Abstract
A chordoma is a slow growing, locally invasive, low-grade tumor belonging to the sarcoma family. It mainly affects the sacrum and skull base. We present a case of thoracic chordoma initially presented with epidural hematoma (EDH), which is a rare clinical entity. We reported this case, and also performed a PRISMA-driven systematic review to summary the similar cases in the literature. This review includes the clinical characteristics and outcome of thoracic chordoma. Our case involves a 60-year-old male who, despite no history of trauma, presented with acute paraparesis. An epidural hematoma was identified at T6 level, leading to a surgical intervention involving T4-6 laminectomy and fixation. Six months subsequent to surgery, the patient experienced progressive lower limb weakness and spasticity. Computed tomography (CT) exhibited erosion of T6 and an associated aggressive mass. Magnetic resonance imaging (MRI) revealed a large heterogenous soft tissue mass arising from the vertebral body and right pedicle of D6, protruding in the epidural space and compressing the spinal cord focally at this level. The mass measured approximately 5 × 4 × 3.5 cm. Magnetic resonance myelography indicated a filling defect at T5–6 level, confirming the intraspinal location of the soft tissue lesion. Complete excision of the mass confirmed the diagnosis of thoracic chordoma. Postoperative follow-up demonstrated notable improvement in the lower limb spasticity and paraparesis, and the patient started adjuvant radiotherapy. This case underscores the importance of maintaining a high index of suspicion when evaluating presentations resembling EDH.
Keywords: Chordoma, thoracic spine, epidural hematoma, surgery, case report
Introduction
Chordoma is a rare malignant tumor arising from embryonic notochord remnants. 1 It occurs mainly in the sacrum, base of the skull, particularly the clival region.2-4 Thoracic chordoma accounts for only 1% of all chordomas, it is characterized by slow growth, local invasion, and a high recurrence rate. 5 The clinical presentation of thoracic chordoma may include back pain, spinal deformity, neurological deficits, and respiratory symptoms. 3 The diagnosis of thoracic chordoma is based on radiological and histopathological findings and molecular markers. 5 The optimal treatment for thoracic chordoma is complete surgical resection with negative margins, which can improve survival and quality of life. 3 However, the surgical approach is challenging due to the complex anatomy and proximity to vital structures. 3 Adjuvant therapies such as radiation and chemotherapy have limited effectiveness and are mainly used for palliation or prevention of recurrence. Therefore, new therapeutic strategies based on targeted therapy and immunotherapy are being explored to improve the outcomes of patients with thoracic chordoma. 5 Here, we report a rare case of a thoracic chordoma, which was initially manifested with epidural hematoma (EDH) causing acute motor deficit. Moreover, we conducted a PRISMA-driven systematic review previous studies reporting thoracic chordomas.
Case Presentation
A 60-year-old man who was previously healthy presented with acute paraparesis. He reported no history of trauma or fever. Clinical examination showed bilateral lower limb weakness (2/5 Medical Research Council grade) without muscle atrophy and preserved deep tendon reflexes. Urgent CT and MRI showed an abnormal epidural density/intensity at the level of D6 (Figure 1A and B), requiring urgent surgical intervention to alleviate spinal cord compression. Intraoperatively, an epidural hematoma was recognized and evacuated. Short segment fusion at the levels of T5−T7 was performed (Figure 1C). Postoperatively, muscle power was restored, and the pain improved. Two months post-surgery, the fixation system was removed due to failure, likely due to technical factors. The patient was advised to wear a dorsolumbar support.
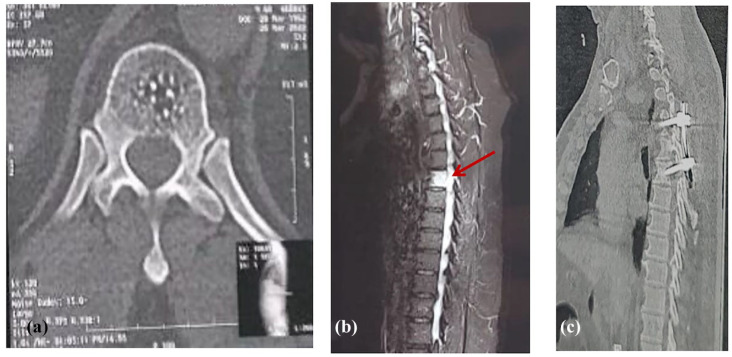
Figure 1.
(A) Pre-operative selected axial CT image at the level of T6 “bone window” demonstrating a subtle poorly-defined abnormal density within the vertebral body, with multiple foci of intra-lesional calcification. (B) Pre-operative sagittal MRI spine, T2 fat-saturated image demonstrating high T2 signal abnormality within the vertebral body of T6. No vertebral body compression, collapse or destruction seen. There is another abnormality of focal intraspinal high T2 signal abnormality at the same level “the arrow,” which represents the EDH. Unfortunately, the T1-sequence “not shown here” was of low imaging quality due to patient-related motion artifact. The hematoma was confirmed intra-operatively. At this point, the Radiology Neurosurgery team interpreted the vertebral body lesion as a hemangioma, with a small extradural hematoma at the same level. Due to the acute presentation of the patient, the decision was made to take the patient to the OR to evacuate the extradural hematoma. (C) A postoperative sagittal CT image “bone window” showing a short-segment vertebral fixation at the levels of T5–T7.
Six months post-surgery, the patient showed bilateral lower limb weakness (3/5 Medical Research Council grade). Spinal MRI showed a heterogenous 5 × 4 × 3.5 cm soft tissue mass from the vertebral body and right pedicle of T6, protruding in the epidural space and compressing the spinal cord. Magnetic resonance myelography demonstrated significant compression of the cord (Figure 2).
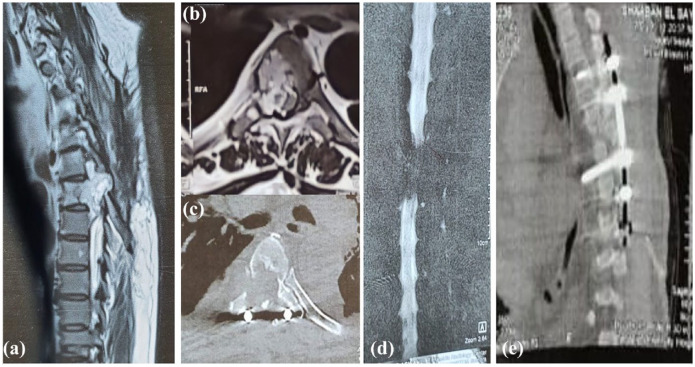
Figure 2.
