Abstract
Background:
We aimed to investigate the patterns of incidence and prevalence of bone sarcoma (BS) and soft tissue sarcoma (STS), morphology as well as geographical distribution in the elderly in Iran.
Methods:
By the primary site of the tumor and the morphological types, whole cases of cancer were classified. Then, the WHO classification (2018) and the third revision of the standard International Classification of Diseases for Oncology (ICD-O-3) were used to assign a code to them. The estimated incidence rates were obtained as the frequency of the newly-diagnosed cases within one year divided by the calculated population of the mid-year Iranian residents as estimated by the Iranian Bureau of Statistics. The age-standardized incidence rates were also estimated for both bone and soft tissue sarcoma.
Results:
The annual crude incidence rates of sarcomas in males (0.80 per 100,000) were more than in females (0.55 per 100,000) in all years. The total combined crude incidence in 2014 years was obtained at 0.67 per 100,000 people. In terms of disease grade majority of the patients were of grade 3 (11.5 %). In terms of tumor location, the Lower extremity was 16.8%, the Visceral (including gastrointestinal & uterus) 15.8%, the Thoracic 12.8%, and the Pelvic & abdominal wall 9.7%.
Conclusion:
Even though such sarcoma is more prevalent in elderly men, its incidence was also observed in lower-aged female groups. In addition, the incidence rate of BS was lower in comparison with that of STS, and the patients often exhibited an unknown degree of sarcoma.
Keywords: Bone sarcoma, Soft tissue sarcoma, Epidemiology, Elderly, Iran
Introduction
Sarcomas are a group of malignant neoplasms of bone and soft tissue with diverse biological and clinical features, most of which occur in tissues of mesenchymal origin such as muscle, adipose tissue, and bone, although the exact cell origin is unknown for most (1). According to the WHO, there are more than 80 distinct subtypes of tissue sarcomas difficult to categorize accurately (2). Despite the complex histology of sarcomas, they can develop at almost any anatomical site and at any age, accounting for about 1–3% of all newly diagnosed malignancies (3, 4). In terms of anatomy, there are two different types of sarcomas: bone sarcoma (BS) and soft tissue sarcoma (STS), which account for 20% and 80% of cases, respectively (5).
STS are a heterogeneous group of malignant neoplasms of mesoderm cellular origin that are widely distributed within organs and other areas designated as connective tissues (1). In Europe, the annual incidence rate of STS is estimated at 5 per 100,000 population (2, 3). More than 40% of cases of STS are diagnosed in people over 65 yr of age, among which people 65–74 yr have the highest rate (19.9%)(4). Elderly patients with STS are significantly different from younger patients. Older patients have a worse prognosis and have higher and larger tumors in the early stages (5). In the case of primary BS, they generally account for 0.2% of all malignancies (6–8). The prevalence and mortality of cancer are increasing rapidly worldwide. There are several reasons for this increase, including aging, radiation, exposure to chemicals, neurofibromatosis, and retinoblastoma (9). In a European study, the incidence rate of crude sarcoma was 5.6 per 100,000 people per year, with the incidence of age-standardized rate (ASR) for soft tissue sarcoma ranging from 3.3 per 100 people in Eastern Europe to 4.7 per 100 people in Northern Europe (10). In Iran, the incidence of combined crude at 3.2 per 100,000 people, which was 2.7 and 0.5 per 100,000 people for STS and BS, respectively, which increased with age. In this study, sarcoma, osteosarcoma, leiomyosarcoma, liposarcoma and spindle cell sarcoma were the most common morphologies (11). Most studies on the incidence of sarcomas have shown that these rates increase with age, especially in STS (1, 11–14). Therefore, it is important to gain a more accurate understanding of the epidemiology of sarcomas in the elderly, especially in advanced cases of the disease, in order to obtain timely information and knowledge of the prevalence and distribution patterns of sarcomas, as well as identify risk factors for care. Provided more effective health and treatment to improve the outcomes of patients suffering from these malignancies.
