Abstract
Gastric cancer (GC) is a prevalent gastrointestinal tumor characterized by high mortality and recurrence rates. Current treatments often have limitations, prompting researchers to explore novel anti-tumor substances and develop new drugs. Flavonoids, natural compounds with diverse biological activities, are gaining increasing attention in this regard. We searched from PubMed, Web of Science, SpringerLink and other databases to find the relevant literature in the last two decades. Using “gastric cancer”, “stomach cancers”, “flavonoid”, “bioflavonoid”, “2-Phenyl-Chromene” as keywords, were searched, then analyzed and summarized the mechanism of flavonoids in the treatment of GC. It was revealed that the anti-tumor mechanism of flavonoids involves inhibiting tumor growth, proliferation, invasion, and metastasis, as well as inducing cell death through various processes such as apoptosis, autophagy, ferroptosis, and pyroptosis. Additionally, combining flavonoids with other chemotherapeutic agents like 5-FU and platinum compounds can potentially reduce chemoresistance. Flavonoids have also demonstrated enhanced biological activity when used in combination with other natural products. Consequently, this review proposes innovative perspectives for the development of flavonoids as new anti-GC agents.
Keywords: flavonoid, gastric cancer, apoptosis, angiogenesis
Introduction
Gastric cancer (GC) ranks as the fifth most prevalent cancer worldwide,1 with approximately 990,000 new cases diagnosed annually.2 It remains one of the leading causes of cancer-related deaths globally.3 While the overall incidence and mortality of GC have declined in most countries over the past few decades,4–6 the disease still poses a significant burden in Asia. GC development is influenced by various common factors, such as family history, diet, alcohol consumption, smoking, Helicobacter pylori infection, and Epstein Barr virus (EBV) infection.7–10 Controllable risk factors, including H. pylori infection, dietary habits and lifestyle, chemical radiation, or viral infections,11,12 can be addressed to potentially prevent GC. Currently, early-stage gastric cancers (GCs) are predominantly managed through endoscopic resection, whereas surgery is employed for non-early-stage operable tumors. Advanced GCs undergo a sequential chemotherapy regimen, typically involving platinum combined with fluoropyrimidine. Nevertheless, this treatment approach is associated with severe side effects, and the median survival rate is less than one year. Notably, cisplatin, a commonly used chemotherapeutic agent, has been linked to thromboembolic disease and renal dysfunction.13 Targeted therapies, such as trastuzumab, ramucirumab, and nivolumab or pembrolizumab, have also emerged as viable options in GC treatment. However, the diverse nature of GC among tumors, patients, and even within a single tumor poses a significant challenge to the development of effective targeted therapeutics.14 Consequently, there is a pressing need for novel treatment regimens or drugs that can effectively manage GC patients while minimizing side effects and drug resistance.
Flavonoids are the most prevalent natural compounds found in plants, including Leguminosae, Brassicaceae, Umbelliferae, Genisteinae, Lioceae, and various plant parts. Flavonoids typically refer to a group of compounds where two benzene rings, each containing phenolic hydroxyl groups, are interconnected by three central carbon atoms to form the C6-C3-C6 unit.15,16 They can be classified into 7 subclasses based on characteristics that include the degree of oxidation of the central three-carbon chain and the position of the B-ring attachment. Examples of these subclasses include chalcones, dihydrochalcones, flavones, and flavanols.17–19 Because of their abundance, flavonoids are commonly present in dietary fibers and find diverse applications in food and medicine.20 Flavonoids in the diet can influence various molecular targets and signaling pathways related to cell growth, proliferation, differentiation, migration, angiogenesis, and hormone activity.21,22 This modulation contributes to the inhibition of different cancers, such as lung, liver, breast, and ovarian cancers.23–26
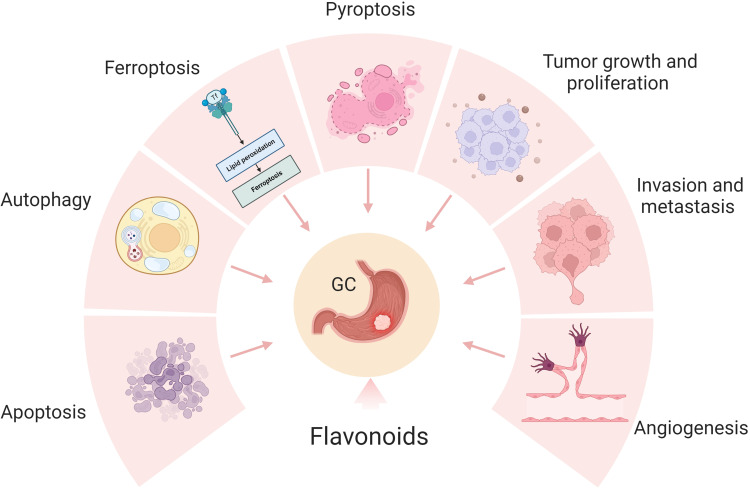
Recent studies have increasingly demonstrated the beneficial effects of flavonoids on inhibiting GC. Numerous studies have provided evidence that flavonoids exert a regulatory role in modulating various phenotypes of GC, encompassing tumor growth, proliferation, invasion, metastasis, angiogenesis, and cell death (Figure 1). Therefore, our review tried to find the mechanism of flavonoids in the treatment of GC and provided new ideas for researchers.
Figure 1.
The potential role of flavonoids for GC therapy. Flavonoids exert a regulatory role in modulating various phenotypes of GC, encompassing tumor growth, proliferation, invasion, metastasis, angiogenesis, and cell death. Created by Biorender.com.
Method
We searched from PubMed (https: //pubmed.ncbi.nlm.nih.gov/), Web of Science (http://apps.webofknowledge.com/) SpringerLink (https://link.springer.com/) and other databases to search the literature in the last two decades. Using “gastric cancer”, “stomach cancers”, “flavonoid”, “bioflavonoid”, “2-Phenyl-Chromene” as keywords, were searched, then excluding literature unrelated to the topic.
The Bioactivity of Flavonoids
Extensive research has revealed that flavonoids possess diverse biological activities, including antioxidant, anti-inflammatory, and anticancer effects.27–29 Numerous mechanisms have been investigated to understand the antioxidant effects of flavonoids. These mechanisms include direct scavenging of oxides, activation of antioxidant enzymes, metal chelating activity, attenuation of oxidative stress, inhibition of natriuretic oxidase, enhancement of antioxidant properties of low molecular weight antioxidants, elevation of uric acid levels, and elevation of deoxygenated oxygen radicals.30 For example, fisetin has been found to inhibit xanthine oxidase activity and reduce oxidative damage.31 Flavonoids’ anti-inflammatory effects often occur through the targeting of regulatory enzymes in signaling pathways, such as phosphatidylinositol kinase and protein kinase C.32 Additionally, flavonoids may exert anti-inflammatory effects by inhibiting transcription factors.29 Many flavonoids also act as potent inhibitors of arachidonic acid, cyclooxygenase, and phospholipase A2, leading to a reduction in the production of prostaglandins, leukotrienes, and nitric oxide, all of which are key inflammatory substances.33–35 For instance, mulberry leaf flavonoids have been shown to decrease lipopolysaccharide-induced production of NO, PGE2, COX-2, and inflammatory factors in RAW 264.7 cells. Flavonoids’ anticancer properties are frequently observed through their inhibitory effects on various types of cancer. Epigallocatechin and genistein, for instance, have been found to inhibit DNA methyltransferases and modify chromatin alterations in breast cancer.25 Icaritin has been shown to enhance mitosis in hepatocellular cancer and synergistically promote immune cell death with doxorubicin.36 Therefore, the current study suggests that flavonoids not only have diverse sources but also exhibit potent biological activities, making them attractive candidates for the development of new drugs (Figures 2 and 3).
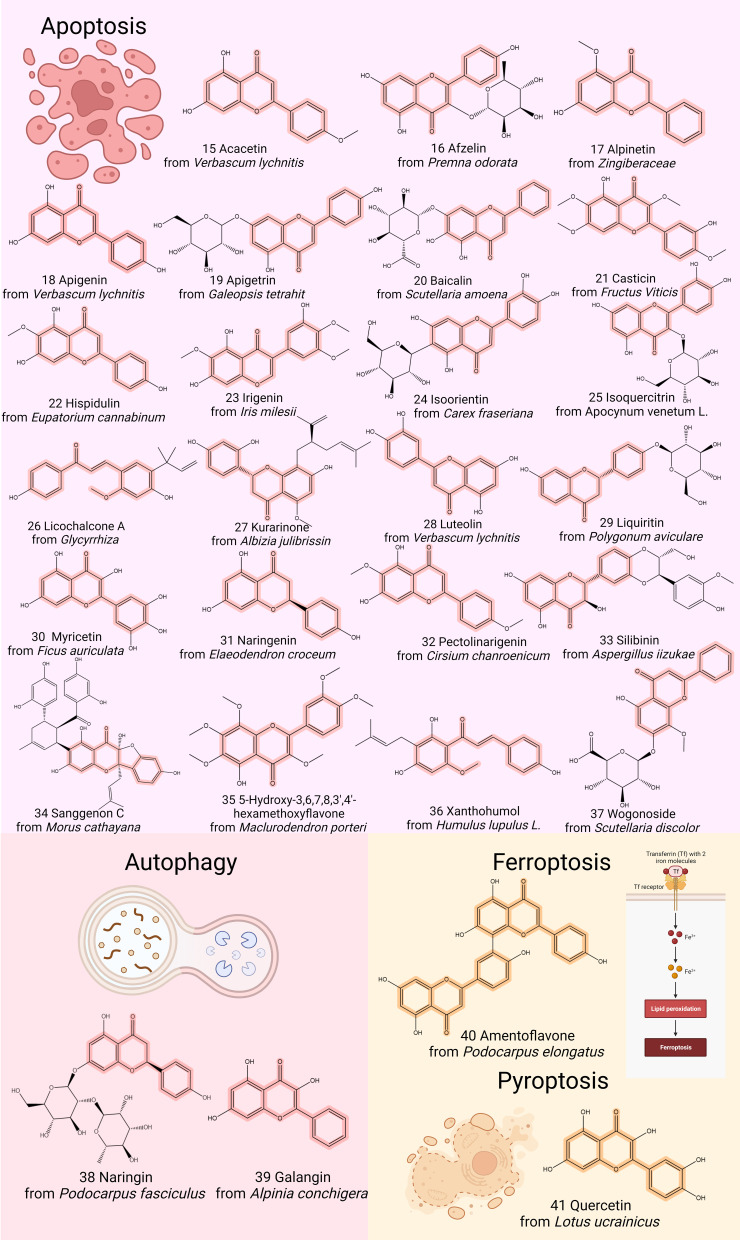
Figure 2.
Chemical structures of representative flavonoids that prevent GC by modulating tumor growth, proliferation, invasion, metastasis, and angiogenesis. Created by Biorender.com.
Figure 3.
Chemical structures of representative flavonoids that prevent GC by modulating cell death. Created by Biorender.com.
Despite flavonoids are safe, some of them also have a prooxidative effect. For example, dietary phenolic substances have been shown to act as prooxidants in systems containing redox active metals. This indicates that different flavonoids may have opposite biological activities.
Flavonoids for Fighting Against GC
Recently, numerous flavonoids have been discovered to possess varying degrees of inhibitory effects on GC, with potential antitumor properties. These flavonoids have the ability to impact tumor growth and proliferation, inhibit tumor invasion and metastasis, affect angiogenesis, and induce cell death. Examining the antitumor effects of flavonoids from these perspectives could generate novel insights for drug development (Table 1).
