Abstract
Objective:
To explore routinely measured markers of systemic inflammation in hypertension (HTN) and type 2 diabetes (T2D) comorbidity, and their association with atherogenicity.
Methods:
This study included a total of 70 patients with T2D which were categorised into 2 groups, that is with T2D and with HTN comorbidity (T2D + HTN) (n = 35/group). All measured laboratory parameters were determined using standardised methods.
Results:
The neutrophil/lymphocyte ratio (NLR) was elevated in patients with T2D + HTN when compared to those with T2D (P = .0494). This was also the case with C-reactive protein (CRP) levels (P < .0001) and systemic immune-inflammation (SII) index (P = .0298). Notably, the majority of patients with T2D + HTN [63% (n = 22)] were classified as having an intermediate or high atherogenic index of plasma (AIP). The correlation analysis of systemic inflammation showed significant associations between CRP and age (r = .24, P = .0477); CRP and red blood cell count (r = −.4, P = .0455), and SII and systolic blood pressure (SBP) (r = .33, P = .0056). However, there was no association between inflammatory profiles and lipograms (P > .05). We further assessed predictors for an elevated AIP using mutivariable regression model adjusted for age, SBP, CRP and SII. Only NLR was a significant predictor of AIP (β = .287, SE: 0.1, P = .0046).
Conclusion:
HTN comorbidity in T2D is associated with exacerbated levels of inflammation and atherogenicity. NLR is a significant independent risk factor for increased atherogenicity in patients with T2D. Therefore, the use of therapeutic strategies that target and alleviate inflammation in patients with T2D and HTN comorbidity is imperative in reducing the initiating and progression of cardiovascular events (CVEs).
Keywords: Atherogenicity, cardiovascular risk, hypertension, inflammation, type 2 diabetes
Introduction
Hypertension (HTN) is an independent risk factor for cardiovascular events (CVEs) in patients with type 2 diabetes (T2D). 1 Obesity, vascular and renal interstitial inflammation are identified as the prominent mechanisms involved in the pathogenesis of HTN.2,3 Patients living with HTN and T2D comorbidity are at a two-fold risk of developing CVEs when compared to normotensive individuals with T2D. 4 Therefore, the early assessment of cardiovascular risk in these patients using routinely measured laboratory biomarkers is instrumental in the identification and monitoring of high risk cases, and reducing disease burden. The lipoproteins derived atherogenic index of plasma (AIP) is a good predictor of CVEs in patients with T2D and metabolic syndrome. 5 Notably, the coexistence of atherogenic dyslipidaemia and low-grade inflammation is a prominent feature in T2D. 6 However, this relationship remains unclear in patients with HTN and T2D comorbidity.
Individually, HTN and T2D are associated with chronic inflammation and immune activation mediated by pro-inflammatory immune cells.7,8 The routinely measured complete blood count and its derived parameters are instrumental in elucidating the patient’s inflammatory state. In the context of T2D, the chronic inflammatory state is characterised by elevated white cell, neutrophil and lymphocytes counts, and a high neutrophil-to-lymphocyte ratio (NLR). 9 This immune activation signature is also observed in patients with HTN. 10 The systemic immune-inflammation index (SII) is a reliable inflammatory index that integrates 3 immune cells (neutrophils, platelets and lymphocytes) and is strongly associated with the pathogenesis of T2D-associated complications including CVEs.11,12 However, the interplay between systemic inflammation and HTN in patients with T2D remains elusive. Therefore, in this study, we explored routinely measured markers of systemic inflammation in HTN and T2D comorbidity, and their association with atherogenicity.
Materials and Methods
Study population
This prospective cross-sectional study included a cohort of outpatients with T2D who visited the Katutura Community Health Centre in Windhoek, Namibia between September, and December 2020. All cases of T2D were clinician diagnosed, based on the American Diabetes Association guidelines. 13 The study was approved by the Namibia University of Science and Technology Ethics committee (FHAS 1/2020) and the Namibia Ministry of Health and Social Services (17/3/3 MN). All included participants provided written informed consent and the study was conducted according to the Declaration of Helsinki code of ethics. 14 One hundred and fifteen adult patients (n = 115) were randomly recruited and a total of 70 participants were included in this sub study. The patients were stratified into 2 equal groups, that is with T2D and those with HTN comorbidity (T2D + HTN) (n = 35 per group). Patients with recent or active infections, pregnancy, malignant diseases or autoimmune diseases were excluded. All included patients underwent a general physical examination and clinical measurements were performed by a qualified health practitioner.