We aimed to investigate the patterns of incidence and prevalence of BS and STS sarcoma, as well as its geographical distribution in the elderly in Iran.
Materials and Methods
The present investigation utilized the data recorded by the Iranian National Cancer Registry (INCR) for the patients suffering from cancer diagnosed with sarcoma during the 2009–2014 period. This registry is responsible for collecting nationwide cancer registry data obtained from 31 provinces. Under the supervision of the Iranian Chief Medical Universities, the local cancer registries gather the provincial data, which includes nearly the whole (≥90%) respective data for the Iranian population. The gathered data were sent to the Iranian National Cancer Registry, which was then pooled and de-identified. Then, they were analyzed for the present investigation. These data consisted of patients’ ID numbers, names, gender (male/female), age, residential province, dates of birth, and year of diagnosis. In addition, they included some information regarding tumors, such as their morphology, grade, and location.
The present investigation observed the whole aspects of ethical standards and received the approval of the Ethical Committee of Shahid Beheshti University of Medical Sciences (IR.SUMS.CRC.1398.019).
The subjects included in this study were individuals meeting the morphologic criteria for BS and STS by the International Agency for Research on Cancer (IARC) classifications (15). Thus, by the above criteria, the present investigation included a population of 3066 patients.
The entire cases of cancer were classified based on the primary site and morphological types of their tumors. Then, they were coded by the WHO classification (2018) and the standard International Classification of Diseases for Oncology (ICD-O-3) (the third revision) (16–18). The codes 8800–8935, 8910, 8920, 8940, 8950–8959, 8963–8964, 8990–8991, 9020–9044, 9120–9133, 9150, 9170, 9180, 9231, 9240, 9251, 9260, 9364–9372, 9540, 9560–9571, 9580–9581, etc. of ICDO-3 M were used to identify the subtypes of the soft tissue sarcoma morphologically. Moreover, the M codes 8800–8920, 9040–9044, 9120–9133, 9150, 9170, 9180–9250, 9260–9261, 9310, 9364, 9370, 9540–9581, etc., were used to identify the subtypes of the bone sarcoma morphologically. Given the anatomic site of origin of tumors, the ICD-O-3 codes C40.0–41.9 and C00–C80 (except C40.0–41.9) were assigned to the bone and STS tumors, respectively.
The incidence rates of cancers were determined as the frequency of new cases diagnosed within one year divided by the calculated population of the mid-year Iranian residents as estimated by the Iranian Bureau of Statistics. This figure is reported as the number of cases in 100,000 individuals. The age-standardized incidence rates were also estimated for both BS and STS through the adjustment for the distribution of age groups among the entire Iranians and the world population of Segi. By dividing the total cases diagnosed with BS or STS by the total cases of diagnosed individuals, the proportions of BS and STS were estimated. In addition, the proportions of every single morphological subtype were calculated as a percentage of the total annual BS/STS diagnoses recorded for every single year during the 2009–2014 period. Moreover, the incident rates were grouped into four 5-year age cohorts of 80–100, 75–79, 70–74, and 65–69. In total, the diagnosed cases of sarcoma were scrutinized for eight categories of tumor locations, 13 categories of morphologies, and six different grades of tumors, including scores 0, grade 1, 2, 3, 4, and 9 concerning the above-said age cohorts and gender of the patients. Thus, by the same variables, the respective incidence rates were determined. Eventually, the incident rates and their respective changes in percent were employed to find the regions with a higher risk of Sarcoma in Iran.
Statistical analysis
Descriptive statistical analysis was carried out by employing the Statistical Package for the Stata Corp 2019 (Stata Statistical Software: release 16. College Station, TX: Stata Corp LLC), ArcGIS Version 10.7 (Redlands, California, USA), and Microsoft Excel 2013 (Office 10, Microsoft Corporation, WA, USA). All calculations were done in Stata, Microsoft Excel, and the maps were designed in ArcGIS software.
