Abstract
BACKGROUND
Chronic hepatitis B (CHB) virus infection is a major cause of liver-associated morbidity and mortality, particularly in low-income countries. A better understanding of the epidemiological, clinical, and virological characteristics of CHB will guide appropriate treatment strategies and improve the control and management of CHB in Ethiopia.
AIM
To investigate the characteristics of CHB in Eastern Ethiopia and assess the efficacy and safety of antiviral treatment.
METHODS
This cohort study included 193 adults who were human immunodeficiency virus-negative with CHB between June 2016 and December 2019. Baseline assessments included chemistry, serologic, and viral markers. χ2 tests, Mann-Whitney U tests, and logistic regression analyses were used to identify the determinants of cirrhosis. Tenofovir disoproxil fumarate (TDF) was initiated using treatment criteria from the Ethiopian CHB pilot program.
RESULTS
A total of 132 patients (68.4%) were men, with a median age of 30 years [interquartile range (IQR): 24-38]. At enrollment, 60 (31.1%) patients had cirrhosis, of whom 35 (58.3%) had decompensated cirrhosis. Khat use, hepatitis B envelope antigen positivity, and a high viral load were independently associated with cirrhosis. Additionally, 66 patients (33.4%) fulfilled the treatment criteria and 59 (30.6%) started TDF. Among 29 patients who completed 24 months of treatment, the median aspartate aminotransferase to platelet ratio index declined from 1.54 (IQR: 0.66-2.91) to 1.10 (IQR: 0.75-2.53) (P = 0.002), and viral suppression was achieved in 80.9% and 100% of patients after 12 months and 24 months of treatment, respectively. Among the treated patients, 12 (20.3%) died within the first 6 months of treatment, of whom 8 had decompensated cirrhosis.
CONCLUSION
This study highlights the high prevalence of cirrhosis, initial mortality, and the efficacy of TDF treatment. Scaling up measures to prevent and control CHB infections in Ethiopia is crucial.
Keywords: Chronic hepatitis B, Cirrhosis, Cohort study, Resource-limited settings, Sub-Saharan Africa
Core Tip: In this study, we investigated the characteristics and efficacy of antiviral treatment in 193 patients who were human immunodeficiency virus-negative and participated in a pilot chronic hepatitis B treatment program in Eastern Ethiopia. Notably, one-third of the patients had cirrhosis at enrollment, reflecting poor access to hepatitis B virus services in the study area. Tenofovir disoproxil fumarate treatment was highly effective in achieving viral suppression and improving the levels of liver fibrosis markers. However, the initial mortality rate was high owing to the high proportion of patients with decompensated cirrhosis. This finding highlights the importance of scaling up national hepatitis B virus prevention and control programs.
INTRODUCTION
Hepatitis B virus (HBV) infection is a public health concern, affecting approximately 296 million people worldwide. Annually, 1.5 million new infections and an estimated 820000 deaths are attributable to HBV infection[1]. The prevalence of HBV infection varies geographically, with East Asia and sub-Saharan Africa having the highest rates[2].
The natural course of chronic hepatitis B (CHB) varies from an inactive carrier state to the development of liver cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC). The estimated 5-year progression rate from CHB to cirrhosis is 12%-20%[3]. In patients with cirrhosis, the 5-year risks of developing hepatic decompensation and HCC are estimated to be 20%-23% and 6%-15%, respectively[4-7]. The greatest risk of progression occurs in patients in the immune clearance phase, those with delayed hepatitis B envelope antigen (HBeAg) seroconversion, and those with reactivation of HBV replication after HBeAg seroconversion[8-12].
The natural course of HBV infection is influenced by viral factors such as HBV DNA level, HBeAg status, hepatitis B surface antigen (HBsAg) level, and genotype, as well as non-HBV related factors such as host factors (e.g., age, sex, obesity, diabetes), lifestyle factors (e.g., alcohol abuse), and co-infection with other viruses, including hepatitis C virus (HCV), hepatitis D virus (HDV), or human immunodeficiency virus (HIV)[8,13-16]. Ethiopia is endemic for HBV infection with an intermediate-to-high prevalence[17]. The estimated prevalence of HBsAg is 7.4%, and HBV and HCV infections account for 60% and 80% of chronic liver disease (CLD) and HCC[18]. In a large Ethiopian CHB cohort in Addis Ababa, the majority (90.7%) of patients were HBeAg-negative, one-third (38.9%) were inactive carriers, and one-third had significant fibrosis at enrollment[19]. However, information on CHB characteristics in Eastern Ethiopia is lacking. In a study of patients with CLD in Eastern Ethiopia, CHB accounted for one-third of the cases[20]. Clinical and virological features of patients with CHB remain poorly documented in this area. Moreover, the limited diagnostic facilities, restrictions on antiviral drugs, and inadequate public funding are barriers to CHB prevention and control in Ethiopia.
Previous studies have shown that antiviral therapy for CHB reduces the risk of disease progression, prevents or reverses liver fibrosis, and prevents HCC development[21-23]. However, in Ethiopia, particularly in the eastern region, treatment for viral hepatitis is rarely available in the public sector. Consequently, patients infected with HBV alone cannot access this life-saving drug and remain untreated. This study aimed to address the gaps in current knowledge by investigating the characteristics of chronic HBV infection and evaluating the efficacy and safety of antiviral treatment in Eastern Ethiopia.
MATERIALS AND METHODS
Study setting and participants
This observational cohort study was conducted at Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia, from June 2016 to December 2019. In June 2016, a CHB treatment program was established, and healthcare providers at other hospitals and clinics were informed of the new services provided. Therefore, patients who were HBsAg-positive were referred to various health facilities for evaluation and treatment. Consenting adults (aged > 18 years) with CHB were included in this study. CHB was defined as a persistently positive HBsAg test result for more than 6 months. Patients without a previous HBsAg test or with the first positive result less than 6 months ago retested to distinguish from acute hepatitis B. Patients with HCC and other terminal illnesses were excluded from the study. Similarly, patients who had HIV infection were not included and were sent to the closest HIV treatment facility.
The study was approved by the National Research Ethics Review Committee (Ref. No. 3.10/829/07) in Ethiopia and by the Regional Committees for Medical and Health Research Ethics (Ref. No. 2014/1146) in Norway. This study was conducted in accordance with the principles of the Declaration of Helsinki[24]. Written informed consent was obtained from all the study participants.
Patient assessment
All the patients were interviewed and examined using a standardized form. Sociodemographic information, including sex, age, marital status, residence area, and occupation, was collected. Pregnancy and breastfeeding statuses were recorded. Past medical history included previous liver disease, hepatitis serology, and ingestion of alcohol, khat, and other substances. Harmful alcohol use was defined as consuming > 30 g/day for men and 20 g/day for women[25], and khat abuse was defined as the current use of khat, regardless of the quantity. Clinical examinations included liver disease stigmata (palmar erythema, spider angiomas, jaundice, ascites, and hepatosplenomegaly) as well as weight or height.
Laboratory tests
Blood samples were collected at enrollment and every 3 months for local testing of hematology, biochemistry, and serology. HBsAg was detected onsite using a World Health Organization (WHO)-approved rapid diagnostic test (Determine, Alere Inc., United States), and an antibody against HCV (anti-HCV) was detected onsite using another rapid diagnostic test (Nantong Diagnos Technology Co., Ltd., China). Routine laboratory investigations were performed using the following kits and assays: Complete blood cell counts were conducted using a hematology analyzer (Sysmex, Kobe, Japan), and biochemical tests were carried out using a semi-automatic biochemistry analyzer DR-7000D (DIRUI, Changchun, China) and a HumaLyzer 3000 (HUMAN, Wiesbaden, Germany).