(A) A sagittal T2W MRI spine of the same patient 6 months postoperative demonstrating significant progression of the signal abnormality in the vertebral body of T6. This is associated with a large heterogeneously high T2 soft tissue component bulging into the epidural spinal canal with extensive compression of the spinal cord and right neural foramen at the same level. There is also minimal caudal migration to the level of mid D7. (B) axial T2W MRI & (C) an axial CT at the T6 level showing the previously described signal abnormality, extending to the right pedicle and lamina with corresponding bony erosion well-demonstrated in the provided CT image. The MRI shows significant compression of the spinal cord, which is compressed and displaced to the left side, but with no intramedullary signal abnormality. (D) A coronal image of MR myelography showing a significant filling defect at the level of T6 and T7 confirming the intraspinal extension of the lesion and significant cord compression. (E) A postoperative sagittal CT image of the dorsal spine demonstrating interval longer fixation of the vertebrae at the levels of T4, T5 and T7, T8.
Under general anesthesia, decompression and mass excision were performed, followed by long-segment fixation via a posterior approach. The fixation consisted of segmental rod-screw constructs 2 levels above (T4, T5) and 2 levels below (T7, T8) (Figure 2E). Histopathological examination confirmed chordoma diagnosis (Figure 3). The patient was referred for radiotherapy and scheduled for regular follow-up. Complete resolution of his symptoms was reported, with no neurological deficits.
Figure 3.
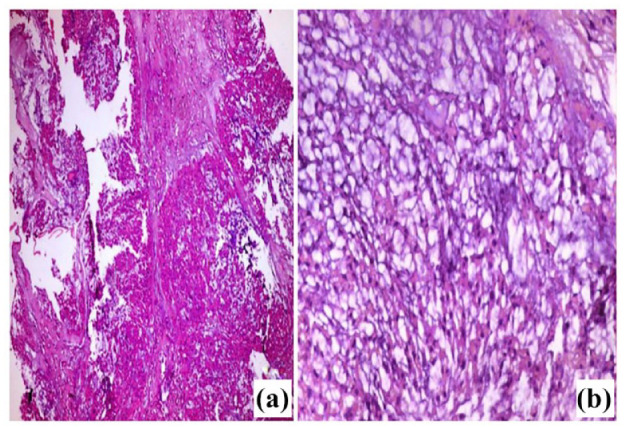
Light microscopic picture of chordoma (A) Tumor with multilobulated growth pattern of epithelioid cells. The lobules showed anastomosing nests or cords of acidophilic tumor cells. (B) Higher magnification of the tumor showing cords of acidophilic, vacuolated small and medium-sized cells with rounded nuclei, most of which had low-grade atypia. The tumor cells arranged in a basophilic fibromyxoid stroma, with no evidence of necrosis. H&E (A) 100x, (B) 400x.
Methods
PRISMA-driven systematic review for thoracic chordoma
Search strategy of systemic reviews
We conducted an extensive literature review was performed following the guidelines published for PRISMA. 6 An expansive computerized systemic review of published randomized controlled trials, cohort studies, and case reports, was performed by searching the following 3 databases: Scopus, PubMed, Science Direct. The key search terms included “chordoma,” “thoracic,” “spinal cord” and “tumor.” The search was limited to human studies and full text, written in English, published in English until May 22, 2022.
Screening and data extraction
The above-mentioned search strategy was completed by May 2022. Two independent investigators reviewed the relevance of all titles and abstracts identified from the different databases. The opinion of a third investigator was considered in articles with discrepancies. Full articles were further assessed when the abstracts met the inclusion criteria. The reviewed data were obtained and entered onto each reviewer’s ad hoc standardized data entry form. We compared the inclusion data for the year of publication, the number of patients studied, the sex and age of every patient, the initial presenting symptoms, the vertebral level of chordoma, the surgical approach deployed, any adjuvant radio or chemotherapy, the outcome of the patient, the follow-up period and the incidence of recurrence.
Assessment of methodological quality
The methodological quality of the included studies was assessed using the Newcastle–Ottawa Quality Assessment Scale, which scores from 1 (poor) to 9 (excellent). 7 Due to the lack of standardized descriptions for intermediate scores on this scale, we categorized studies with a total score of 7 or higher as high-quality.
Results
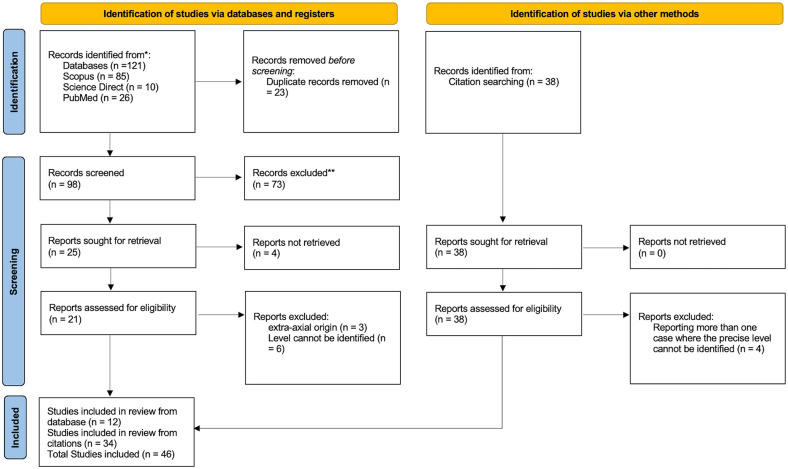
Our search yielded 160 citations. After reviewing the title and abstract and the entire text, 113 were discarded as they did not meet the proposed criteria. In total, 46 papers were identified for review, as illustrated in the PRISMA flow diagram (Figure 4). The clinical characteristics of the included articles and the current case report are summarized in Table 1 where 54 patients including the current case were described. The age ranged between 8 and 89, with a mean of 46.8 (20.5) years. Male patients slightly outnumbered female patients (=28; 51.9%, vs 26; 46.3%), and the sex of 1 patient was not mentioned. The most frequently reported tumor level was T3 (n = 18; 33.3%), followed by T2 (n = 15; 27.8%) and T4 (n = 14;25.9%). T8 and T9 were the least frequently reported levels in 2 patients (3.7% for each). Associated cervical involvement was reported in 3 patients, and in 1 patient, the sacral segments were involved. In 9 patients, the tumor was discovered incidentally on chest radiography. Sensory affection was the predominant complaint. Surgical resection was conducted in most studied patients (n = 50; 92.6%). However, in 1 patient, the tumor was too extensive for surgical treatment. Another patient was still under evaluation for surgery, and we could not identify if other 2 patients underwent surgical procedures.
Figure 4.
PRISMA flow diagram for systematic review of thoracic chordoma.
Table 1.
