Abstract
BACKGROUND
General anesthesia is commonly used in the surgical management of gastrointestinal tumors; however, it can lead to emergence agitation (EA). EA is a common complication associated with general anesthesia, often characterized by behaviors, such as crying, struggling, and involuntary limb movements in patients. If treatment is delayed, there is a risk of incision cracking and bleeding, which can significantly affect surgical outcomes. Therefore, having a proper understanding of the factors influencing the occurrence of EA and implementing early preventive measures may reduce the incidence of agitation during the recovery phase from general anesthesia, which is beneficial for improving patient prognosis.
AIM
To analyze influencing factors and develop a risk prediction model for EA occurrence following general anesthesia for primary liver cancer.
METHODS
Retrospective analysis of clinical data from 200 patients who underwent hepatoma resection under general anesthesia at Wenzhou Central Hospital (January 2020 to December 2023) was conducted. Post-surgery, the Richmond Agitation-Sedation Scale was used to evaluate EA presence, noting EA incidence after general anesthesia. Patients were categorized by EA presence postoperatively, and the influencing factors were analyzed using logistic regression. A nomogram-based risk prediction model was constructed and evaluated for differentiation and fit using receiver operating characteristics and calibration curves.
RESULTS
EA occurred in 51 (25.5%) patients. Multivariate analysis identified advanced age, American Society of Anesthesiologists (ASA) grade III, indwelling catheter use, and postoperative pain as risk factors for EA (P < 0.05). Conversely, postoperative analgesia was a protective factor against EA (P < 0.05). The area under the curve of the nomogram was 0.972 [95% confidence interval (CI): 0.947-0.997] for the training set and 0.979 (95%CI: 0.951-1.000) for the test set. Hosmer-Lemeshow test showed a good fit (χ2 = 5.483, P = 0.705), and calibration curves showed agreement between predicted and actual EA incidence.
CONCLUSION
Age, ASA grade, catheter use, postoperative pain, and analgesia significantly influence EA occurrence. A nomogram constructed using these factors demonstrates strong predictive accuracy.
Keywords: Primary hepatocellular carcinoma resection, General anesthesia, Emergence agitation, Risk factors, Forecast, Nomograph
Core Tip: In this study, we retrospectively analyzed clinical data from 200 patients with primary liver cancer undergoing general anesthesia. The aim was to identify key factors influencing postoperative emergence agitation (EA) occurrence and to construct a risk prediction model. The findings revealed advanced age, American Society of Anesthesiologists grade III, indwelling catheter, and postoperative pain as risk factors for EA, whereas postoperative analgesia emerged as a protective factor. Successful construction of a nomogram risk prediction model demonstrated good predictive efficacy, offering a practical tool for the clinical evaluation and prevention of EA.
INTRODUCTION
General anesthesia involves the administration of narcotic drugs to temporarily suppress the central nervous system, leading to reversible loss of consciousness, sensation, and reflexes[1]. Emergence agitation (EA) refers to a transient state of consciousness and behavior separation during the transition from anesthesia to full consciousness. It is a common postoperative complication in patients undergoing general anesthesia[2], with an incidence of 17.09%-24.80%[3]. The symptoms of EA mainly manifest as emotional agitation, restlessness, and disorientation[4]. Prolonged agitation may result in hazardous behaviors, such as self-removal of masks and catheters, resulting in hypoxia, falls, bleeding from surgical incisions, and limb injuries, which compromises surgical outcomes and patient safety. Primary liver cancer ranks fifth globally and third in Asia-Pacific cancer-related deaths[5], with surgery being the foremost treatment option[6]. Hepatocellular carcinoma resection, characterized by prolonged operation time, extensive drug use, and large wound area, predisposes patients to higher EA risk. Thus, early identification of EA risk factors in patients with primary liver cancer after surgery is crucial for timely intervention. Currently, clinical factors influencing EA remain unclear and may be related to factors such as catheter irritation, pain, or drugs[7]. In view of this, our study aimed to analyze factors influencing the occurrence of EA in patients with primary liver cancer after surgery and construct a risk prediction model to aid the prevention of EA in this population.
