42%) samples. The most common pattern detected in the confirmatory test was the co-positivity (18.2%) of antibodies against gp160 - gp41 envelope glycoprotein. The HIV-2 antibody result was indeterminate in only one patient (Table 1).
Table 1:
Confirmation test band positivity distribution
| Band | Antigen | *n,(%) |
|---|---|---|
| 4–6 Positive (+) | gp160,gp41 | 27(18.2) |
| 4–5–6 Positive (+) | gp160,p24,gp41 | 23(15.5) |
| 3–4–5–6 Positive (+) | p31,gp160,p24,gp41 | 22(14.8) |
| 3–4–6 Positive (+) | p31,gp160, gp41 | 8(5.4) |
| Indeterminate [6(+)] | gp41 | 3(2) |
| HIV 2 (2. Band) | 1(0.7) | |
| Negative | - | 63(42.6) |
| Total | 148(100) |
The HIV-1 RT-qPCR test was applied to 97 samples. Of these, 67 were positive with the antibody confirmation test, and 95.5% (64/67) of them were HIV-1 RNA positive.
HIV-1 RT-qPCR was performed on 29 of 64 samples with a negative confirmation test. Of these, 93.1% (27/29) were found to be negative (Fig. 1).
Fig. 1:
Test results of the samples
We detected three samples with reactive ELISA test, positive antibody confirmation test and negative HIV-1 RNA PCR test. There were two samples with reactive ELISA tests, negative anti-body confirmation test and positive HIV-1 RNA PCR test. (Table 2, Fig. 1).
Table 2:
The patients with ELISA reactive, Geenius negative and HIV-1 RT-PCR-positive results
| ELISA (S/CO) | Geenius | Geenius (repeated 2 months later) | HIV-1 RT-PCR | |
|---|---|---|---|---|
| Patient 1 | 1.09 | Negative | - | 115 356 copy/mL |
| Patient 2 | 13.16 | Negative | Positive (gp160, p24, gp41) | 72862 copy/mL |
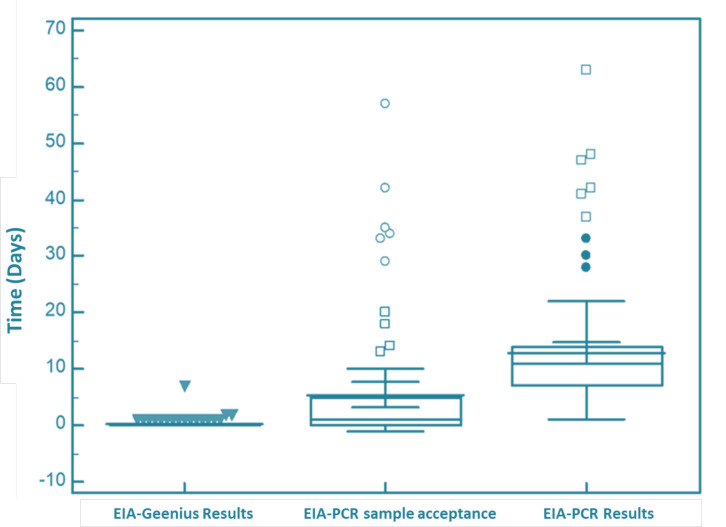
When we examined the reporting times of the test results, the confirmatory test was reported 3 h and 50 min after the ELISA test result. HIV-1 RNA PCR test results were reported 12.79 d (±10.22) after the ELISA result and 12.63 (±10.12) days after the confirmatory test result. There was an average of 7.34 (±4.80) d between the acceptance of the samples to the laboratory and the reporting of the result (Fig. 2).
Fig. 2:
Differences between test reporting and sample acceptance time
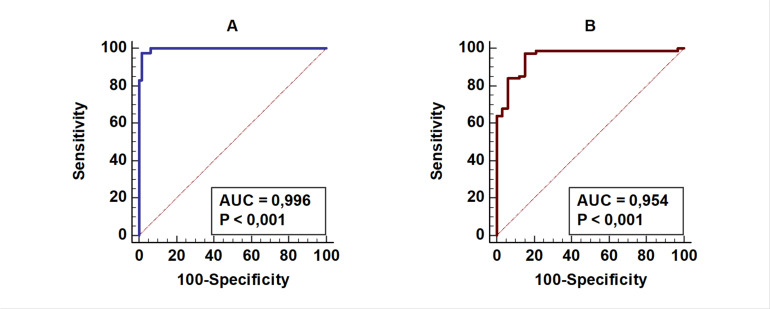
The significance of the S/CO ratio in the ELISA test in predicting confirmatory test positivity was analyzed by ROC analysis. The highest sensitivity and specificity were in S/CO >13.16 (AUC: 0.996) ratio, (Sensitivity: 97.59%, Specificity: 98.39% +LR: 60.51 −LR: 0.024) (Table 3, Fig. 3A).