The characteristics of included studies.
| Study number | Study, et al. | Number | Sex | Age | Level | Initial presentation | Surgical approach | Chemotherapy | Radiotherapy | Outcome | Death | Residual symptoms and/or recurrence | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | The current study | 1 | Male | 60 | T6 | Acute paraparesis and bilateral lower limb weakness | Decompression and complete surgical excision followed by fixation | No | Yes | Favorable | No | No | 1 y |
| 2 | Willatt et al 8 | 1 | Female | 72 | T2−T3 | 2-year of progressive pain in the upper thoracic spine radiating to the left arm and leg to her left arm and leg. She was non-responsive to pain relief but had no functional deficits |
Robot-assisted para-vertebral en-bloc tumor resection | No | No | Favorable | No | No | |
| 3 | Dokponou et al 9 | 1 | Male | 56 | T1−T3 | Dorsal back pain, LL fatigue, intermittent claudication complicated by bladder and bowel disturbances | Laminectomy with en bloc resection of the lesion | No | No | Favorable | No | No | 6 mo |
| 4 | Mihara et al 10 | 1 | Male | 75 | T2−T3 | 3-month history of progressive bilateral lower extremity numbness, hypesthesia, and spastic gait. | Laminectomy and complete resection of the tumor in a piece-by-piece manner | NM | NM | Favorable | No | Spasticity | 3 y |
| 5 | Kassels et al 1 | 1 | Female | 36 | T2−3 | Chest pain, dizziness, abdominal pain | Rt sided video-assisted thoracoscopic surgical resection | No | Yes | Favorable | No | No | 16 mo |
| 6 | Moune et al 11 | 1 | Male | 56 | T12 | T12-dorsal/intercostal pain | Laminectomy for tumor debulking, followed by gross total en bloc resection with fusion | Yes | Yes | Unfavorable | No | Recurrence | 2 y |
| 7 | Mahmoud et al 12 | 1 | Female | 18 | Extending from the left lower neck into the upper anterior mediastinum (cervicothoracic vertebrae) | Progressively worsening shortness of breath and dysphagia. | Extensive palliative debulking | No | Yes | Favorable | No | No | NM |
| 8 | Yoon et al 13 | 1 | Female | 52 | T4–5 | 3-month history of dysphagia | Video-assisted thoracotomy | Yes | Yes | Favorable | No | No | 1 y |
| 9 | Costanzo et al 14 | 1 | Male | 62 | T3−T4 | Thoracic pain, gait disturbance, bilateral lower limb weakness, and paresthesias below T2 level | Aggressive transthoracic resection of tumor | No | No | Favorable | No | No | 6 mo |
| 10 | Sobash et al 15 | 1 | Female | 62 | Right lung apex at levels of C7 to T2 | Right arm and hand numbness with a history of previous carpal tunnel release. | Incomplete resection | No | Yes | Unfavorable | No | Recurrence and regrowth of residual | 8 mo |
| 11 | Leary et al 16 | 2 | Male | 70 | T10–T12 | NM | 2-staged approach of en bloc resection and anterior column reconstruction with segmental fixation and fusion. | NM | NM | NM | NM | NM | NM |
| Male | 68 | C6−T4 | NM | A anterior trapped-door thoracotomy and parasagittal osteotomies followed by en bloc resection of the tumor, fixation and fusion | NM | NM | NM | NM | NM | NM | |||
| 12 | Supreeth et al 17 | 1 | Female | 52 | T4−5 | Incidental finding of posterior mediastinal mass with no significant medical history | VATS-assisted thoracotomy for mass excision and vertebral body en-bloc resection | Yes | No | Favorable | No | No | 1 y |
| 13 | Bai et al 18 | 1 | Male | 73 | Sacral (S1, S2) and thoracic (T11) | Intermittent lumbosacral pain and root pain of the lower limbs, which severely affected his gait and gradually led to difficulty in defecating and urinating | Posterior decompression followed by extended resection | No | No | Favorable | No | No | 24 mo |
| 14 | Liu et al 19 | 1 | Male | 64 | T5, T6 | 4-month history of continuous and progressive back pain | The patient underwent one-stage thoracoscopic release of vertebral and paravertebral tumors. | No | No | Favorable | No | No | 6 mo |
| 15 | Goomany et al 20 | 1 | Male | 46 | T10−T12 | 18-month history of progressively worsening thoracic back pain |
En bloc resection of a thoracic chordoma via a minimally invasive extreme lateral interbody fusion approach | No | No | Favorable | No | No | 8 y |
| 16 | Faheem et al 21 | 1 | Male | 8 | T11−L1 | Insidious onset, non-traumatic low back ache of 1-year duration along with paraparesis and urinary incontinence of 3 mo duration. |
Laminectomy. The tumor was decompressed with the help of a cavitron ultrasonic aspirator, and near-total excision was performed. A watertight dural closure was performed | NM | Yes | Favorable | No | Urinary incontinence. Lower limb muscle weakness | 10 mo |
| 17 | Pu et al 22 | 1 | Male | 41 | T10−L2 | Progressive low back pain at T10-L2 for 2 y. | Complete resection of the tumor and internal fixation of the vertebral bodies | No | No | Favorable | No | No | 30 mo |
| 18 | Kim et al 23 | 1 | Male | 60 | T7, T8, T9 | Unsuccessful attempted resection of a left-sided paraspinal mass spanning the level T7−T9 for a presumed diagnosis of schwannoma. | 2-staged en bloc surgical resection | No | No | Favorable | No | No | 1 y |
| 19 | Rena, et al 24 | 1 | Female | 69 | T2, T3 | Incidentally diagnosed in on routine chest X-ray as a left paravertebral mass. 8 y later she was presented with acute back pain associated with increasing paraparesis. | Thoracotomy and staged decompressive laminectomy | No | No | Favorbale | No | No | 30 d |
| 20 | Royo Crespo et al 25 | 1 | Male | 52 | T5, T6 | 6-month history of dorsal thoracic pain | Laminectomy and en bloc excision resection of the tumor, together with reconstruction | Yes | Yes | Favorable | No | No | 7 d |
| 21 | Conzo et al 26 | 1 | Male | 47 | T1, T2 | An asymptomatic patient incidentally misdiagnosed as a paraganglioma | Radical removal through a right extended cervicotomy | No | No | Favorable | No | No | 18 mo |
| 22 | Matsubayashi et al 27 | 1 | Female | 47 | Paravertebral thoracic posterior mediastinal not invading the bone | Incidentally detected on a chest radiograph | Surgically removal with thoracoscopic assistance | No | No | Favorable | No | No | 5 mo |
| 23 | Fernández Carballal et al 28 | 1 | Female | 31 | T1−T2 | 8-week history of gait disturbance. | Surgical resection using a laminectomy and complete facetectomy | NM | Yes | Favorable | No | No | 6 mo |
| 24 | Fontes and O’Toole 5 | 1 | Male | 89 | T10 | Incapacitating pain and early signs of thoracic myelopathy. | Intralesional posterolateral resection and reconstruction | No | Yes | Favorbale | No | No | 13 mo |