MATERIALS AND METHODS
Research object
Two hundred patients who underwent hepatic cancer resection under general anesthesia and were admitted to Wenzhou Central Hospital between January 2020 and December 2023 were selected for the study. Inclusion criteria were as follows: (1) Mentally normal and able to cooperate before anesthesia; (2) No contraindications to anesthesia and no history of allergy to anesthetic drugs; and (3) Age ≥ 18 years old. Exclusion criteria were: (1) Incomplete medical records; (2) Transfer to the intensive care unit after surgery; and (3) Renal failure. The study was approved by the Institutional Review Board of Wenzhou Central Hospital, and the need for informed consent was waived.
Diagnostic criteria
The occurrence of EA was evaluated using the Richmond Agitation-Sedation Scale[8]. The scale is divided into 10 sedation levels, with a score of 4 to -5 indicating the patient’s level of consciousness from “aggressive” to “unconscious”. Coma was -5 points; severe sedation -4 points; moderate sedation -3 points; light sedation -2 points; drowsy -1 point; awake and calm 0 points; restless and anxious 1 point; agitated anxiety 2 points, very agitated 3 points; and aggressive 4 points. A score of ≥ 1 was considered indicative of EA.
Research method
Demographic information including, sex, age, duration of surgery, duration of anesthesia, American Society of Anesthesiologists (ASA)[9] classification, indwelling catheter use, postoperative analgesia, time to recovery, and postoperative pain, were collected. Postoperative pain was assessed using the Digital Pain Rating Scale[10], ranging from 0 to 10 points, where a higher score indicated stronger pain sensation.
Statistical analysis
Statistical analysis was performed using SPSS 23.0 and R software. Measurement information, such as age, was expressed as mean ± SD, and a t-test was used for group comparison. Count data, such as sex, were expressed as cases (%), and comparisons were made using the chi-square test. Logistic regression analysis was used to analyze influencing factors, with P < 0.05 considered statistically significant. Column line graphs were constructed using R software, and their performance was assessed using the area under the curve (AUC) of the receiver operating characteristic (ROC) curves and calibration curves of the subjects’ work characteristics.
RESULTS
Clinical characteristics
A total of 200 patients were included in this study, 51 of whom developed EA postoperatively, resulting in an incidence rate of 25.5%. The EA group comprised 51 patients who experienced EA after surgery, whereas the remaining patients were categorized into the group without EA. As shown in Table 1, significant differences were observed between the two groups in terms of age, operation time, anesthesia duration, ASA grade, indwelling catheter use, postoperative analgesia, and postoperative pain (P < 0.05).
Table 1.
Comparison of general information between the two groups, n (%)
|
Factor
|
EA group (n = 51)
|
Non-EA group (n = 149)
|
χ2/t
|
P value
|
| Gender | ||||
| Female | 22 (43.14) | 67 (44.97) | 0.051 | 0.821 |
| Male | 29 (56.86) | 82 (55.03) | ||
| Age (years) | 55.67 ± 7.46 | 50.23 ± 6.15 | -5.145 | < 0.001 |
| Smoking history | ||||
| No | 24 (47.06) | 76 (51.01) | 0.237 | 0.626 |
| Yes | 27 (52.94) | 73 (48.99) | ||
| Drinking history | ||||
| No | 25 (49.02) | 85 (57.05) | 0.989 | 0.320 |
| Yes | 26 (50.98) | 64 (42.95) | ||
| Hypertension | ||||
| No | 32 (62.75) | 106 (71.14) | 1.252 | 0.263 |
| Yes | 19 (37.25) | 43 (28.86) | ||
| Diabetes | ||||
| No | 34 (66.67) | 109 (73.15) | 0.785 | 0.376 |
| Yes | 17 (33.33) | 40 (26.85) | ||
| ASA grade | ||||
| I | 11 (21.57) | 70 (46.98) | 21.096 | < 0.001 |
| II | 18 (35.29) | 58 (38.93) | ||
| III | 22 (43.14) | 21 (14.09) | ||
| Indwelling catheter | ||||
| No | 18 (35.29) | 104 (69.80) | 19.014 | < 0.001 |
| Yes | 33 (64.71) | 45 (30.20) | ||
| Postoperative analgesia | ||||
| No | 33 (64.71) | 55 (36.91) | 11.911 | 0.001 |
| Yes | 18 (35.29) | 94 (63.09) | ||
| Operation time (minute) | 302.76 ± 65.51 | 264.38 ± 61.81 | -3.770 | < 0.001 |
| Anesthesia time (minute) | 348.14 ± 65.09 | 313.48 ± 62.24 | -3.392 | < 0.001 |
| Awakening time (minute) | 42.62 ± 3.58 | 41.91 ± 3.87 | -1.129 | 0.260 |
| Postoperative pain (scores) | 4.43 ± 1.20 | 2.60 ± 0.76 | -10.201 | < 0.001 |
EA: Emergence agitation; ASA: American Society of Anesthesiologists.