Laboratory measurements and clinical parameters
Venous blood (2-3 mL) was collected by a qualified registered nurse into ethylenediaminetetraacetic acid (EDTA), sodium fluoride, and serum separator tubes (BD; San Jose, USA). All laboratory tests were performed at the Namibia Institute of Pathology, an ISO 15189 accredited laboratory. The levels of glycated haemoglobin (Hb1Ac) and plasma glucose were measured the Cobas c501 analyser (Roche, Basel, Switzerland). The haematological indices were determined using the Sysmex 1000 XN automated haematology analyser (Sysmex Corporation, Kobe, Japan). The levels of CRP and serum globulins were measured using the Alinity c analyser (Abbot, Illinois, USA), whilst the Test 1 THL Alifax S.p.A (Alifax, Udine, Italy) was used to determine the erythrocyte sedimentation rate (ESR). We also calculated the NLR, albumin to globulins (A/G) ratio and the SII as surrogate markers of inflammation. The SII was calculated using the formula: SII = . 12
In order to assess the cardiovascular risk, we determined the lipid measurements (cholesterol and triglycerides levels) using the Alinity c analyser (Abbot, Illinois, USA). In addition, we estimated high-density lipoprotein cholesterol (HDL-c)/cholesterol ratio and AIP as surrogate markers of cardiovascular risk. The AIP was calculated using the formula; AIP = and the patients were stratified into 3 categories viz low, intermediate and high risk. 15
Statistical analysis
We estimated the minimum sample size using G*power software version 8.01 16 based on a previous study. 17 The following assumptions were made; the power of the study (1-βerr prob) was set at 0.95, an alpha value (αerr prob) of 0.05, an effect size (d) of 0.95 and group allocation ratio 1:1. The D’Agostino & Pearson test was used to test for normality. A two-tailed unpaired student’s t-test was used to compare the means between the groups for all parametric variables and data was presented as mean ± standard deviation (SD). The Welch’s test was also applied to correct for unequal variance. For non-parametric data, the Mann Whitney U test was used, and data were presented as median and interquartile range [IQR]. Correlation analyses were performed using the Spearman coefficient and the multivariable regression analysis was conducted to adjust for factors that explain the associations between AIP and NLR, SII, CRP, SBP and age variables. A P-value of <.05 was considered statistically significant. All statistical analyses will be performed using GraphPad Prism version 8 software version 8.0.2 (GraphPad Software Inc, CA, USA).
Results
Clinical characteristics of included participants
A total of 70 outpatients with T2D were included in the study. The participants were from the same socio-economic and ethnic background with a male-to-female ratio of 0.56 (Table 1). The duration of T2D differed significantly between the groups with the T2D + HTN group having a longer duration than the T2D group (P = .0212). All included participants were on anti-diabetic treatment and were mostly (91%) on metformin, and a few were on insulin or sulphonylureas or moduretic (Table 1). The T2D + HTN group had significantly elevated systolic blood pressure (SBP) (140.20 ± 21.24) and diastolic blood pressure (DBP) (88.66 ± 11.79) when compared to the T2D group (127.10 ± 15.07) (P = .0040) and (82.34 ± 9.17) (P = .0149), respectively (Table 1). The majority of the patients with HTN (58%) were in stage 2 with a SBP of ⩾140 mm/Hg and a DBP ⩾90 mm/Hg, and 20% had stage 1 (SBP: 130-139 mm/Hg and DBP: 80-89 mm/Hg). The remaining 8% and 18% of the patients had elevated (SBP: 120-129 mm/Hg and DBP < 80 mm/Hg) and normal (SBP < 120 mm/Hg and DBP <80 mm/Hg) blood pressures, respectively. Patients with stage 1 HTN had a mean age of 54.63 ± 11.34 years whilst those with stage 2 was 47.80 ± 12.13 years. Although the BMI was comparable between the 2 groups (P > .05), the participants in both groups were overweight (BMI > 25 kg/m2 (Table 1)).
Table 1.