| 25 | Delgado et al 29 | 1 | Female | 68 | T7 | Moderate to intense pain in the upper abdomen, irradiating to the flanks, followed by weakness of the lower limbs, rapidly evolving to paralysis and urinary incontinence. Around admission, the patient presented flaccid paraplegia, global anesthesia below T4 and atonia of the anal sphincter. | Initial right costotransversectomy to decompress the fracture and remove material for pathological study followed by a trans pleural thoracotomy for corpectomy and anterior arthrodesis | NM | NM | Unfavorable | Yes | Circulatory instability and ischemia of the right hand followed by necrosis and amputation of the first, second and third finger followed by sepsis | <1 mo |
| 26 | Miyazawa et al 30 | 1 | Male | 37 | Thoracic vertebrae | Back pain | Surgical resection | No | Yes | Favorable | No | Backache and recurrence | 15 mo |
| 27 | Wang et al 31 | 1 | Female | 25 | T1−T5 | Incidentally, a huge upper mediastinal mass was found on chest radiographic examination. The only symptom probably associated with the mass was anhidrosis of her left hand. | The patient is still under evaluation for surgery. | NM | NM | NM | NM | NM | NM |
| 28 | Van Kollenburg et al 32 | 1 | Female | 51 | T3−T4 | Paresthesia in the face and the upper thoracic area, dizziness, transpiration, and other mild signs of autonomic system disorders. Vague pain in the thoracic spine region and both legs for 1 y | Preoperatively embolization followed by palliative anterior resection with decompression of the spinal cord. Next, en bloc spondylectomy with the left second rib resection, followed by anterior fusion. | No | Yes | Favorable | No | Minor neurological symptoms and pain at the site of operation | 2 y |
| 29 | Bisceglia et al 33 | 1 | Male | 70 | T9 | 6-month history of backache, neurological symptoms and bilateral lower limb weakness and the patient was misdiagnosed as poorly differentiated metastatic large cell carcinoma was. 3 y later he was presented with back pain and hyposthenia of both lower extremities recurred, with the severity of the symptoms markedly increasing and progressing to complete paraparesis with bladder and bowel dysfunction. He was misdiagnosed as an irradiated metastatic adenocarcinoma to the thoracic vertebra | Bilateral laminectomy followed 3 y later by second palliative surgery for subtotal piecemeal removal of the tumor followed by reconstruction of the bony defect and stabilization | No | Yes | Unfavorable | Yes | Recurrence | 5 mo |
| 30 | Selvaraj and Wood 34 | 1 | Female | 33 | T2−T3 | Incidental finding on chest X-ray of bilobed paravertebral mass in the upper mediastinum. She subsequently developed swelling and redness of the face on lifting her hands to dress her hair and tingling sensation along the medial side of the left forearm. | Initial excision biopsy through thoracotomy followed by re-admission for a left posterolateral thoracotomy where the mass was excised. A third procedure was undertaken to excise the remnant of tissue. | No | No | Favorable | No | No | 6 |
| 31 | Smolders et al 35 | 1 | NM | NM | T7 | NM | Not mentioned | NM | NM | NM | NM | NM | NM |
| 32 | Topsakal et al 36 | 1 | Female | 44 | Backache, lower limbs weakness and sphincter incontinence | Total resection combined with anterior interbody fusion through left trans pleural transthoracic approach | No | No | Favorable | No | Recurrence | 2 y | |
| 33 | Pai 37 | 1 | Male | 49 | T2−T3 | Pins and needles sensations from the chest down and unsteadiness on feet. Thoracic backache for many months and the spine had been manipulated by an osteopath on several occasions. Worsening of back pain in the days before being seen. | The tumor was too extensive for surgical treatment | Yes | Yes | Unfavorable | No | Grade II weakness in the lower limbs (wheelchair-bound with) and urinary incontinence. | 2 mo |
| 34 | Murphy et al 38 | 1 | Female | 39 | T1, T2, T3 | Gradual onset of hoarseness, dysphagia and increasing shortness of breath | Surgical resection of the tumor | No | No | Unfavorable | Yes | 10 d | |
| 35 | Holden et al 39 | 1 | Male | 20 | T4, T5 | 4-month history of worsening numbness in both thighs and a 1-month history of worsening ataxia | Thoracic laminectomy and uncomplicated removal of the tumor. | No | No | Favorable | No | No | NM |
| 36 | Levowitz et al 40 | 1 | Male | 64 | T1−T6 | Nonproductive cough of 3 mo duration. For many years the patient experienced a dull substernal pain unrelated to activity and occasional episodes of dysphagia. Later on he was admitted for pain in the right upper arm and cervical region of 1 month’s duration. The third admission was with bilateral upper arm pain associated with motor and sensory impairment involving the left hand. | Right thoracotomy | Yes | Yes | Unfavorable | No | Recurrence | 8 y |
| 37 | Suster and Moran 41 | 6 | Male | 8 | T7−T7 | Difficulty in swallowing | Piecemeal resection | No | No | Unfavorable | Yes | Recurrence | 3 y |
| Female | 14 | T1−T4 | Incidental X-ray finding secondary to sports injury | Surgical excision | No | No | NM | No | No | LFU | |||
| Female | 33 | Chest discomfort | Complete surgical excision | No | No | Favorable | No | No | 16 y | ||||
| Male | 61 | Chest pain, SOB, left pleural effusion | Partial excision | No | No | Unfavorable | Yes | Recurrence | 1 y | ||||
| Female | 63 | T5−T6 | Backache and SOB for 1-year | Complete surgical excision | No | No | NM | No | No | LFU | |||
| Male | 65 | T3−T4 | Chest discomfort and SOB | Complete surgical excision | No | Yes | Favorable | No | No | 3 y | |||
| 38 | Taki et al 42 | 1 | Female | 15 | Left side of the upper posterior mediastinum | Asymptomatic with only left side Horner syndrome, and when chest radiograph was taken, it was incidental finding | Subtotal resection | NM | NM | NM | No | NM | NM |
| 39 | Ahrendt and Wesselhoeft 43 | 1 | Male | 9 | T3 | Right-sided chest pain during an upper respiratory infection. Chest x-ray demonstrated a right posterior mediastinal mass. | An exploratory thoracotomy and complete tumor excision leaving no evidence of residual gross disease | No | Yes | Favorable | No | No | 4 y 8 mo |
| 40 | Walsh and Mayer 44 | 1 | Female | 69 | T11 | Paresthesia from the back to mid waist | Right-sided transthoracic partial resection then posterior approach for total resection | No | Yes | Favorable | No | No | 7 y |
| 41 | Amendola et al 45 | 1 | Female | 66 | Thoracic vertebrae | Pain and soft tissue mass | Subtotal resection | No | Yes | Unfavorable | Yes | 6.5 y | |
| 42 | Ramiro et al 46 | 1 | Female | 33 | T4 | 3-month history of pain across the shoulders and progressive disturbance of gait. | Laminectomy and complete resection | NM | NM | Favorable | No | No | 1 y |
