Abstract
BACKGROUND
The modified Xiaoyao San (MXS) formula is an adjuvant drug recommended by the National Health Commission of China for the treatment of liver cancer, which has the effect of preventing postoperative recurrence and metastasis of hepatocellular carcinoma and prolonging patient survival. However, the molecular mechanisms underlying that remain unclear.
AIM
To investigate the role and mechanisms of MXS in ameliorating hepatic injury, steatosis and inflammation.
METHODS
A choline-deficient/high-fat diet-induced rat nonalcoholic steatohepatitis (NASH) model was used to examine the effects of MXS on lipid accumulation in primary hepatocytes. Liver tissues were collected for western blotting and immunohistochemistry (IHC) assays. Lipid accumulation and hepatic fibrosis were detected using oil red staining and Sirius red staining. The serum samples were collected for biochemical assays and NMR-based metabonomics analysis. The inflammation/lipid metabolism-related signaling and regulators in liver tissues were also detected to reveal the molecular mechanisms of MXS against NASH.
RESULTS
MXS showed a significant decrease in lipid accumulation and inflammatory response in hepatocytes under metabolic stress. The western blotting and IHC results indicated that MXS activated AMPK pathway but inhibited the expression of key regulators related to lipid accumulation, inflammation and hepatic fibrosis in the pathogenesis of NASH. The metabonomics analysis systemically indicated that the arachidonic acid metabolism and steroid hormone synthesis are the two main target metabolic pathways for MXS to ameliorate liver inflammation and hepatic steatosis. Mechanistically, we found that MXS protected against NASH by attenuating the sex hormone-related metabolism, especially the metabolism of male hormones.
CONCLUSION
MXS ameliorates inflammation and hepatic steatosis of NASH by inhibiting the metabolism of male hormones. Targeting male hormone related metabolic pathways may be the potential therapeutic approach for NASH.
Keywords: Hepatic steatosis, Inflammation, Sex hormone metabolism, Male hormone, Phosphatase and tensin homolog deleted on chromosome ten
Core Tip: In this study, the modified Xiaoyao San (MXS) formula was found can alleviated inflammation and hepatic steatosis in nonalcoholic steatohepatitis (NASH) by suppressing male hormone metabolism and modulating inflammation/lipid metabolism-related signaling and factors. It suggested that the regulation of sex hormone metabolism and associated signaling could be new avenues for mechanistic research on NASH and for early diagnosis and treatment. This study offers substantial evidence for the therapeutic potential of MXS in NASH and makes a valuable contribution to the development of new drugs for this condition.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has emerged as a significant global health concern, acknowledged as a chronic liver manifestation of metabolic syndrome. It covers a range extending from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) marked by advancing fibrosis[1]. NASH, marked by hepatic steatosis, inflammation, and liver damage, stands as an advanced manifestation of NAFLD and acts as a clinical predisposition for hepatocellular carcinoma (HCC) and cirrhosis[2]. Currently, NAFLD impacts 25% to 30% of adults worldwide, with an especially elevated prevalence rate of 29% in China[3,4]. NASH represents an advanced stage of NAFLD, with approximately one-third of patients progressing to liver failure, cirrhosis, and HCC, resulting in increased mortality. Notably, NASH has emerged as a primary reason for liver transplants due to the advanced stage at which most patients are diagnosed[5]. Despite the severity of this disease, there are no medications approved by the FDA for treating it, and efforts to mitigate its progression in NASH patients remain largely unsatisfactory[6].
The slow progress in drug development for NASH is primarily attributed to the complex pathogenesis of NASH and the lack of related research. Many aspects of NASH development remain unclear and subject to controversy, both at the molecular and pathological levels[7]. Compelling evidence indicates a robust pathophysiological connection between metabolic irregularities and NASH. Metabolic disorders such as hepatic steatosis and low-grade inflammation are significant contributing factors during the transition from NAFLD to NASH[8]. Clinical data indicate that NASH is a multisystem disease, and patients often have other metabolism-related conditions[9]. Therefore, a multidisciplinary and comprehensive approach to managing NASH, along with its associated complications, with a focus on metabolic dysfunction, appears to be a more rational and promising direction. Regulating metabolic processes is the most effective treatment for diseases like NASH, which stem from metabolic disorders[10]. As a holistic multi-objective intervention, traditional Chinese medicine (TCM) demonstrates greater effectiveness in addressing NASH and other complex metabolic conditions. Exploring potential effective and safe therapies derived from natural compounds is also a prominent approach in NASH therapeutics[11-13]. Modified Xiaoyao San (MXS), available as an over-the-counter medicine, boasts excellent hepatoprotective properties and has demonstrated clinical effectiveness in the regulation of liver metabolism and inflammation. The National Administration of TCM has recommended it as an adjunctive treatment to prevent postoperative recurrence of HCC[14]. Nevertheless, the precise regulatory mechanisms by which MXS combats the progression of NAFLD/NASH remain unclear. Further exploration into the molecular mechanisms through which MXS modulates hepatic metabolism and inflammation will contribute to the creation of innovative NASH treatments and enhance the clinical utility of MXS in the postoperative rehabilitation of HCC patients.
In the study, we demonstrated that MXS alleviated inflammation and hepatic steatosis in NASH by modulating sex hormone-related metabolism, specifically the metabolism of male hormones. Analysis of inflammation and lipid metabolism-related signaling and factors in liver tissues also revealed that MXS treatment had activated AMPK signaling while suppressing the expression of COX2, PPARγ, and α-SMA. These findings supported the notion that regulating sex hormone metabolism could have provided a novel avenue for mechanistic research into NASH.
MATERIALS AND METHODS
Animals
We obtained 30 male SD rats, each weighing 150 ± 20 g, from HFK Bioscience Co., Ltd. (Beijing, China) under license number: SCXK (2019-0008; Beijing, China). These rats underwent evaluation by the China Medical Laboratory Animal Research Institute before inclusion in the study. They were accommodated in a controlled environment with a consistent humidity level of 55% ± 5%, a temperature of 22 °C ± 1 °C, and 12-hour light and dark cycle. The rats had unrestricted access to water and food.
Drug and experimental design
MXS was purchased from Chongqing Traditional Chinese Medicine Hospital, national medicine permission number: Z20013060. The rats were divided into three groups (n = 10/group) in a random and equitable manner: Control, NASH, and MXS groups. While the remaining groups were supplied with a choline-deficient/high-fat (CDHF) diet (D09100310), which contained 2% calories from cholesterol, 20% from fructose, and 40% from fat, the control group was provided with a standard diet. The precise dietary composition is outlined in Supplementary Table 1. Throughout the experiment, the body weight of the rats was assessed on a weekly basis, and they were provided with sustenance for a period of 12 weeks in order to establish the NASH model. After that, the rats in the MXS group received MXS intragastrically at a daily dose of 0.9 g/kg (MXS/body weight) for a duration of 6 weeks (except Saturdays and Sundays) before being transitioned back to the standard diet. The control group was given an equivalent volume of distilled water, while the NASH group received the normal diet.
Sample collection
After the experimental period, all rats underwent anesthesia and were sacrificed. Anesthesia was achieved by intraperitoneal injection of 0.9% pentobarbital sodium solution (45 mg/kg) at 0.5 mL/100 g. The rats lost consciousness in the shortest possible time, minimizing the animal's panic and pain. For the biochemical assessments, we collected serum samples. 8 rats were randomly selected from each group and liver tissues from the identical area were collected, preserved in 4% paraformaldehyde, encased in paraffin, and subsequently sliced into sections. 7 paraffin sections were prepared from liver tissue of each rat, including 2 for hematoxylin and eosin (HE staining), 3 for immunohistochemistry (IHC) detection, and 2 for Sirius staining (total = 168 sections). The unused serum and liver samples remained at -80 °C until they were necessary for additional investigations. Serum biochemical markers were analyzed using an automated biochemical analyzer provided by Beckman Coulter Inc, located in California, United States. The animal experiments were carried out in full accordance with the ethical standards for animal research and received approval from the Animal Ethics Committee of the Chongqing Academy of Chinese Materia Medica.