Multi-factor analysis of EA occurrence
Variables showing statistical significance in the univariate analysis were considered independent variables, with the occurrence of restlessness during the recovery period after general anesthesia deemed the dependent variable (yes = 1, no = 0). Table 2 displays the variable assignment tables. Results revealed that old age, ASA class III status, indwelling catheter use, and postoperative pain were independent risk factors for EA. Additionally, postoperative analgesia was found to provide protection against EA, as shown in Table 3.
Table 2.
Assignment table of factors affecting emergence agitation in patients after general anesthesia
|
Factor
|
Assign
|
| Age | Original input |
| Operation time | Original input |
| Anesthesia time | Original input |
| ASA grade | 0 = I, 1 = II, 2 = III |
| Indwelling catheter | 0 = No, 1 = Yes |
| Postoperative analgesia | 0 = No, 1 = Yes |
| Postoperative pain | Original input |
ASA: American Society of Anesthesiologists.
Table 3.
Logistic regression analysis of emergence agitation-related factors
|
Factor
|
B
|
SE
|
Wald
|
P value
|
OR value (95%CI)
|
| Age | 0.202 | 0.065 | 9.693 | 0.002 | 1.224 (1.078-1.391) |
| Operation time (minute) | 0.010 | 0.006 | 3.065 | 0.080 | 1.011 (0.999-1.022) |
| Anesthesia time (minute) | -0.003 | 0.005 | 0.259 | 0.611 | 0.997 (0.987-1.008) |
| ASA grade (Class I as reference) | - | - | 6.180 | 0.045 | - |
| II | 0.076 | 0.813 | 0.009 | 0.925 | 1.079 (0.219-5.309) |
| III | 2.165 | 0.937 | 5.338 | 0.021 | 8.713 (1.389-54.671) |
| Indwelling catheter | 1.529 | 0.730 | 4.388 | 0.036 | 4.613 (1.103-19.290) |
| Postoperative analgesia | -3.516 | 1.009 | 12.145 | < 0.001 | 0.030 (0.004-0.215) |
| Postoperative pain | 4.144 | 0.989 | 17.543 | < 0.001 | 63.079 (9.070-438.679) |
SE: Standard error; OR: Odds ratio; CI: Confidence interval; ASA: American Society of Anesthesiologists.