Table 1.
Profiles of Flavonoids Inhibiting GC
| Flavonoid | Source | Cell Line | Targets | Reference |
|---|---|---|---|---|
| Acacetin | Verbascum lychnitis (mullein) | AGS (in vitro) | ↑Bax, p53; ↓Bcl-2 | [37] |
| MKN-45, MGC-803 (in vitro) | ↓MMP2, MMP9 | [38] | ||
| Afzelin | Premna odorata (fragrant premna) | AGS | ↑GalNAcTL5; ↓C1GalT1 | [39] |
| Alpinetin | Zingiberaceae | AGS, N-87 | ↑Bax; ↓Bcl-2 | [40] |
| Amentoflavone | Podocarpus elongatus | AGS, HGC-27 (in vivo) | ↑miR-496; ↓ATF2 | [41] |
| Apigenin |
Verbascum lychnitis (mullein) |
AGS, SNU-216, NCI-N87, SNU-638, MKN-7, MKN-74 |
↓p-mTOR, p62, HIF-1α, Ezh2 | [42] |
| HGC-27, SGC-7901 | ↑Caspase-3, Bax; ↓Bcl-2 | [43] | ||
| SGC-7901 | Inhibits the cell proliferation | [44] | ||
| MKN-45 | ↑MUC-2, IκBα; ↓NF-κB | [45] | ||
| Apigetrin |
Galeopsis tetrahit (hempnettle) |
SGC-7901, MGC-803 | ↑Caspase-9/-3, Bax; ↓Bcl-2 | [46] |
| AGS | ↑Caspase-3, PARP, LC3B-II, beclin-1; ↓CDK1, cyclin B1, cdc25c proteins | [47] | ||
| Baicalein |
Stachys annua (annual hedgenettle) |
HGC-27, SGC-7901 | ↓FAK, PI3K, AKT, mTOR | [48] |
| AGS | ↓N-cadherin, vimentin, ZEB1 and ZEB2 | [49] | ||
| HGC-27, SGC-7901, MGC-803, BGC-823 | ↑miR-7; ↓p-FAK | [50] | ||
| SGC-7901 | ↑Bax; ↓Bcl-2 | [51] | ||
| HGC-27, AGS | ↓PI3K, AKT | [52] | ||
| SGC-7901, MGC-803 | ↓MMP-2/-9, p38 | [53] | ||
| AGS | ↑PTEN; ↓Akt, HIF-1α | [54] | ||
| Baicalin |
Scutellaria amoena (Scutellaria) |
BGC-823, MGC-803 | ↑Caspase-9/-3, Bax; ↓Bcl-2 | [55] |
| AGS (in vivo) | ↓IL-1β, IL-8, IgM, IgA | [56] | ||
| AGS, SGC-7901 | ↑ROS | [57] | ||
| Casticin | Fructus Viticis | BGC-823, SGC-7901, MGC-803 | ↓cFLIP, Bcl-2, XIAP, survivin | [58] |
| Cardamonin | Amomum subulatum | AGS, MGC-803, BGC-823 | ↑Caspase-3, Bax; ↓Bcl-2 | [59] |
| Chrysin |
Scutellaria amoena (Scutellaria) |
AGS | ↑let-7a, miR-9, miR-22, miR-34a, miR-126; ↓miR-18, miR-21, miR-221 | [60] |
| SGC-7901, MKN-45, BGC-823 | ↑p53, E-cadherin; ↓COPB2 | [61] | ||
| MKN-45 | ↑TET1,5hmC | [62] | ||
| AGS | ↑miR-22, miR-34a, miR-126 | [63] | ||
| AGS | ↓ MMP9 | [64] | ||
| AGS | ↓NF-κB, Egr-1 | [65] | ||
| Deguelin | Derris montana | SNU-484, AGS, MKN-28 | ↓AKT, HIF-1α | [66] |
| EGCG | Limoniastrum guyonianum (Limonium) | MKN28, AGS | ↓RON | [67] |
| SGC-7901 | ↓VEGF | [68] | ||
| AGS | ↓STAT6, VEGF | [69] | ||
| Farrerol | Daphne aurantiaca | SGC-7901 | ↑caspase-9/-3, Bax; ↓Bcl-2 | [70] |
| SGC-7901 (in vitro) | ↑p27KIP1, ERK1/2, p38 MAPK | [71] | ||
| Formononetin |
Pterocarpus indicus (Purple sandalwood) |
SGC-7901, MKN-45, MGC-803 | ↓SIRT1 | [72] |
| Galangin | Alpinia conchigera | MGC-803 | ↑Caspase-3, Bax, PARP; ↓Bcl-2 | [73] |
| SNU-484 | ↑Caspase-9/-3, PARP | [74] | ||
| MGC-803 | ↑LC3BII; ↓NF-κB | [75] | ||
| Hispidulin |
Eupatorium cannabinum (hemp agrimony) |
AGS | ↑p21/WAF1, p16; ↓COX-2, cyclin D1/E | [76] |
| Icariin | Epimedium pubescens | BGC-823 | ↓Rac1, VASP | [77] |
| SGC-7901, BGC-823, MKN-7, HGC-27, NUGC-3, AGS, Hs-746T, NCl-N87 | ↓Hsa_cir_0003159 | [78] | ||
| Iridin | Iris milesii | AGS | ↓Cdc25C, CDK1, Cyclin B1, p-PI3K, p-AKT | [79] |
| Irigenin | Iris milesii | NCI-N87 | ↑FADD, DR5, Bax | [80] |
| Isoliquiritigenin | Pterocarpus indicus (Purple sandalwood) | MKN-28 | ↑LC3II/LC3I, Beclin 1 | [81] |
| Isoquercitrin | Apocynum venetum L. (kender) | AGS, HGC-27 | ↑HMGB1, HSP70, HSP90 | [82] |
| Isoorientin | Carex fraseriana | AGS | ↓p-AKT, p-GSK-3β, β-catenin | [83] |
| Kurarinone | Albizia julibrissin (acacia) | SGC-7901 | ↑Mcl-1, c-FLIP | [84] |
| Liquiritin |
Polygonum aviculare (knotgrass) |
AGS, SNU-216 | ↑Caspase-9/-3, PARP | [85] |
| Licochalcone A |
Glycyrrhiza (Licorice) |
BGC-823 | ↑ROS, ERK, JNK | [86] |
| Licoflavone A |
Glycyrrhiza Glabra (Licorice) |
SGC-7901, MKN-45, MGC-803 | ↓VEGFR-2, PI3K/AKT, MEK/ERK | [87] |
| Luteolin | Verbascum lychnitis | MKN-45 | ↑Bax, Caspase-3, cytochrome C; ↓Bcl-2, p-Akt | [88] |
| Hs-746T | ↓VEGF | [89] | ||
| Hs-746T, MKN-28 | ↓Notch1 | [90] | ||
| BGC-823, SGC-7901 | ↑miR-34a; ↓Bcl-2 | [91] | ||
| CRL-1739 | ↓MUC1, ADAM-17 | [92] | ||
| BGC-823 | ↑Caspase-9/-3, Bax; ↓Bcl-2 | [93] | ||
| SGC7901/DDP, BGC-823, HGC-27 | ↓Mcl-1, Survivin, Bcl-xl | [94] | ||
| MKN-45, SGC-7901 | ↑Caspase-3, PARP-1; ↓MMP9, cMet | [95] | ||
| AGS, BGC-823, SGC-7901 | ↓HK1, miR-34a | [96] | ||
| HGC-27, MFC, MKN-45 | ↑ROS | [97] | ||
| Myricetin | Ficus auriculata | AGS | ↓PI3K/Akt/mTOR | [98] |
| HGC-27, SGC-7901 | ↑Mad1 | [99] | ||
| Naringin | Podocarpus fasciculus | AGS | ↑Bad; ↓Bcl-xL | [100] |
| MGC-803, MKN-45 | ↑E-cadherin; ↓Zeb1, P-AKT | [101] | ||
| SNU-1 | ↑Caspase-3, Bax; ↓Bcl-2 | [102] | ||
| Naringenin | Elaeodendron croceum | SGC-7901 | ↑Caspase-3, Bax; ↓Bcl-2 | [103] |
| Nobiletin | Citrus tankan | SGC-7901 | ↑Caspase-3; ↓Bcl-2, Survivin | [104] |
| Pectolinarigenin | Cirsium chanroenicum | AGS, MKN-28 | ↓PI3K/AKT/mTOR | [105] |
| Quercetin | Lotus ucrainicus | AGS | ↑CASP3, PARP | [106] |
| Scutellarin | Erigeron breviscapus | MGC-803, AGS | ↑PTEN; ↓PI3K | [107] |
| Sanggenon C | Morus cathayana(voasun) | HGC-27, AGS | ↓ERK | [108] |
| Silibinin | Aspergillus iizukae | SGC-7901 | ↑p53, p21; ↓p34cdc2 | [109] |
| Wogonin | Scutellaria likiangensis | BGC-823, MGC-803, MKN-45, HGC-27 | ↑Caspase-3, Bax; ↓Bcl-2 | [110] |
| MGC-803 | ↓NF-κB | [111] | ||
| SGC-7901 | ↓HIF-1α, MCT-4 | [112] | ||
| Wogonoside | Scutellaria discolor | AGS, MKN-45 | ↑ASK1, JNK, Caspase-3/-9 | [113] |
| Xanthohumol |
Humulus lupulus L. (common hop) |
AGS | ↑ROS; ↓NF-κB | [114] |
| 5-Hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone | Maclurodendron porteri | BGC-7901 | ↑Caspase-3/-9, PARP, Bax/Bcl-2 | [115] |
Inhibition of Tumor Growth and Proliferation
A hallmark of tumorigenesis is the rapid growth and extensive proliferation capacity of tumors. Malignant tumors demonstrate virtually unlimited growth and proliferation, while benign tumors display more restricted expansion. Among the most prevalent natural compounds, flavonoids frequently exert inhibitory effects on the growth and proliferation of GC cells. For instance, farrerol (Figure 2, 2), a representative natural flavanone extracted from Rhododendron dauricum L., serves as an illustration. Treating SGC7901 cells with farrerol demonstrated its ability to suppress cancer cell growth by inducing sustained ERK activation-mediated G0/G1 phase cell cycle arrest and significantly upregulating the p27KIP1 protein.71 Additionally, isoliquiritigenin (Figure 2, 9), derived from licorice root, is a bioactive compound characterized by its chalcone structure.116 It possesses the capability to modulate the tumor microenvironment and inhibit tumor stemness, effectively suppressing GC stem-like properties by down-regulating GRP78 through CREB3L-mediated pathways.117
Furthermore, nano-encapsulation of chrysin (Figure 2, 7) in the PLGA-PEG-chrysin complex inhibited the growth of AGS cells. This inhibition was achieved by promoting increased expression of miR-22, miR-34a, and miR-126, resulting in the suppression of GC cell growth.63 Nano-encapsulated bryostatin exhibited more consistent antitumor activity compared to the unencapsulated form.