Results
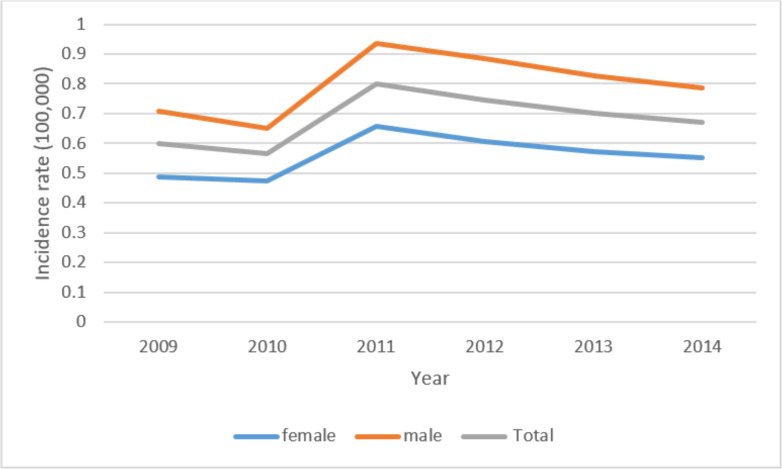
Overall, 3076 cases of age higher than 65 yr with either STS or BS were identified nationwide between 2009 and 2014. Of these, 40.6% were women and 59.4% were men. The highest incidence of men is in the age group of 80–100 yr with 16.3% and women in the age group of 65–69 with 13.1%. As shown in Fig. 1, the annual crude incidence rates of sarcomas by gender were calculated, and the incidence rate in males (0.80 per 100,000) is more than in females (0.55 per 100,000) in all years. The total combined crude incidence in 2014 years was obtained at 0.67 per 100000 people. And 5.6% of the type of tumor was BS and 94.4% of STS (Table 1).
Fig. 1:
Trend of the incidence rate of sarcoma cancer by gender
Table 1:
Demographic variables of patients
| Variables | N (%) | |
|---|---|---|
| Year | 2009 | 430 (14.0) |
| 2010 | 408 (13.3) | |
| 2011 | 600 (19.6) | |
| 2012 | 567 (18.5) | |
| 2013 | 539 (17.6) | |
| 2014 | 522 (17.0) | |
| Age group | 65–69 | 878 (28.6) |
| 70–74 | 768 (25) | |
| 75–79 | 665 (21.7) | |
| 80–100 | 755 (24.6) | |
| Gender | female | 1246 (40.6) |
| male | 1820 (59.4) | |
| Location of tumor code | Head and neck | 243 (7.9) |
| Upper extremity | 207 (6.8) | |
| Thoracic | 393 (12.8) | |
| Visceral (including gastrointestinal & uterus) | 478 (15.6) | |
| Retroperitoneal / intra abdominal | 201 (6.6) | |
| Pelvic & abdominal wall | 297 (9.7) | |
| Lower extremity | 515 (16.8) | |
| Other* | 732 (23.9) | |
| Tumor type | BS | 173 (5.6) |
| STS | 2893 (94.4) | |
| Grading | 0 | 4 (0.1) |
| 1 | 263 (8.6) | |
| 2 | 116 (3.8) | |
| 3 | 354 (11.5) | |
| 4 | 65 (2.1) | |
| 9 | 2264 (73.8) | |
| Morphology code | Fibroblastic / myofibroblastic tumours | 223 (7.3) |
| Malignant Tumors of Peripheral Nerves | 78 (2.5) | |
| Malignant Tumors of Adipose Tissue | 226 (7.4) | |
| Malignant Tumors of Blood and Lymph Vessels | 344 (11.2) | |
| Malignant Tumors of Smooth Muscle | 210 (6.8) | |
| Malignant Tumors of Striated Muscle | 0 (0) | |
| Malignant Tumors of Hematopoietic Tissue | 0 (0) | |
| Gastrointestinal stromal sarcoma | 138 (4.5) | |
| Malignant Osseous or Cartilaginous Tumors | 281 (9.2) | |
| Gynecologic Sarcomas | 58 (1.9) | |
| Undifferentiated/unclassified sarcomas | 923 (30.1) | |
| Malignant Tumors of Uncertain Cell Type | 244 (8) | |
| Others** | 340 (11.1) |
*Unknown primary sites, upper and lower extremities, other ill-defined and overlapping sites, overlapping lesions of connective tissue, other endocrine glands, skin, peripheral nerve, bone except limbs, bone of limbs.