HIV testing was performed following the national algorithm using a WHO-approved rapid test kit [HIV 1 + 2 Antibody Colloidal Gold (KHB), Shanghai Kehua Bio-engineering Co., China] for screening and another rapid test kit (HIV 1/2 STAT-PAK, Chembio Diagnostics, United States) for confirmation.
Aspartate aminotransferase to platelet ratio index (APRI) was calculated from standard blood test results using the following formula 1.
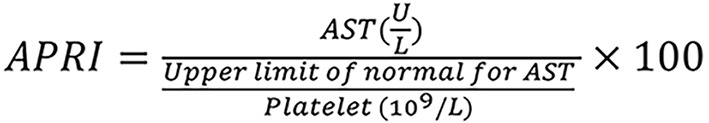
Using 1.5 as an indicator of significant fibrosis[26] and the fibrosis-4 (FIB-4) score was calculated as using a threshold of 3.25 as the following formula 2, an indicator of advanced fibrosis or cirrhosis[27].
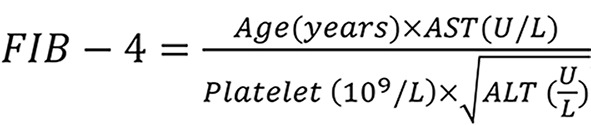
Serological tests for HBV markers were conducted at the International Clinical Laboratory of Addis Ababa. HBeAg and anti-HBeAg antibodies (anti-HBe) were analyzed using an enzyme-linked fluorescent immunoassay (VIDAS HBe/anti-HBe; BioMérieux, Marcy l’Etoile, France). Viral load testing was performed using the Abbott Real-time HBV assay (Abbott Molecular, Des Moines, United States), following the manufacturer’s instructions. A complete blood count, routine chemistry [bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine], and serological tests (HIV antibody, HBsAg, anti-HCV, HBV DNA viral load, HBeAg, and anti-HBe antibody) were performed on all patients at the first visit.
Radiology
Abdominal ultrasound was performed locally in order to detect cirrhosis, ascites, and/or HCC.
Criteria to start antiviral treatment
Treatment decisions were made by a dedicated physician using the treatment criteria provided by a simplified CHB treatment protocol adopted from an Ethiopian CHB pilot study[28]. Patients who met any of the following criteria were considered eligible for treatment: (1) Decompensated cirrhosis; (2) Compensated cirrhosis (confirmed with ultrasound, APRI score > 2 or both); (3) Significant fibrosis (APRI score > 1.5) and viral load > 2000 IU/mL; (4) Moderate or severe liver inflammation (ALT > 80 U/L) and viral load > 2000 IU/mL; and (5) HCC among first-grade relatives and viral load > 2000 IU/mL.
Participants eligible for treatment were counseled on adherence and education about the disease (the natural course of HBV infection, its long-term consequences, the benefits and possible side effects of therapy, the risk of resistance to irregular treatment, the risk of hepatitis flares if treatment was interrupted, and the need for life-long follow-up). The patients were advised to consume excess alcohol. Preventive measures such as condom use and HBV vaccination for patients’ partners and children are recommended. Tenofovir disoproxil fumarate (TDF) 300 mg once daily was used in this program because of its efficacy, safety, and high resistance barrier. This program does not offer treatment for HCV or HDV co-infections.
Follow-up of patients
Patients with signs or symptoms of decompensated liver disease (ascites, jaundice, history of upper gastrointestinal (GI) bleeding, and confusion) were re-examined 1-2 weeks after the initial examination. Patients without signs or symptoms of advanced liver disease were included after 3 months, following the availability of viral load test results. Individuals receiving treatment were re-evaluated after 2 weeks and 4 weeks, and subsequently every 3 months. Monitoring adherence and side effects were prioritized at each visit. Untreated individuals were followed up at 3-month intervals. The laboratory tests performed during the follow-up period are summarized in Table 1. If untreated patients at a later stage during follow-up fulfilled the treatment criteria, antiviral treatment was initiated after proper adherence counseling, and HIV infection was excluded.
Table 1.
Follow up schedule of enrolled chronic hepatitis B patients, Harar, Eastern Ethiopia
|
Laboratory test
|
On treatment
|
Not on treatment
|
| After 4 weeks | CBC, ALT, AST, creatinine | |
| Every 3 months | CBC, ALT, AST, creatinine HIV rapid test |
CBC, ALT, AST, creatinine |
| Every 6 months | HBsAg, HBV DNA viral load, HBeAg/anti-HBe (only if HBeAg-positive previously) | HBsAg, HBV DNA viral load |
| Every 12 months | Abdominal ultrasound as screening for HCC |
ALT: Alanine aminotransferase; anti-HBe: Hepatitis B envelope antigen antibody; AST: Aspartate aminotransferase; CBC: Complete blood count; HBeAg: Hepatitis B envelope antigen; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma.
Assessment of adherence to therapy
TDF was initially dispensed for 1 month and subsequently at 3-month intervals. The ‘pharmacy refill’ or ‘pill count’ method was used to assess adherence. Adherence was classified into three categories: Excellent adherence, if they took more than 95% of their tablets; medium adherence, if they took 80%-95% of their tablets; and poor adherence, if they took less than 80% of their tablets. Patients who missed scheduled appointments for more than 1 month were traced via telephone or through questioning their relatives. Participants were recorded as dropping out of the study if they refused or withdrew their consent. If a patient died, the cause and approximate date of death were registered in their file.
Classification of patients with CHB into the various phases
The diagnostic criteria from the EASL 2017 guidelines were used to define the phases of patients with CHB as follows[29]: (1) HBeAg-positive chronic infection: HBeAg-positive, HBV DNA levels > 20000 IU/mL, persistently normal ALT levels; (2) HBeAg-positive chronic hepatitis: HBeAg-positive, HBV DNA levels > 10000 IU/mL, and ALT levels intermittently or persistently elevated above the upper limit of normal; (3) HBeAg-negative chronic infection: HBeAg-negative, anti-HBe-positive, HBV DNA levels < 2000 IU/mL, and persistently normal ALT levels; and (4) HBeAg negative chronic hepatitis: HBeAg-negative, HBV DNA level > 2000 IU/mL, and ALT level intermittently or persistently elevated above the upper limit of normal. The upper limit of normal for ALT was defined according to the traditional cutoff value of 40 U/L.
Definition of terms
Cirrhosis is defined as any of the following: (1) Current or past evidence of ascites, confirmed by ultrasound or clinical examination; (2) Nodular or irregular liver surface on ultrasound; and (3) APRI score ≥ 2. Decompensated cirrhosis is defined as the current or past evidence of ascites, detected through ultrasonography or clinical examination, or the current or past evidence of upper GI bleeding, jaundice, encephalopathy, or coagulopathy. Compensated cirrhosis is defined as a cirrhotic liver with a U/S, APRI score ≥ 2, or both in the absence of clinical signs of decompensation. Virological suppression is achieved when HBV DNA level < 69 IU/mL. Virologic failure or persistent viremia is indicated by HBV DNA level > 69 IU/mL 12 months after treatment. Major virologic failure is characterized by HBV DNA level > 1000 IU/mL at 12 months after treatment.
Statistical analysis
Descriptive statistics summarized the patient demographics and baseline characteristics. Categorical variables were reported as frequencies, and continuous variables as medians and interquartile ranges (IQR). Comparisons between groups were performed using the χ2 test for categorical variables and Mann-Whitney U-tests for continuous variables. Logistic regression analyses identified the factors that were independently associated with cirrhosis. Variables with a P value < 0.2 in the bivariable analysis were exported to the multivariable analysis using a forward stepwise method. Statistical significance level was set at P < 0.05. Statistical analyses were performed using SPSS 27 software (SPSS Inc., Chicago, IL, United States).