Characteristics of included patients.
| Parameter | T2D (n = 35) | T2D + HTN (n = 35) | P-value |
|---|---|---|---|
| Clinical characteristics | |||
| Age | 42.71 ± 10.88 | 49.49 ± 10.67 | .0106 |
| Male n (%) | 13 (37%) | 10 (29%) | .4452 |
| Systolic blood pressure (mm/Hg) | 127.10 ± 15.07 | 140.20 ± 21.24 | .0040 |
| Diastolic blood pressure (mm/Hg) | 82.34 ± 9.17 | 88.66 ± 11.79 | .0149 |
| Body mass index (kg/m2) | 27.52 ± 5.13 | 28.96 ± 5.41 | .2552 |
| Duration of type 2 diabetes (years) | 3.69 ± 2.87 | 6.40 ± 6.10 | .0212 |
| <2 y n (%) | 8 (22.86%) | 10 (28.57%) | |
| 2-4 y n (%) | 17 (48.57%) | 8 (28.57%) | |
| >4 y n (%) | 10 (28.57%) | 17 (48.57%) | |
| Treatment | |||
| Metformin n (%) | 27 (77.1%) | 16 (45.71%) | |
| Insulin n (%) | 3 (8.57%) | 4 (11.43%) | |
| Metformin + Insulin n (%) | 5 (14.29%) | 7 (20.00%) | |
| Sulphonylurea n (%) | 0 (0%) | 4 (11.43%) | |
| Moduretic n (%) | 0 (0%) | 4 (11.43%) | |
| Glucose profiles | |||
| Glycated haemoglobin (%) | 8.58 ± 1.45 | 8.31 ± 1.54 | .4587 |
| Fasting plasma glucose (mmol/L) | 10.12 ± 3.55 | 9.53 ± 2.93 | .4454 |
| Haematological profiles | |||
| White cell count (109/L) | 6.10 ± 1.43 | 6.70 ± 1.41 | .0807 |
| Red cell count (1012/L) | 4.97 ± 0.45 | 4.74 ± 0.45 | .0364 |
| Platelets (109/L) | 293.30 ± 73.52 | 302.70 ± 31.48 | .5637 |
| Mean platelet volume (fL) | 10.80 ± 0.99 | 10.65 ± 0.84 | .4791 |
P-value <.05 was significant and is presented in bold.
Glucose and haematological profiles
The HbA1c and the plasma FPG levels were comparable between the groups (P > .05) and above the normal range despite the patients being on treatment (Table 1). An assessment of haematological indices showed a reduction in the red cell count in the T2D + HTN group (4.74 ± 0.45) when compared to the T2D group (4.97 ± 0.45), P = .0364. However, there was no significant difference in the white cell nor platelet counts between the 2 groups (P > .05) (Table 1).
Inflammatory profiles
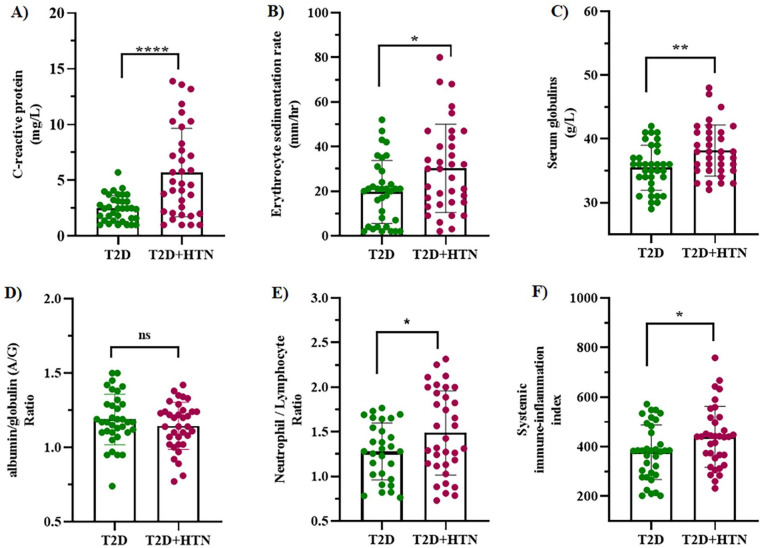
CRP is a sensitive and reliable marker of systemic inflammation that is associated with increased risk of HTN. In this study, we report elevated CRP levels in the T2D + HTN (5.71 ± 3.97) in comparison to the T2D group (2.48 ± 1.17), P < .0001 (Figure 1A). Similarly, the ESR was higher in the T2D + HTN (30.29 ± 19.78) when compared to the T2D group (19.66 ± 14.18), P = .0119 (Figure 1B). The serum globulins were elevated in the T2D + HTN group (38.14 ± 4.01) when compared to the T2D group (35.46 ± 3.53), P = .0004 (Figure 1C). However, there was no difference between the A/G ratio of the T2D + HTN group (1.14 ± 0.16) and the T2D group (1.19 ± 0.17), P = .2699 (Figure 1D). The assessment of the N/L ratio showed significant difference (P = .0494) between the T2D + HTN group (1.43 [1.13-1.94]) and the T2D (1.26 [1.03-1.51]) (Figure 1E). The SII of patients with T2D + HTN (439.30 ± 122.7) was significantly elevated in comparison to those with T2D (377.50 ± 110.20), P = .0298 (Figure 1F). The mean platelet volume/lymphocyte ratio (P = .8363) and the CRP to albumin ratio (0.1930) were comparable between the 2 groups.