** Phyllodes tumor malignant, carcinosarcoma, Mullerian mixed tumor, mesothelioma malignant, gliosarcoma, myxosarcoma, mast cell sarcoma, histiocytic sarcoma, myofibroblastic tumor, malignant histiocytosis, rhabdomyosarcoma, pleomorphic rhabdomyosarcoma, embryonal rhabdomyosarcoma, malignant rhabdoid tumor, rhabdoid sarcoma, pleomorphic rhabdomyosarcoma
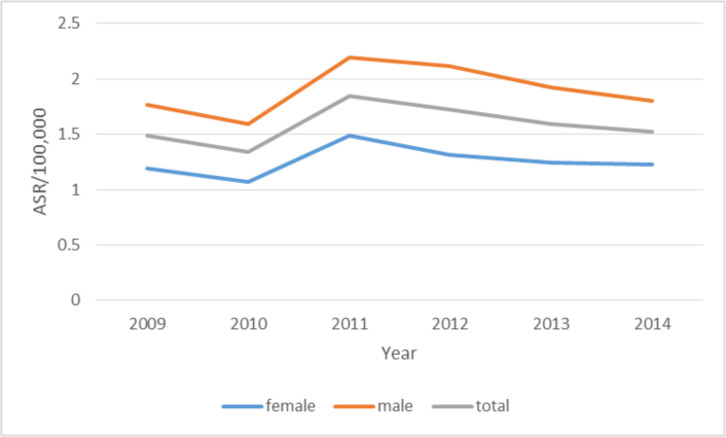
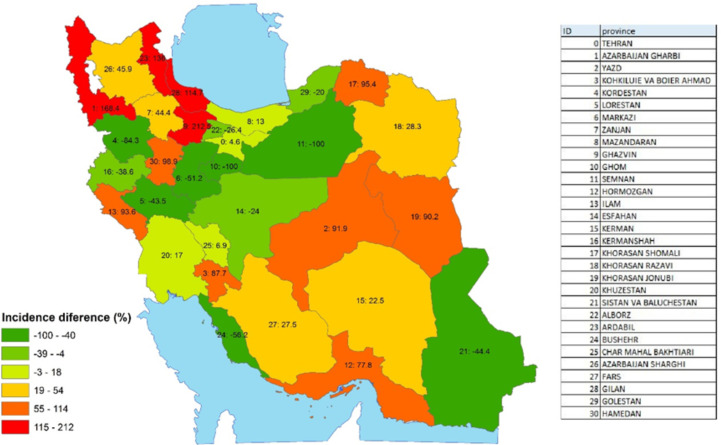
According to Fig. 2, the age-standardized incidence rates were higher in males than females. As illustrated in Fig. 3, the incidence of sarcoma cancer in the northwest of Iran has increased significantly. During these 6 years, the highest change in the incidence of sarcoma cancer occurred in Ghazvin (212.5%). In the following, the incidence of West Azarbaijan (168.4%), Ardabil (136.0%), and Gilan (114.7%) changed significantly.