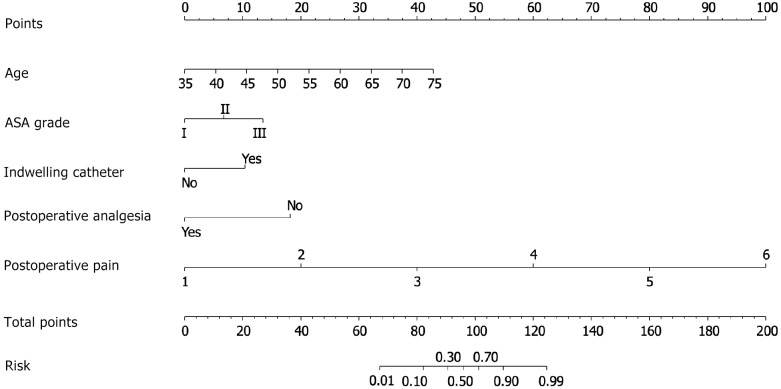
Construction of EA nomogram after operation
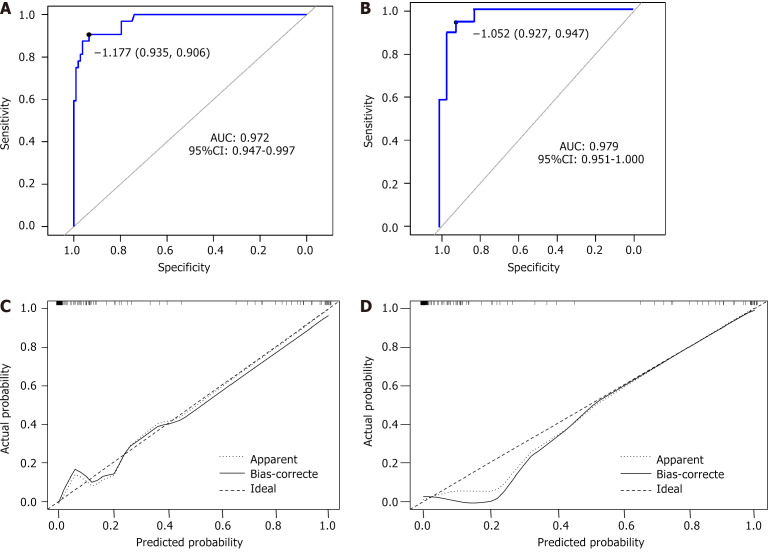
Using a sample of 140 patients from the training set, the five factors influencing EA were incorporated into a risk assessment model for EA after general anesthesia for primary liver cancer, represented as a column graph (Figure 1). To further verify the predictive efficiency of the model, ROC curves were plotted for both the training and test sets (Figure 2A and B). The model demonstrated high predictive accuracy in both sets, with an AUC of 0.972 [95% confidence interval (CI): 0.947-0.997] for the training set and 0.979 (95%CI: 0.951-1.000) for the test set. The Hosmer-Lemeshow test showed an excellent fit (χ2 = 5.483, P = 0.705), and the calibration curve (Figure 2C and D) revealed good agreement between the predicted probability and the actual incidence of EA in both the training and test sets.
Figure 1.
Nomogram prediction model. This is a column graph illustrating the integration of five factors influencing emergence agitation (EA) into a risk assessment model for EA following general anesthesia for primary liver cancer. The graph was constructed using data from a sample of 140 patients from the training set. ASA: American Society of Anesthesiologists.
Figure 2.
Receiver operating characteristic and calibration curve analysis. A and B: It displays receiver operating characteristic curves plotted to further validate the predictive efficiency of the model. The curves represent both the training and test sets; C and D: The calibration curve illustrates the agreement between the predicted probability and the actual incidence of emergence agitation in both the training and test sets. A and C are training set, B and D are test set. AUC: Area under the curve.