| 43 | Schwarz et al 47 | 1 | Male | 59 | T8 | Back pain and bilateral lower limb weakness | Initial posterior decompression, followed by left trans pleural transthoracic subtotal resection | No | Yes | Favorable | No | Expected to do well due to aggressive resection and lack of MetS | NM |
| 44 | Cotler et al 48 | 1 | Female | 14 | T3, T4, T5 | Whooping cough for 2 wk, then incidentally diagnosed when a roentgenogram was requested. | Transthoracic excision of the tumor and excision of anterior aspects of affected vertebrae | NM | NM | Favorable | No | No | 3 y |
| 45 | Clemons et al 49 | 1 | Female | 14 | Upper thoracic vertebrae | Asymptomatic incidentally diagnosed after sports injury, an X-ray revealed a right superior posterior mediastinal mass | Right thoracotomy and surgical resection. | No | No | NM | NM | NM | LFU |
| 46 | Burauzewski and Rudowski 50 | 2 | Male | 34 | T3, T4, T5 | 4 y earlier, the patient had received X-ray treatment because of pains in the chest, which had been present at that time for about a year. 4 y later symptoms reappeared and gradually increased. after radiotherapy and refusal of surgery, 6 mo later, he was brought with paralysis of the lower limbs | Laminectomy and decompression | No | Yes | Unfavorable | Yes | Acute circulatory failure | 2 d |
| Male | 37 | T4 | Pains in the chest located in the lower left scapular area, followed a month later by pulmonary hemorrhages, disturbances of gait, and sensory changes in the lower limbs. | Laminectomy | NM | NM | Favorable | No | No | NM | |||
| 47 | Crowe and Muldoon 51 | 1 | Male | 30 | NM | Cough, anemia, weight loss, and right arm deficit | NM | NM | NM | NM | NM | NM | NM |
Abbreviations: LFU, lost to follow-up; NM, not mentioned; SOB, shortness of breathing.
Six patients underwent chemotherapy (11.1%), 35 patients did not (64.8%), and in 13 patients, the use of chemotherapy was not mentioned. Moreover, 21 patients underwent radiotherapy (38.9%), 22 patients (40.7%) did not, and in 11 patients, radiotherapy use was not mentioned. Complete cure with no residual symptoms or recurrence was reported in 30 patients (55.46%), and the identified follow-up period ranged between 2 days and 16 years. The majority of the studied patients (n = 34; 63.0%) were described as having favorable outcomes, while 11 patients (20.4%) were considered to have unfavorable outcomes. Recurrence was reported in 6 patients (10.9%), and residual regrowth was reported in another patient. Seven patients died, representing 13.0% of the studied patients; 421 patients survived (75.9%), while we could not identify the fate of 6 patients.
Discussion
A chordoma is uncommon malignant tumor of the axial skeleton that originate from embryonic remnants of the primitive notochord (earliest fetal axial skeleton, extending from the Rathke’s pouch to the tip of the coccyx): 50% in the sacral region, 35% in the skull base, and 15% in the vertebral bodies, lumbar spine, the thoracic spine.52,53 The exact etiology of chordoma is related to a genetic malformation of the notochord, which is the origin of the nucleus pulposus in humans. These genes are still being tested, but PTEN deficiency (mTOR) and the Brachyury gene are considered the most important. 54 Chordomas account for 20% of primary spinal tumors and 3% of all bone tumors and affect men and women in a ratio of 2:1. Although it occurs more frequently in the 40- to 60-year-old age group.52,53 The . Faheem et al. discussed a case of an 8-year-old boy with intramedullary chordoma without bone involvement. 21 Early favorable prognosis depends on how early we can detect the tumor and start its management. 55
Usually, the chordoma has a direct effect through nerve root compression, leading to chronic back pain or urinary and bowel disorders. 56 In this case, we discussed a 60-year-old male patient who presented with acute paraparesis due to cord compression by an epidural hematoma. Six months later, there was an increasing paraparesis due to the soft tissue component, which was later demonstrated as thoracic chordoma by histopathology examination. This paraparesis is not commonly associated with chordomas. However, suppose a chordoma tumor grows large enough or extends into the spinal canal. In that case, it can compress or impinge on the spinal cord or nerve roots, leading to neurological symptoms such as weakness, numbness, or paralysis in the legs. The severity of paraparesis or other neurological deficits associated with a chordoma can vary depending on the size and location of the tumor. 57
It was mentioned that the most common presenting complaint was chest or back pain, followed by anterior mediastinal compression syndrome, hoarseness, dysphagia, and cough. 5 One-third of reported patients were asymptomatic and detected during routine chest examinations 20 Many preliminary reports described several presentations for thoracic chordoma10,12,15 Among these reports, chordomas were initially misdiagnosed as different neoplasia, including hemangioma, benign neurinomas, 23 and adenocarcinomas. 13 Others reported chordomas metastasizing to lymph nodes, lungs, 18 bones, brain, and viscera. Nonetheless, this type of chordoma is more aggressive. 52
Though few authors proposed primary mechanisms for the association of hemorrhage with chordomas, the exact mechanism is not fully understood. Chordoma can cause dural vessel proliferation if they are in contact with each other. 58 It was hypothesized that rapid tumor growth may cause small friable blood vessels to rupture. Also, subsequent occlusion of small vessels can cause necrosis and hemorrhage in the tumor. 59 We assume a possibility for a chordoma to directly affect nearby blood vessels or disrupt the normal vascular supply to the spine, which could potentially lead to bleeding or vascular complications, but this would be a rare and secondary effect. All these causes may explain the presence of an initial epidural hematoma even before the diagnosis of chordoma in our case. In the present case, the hematoma demonstrated hyperintensity on T2-weighted sequences, indicating a subacute nature and reflecting the paramagnetic properties of hemoglobin breakdown products. The noticed high T2 signal within the vertebral body of T6 and the corresponding abnormal density on CT were thought to be related to a vertebral hemangioma, which is the most common primary tumor of the spine. 60 In response to the emergent presentation of the patient with acute paraparesis, a prompt decision was taken to proceed with surgical intervention. The objective of the surgery was the expeditious decompression of the spinal cord through hematoma evacuation. Intraoperatively, no masses were seen at that time.