Fig. 2:
The trend of age-standardized incidence rates of sarcoma cancer by gender
Fig. 3:
Percentage of 6-year changes in the incidence of sarcoma cancer by province
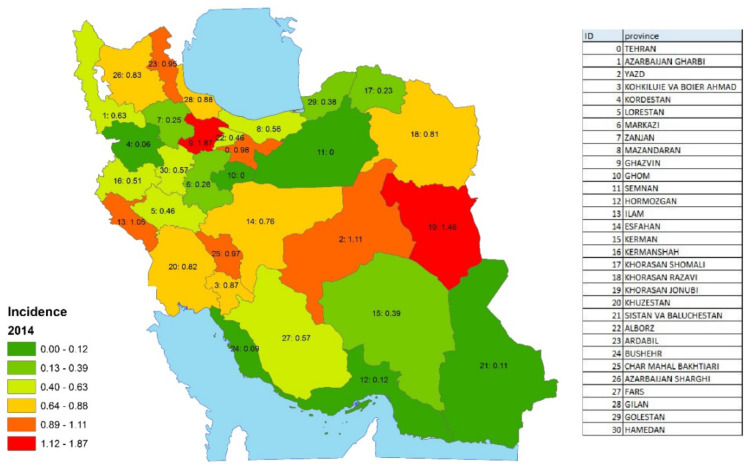
According to Fig. 4, the north and east of Iran reported the highest incidence rate of sarcoma cancer. In Ghazvin (1.87 per 100,000) and South Khorasan (1.46 per 100,000) for instance, is the highest incidence rate.
Fig. 4:
Incidence rate of sarcoma cancer in 2014
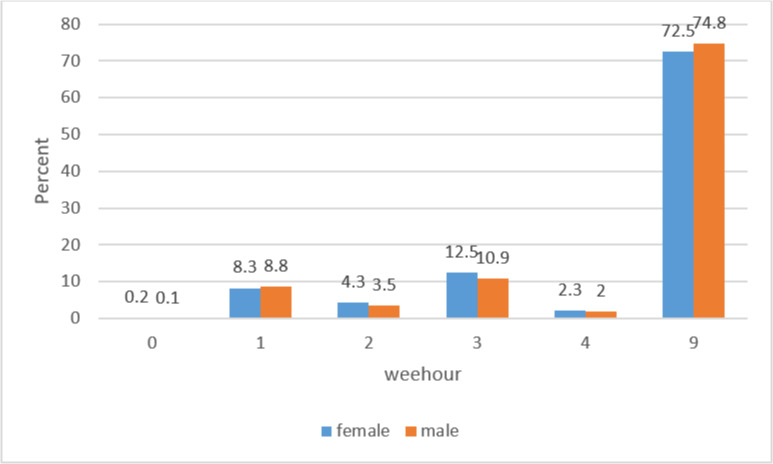
According to Fig. 5, the majority of the patients were of grade 9 (unknown/undifferentiated grade). Regardless of tumors with grade 9, tumors with grade 3, 1, 2, 4, and score 0 had the highest frequencies with the mentioned.
Fig. 5:
Grade of sarcoma cancer by gender
In terms of tumor location, the highest frequency in both sexes was related to the others (unknown primary sites, upper and lower extremity, other ill-defined and overlapping sites, connective tissue of all of the body, overlapping lesion of connective tissue, other endocrine glands, ski, peripheral nerve, bone except limbs, bone of limbs). In terms of tumor morphology, the highest frequency in both sexes was related to the others (phyllodes tumor malignant, carcinosarcoma, Mullerian mixed tumor, mesothelioma malignant, gliosarcoma, myxosarcoma, clear cell sarcoma of kidney, myxosarcoma, mast cell sarcoma, follicular dendritic cell sarcoma, meningeal carcinomatosis, histiocytic sarcoma, myofibroblastic tumor, malignant histiocytosis, rhabdomyosarcoma, pleomorphic rhabdomyosarcoma, embryonal rhabdomyosarcoma, alveolar rhabdomyosarcoma, malignant rhabdoid tumor, rhabdoid sarcoma, mixed type rhabdomyosarcoma, pleomorphic rhabdomyosarcoma). Category 6. Malignant Tumors of Striated Muscle and 7. Malignant Tumors of Hematopoietic Tissue were equal to zero.