Table 3:
Sensitivity, specificity and the positive likelihood ratio and negative likelihood ratio of ELISA results for the prediction Geenius test results (n:144)
| S/CO value | Sensitivity | Specificity | PLR | NLR |
|---|---|---|---|---|
| ≥1.01 | 100.00 | 0.00 | 1.00 | |
| >7.03 | 100.00 | 93.55 | 15.50 | 0.00 |
| >8.54 | 97.59 | 93.55 | 15.13 | 0.026 |
| >13.16 | 97.59 | 98.39 | 60.51 | 0.024 |
| >102 | 83.13 | 98.39 | 51.54 | 0.17 |
*PLR: The likelihood ratio for being positive, NLR: The likelihood ratio for being negative
Fig. 3:
Prediction of ELISA test results by Geenius test results (A) and HIV-1 RT-PCR (B) using ROC curve analysis
(A) Prediction of the ELISA test results with Geenius test results using ROC curve analysis (n:145), AUC: Area under the curve: 0.996, P<0,001
(B) Prediction of ELISA test results with HIV-1 RT-PCR test results using ROC curve analysis (n:97) AUC: Area under the curve: 0.954, P<0,001
A moderately significant positive correlation (R: 0.582) was found between ELISA and NAAT results (P<0.001). We determined that as the ELISA index increased, the viral load increased in the same direction
The highest sensitivity and specificity for the ELISA test to predict viremia were found in samples with S/CO >11.61 (AUC: 0.954) (Sensitivity: 97.3%, Specificity: 84.85% +LR: 6.42 −LR: 0.031) (Table 4, Fig. 3B).
Table 4:
Sensitivity, specificity and the positive likelihood ratio and negative likelihood ratio of ELISA results for the prediction HIV-1 RT-PCR results (n:108)
| S/CO value | Sensitivity | Specificity | PLR | NLR |
|---|---|---|---|---|
| >7.03 | 98.67 | 78.79 | 4.65 | 0.017 |
| >8.54 | 97.33 | 78.79 | 4.59 | 0.034 |
| >11.61 | 97.33 | 84.85 | 6.42 | 0.031 |
| >90.02 | 85.33 | 84.85 | 5.63 | 0.17 |
| >93.13 | 85.33 | 87.88 | 7.04 | 0.17 |
| >102 | 84.00 | 87.88 | 6.93 | 0.18 |
*PLR: The likelihood ratio for being positive, NLR: The likelihood ratio for being negative
Discussion
We detected repetitive ELISA reactiveness as 0.30% in our study. In our country, anti-HIV reactiveness was 0.17%–0.20% in studies with blood donors expected to represent the community (19, 20). We had a higher reactive rate in our study because our study group was people with a risky contact history or symptoms for HIV infection. Thus, blood donors are selected from healthy individuals by excluding those with suspicious sexual contact may have led to this difference. The reactive test rate was 0.7% in a study of 3043 Asian asylum seekers (21). Asylum seekers in this study were from the countries with probably higher HIV prevalence than in Turkey.
In our study, the detection of S/CO value of >13.16 in the ELISA test is the ideal to be a predictive value in predicting positivity in the HIV verification test. ELlSA estimation rate depends on the characteristics of the tests used. Although in a study comparing the results, the ideal predictive value was 32.7 S/CO for Architect, LIA (INNO-LIA® HIV I/II Score, Fujirebo) and NAAT (kPCR Versant HIV-1 RNA, Siemens Healthcare Diagnostics, Spain) (22). Another study that used the Architect and Western Blot test (HIV Blot version 2.2; Genelabs Diagnostics, Singapore) the predictive value founded to be 6.6 S/CO (23).