In addition to influencing the cell cycle through miRNAs, flavonoids affect tumor growth by targeting various pathways. One commonly targeted pathway is the PI3K signaling pathway. For instance, scutellarin (Figure 2, 3) inhibited the growth and epithelial mesenchymal transition (EMT) of GC by up-regulating PTEN to inactivate the PI3K signaling pathway.107 Similarly, iridin (Figure 2, 4) blocked the PI3K/AKT signaling pathway in AGS cells, leading to G2/M cell cycle arrest and affecting cancer cell proliferation.79 Galangin (Figure 3, 39), a natural flavonol found in galangal, inhibited MGC-803 cells growth through the JAK2/STAT3 pathway, increasing ROS accumulation and reversing the abnormal expression of proteins such as p-JAK2, p-STAT3, Bcl-2, cleaved PARP, and Ki67. This induced apoptosis and reduced cell proliferation in GC cells.73 Cardamonin (Figure 2, 1), a chalcone compound with effects similar to galangin (Figure 3, 39), affected GC cell (AGS, MGC-803, BGC-823) proliferation and the cell cycle by suppressing LncRNA-PVT1 expression, down-regulating p-STAT3, and inhibiting STAT3 activation.59 Additionally, icariin (Figure 2, 5), a glycosidic flavonoid from the flavonol family, hindered the viability and growth of GC cells (SGC-7901, BGC-823, MKN-7, HGC-27, NUGC-3, AGS, Hs-746T, NCl-N87) by controlling the hsa_cir_0003159/miR-223-3p/NLRP3 axis.78
In summary, the available research suggests that flavonoids inhibit the growth of GC cells through multiple pathways, particularly targeting PI3K, STAT, and other signaling pathways. These mechanisms encompass regulation of the cell cycle, gene expression, protein expression, and more, resulting in significant anti-growth and anti-proliferative effects (Figure 4).
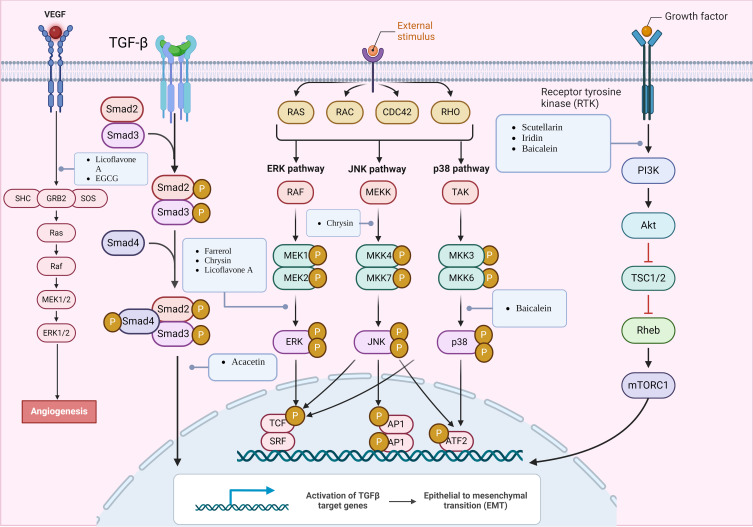
Figure 4.
Mechanisms of flavonoids suppressing GC by inhibiting tumor growth and proliferation, invasive migration, and angiogenesis. Created by Biorender.com.
Inhibition of Invasion and Metastasis
It is widely recognized that an increase in tumor aggressiveness and metastasis indicates unfavorable disease progression. One complication following surgery or chemotherapy is tumor metastasis, making it crucial to explore avenues for reducing tumor aggressiveness and inhibiting metastasis. EMT has been identified as a key player in tumor progression, invasion, and metastasis, enabling cancer cells to acquire increased aggressiveness.118,119 As a methoxyflavone, acacetin (Figure 3, 15) exhibited potent antitumor activity, primarily manifested in its ability to suppress tumor invasion and metastasis.120,121 Studies have demonstrated that acacetin inhibited the PI3K/Akt/Snail signaling pathway induced by TGF-β1-mediated EMT, leading to changes in the expression of EMT-related proteins and subsequently influencing the invasion and metastasis of GC.38 Additionally, luteolin (Figure 3, 28) derived from various plants affects Notch1 signaling and EMT, which in turn inhibited tumor progression. When the Notch receptor binds to the ligand, the activated Notch intracellular domain (NICD) forms a complex with activated β-conjugated proteins. Luteolin blocked this complex formation and inhibited cell proliferation and metastasis, thereby impeding tumor progression.90
Flavonoids, including baicalein (Figure 2, 6), isoliquiritigenin (Figure 2, 9), and others, not only affect EMT but also commonly inhibit the invasion and metastasis of GC cells by modulating the AKT/mTOR signaling pathway.48,81 The suppression of tumor invasion and metastasis by chrysin has garnered significant attention among the numerous flavonoids. Chrysin (Figure 2, 7) inhibited the phosphorylation of c-Jun and c-Fos in AGS cells by inhibiting the JNK1/2 and ERK1/2 signaling pathways. Consequently, it blocked AP-1 and regulated MMP-9 production, demonstrating an anti-invasive effect.64 Ten-eleven translocation (TET) enzymes can catalyze the production of 5-hydroxymethylcytosine (5hmC).122 Previous studies have shown a reduction in TET3 and 5hmC expression in GC cells.123 Researchers observed a significant increase in the expression of TET1 and 5hmC in chrysin-treated MKN-45 cells. Furthermore, the overexpression of TET1 promoted apoptosis and inhibited cell migration and invasion.62 Recepteur d’origine Nantais (RON), a member of the c-Met family, plays an important role in the progression, invasion, and metastasis of GC. Chrysin significantly inhibited the expression of RON by suppressing the activity of Egr-1 and NF-κB transcription factors, thereby inhibiting cell invasion.65 Consequently, based on the findings of this study, chrysin may possess an advantage in terms of drug selection for inhibiting GC invasion when compared to other flavonoids. Inhibiting EMT may serve as a promising therapeutic target for GC invasion (Figure 4).
Inhibition of Angiogenesis
Angiogenesis, an essential factor in cancer development and tumor metastasis, is associated with vascular endothelial growth factor (VEGF) and its receptor (VEGFR-2), making them important targets for treating GC. Moreover, natural compounds called flavonoids have been found to possess anti-angiogenic properties. Scutellaria baicalensis, a traditional Chinese medicine rich in flavonoids such as baicalein and wogonin,124 exhibits both anticancer and antiviral properties. For instance, wogonin (Figure 2, 13) can suppress angiogenesis in SGC-7901 cells by downregulating HIF-1α and MCT-4 expression.112 Similarly, baicalein (Figure 2, 6) inhibited angiogenesis in GC by upregulating miR-7, leading to the blockade of the FAK/PI3K/AKT pathway. As a result, angiogenesis in GC (HGC-27, SGC-7901, MGC-803, BGC-823) was suppressed.50 In addition to the aforementioned targets, VEGF and VEGFR-2, flavonoids such as licoflavone A (Figure 2, 12), luteolin (Figure 3, 28), and deguelin (Figure 2, 11) exhibit potent antitumor effects. Licoflavone A (Figure 2, 12) affected the proliferation, cycling, apoptosis, migration, invasion, and EMT of VEGF-stimulated MKN-45 cells by targeting VEGFR-2 and inhibiting the PI3K/AKT and MEK/ERK signaling pathways.87 Furthermore, in AGS cells, epigallocatechin-3-gallate (EGCG) (Figure 2, 14) suppressed IL-3-induced VEGF production and angiogenesis by reducing STAT6 activity, presenting a novel approach for anti-tumor angiogenesis.69 Although studies have consistently identified the involvement of VEGF in tumor angiogenesis, further investigations are needed to elucidate the precise pathways and mechanisms (Figure 4).
Induction of Cell Death
During tumor development, cell death is an inevitable and significant aspect of the life cycle of cancer cells. Various forms of cell death, including necrosis, apoptosis, autophagy, ferroptosis, and pyroptosis, have been identified, each with distinct biological processes and pathophysiological characteristics.125 Initially, research on flavonoids’ mechanisms against GC cells primarily focused on understanding processes like apoptosis. Consequently, most current studies investigate the apoptotic effects induced by flavonoids and their impact on signaling pathways such as PI3K/AKT, TRAIL, and MAPK. However, as research progresses, a few studies have emerged exploring the induction of GC autophagy, ferroptosis, and pyroptosis by flavonoids (Figure 5).
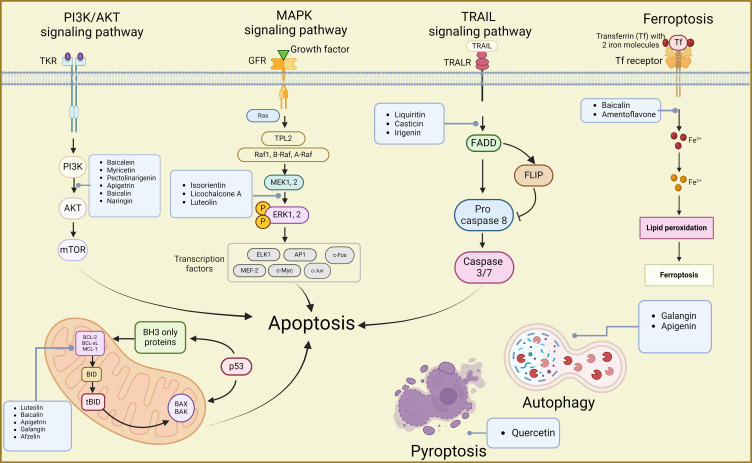
Figure 5.
Mechanisms of flavonoids suppressing GC by inducing cell death inducing apoptosis, autophagy, ferroptosis, and pyroptosis. Created by Biorender.com.
Induction of Apoptosis
Targeting the PI3K/Akt Signaling Pathway
PI3Ks are a family of lipoprotein kinases capable of phosphorylating the 3-OH moiety of inositol phospholipids. They are classified into three classes: I, II, and III, with class I being more relevant to cancer. Akt proteins, important downstream of PI3K, regulate processes such as cell survival, cell cycle progression, and cell growth.126,127 In GC cells, the PI3K/AKT pathway, one of the most common pathways, is frequently studied to understand the mechanism of action of flavonoid agents.
Numerous flavonoids have been reported to induce apoptosis in GC cells through the PI3K/AKT signaling pathway. For instance, overexpression of the B-cell translocation gene 3 (BTG3) suppresses PI3K/AKT/mTOR pathway activation, thereby regulating GC cell proliferation, migration, and apoptosis.128,129 Baicalein (Figure 2, 6) inhibited the PI3K/AKT pathway by activating BTG3, resulting in endoplasmic reticulum stress (ERS) and apoptosis.52 Myricetin (Figure 3, 30), found in various natural plants including bayberry, induced apoptosis by inhibiting the PI3K/Akt/mTOR pathway, thereby reducing the expression of related proteins and affecting the viability of AGS cells.98 Similarly, pectolinarigenin (Figure 3, 32), present in Cirsium chanroenicum, led to autophagy and apoptosis in AGS and MKN-28 cells by downregulating the PI3K/AKT/mTOR pathway.105 Whereas apigetrin (Figure 3, 19)-treated AGS cells inhibited cancer progression by enhancing exogenous apoptosis and autophagic cell death via the PI3K/AKT/mTOR pathway.47 Naringin (Figure 3, 38), isolated from citrus fruits, stimulated the expression of apoptosis-associated proteins Bax, decreased the expression of Bcl-2, and affected the apoptosis of SNU-1 cells by blocking the PI3K/AKT pathway.102 In another study, naringin (Figure 3, 38) was found to block the cell cycle, induced apoptosis, and inhibited the EMT process by inhibiting the PI3K-AKT/Zeb1 pathway in GC cells.101 These findings suggest that naringin has multiple targets on the PI3K/AKT signaling pathway, contributing to its multifaceted anti-tumor activities. In conclusion, various flavonoids can induce cell death by acting on the PI3K/AKT signaling pathway, indicating their potential against GC.