RESULTS
Baseline patient characteristics
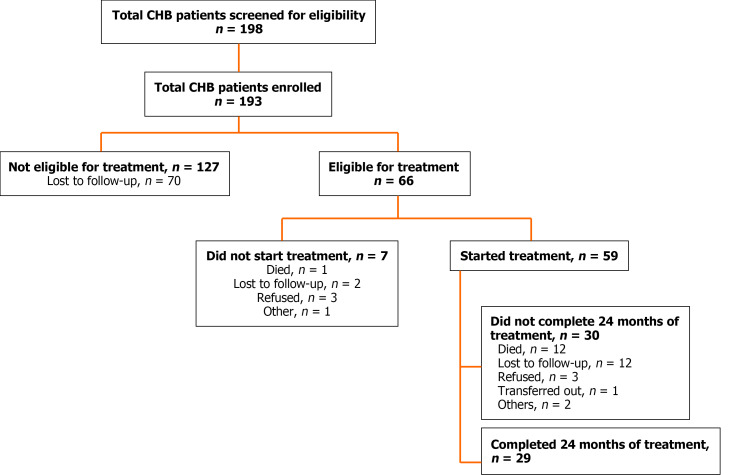
A total of 198 patients were screened; among the 5 who were excluded, 2 did not meet the inclusion criteria, and 3 were retrospectively diagnosed with resolved acute hepatitis. The final study population comprised 193 patients (Figure 1). Overall, the median age was 30 years (IQR: 24-38), and the majority of patients were men (68.4%), derived from the Oromia region (36.8%), married (63.7%), and working as farmers (17%). Alcohol abuse was reported in 35 (18.1%) patients, and 105 (54.4%) patients reported khat chewing (Table 2). Cirrhosis was more common among males, khat users, farmers, and those from the Oromia region. Otherwise, no significant difference was observed in the demographic features between the groups with and without cirrhosis.
Figure 1.
Profile of chronic hepatitis B cohort study, Harar, Eastern Ethiopia. CHB: Chronic hepatitis B.
Table 2.
Baseline characteristics of patients with chronic hepatitis B, by cirrhosis status, Harar, Eastern Ethiopia
|
Variable
|
All patients, n = 193
|
No cirrhosis, n = 133 |
Cirrhosis, n = 60 |
P value
|
| Sex, n (%) | 0.020a | |||
| Male | 132 (68.4) | 84 (63.2) | 48 (80.0) | |
| Female | 61 (31.6) | 49 (36.8) | 12 (20.0) | |
| Age group by years, n (%) | 0.852 | |||
| 18-25 | 69 (35.8) | 47 (35.3) | 22 (36.7) | |
| 26-35 | 70 (36.3) | 47 (35.3) | 23 (38.3) | |
| 36-45 | 36 (18.7) | 18 (13.5) | 18 (30.0) | |
| > 45 | 18 (9.3) | 14 (10.5) | 4 (6.7) | |
| Marital status1, n (%) | 0.597 | |||
| Single | 61 (31.6) | 45 (33.8) | 16 (26.6) | |
| Married | 123 (63.7) | 81 (60.9) | 42 (70.0) | |
| Divorced/widowed | 3 (1.6) | 2 (1.5) | 1 (1.7) | |
| Address, n (%) | 0.032a | |||
| Harar | 48 (24.9) | 40 (30.0) | 8 (13.3) | |
| Dire Dawa | 56 (29.0) | 42 (31.6) | 14 (23.3) | |
| Oromia | 71 (36.8) | 34 (25.6) | 37 (61.7) | |
| Somali | 18 (9.3) | 17 (12.8) | 1 (1.7) | |
| Occupation2, n (%) | 0.009a | |||
| Farmer | 33 (17.1) | 14 (10.5) | 19 (31.7) | |
| Housewife | 29 (15.0) | 22 (16.5) | 7 (11.7) | |
| Student | 23 (11.9) | 14 (10.5) | 9 (15.0) | |
| Public servant | 31 (16.1) | 26 (19.5) | 5 (8.3) | |
| Self employed | 18 (9.3) | 12 (9.0) | 6 (10.0) | |
| Others | 19 (9.8) | 14 (10.5) | 5 (8.3) | |
| Family history of liver cancer3, n (%) | 0.569 | |||
| No | 183 (94.8) | 125 (93.9) | 58 (96.7) | |
| Yes | 9 (4.7) | 7 (5.3) | 2 (3.3) | |
| Traditional herbal medicine4, n (%) | 0.220 | |||
| No | 169 (87.6) | 120 (90.2) | 49 (81.7) | |
| Yes | 21 (10.9) | 11 (8.3) | 10 (16.7) | |
| Pregnant5, n (%) | 0.085 | |||
| No | 51 (89.4) | 39 (86.7) | 12 (100) | |
| Yes | 6 (10.5) | 6 (13.3) | 0 | |
| Breast feeding, n (%) | 0.110 | |||
| No | 54 (94.7) | 43 (95.6) | 11 (91.7) | |
| Yes | 3 (5.3) | 2 (4.4) | 1 (8.3) | |
| Alcohol abuse, n (%) | 0.172 | |||
| No | 158 (81.9) | 105 (78.9) | 53 (88.3) | |
| Yes | 35 (18.1) | 28 (21.1) | 7 (11.7) | |
| Khat abuse, n (%) | 0.001a | |||
| No | 88 (45.6) | 71 (53.4) | 17 (28.3) | |
| Yes | 105 (54.4) | 62 (46.6) | 43 (71.7) |
P < 0.05.
A total of 6 missing values, of whom 1 had cirrhosis.
A total of 40 missing values, of whom 9 had cirrhosis.
Data missing in 1 patient without cirrhosis.
A total of 3 missing values, of whom 1 had cirrhosis.
A total of 2 missing values, of whom none had cirrhosis.
Clinical presentation
A total of 115 (59.6%) patients were symptomatic, and the most common complaint was abdominal pain, followed by abdominal swelling. The majority (n = 159, 82.4%) had a normal physical examination; the most common abnormal clinical findings were underweight (body mass index < 18.5 kg/m2), splenomegaly, and ascites. Abdominal ultrasound was performed for 134 patients; among them, 64 (47.8%) had a normal liver surface, 49 (36.6%) had a nodular and irregular surface, and 11 (8.2%) had a mildly uneven surface (Table 3). Patients with cirrhosis are more likely to present with symptoms and signs of liver disease.
Table 3.