DISCUSSION
Patients undergoing general anesthesia pass through three stages: Light anesthesia, non-anesthesia, and awakening during the recovery of normal physiological functions after surgery. During this process, protective physiological reflexes gradually return. However, some abnormal changes may occur, with the most significant neurological manifestation being EA[11]. Patients with EA often exhibit ambiguous consciousness, drowsiness, failure to follow instructions, involuntary movements, extreme disorientation, restlessness, and other agitations. If left untreated, EA can lead to complications such as increased internal bleeding, cerebrovascular disease, myocardial infarction, endangerment of suture lines, and in severe cases, surgical failure, pipeline dislodgement, falls, and other accidental injuries[12]. These consequences not only pose a serious threat to medical safety but also endanger patients’ lives and health, leading to potential disputes between doctors and patients. Currently, the clinical factors contributing to EA are not fully understood. General anesthetic drugs are believed to exert varying degrees of inhibition on the central nervous system, leading to the emergence of reflex confrontation and an abnormal state of consciousness[13,14]. The results of this study indicate a postoperative EA incidence of 25.5%. A study by Kang et al[15] reported a postoperative EA incidence of 14.1% among 1950 adult patients operated under general anesthesia, while Abitağaoğlu et al[16] observed a 15.4% incidence among 102 postoperative patients. The relatively high incidence observed in our study suggests that EA remains a significant concern in postoperative patients recovering from general anesthesia. Discrepancies with other studies may stem from differences in population inclusion criteria, age demographics, and geographical variations. This study identified potential influencing factors, revealing postoperative analgesia as a protective factor against EA, while old age, indurating catheter use, ASA grade III, and postoperative pain emerged as risk factors. The incidence of EA increased with age, possibly due to abnormal melatonin secretion. Melatonin, an amine hormone secreted by the pineal glands of both mammals and humans, decreases with age, leading to dysfunctions that hinder postoperative recovery and increase EA incidence[17]. Some scholars also believe[18] that older people may have poorer physical fitness and lower pain tolerance than younger people. Similarly, due to reduced physical function, these patient populations often lack confidence in treatment and are prone to anxiety, thereby contributing to an increased occurrence of EA. Indwelling catheter use was associated with an elevated EA risk, potentially due to unconscious urethral mucosal injury during surgery and subsequent pain and discomfort during recovery[19]. The ASA grade is also a risk factor for EA. Patients with higher ASA grades are more susceptible to fluctuations in respiration, circulation, and the internal environment during anesthesia and surgery[20]. In addition, patients with higher ASA grades often require larger doses of anesthesia, which can lead to excessive anesthetic drug levels in the body. This can inhibit the cardiovascular system, resulting in an increased risk of EA for various reasons[21]. The incidence of EA notably rises in patients experiencing intense postoperative pain. This is primarily because pain, discomfort, and other stimuli can trigger defensive reflexes, resulting in an increased incidence of EA[22-24]. Healthcare professionals can alleviate patients’ fear of pain through psychological support, effective communication, and other methods. Additionally, they can provide personalized analgesic intervention based on patients’ individual conditions. The results of this study showed that postoperative analgesia serves as a protective factor against EA. A study by Yang et al[25] further supports this, indicating that postoperative analgesia significantly reduces the incidence of postoperative EA among patients undergoing general anesthesia. This underscores the importance of postoperative analgesia as a protective measure against EA occurrence. The study has some limitations worth noting. Firstly, its focus solely on patients undergoing general anesthesia may overlook factors relevant to other anesthesia types. Secondly, as a retrospective analysis conducted in a single center, the findings may not be fully generalizable to broader patient populations. Additionally, although the sample size was adequate, larger multicenter studies could offer more robust insights. The complexity of risk factors and reliance on retrospective data pose further limitations, alongside the study’s primary reliance on EA as the outcome measure, potentially neglecting other clinical endpoints. Furthermore, limited follow-up post-discharge restricts understanding of long-term complications. Addressing these limitations in future research could advance our understanding and improve preventive strategies for EA.
CONCLUSION
In conclusion, age, indwelling catheter use, ASA grade, and postoperative pain significantly affect the occurrence of EA. Therefore, clinical attention should be directed towards these factors, and appropriate prevention and control measures should be implemented. In addition, postoperative analgesia serves as a protective factor against EA. Hence, tailored analgesia programs can be offered based on the individual clinical circumstances of the patients. Future studies should explore the comprehensive understanding of EA in patients undergoing general anesthesia, focusing on refining preventive strategies and improving patient outcomes. Investigations into additional influencing factors, long-term complications, and the efficacy of personalized analgesic interventions could further enhance EA management and patient care.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Wenzhou Central Hospital (Approval No. L2024-02-030).