Histopathology, immunohistochemical analysis, and western blotting
For the evaluation of liver histopathological changes, we stained tissue sections with HE staining and examined them using a light microscope. To conduct immunohistochemical analysis, we examined the levels of PTEN, αSMA, and FASN in the hepatic tissues. For this experiment, we utilized the Biotin-Streptavidin HRP Detection System provided by ZSGB-Bio, located in China and followed the instructions provided in the manual. The hepatic tissue sections were exposed to primary antibodies in a suitable 1:200 ratio and then refrigerated at 4 °C overnight (ON). On the following day, we subjected the sections to secondary antibodies, and subsequently, a streptavidin-biotin-peroxidase complex was administered to the slices at 37 °C for a duration of 15 minutes. To visualize the signal, we employed diaminobenzidine. Hematoxylin was employed for nuclear staining, and target proteins were observed under a light microscope. The following antibodies were used: Α-SMA (Cell Signaling Tech 19245) at 1:400 dilution and FASN (Signalway Antibody 38133) at 1:100 dilution.
For the western blotting analysis, we initiated by lysing the liver tissues in RIPA buffer. We determined the protein concentration utilizing the Enhanced BCA protein assay kit (P0010S, Beyotime, Shanghai, China). Subsequently, we blocked the blotting membranes for an hour in a solution comprising 5% non-fat milk in phosphate-buffered saline (PBS) supplemented with 0.05% Tween-20. Next, the membranes underwent ON probing with primary antibodies, followed by the application of secondary antibodies from ZEN-Bioscience, Chengdu, China. The primary antibodies utilized included PPARγ (Signalway Antibody 49371) at 1:1000 dilution, COX2 (Signalway Antibody 33345) at 1:2000 dilution, AMPKα (Cell Signaling Tech 2603) at 1:2000 dilution, p-AMPKα (Cell Signaling Tech 2535) at 1:2000 dilution, and GAPDH (Signalway Antibody 37859) at 1:10000 dilution.
Oil red O staining and sirius red staining
To evaluate hepatic lipid accumulation, we began by embedding liver tissues in optimal cutting temperature compound-freeze medium and subsequently cutting them into thin sections with a thickness of 12 µm (8 rats were randomly selected from each group and liver tissues from the identical area were collected). These sections were subsequently subjected to staining using the Oil Red O (ORO) working solution (Absin Bioscience, Inc., Shanghai, China) for a duration of 15 to 20 minutes, followed by rinsing in distilled water. Then, the sections were stained with hematoxylin for a duration of 40 seconds. Following a meticulous 5-minute rinsing in flowing tap water, the slides underwent an additional purification step using distilled water and were ultimately sealed with glycerin jelly.
The NAS score serves as a semi-quantitative scoring system, not a diagnostic tool. A score below than 3 indicates the exclusion of NASH, while a score above 4 signifies a diagnosis of NASH. A score falling between these values suggests a potential presence of NASH. NAFL is defined as having no lobular inflammation, ballooning, or fibrosis but with liver steatosis less than 33%. Individuals with steatosis below this threshold are simply classified as having hepatocellular steatosis.
For Sirius red staining, the tissue sections underwent deparaffinization before being immersed in Sirius red dye for 1 hour. Following this, the tissue sections were washed with distilled water or PBS to eliminate any excess dye and prevent nonspecific staining. The sections were then dehydrated by sequentially immersing them for 1 minute in increasing concentrations of ethanol (such as 70%, 95%, and 100%), followed by three rounds of 1 to 2-minute immersions in xylene. Neutral balata was applied for fixation (mounting). Images were then examined and captured with the assistance of a Zeiss light microscope from Germany.
Metabolomic profiling
Untargeted metabolomics sequencing was carried out by Novogene Co., Ltd. (Beijing, China). In brief, individual tissue samples (100 mg) were first ground using liquid nitrogen. The resulting homogenate was then thoroughly resuspended by vortexing in precooled 80% methanol. After incubation on ice for 5 minutes, the samples were centrifuged at 15000 xg for 20 minutes at 4 °C. Dilution of a portion of the supernatant to reach a 53% methanol concentration was carried out using liquid chromatograph-mass spectrometer (LC-MS) grade water. The prepared samples were moved to new Eppendorf tubes and underwent additional centrifugation at 15000 g for 20 minutes at 4 °C. Subsequently, the obtained supernatant was then analyzed using the LC-MS/MS system.
Statistical analysis
Results were expressed as mean ± SD. To assess significant differences between two groups, we utilized either student’s t-test or the Mann-Whitney U test. We performed multiple group comparisons using either a one-way analysis of variance (ANOVA) or Kruskal-Wallis test. Using GraphPad Prism V. 5.1.0 (GraphPad Software, Inc., United States), we performed all statistical analyses. A significance level of P value below than 0.05 was considered statistically significant.
RESULTS
MXS mitigates hepatic steatosis and inflammation induced by the CDHF diet
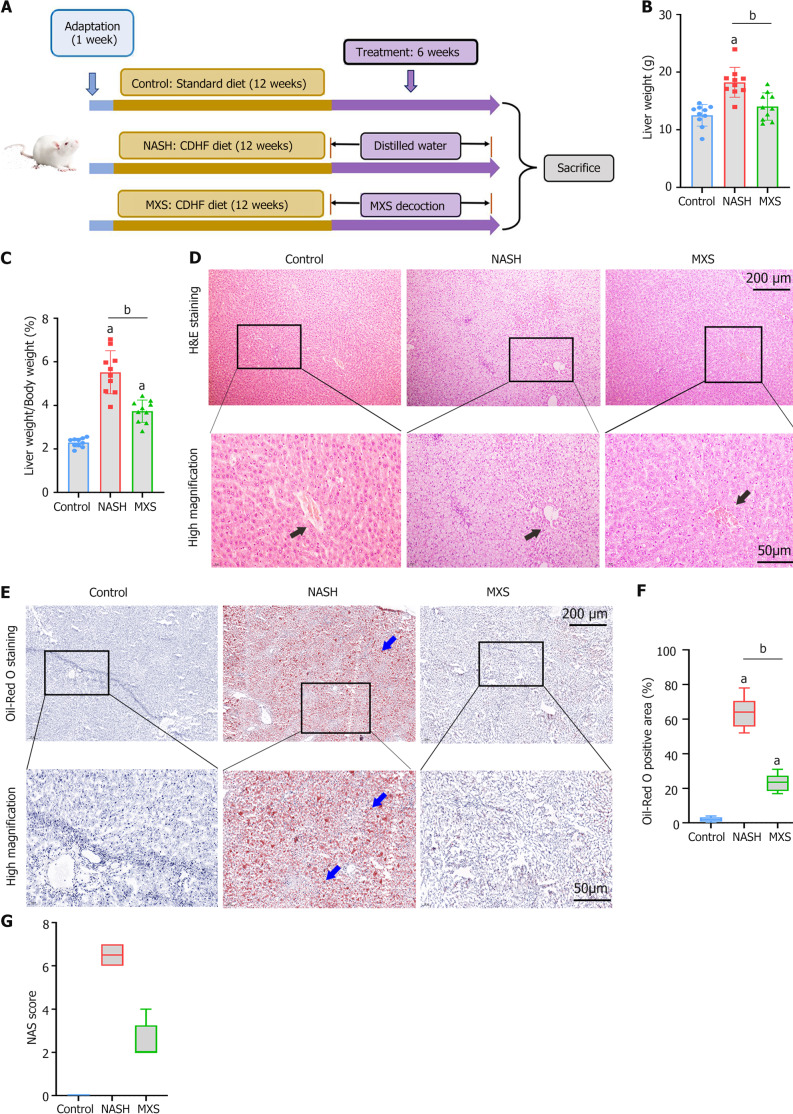
To assess the pharmacological impact of MXS on inflammation and liver steatosis, we employed a NASH rat model induced by a CDHF diet, leading to lipid buildup and hepatocyte inflammation. Rats were subjected to a CDHF diet for 12 weeks, followed by an extra 6 weeks of intragastric administration of MXS alongside a standard diet (Figure 1A). When the animal protocol concluded, MXS group resulted in a substantial reduction in the elevated hepatic weight and percentage of hepatic weight to body weight observed in CDHF-fed rats (NASH group) (Figure 1B and C). In the control group, the hepatocytes were of uniform size and the cytoplasm was homogeneous, and there was no swelling and fatty degeneration. In NASH group, hepatocyte cords were disorganized and the hepatocytes were obviously swollen and lipid droplet vacuoles of different sizes and more balloon-like changes were seen. By comparison, ballooning and liver steatosis, as evidenced by HE staining, were significantly attenuated by MXS in CDHF-fed rat livers (Figure 1D). This effect was further confirmed through ORO staining, including quantification of ORO-positive areas (Figure 1E and F) and the NAFLD activity score (Figure 1G).