Clinical presentation of patients with chronic hepatitis B, by cirrhosis status, Harar, Eastern Ethiopia
|
Variables
|
All patients, n = 193 |
No cirrhosis, n = 133 |
Cirrhosis, n = 60 |
P value
|
| Symptoms, n (%) | 69 (35.8) | 58 (43.6) | 57 (95.0) | < 0.001a |
| Jaundice | 34 (17.6) | 14 (10.5) | 20 (33.3) | 0.001a |
| Abdominal pain | 69 (35.8) | 38 (28.6) | 31 (51.7) | 0.003a |
| Abdominal swelling | 57 (29.5) | 20 (15) | 37 (61.7) | < 0.001a |
| Edema | 32 (16.6) | 6 (4.5) | 26 (43.3) | < 0.001a |
| Nausea/vomiting | 36 (18.7) | 13 (9.8) | 23 (38.3) | < 0.001a |
| Hematemesis | 24 (12.4) | 8 (6.0) | 16 (26.7) | < 0.001a |
| Pruritus | 18 (9.3) | 10 (7.5) | 8 (13.3) | 0.199 |
| Muscle/joint aches | 44 (22.8) | 21 (15.8) | 23 (38.3) | 0.001a |
| Confusion | 13 (6.7) | 9 (6.8) | 4 (6.7) | 0.979 |
| Weight loss | 51 (26.4) | 18 (13.5) | 33 (55.0) | < 0.001a |
| Signs, n (%) | ||||
| BMI < 18.5 kg/m2 | 34 (17.6) | 6 (4.5) | 49 (81.7) | < 0.001a |
| Edema | 30 (15.5) | 18 (13.5) | 12 (20.0) | 0.033a |
| Jaundice | 12 (6.2) | 0 (0) | 12 (20.0) | < 0.001a |
| Pale conjunctiva | 10 (5.2) | 1 (0.8) | 9 (15.0) | < 0.001a |
| Palmar erythema | 14 (7.3) | 2 (1.5) | 12 (20.0) | < 0.001a |
| Gynecomastia | 8 (4.1) | 0 (0) | 8 (13.3) | 0.011a |
| Ascites | 29 (15.0) | 0 (0) | 29 (48.3) | < 0.001a |
| Splenomegaly | 34 (17.6) | 1 (0.8) | 33 (55.0) | < 0.001a |
| Ultrasound findings1, n (%) | ||||
| Normal liver surface | 64 (47.8) | 62 (80.5) | 2 (3.5) | 0.022a |
| Mild uneven liver surface | 11 (8.2) | 5 (6.5) | 6 (10.5) | 0.478 |
| Nodular and irregular surface | 49 (36.6) | 0 (0) | 49 (86.0) | < 0.001a |
| Other | 10 (7.5) | 10 (13.0) | 0 (0) | 0.057 |
P < 0.05.
A total of 59 missing values, of whom 3 patients had clinical signs of cirrhosis. BMI: Body mass index.
Baseline biochemical, serological, and virological data
Overall, the alterations in laboratory variables were mild (Table 3). Among the 187 patients with platelet counts recorded at presentation, 60 (32.1%) were below the reference range. The median ALT level was 36 U/L (IQR: 23-58), and of the 183 patients with ALT values determined at presentation, 87 (47.5%) had an ALT level > 40 U/L. The median AST level was 40 U/L (IQR: 29-65); among the 182 patients with AST values determined at presentation, 92 (50.5%) had an AST level > 40 U/L. The median APRI value was 0.52 (IQR: 0.29-1.35), and 39 (22.3%) patients had an APRI score ≥ 1.5, indicative of liver fibrosis. The median FIB-4 score was 1.06 (IQR: 0.60-198), and 30 (17.4%) patients had a FIB-4 score ≥ 3.25, indicating advanced fibrosis. The HBeAg status was determined in 133 patients, 28 (21.1%) of whom were HBeAg-positive. A viral load test was performed on 177 patients; among them, 114 (64.4%) had a viral load below 2000 IU/mL, with a median viral load of 677 IU/mL (IQR: 135-7580) (Table 4). None of the patients were co-infected with HCV or HIV (patients with HIV were not included in this program). Patients with cirrhosis had lower platelets, higher liver transaminases, higher FIB-4 score, and APRI score (1.53 vs 0.38) compared to those without cirrhosis. Moreover, patients with cirrhosis were more likely to be HBeAg-positive and had a higher viral load than those without cirrhosis (Table 4).
Table 4.
Baseline biochemical, serologic, and virological data of patients with chronic hepatitis B, by cirrhosis status, Harar, Eastern Ethiopia
|
Variables
|
All patients, n = 193 | No cirrhosis, n = 133 | Cirrhosis, n = 60 |
P value
|
| WBC as 106/L | 5.9 (4.1-7.7) | 6.5 (4.9-7.8) | 4.2 (3.0-6.3) | <0.001a |
| Hemoglobin in g/L | 14.7 (12.4-16.6) | 15.5 (13.8-16.9) | 11.8 (10.0-15.2) | < 0.001a |
| Platelet count as 109/L1 | <0.001a | |||
| Median (IQR) | 218 (119-294) | 253 (184-344) | 118 (54-180.3) | |
| < 150, n (%) | 60 (32.1) | 20 (15.7) | 40 (66.7) | |
| ≥ 150, n (%) | 127 (67.9) | 107 (84.3) | 20 (33.3) | |
| ALT (U/L)2 | 0.033a | |||
| Median (IQR) | 36 (23-58) | 35 (22-52) | 45 (27-84) | |
| < 40, n (%) | 96 (52.5) | 70 (55.6) | 26 (45.6) | |
| ≥ 40, n (%) | 87 (47.5) | 56 (44.4) | 31 (54.4) | |
| AST as IU/L3 | 0.016a | |||
| Median (IQR) | 40 (29-65) | 36 (28-57) | 51 (34-81) | |
| < 40, n (%) | 90 (49.5) | 67 (53.6) | 23 (40.4) | |
| ≥ 40, n (%) | 92 (50.5) | 58 (46.4) | 34 (59.6) | |
| Creatinine in mg/dL4 | 0.750 | |||
| Median (IQR) | 0.8 (0.6-1.0) | 0.8 (0.6-1.0) | 0.8 (0.6-1.0) | |
| ≤ 1.2, n (%) | 150 (90.4) | 101 (91.0) | 49 (89.1) | |
| > 1.2, n (%) | 16 (9.6) | 10 (9.0) | 6 (10.9) | |
| APRI score5 | < 0.001a | |||
| Median (IQR) | 0.52 (0.29-1.35) | 0.38 (0.26-0.70) | 1.53 (0.66-2.84) | |
| < 1.5, n (%) | 136 (77.7) | 108 (91.5) | 28 (49.1) | |
| ≥ 1.5, n (%) | 39 (22.3) | 10 (8.5) | 29 (50.9) | |
| FIB-4 score6 | < 0.001a | |||
| Median (IQR) | 1.06 (0.60-1.98) | 0.85 (0.55-1.41) | 1.93 (1.03-4.08) | |
| ≤ 3.25, n (%) | 142 (82.6) | 106 (90.6) | 36 (65.5) | |
| > 3.25, n (%) | 30 (17.4) | 11 (9.4) | 19 (34.5) | |
| HBeAg7 | 0.001a | |||
| Negative, n (%) | 105 (78.9) | 80 (87.9) | 25 (59.5) | |
| Positive, n (%) | 28 (21.1) | 11 (12.8) | 17 (40.5) | |
| HBV viral load in IU/mL8 | < 0.001a | |||
| Median (IQR) | 677 (135-7580) | 486 (105-2863) | 2843 (313-805407) | |
| < 2000, n (%) | 114 (64.4) | 91 (72.2) | 23 (45.1) | |
| 2000-19999, n (%) | 24 (13.6) | 15 (11.9) | 9 (17.6) | |
| ≥ 20000, n (%) | 39 (22.0) | 20 (15.9) | 19 (37.3) |
P < 0.05.
A total of 6 missing values, of whom none had cirrhosis.
A total of 10 missing values, of whom 3 had cirrhosis.
A total of 11 missing values, of whom 3 had cirrhosis.
A total of 27 missing values, of whom 3 had cirrhosis.
A total of 18 missing values, of whom 3 had cirrhosis.
A total of 21 missing values, of whom 5 had cirrhosis.
A total of 60 missing values, of whom 18 had cirrhosis.
A total of 16 missing values, of whom 9 had cirrhosis. ALT: Alanine aminotransferase; APRI: Aspartate aminotransferase to platelet ratio index; AST: Aspartate aminotransferase; FIB-4: Fibrosis-4; HBeAg: Hepatitis B envelope antigen; HBV: Hepatitis B virus; IQR: Interquartile range; WBC: White blood cell count.