Targeting TRAIL Signaling Pathway
TRAIL is a pro-apoptotic protein that initiates apoptosis by binding to death receptors (DR).130,131 However, GC cells exhibit reduced sensitivity to TRAIL-induced apoptosis. Therefore, several scientific studies have focused on targeting TRAIL-induced apoptosis in GC cells using natural products like flavonoids. Liquiritin (Figure 3, 29) is a flavanone glycoside present in licorice,132 while irigenin (Figure 3, 23) is an isoflavonoid isolated from the roots of Belamcanda chinensis.133 Both of them could induce apoptosis by sensitizing TRAIL, which in turn promoted the enhancement of pro-apoptotic proteins and the generation of ROS.80,85 Furthermore, casticin (Figure 3, 21), isolated from Fructus Viticis, enhanced TRAIL-induced apoptosis by down-regulating cell survival proteins and up-regulating the DR5 receptor via the ROS-ER stress-CHOP pathway.58 Although there are limited studies on flavonoid-targeted TRAIL-induced apoptosis in GC, the current study presents a novel approach for GC treatment.
Targeting Bcl-2 Family
The Bcl-2 family plays a crucial role in apoptosis. Bcl-2, a pro-survival protein, regulates apoptosis by inhibiting the release of pro-apoptotic factors and the subsequent activation of the caspase cascade.134 Flavonoids such as baicalin (Figure 3, 20), apigetrin (Figure 3, 19) and afzelin (Figure 3, 16) can interact with this protein.39,46,55 For instance, baicalin (Figure 3, 20) has been shown to induce apoptosis in BGC-823 and MGC-803 cells. This effect is achieved by up-regulating Bcl-2-associated X (Bax) protein, down-regulating Bcl-2, and activating caspase-3 and caspase-9 at both protein and mRNA levels.55 Apigetrin (Figure 3, 19) acted as an apoptosis inducer by reducing Bcl-2 and enhancing Bax, caspase-9/-3, and PARP cleavage in SGC-7901, MGC-803.46 In another study, afzelin (Figure 3, 16) was found to stimulate the apoptotic response by increasing the expression of Bax and caspase-8/-9/-3 mRNA, while simultaneously decreasing the expression of the extracellular structural domain of MUC1 (a transmembrane glycoprotein) and the extracellular expression of galectin-3 (a galactose-binding protein).39 Therefore, targeting the Bcl-2 family is a potential pathway for flavonoids to combat GC.
Targeting MAPK Signaling Pathway
The mitogen-activated protein kinase (MAPK) signaling pathway is an evolutionarily conserved pathway that plays a vital role in carcinogenesis. It consists of five cascades: ERK1/2, SAPK/JNK, p38 MAPK, ERK5, and ERK3/4.135 The MAPK pathway has been implicated in various cellular processes such as proliferation, differentiation, migration, senescence, and apoptosis.136 Therefore, researchers have investigated the interplay between flavonoids’ anticancer properties and the MAPK signaling pathway.
Isoorientin (Figure 3, 24), a C-glucosyl flavone naturally occurring in various foods and beverages, has been found to inhibit the p-ERK, p-STAT3, and NF-κB signaling pathways through ROS-mediated MAPK/STAT3/NF-κB signaling. This resulted in increased expression levels of STAT3 and NF-κB and induced apoptosis in AGS cells.83,137 Likewise, licochalcone A (Figure 3, 26) isolated from Glycyrrhiza glabra suppressed the MAPK signaling cascade, induced ROS generation and oxidative stress in BGC cells, leading to cell death.86 In addition, luteolin (Figure 3, 28) inhibited the MAPK and PI16K signaling pathways at the mRNA level by up-regulating specific dual-specificity phosphatases and down-regulating chemokine (C-X-C motif) ligand 3, leading to apoptosis.93 Therefore, these flavonoids have shown the potential to induce apoptosis and exhibit antitumor effects in GC cells through the modulation of the MAPK signaling pathway.
Induction of Autophagy
Autophagy is a process of programmed cell death whereby dead cells trigger the formation of autophagosomes, followed by degradation.138,139 There is increasing evidence supporting the role of flavonoids in inducing autophagy in GC cells. Notably, the citrus flavonoid naringin (Figure 3, 38) was found to induce autophagic cell death by promoting ROS production. This, in turn, activated the ERK1/2-p38 MAPK pathway and caused LMP-mediated lysosomal damage, ultimately leading to autophagy.100 Similarly, treatment of AGS cells with apigenin (Figure 3, 18) resulted in increased autophagic cell death. This was evidenced by the upregulation of LC3B-II and beclin-1, the formation of autophagic vesicles and acidic vesicular organelles, as well as the elevated expression of the autophagic flux marker protein p62, all of which were induced by ERS.47 Mechanistically, it has been reported that apigenin induced autophagic cell death in GC cells through the mTOR/AMPK/ULK1 pathway, leading to the down-regulation of p-mTOR, while increasing AMPK and ULK1 phosphorylation. Furthermore, under both normoxic and hypoxic conditions, apigenin induced ERS and autophagic cell death by inhibiting HIF-1α and Ezh2.42 Conversely, in a tumor tissue model using nude mice, galangin (Figure 3, 39) reduced the phosphorylation level of proteins related to the NF-κB signaling pathway, down-regulated the expression of the autophagy marker protein LC3B-I, up-regulated LC3B-II, and induced autophagy in MGC803 tumor tissues.75
Induction of Ferroptosis and Pyroptosis
Ferroptosis is an iron-dependent, non-apoptotic form of cell death characterized by an accumulation of iron and ROS.125,140 Substances that induce ferroptosis can directly or indirectly modulate glutathione peroxidase, leading to a reduction in antioxidant capacity, an increase in ROS, and ultimately oxidative cell death.141 A limited number of studies have investigated the potential anti-GC activity of flavonoids through ferroptosis. For instance, baicalin (Figure 3, 20) promoted ROS-associated ferroptosis in AGS and SGC-7901 cells.57 Amentoflavone (Figure 3, 40), a naturally occurring multifunctional biflavonoid, was found to inhibit activating transcription factor 2 (ATF2) by up-regulating miR-496 and subsequently malondialdehyde (MDA), ROS, and glutathione down-regulation. As a result, amentoflavone inhibited GC cell proliferation and induced ferroptosis.41
In contrast to ferroptosis, pyroptosis is an inflammatory vesicle-triggered, lysogenic programmed cell death characterized by cell swelling, lysis, and the release of various pro-inflammatory factors such as IL-1β, IL-18, ATP, and HMGB1.142,143 In one study, quercetin (Figure 3, 41), a dietary flavonol, exerted its pyroptosis effect on AGS cells by activating the core pyroptosis gene and significantly upregulating the expression levels of pyroptosis makers (GSDMD, GSDME, Cleaved CASP1, NLRP3).106 Although there are fewer studies investigating the impact of flavonoids on these two pathways, further research can explore additional flavonoids’ effects on GC cell activity through ferroptosis and pyroptosis.
Combination with Other Drugs
In current treatments for GC, 5-FU and platinum compounds are frequently selected as chemotherapeutic agents. Consequently, combinations of these agents with flavonoids have emerged as a common approach to investigate their synergistic antitumor effects (Table 2).
Table 2.
Profiles of Flavonoids Combined Other Drugs Inhibiting GC
| Flavonoid | Combination of Drugs | Cell Line | Targets | Reference |
|---|---|---|---|---|
| Baicalein | Baicalin, berberine chloride | AGS | ↓IL-8, COX-2, iNOS | [144] |
| Cisplatin | MGC-803, HGC-27, SGC-7901, SGC-7901/DDP | ↑Nrf2; ↓Keap1, MDR1 | [145] | |
| 5-FU | AGS | ↑PTEN; ↓HIF-1α | [54] | |
| Cardamonin | 5-FU | BGC-823 | ↓P-glycoprotein, β-catenin, TCF4 | [146] |
| Catechin | 5-FU | SNU-620, SNU-620/5FU | ↑Caspase-3, Bax; ↓Bcl-2 | [147] |
| Chrysin | 5-FU | AGS, AGS/FR | ↓MDR1 | [148] |
| Isoliquiritigenin | 5-FU | MKN-45 | ↓GRP78 | [117] |
| Licochalcone A | 5-FU | SGC-7901, MKN-45 | ↑Caspase-3, Bax; ↓Bcl-2 | [149] |
| Troxerutin | 5-FU | SGC-7901 | ↓STAT3/NF-κB, Bcl-2 | [150] |
| Kaempferol | Cisplatin/paclitaxel | AGS, SNU-216, NCI-N87, SNU-638 | ↑LC3-II; ↓G9a, p62 | [151] |
| Liquiritin | Cisplatin | SGC7901/DDP (in vivo) | ↑p53, p21; ↓cyclin D1, cyclin A, CDK4 | [152] |
| Luteolin | Cisplatin | AGS | ↑p21/cip1;↓Cdc2,Cyclin B1,Cdc25C | [153] |
| Oxaliplatin | SGC-7901 | Inhibit cell proliferation | [154] | |
| Oxaliplatin | MFC | ↑Bad; ↓Bcl-xL | [155] | |
| Naringenin | ABT-737 | SGC-7901 | ↑p53; ↓Akt | [156] |
| Nobiletin | Cisplatin | TMK-1, MKN-45, MKN-74, KATO-III | Inhibit cell proliferation | [157] |
| Quercetin | Irinotecan | AGS (in vivo) | ↓VEGF | [158] |
| Rutin | Cisplatin, isoquercetin | AGS | Increase cytotoxicity | [159] |
| Oxaliplatin | SGC-7901 | ↑p38/Caspase | [160] | |
| Lobetyolin, calycosin-7-glucoside | MGC-803, SGC-7901, BGC-823 | ↑HIF-1α | [161] | |
| Quercetin, d-chiro-inositol | MGC-803 | Inhibit cell proliferation | [162] | |
| Wogonin | Oxaliplatin | BGC-823 | ↑JNK | [163] |
Combination with 5-FU
5-FU, a uracil analog, functions by inhibiting thymidylate synthase, thus reducing DNA synthesis. It is widely employed as a chemotherapeutic drug in the treatment of GC.164 However, the emergence of resistance poses a significant challenge in chemotherapy. Catechin, an antioxidant flavonoid predominantly found in woody plants as (+)-catechin and (-)-epicatechin165, was found to effectively inhibit lactic acid production and lactate dehydrogenase A (LDHA) activity. It specifically targeted lactate dehydrogenase A to enhance the susceptibility of SNU620 GC cells to 5-FU.147 This suggests that combining catechin with 5-FU enhances the cytotoxic effect on GC cells. Furthermore, similar resistance-reversing effects of flavonoids and 5-FU combinations were observed in other types of GC cells, such as AGS and MGS cells. For instance, the combination of chrysin (figure 2, 7) and 5-FU resulted in enhanced chemotherapeutic effects by blocking the G2/M phase in 5-FU-resistant AGS cells.148 Under hypoxic conditions, baicalein (Figure 2, 6) inhibited glycolysis by modulating the PTEN/Akt/HIF-1α signaling pathway and increased the sensitivity of AGS cells to 5-FU. It also reversed the resistance of cancer cells to 5-FU under hypoxic conditions.54 In the case of MGC-803 cells, wogonin (Figure 2, 13) modulated metabolizing enzymes of 5-FU, prolonging catabolism and sensitizing MGC-803 cells to 5-FU-induced apoptosis by inhibiting NF-κB nuclear translocation.111 Additional studies on flavonoids combined with 5-FU in GC are listed in Table 2.117,146,149,150
Combination with Platinum Compounds
Platinum compounds, being dominant and critical metalloids, find extensive application in the treatment of solid malignant tumors. They are commonly used in clinical practice as anticancer drugs for both monotherapy and combination therapy.166,167 However, flavonoids obtained from natural products often combine with platinum compounds to produce specific antitumor effects. For instance, when liquiritin (Figure 3, 29) and cisplatin were combined, they effectively induced apoptosis and autophagy both in vitro and in vivo in a GC cell model using nude mice. This combination enhanced the cleavage of caspase8/-9/-3 and PARP, as well as the expression of LC3B and Beclin 1.152 Luteolin (Figure 3, 28) impaired the cellular mitochondrial membrane potential and hindered the growth and proliferation of GC cells when MFC cells were treated with low-dose oxaliplatin.155 Additionally, wogonin (Figure 2, 13) enhanced not only the cytotoxic effect of 5-FU but also the cytotoxicity of oxaliplatin. Wogonin further aggravated the damage to the mitochondrial membrane potential induced by oxaliplatin in BGC-823 cells. Furthermore, when used in combination, wogonin allowed for a reduction in the dosage of oxaliplatin, thereby reducing the side effects of chemotherapeutic agents.163 More flavonoids in conjunction with platinum chemicals for GC are listed in Table 2.145,151,153,154,157,159,160
Combination with Other Natural Products
In addition to these two commonly used chemotherapeutic agents, flavonoids can be combined with other natural products to combat GC. For example, Hwanglyeonhaedok-tang, a traditional medicine, contains baicalein (Figure 2, 6) along with small amounts of alkaloids. This compound has been shown to inhibit H. pylori adhesion, as well as the increase of IL-8 and COX-2 in AGS cells 144 Another example is Modified Spleen and Nutritional Soup, which contains mangostensin and rutin. These compounds have been found to inhibit GC progression by regulating tumor-associated macrophages and decreasing aerobic glycolysis in GC cells.161
Overall, these findings indicate that flavonoids alone possess certain antitumor effects on GC cells. However, when combined with chemotherapeutic drugs, they can help reduce drug resistance. Additionally, when combined with natural products, they can enhance their cytotoxic effects.