Six months after surgery, the patient showed recurrence of the lower limb motor deficit. An urgent CT was requested, which demonstrated a lytic lesion within the vertebral body, right pedicle and lamina of T6 associated with a large soft tissue lesion expanding the spinal canal. The MRI scan confirmed the epidural extent of the lesion, and MR myelography demonstrated the significant compression of the cord which helped for presurgical evaluation. At this point, the preliminary diagnosis of vertebral hemangioma was refuted due to presence of the aggressive features and soft tissue component. However, these features did not confirm chordomas, as some chordomas may mimic other neoplasia. 13 So, a biopsy should be examined under the microscope to ensure the specific pattern of physaliferous bubbly cells within the myxoid stroma, which indicates a chordoma lesion. A tissue sample should also be examined for a high Brachyury gene value manifesting in high concentrations. 61
The first line of treatment for chordomas is surgical resection. Stacchiotti et al, 62 reported multiple variations in resection margins according to the chordoma consensus group approach, such as wide resection, marginal resection, and intralesional resection. Also, the posterior approach for spinal decompression was recommended in giant chordomas with neurological deficits. 19 The present case underwent short and long-segment fixation. The short segment fixation was conducted prior to the diagnosis of chordoma. Following an unsuccessful outcome, the fixation device was removed. Nevertheless, the long-segment fixation was successful, and the patient improved. McLain et al, acknowledged some multifactorial causes of short-segment instrumentation failure in thoracolumbar spines, including the limited ability to maintain sagittal correction in the face of axial instability and the undue bending moments generated at the screw hub. Once initial bending failure happens, additional collapse is likely, developing loss of lordosis, predisposing to a higher incidence of clinical failure and associated pain. 63 On the other hand, literature supports the use of long constructs in thoracic and thoracolumbar segments for greater force reduction and better stabilization. 64 The thoracic spine is comparatively immobile and extending the construct into these segments has little mechanical cost, while offering more extensive fixation. Contemporary instrumentation systems are versatile, allowing fixation with pedicle screws to permit the surgeon to directly instrument vertebrae and provide 3-column fixation in unstable injuries and to minimize the length of fusion. 65
Radiotherapy plays a vital role in the management of the tumor, even in disease control or after surgery to prevent a recurrence. A clinical trial conducted on 50 patients who underwent spinal chordoma resection to examine the effect of proton radiotherapy on recurrence rate and local control showed that a recurrence rate was less common for primary tumors (11%), and the local control was high. 66 Therefore, the ideal treatment protocol is resection of the tumor followed by proton irradiation.45,67 A 5-year survival after this protocol was estimated to be 50%–60%, and the median overall survival for the tumor was about 7 years. However, the recurrence rate, tumor metastasis, and tumor extent mainly determine the overall survival rate. 68 Conventional chemotherapy plays no role in the management of chordomas; however, a small study suggested that imatinib mesylate may have antitumor activity in patients with chordomas. 69 A later study showed that the combination of imatinib mesylate and metronidazole cyclophosphamide had a synergistic anti-angiogenic effect on pericytes and endothelial cells. 70 Royo Crespo et al 25 reported good response of thoracic chordoma to chemotherapy in an elderly male. Contradicting these studies, Pai, 37 reported unfavorable outcomes and long-term disability and neurological deficits in the form of urinary incontinence in 49-year inoperable male patient diagnosed with thoracic chordoma.
In advanced cases where surgery is not feasible, radiofrequency ablation may be considered as a palliative alternative. 71 In the current study, several key factors influenced the decision to initiate adjuvant therapy. Histopathological examination confirmed the diagnosis of chordoma and showed low-grade atypia, suggesting a risk of aggressive tumor behavior. Following meticulous intraoperative tumor resection and spinal cord decompression, the surgical team aimed to achieve complete tumor removal with negative surgical margins, which is associated with favorable long-term outcomes in chordoma cases. 72
Patient Perspective
It was a startling revelation for me to discover that I had a tumor in my spine and to undergo 2 surgeries within 1 year. Following the surgery, I can now carry out my daily tasks. I was worried when the doctor indicated that I might not fully regain my muscle power, but fortunately enough, I did.
Conclusion
Chordomas of the thoracic spine are rare neoplasms that exhibit diverse clinical presentations. Most cases are asymptomatic and discovered incidentally. Early management is associated with a better prognosis. So, early warning signs, including EDH should be considered critically. An EDH should not be exclusively attributed to trauma unless tumors, including chordoma are ruled out. We reported an atypical initial presentation of chordoma with EDH that was successfully treated by decompression and fixation.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed to the study conceptualization, Methodology, Formal analysis, Writing, Reviewing and Editing the manuscript.
Ethical Consideration: The current case report was implemented following the CARE (CAse REport) guidelines, 73 following the declaration of Helsinki and its later amendment, 1964. An approval from the institutional review board of the Faculty of Medicine Hospitals, Zagazig University, was obtained before commencing this work.
Informed Consent: Informed consent was obtained from the patient, including his consent to publish radiological and histopathological images. However, we concealed the patient’s identity, and no data revealing his identity was published.