Phases of CHB
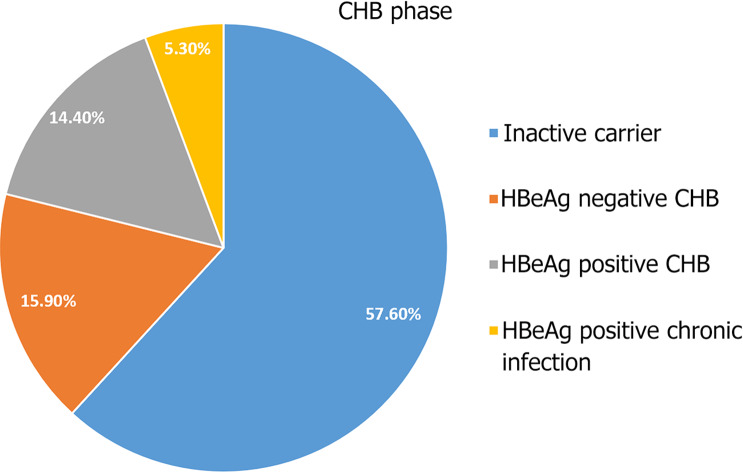
Individualized analysis of HBV DNA, ALT, and HBeAg status allowed phase classification of chronic hepatitis in 132 (68.4%) patients, as follows: 76 (57.6%) presented with HBeAg-negative chronic infection (inactive carrier), 7 (5.3%) with HBeAg-positive chronic infection, 19 (14.4%) with HBeAg-positive chronic hepatitis, and 21 (15.9%) with HBeAg-negative chronic hepatitis (Figure 2). In 61 (31.6%) patients, phase classification was not possible because of missing HBeAg or viral load data. Of the 60 (31.1%) patients diagnosed with cirrhosis at presentation, 35 (58.3%) had decompensated cirrhosis.
Figure 2.
Clinical phase of 132 chronic hepatitis B patients with full data available, Harar, Eastern Ethiopia. Individualized analysis of hepatitis B virus DNA, alanine aminotransferase, and hepatitis B envelope antigen (HBeAg) status allowed phase classification of chronic hepatitis in 132 (68.4%) patients: 76 (57.6%) presented with HBeAg-negative chronic infection (inactive carrier), 7 (5.3%) with HBeAg-positive chronic infection, 19 (14.4%) with HBeAg-positive chronic hepatitis, and 21 (15.9%) with HBeAg-negative chronic hepatitis. In 61 (31.6%) patients, phase classification was not possible because of missing HBeAg or viral load data. CHB: Chronic hepatitis B.
Factors associated with cirrhosis
Male sex, khat abuse, HBeAg positivity, and viral load > 2000 IU/mL were significantly associated with cirrhosis in the bivariable analysis. In the multivariate analysis, only khat abuse [adjusted odds ratio (AOR) = 2.86, P = 0.003], HBeAg positivity (AOR = 3.81, P = 0.022), and HBV DNA viral load > 2000 IU/mL (AOR = 5.29, P = 0.005) were independently associated with cirrhosis (Table 5).
Table 5.
Factors associated with cirrhosis among patients with chronic hepatitis B, Harar, Eastern Ethiopia
| Baseline variables | No cirrhosis, n = 133 | Cirrhosis, n = 60 |
Bivariable
|
Multivariable
|
||
|
COR (95%CI)
|
P value
|
AOR (95%CI)
|
P value
|
|||
| Sex, n (%) | 0.022 | 0.308 | ||||
| Female | 49 (36.8) | 12 (20.0) | 1 | 1 | ||
| Male | 84 (63.2) | 48 (80.0) | 2.33 (1.13-4.81) | 1.74 (0.72-4.20) | ||
| Age group by years, n (%) | 0.635 | |||||
| < 40 | 100 (75.2) | 47 (78.3) | 1 | |||
| ≥ 40 | 33 (24.8) | 13 (26.7) | 0.84 (0.40-1.74) | |||
| Alcohol abuse, n (%) | 0.122 | |||||
| No | 105 (78.9) | 53 (88.3) | 1 | |||
| Yes | 28 (21.1) | 7 (11.7) | 0.49 (0.20-1.21) | |||
| Khat abuse, n (%) | 0.002 | 0.003a | ||||
| No | 71 (53.4) | 17 (28.3) | 1 | 1 | ||
| Yes | 62 (46.6) | 43 (71.7) | 2.89 (1.50-5.59) | 2.86 (1.44-5.68) | ||
| ALT in U/L1, n (%) | 0.213 | |||||
| < 40 | 70 (55.6) | 26 (45.6) | 1 | |||
| ≥ 40 | 56 (44.4) | 31 (54.4) | 1.49 (0.79-2.79) | |||
| AST in U/L2, n (%) | 0.099 | |||||
| < 40 | 67 (53.6) | 23 (40.4) | 1 | |||
| ≥ 40 | 58 (46.4) | 34 (59.6) | 1.71 (0.91-3.22) | |||
| HBeAg3, n (%) | 0.007 | 0.022a | ||||
| Negative | 80 (87.9) | 25 (59.5) | 1 | 1 | ||
| Positive | 11 (12.8) | 17 (40.5) | 3.61 (1.41-9.21) | 3.81 (1.21-11.98) | ||
| HBV viral load in IU/mL4, n (%) | 0.001 | 0.004a | ||||
| < 2000 | 91 (79.8) | 23 (45.1) | 1 | 1 | ||
| ≥ 2000 | 35 (20.2) | 28 (54.9) | 3.17 (1.61-6.23) | 5.29 (1.72-16.33) | ||
P < 0.05.
A total of 10 missing values, of whom 3 had cirrhosis at baseline.
A total of 11 missing values, of whom 3 had cirrhosis at baseline.
A total of 61 missing values, of whom 18 had cirrhosis at baseline.
A total of 16 missing values, of whom 9 had cirrhosis at baseline. ALT: Alanine aminotransferase; AOR: Adjusted odds ratio; AST: Aspartate aminotransferase; CI: Confidence interval; COR: Crudes odds ratio; HBeAg: Hepatitis B envelope antigen; HBV: Hepatitis B virus.
Antiviral treatment
Of the 193 patients in this analysis, 66 (34.2%) met the treatment eligibility criteria and 59 (30.6%) started treatment (Figure 1). The majority (84.7%) started treatment based on a diagnosis of cirrhosis; among them, 32 (54.2%) had decompensated cirrhosis, whereas 18 (30.5%) had compensated cirrhosis. The remaining treatment was started based on moderate or severe liver inflammation in 5 (8.5%) patients, significant fibrosis in 2 (3.4%) patients, and a family history of first-degree relatives with HCC in 2 (3.4%) patients. Additionally, 4 patients reported previous exposure to antiviral treatment in the private sector; however, all had discontinued the therapy for financial reasons. Among those who started treatment, 56 (94.9%) started treatment immediately (i.e. within 4 weeks of enrollment), and the remaining 3 (5.1%) started more than 6 months later.
A total of 12 (20.3%) patients on treatment died, 8 of whom had decompensated cirrhosis at baseline. All died within 6 months after starting treatment. The causes of death was identified in 4 patients and were as follows: 3 from upper GI bleeding, and 1 from spontaneous bacterial peritonitis. A total of 18 (30.5%) patients did not complete 24 months of antiviral therapy; among them, 12 (20.3%) were lost to follow-up, 3 (5.1%) refused to continue treatment for various reasons, 2 (3.4%) were excluded from the study after 6 months of therapy because they were retrospectively diagnosed with resolved acute hepatitis, and 1 (1.7%) was transferred after being diagnosed with HCC.