Figure 1.
A comparison of inflammatory profiles between patients with type 2 diabetes (T2D) and those with T2D and hypertension comorbidity (T2D + HTN). The levels of C-reactive protein (A), erythrocyte sedimentation rate (ESR) (B), serum globulins (C), neutrophil/lymphocyte ratio (E) and systemic immune-inflammation index (F) were significantly higher in the T2D + HTN group when compared to the T2D group (P < .05). However, the albumin/globulins ratios (D) were comparable between the 2 groups (P > .05). All results are expressed as mean ± standard deviation except for the neutrophil/lymphocyte ratio which is reported as median interquartile range.
Abbreviation: ns, non-significant.
****P < .0001. **P < .001. *P < .05.
Assessment of lipid profiles and cardiovascular risk
In order to assess the risk of atherogenesis, we measured lipid profiles and determined the AIP of the included patients. The levels of triglycerides were higher in the T2D + HTN group (1.48 ± 0.54) when compared to the T2D (1.21 ± 0.37) group, P = .0177 (Table 2). Similarly, low-density lipoprotein cholesterol (LDL-c) levels were elevated in the T2D + HTN group (3.13 ± 0.89) when compared to the T2D (2.64 ± 0.84) group, P = .0189 (Table 2). However, total cholesterol, HDL-c levels and the HDL-c/cholesterol ratio were comparable between the 2 groups (P > .05) (Table 2). Since atherosclerosis is the hallmark of many CVD events such as stroke, myocardial infarction, coronary artery disease, and peripheral artery disease, the AIP of included patients was calculated. 18 There were no differences in the AIP scores between the 2 groups (P > .05), and 39% of the included patients had high risk AIP (Table 2).
Table 2.
Comparison of lipogram and cardiovascular risk in T2D versus those with T2D + HTN.
| Parameter | T2D (n = 35) | T2D + HTN (n = 35) | P-value |
|---|---|---|---|
| Lipid profiles | |||
| Triglycerides (mmol/L) | 1.21 ± 0.37 | 1.48 ± 0.54 | .0177 |
| Total cholesterol (mmol/L) | 4.60 ± 1.04 | 4.64 ± 0.76 | .8629 |
| LDL-cholesterol (mmol/L) | 2.64 ± 0.84 | 3.13 ± 0.89 | .0189 |
| HDL-cholesterol (mmol/L) | 1.05 ± 0.26 | 1.04 ± 0.26 | .9042 |
| HDL/Cholesterol ratio | 0.24 ± 0.07 | 0.22 ± 0.05 | .2705 |
| Trig/HDL ratio | 1.37 [0.95-2.14] | 1.48 [0.97-1.76] | .9326 |
| Cardiovascular risk | |||
| Atherogenic Index of plasma (Overall) | 0.11 ± 0.25 | 0.16 ± 0.23 | .4113 |
| AIP low risk (<0.11) n (%) | 16 (46%) | 13 (37%) | .4667 |
| AIP intermediate risk (⩾0.11, ⩽0.24) n (%) | 4 (11%) | 10 (29%) | .073 |
| AIP high risk (>0.24) n (%) | 15 (43%) | 12 (34%) | .4613 |
Abbreviations: HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol.
P-value <.05 was significant and is presented in bold.