Figure 1.
Modified Xiaoyao San demonstrated its capacity to alleviate inflammation and hepatic steatosis in rats subjected to a choline-deficient/high-fat diet. A: The diagram illustrating the experimental procedure outlined the systematic investigation of the protective role of modified Xiaoyao San (MXS) in rats following a 12-week choline-deficient/high-fat (CDHF) diet regimen. Starting at week 13, rats that had been fed the CDHF diet received daily intragastric administration of either saline or MXS decoction for a duration of 6 weeks; B and C: The liver weight and liver/body weight ratio of the rat groups were presented; D and E: The images from hepatic sections were stained with hematoxylin and eosin and Oil Red O (ORO). Black arrows indicate the liver portal areas. The lipid droplets (indicated by blue arrows) in the tissue are orange-red and the nuclei are blue; F and G: Quantitative data pertaining to ORO-positive areas and the nonalcoholic fatty liver disease activity score for each group were displayed. Data were presented as mean ± standard error of mean (n = 10/group), and were analyzed by ANOVA. aP < 0.05 Control vs nonalcoholic steatohepatitis (NASH) group. bP < 0.05 NASH vs MXS group. MXS: Modified Xiaoyao San; NASH: Nonalcoholic steatohepatitis.
MXS altered the overall metabolite profile induced by CDHF diet
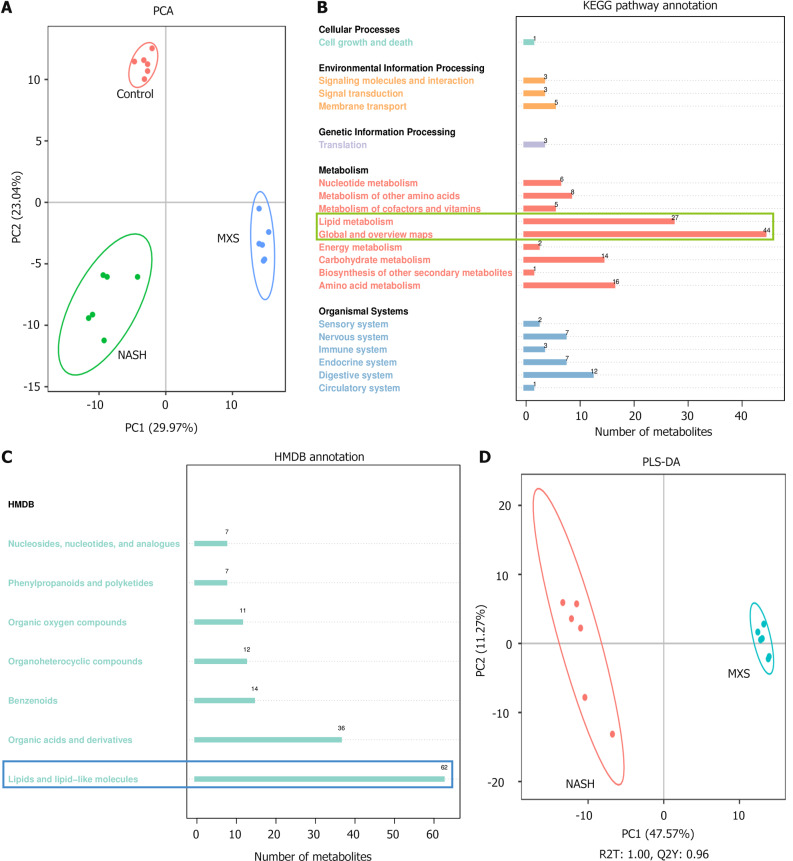
To acquire a comprehensive understanding of the molecular mechanism responsible for the reduction of liver fat deposition by MXS, we collected rat serum after MXS treatment and conducted non-targeted metabolomics analysis. As shown in the results of principal component analysis, the metabolite data of serum in the control, NASH, and MXS groups were well separated (Figure 2A and Supplementary Figure 1). The analysis of differential metabolites using Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment unveiled that lipid metabolism was the most significantly impacted pathway by MXS treatment, which was also confirmed by Human Metabolome Database classification of differential metabolites (Figure 2B and C). Partial least squares discrimination analysis was then conducted on differential metabolites after MXS treatment, and the results indicated a clear differentiation between NASH and MXS groups (Figure 2D). These results indicated that MXS exerted a substantial regulatory influence on lipid disorders induced by the CDHF diet-induced NASH model.
Figure 2.
Significant changes in metabolites after modified Xiaoyao San treatment were mainly related to lipid metabolism. A: Principal component analysis score map of each group; B: Gene ontology enrichment analyses; C: Human Metabolome Database classification notes; D: Partial least squares discrimination analysis between nonalcoholic steatohepatitis and modified Xiaoyao San groups. The scores of the first-ranked principal component (PC1, horizontal) and the second-ranked principal component (PC2, vertical) were used to represent the data. Samples from various experimental cohorts were represented by differently colored scatter plots, while 95% confidence intervals were visually depicted using ellipses. PCA: Principal component analysis; PLS-DA: Partial least squares discrimination analysis; HMDB: Human Metabolome Database; MXS: Modified Xiaoyao San; NASH: Nonalcoholic steatohepatitis.