In a study evaluating ELISA (Architect and VIDAS) and Geenius results, when the result is S/CO >33 with both ELISA tests, Geenius test is always positive and if S/CO ≤ 33, ELISA results can be false positive and S/CO <3 in Architect, the reactive test is always found to be a false positive (24). In our study, 45 patient samples with ELISA results of ≤3 S/CO were negative with Geenius. Antibody verification was positive in only three (17.6%) of 17 patient samples with a value between 3 and 33 S/CO. The architect HIV combo test can detect acute infection by scanning for p24 antigen. In our study, two patients with negative validation tests on clinical suspicion NAAT was positive. The Geenius test, which is an antibody verification test, may give false-negative results in individuals with acute infections who have not yet produced antibodies. The diagnosis of the acute infection is ensured by NAAT verification of such instances.
There are some problems with the verification tests and NAAT. To produce results NAAT takes a long period compared to distinctive verification tests, high cost and often samples grouped before testing, requiring experienced technicians and laboratory conditions for the operation, available commercial tests are only for HIV-1 are disadvantages. The time factor could be exceeded using NAAT, which do not need to accumulate samples and give fast results. In our study, both verification and viral load determination were performed with HIV-1 RT-PCR test without Geenius test in only one case.
The distinctive antibody verification test used in the study is easy to use and even possible to evaluate with the eye without a reading device. The antibody verification test was indefinite for HIV-2 antibodies in one patient in the examined group in our study. This case was a 38-year-old female patient waiting for a liver transplant due to auto-immune hemolytic anemia and Budd–Chiari Syndrome, and recurrent reactiveness (S/CO: 5.79) was detected by ELISA, but HIV-2 RNA PCR result was evaluated as a non-specific reaction upon negative results of HIV-2 RNA PCR studied in the S.B. HSGM Virology Reference laboratory.
In our study, we had two cases that tested positive for Geenius and negative for HIV-1 RNA. Our first case was a 33-year-old female patient who had chemotherapy for cervical carcinoma and was under antiretroviral therapy (ART). The other case is a baby born to an HIV-1-positive mother. Upon the negative results of NAAT tests performed on the 14th day, 1st month and 4th month after birth, the case was considered HIV-1 negative.
In our analysis, 16 patients tested positive for Geenius and were reactive by ELISA but did not receive an HIV-1 PCR test. When these patients applied to our center owing to another health issue, they were already receiving ART elsewhere, and the verification test was not required because HIV Ag/Ab ELISA reactiveness had been verified through routine examinations. This issue will be resolved and assist in reducing the workload and overall cost of laboratories through inter-laboratory networks, which may include data from patients with proven HIV diagnosis.
The Ministry of Health’s 2019 “HIV/AIDS Diagnostic Guide” recommends two different algorithms for diagnosing newborns of HIV-positive mothers, depending on whether the mother receives the ART before birth and virally suppressed (3). In our study, recurrent ELISA positivity was detected with 124.46 S/CO in the baby of a 27-year-old mother who received ART and had viral suppression, antibodies against gp160, p24, gp41 antigens were detected in the verification test due to maternal antibodies. According to the proposed algorithm, the baby was accepted as HIV-1 negative after HIV-1 RT-PCR negative was detected in all three of the samples taken on the 14th day, 1st month and 4th month.
False positivity is more common in low S/CO indexes in ELISA tests. Technical causes due to laboratory methods of study, rheumatological diseases, viral hepatitis, malignancy, multiple pregnancy history, tuberculosis and Rickettsia infections can cause false positives (25–27). In a study of 264 samples with recurrent reactivity with the Abbott ARCHITECT HIV Ag/Ab combo test, 95% of the samples were found to be non-reactive and false positive when reassessed using different ELISA methods (Siemens ADVIA Centaur HIV Ag/Ab), stating that heterophile antibody interference could lead to this reactivity (28). In our study, 61 samples showing low S/CO values, which negative validations were identified. These samples could not be compared with another commercial ELISA method. Determining which causes lead to repetitive false positivity is beyond the objectives and possibilities of this study. In our study, Geenius and HIV-1 RT-PCR tests were negative despite repeated ELISA reactivity with an index of 100 and 105 S/CO in a blood donor, unlike other samples showing false positive with low ELISA index. The donor’s inquiry did not identify any recent infections or diseases. In the literature, recurrent high S/CO (415–493) indexes were detected with elecsys HIV combi PT (Roche Diagnostics, Mannhein, Germany) test in a patient with ameloblastoma and a history of schistosomiasis infection, but it was interpreted as false positivity with validation tests and molecular tests being negative (29).