Discussion
GC, as a tumor with high morbidity and mortality, often faces major challenges in its treatment. We reviewed the mechanisms of action of flavonoids against gastric cancer from different pathways, such as inhibition of tumor growth and proliferation, invasion and metastasis, neovascularization, and induction of cell death. Several studies have found that flavonoids act on a variety of different biological pathways to inhibit GC, especially inducing cell death in gastric cancer. Flavonoids target pathways such as PI3K/Akt, TRAIL, MAPK, and the Bcl-2 family. In addition, it is worth our attention that flavonoids also act on common gastric cancer targets HER2, cMet, and VEGF. However, only some of the flavonoids have been found to inhibit these common gastric cancer targets, but the strength of the inhibitory effect needs to be verified by other experiments, and it remains to be explored whether other flavonoids also act on these targets. Moreover, compared with the studies that are associated with the signaling pathway, the research about flavonoids inhibiting HER2, Met, and PD-1 targets seems less, and even studies of flavonoids on Claudin 18.2 targets are lacking.
Flavonoids are mostly extracted from plants and often have multi-target effects. In addition to the common signaling pathways such as PI3K/Akt, there are some other targets that deserve our attention, one of which is short-chain ribonucleic acid. miR-7 and miR-496 are the two flavonoid drug targets that have been discovered so far, but their specific effects and strengths of action have not yet been explored. In addition, other solid tumor targets may be of interest. MUC1, a glycosylated type I transmembrane protein, is a potential drug target. Direct methods, including biotin labeling technology, click chemistry, and photocrosslinking reaction labeling technology, and indirect methods, including proteomics, metabolomics, and computer-aided drug design, are used for validation. Currently, there are many drug targets for gastric cancer, and the most common ones are HER2 and VEGF, which are mentioned above, but they are far from being sufficient for the treatment of tumors, so it is necessary to seek more new drug targets.
In addition to their antitumor effects through targeting, flavonoids may act in combination with other drugs. Most gastric cancers are insensitive to immune checkpoint inhibitor monotherapy, so patients with gastric cancer may need combination therapy to improve the response to immune checkpoint inhibitors. ADC drugs are a class of targeted biopharmaceuticals consisting of antibodies, linkers, and cytotoxic drugs, which can be used in tandem with small-molecule drugs to exert antitumor effects. Flavonoids are mostly natural small molecules, and more monomers have inhibitory effects on certain targets. Whether it is possible to develop flavonoids as new ADC drugs for gastric cancer treatment in the future, alone or in combination with PD-1 inhibitor K drugs, requires more research to explore.
However, even though there are more studies demonstrating the anticancer activity of flavonoids, their clinical aspects of research still need to be further deepened. In addition, age, gender, and genotype affect the absorption, distribution, metabolism, and elimination of flavonoids, resulting in low absorption, extensive metabolism, and rapid elimination, which affects the bioavailability and bioactivity of flavonoids, among others. Moreover, flavonoids are categorized into different subgroups, including isoflavonols, flavanones, flavones, flavonols, anthocyanins, chalcones and so on. One of the representative compounds of flavonols is quercetin (Figure 3, 41), which may be detrimental to athletes, the elderly, or people with enzyme problems. Therefore, researchers should explore how to maximize the retention of flavonoids’ bioactivity while reducing their side effects and maximizing the use of the drug in the future.
In conclusion, flavonoids have a wide range of biological effects, with particularly prominent anti-cancer effects. Due to the advantages of flavonoids’ wide source, safety, and certain anticancer effects, in the future, flavonoids can be used in tumor therapy as drug formulations alone or in combination with existing antitumor drugs for multi-targeted or directed-targeted therapies. Therefore, flavonoids can be a good research direction for exploring GC therapeutic options and providing new possibilities for GC treatment.
Acknowledgments
The authors would like to thank all reviewers and also the authors of all references. The reviewer’s advice really makes the great improvement of this paper.
Funding Statement
This paper was supported by the Major Program of the National Natural Science Foundation of China (82192915), National Natural Science Foundation of China (82274187) (82174346), Major scientific research problems and key topics of medical technology problems of China Medical Education Association (2022KTZ016), the Science and Technology Project of Sichuan Province (Grant No. 2022YFS0399), Xinglin Scholar Research Project of Chengdu University of TCM (Grant no. QJRC2022028)(Grant No. QJJJ2022010), the Program of Science and Technology Department of Sichuan Province (Grant No. 2023NSFSC0039) (grant no. 2023NSFSC1761), “Hundred Talents Program” of the Hospital of the Chengdu University of Traditional Chinese Medicine (Grant No. 22-B09), Young Elite Scientists Sponsorship Program by CACM (CACM-(2023-QNRC2-A01)).
Abbreviations
5hmC, 5-hydroxymethylcytosine; ASK1, apoptosis signal-regulating kinase 1; ATF2, activating transcription factor 2; ATF2, activating transcription factor 2; Bax, Bcl-2-associated X; BTG3, B-cell translocation gene 3; CDK1, cyclin-dependent kinase 1; cFLIP, cellular FLICE-like inhibitory protein; DR, death receptors; EBV, Epstein Barr virus; EGCG, epigallocatechin-3-gallate; EMT, epithelial mesenchymal transition; ERK, extracellular signal-regulated kinase; ERS, endoplasmic reticulum stress; EZH2, The enhancer of zeste homolog 2; FADD, Fas-associated protein with death domain; FAK, focal adhesion kinase; GC, gastric cancer; GCs, gastric cancers; H. pylori, Helicobacter pylori; HIF-1alpha, hypoxia-inducible factor-1alpha; LDHA, lactate dehydrogenase A; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MMP-9, matrix metalloproteinase 9; MUC, mucin; NICD, Notch intracellular domain; PARP, polymerase; PI3K, phosphatidylinositol 3 kinase; PTEN, phosphatase and tensin homolog; RON, Recepteur d’origine Nantais; SIRT1, silent information regulator 1; TET, ten-eleven translocation; VEGF, vascular endothelial growth factor.
Consent for Publication
All authors read and approved the final manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that there are no conflicts of interest and agree to publish this paper.
References
- 1.Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167–192. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Internat J Can. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 3.Ashrafizadeh M, Rafiei H, Mohammadinejad R, Farkhondeh T, Samarghandian S. Anti-tumor activity of resveratrol against gastric cancer: a review of recent advances with an emphasis on molecular pathways. Cancer Cell Int. 2021;21(1):66. doi: 10.1186/s12935-021-01773-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020;69(5):823–829. doi: 10.1136/gutjnl-2019-320234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferro A, Peleteiro B, Malvezzi M, et al. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50(7):1330–1344. doi: 10.1016/j.ejca.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 6.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 7.Bouras E, Tsilidis KK, Triggi M, Siargkas A, Chourdakis M, Haidich AB. Diet and risk of gastric cancer: an umbrella review. Nutrients. 2022;14:9. doi: 10.3390/nu14091764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J, Thrift AP. Alcohol, smoking and risk of oesophago-gastric cancer. Best practice & research. Clin Gastroenterol. 2017;31(5):509–517. doi: 10.1016/j.bpg.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21(11):4012. doi: 10.3390/ijms21114012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. doi: 10.1016/j.canlet.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 11.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. doi: 10.5114/pg.2018.80001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun DQ, Yang F, Li H, et al. 1990—2019年全球胃癌发病率和死亡率变化趋势及地区差异 [Regional disparities in trends of global gastric cancer incidence and mortality from 1990 to 2019]. Zhonghua zhong liu za zhi. 2022;44(9):950–954. Chinese. doi: 10.3760/cma.j.cn112152-20220120-00049 [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Anna Oncol. 2015;26(1):141–148. doi: 10.1093/annonc/mdu472 [DOI] [PubMed] [Google Scholar]
- 14.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi: 10.1016/S0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- 15.Ullah A, Munir S, Badshah SL, et al. Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badshah SL, Faisal S, Muhammad A, Poulson BG, Emwas AH, Jaremko M. Antiviral activities of flavonoids. Biomed Pharmacothe. 2021;140:111596. doi: 10.1016/j.biopha.2021.111596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Torres I, Castrejón-Téllez V, Soto ME, Rubio-Ruiz ME, Manzano-Pech L, Guarner-Lans V. Oxidative stress, plant natural antioxidants, and obesity. Int J Mol Sci. 2021;22(4):1786. doi: 10.3390/ijms22041786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki N, Nakayama T. Achievements and perspectives in biochemistry concerning anthocyanin modification for blue flower coloration. Plant Cell Physiol. 2015;56(1):28–40. doi: 10.1093/pcp/pcu097 [DOI] [PubMed] [Google Scholar]
- 19.Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126(2):485–493. doi: 10.1104/pp.126.2.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. TheScientificWorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Tan X, Zhang W, et al. Natural products targeting macroautophagy signaling in hepatocellular carcinoma therapy: Recent evidence and perspectives. Phytother Res. 2024;38(3):1623–1650. doi: 10.1002/ptr.8103 [DOI] [PubMed] [Google Scholar]
- 22.Luo S, Huang M, Lu X, et al. Optimized therapeutic potential of Yinchenhao decoction for cholestatic hepatitis by combined network meta-analysis and network pharmacology. Phytomedicine. 2024;129:155573. doi: 10.1016/j.phymed.2024.155573 [DOI] [PubMed] [Google Scholar]