References
- 1. Kassels AC, Melamed J, Rogers A, Johnstone D. Thoracic chordoma in a 36-year-old female. J Surg Case Rep. 2022;2022:rjac541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez-Miranda JC, Gardner PA, Snyderman CH, et al. Clival chordomas: a pathological, surgical, and radiotherapeutic review. Head Neck. 2014;36:892-906. [DOI] [PubMed] [Google Scholar]
- 3. Ulici V, Hart J. Chordoma: a review and differential diagnosis. Arch Pathol Lab Med. 2021;146:386-395. [DOI] [PubMed] [Google Scholar]
- 4. Kawahara N, Tomita K, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: surgical techniques and related basic background. Orthop Clin North Am. 2009;40:47-63, vi. [DOI] [PubMed] [Google Scholar]
- 5. Fontes R, O’Toole JE. Chordoma of the thoracic spine in an 89-year-old. Eur Spine J. 2012;21:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, Altman DG; and the PRISMA Group. Preferred Reporting Items for systematic reviews and meta-analyses: the PRISMA Statement. Phys Ther. 2009;89:873-880. [PubMed] [Google Scholar]
- 7. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willatt LFS, Wollheim MH, Ehrsam JP, et al. A rare posterior mediastinal mass: chordoma. J Surg Case Rep. 2024;2024:rjae299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dokponou Y, Mohcine S, Obame F, et al. Upper thoracic chordoma: case report and review of the literature. J Neurosurg Spine. 2023;1:94-98. [Google Scholar]
- 10. Mihara A, Kanchiku T, Imajo Y, et al. A rare case of epidural chordoma without bone involvement within the thoracic spinal canal: a case report with a review of literature. J Orthop Sci. 2023;28:1407-1411. [DOI] [PubMed] [Google Scholar]
- 11. Moune MY, Sayore CM, Hemama M, El Fatemi N, El Maaqili MR. Surgical management of a thoracic chordoma: a case report and literature review. Surg Neurol Int. 2022;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahmoud AA, Wei ET, Naser-Tavakolian K, Gupta A. Mediastinal extraosseous chordoma in a teenager: diagnosis by ultrasound-guided percutaneous biopsy. Radiol Case Rep. 2022;17:3859-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoon SK, Moon MH, Moon SW. Thoracic chordoma misdiagnosed as primary adenocarcinoma of the Mediastinum. J Chest Surg. 2021;54:158-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costanzo R, Scalia G, Marrone S, et al. Thoracic dumbbell spinal chordoma mimicking a schwannoma. Surg Neurol Int. 2021;12:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sobash PT, Vedala K, Alfano D, et al. A rare case of chordoma presenting as a pancoast tumor. Rare Tumors. 2021;13:2-5. doi: 10.1177/20363613211029493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leary OP, Crozier J, Liu DD, et al. Three-dimensional printed natomic modeling for surgical planning and real-time operative guidance in complex primary spinal column tumors: single-center experience and case series. World Neurosurg. 2021;145:e116-e126. [DOI] [PubMed] [Google Scholar]
- 17. Supreeth S, Cho CH, Kim SI, Kim YH. Thoracic chordoma in an atypical location mimicking an esophageal adenocarcinoma: a case report. J Korean Soc Spine Surg. 2020;27:109-114. [Google Scholar]
- 18. Bai R, Zhao ZQ, Wang YX, et al. Sacral and thoracic chordoma with pulmonary metastases: a case report and review of the literature. Mol Clin Oncol. 2021;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu S, Zhou X, Song A, et al. Surgical treatment of giant chordoma in the thoracic spine combining thoracoscopic and posterior spinal surgery. Medicine. 2019;98:e16990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goomany A, Timothy J, Robson C, Rao A. En bloc resection of a thoracic chordoma is possible using minimally invasive anterior access: an 8-year follow-up. J Neurosci Rural Pract. 2016;7:138-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faheem M, Zeeshan Q, Ojha B, Agrawal P. Thoracic intramedullary chordoma without bone involvement: a rare clinical entity. BMJ Case Rep. 2016;2016:1-3. doi: 10.1136/bcr-2016-216101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pu F, Wang B, Liu J, Chen F, Shao Z. Giant chordoma in the thoracolumbar spine: a case report and literature review. Eur Spine J. 2017;26:95-S99. [DOI] [PubMed] [Google Scholar]
- 23. Kim S, Yoo B, Baaj A, Little AG. Resection of a posterior mediastinal mass: lessons learned from a failed exploration for presumed schwannoma. J Thorac Cardiovasc Surg. 2016;152:e75-e77. [DOI] [PubMed] [Google Scholar]
- 24. Rena O, Davoli F, Allegra G, Casadio C, Turello D. Giant chordoma of the upper thoracic spine with mediastinal involvement: a surgical challenge. Asian Spine J. 2014;8:353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Royo Crespo I, Rivas de, Andrés JJ, Embún Flor R, Cortés Franco S. Chondroid chordoma in an atypical location. Arch Bronconeumol. 2013;49:491-493. [DOI] [PubMed] [Google Scholar]
- 26. Conzo G, Gambardella C, Pasquali D, et al. Multifocal thoracic chordoma mimicking a paraganglioma. J Cancer Res Ther. 2013;9:497-499. [DOI] [PubMed] [Google Scholar]
- 27. Matsubayashi J, Sato E, Nomura M, et al. A case of paravertebral mediastinal chordoma without bone destruction. Skeletal Radiol. 2012;41:1641-1644. [DOI] [PubMed] [Google Scholar]
- 28. Fernández Carballal C, González Rodrigalvarez R, López De La Riva M, Ares C. Dumbbell-shaped thoracic chondroid chordoma mimicking a neurinoma. Acta Neurochir. 2010;152:325-326. [DOI] [PubMed] [Google Scholar]
- 29. Delgado R, De Pádua A, Bonatelli F, et al. Thoracic chordoma. Arq Neuropsiquiatr. 2008;66:405-407. [DOI] [PubMed] [Google Scholar]
- 30. Miyazawa N, Ishigame K, Kato S, et al. Thoracic chordoma: review and role of FDG-PET/Comment. J Neurosurg Sci. 2008;52:117. [PubMed] [Google Scholar]
- 31. Wang TJ, Shu SH, Lin CW, et al. Thoracic chordoma: an unusual presentation of the spinal tumor. Am J Med Sci. 2008;335:239-241. [DOI] [PubMed] [Google Scholar]
- 32. Van Kollenburg JA, De Waal Malefijt J, Gosens T, Spoor AB, Van der Wurff AA. Chordoma of a thoracic vertebra. A case report. Acta Orthop Belg. 2007;73:812-816. [PubMed] [Google Scholar]
- 33. Bisceglia M, D’Angelo VA, Guglielmi G, Dor DB, Pasquinelli G. Dedifferentiated chordoma of the thoracic spine with rhabdomyosarcomatous differentiation. Report of a case and review of the literature. Ann Diagn Pathol. 2007;11:262-273. [DOI] [PubMed] [Google Scholar]
- 34. Selvaraj A, Wood AJ. Superior mediastinal chordoma presenting as a bilobed paravertebral mass. Eur J Cardio-Thorac Surg. 2003;23:248-250. [DOI] [PubMed] [Google Scholar]
- 35. Smolders D, Wang X, Drevelengas A, Vanhoenacker F, De Schepper AM. Value of MRI in the diagnosis of non-clival, non-sacral chordoma. Skeletal Radiol. 2003;32:343-350. [DOI] [PubMed] [Google Scholar]