The biosynthesis of steroid hormones was one of the main target pathways regulated by MXS
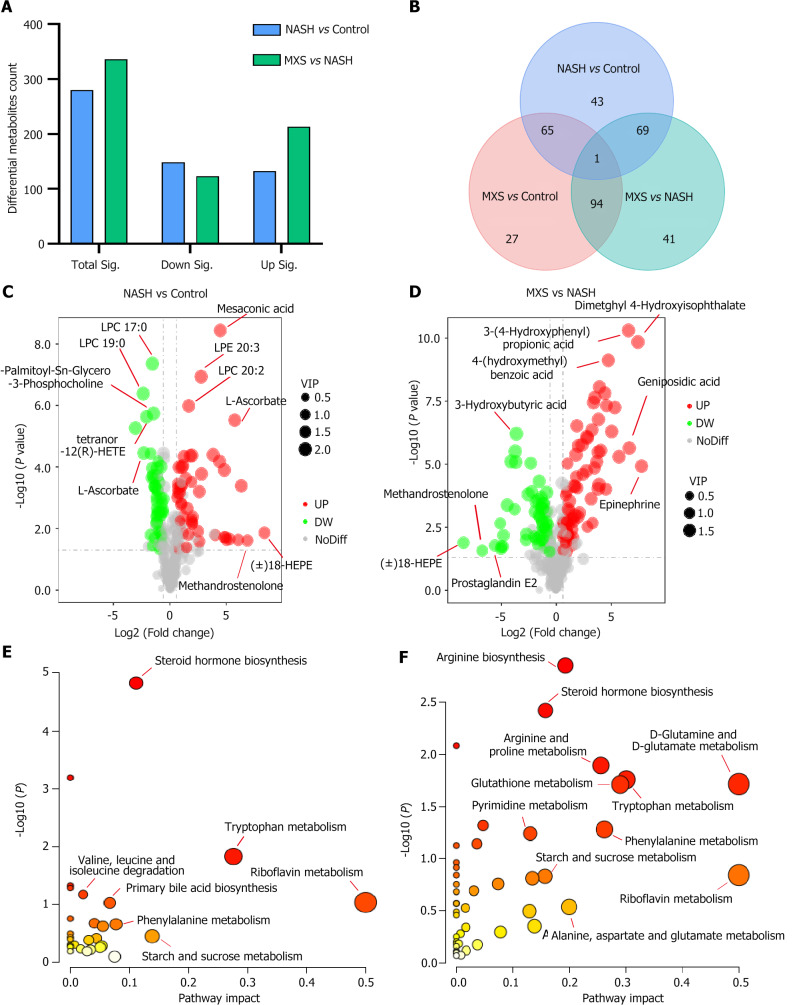
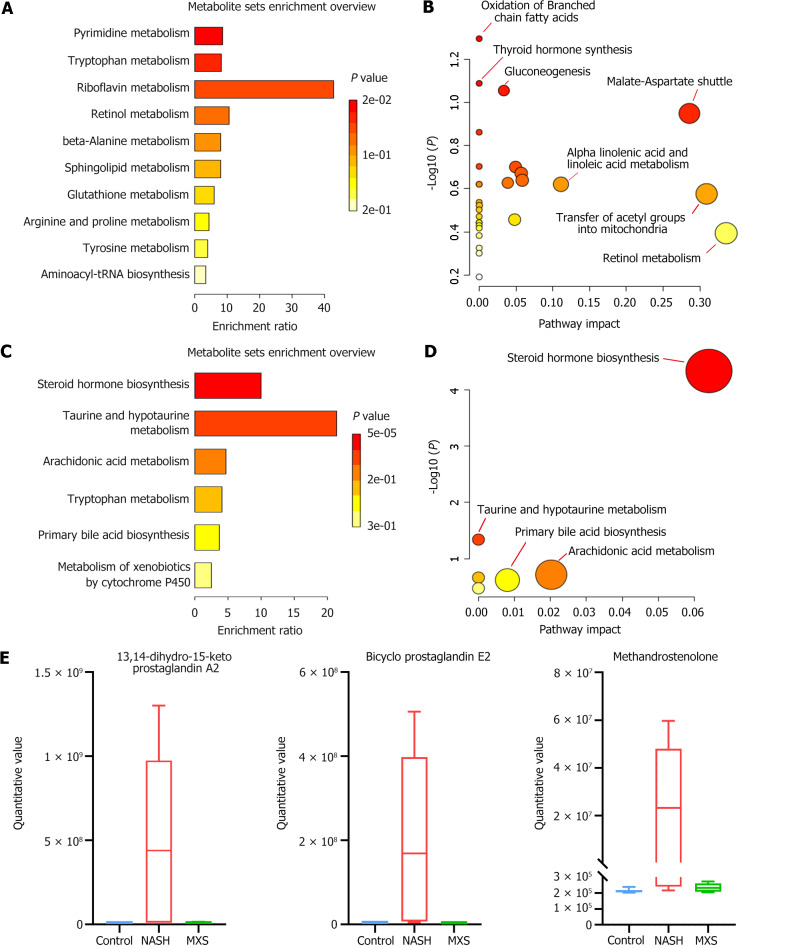
We screened and classified the 3090 identified differential serum metabolites, establishing criteria with fold change (FC) below than 0.667, FC greater than 1.5, or variable importance in the projection greater than 1.0, and P value below than 0.05. Following the criteria above, a total of 920 significantly changed metabolites were screened, classified, and annotated according to different comparisons between groups (NASH vs control; MXS vs NASH). Compared to the NASH model (NASH vs control), the MXS treatment (MXS vs NASH) induced more upregulated metabolites, but the numbers of differential metabolites between the different comparisons were very similar (Figure 3A and B; Supplementary Figures 2 and 3). This indicates that the majority of metabolites that underwent changes in the NASH model were regulated by MXS treatment. Further analysis was carried out on the metabolites based on their FC and P value from various comparisons (NASH vs Control; MXS vs NASH). As shown in the volcano plot, the top 3 metabolites with the highest fold reduction after MXS treatment were 18-HEPE, methandrostenolone, and Prostaglandin E2. It is worth noting that 18-HEPE and methandrostenolone were also the top 2 metabolites with the highest upregulation FC in the NASH model (Figure 3C and D). Metabolic pathway analysis was then used to analyze the metabolites from different comparisons between groups. Among the significantly altered pathways, we observed that four main pathological processes were shared between both comparisons (NASH vs Control; MXS vs NASH): Riboflavin metabolism, starch and sucrose metabolism, steroid biosynthesis, and tryptophan metabolism.
Figure 3.
Steroid hormone synthesis was the primary target pathway affected by modified Xiaoyao San treatment. A: Statistical analysis of differential metabolites. Total Sig denoted the total count of metabolites that exhibited significant changes. Down/up Sig represented the overall number of metabolites that underwent significant downregulation or upregulation; B: A Venn diagram illustrated the comparative analysis of metabolites among the three groups; C and D: Volcano plots displayed significantly changed metabolites, indicated by red and blue-colored dots. The criteria for significance were fold change (FC) below than 0.667, FC greater than 1.5, or variable importance in the projection greater than 1.0, and P value below than 0.05; E and F: Pathway enrichment analysis of differential metabolites in different comparisons. The pathway impact value (X-axis) was denoted by the size of the circles, and the -log10 P value weight (ranging from white to red, Y-axis) was indicated by the color intensity. MXS: Modified Xiaoyao San; NASH: Nonalcoholic steatohepatitis.
Within these four pathways, steroid hormone synthesis displayed the most substantial differential change (-log10 P value) in both comparisons (Figure 3E and F). These findings suggest that steroid hormone synthesis played a pivotal role in the amelioration of hepatic steatosis and liver inflammation by MXS.