- 23.Jiang ZB, Wang WJ, Xu C, et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021;515:36–48. doi: 10.1016/j.canlet.2021.05.019 [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Yao Q, An Y, Fan L, Wang J, Li H. Baicalin suppresses the progression of Type 2 diabetes-induced liver tumor through regulating METTL3/m(6)A/HKDC1 axis and downstream p-JAK2/STAT1/clevaged Capase3 pathway. Phytomedicine. 2022;94:153823. doi: 10.1016/j.phymed.2021.153823 [DOI] [PubMed] [Google Scholar]
- 25.Selvakumar P, Badgeley A, Murphy P, et al. Flavonoids and other polyphenols act as epigenetic modifiers in breast cancer. Nutrients. 2020;12:3. doi: 10.3390/nu12030761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banik K, Khatoon E, Harsha C, et al. Wogonin and its analogs for the prevention and treatment of cancer: a systematic review. Phytoth Res. 2022;36(5):1854–1883. doi: 10.1002/ptr.7386 [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Arce E, Saldías M. Antioxidant properties of flavonoid metal complexes and their potential inclusion in the development of novel strategies for the treatment against neurodegenerative diseases. Biomed Pharmacothe. 2021;143:112236. doi: 10.1016/j.biopha.2021.112236 [DOI] [PubMed] [Google Scholar]
- 28.Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as Anticancer Agents. Nutrients. 2020;12:2. doi: 10.3390/nu12020457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124 [DOI] [PubMed] [Google Scholar]
- 30.Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531. doi: 10.1016/j.foodchem.2022.132531 [DOI] [PubMed] [Google Scholar]
- 31.Zeng N, Zhang G, Hu X, Pan J, Gong D. Mechanism of fisetin suppressing superoxide anion and xanthine oxidase activity. J Funct Food. 2019;58:1–10. doi: 10.1016/j.jff.2019.04.044 [DOI] [Google Scholar]
- 32.Hou DX, Kumamoto T. Flavonoids as protein kinase inhibitors for cancer chemoprevention: direct binding and molecular modeling. Antioxid Redox Signaling. 2010;13(5):691–719. doi: 10.1089/ars.2009.2816 [DOI] [PubMed] [Google Scholar]
- 33.Kaushal N, Singh M, Singh Sangwan R. Flavonoids: food associations, therapeutic mechanisms, metabolism and nanoformulations. Food Res Internat. 2022;157:111442. doi: 10.1016/j.foodres.2022.111442 [DOI] [PubMed] [Google Scholar]
- 34.Hanáková Z, Hošek J, Kutil Z, et al. Anti-inflammatory activity of natural geranylated flavonoids: cyclooxygenase and lipoxygenase inhibitory properties and proteomic analysis. J Natural Prod. 2017;80(4):999–1006. doi: 10.1021/acs.jnatprod.6b01011 [DOI] [PubMed] [Google Scholar]
- 35.Zeinali M, Rezaee SA, Hosseinzadeh H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed Pharmacothe. 2017;92:998–1009. doi: 10.1016/j.biopha.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 36.Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS nano. 2020;14(4):4816–4828. doi: 10.1021/acsnano.0c00708 [DOI] [PubMed] [Google Scholar]
- 37.Pan MH, Lai CS, Hsu PC, Wang YJ. Acacetin induces apoptosis in human gastric carcinoma cells accompanied by activation of caspase cascades and production of reactive oxygen species. J Agricul Food Chem. 2005;53(3):620–630. doi: 10.1021/jf048430m [DOI] [PubMed] [Google Scholar]
- 38.Zhang G, Li Z, Dong J, et al. Acacetin inhibits invasion, migration and TGF-β1-induced EMT of gastric cancer cells through the PI3K/Akt/Snail pathway. BMC Complement Med Therap. 2022;22(1):10. doi: 10.1186/s12906-021-03494-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radziejewska I, Supruniuk K, Czarnomysy R, Buzun K, Bielawska A. Anti-cancer potential of afzelin towards AGS gastric cancer cells. Pharmaceut. 2021;14(10). doi: 10.3390/ph14100973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Lu W, Li Y, Tang B. Alpinetin promotes Bax translocation, induces apoptosis through the mitochondrial pathway and arrests human gastric cancer cells at the G2/M phase. Molec Med Rep. 2013;7(3):915–920. doi: 10.3892/mmr.2012.1243 [DOI] [PubMed] [Google Scholar]
- 41.Tang F, Xu Y, Gao E, et al. Amentoflavone attenuates cell proliferation and induces ferroptosis in human gastric cancer by miR −496/ ATF2 axis. Chem. Biol. Drug Des. 2023;102:782–792. doi: 10.1111/cbdd.14288 [DOI] [PubMed] [Google Scholar]
- 42.Kim TW, Lee HG. Apigenin induces autophagy and cell death by targeting EZH2 under hypoxia conditions in gastric cancer cells. Int J Mol Sci. 2021;22:24. doi: 10.3390/ijms222413455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Chen J, Li Z, Liu C, Yin L. The apoptotic effect of apigenin on human gastric carcinoma cells through mitochondrial signal pathway. Tumour Biol. 2014;35(8):7719–7726. doi: 10.1007/s13277-014-2014-x [DOI] [PubMed] [Google Scholar]
- 44.Wu K, Yuan LH, Xia W. Inhibitory effects of apigenin on the growth of gastric carcinoma SGC-7901 cells. World J Gastroenterol. 2005;11(29):4461–4464. doi: 10.3748/wjg.v11.i29.4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang YC, Huang KM. In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem Toxicol. 2013;53:376–383. doi: 10.1016/j.fct.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 46.Sun Q, Lu NN, Feng L. Apigetrin inhibits gastric cancer progression through inducing apoptosis and regulating ROS-modulated STAT3/JAK2 pathway. Biochem Biophys Res Commun. 2018;498(1):164–170. doi: 10.1016/j.bbrc.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 47.Kim SM, Vetrivel P, Ha SE, Kim HH, Kim JA, Kim GS. Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell. J Nutr Biochem. 2020;83:108427. doi: 10.1016/j.jnutbio.2020.108427 [DOI] [PubMed] [Google Scholar]
- 48.Qiao D, Jin J, Xing J, et al. Baicalein Inhibits Gastric Cancer Cell Proliferation and Migration through a FAK Interaction via AKT/mTOR Signaling. Am J Chin Med. 2021;49(2):525–541. doi: 10.1142/S0192415X21500245 [DOI] [PubMed] [Google Scholar]
- 49.Chen F, Zhuang M, Peng J, et al. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-β signaling pathway. Molec Med Rep. 2014;10(4):1999–2003. doi: 10.3892/mmr.2014.2452 [DOI] [PubMed] [Google Scholar]
- 50.Qiao D, Xing J, Duan Y, et al. The molecular mechanism of baicalein repressing progression of gastric cancer mediating miR-7/FAK/AKT signaling pathway. Phytomedicine. 2022;100:154046. doi: 10.1016/j.phymed.2022.154046 [DOI] [PubMed] [Google Scholar]
- 51.Mu J, Liu T, Jiang L, et al. The traditional Chinese medicine baicalein potently inhibits gastric cancer cells. J Cancer. 2016;7(4):453–461. doi: 10.7150/jca.13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen J, Yang Z, Wu X, Yao G, Hou M. Baicalein facilitates gastric cancer cell apoptosis by triggering endoplasmic reticulum stress via repression of the PI3K/AKT pathway. Appl Biol Chem. 2023;66(1):10. doi: 10.1186/s13765-022-00759-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan X, Rui X, Zhang K. Baicalein inhibits the invasion of gastric cancer cells by suppressing the activity of the p38 signaling pathway. Oncol Rep. 2015;33(2):737–743. doi: 10.3892/or.2014.3669 [DOI] [PubMed] [Google Scholar]
- 54.Chen F, Zhuang M, Zhong C, et al. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol Rep. 2015;33(1):457–463. doi: 10.3892/or.2014.3550 [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Li H, Chen F, et al. Baicalin extracted from Huangqin (Radix Scutellariae Baicalensis) induces apoptosis in gastric cancer cells by regulating B cell lymphoma (Bcl-2)/Bcl-2-associated X protein and activating caspase-3 and caspase-9. J Trad Chin Med. 2017;37(2):229–5. doi: 10.1016/s0254-6272(17)30049-3 [DOI] [PubMed] [Google Scholar]
- 56.Chen ME, Su CH, Yang JS, et al. Baicalin, baicalein, and lactobacillus rhamnosus jb3 alleviated helicobacter pylori infections in vitro and in Vivo. J Food Sci. 2018;83(12):3118–3125. doi: 10.1111/1750-3841.14372 [DOI] [PubMed] [Google Scholar]
- 57.Yuan J, Khan SU, Yan J, Lu J, Yang C, Tong Q. Baicalin enhances the efficacy of 5-Fluorouracil in gastric cancer by promoting ROS-mediated ferroptosis. Biomed Pharmacothe. 2023;164:114986. doi: 10.1016/j.biopha.2023.114986 [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Tian L, Long L, Quan M, Liu F, Cao J. Casticin potentiates TRAIL-induced apoptosis of gastric cancer cells through endoplasmic reticulum stress. PLoS One. 2013;8(3):e58855. doi: 10.1371/journal.pone.0058855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Tang X, Wu X, et al. Cardamonin exerts anti-gastric cancer activity via inhibiting LncRNA-PVT1-STAT3 axis. Biosci Rep. 2019;39:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohammadian F, Pilehvar-Soltanahmadi Y, Alipour S, Dadashpour M, Zarghami N. Chrysin Alters microRNAs expression levels in gastric cancer cells: possible molecular mechanism. Drug Res. 2017;67(9):509–514. doi: 10.1055/s-0042-119647 [DOI] [PubMed] [Google Scholar]
- 61.Chen L, Li Q, Jiang Z, et al. Chrysin Induced Cell Apoptosis Through H19/let-7a/COPB2 axis in gastric cancer cells and inhibited tumor growth. Front Oncol. 2021;11:651644. doi: 10.3389/fonc.2021.651644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong X, Liu D, Jiang Z, et al. Chrysin induced cell apoptosis and inhibited invasion through regulation of TET1 expression in gastric cancer cells. Onco Targets Ther. 2020;13:3277–3287. doi: 10.2147/OTT.S246031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohammadian F, Abhari A, Dariushnejad H, Nikanfar A, Pilehvar-Soltanahmadi Y, Zarghami N. Effects of Chrysin-PLGA-PEG nanoparticles on proliferation and gene expression of miRNAs in gastric cancer cell line. Iran J Cancer Prev. 2016;9(4):e4190. doi: 10.17795/ijcp-4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia Y, Lian S, Khoi PN, et al. Chrysin inhibits tumor promoter-induced MMP-9 expression by blocking AP-1 via suppression of ERK and JNK pathways in gastric cancer cells. PLoS One. 2015;10(4):e0124007. doi: 10.1371/journal.pone.0124007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia Y, Lian S, Khoi PN, et al. Chrysin inhibits cell invasion by inhibition of Recepteur d’origine Nantais via suppressing early growth response-1 and NF-κB transcription factor activities in gastric cancer cells. Int j Oncol. 2015;46(4):1835–1843. doi: 10.3892/ijo.2015.2847 [DOI] [PubMed] [Google Scholar]
- 66.Lee H, Lee JH, Jung KH, Hong SS. Deguelin promotes apoptosis and inhibits angiogenesis of gastric cancer. Oncol Rep. 2010;24(4):957–963. doi: 10.3892/or.2010.957 [DOI] [PubMed] [Google Scholar]
- 67.Park JS, Khoi PN, Joo YE, et al. EGCG inhibits recepteur d’origine nantais expression by suppressing Egr-1 in gastric cancer cells. Int j Oncol. 2013;42(3):1120–1126. doi: 10.3892/ijo.2013.1775 [DOI] [PubMed] [Google Scholar]