Another important point in the diagnosis of HIV infection is to minimize the loss of follow-up of cases with the rapid diagnosis and thus reduce the risk of transmission by rapidly starting anti-retroviral treatment. In a US study, the time it took for the results to be reported was calculated as an average of 8 d using the current algorithm and an average of 14 d in the modified algorithm where verification was performed using “Western Blot.” (30). In our study, there was an average of 3 h and 50 min between ELISA and Geenius results and an average of 12.79 days between ELISA and PCR result. The fact that all tests can be done in our laboratory has positively affected the time required for the result. The most important factor that extends the duration is the receipt of the appropriate sample (plasma) for NAAT and its delivery to the laboratory. With the introduction of quick and simple-to-use (Geenius) assays, the entire HIV diagnostic algorithm can be performed in a matter of hours. For the diagnosis of acute HIV infection, NAAT is also necessary. By allowing the samples to be analyzed in the institution or province where the patient is located rather than shipping them to NAAT verification facilities, the time needed for viral tests in diagnosis/follow-up will be decreased.
Although ELISA and PCR tests examined different parameters, our study found a moderately significant correlation between ELISA S/CO values and viral loads detected in PCR. The study, in which Brennan and colleagues compared viral load with Architect HIV Combo, found an excellent correlation (R: 0.902) (31). The most important factor affecting the correlation is the necessity of having treatment information of the patients since the viral loads of the patients under treatment are suppressed.
As limitations of our study, it was a retrospective study, and repetitive ELISA reactivity was not repeated with another ELISA test. The NAAT test was not performed on every patient whose confirmatory test was studied.
Conclusion
With the confirmation test in the current algorithm, results are obtained in a short time, HIV infection can be diagnosed and treatment can be started early. However, false-negative results can be obtained with the confirmatory test in cases in the early stage of the infection but who have not developed antibodies yet, and in case of doubt, NAAT should be applied. In order for the new algorithm to be used effectively, it would be appropriate to decentralize the validation tests.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.WHO HIV/AIDS Fact sheets, Available from: https://www.who.int/news-room/fact-sheets/detail/hiv-aids
- 2.European Centre for Disease Prevention and Control, WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2020, Available from: https://www.ecdc.europa.eu/sites/default/files/documents/hiv-surveillance-report-2020.pdf
- 3.Republic of Turkey Ministry of Health, General Directorate of Public Health, Department of Infectious Diseases /HIV AIDS Statistics. Available from: https://hsgm.saglik.gov.tr/tr/
- 4.Cohen MS, Chen YQ, McCauley M, et al. (2016). Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med, 375(9):830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller WC, Rosenberg NE, Rutstein SE, et al. (2010). Role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS, 5(4):277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wawer MJ, Gray RH, Sewankambo NK, et al. (2005). Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis, 191(9):1403–9. [DOI] [PubMed] [Google Scholar]
- 7.Busch MP, Satten GA. (1997). Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am J Med, 102(5b):117–24; discussion 25–6. [DOI] [PubMed] [Google Scholar]
- 8.Fiebig EW, Wright DJ, Rawal BD, et al. (2003). Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids, 17(13):1871–9. [DOI] [PubMed] [Google Scholar]
- 9.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. (2008). Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A, 105(21):7552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HY, Giorgi EE, Keele BF, et al. (2009). Modeling sequence evolution in acute HIV-1 infection. J Theor Biol, 261(2):341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masciotra S, McDougal JS, Feldman J, et al. (2011). Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol, 52 Suppl 1:S17–22. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen M, Coleman C, Mitchel J, et al. (2013). Comparison of human immunodeficiency virus assays in window phase and elite controller samples: viral load distribution and implications for transmission risk. Transfusion, 53(10 Pt 2):2384–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC: Laboratory Testing for the Diagnosis of HIV Infection Updated Recommendations 2014, Available from: https://stacks.cdc.gov/view/cdc/23447
- 14.Republic of Turkey Ministry of Health, General Directorate of Public Health, Department of Infectious Diseases “HIV diagnosis -Treatment guide 2019”, Available from: https://hsgm.saglik.gov.tr/depo/birimler/bulasici-hastaliklar-ve-erken-uyari-db/Dokumanlar/Rehberler/HIV-AIDS_Tani-Tedavi_Rehberi_2019.pdf
- 15.ABBOTT. ARCHITECT HIV Ag/Ab Combo Reagent Insert 2009.