MXS downregulates the levels of metabolites involved in steroid hormone synthesis pathways
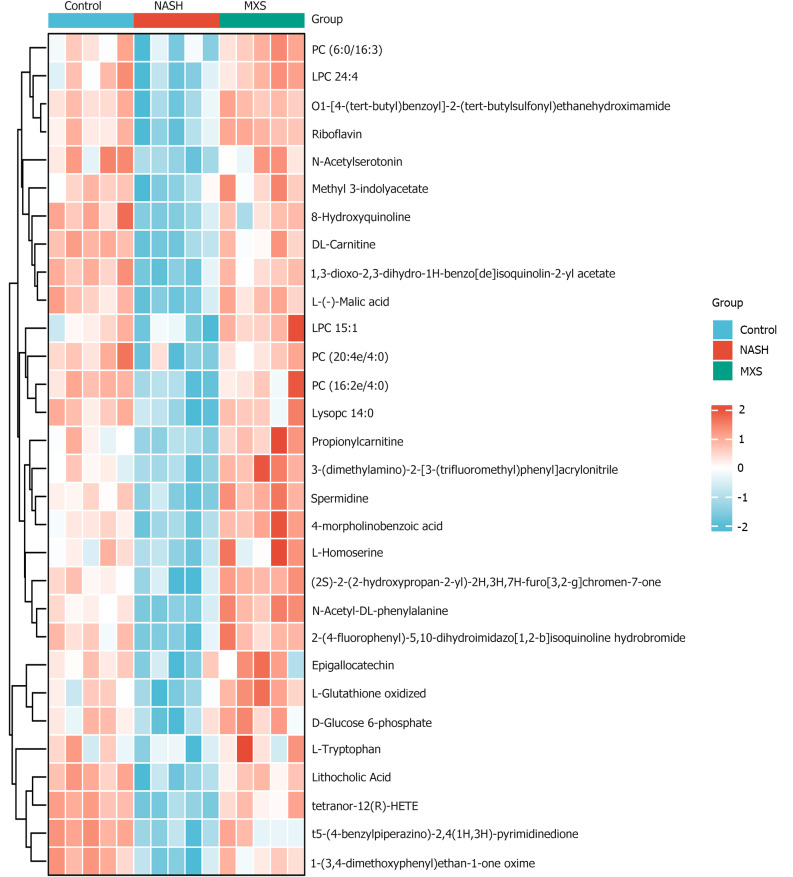
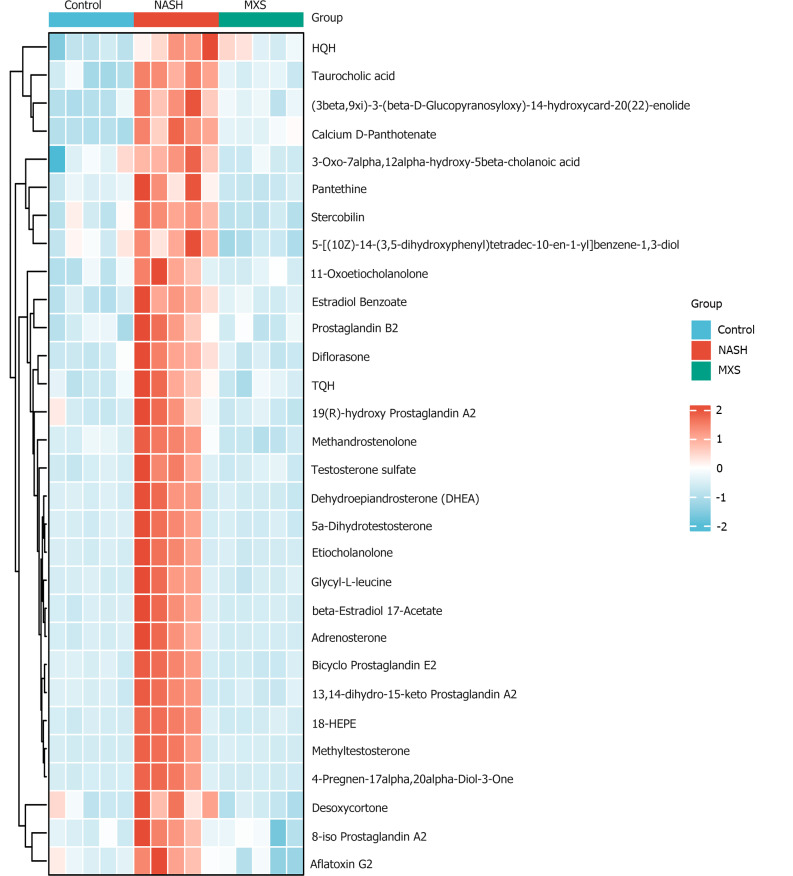
To understand how MXS regulates steroid hormone pathways, we screened and analyzed the top 30 metabolites that exhibited a decrease in the NASH model group but showed recovery or increase following MXS treatment. However, the trends of these 30 metabolites were not sufficiently clear and homogeneous (Figure 4 and Supplementary Figure 4).
Figure 4.
Heatmap of the top 30 metabolites that decreased in the nonalcoholic steatohepatitis model group but recovered or increased after modified Xiaoyao San treatment. MXS: Modified Xiaoyao San; NASH: Nonalcoholic steatohepatitis.
For the same reasons, the top 30 metabolites that had unusually increased in the NASH model group but recovered or downregulated after MXS treatment were also screened and analyzed. As the heatmap shows, the changing trends of the 30 metabolites were significant and homogeneous (Figure 5 and Supplementary Figure 5). In addition, the KEGG enrichment and pathway analysis showed that more than 10 pathways were related to the 30 metabolites, such as oxidation of branched-chain fatty acids, tryptophan metabolism, pyrimidine metabolism, gluconeogenesis (Figure 6A and B; Supplementary Figure 6A). Consistently, both the KEGG enrichment and pathway analysis showed that the pathways enriched by these 30 metabolites were more concentrated, with only 6 pathways being enriched. Notably, steroid hormone biosynthesis was the most significantly altered process in both methods of analysis (Figure 6C and Supplementary Figure 6B).
Figure 5.
Heatmap depicted the 30 most prominent metabolites that unusually increased in the nonalcoholic steatohepatitis model group but recovered or downregulated after modified Xiaoyao San treatment. MXS: Modified Xiaoyao San; NASH: Nonalcoholic steatohepatitis.
Figure 6.
Modified Xiaoyao San inhibited steroid synthesis and inflammation-related metabolic pathways. A and B: Metabolite set enrichment and pathway analysis of the 30 identified metabolites that decreased in the nonalcoholic steatohepatitis (NASH) model group but recovered or increased after modified Xiaoyao San (MXS) treatment; C and D: Metabolite set enrichment and pathway analysis of the 30 identified metabolites that unusually increased in the NASH model group but recovered or were downregulated after MXS treatment; E: Histogram of the top 3 metabolites related to steroid hormones and the inflammation process. MXS: Modified Xiaoyao San; NASH: Nonalcoholic steatohepatitis.
The cluster analysis and correlation analysis were also conducted for significantly changed metabolites between groups. After MXS treatment (MXS vs NASH), a clear clustering pattern emerged, and the correlation analysis results revealed that almost all of the top 20 significantly changed metabolites exhibited positive correlations with each other (Supplementary Figures 7 and 8). Arachidonic acid metabolism was the second most significantly affected pathway that was downregulated by MXS treatment (Figure 6C and D). Consequently, we statistically categorized the 52 metabolites that had unusually increased in the NASH model group but showed recovery or downregulation after MXS treatment. Among these 52 metabolites, 14 were associated with inflammation pathways. Prostaglandin A2, E2, and methandrostenolone were the three most significantly downregulated metabolites associated with inflammation and steroid metabolism processes after MXS treatment (Figure 6E and Supplementary Figure 9). These findings suggested that MXS exerted a regulatory effect on both steroid hormone biosynthesis and arachidonic acid metabolism by inhibiting the metabolic processes in these pathways.