- 68.Zhu BH, Zhan WH, Li ZR, et al. (-)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol. 2007;13(8):1162–1169. doi: 10.3748/wjg.v13.i8.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu BH, Chen HY, Zhan WH, et al. (-)-Epigallocatechin-3-gallate inhibits VEGF expression induced by IL-6 via Stat3 in gastric cancer. World J Gastroenterol. 2011;17(18):2315–2325. doi: 10.3748/wjg.v17.i18.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu E, Liang T, Wang X, Ban S, Han L, Li Q. Apoptosis induced by farrerol in human gastric cancer SGC-7901 cells through the mitochondrial-mediated pathway. Europ J Canc Prevent. 2015;24(5):365–372. doi: 10.1097/CEJ.0000000000000104 [DOI] [PubMed] [Google Scholar]
- 71.Liu E, Li J, Shi S, et al. Sustained ERK activation-mediated proliferation inhibition of farrerol on human gastric carcinoma cell line by G0/G1-phase cell-cycle arrest. Europ J Canc Prevent. 2016;25(6):490–499. doi: 10.1097/CEJ.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 72.Yao JN, Zhang XX, Zhang YZ, et al. Discovery and anticancer evaluation of a formononetin derivative against gastric cancer SGC7901 cells. Invest New Drugs. 2019;37(6):1300–1308. doi: 10.1007/s10637-019-00767-7 [DOI] [PubMed] [Google Scholar]
- 73.Liang X, Wang P, Yang C, et al. Galangin inhibits gastric cancer growth through enhancing STAT3 Mediated ROS production. Front Pharmacol. 2021;12:646628. doi: 10.3389/fphar.2021.646628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim DA, Jeon YK, Nam MJ. Galangin induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P. Food Chem Toxicol. 2012;50(3–4):684–688. doi: 10.1016/j.fct.2011.11.039 [DOI] [PubMed] [Google Scholar]
- 75.Liang XH, Yu MZ, Shi HL, Wu XJ. [Galangin enhances autophagy by inhibiting NF-κB pathway in gastric cancer MGC-803 cells]. Zhongguo Zhong yao za zhi. 2021;46(16):4167–4174. Chinese doi: 10.19540/j.cnki.cjcmm.20210406.401 [DOI] [PubMed] [Google Scholar]
- 76.Yu CY, Su KY, Lee PL, et al. Potential therapeutic role of hispidulin in gastric cancer through induction of apoptosis via NAG-1 Signaling. Evid Bas Complem Altern Med. 2013;2013:518301. doi: 10.1155/2013/518301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Dong H, Zhu M, et al. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur J Pharmacol. 2010;635(1–3):40–48. doi: 10.1016/j.ejphar.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 78.Zhang F, Yin Y, Xu W, et al. Icariin inhibits gastric cancer cell growth by regulating the hsa_circ_0003159/miR-223-3p/NLRP3 signaling axis. Hum Exp Toxicol. 2022;41:9603271221097363. doi: 10.1177/09603271221097363 [DOI] [PubMed] [Google Scholar]
- 79.Bhosale PB, Vetrivel P, Ha SE, et al. Iridin Induces G2/M phase cell cycle arrest and extrinsic apoptotic cell death through PI3K/AKT signaling pathway in AGS gastric cancer cells. Molecules. 2021;26:9. doi: 10.3390/molecules26092802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Y, Gao CC, Pan ZG, Zhou CW. Irigenin sensitizes TRAIL-induced apoptosis via enhancing pro-apoptotic molecules in gastric cancer cells. Biochem Biophys Res Commun. 2018;496(3):998–1005. doi: 10.1016/j.bbrc.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 81.Zhang XR, Wang SY, Sun W, Wei C. Isoliquiritigenin inhibits proliferation and metastasis of MKN28 gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling pathway. Molec Med Rep. 2018;18(3):3429–3436. doi: 10.3892/mmr.2018.9318 [DOI] [PubMed] [Google Scholar]
- 82.Liu J, Ren L, Wang H, Li Z. Isoquercitrin induces endoplasmic reticulum stress and immunogenic cell death in gastric cancer cells. Biochem Genet. 2023;61(3):1128–1142. doi: 10.1007/s10528-022-10309-1 [DOI] [PubMed] [Google Scholar]
- 83.Zhang T, Xiu YH, Xue H, et al. A mechanism of isoorientin-induced apoptosis and migration inhibition in gastric cancer AGS cells. Pharmaceuticals. 2022;15(12):1541. doi: 10.3390/ph15121541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou W, Cao A, Wang L, Wu D. Kurarinone synergizes TRAIL-induced apoptosis in gastric cancer cells. Cell Biochem Biophys. 2015;72(1):241–249. doi: 10.1007/s12013-014-0444-0 [DOI] [PubMed] [Google Scholar]
- 85.Xie R, Gao CC, Yang XZ, et al. Combining TRAIL and liquiritin exerts synergistic effects against human gastric cancer cells and xenograft in nude mice through potentiating apoptosis and ROS generation. Biomed Pharmacothe. 2017;93:948–960. doi: 10.1016/j.biopha.2017.06.095 [DOI] [PubMed] [Google Scholar]
- 86.Hao W, Yuan X, Yu L, et al. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci Rep. 2015;5:10336. doi: 10.1038/srep10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hongxia G, Xiaojie J, Guangxian L, et al. Licoflavone A suppresses gastric cancer growth and metastasis by blocking the VEGFR-2 signaling pathway. J Oncol. 2022;2022:5497991. doi: 10.1155/2022/5497991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yajie D, Feng L, Zhaoyan LI, et al. Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism. J Trad Chin Med. 2023;43(1):34–41. doi: 10.19852/j.cnki.jtcm.2023.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zang M, Hu L, Zhang B, et al. Luteolin suppresses angiogenesis and vasculogenic mimicry formation through inhibiting Notch1-VEGF signaling in gastric cancer. Biochem Biophys Res Commun. 2017;490(3):913–919. doi: 10.1016/j.bbrc.2017.06.140 [DOI] [PubMed] [Google Scholar]
- 90.Zang MD, Hu L, Fan ZY, et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J Transl Med. 2017;15(1):52. doi: 10.1186/s12967-017-1151-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu H, Huang M, Liu Y, Shu Y, Liu P. Luteolin induces apoptosis by up-regulating mir-34a in human gastric cancer cells. Technol Cancer Res Treat. 2015;14(6):747–755. doi: 10.7785/tcrt.2012.500434 [DOI] [PubMed] [Google Scholar]
- 92.Radziejewska I, Borzym-Kluczyk M, Leszczyńska K. Luteolin alters MUC1 extracellular domain, sT antigen, ADAM-17, IL-8, IL-10 and NF-κB expression in Helicobacter pylori-infected gastric cancer CRL-1739 cells: a preliminary study. Biomed Rep. 2021;14(2):19. doi: 10.3892/br.2020.1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu X, Li Y, Li X, Aisa HA. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer. Oncol Lett. 2017;14(2):1993–2000. doi: 10.3892/ol.2017.6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song S, Su Z, Xu H, et al. Correction: luteolin selectively kills STAT3 highly activated gastric cancer cells through enhancing the binding of STAT3 to SHP-1. Cell Death Dis. 2018;9(8):787. doi: 10.1038/s41419-018-0827-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu J, Li G, He K, et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J Transl Med. 2015;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Y, Ding BZ, Lin YP, Wang HB. MiR-34a, as a suppressor, enhance the susceptibility of gastric cancer cell to luteolin by directly targeting HK1. Gene. 2018;644:56–65. [DOI] [PubMed] [Google Scholar]
- 97.Ma J, Pan Z, Du H, et al. Luteolin induces apoptosis by impairing mitochondrial function and targeting the intrinsic apoptosis pathway in gastric cancer cells. Oncol Lett. 2023;26(2):327. doi: 10.3892/ol.2023.13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han SH, Lee JH, Woo JS, et al. Myricetin induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Heliyon. 2022;8(5):e09309. doi: 10.1016/j.heliyon.2022.e09309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng J, Chen X, Wang Y, et al. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol Cell Biochem. 2015;408(1–2):163–170. doi: 10.1007/s11010-015-2492-1 [DOI] [PubMed] [Google Scholar]
- 100.Raha S, Kim SM, Lee HJ, et al. Naringin induces lysosomal permeabilization and autophagy cell death in AGS gastric cancer cells. Am J Chin Med. 2020;48(3):679–702. doi: 10.1142/S0192415X20500342 [DOI] [PubMed] [Google Scholar]
- 101.Zhu L, Shi J, Mu M, et al. Naringin inhibits the proliferation, migration, invasion and epithelial-to-mesenchymal transition of gastric cancer cells via the PI3K/AKT signaling pathway. Alternat Therap Health Med. 2023;29(1):191–197. [PubMed] [Google Scholar]
- 102.Xu C, Huang X, Huang Y, et al. Naringin induces apoptosis of gastric carcinoma cells via blocking the PI3K/AKT pathway and activating pro‑death autophagy. Molec Med Rep. 2021;24:5. doi: 10.3892/mmr.2021.12412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bao L, Liu F, Guo HB, et al. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumour Biol. 2016;37(8):11365–11374. doi: 10.1007/s13277-016-5013-2 [DOI] [PubMed] [Google Scholar]
- 104.Lee YC, Cheng TH, Lee JS, et al. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell Biochem. 2011;347(1–2):103–115. doi: 10.1007/s11010-010-0618-z [DOI] [PubMed] [Google Scholar]
- 105.Lee HJ, Venkatarame Gowda Saralamma V, Kim SM, et al. Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling pathway. Nutrients. 2018;10:8. doi: 10.3390/nu10081043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rong Y, Liu SH, Tang MZ, Yang XJ. Quercetin inhibits the proliferative effect of gastric cancer cells by activating the pyroptosis pathway. Asian J Surg. 2023;46:5286–5288. doi: 10.1016/j.asjsur.2023.07.051 [DOI] [PubMed] [Google Scholar]
- 107.Li F, Wang S, Niu M. Scutellarin inhibits the growth and EMT of gastric cancer cells through regulating PTEN/PI3K Pathway. Biol Pharmac Bull. 2021;44(6):780–788. doi: 10.1248/bpb.b20-00822 [DOI] [PubMed] [Google Scholar]
- 108.Chen XJ, Cui QX, Wang GL, et al. Sanggenon C suppresses tumorigenesis of gastric cancer by blocking ERK-Drp1-mediated mitochondrial fission. J Natural Prod. 2022;85(10):2351–2362. doi: 10.1021/acs.jnatprod.2c00524 [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Li Q, Ge Y, et al. Silibinin triggers apoptosis and cell-cycle arrest of SGC7901 cells. Phytoth Res. 2013;27(3):397–403. doi: 10.1002/ptr.4733 [DOI] [PubMed] [Google Scholar]
- 110.Wang T, Gao J, Yu J, Shen L. Synergistic inhibitory effect of wogonin and low-dose paclitaxel on gastric cancer cells and tumor xenografts. Chin J Can Res. 2013;25(5):505–513. doi: 10.3978/j.issn.1000-9604.2013.08.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao Q, Wang J, Zou MJ, et al. Wogonin potentiates the antitumor effects of low dose 5-fluorouracil against gastric cancer through induction of apoptosis by down-regulation of NF-kappaB and regulation of its metabolism. Toxicol Lett. 2010;197(3):201–210. doi: 10.1016/j.toxlet.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 112.Wang SJ, Zhao JK, Ren S, Sun WW, Zhang WJ, Zhang JN. Wogonin affects proliferation and the energy metabolism of SGC-7901 and A549 cells. Exp Ther Med. 2019;17(1):911–918. doi: 10.3892/etm.2018.7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gu Q, Zhu C, Wu X, Peng L, Huang G, Hu R. Wogonoside promotes apoptosis and ER stress in human gastric cancer cells by regulating the IRE1α pathway. Exp Ther Med. 2021;21(4):411. doi: 10.3892/etm.2021.9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei S, Sun T, Du J, Zhang B, Xiang D, Li W. Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro. Oncol Rep. 2018;40(6):3213–3222. doi: 10.3892/or.2018.6723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X, Xia M. 5-Hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone, a polymethoxyflavone, exerts antitumor effect on PI3K/Akt signaling pathway in human gastric cancer cell BGC-7901. J Recep Sig Transd Res. 2016;36(5):471–477. doi: 10.3109/10799893.2015.1122046 [DOI] [PubMed] [Google Scholar]