- 36. Topsakal C, Bulut S, Erol S, et al. Chordoma of the thoracic spine-case report. Neurol Med Chir. 2001;42:175-180. [DOI] [PubMed] [Google Scholar]
- 37. Pai V. Persistent back pain in adults: four case reports. J Orthop Surg. 2000;8:65-68. [DOI] [PubMed] [Google Scholar]
- 38. Murphy JM, Wallis F, Toland J, Toner M, Wilson GF. CT and MRI appearances of a thoracic chordoma. Eur Radiol. 1998;8:1677-1679. [DOI] [PubMed] [Google Scholar]
- 39. Holden A, Khangure M, Robins P. Thoracic chordoma mimicking a neurogenic tumour. Australas Radiol. 1998;42:385-387. [DOI] [PubMed] [Google Scholar]
- 40. Levowitz BS, Khan MY, Rand E, Hurwitz A. Thoracic vertebral chordoma presenting as a posterior mediastinal tumor. Ann Thorac Surg. 1966;2:75-81. [DOI] [PubMed] [Google Scholar]
- 41. Suster S, Moran CA. Chordomas of the mediastinum: clinicopathologic, immunohistochemical, and ultrastructural study of Six cases presenting as posterior mediastinal masses. Hum Pathol. 1995;26:1354-1362. [DOI] [PubMed] [Google Scholar]
- 42. Taki S, Kakuda K, Kakuma K, et al. Posterior mediastinal chordoma: MR imaging findings. AJR Am J Roentgenol. 1996;166:26-27. [DOI] [PubMed] [Google Scholar]
- 43. Ahrendt MN, Wesselhoeft CW. Chordoma presenting as a posterior mediastinal mass in a pediatric patient. J Pediatr Surg. 1992;27:1515-1518. [DOI] [PubMed] [Google Scholar]
- 44. Walsh TM, Mayer PJ. Chordoma of the thoracic spine presenting as a second primary malignant lesion: a case report. Spine. 1992;17:1524-1528. [DOI] [PubMed] [Google Scholar]
- 45. Amendola BE, Amendola MA, Oliver E, McClatchey KD. Chordoma: role of radiation therapy. Radiology. 1986;158:839-843. [DOI] [PubMed] [Google Scholar]
- 46. Ramiro J, Ferreras B, Calvo JP, Zafra A. Thoracic intradural chordoma. Surg Neurol. 1986;26:571-272. [DOI] [PubMed] [Google Scholar]
- 47. Schwarz SS, Fisher WS, Pulliam MW, Weinstein ZR. Thoracic chordoma in a patient with paraparesis and ivory vertebral body. Neurosurg. 1985;16:100-102. [PubMed] [Google Scholar]
- 48. Cotler HB, Cotler JM, Cohn HE, Israel HI, Gartland JJ. Intrathoracic chordoma presenting as a posterior superior mediastinal tumor. Spine. 1983;8:781-786. [DOI] [PubMed] [Google Scholar]
- 49. Clemons RL, Blank RH, Hutcheson JB, Ruffolo EH. Chordoma presenting as a posterior mediastinal mass. J Thorac Cardiovasc Surg. 1972;63:922-924. [PubMed] [Google Scholar]
- 50. Burauzewski J, Rudowski W. Chordoma of the thoracic spine appearing as a mediastinal tumor. J Thorac Surg. 1957;34:75-84. [PubMed] [Google Scholar]
- 51. Crowe GG, Muldoon PB. Thoracic chordoma. Thorax. 1951;6:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Young VA, Curtis KM, Temple HT, et al. Characteristics and patterns of metastatic disease from chordoma. Sarcoma. 2015;2015:517657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. George B, Bresson D, Herman P, Froelich S. Chordomas: a review. Neurosurg Clin N Am. 2015;26:437-452. [DOI] [PubMed] [Google Scholar]
- 54. Gulluoglu S, Turksoy O, Kuskucu A, Ture U, Bayrak OF. The molecular aspects of chordoma. Neurosurg Rev. 2016;39:185-196. [DOI] [PubMed] [Google Scholar]
- 55. Huang J-F, Chen D, Zheng X-Q, et al. Conditional survival and changing risk profile in patients with chordoma: a population-based longitudinal cohort study. J Orthop Surg Res. 2019;14:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. World Health Organization. WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours. Vol. 5. World Health Organization; 2013. [Google Scholar]
- 57. Erlemann R. Imaging and differential diagnosis of primary bone tumors and tumor-like lesions of the spine. Eur J Radiol. 2006;58:48-67. [DOI] [PubMed] [Google Scholar]
- 58. Kim SK, Kim YH, Park CK, Kim MA, Park SH. Intracranial intradural chordoma presenting with intraventricular hemorrhage. Clin Neurol Neurosurg. 2012;114:1189-1192. [DOI] [PubMed] [Google Scholar]
- 59. Lee HJ, Kalnin AJ, Holodny AI, et al. Hemorrhagic chondroid chordoma mimicking pituitary apoplexy. Neuroradiol. 1998;40:720-723. [DOI] [PubMed] [Google Scholar]
- 60. Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World J Orthop. 2016;7:109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sheppard HE, Dall’Agnese A, Park WD, et al. Targeted brachyury degradation disrupts a highly specific autoregulatory program controlling chordoma cell identity. Cell Rep Med. 2021;2:100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stacchiotti S, Gronchi A, Fossati P, et al. Best practices for the management of local-regional recurrent chordoma: a position paper by the Chordoma Global Consensus Group. Ann Oncol. 2017;28:1230-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McLain RF, Sparling E, Benson DR. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures. A preliminary report. JBJS. 1993;75:162-167. [DOI] [PubMed] [Google Scholar]
- 64. McLain RF. The biomechanics of long versus short fixation for Thoracolumbar Spine fractures. Spine. 2006;31:S70-S79. doi: 10.1097/01.brs.0000218221.47230.dd [DOI] [PubMed] [Google Scholar]
- 65. Gurr KR, McAfee PC, Shih CM. Biomechanical analysis of anterior and posterior instrumentation systems after corpectomy. A calf-spine model. JBJS. 1988;70:1182-1191. [PubMed] [Google Scholar]
- 66. DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110:115-122. [DOI] [PubMed] [Google Scholar]
- 67. Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493-503. [DOI] [PubMed] [Google Scholar]
- 68. Pan Y, Lu L, Chen J, Zhong Y, Dai Z. Analysis of prognostic factors for survival in patients with primary spinal chordoma using the SEER Registry from 1973 to 2014. J Orthop Surg Res. 2018;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Casali PG, Messina A, Stacchiotti S, et al. Imatinib mesylate in chordoma. Cancer. 2004;101:2086-2097. [DOI] [PubMed] [Google Scholar]
- 70. Adenis A, Ray-Coquard I, Italiano A, et al. A dose-escalating phase I of imatinib mesylate with fixed dose of metronomic cyclophosphamide in targeted olid tumours. Br J Cancer. 2013;109:2574-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anis N, Chawki N, Antoine K. Use of radio-frequency ablation for the palliative treatment of sacral chordoma. Am J Neuroradiol. 2004;25:1589-1591. [PMC free article] [PubMed] [Google Scholar]
- 72. Baig Mirza A, Bartram J, Okasha M, et al. Surgical management of spinal chordoma: a systematic review and single-center experience. World Neurosurg. 2021;156:e111-e129. [DOI] [PubMed] [Google Scholar]
- 73. Gagnier JJ, Kienle G, Altman DG, et al. The CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Forsch Komplementarmed. 2013;7:385-386. [DOI] [PMC free article] [PubMed] [Google Scholar]