MXS inhibited the metabolic pathways related to male hormones and the key regulators associated with lipid metabolism
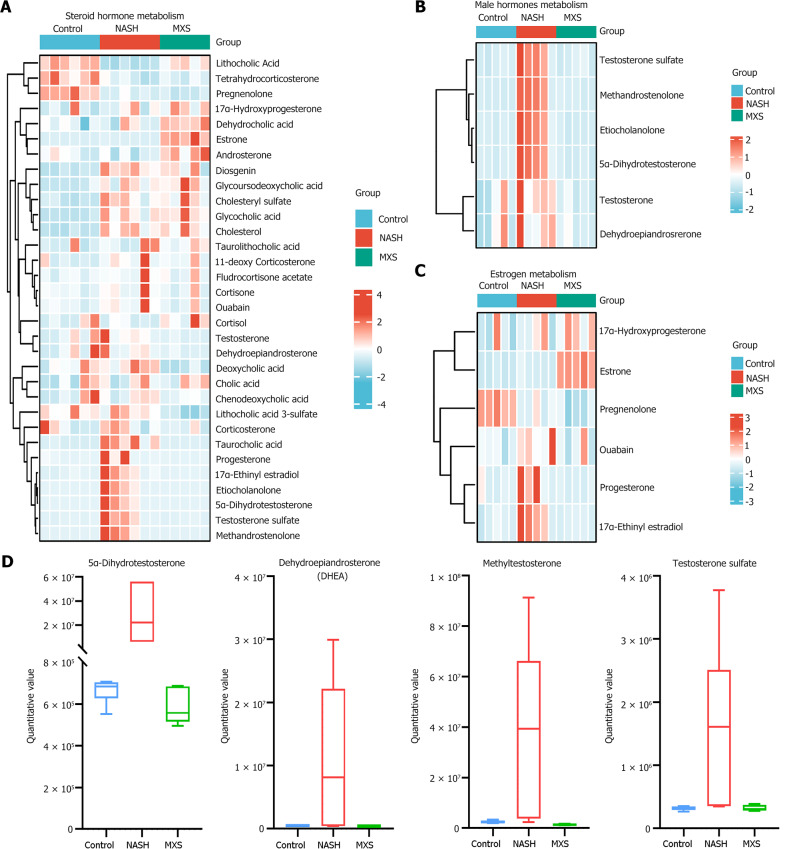
To investigate the mechanism by which MXS regulated steroid metabolism and alleviated hepatic steatosis, all metabolites related to steroid metabolism among the total differential metabolites were identified for further analysis. The results revealed an interesting phenomenon: MXS primarily suppressed steroid metabolic pathways by inhibiting sex hormone-related metabolism within them (Figure 7A). Subsequently, metabolites related to male hormones and estrogens were identified and analyzed separately (Figure 7B and C). The findings indicated that metabolites associated with male hormones were the primary targets through which MXS inhibited steroid metabolic pathways and mitigated hepatic steatosis (Figure 7B and D).
Figure 7.
Metabolites related to male hormones were the primary targets influenced by modified Xiaoyao San. A: Heatmap of metabolites related to steroid hormone metabolism; B and C: Heatmaps of metabolites related to male hormones and estrogen metabolism; D: Histogram of the top 4 metabolites related to male hormones metabolism. MXS: Modified Xiaoyao San; NASH: Nonalcoholic steatohepatitis.
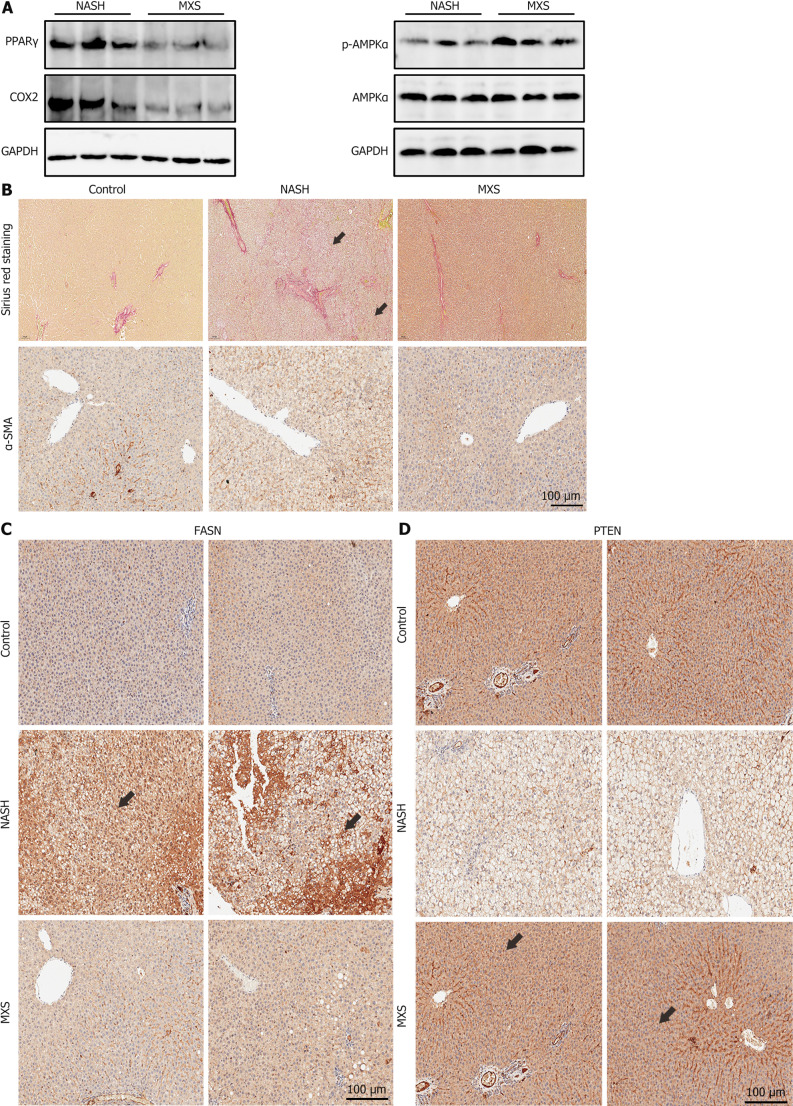
To further validate this mechanism, molecules related to inflammation, liver fibrosis, and fatty acid synthesis in liver tissues, including PPARγ, COX2, AMPKα, FASN, and α-SMA, were detected.
The findings indicated that MXS treatment had a significant regulatory impact on the expression and stimulation of signaling pathways and components related to inflammation and lipid metabolism. IHC results also demonstrated that MXS alleviated CDHF diet-induced hepatic fibrosis and down-regulated α-SMA expression (Figure 8A-C). Additionally, the examination of PTEN expression levels in hepatic tissues, a critical protein central to the link between hormone and lipid metabolism, was conducted. As shown in the results, PTEN expressions were restored after MXS treatment (Figure 8D). These findings suggested that MXS primarily alleviated hepatic steatosis and inflammation by inhibiting male hormone metabolism.
Figure 8.
Modified Xiaoyao San treatment regulated the signaling pathways and factors related to inflammation and lipid metabolism. A: In hepatic tissues, PPARγ and COX2 expressions were decreased by modified Xiaoyao San (MXS) treatment and MXS also activated the AMPK signaling; B: MXS treatment attenuated hepatic fibrosis and α-SMA expression. Collagen fibers appear red (black arrows) under normal light microscopy and other tissue components were dyed yellow; C: The MXS treatment inhibited the levels of FASN in liver tissues; D: The PTEN expression in liver tissues was restored after MXS treatment (immunohistochemistry detection reagents yielding a brown reaction product, indicated by black arrows). The brown portion is the portion of the antigen that expresses coloration. MXS: Modified Xiaoyao San; NASH: Nonalcoholic steatohepatitis.