- 16.Malloch L, Kadivar K., Putz J, et al. (2013). Comparative evaluation of the Bio-Rad Geenius HIV-1/2 Confirmatory Assay and the Bio-Rad Multispot HIV-1/2 Rapid Test as an alternative differentiation assay for CLSI M53 algorithm-I. Journal of Clinical Virology, 58:e85–e91. [DOI] [PubMed] [Google Scholar]
- 17.Artus HI Virus-1 RG RT-PCR Kit Hand Book 03/2015, Available from: http://rcostoya.com/uploads/galerias/con814/hb-0129-005-1-artus-hiv1-rg-rtpcr-ce-kit-24-96.pdf
- 18.Qiagen EZ1 Advanced XL User Manual, Available from: https://www.qiagen.com/us/resources/download.aspx?id=471d7f96-9a1d-4a31-b121-01b4367bae33&lang=en
- 19.Acar A, Kemahli S, Altunay H, et al. (2010). HBV, HCV and HIV seroprevalence among blood donors in Istanbul, Turkey: how effective are the changes in the national blood transfusion policies? Braz J Infect Dis, 14(1):41–6. [DOI] [PubMed] [Google Scholar]
- 20.Şaşmaz C, Kurt A, Öner S, et al. (2011). Hepatitis B, hepatitis C, HIV, and VDRL seroprevalence of blood donors in Mersin, Turkey. Turkish Journal of Medical Sciences, 41. [Google Scholar]
- 21.Kart Yaşar K. (2014). Seroprevalence of Hepatitis B, C and HIV/AIDS in Asylum Seekers in Istanbul. Journal of Microbiology and Infectious Diseases, 4:20–5. [Google Scholar]
- 22.Chacon L, Mateos ML, Holguin A, et al. (2017). Relevance of cutoff on a 4th generation ELISA performance in the false positive rate during HIV diagnostic in a low HIV prevalence setting. J Clin Virol, 92:11–3. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Lee J-H., Choi JY, et al. (2010). False-Positive Rate of a “Fourth-Generation” HIV Antigen/Antibody Combination Assay in an Area of Low HIV Prevalence. Clin Vaccine Immunol, 17(10):1642–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakovsky A, Gozlan Y, Bassal R, et al. (2018). Diagnosis of HIV-1 infection: Performance of Xpert Qual and Geenius supplemental assays in fourth generation ELISA-reactive samples. J Clin Virol, 101:7–10. [DOI] [PubMed] [Google Scholar]
- 25.CDC HIV Factsheet False Positive Results, Available from: https://www.cdc.gov/hiv/pdf/testing/cdchiv-factsheet-false-positive-test-results.pdf
- 26.Crucitti T, Taylor D, Beelaert G, et al. (2011). Performance of a rapid and simple HIV testing algorithm in a multicenter phase III microbicide clinical trial. Clin Vaccine Immunol, 18(9):1480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber B, Fall EH, Berger A, et al. (1998). Reduction of diagnostic window by new fourth-generation human immunodeficiency virus screening assays. J Clin Microbiol, 36(8):2235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavoie S, Caswell D, Gill MJ, et al. (2018). Heterophilic interference in specimens yielding false-reactive results on the Abbott 4th generation ARCHITECT HIV Ag/Ab Combo assay. J Clin Virol, 104:23–8. [DOI] [PubMed] [Google Scholar]
- 29.Reid J, Van Zyl G, Linström M, et al. (2020). High positive HIV serology results can still be false positive. IDCases, 21:e00849–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasrullah M, Wesolowski LG, Ethridge SF, et al. (2016). Acute infections, cost and time to reporting of HIV test results in three U.S. State Public Health Laboratories. J Infect, 73(2):164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennan CA, Yamaguchi J, Vallari A, et al. (2013). ARCHITECT(R) HIV Ag/Ab Combo assay: correlation of HIV-1 p24 antigen sensitivity and RNA viral load using genetically diverse virus isolates. J Clin Virol, 57(2):169–72. [DOI] [PubMed] [Google Scholar]