Discussion
In the present study, we performed a population-based epidemiological analysis of elderly Iranian patients with STS and BS from 2009 to 2014. The incidence of sarcoma is higher in elderly men in Iran. In men, the highest incidence is in the age group of 80–100 yr, and in women in the age group of 65–69 yr. Although the incidence is higher in men, the age of onset in women is lower than in men. Most tumors in the elderly are STS type. The incidence of this cancer has increased significantly in the northwest of Iran, and in general, the north and east of Iran have reported the highest incidence of sarcoma. The majority of Iranian elderly have shown grade 9 sarcoma (unknown, differentiated). Occurrence of tumors with others were seen more. In terms of morphology, most of the tumors are of other types.
Based on our results, an increasing trend of age-standardized incidence rates of sarcoma cancer was observed in 2011. We think that maybe the reason for this increase is the start of cancer registries in Iran; And due to more investigation and screening in the registries, this increase has been seen.
According to the results obtained in our investigation, such cancer shows more prevalence among male Iranian’s elderly. Such a result is in agreement with that of an earlier investigation conducted in Iran (11). A noticeable inconsistency is observed in the predominance of sarcoma incidence in males over that in females and vice versa. In this respect, in Shanghai, Chinese females and males showed a similar incidence rate of sarcoma (12). Nonetheless, according to an investigation carried out in Taiwan, bone sarcoma was more incident among men (19). By contrast, the investigations carried out in China, Ireland, and European Union countries reported more sarcoma cases in females (20, 21). The reason for higher sarcoma incidence among females in these investigations has been ascribed to the more frequency of the events occurring in their breasts and genital organs (uterus). In addition, most of the diagnosed cases of a subtype, such as leiomyosarcoma, predominantly take place in the female uterus and genital organs (11). Apparently, a higher risk of developing sarcoma is expected in elderly people. This is attributable to a variety of physiological changes as well as their potentially compromised immune systems (11). After the age of 50, the incidence of sarcoma is greatly increased, and thus, the increasing population of older adults may potentially contribute to such an increased incidence (22).
A tumor grade affects the patients’ outcomes significantly. Moreover, it is directly associated with total survival and disease-free survival in patients suffering from cancer. Additionally, compared to the tumors of unknown grades, those featuring a known grade are more manageable. As a result, we classified the sarcoma patients by their tumor grades. The cases investigated in this research were predominantly of undifferentiated/unknown grades. These results are in good agreement with those of the Surveillance, Epidemiology, and End Results (SEER) study (23). This program is administered by the National Cancer Institute (NCI). This program is designed to collect and record information about cancer patients in the United States of America. Nevertheless, according to the SEER program results, sarcomas of unknown mixed grades are disproportionate to other grades and instead might exhibit a rare disproportionate subset of sarcoma tumors, which are graded insufficiently and, as a result, are potentially managed inadequately.
The most significant finding of our investigation was the proportion of patients characterized by an unknown grade. Histologic analyses and proper grading are regarded as crucial stages of the diagnostic workup for sarcoma. This is because they are considered the most significant risk factors for various disease outcomes. An earlier investigation approved the same result (24). Another research reporting the same result claims that the crucial parameters related to an increased chance of having an unknown grade are the tumors belonging to the “other” primary site and the “other” histological subtype. The “other” histologic subtype is composed of at least 20 very rare types of tumors, each of which accounts for less than 0.5% of soft tissue sarcoma. The grading and diagnosis of such very rarely-reported tumors necessitate multidisciplinary expertise (25). Furthermore, the tumors featuring an unknown grade are characterized by local spread, unknown size, and status of the lymph node. One of the reasons for the comparatively high proportion of soft tissue sarcoma with unknown grades observed in large national databases can be the fact that such tumors do not demand a grade to guide treatment decisions (24). Nonetheless, the patients suffering from tumors of unknown grades may remain undertreated and are 1.5- and 2.6-fold more likely to not undergo radiation therapy and surgery, respectively, when different risk factors are considered (26). The tumors characterized by an unknown grade may not be a proportional mixture of other grades. Instead, they indicate a rarely-observed disproportionate subset of sarcoma tumors, which are not graded adequately and, as a result, potentially managed poorly.