DISCUSSION
In recent years, NASH has emerged as a leading cause of liver transplantation and mortality, distinguished by inflammation, liver steatosis, and fibrosis[15,16]. However, current treatments for NASH provide only limited relief from symptoms[17-19], and there is significant importance in identifying more effective therapeutics for NASH using novel strategies. This study demonstrated that MXS effectively alleviated inflammation and lipid buildup in hepatocytes, and it significantly impeded the advancement of NASH by inhibiting both male hormone metabolism and the arachidonic acid metabolism pathways. As a clinical drug recommended by the National Health Commission (NHS), MXS is effective in treating metabolic diseases like hyperglycemia and hyperlipidemia. It has also found extensive application in preventing and treating postoperative recurrence and metastasis of primary liver cancer[14]. Refer to the Diagnosis and Treatment Guidelines for Primary Liver Cancer, NHS [2022] No. 12 for more information. Our findings suggest that MXS had the potential to regulate male hormone metabolism, offering a novel discovery that could contribute to the development of MXS as a novel treatment option for enhancing outcomes in NASH patients.
As a central aspect of NASH pathogenesis, the inflammatory reaction encompasses two critical elements: Release of multiple pro-inflammatory cytokines and hepatocyte injury[16,20-22]. A heightened presence of arachidonic acid is believed to be responsible for connecting lipo-toxicity to inflammation and liver disease[23,24]. Our data clarified that arachidonic acid metabolism, along with steroid hormone synthesis, is one of the two main pathways significantly inhibited by MXS treatment. We identified 52 metabolites that displayed unusual increases in the NASH model group but were restored or downregulated after MXS treatment. Among these metabolites, at least 14 were related to the arachidonic acid metabolic pathway, constituting nearly 30% of the total. Moreover, our analysis of signaling pathways and factors related to lipid metabolism and inflammation in liver tissues showed that MXS treatment triggered the AMPK signaling pathway activation while suppressing the expressions of COX2 and PPARγ. These findings corroborated the substantial influence of MXS on the inflammatory response and hepatic lipid metabolism. Rather than solely focusing on the NASH disease model, we conducted a systematic and comprehensive investigation into how MXS ameliorated metabolic disorders induced by NASH. AMPK, a crucial molecule responsible for regulating biological energy metabolism, is expressed in different organs related to metabolism and plays a vital role in maintaining glucose homeostasis[25-27]. The activation of AMPK by pharmacological agents presents a significant challenge due to the intricate molecular mechanisms involved[28,29]. These results align with our study and further confirm the potential of MXS as an innovative therapeutic choice for NASH.
Steroid hormones, as a crucial class of endocrine regulators, have the capacity to influence a range of physiological processes, including cellular proliferation, differentiation, and metabolism. By binding to intracellular receptors, they can govern gene expression and protein synthesis, thereby exerting their biological functions through diverse mechanisms[30-32]. Our results provided evidence that steroid hormone synthesis represented the primary target pathway of MXS and was the pathway most significantly inhibited by MXS treatment. Upon conducting a specialized analysis of steroid hormone metabolism, we observed a captivating phenomenon. MXS treatment predominantly suppressed the steroid hormone metabolic pathway, particularly by inhibiting sex hormone-related metabolism, including the metabolism of male hormones. This inhibitory effect resulted in a significant increase in androgen levels following MXS treatment (Figure 7A). As recently reviewed, alterations in sex hormone levels have been associated with sex-specific differences in lipid metabolism. Research conducted on individuals with biopsy-confirmed NAFLD and progressed liver fibrosis has indicated that older age and being male are associated with increased mortality and a higher occurrence of HCC[33,34]. Although we currently possess limited knowledge about the molecular mechanisms, sex differences indeed play a role in the development of NASH[35]. However, it is undeniable that MXS had the capacity to impact the NASH process by regulating sex hormone metabolism, positioning MXS as a promising candidate for the development of a new anti-NASH medication. We also administered MXS to healthy rats (for 4 weeks), and HE results showed no toxicity or other adverse effects of MXS on the major organs of the rats (Supplementary Figure 10). Furthermore, our findings indicated that MXS treatment led to the restoration of PTEN expression compared to the NASH model group. In the past few years, studies have shed light on the critical role of PTEN in glucose and lipid metabolism, along with its involvement in regulating mitochondrial functions. As a key protein in the interface between hormone and lipid metabolism, PTEN protein expression and activity changes underlie sex differences in NASH[36-40]. We are currently uncertain whether the increased PTEN protein expression observed after MXS treatment is a consequence or a causal factor in the suppression of male hormone metabolism. Nonetheless, our study has demonstrated that MXS treatment can enhance PTEN protein expression and mitigate hepatic steatosis by modulating the male hormone metabolic pathway.
CONCLUSION
Collectively, our findings support the notion that MXS alleviated inflammation and hepatic steatosis in NASH by suppressing male hormone metabolism and modulating inflammation/lipid metabolism-related signaling and factors. This suggested that the regulation of sex hormone metabolism and associated signaling could be new avenues for mechanistic research on NASH and for early diagnosis and treatment. This study offers substantial evidence for the therapeutic potential of MXS in NASH and makes a valuable contribution to the development of new drugs for this condition.
ACKNOWLEDGEMENTS
We would like to thank Professor Tao Feng (College of Pharmacy, Chongqing Medical University) for providing the site and instruments for the experiments related to the detection of MXS.
Footnotes
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Chongqing Academy of Chinese Materia Medica, approval No. CTCM 2022YN012.
Conflict-of-interest statement: All authors have no conflicts of interest to declare.
ARRIVE guidelines statement: The authors have read the ARRIVE Guidelines, and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade D
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Perse M, Slovenia S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
Contributor Information
Xiao-Li Mei, Department of Pharmacology and Toxicology, Sichuan-Chongqing Joint Key Laboratory of New Chinese Medicine Creation Laboratory, Chongqing Academy of Chinese Materia Medica, Chongqing 400061, China.
Shu-Yi Wu, College of Chinese Medicine, Chongqing College of Traditional Chinses Medicine, Chongqing 402760, China.
Si-Lan Wu, Department of Pharmacology and Toxicology, Sichuan-Chongqing Joint Key Laboratory of New Chinese Medicine Creation Laboratory, Chongqing Academy of Chinese Materia Medica, Chongqing 400061, China.
Xiao-Lin Luo, Department of Pharmacology and Toxicology, Sichuan-Chongqing Joint Key Laboratory of New Chinese Medicine Creation Laboratory, Chongqing Academy of Chinese Materia Medica, Chongqing 400061, China.
Si-Xing Huang, Department of Pharmacology and Toxicology, Sichuan-Chongqing Joint Key Laboratory of New Chinese Medicine Creation Laboratory, Chongqing Academy of Chinese Materia Medica, Chongqing 400061, China.
Rui Liu, Department of Oncology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China.
Zhe Qiang, Department of Pharmacology and Toxicology, Sichuan-Chongqing Joint Key Laboratory of New Chinese Medicine Creation Laboratory, Chongqing Academy of Chinese Materia Medica, Chongqing 400061, China; College of Chinese Medicine, Chongqing College of Traditional Chinses Medicine, Chongqing 402760, China; College of Pharmacy, Chongqing Medical University, Chongqing 400010, China. qiangzhe@cqctcm.edu.cn.
Data sharing statement
The data that support the findings of this study are available from the corresponding author at qiangzhe@cqctcm.edu.cn upon reasonable request.