Among the remaining 29 patients who completed 24 months of antiviral treatment, the median ALT levels were 49 U/L (IQR: 28-88) at baseline, 35 U/L (IQR: 22-63) at 12 months, and 41 U/L (IQR: 30-48) at 24 months. The median APRI score steadily declined during treatment as follows: 1.54 (IQR: 0.66-2.91) at baseline, 1.41 (IQR: 0.66-3.1) at 12 months, and 1.10 (IQR: 0.75-2.53) at 24 months (P = 0.002). Among the 17 patients who were HBeAg-positive and started treatment, 4 (23.5%) experienced HBeAg loss at 24 months, while 1 (3.4%) experienced HBsAg seroconversion, defined as having two consecutive negative HBsAg results and positive anti-HB antibody results. Among the 21 patients who underwent a viral load test at 12 months, 17 (80.9%) experienced suppressed viremia (viral load 69 IU/mL), and 4 (19.1%) experienced virologic failure (viral load 69 IU/mL), including 1 patient with major virologic failure (viral load > 1000 IU/mL). However, virological suppression was achieved in all 15 patients based on viral load test data after 24 months of treatment. In the remaining 14 patients, viral load at 24 months of age was not determined. Of the 29 patients who completed 24 months of treatment, 21 (72.4%) had excellent adherence, 6 (20.7%) had medium adherence, and 2 (6.9%) had poor adherence.
DISCUSSION
In this study, we described the characteristics of 193 patients who were HIV-negative and participated in a CHB pilot treatment program in Eastern Ethiopia. Notably, cirrhosis was diagnosed in nearly one-third of the patients at enrollment, with 58% exhibiting hepatic decompensation. Previous studies have found that 4%-13% of individuals who were HBV-infected in sub-Saharan Africa have cirrhosis[30]. A study from an early treatment program in Ethiopia found that 17.6% of patients were diagnosed with cirrhosis[31]. The higher proportion of patients with cirrhosis in our cohort might reflect poor access to HBV services in Eastern Ethiopia; hence, patients with symptoms desperate for life-saving antiviral treatment were overrepresented in our treatment program. This finding confirms the importance of scaling up national HBV prevention and treatment programs to achieve the WHO elimination goals by 2030.
The high prevalence of HBeAg-negative chronic HBV infection in our study is consistent with the findings of previous HBV studies. Aberra et al[19] reported that 90% of Ethiopian patients with CHB were HBeAg negative. Similarly, studies conducted in other sub-Saharan Africa countries found that the prevalence of HBeAg-negative chronic HBV infection ranged from 80%-93%[32,33]. Previous studies have reported a lower rate of HBeAg-positive chronic HBV infection in African patients with CHB than in those in the Far East, possibly attributed to geographical variability in HBV genotypes[21,22,34]. This difference in genotype distribution and natural history underscores the need for longitudinal studies in Africa to inform local treatment guidelines.
Most patients in our CHB cohort were diagnosed in the inactive carrier phase, consistent with the findings of other studies from sub-Saharan Africa[35-38]. Based on studies from Asia, up to 20% of patients have one or more reversions to HBeAg-positive CHB (immune-reactive phase), and 20%-30% reactivate into HBeAg-negative CHB (immune-active phase)[10]. However, whether this occurs in African patients with CHB is unknown. Antiviral therapy is usually not indicated in these patients because of their excellent long-term prognosis.
In this study, khat use, HBeAg positivity, and HBV viral load greater than 2000 IU/mL were independently associated with cirrhosis. The strong association between HBV DNA level and cirrhosis observed in our study is similar to reports from other African studies[39,40]. In the REVEAL-HBV cohort study, the HBV DNA level was the strongest predictor of progression to cirrhosis, and the risk increased significantly with increasing HBV DNA level, irrespective of HBeAg status and ALT levels[41]. Early initiation of antiviral therapy in patients with CHB with sustained viral suppression has been shown to reduce the risk of cirrhosis, HCC, and liver-related death[42]. In this study, HBeAg positivity was associated with a four-fold increased risk of cirrhosis. Similar results were observed in other studies[39,43], although some found no association between HBeAg status and cirrhosis[19,44]. Overall, consensus is lacking regarding the effect of HBeAg status on liver disease progression to cirrhosis in Africa[41,45]. Whether HBeAg plays a direct biological role or merely represents a surrogate marker of HBV replication remains unclear[46]. Nevertheless, the worse prognosis in patients with HBeAg-positive CHB may be related to necroinflammation of the liver caused by recurrent episodes of hepatitis, which increases the risk of fibrosis, cirrhosis, and potentially carcinogenesis[47].
Interestingly, our study found an independent association between khat consumption and an increased risk of cirrhosis. Khat consumption has been implicated in the development of acute and CLD[48-51]. In a case-control study conducted in Eastern Ethiopia, Orlien et al[52] reported a strong dose-dependent association between chewing khat and the risk of developing CLD. CHB was noted in approximately 37% of the patients who chewed khat in this study. Our findings suggest that khat consumption may potentiate CHB-mediated liver damage. However, longitudinal studies are required to assess the causality and dose-response relationship between khat use and the risk of developing cirrhosis in patients with CHB. Although the exact mechanism underlying khat-related hepatotoxicity is unknown, possible explanations include the direct toxic effects of khat alkaloids and their metabolites[53], induction of immune-mediated liver injury[54-56], and generation of reactive oxygen species[57].
In our study, 80.9% and 100% of the patients achieved viral suppression 12 months and 24 months after treatment initiation, respectively. This finding aligns with a previous Ethiopian CHB cohort study where 85.2% of patients achieved viral suppression below 69 IU/L after 12 months of treatment[28]. Additionally, studies from high-, low-, and middle-income countries have reported similar results[58-60]. In this study, the 2-year mortality rate among treated patients was 20.3%, consistent with previous findings[28]. The high mortality rate might be attributed to limited access to HBV services in Eastern Ethiopia; consequently, patients with advanced cirrhosis in critical need of life-saving antiviral treatment were overrepresented in our treatment program. Notably, 8 of the 12 patients who died had decompensated cirrhosis.
Our study has certain limitations. First, we used the APRI, an imperfect tool for assessing liver fibrosis and cirrhosis. The elevated thresholds used to identify significant fibrosis and cirrhosis were high and may have led to underestimating their prevalence. In a large CHB cohort study in Ethiopia, the non-invasive fibrosis test showed low sensitivity, detecting 10% of cirrhosis cases using an APRI threshold of 2.0, as recommended by the WHO[61]. Second, the study was not sufficiently equipped to define the predictors of treatment response, mortality, and adherence to therapy because the proportion of patients on antiviral therapy was small and the study dropout rate was high. Third, co-infection with HDV and HBV genotypes was not determined, which would have been a useful addition to our results.
CONCLUSION
In this CHB cohort study conducted in Eastern Ethiopia, the prevalence of advanced liver disease, including decompensated cirrhosis, was high. Khat use, HBeAg positivity, and high HBV viral load were independently associated with cirrhosis. Treatment with TDF effectively achieved viral suppression and improved the levels of liver fibrosis markers. However, the initial mortality rate was high, which was attributed to the large proportion of patients with advanced cirrhosis. These findings are expected to guide appropriate treatment strategies and improve the control and management of this disease in Ethiopia.
ACKNOWLEDGEMENTS
We are indebted to the patients who participated in the study. We are thankful to the hepatitis clinic and laboratory staff at Hiwot Fana Specialized University Hospital, as well as the laboratory staff at Aklilu Lemma Institute of Pathobiology for their dedication and hard work.