Given the heterogeneous composition of Iran’s population (composed of different races, such as Turks, Persians, Balochs, Kurds, and Arabs) and the different lifestyles and potential genetic features, the difference between the incidences of different people can be affected by the above facts. Besides, the genetic-environmental variables and population heterogeneity that may serve as potential factors contributing to the provincial incidence rates, these rates can also be affected as a result of different protocol capabilities (11). For instance, the economically poor statuses of Sistan and Baluchistan, and Bushehr provinces may also affect their provincial registration systems. The lower rates recorded for sarcoma incidence in these provinces could be resulting from the poor capacity of their provincial cancer registrations. Thus, these confounding parameters may result in decreased incidence of malignancy, and therefore, such registration facilities ought to be enhanced to estimate the nationwide incidence of malignancy more accurately. Other contributing variables, including economic and cultural status and climatic and environmental conditions, can also contribute to the differences observed between incidence rates (27).
The present research is faced with several constraints, such as the ones associated with the Iranian National Cancer Registry (INCR) database because it did not gather the data on local recurrence or surgical margins, patient comorbidities, climatic conditions, and some confounding data. In addition, the grouping of histological types prevented us from commenting on more than 80 subtypes of sarcoma. Nevertheless, such grouping assisted us in providing general epidemiologic information on sarcoma. In addition, some of the other limitations of the present investigation include the differences observed in diagnoses in various geographical regions across the country and the accuracy of data recording. Despite the cited limitations, the present research has provided a thorough update on the sarcoma epidemiology in the elderly population of Iran.
Conclusion
Even though such sarcoma is more prevalent in elderly men, its incidence was also observed in lower-aged female groups. In addition, the incidence rate of BS was lower in comparison with that of STS, and the patients often exhibited an unknown degree of sarcoma. Different provinces showed noticeable differences in sarcoma occurrence. According to the mentioned cases and the prevalence of sarcoma in the elderly population of Iran, it is suggested to pay more attention to changing the lifestyle, especially in this population, to encourage the elderly to do physical activities, pay attention to their diet, and to pay attention to the state of their diseases. Chronic diseases of this population, especially diabetes and high blood pressure, can reduce other complications and health problems of the elderly. Encouraging early screening as well as providing access to health care as well as educating and informing them can help to reduce various diseases as well as the risk factors of these diseases. It is recommended to carry out more investigations to study the survival rate of elderly people suffering from two types of BS and STS and the etiological parameters potentially contributing to sarcoma incidence during old age in various provinces.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The authors would like to express their deepest gratitude to all healthcare personnel at Shahid Beheshti University of Medical Sciences, and special thanks to the staff of the Cancer Research Center.