Correlation and multivariable regression analysis of inflammatory and CVD risk profiles in patients with T2D
We performed correlations to determine whether there are any associations between age, duration of T2D, inflammatory profiles and CVD risk. The duration of T2D was directly associated with age (r = .321, P = .0068). The assessment of inflammatory profiles showed significant associations between CRP and age (r = .24, P = .0477), CRP and RBC (r = −.4, P = .0455), and SII and SBP (r = .33, P = .0056). As expected, there was a direct association between CRP and ESR (r = .27, P = .0234), SII and ESR (r = .28, P = .0176), globulins and ESR (r = .28, P = .0199), NLR and SII (r = .65, P < .0001), NLR and ESR (r = .25, P = .0461). There was no association between lipograms and inflammatory profiles (P > .05). We further assessed the significant predictors of AIP using NLR, SII, CRP, SBP and age in patients with T2D using multivariable regression analyses. Only NLR was a significant predictor of AIP (β = .287, SE: 0.1, P = .0046) (Table 3).
Table 3.
Multivariable linear regression analysis of predictors of atherogenic index of plasma in type 2 diabetes.
| Variable | Beta | Standard error | P-value |
|---|---|---|---|
| Neutrophil to Lymphocyte ratio | .287 | 0.100 | .0046 |
| Systemic Immune-Inflammation Index | −.001 | 0.000 | .0505 |
| C-reactive protein | .007 | 0.010 | .4470 |
| Systolic Blood Pressure | −.002 | 0.002 | .2350 |
| Age | −.004 | 0.003 | .1885 |
P-value <.05 was significant and is presented in bold.
Discussion
In this study, we explored routinely measured markers systemic inflammation in T2D and HTN comorbidity, and their association with atherogenicity. Our results show that HTN and T2D comorbidity is associated with an exaggerated systemic inflammatory state and cardiovascular risk. The NLR is the only significant independent risk factor for elevated cardiovascular risk in HTN and T2D comorbidity. Therefore, the modulation of inflammation and atherogenic dyslipidaemia is crucial in the primary prevention of CVEs, especially in patients at high risk.
Although all other indices were comparable in the assessment of complete blood count, red cell counts were lower in in the T2D + HTN group when compared to T2D group, albeit within normal range. This may be attributed to exacerbated inflammatory levels observed in the HTN comorbidity group which are associated with anaemia of chronic disease as we previously described.19,20 Patients with HTN and T2D comorbidity may need to be screened since they are at risk of developing overt anaemia. The NLR is a rapidly measurable biomarker that consolidates differential counts into 1 inflammatory variable that reliably stratifies patients into cardiovascular risk categories. 21 Patients with HTN and T2D comorbidity had significantly elevated NLR, which was associated with poor atherogenicity. The SII is another novel biomarker marker of systemic inflammation that further combines the NLR with the platelet count. 22 A recent systematic review and meta-analysis showed that elevated SII is associated with a broad range of CVEs, including ischaemic stroke, myocardial infarction, and arterial disease. 23 However, it remained to be elucidated whether this is applicable to patients with T2D and HTN commodity. Here, we report high SII in patients with T2D + HTN which positively correlated with SBP, thus alluding to the findings of the meta-analysis. Although the underlying mechanisms in the pathogenesis of CVEs may be multifactorial, systemic inflammation seems to play a central role in the development and progression of CVD in patients with T2D. 24 This is further supported by increased CRP levels, ESR and serum globulins in the HTN comorbidity group when compared to the T2D. The CRP to albumin ratio and the mean platelet volume/lymphocyte ratio have been well described as excellent inflammatory predictors in patients with diabetic nephropathy.25,26 However, in this study, the ratios were comparable between the 2 groups. The efficacy of the ratios therefore, seems to be dependent on the T2D-associated complication.