Footnotes
Institutional review board statement: The study was reviewed and approved by the National Research Ethics Review Committee (Ref. No. 3.10/829/07) in Ethiopia and by the Regional Committees for Medical and Health Research Ethics (Ref. No. 2014/1146) in Norway, as well as the pertinent institutional ethical review boards.
Informed consent statement: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Ethiopia
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Lv J S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Cai YX
Contributor Information
Nejib Y Ismael, Department of Internal Medicine, Haramaya University, College of Health and Medical Sciences, Harar 252, Ethiopia.
Semir A Usmael, Department of Internal Medicine, Haramaya University, College of Health and Medical Sciences, Harar 252, Ethiopia. semirabdi61@gmail.com.
Nega B Belay, Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa 1000, Ethiopia; Regional Centre for Imported and Tropical Diseases, Oslo University Hospital, Oslo 0450, Ullevål, Norway.
Hailemichael Desalegn Mekonen, Internal Medicine, Gastroenterology and Hepatology Unit, Saint Paul’s Hospital Millennium Medical College, Addis Ababa 1000, Ethiopia; Department of Infectious Disease, Vestfold Hospital Trust, Tønsberg 3103, Norway.
Asgeir Johannessen, Regional Centre for Imported and Tropical Diseases, Oslo University Hospital, Oslo 0450, Ullevål, Norway; Department of Infectious Disease, Vestfold Hospital Trust, Tønsberg 3103, Norway; Institute of Clinical Medicine, Oslo University, Oslo 0318, Norway.
Stian MS Orlien, Regional Centre for Imported and Tropical Diseases, Oslo University Hospital, Oslo 0450, Ullevål, Norway; Department of Infectious Disease, Vestfold Hospital Trust, Tønsberg 3103, Norway; Department of Pediatrics, Oslo University, Oslo 0450, Ullevål, Norway.
Data sharing statement
No additional data are available.
References
- 1.World Health Organization. Hepatitis B. [cited 9 April 2024]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b .
- 2.GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796–829. doi: 10.1016/S2468-1253(22)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, Christensen E, Krogsgaard K, Degos F, Carneiro de Moura M. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21:77–82. doi: 10.1002/hep.1840210114. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF, Lin DY, Chen TJ, Chu CM. Natural course after the development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Liver. 1989;9:235–241. doi: 10.1111/j.1600-0676.1989.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 6.de Jongh FE, Janssen HL, de Man RA, Hop WC, Schalm SW, van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology. 1992;103:1630–1635. doi: 10.1016/0016-5085(92)91188-a. [DOI] [PubMed] [Google Scholar]
- 7.Realdi G, Fattovich G, Hadziyannis S, Schalm SW, Almasio P, Sanchez-Tapias J, Christensen E, Giustina G, Noventa F. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP) J Hepatol. 1994;21:656–666. doi: 10.1016/s0168-8278(94)80115-0. [DOI] [PubMed] [Google Scholar]
- 8.Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK Hong Kong Liver Fibrosis Study Group. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395–401. doi: 10.1002/hep.21724. [DOI] [PubMed] [Google Scholar]
- 9.McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759–768. doi: 10.7326/0003-4819-135-9-200111060-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 11.Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat. 2007;14:147–152. doi: 10.1111/j.1365-2893.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 12.Chu CM, Liaw YF. Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B. Gastroenterology. 2007;133:1458–1465. doi: 10.1053/j.gastro.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 14.Krajden M, McNabb G, Petric M. The laboratory diagnosis of hepatitis B virus. Can J Infect Dis Med Microbiol. 2005;16:65–72. doi: 10.1155/2005/450574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002;9:52–61. doi: 10.1046/j.1365-2893.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro RM, Germanidis G, Powers KA, Pellegrin B, Nikolaidis P, Perelson AS, Pawlotsky JM. Hepatitis B virus kinetics under antiviral therapy sheds light on differences in hepatitis B e antigen positive and negative infections. J Infect Dis. 2010;202:1309–1318. doi: 10.1086/656528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Global hepatitis report 2017. [cited 6 March 2024]. Available from: https://www.who.int/publications/i/item/9789241565455 .
- 18.Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:761. doi: 10.1186/s12879-016-2090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aberra H, Desalegn H, Berhe N, Medhin G, Stene-Johansen K, Gundersen SG, Johannessen A. Early experiences from one of the first treatment programs for chronic hepatitis B in sub-Saharan Africa. BMC Infect Dis. 2017;17:438. doi: 10.1186/s12879-017-2549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orlien SMS, Ismael NY, Ahmed TA, Berhe N, Lauritzen T, Roald B, Goldin RD, Stene-Johansen K, Dyrhol-Riise AM, Gundersen SG, Morgan MY, Johannessen A. Unexplained chronic liver disease in Ethiopia: a cross-sectional study. BMC Gastroenterol. 2018;18:27. doi: 10.1186/s12876-018-0755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 22.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 23.Kim WR, Loomba R, Berg T, Aguilar Schall RE, Yee LJ, Dinh PV, Flaherty JF, Martins EB, Therneau TM, Jacobson I, Fung S, Gurel S, Buti M, Marcellin P. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121:3631–3638. doi: 10.1002/cncr.29537. [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 25.Schiff ER, Sorrell MF, Maddrey WC. Schiff’s diseases of the liver. 10th ed. Philadelphia: Lippincott Williams and Wilkins, 2007: 258-276. [Google Scholar]
- 26.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 27.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 28.Desalegn H, Aberra H, Berhe N, Mekasha B, Stene-Johansen K, Krarup H, Pereira AP, Gundersen SG, Johannessen A. Treatment of chronic hepatitis B in sub-Saharan Africa: 1-year results of a pilot program in Ethiopia. BMC Med. 2018;16:234. doi: 10.1186/s12916-018-1229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Surial B, Wyser D, Béguelin C, Ramírez-Mena A, Rauch A, Wandeler G. Prevalence of liver cirrhosis in individuals with hepatitis B virus infection in sub-Saharan Africa: Systematic review and meta-analysis. Liver Int. 2021;41:710–719. doi: 10.1111/liv.14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aberra H, Desalegn H, Berhe N, Mekasha B, Medhin G, Gundersen SG, Johannessen A. The WHO guidelines for chronic hepatitis B fail to detect half of the patients in need of treatment in Ethiopia. J Hepatol. 2019;70:1065–1071. doi: 10.1016/j.jhep.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Mohammed EH, Saud MR, Yemane S, Isam Mohammed E, Freweini T. Serological and virological profile of patients with chronic hepatitis B infection in Eritrea. Int J Clin Virol. 2020;4:96–101. [Google Scholar]
- 33.Lesi OA, Audu RA, Okwuraiwe AP, Adeleye OO, Ige FA, Iwuorah JC. Serological and virological markers of nigerian patients with hepatitis B infection. Niger J Clin Pract. 2019;22:534–538. doi: 10.4103/njcp.njcp_273_17. [DOI] [PubMed] [Google Scholar]