Footnotes
Conflicts of interest
The authors declare that there is no conflict of interest
References
- 1.Saltus CW, Calingaert B, Candrilli S, et al. (2018). Epidemiology of adult soft-tissue sarcomas in Germany. Sarcoma, 2018:5671926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaghmaii B, Nili F, Najafi Z, et al. (2022). Renal involvement in a child with COVID-19 infection and its management with CRRT. Int J Prev Med, 13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hejazi S, Noroozi M, Kuhestani MA, et al. (2020). Effective factors on clinical outcomes of children with osteosarcoma and Ewing bone tumors. Immunopathol Persa, 6(2):e26–e26 [Google Scholar]
- 4.Sahraei Z, Panahi P, Solhjoukhah K, et al. (2023). The Efficacy of High-Dose Pulse Therapy vs. Low-Dose Intravenous Methylprednisolone on Severe to Critical COVID-19 Clinical Outcomes: A Randomized Clinical Trial. Iran J Pharm Res, 22: e137838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garbay D, Maki R, Blay J, et al. (2013). Advanced soft-tissue sarcoma in elderly patients: patterns of care and survival. Ann Oncol, 24:1924–30. [DOI] [PubMed] [Google Scholar]
- 6.Ebrahimpour A, Chehrassan M, Sadighi M, et al. (2020). The survival and incidence rate of Ewing sarcoma; a national population-based study in Iran (2008–2015). Arch Bone Jt Surg, 8: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zabihiyeganeh M, Bozorgi MHA, Hatami S. (2022). Multiple long bone fractures in a man with pycnodysostosis; a case report. Immunopathol Persa, 9:e24239–e. [Google Scholar]
- 8.Arabizadeh N, Zakerzadeh D, Nakhaie R, et al. (2023). The pathology of brown tumor in a-36-year-old man on maintenance hemodialysis. J Parathyr Dis, 11:e11200. [Google Scholar]
- 9.Bray F, Ferlay J, Soerjomataram I, et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 68:394–424. [DOI] [PubMed] [Google Scholar]
- 10.Stiller C, Trama A, Serraino D, et al. (2013). Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer, 49:684–95. [DOI] [PubMed] [Google Scholar]
- 11.Asef-Kabiri L, Moradi A, Khayamzadeh M, et al. (2021). Sarcomas Epidemiology in Iran: Analysis of Data from Iranian National Cancer Registry between 2009 and 2014. Int J Cancer Manag, 14: e110504. [Google Scholar]
- 12.Pingping B, Yuhong Z, Weiqi L, et al. (2019). Incidence and mortality of sarcomas in Shanghai, China, during 2002–2014. Front Oncol, 9:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasanpour Dehkordi A, Keikhaei B, Bahadoram M, et al. (2019). Keep the corners; impact of chemotherapy on renal function. J Nephropathol, 9: e02–e02. [Google Scholar]
- 14.Toumaj S. (2023). Brown tumor in hyperparathyroidism; a new look at an old problem. J Parathyr Dis, 11:e11207. [Google Scholar]
- 15.Fletcher C, Bridge JA, Hogendoorn PCW, Mertens F. (2013). WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours, 5:468. [Google Scholar]
- 16.Doyle LA. (2014). Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer, 120:1763–74. [DOI] [PubMed] [Google Scholar]
- 17.Kleihues P, Cavenee W. (2000). WHO classification of tumours. Pathology & genetics Tumors of the nervous system Lyon, France, IARCpress, 4:245. [Google Scholar]
- 18.Fletcher CD. (2014). The evolving classification of soft tissue tumours–an update based on the new 2013 WHO classification. Histopathology, 64:2–11. [DOI] [PubMed] [Google Scholar]
- 19.Hung G-Y, Horng J-L, Yen H-J, et al. (2014). Incidence patterns of primary bone cancer in Taiwan (2003–2010): a population-based study. Ann Surg Oncol, 21:2490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatt N, Deady S, Gillis A, Bertuzzi A, et al. (2016). Epidemiological study of soft-tissue sarcomas in Ireland. Cancer Med, 5:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z, Zheng R, Zhang S, et al. (2019). Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China. Cancer Biol Med, 16:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burningham Z, Hashibe M, Spector L, Schiffman JD. (2012). The epidemiology of sarcoma. Clin Sarcoma Res, 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardenas C. (2023). Sarcomas: A comprehensive review of classification, diagnosis, treatment, and psychosocial aspects. Clin Oncol Case Rep, 6:2–8. [Google Scholar]
- 24.Gadgeel SM, Harlan LC, Zeruto CA, et al. (2009). Patterns of care in a population-based sample of soft tissue sarcoma patients in the United States. Cancer, 115:2744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan MF, Antonescu CR, Moraco N, Singer S. (2014). Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg, 260:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gage MM, Nagarajan N, Ruck JM, et al. (2019). Sarcomas in the United States: Recent trends and a call for improved staging. Oncotarget, 10:2462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas DM, Savage SA, Bond GL. (2012). Hereditary and environmental epidemiology of sarcomas. Clin Sarcoma Res, 2(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]