Informed consent statement: The ethics committee granted an exemption from obtaining informed consent because this is a retrospective study that does not involve patient privacy.
Conflict-of-interest statement: The authors declare no conflict of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Arun S, India S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
Contributor Information
Shu-Shu Song, Department of Anesthesia and Surgery, Wenzhou Central Hospital, Wenzhou 325099, Zhejiang Province, China.
Li Lin, Department of Anesthesia and Surgery, Wenzhou Central Hospital, Wenzhou 325099, Zhejiang Province, China.
Li Li, Department of Anesthesia and Surgery, Wenzhou Central Hospital, Wenzhou 325099, Zhejiang Province, China.
Xiao-Dong Han, Department of Anesthesia and Surgery, Wenzhou Central Hospital, Wenzhou 325099, Zhejiang Province, China. hxd1980115@sina.com.
Data sharing statement
Anonymous datasets can be obtained from the corresponding author upon request.
References
- 1.Rogobete AF, Sandesc D. General Anesthesia as a Multimodal Individualized Clinical Concept. Medicina (Kaunas) 2022;58 doi: 10.3390/medicina58070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S, Sohn JY, Hwang IE, Lee HJ, Yoon S, Bahk JH, Kim BR. Effect of a repeated verbal reminder of orientation on emergence agitation after general anaesthesia for minimally invasive abdominal surgery: a randomised controlled trial. Br J Anaesth. 2023;130:439–445. doi: 10.1016/j.bja.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020;73:471–485. doi: 10.4097/kja.20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bağcaz A, Ayhan A. Emergence Agitation with Earthquake-Related Traumatic Stress Symptoms After Intravenous Sedation. Turk Psikiyatri Derg. 2023;34:136–139. doi: 10.5080/u27347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi JF, Cao M, Wang Y, Bai FZ, Lei L, Peng J, Feletto E, Canfell K, Qu C, Chen W. Is it possible to halve the incidence of liver cancer in China by 2050? Int J Cancer. 2021;148:1051–1065. doi: 10.1002/ijc.33313. [DOI] [PubMed] [Google Scholar]
- 6.Orcutt ST, Anaya DA. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control. 2018;25:1073274817744621. doi: 10.1177/1073274817744621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Hao J, Zhang J, Du J, Luo Z. Risk factors for emergence agitation during the awakening period in elderly patients after total joint arthroplasty: a retrospective cohort study. BMJ Open. 2023;13:e068284. doi: 10.1136/bmjopen-2022-068284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medlej K. Calculated decisions: Richmond Agitation-Sedation Scale (RASS) Emerg Med Pract. 2021;23:CD3–CD4. [PubMed] [Google Scholar]
- 9.Apfelbaum JL, Connis RT. The American Society of Anesthesiologists Practice Parameter Methodology. Anesthesiology. 2019;130:367–384. doi: 10.1097/ALN.0000000000002551. [DOI] [PubMed] [Google Scholar]
- 10.Thong ISK, Jensen MP, Miró J, Tan G. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain. 2018;18:99–107. doi: 10.1515/sjpain-2018-0012. [DOI] [PubMed] [Google Scholar]
- 11.Tolly B, Waly A, Peterson G, Erbes CR, Prielipp RC, Apostolidou I. Adult Emergence Agitation: A Veteran-Focused Narrative Review. Anesth Analg. 2021;132:353–364. doi: 10.1213/ANE.0000000000005211. [DOI] [PubMed] [Google Scholar]
- 12.Talih G, Yüksek A, Şahin E. Evaluation of emergence agitation after general anaesthesia in rhinoplasty patients: Inhalation anaesthesia versus total intravenous anaesthesia. Am J Otolaryngol. 2020;41:102387. doi: 10.1016/j.amjoto.2020.102387. [DOI] [PubMed] [Google Scholar]
- 13.Wei B, Feng Y, Chen W, Ren D, Xiao D, Chen B. Risk factors for emergence agitation in adults after general anesthesia: A systematic review and meta-analysis. Acta Anaesthesiol Scand. 2021;65:719–729. doi: 10.1111/aas.13774. [DOI] [PubMed] [Google Scholar]