- 116.Wang KL, Yu YC, Hsia SM. Perspectives on the role of isoliquiritigenin in cancer. Cancers. 2021;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee CH, Tsai HY, Chen CL, et al. Isoliquiritigenin inhibits gastric cancer stemness, modulates tumor microenvironment, and suppresses tumor growth through glucose-regulated protein 78 downregulation. Biomedicines. 2022;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Babaei G, Aziz SG, Jaghi NZZ. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed Pharmacothe. 2021;133:110909. [DOI] [PubMed] [Google Scholar]
- 119.Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fong Y, Shen KH, Chiang TA, Shih YW. Acacetin inhibits TPA-induced MMP-2 and u-PA expressions of human lung cancer cells through inactivating JNK signaling pathway and reducing binding activities of NF-kappaB and AP-1. J Food Sci. 2010;75(1):H30–8. doi: 10.1111/j.1750-3841.2009.01438.x [DOI] [PubMed] [Google Scholar]
- 121.Shen KH, Hung SH, Yin LT, et al. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem. 2010;333(1–2):279–291. doi: 10.1007/s11010-009-0229-8 [DOI] [PubMed] [Google Scholar]
- 122.He YF, Li BZ, Li Z, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333(6047):1303–1307. doi: 10.1126/science.1210944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Du C, Kurabe N, Matsushima Y, et al. Robust quantitative assessments of cytosine modifications and changes in the expressions of related enzymes in gastric cancer. Gast Can. 2015;18(3):516–525. doi: 10.1007/s10120-014-0409-4 [DOI] [PubMed] [Google Scholar]
- 124.Zhao T, Tang H, Xie L, et al. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmacol. 2019;71(9):1353–1369. doi: 10.1111/jphp.13129 [DOI] [PubMed] [Google Scholar]
- 125.Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204. doi: 10.1016/j.ctrv.2003.07.007 [DOI] [PubMed] [Google Scholar]
- 127.Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017;45:62–71. doi: 10.1016/j.ceb.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan R, Xu H, Fu X. Salidroside protects hypoxia-induced injury by up-regulation of miR-210 in rat neural stem cells. Biomed Pharmacothe. 2018;103:1490–1497. doi: 10.1016/j.biopha.2018.04.184 [DOI] [PubMed] [Google Scholar]
- 129.Ren XL, Zhu XH, Li XM, et al. Down-regulation of BTG3 promotes cell proliferation, migration and invasion and predicts survival in gastric cancer. J Cancer Res Clin Oncol. 2015;141(3):397–405. doi: 10.1007/s00432-014-1826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pan G, O’Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276(5309):111–113. doi: 10.1126/science.276.5309.111 [DOI] [PubMed] [Google Scholar]
- 131.Walczak H, Degli-Esposti MA, Johnson RS, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16(17):5386–5397. doi: 10.1093/emboj/16.17.5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qin J, Chen J, Peng F, et al. Pharmacological activities and pharmacokinetics of liquiritin: a review. J Ethnopharmacol. 2022;293:115257. doi: 10.1016/j.jep.2022.115257 [DOI] [PubMed] [Google Scholar]
- 133.Zhang G, Liao Y, Yang H, Tao J, Ma L, Zuo X. Irigenin reduces the expression of caspase-3 and matrix metalloproteinases, thus suppressing apoptosis and extracellular matrix degradation in TNF-α-stimulated nucleus pulposus cells. Chem Biol Interact. 2021;349:109681. [DOI] [PubMed] [Google Scholar]
- 134.Tomek M, Akiyama T, Dass CR. Role of Bcl-2 in tumour cell survival and implications for pharmacotherapy. J Pharm Pharmacol. 2012;64(12):1695–1702. doi: 10.1111/j.2042-7158.2012.01526.x [DOI] [PubMed] [Google Scholar]
- 135.Lee S, Rauch J, Kolch W. Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int J Mol Sci. 2020;21:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007. doi: 10.3892/etm.2020.8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ziqubu K, Dludla PV, Joubert E, et al. Isoorientin: a dietary flavone with the potential to ameliorate diverse metabolic complications. Pharmacol Res. 2020;158:104867. doi: 10.1016/j.phrs.2020.104867 [DOI] [PubMed] [Google Scholar]
- 138.Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19(1):12. doi: 10.1186/s12943-020-1138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kovacs SB, Miao EA. Gasdermins: effectors of Pyroptosis. Trends Cell Biol. 2017;27(9):673–684. doi: 10.1016/j.tcb.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fang Y, Tian S, Pan Y, et al. Pyroptosis: a new frontier in cancer. Biomed pharmacothe. 2020;121:109595. doi: 10.1016/j.biopha.2019.109595 [DOI] [PubMed] [Google Scholar]
- 144.Park HS, Wijerathne CUB, Jeong HY, Seo CS, Ha H, Kwun HJ. Gastroprotective effects of Hwanglyeonhaedok-tang against Helicobacter pylori-induced gastric cell injury. J Ethnopharmacol. 2018;216:239–250. doi: 10.1016/j.jep.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 145.Li P, Hu J, Shi B, Tie J. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem Biophys Res Commun. 2020;531(3):320–327. doi: 10.1016/j.bbrc.2020.07.045 [DOI] [PubMed] [Google Scholar]
- 146.Hou G, Yuan X, Li Y, Hou G, Liu X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway. Invest New Drugs. 2020;38(2):329–339. doi: 10.1007/s10637-019-00781-9 [DOI] [PubMed] [Google Scholar]
- 147.Han JH, Kim M, Kim HJ, et al. Targeting lactate dehydrogenase A with catechin resensitizes SNU620/5FU gastric cancer cells to 5-Fluorouracil. Int J Mol Sci. 2021;22:10. doi: 10.3390/ijms22105406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee S, Lee SK, Jung J. Potentiating activities of chrysin in the therapeutic efficacy of 5-fluorouracil in gastric cancer cells. Oncol Lett. 2021;21(1):24. doi: 10.3892/ol.2020.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin X, Tian L, Wang L, Li W, Xu Q, Xiao X. Antitumor effects and the underlying mechanism of licochalcone A combined with 5-fluorouracil in gastric cancer cells. Oncol Lett. 2017;13(3):1695–1701. doi: 10.3892/ol.2017.5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu GY, Tang XJ. Troxerutin (TXN) potentiated 5-Fluorouracil (5-Fu) treatment of human gastric cancer through suppressing STAT3/NF-κB and Bcl-2 signaling pathways. Biomed Pharmacothe. 2017;92:95–107. doi: 10.1016/j.biopha.2017.04.059 [DOI] [PubMed] [Google Scholar]
- 151.Kim TW, Lee SY, Kim M, Cheon C, Ko SG. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9(9):875. doi: 10.1038/s41419-018-0930-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wei F, Jiang X, Gao HY, Gao SH. Liquiritin induces apoptosis and autophagy in cisplatin (DDP)-resistant gastric cancer cells in vitro and xenograft nude mice in vivo. Int j Oncol. 2017;51(5):1383–1394. doi: 10.3892/ijo.2017.4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu B, Zhang Q, Shen W, Zhu J. Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol Cell Biochem. 2008;313(1–2):125–132. doi: 10.1007/s11010-008-9749-x [DOI] [PubMed] [Google Scholar]
- 154.Ren LQ, Li Q, Zhang Y. Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. Biomed Res Int. 2020;2020:9396512. doi: 10.1155/2020/9396512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ma J, Chen X, Zhu X, et al. Luteolin potentiates low-dose oxaliplatin-induced inhibitory effects on cell proliferation in gastric cancer by inducing G(2)/M cell cycle arrest and apoptosis. Oncol Lett. 2022;23(1):16. doi: 10.3892/ol.2021.13134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang H, Zhong X, Zhang X, Shang D, Zhou YI, Zhang C. Enhanced anticancer effect of ABT-737 in combination with naringenin on gastric cancer cells. Exp Ther Med. 2016;11(2):669–673. doi: 10.3892/etm.2015.2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yoshimizu N, Otani Y, Saikawa Y, et al. Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment Pharmacol Ther. 2004;20(1):95–101. doi: 10.1111/j.1365-2036.2004.02082.x [DOI] [PubMed] [Google Scholar]
- 158.Lei CS, Hou YC, Pai MH, Lin MT, Yeh SL. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J Nutr Biochem. 2018;51:105–113. [DOI] [PubMed] [Google Scholar]
- 159.Ghavami G, Muhammadnejad S, Amanpour S, Sardari S. Bioactivity screening of mulberry leaf extracts and two related flavonoids in combination with cisplatin on human gastric adenocarcinoma cells. Iran J Pharmac Res. 2020;19(2):371–382. doi: 10.22037/ijpr.2020.1101087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Q, Ren L, Zhang Y, et al. P38 signal transduction pathway has more cofactors on apoptosis of SGC-7901 gastric cancer cells induced by combination of rutin and oxaliplatin. Biomed Res Int. 2019;2019:6407210. doi: 10.1155/2019/6407210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun Q, Yuan M, Wang H, et al. PKM2 is the target of a multi-herb-combined decoction during the inhibition of gastric cancer progression. Front Oncol. 2021;11:767116. doi: 10.3389/fonc.2021.767116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou XL, Chen ZD, Zhou YM, Shi RH, Li ZJ. The effect of tartary buckwheat flavonoids in inhibiting the proliferation of MGC80-3 cells during seed germination. Molecules. 2019;24:17. doi: 10.3390/molecules24173092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hong ZP, Wang LG, Wang HJ, Ye WF, Wang XZ. Wogonin exacerbates the cytotoxic effect of oxaliplatin by inducing nitrosative stress and autophagy in human gastric cancer cells. Phytomedicine. 2018;39:168–175. doi: 10.1016/j.phymed.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 164.Blondy S, David V, Verdier M, Mathonnet M, Perraud A, Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: from classical pathways to promising processes. Can Sci. 2020;111(9):3142–3154. doi: 10.1111/cas.14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bernatoniene J, Kopustinskiene DM. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules. 2018;23:4. doi: 10.3390/molecules23040965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lv P, Man S, Xie L, Ma L, Gao W. Pathogenesis and therapeutic strategy in platinum resistance lung cancer. Biochim Biophys Acta Rev Cancer. 1876;2021(1):188577. [DOI] [PubMed] [Google Scholar]
- 167.Xiao X, Oswald JT, Wang T, Zhang W, Li W. Use of anticancer platinum compounds in combination therapies and challenges in drug delivery. Curr Med Chem. 2020;27(18):3055–3078. doi: 10.2174/0929867325666181105115849 [DOI] [PubMed] [Google Scholar]