CVEs are number one cause of death in patients with T2D, thus it is important to stratify patients according to their cardiovascular risk. A staggering two-thirds of patients with T2D have HTN comorbidity. 27 The elevated arterial blood pressure in HTN contributes to the increased incidence of CVEs in these patients. 28 Upon activation of the renin-angiotensin-aldosterone system (RAAS), there is initiation of several neuro-hormonal responses that induce vasoconstriction, activation of the endothelium and ultimately HTN. 29 In inflammatory conditions, the activation of RAAS is exacerbated, and this results in increased release of angiotensin II, a proatherogenic hormone that promotes the secretion of proinflammatory cytokines, adhesion molecules and reactive oxygen species.30,31 These factors induce vascular inflammation, such as endothelial dysfunction and promote atherosclerosis. 32 The infiltration of activated T-cells and monocytes/macrophages in the atherosclerotic plaque promotes foam cell formation which with the help of angiotensin II, fuels vascular LDL oxidation. 33 The latter plays a huge role in dyslipidaemia, a common feature in T2D that is characterised by elevated TG and LDL-c coupled with reduced levels of HDL-c. 34 In this study, we report elevated levels of the atherogenic LDL-c and triglycerides in patients with the HTN comorbidity. Since this dyslipidaemia is closely associated with obesity and CVEs in patients with T2D, 35 we calculated and assessed the AIP. Although the values were comparable between the 2 groups, it is worth noting that almost two-thirds of the patients with HTN comorbidity had an intermediate to high AIP. This finding is important since it is a surrogate marker of atherosclerosis, a hallmark of many CVD events such as stroke, myocardial infarction, coronary artery disease, and peripheral artery disease.36,37
The cross-sectional nature of this study was a limitation since the design lack follow-up examinations of included patients. As a result, causal effect between HTN and reported outcomes could therefore not be established. A longitudinal study will be required in stratifying the cardiovascular risk over time. Other novel leucocyte and thrombocyte indexes reported elsewhere 38 may need to be analysed in patients with T2D and HTN comorbidity. Nonetheless, in this study, we report on systemic inflammation that is associated with cardiovascular risk and NLR being a routinely measured reliable biomarker to predict atherogenicity in T2D.
Conclusion
The coexistence of HTN in T2D is characterised by aggravated systemic inflammation which is closely associated with elevated atherogenicity. Although the underlying mechanisms in the pathophysiology of CVD in these patients may be multifactorial, it is apparent that low-grade inflammation plays a central role in the process. Therefore, understanding the role of chronic inflammation is important in the designing of therapeutic strategies that aim to reducing the incidence of CVEs. Most importantly, the NLR is a reliable independent predictor of increased atherogenicity in patients with T2D. This finding is extremely relevant, particularly in developing countries where resources are scare, as the parameter can be derived from routinely measured test.
Acknowledgments
None.
Footnotes
ORCID iD: Tawanda M Nyambuya  https://orcid.org/0000-0002-3288-9524
https://orcid.org/0000-0002-3288-9524
Declarations
Ethics Approval and Consent to Participate: The study was approved by the Namibia University of Science and Technology Ethics committee (FHAS 1/2020) and the Namibia Ministry of Health and Social Services (17/3/3 MN). All included participants provided written informed consent.
Consent for publication: Not applicable.
Author contributions: EJG and TMN conceptualised, designed the study, and drafted the manuscript. EJG, BBN and TMN performed formal analysis, methodology and validation as well as visualisation. All authors reviewed, edited, and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Namibia University of Science and Technology (NUST), Grant Number: MG/2017; and NUST Postgraduate Research Fund (EJG/2020).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data and Materials: All data and materials are included in the manuscript.
References
- 1. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34: 575-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leggio M, Lombardi M, Caldarone E, et al. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res. 2017;40:947-963. [DOI] [PubMed] [Google Scholar]
- 3. Barrows IR, Ramezani A, Raj DS. Inflammation, immunity, and oxidative stress in hypertension—partners in crime? Adv Chronic Kidney Dis. 2019;26:122-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yen FS, Wei JC, Chiu LT, Hsu CC, Hwu CM. Diabetes, hypertension, and cardiovascular disease development. J Transl Med. 2022;20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pourfarzam M, Zadhoush F, Sadeghi M. The difference in correlation between insulin resistance index and chronic inflammation in type 2 diabetes with and without metabolic syndrome. Adv Biomed Res. 2016;5:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lan Y, Chen G, Wu D, et al. Temporal relationship between atherogenic dyslipidemia and inflammation and their joint cumulative effect on type 2 diabetes onset: a longitudinal cohort study. BMC Med. 2023;21:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patrick DM, Van Beusecum JP, Kirabo A. The role of inflammation in hypertension: novel concepts. Curr Opin Physiol. 2021;19:92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H, Yang Z, Zhang W, et al. White blood cell subtypes and risk of type 2 diabetes. J Diabetes Complications. 2017;31:31-37. [DOI] [PubMed] [Google Scholar]