- 34.Buti M, Fung S, Gane E, Afdhal NH, Flisiak R, Gurel S, Flaherty JF, Martins EB, Yee LJ, Dinh P, Bornstein JD, Mani Subramanian G, Janssen HL, George J, Marcellin P. Long-term clinical outcomes in cirrhotic chronic hepatitis B patients treated with tenofovir disoproxil fumarate for up to 5 years. Hepatol Int. 2015;9:243–250. doi: 10.1007/s12072-015-9614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamida ME, Raja SM, Seyoum Y, Elkhidir IM, Tekle F. Prevalence of chronic hepatitis B phases in Eritrean patients: a laboratory-based cross-sectional study. BMC Gastroenterol. 2021;21:198. doi: 10.1186/s12876-021-01789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tufon KA, Anong DN, Meriki HD, Georges TD, Maurice M, Kouanou YS, Bolimo AF, Tony NJ, Kwenti TE, Wung NH, Nkuo-Akenji T. Characterization and assessment of HBV chronically infected patients: Identification of those eligible for treatment in the South West region of Cameroon. PLoS One. 2018;13:e0203312. doi: 10.1371/journal.pone.0203312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roos RAN, Sonderup M, Smuts HE, Gogela N, Setshedi M, Hairwadzi HN, Spearman W. A cross sectional study of HBeAg negative chronic hepatitis B virus infection in Cape Town, South Africa. J Viral Hepatitis. 2015;22:53–54. [Google Scholar]
- 38.Lakoh S, Firima E, Jiba DF, Kamara MN, Gashau W, Deen GF, Adekanmbi O, Yendewa GA. Prevalence of sero-markers and non-invasive assessment of liver cirrhosis in patients with Hepatitis B virus infection in Freetown, Sierra Leone: a cross-sectional study. BMC Gastroenterol. 2021;21:320. doi: 10.1186/s12876-021-01892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendy ME, Welzel T, Lesi OA, Hainaut P, Hall AJ, Kuniholm MH, McConkey S, Goedert JJ, Kaye S, Rowland-Jones S, Whittle H, Kirk GD. Hepatitis B viral load and risk for liver cirrhosis and hepatocellular carcinoma in The Gambia, West Africa. J Viral Hepat. 2010;17:115–122. doi: 10.1111/j.1365-2893.2009.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramírez Mena A, Ngom NF, Tine J, Ndiaye K, Fortes L, Ndiaye O, Fall M, Gaye A, Ka D, Seydi M, Wandeler G On Behalf Of The Sen-B Study Group. Prevalence and Predictors of Liver Fibrosis in People Living with Hepatitis B in Senegal. Viruses. 2022;14 doi: 10.3390/v14081614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, Wang CC, Su WW, Chen MY, Peng CY, Chien RN, Huang YW, Wang HY, Lin CL, Yang SS, Chen TM, Mo LR, Hsu SJ, Tseng KC, Hsieh TY, Suk FM, Hu CT, Bair MJ, Liang CC, Lei YC, Tseng TC, Chen CL, Kao JH C-TEAM study group and the Taiwan Liver Diseases Consortium. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36:1755–1764. doi: 10.1111/liv.13253. [DOI] [PubMed] [Google Scholar]
- 43.Shimakawa Y, Lemoine M, Njai HF, Bottomley C, Ndow G, Goldin RD, Jatta A, Jeng-Barry A, Wegmuller R, Moore SE, Baldeh I, Taal M, D'Alessandro U, Whittle H, Njie R, Thursz M, Mendy M. Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from The Gambia. Gut. 2016;65:2007–2016. doi: 10.1136/gutjnl-2015-309892. [DOI] [PubMed] [Google Scholar]
- 44.Liu SQ, Zhu XJ, Sun XH, Li M, Gao YQ. [Characteristic of liver pathology in HBeAg-positive and HBeAg-negative chronic hepatitis B patients with mildly elevated ALT] Zhonghua Gan Zang Bing Za Zhi. 2012;20:348–352. doi: 10.3760/cma.j.issn.1007-3418.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Keshvari M, Alavian SM, Sharafi H. Comparison of Serum Hepatitis B Virus DNA and HBsAg Levels Between HBeAg-Negative and HBeAg-Positive Chronic Hepatitis B Patients. Jundishapur J Microbiol. 2015;8:e21444. doi: 10.5812/jjm.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38:1075–1086. doi: 10.1053/jhep.2003.50453. [DOI] [PubMed] [Google Scholar]
- 47.Liaw YF, Tai DI, Chu CM, Pao CC, Chen TJ. Acute exacerbation in chronic type B hepatitis: comparison between HBeAg and antibody-positive patients. Hepatology. 1987;7:20–23. doi: 10.1002/hep.1840070106. [DOI] [PubMed] [Google Scholar]
- 48.Chapman MH, Kajihara M, Borges G, O'Beirne J, Patch D, Dhillon AP, Crozier A, Morgan MY. Severe, acute liver injury and khat leaves. N Engl J Med. 2010;362:1642–1644. doi: 10.1056/NEJMc0908038. [DOI] [PubMed] [Google Scholar]
- 49.Mahamoud HD, Muse SM, Roberts LR, Fischer PR, Torbenson MS, Fader T. Khat chewing and cirrhosis in Somaliland: Case series. Afr J Prim Health Care Fam Med. 2016;8:e1–e4. doi: 10.4102/phcfm.v8i1.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peevers CG, Moorghen M, Collins PL, Gordon FH, McCune CA. Liver disease and cirrhosis because of Khat chewing in UK Somali men: a case series. Liver Int. 2010;30:1242–1243. doi: 10.1111/j.1478-3231.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 51.Stuyt RJ, Willems SM, Wagtmans MJ, van Hoek B. Chewing khat and chronic liver disease. Liver Int. 2011;31:434–436. doi: 10.1111/j.1478-3231.2010.02440.x. [DOI] [PubMed] [Google Scholar]
- 52.Orlien SMS, Sandven I, Berhe NB, Ismael NY, Ahmed TA, Stene-Johansen K, Gundersen SG, Morgan MY, Johannessen A. Khat chewing increases the risk for developing chronic liver disease: A hospital-based case-control study. Hepatology. 2018;68:248–257. doi: 10.1002/hep.29809. [DOI] [PubMed] [Google Scholar]
- 53.Forbes MP, Raj AS, Martin J, Lampe G, Powell EE. Khat-associated hepatitis. Med J Aust. 2013;199:498–499. doi: 10.5694/mja13.10951. [DOI] [PubMed] [Google Scholar]
- 54.Riyaz S, Imran M, Gleeson D, Karajeh MA. Khat (Catha Edulis) as a possible cause of autoimmune hepatitis. World J Hepatol. 2014;6:150–154. doi: 10.4254/wjh.v6.i3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D'Souza R, Sinnott P, Glynn MJ, Sabin CA, Foster GR. An unusual form of autoimmune hepatitis in young Somalian men. Liver Int. 2005;25:325–330. doi: 10.1111/j.1478-3231.2005.01088.x. [DOI] [PubMed] [Google Scholar]
- 56.Abid MD, Chen J, Xiang M, Zhou J, Chen X, Gong F. Khat (Catha edulis) generates reactive oxygen species and promotes hepatic cell apoptosis via MAPK activation. Int J Mol Med. 2013;32:389–395. doi: 10.3892/ijmm.2013.1394. [DOI] [PubMed] [Google Scholar]
- 57.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 58.Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in 'real-life' settings: from clinical trials to clinical practice. J Viral Hepat. 2012;19:377–386. doi: 10.1111/j.1365-2893.2012.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goyal SK, Dixit VK, Shukla SK, Ghosh J, Behera M, Tripathi M, Gupta N, Ranjan A, Jain AK. Prolonged use of tenofovir and entecavir in hepatitis B virus-related cirrhosis. Indian J Gastroenterol. 2015;34:286–291. doi: 10.1007/s12664-015-0576-1. [DOI] [PubMed] [Google Scholar]
- 60.Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Brückner S, Zeissig S, Stephan AM, Wodarz N, Devière J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Völzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nöthen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 61.Desalegn H, Aberra H, Berhe N, Gundersen SG, Johannessen A. Are non-invasive fibrosis markers for chronic hepatitis B reliable in sub-Saharan Africa? Liver Int. 2017;37:1461–1467. doi: 10.1111/liv.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.