References
- 1.Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. 2021;3:1596–1607. doi: 10.1038/s42255-021-00501-9. [DOI] [PubMed] [Google Scholar]
- 2.Farrell GC, Haczeyni F, Chitturi S. Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease. Adv Exp Med Biol. 2018;1061:19–44. doi: 10.1007/978-981-10-8684-7_3. [DOI] [PubMed] [Google Scholar]
- 3.Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Liu C, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809–2817.e28. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71:1851–1864. doi: 10.1002/hep.31150. [DOI] [PubMed] [Google Scholar]
- 5.Lonardo A, Mantovani A, Petta S, Carraro A, Byrne CD, Targher G. Metabolic mechanisms for and treatment of NAFLD or NASH occurring after liver transplantation. Nat Rev Endocrinol. 2022;18:638–650. doi: 10.1038/s41574-022-00711-5. [DOI] [PubMed] [Google Scholar]
- 6.Abdelmalek MF. Nonalcoholic fatty liver disease: another leap forward. Nat Rev Gastroenterol Hepatol. 2021;18:85–86. doi: 10.1038/s41575-020-00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sodum N, Kumar G, Bojja SL, Kumar N, Rao CM. Epigenetics in NAFLD/NASH: Targets and therapy. Pharmacol Res. 2021;167:105484. doi: 10.1016/j.phrs.2021.105484. [DOI] [PubMed] [Google Scholar]
- 8.Zhao P, Saltiel AR. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J Biol Chem. 2020;295:12279–12289. doi: 10.1074/jbc.REV120.011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Schwabe RF, Tabas I, Pajvani UB. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan J, Zhang Y, Yang D, Liang Y, Yang L, Hu S, Liu Z, Fang Q, Tian S, Ding Y. Gastrodin Improves Nonalcoholic Fatty Liver Disease Through Activation of the Adenosine Monophosphate-Activated Protein Kinase Signaling Pathway. Hepatology. 2021;74:3074–3090. doi: 10.1002/hep.32068. [DOI] [PubMed] [Google Scholar]
- 12.Lan T, Yu Y, Zhang J, Li H, Weng Q, Jiang S, Tian S, Xu T, Hu S, Yang G, Zhang Y, Wang W, Wang L, Zhu Q, Rong X, Guo J. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology. 2021;74:686–703. doi: 10.1002/hep.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan T, Jiang S, Zhang J, Weng Q, Yu Y, Li H, Tian S, Ding X, Hu S, Yang Y, Wang W, Wang L, Luo D, Xiao X, Piao S, Zhu Q, Rong X, Guo J. Breviscapine alleviates NASH by inhibiting TGF-β-activated kinase 1-dependent signaling. Hepatology. 2022;76:155–171. doi: 10.1002/hep.32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bureau of Medical Administration, National Health Commission of the People's Republic of China. [Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition)] Zhonghua Gan Zang Bing Za Zhi. 2022;30:367–388. doi: 10.3760/cma.j.cn501113-20220413-00193. [DOI] [PubMed] [Google Scholar]
- 15.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology. 2019;70:1119–1133. doi: 10.1002/hep.30702. [DOI] [PubMed] [Google Scholar]
- 18.Lee HW, Wong VW. Changing NAFLD Epidemiology in China. Hepatology. 2019;70:1095–1098. doi: 10.1002/hep.30848. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, Xie X, Feng Y, Stave CD, Zhu Q, Cheung R, Nguyen MH. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta-analysis. Hepatol Int. 2020;14:259–269. doi: 10.1007/s12072-020-10023-3. [DOI] [PubMed] [Google Scholar]
- 20.Kim DY, Park JY. Overview of emerging treatment of non-alcoholic fatty liver disease: more than one drug needed? Hepatobiliary Surg Nutr. 2019;8:522–524. doi: 10.21037/hbsn.2019.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Li YZ, Hylemon PB, Zhang LY, Zhou HP. Cordycepin inhibits LPS-induced inflammatory responses by modulating NOD-Like Receptor Protein 3 inflammasome activation. Biomed Pharmacother. 2017;95:1777–1788. doi: 10.1016/j.biopha.2017.09.103. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Mehal W, Nagy LE, Rotman Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol Immunol. 2021;18:73–91. doi: 10.1038/s41423-020-00579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, Guan KL, Xiong Y, Liu J, Yuan HX. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 2020;40:1378–1394. doi: 10.1111/liv.14428. [DOI] [PubMed] [Google Scholar]
- 24.Bianco C, Casirati E, Malvestiti F, Valenti L. Genetic predisposition similarities between NASH and ASH: Identification of new therapeutic targets. JHEP Rep. 2021;3:100284. doi: 10.1016/j.jhepr.2021.100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju L, Wen X, Wang C, Wei Y, Peng Y, Ding Y, Feng L, Shu L. Salidroside, A Natural Antioxidant, Improves β-Cell Survival and Function via Activating AMPK Pathway. Front Pharmacol. 2017;8:749. doi: 10.3389/fphar.2017.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng T, Yang X, Li W, Wang Q, Chen L, Wu D, Bian F, Xing S, Jin S. Salidroside Attenuates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via AMPK-Dependent TXNIP/NLRP3 Pathway. Oxid Med Cell Longev. 2018;2018:8597897. doi: 10.1155/2018/8597897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SC, Hardie DG. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018;27:299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Spaulding HR, Yan Z. AMPK and the Adaptation to Exercise. Annu Rev Physiol. 2022;84:209–227. doi: 10.1146/annurev-physiol-060721-095517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg GR, Carling D. AMP-activated protein kinase: the current landscape for drug development. Nat Rev Drug Discov. 2019;18:527–551. doi: 10.1038/s41573-019-0019-2. [DOI] [PubMed] [Google Scholar]
- 30.D'Uva G, Lauriola M. Towards the emerging crosstalk: ERBB family and steroid hormones. Semin Cell Dev Biol. 2016;50:143–152. doi: 10.1016/j.semcdb.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Lappano R, Todd LA, Stanic M, Cai Q, Maggiolini M, Marincola F, Pietrobon V. Multifaceted Interplay between Hormones, Growth Factors and Hypoxia in the Tumor Microenvironment. Cancers (Basel) 2022;14 doi: 10.3390/cancers14030539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Northey JJ, Weaver VM. Mechanosensitive Steroid Hormone Signaling and Cell Fate. Endocrinology. 2022;163 doi: 10.1210/endocr/bqac085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, Suzuki A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology. 2019;70:1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Alvarez-Quiñones Sanz M, Conde-Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero-Gomez M. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443–457.e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Lonardo A, Suzuki A. Nonalcoholic fatty liver disease: does sex matter? Hepatobiliary Surg Nutr. 2019;8:164–166. doi: 10.21037/hbsn.2018.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anezaki Y, Ohshima S, Ishii H, Kinoshita N, Dohmen T, Kataoka E, Sato W, Iizuka M, Goto T, Sasaki J, Sasaki T, Suzuki A, Ohnishi H, Horie Y. Sex difference in the liver of hepatocyte-specific Pten-deficient mice: A model of nonalcoholic steatohepatitis. Hepatol Res. 2009;39:609–618. doi: 10.1111/j.1872-034X.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 37.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci U S A. 2020;117:31189–31197. doi: 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson DL, Stiles BL. Maf1, A New PTEN Target Linking RNA and Lipid Metabolism. Trends Endocrinol Metab. 2016;27:742–750. doi: 10.1016/j.tem.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CY, Chen J, He L, Stiles BL. PTEN: Tumor Suppressor and Metabolic Regulator. Front Endocrinol (Lausanne) 2018;9:338. doi: 10.3389/fendo.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li A, Qiu M, Zhou H, Wang T, Guo W. PTEN, Insulin Resistance and Cancer. Curr Pharm Des. 2017;23:3667–3676. doi: 10.2174/1381612823666170704124611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author at qiangzhe@cqctcm.edu.cn upon reasonable request.