- 10. Hou M, Cao L, Ding Y, et al. Neutrophil to lymphocyte ratio is increased and associated with left ventricular diastolic function in newly diagnosed essential hypertension children. Front Pediatr. 2021;9:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo W, Song Y, Sun Y, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011-2018. Front Endocrinol. 2022;13:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S14-S31. [DOI] [PubMed] [Google Scholar]
- 14. World Medical Association. World medical association declaration of helsinki ethical principles for medical research involving human subjects. JAMA. 2013; 310:2191-2194. [DOI] [PubMed] [Google Scholar]
- 15. Bo MS, Cheah WL, Lwin S, et al. Understanding the relationship between atherogenic index of plasma and cardiovascular disease risk factors among staff of an university in Malaysia. J Nutr Metab. 2018;2018:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-191. [DOI] [PubMed] [Google Scholar]
- 17. Govind S, Saha S, Brodin LA, et al. Impaired myocardial functional reserve in hypertension and diabetes mellitus without coronary artery disease: searching for the possible link with congestive heart failure in the myocardial doppler in diabetes (MYDID) study II. Am J Hypertens. 2006;19:851-857. [DOI] [PubMed] [Google Scholar]
- 18. Zhu X, Yu L, Zhou H, et al. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids Health Dis. 2018;17:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ndevahoma F, Nkambule BB, Dludla PV, et al. The effect of underlying inflammation on iron metabolism, cardiovascular risk and renal function in patients with type 2 diabetes. EJHaem. 2021;2:357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ndevahoma F, Mukesi M, Dludla PV, et al. Body weight and its influence on hepcidin levels in patients with type 2 diabetes: a systematic review and meta-analysis of clinical studies. Heliyon. 2021;7:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He J, Bian X, Song C, et al. High neutrophil to lymphocyte ratio with type 2 diabetes mellitus predicts poor prognosis in patients undergoing percutaneous coronary intervention: a large-scale cohort study. Cardiovasc Diabetol. 2022;21:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212-6222. [DOI] [PubMed] [Google Scholar]
- 23. Ye Z, Hu T, Wang J, et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5:444-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zahid Kocak M, Aktas G, Erkus E, et al. Mean platelet volume to lymphocyte ratio as a novel marker for diabetic nephropathy. J Coll Physicians Surg Pak. 2018; 28:844-847. [DOI] [PubMed] [Google Scholar]
- 26. Bilgin S, Kurtkulagi O, Atak Tel BM, et al. Does C-reactive protein to serum albumin ratio correlate with diabEtic nephropathy in patients with type 2 dIabetes mellitus? The CARE TIME study. Prim Care Diabetes. 2021;15:1071-1074. [DOI] [PubMed] [Google Scholar]
- 27. Pavlou DI, Paschou S, Anagnostis P, et al. Hypertension in patients with type 2 diabetes mellitus: targets and management. Maturitas. 2018;112:71-77. [DOI] [PubMed] [Google Scholar]
- 28. Adler AI, Stratton IM, Neil HAW, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. Br Med J. 2000;321:412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gallo G, Volpe M, Savoia C. Endothelial dysfunction in hypertension: current concepts and clinical implications. Front Med. 2022;8:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques potential implications for inflammation and plaque instability. Circulation. 2000;101:1372-1378. [DOI] [PubMed] [Google Scholar]
- 31. Pueyo ME, Gonzalez W, Nicoletti A, et al. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-κB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645-651. [DOI] [PubMed] [Google Scholar]
- 32. Ekholm M, Kahan T. The impact of the renin-angiotensin-aldosterone system on inflammation, coagulation, and atherothrombotic complications, and to aggravated COVID-19. Front Pharmacol. 2021;12:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019; 124:315-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pitso L, Mofokeng TRP, Nel R. Dyslipidaemia pattern and prevalence among type 2 diabetes mellitus patients on lipid-lowering therapy at a tertiary hospital in central South Africa. BMC Endocr Disord. 2021;21:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard lecture 2009. Diabetologia. 2010;53: 1270-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Z, Huang Q, Sun L, Bao T, Dai Z. Atherogenic index in type 2 diabetes and its relationship with chronic microvascular complications. Int J Endocrinol. 2018; 2018:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fu L, Zhou Y, Sun J, et al. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klisic A, Scepanovic A, Kotur-Stevuljevic J, Ninic A. Novel leukocyte and thrombocyte indexes in patients with prediabetes and type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2022;26:2775-2781. [DOI] [PubMed] [Google Scholar]