- 14.Shen QH, Xu-Shen, Lai L, Chen YJ, Liu K, Sun LJ. The effect of magnesium sulfate on emergence agitation in children undergoing general anesthesia: A systematic review and meta-analysis. J Clin Anesth. 2022;78:110669. doi: 10.1016/j.jclinane.2022.110669. [DOI] [PubMed] [Google Scholar]
- 15.Kang X, Lin K, Tang H, Tang X, Bao F, Gan S, Zhu S. Risk Factors for Emergence Agitation in Adults Undergoing Thoracoscopic Lung Surgery: A Case-Control Study of 1,950 Patients. J Cardiothorac Vasc Anesth. 2020;34:2403–2409. doi: 10.1053/j.jvca.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Abitağaoğlu S, Köksal C, Alagöz S, Karip CŞ, Arı DE. Effect of ketamine on emergence agitation following septoplasty: a randomized clinical trial. Braz J Anesthesiol. 2021;71:381–386. doi: 10.1016/j.bjane.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Jia X, Lin D, Ma J. Melatonin or its analogs as premedication to prevent emergence agitation in children: a systematic review and meta-analysis. BMC Anesthesiol. 2023;23:392. doi: 10.1186/s12871-023-02356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XL, Xu WF, Wang LL, Wu YF, Ma HP. Analysis on the Incidence and Risk Factors of Emergence Agitation in Patients with Gastric Cancer after Laparoscopic Surgery in Anesthesia Recovery Room. Xiandai Shengwuyixue Jinzhan. 2022;22:2879–2882, 2964. [Google Scholar]
- 19.Zhang M, Hu XW, Li R, Jia JX, Zhang Q. Analysis of Restlessness and its Influencing Factors in Patients after General Anesthesia. Xiandai Shengwuyixue Jinzhan. 2022;22:397–400. [Google Scholar]
- 20.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, Browndyke JN, Wright CB, Culley DJ, Evered L, Scott DA, Wang NY, Brown CH 4th, Oh E, Purdon P, Inouye S, Berger M, Whittington RA, Price CC, Deiner S. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth. 2019;123:464–478. doi: 10.1016/j.bja.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mevorach L, Forookhi A, Farcomeni A, Romagnoli S, Bilotta F. Perioperative risk factors associated with increased incidence of postoperative delirium: systematic review, meta-analysis, and Grading of Recommendations Assessment, Development, and Evaluation system report of clinical literature. Br J Anaesth. 2023;130:e254–e262. doi: 10.1016/j.bja.2022.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Sun X, Li P, Deng X. Prevalence and risk factors of emergence agitation among pediatric patients undergo ophthalmic and ENT Surgery: a cross-sectional study. BMC Pediatr. 2023;23:598. doi: 10.1186/s12887-023-04434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barreto ACTP, Rangel da Rocha Paschoal AC, Barbosa Farias C, Gomes Nogueira Borges PS, Gonelli Albanez da Cunha Andrade R, de Orange FA. [Risk factors associated with anesthesia emergence delirium in children undergoing outpatient surgery] Braz J Anesthesiol. 2018;68:162–167. doi: 10.1016/j.bjane.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Sherbiny SM, Kamal RA, Sadik N, Elshahat A. Effect of Dexmedetomidine in Sub-Tenon's Block on Emergence Agitation in Pediatric Strabismus Surgery under Sevoflurane Anesthesia. Anesth Essays Res. 2022;16:160–166. doi: 10.4103/aer.aer_99_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Hu Z, Peng F, Chen G, Zhou Y, Yang Q, Yang X, Wang M. Effects of Dexmedetomidine on Emergence Agitation and Recovery Quality Among Children Undergoing Surgery Under General Anesthesia: A Meta-Analysis of Randomized Controlled Trials. Front Pediatr. 2020;8:580226. doi: 10.3389/fped.2020.580226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymous datasets can be obtained from the corresponding author upon request.