Abstract
Wnt family proteins are secreted glycolipoproteins that signal through multitude of signal transduction pathways. The Wnt-pathways are conserved and critical in all metazoans. They are essential for embryonic development, organogenesis and homeostasis, and associated with many diseases when defective or deregulated. Wnt signaling pathways comprise the canonical Wnt pathway, best known for its stabilization of β-catenin and associated nuclear β-catenin activity in gene regulation, and several non-canonical signaling branches. Wnt–Planar Cell Polarity (PCP) signaling has received the most attention among the non-canonical Wnt pathways. The relationship of cilia to Wnt-signaling is complex. While it was suggested that canonical Wnt signaling requires cilia this notion was always challenged by results suggesting the opposite. Recent developments provide insight and clarification to the relationship of Wnt signaling pathways and cilia. First, it has been now demonstrated that while ciliary proteins, in particular the IFT-A complex, are required for canonical Wnt/β-catenin signaling, the cilium as a structure is not. In contrast, recent work has defined a diverged canonical signaling branch (not affecting β-catenin) to be required for ciliary biogenesis and cilia function. Furthermore, the non-canonical Wnt–PCP pathway does not affect cilia biogenesis per se, but it regulates the position of cilia within cells in many cell types, possibly in all cells where it is active, with cilia being placed near the side of the cell that has the Frizzled–Dishevelled complex. This Wnt/PCP feature is conserved with both centrioles and basal bodies/cilia being positioned accordingly, and it is also used to align mitotic spindles within the Wnt–PCP polarization axis. It also coordinates the alignment of cilia in multiciliated cells. This article addresses these new insights and different links and relationships between cilia and Wnt signaling.
1. Introduction
The Wnt signaling pathways comprise a major cellular signaling system. They are used across all metazoans for many aspects of animal development (A–P axis establishment), organogenesis and organ growth (with Wnts often acting as morphogens), cell fate induction, and the regulation of cell proliferation and cell death (Cadigan & Waterman, 2012; Clevers, 2006; Eisenmann, 2005; Logan & Nusse, 2004; MacDonald, Tamai, & He, 2009; Niehrs, 2012). The founding member of the Wnt gene family was discovered in Drosophila, with its original mutant allele lacking wings, and hence it was named wingless (wg) (Sharma & Chopra, 1976) and the name “Wnt” is derived from Drosophila Wg and mouse Int1, which was identified as the first mammalian homolog due to a viral insertion causing mammary gland tumors in the mouse (Nusse, van Ooyen, Cox, Fung, & Varmus, 1984). Drosophila genetics revealed that wg was an essential gene and required throughout development including embryogenesis (Nusslein-Volhard & Wieschaus, 1980). Subsequently, it was shown that the family of Wnt/Wg genes is conserved in all metazoans, with multiple members in all species, and encodes proteins of a secreted glycolipoprotein class. Wnt genes are both essential and critical for development, organogenesis, and homeostasis of all metazoans, and are also critically linked to regeneration and stem cell maintenance; moreover, defective and/or mis-regulated Wnt-signaling is linked to many diseases ranging from birth defects to cancer (Cadigan & Waterman, 2012; Clevers, 2006; Logan & Nusse, 2004; MacDonald et al., 2009; Simons & Mlodzik, 2008).
Signaling pathways associated with the Wnt protein family comprise a fairly large set of distinct signaling branches, which share the Wnt ligands and Frizzled (Fz, or Fzd in mammals, for short) receptor family and the downstream effector Dishevelled (Dsh, a.k.a. Dvl in mammals) (see below for review references). The canonical Wnt/β-catenin signaling pathway is the most studied and overall best understood (Cadigan & Waterman, 2012; Clevers, 2006; Eisenmann, 2005; Logan & Nusse, 2004; MacDonald et al., 2009; Niehrs, 2012) despite several gaps in our knowledge. For a comprehensive overview see the “Wnt homepage”: (www.stanford.edu/~rnusse/wntwindow.html), which has been curated by the lab of Roel Nusse.
In addition to the canonical pathway, there exist several so-called non-canonical Wnt signaling pathways; for a complete listing of additional non-canonical Wnt-pathways see (Semenov, Habas, Macdonald, & He, 2007). Among those non-canonical pathways, Wnt-Planar Cell Polarity (PCP) signaling stands out as the best understood and most conserved (Adler, 2002; Carvajal-Gonzalez & Mlodzik, 2014; Devenport, 2014; Goodrich & Strutt, 2011; Harrison, Shao, Strutt, & Strutt, 2020; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012; Wang & Nathans, 2007; Yang & Mlodzik, 2015). Both, the canonical Wnt pathway and Wnt/PCP signaling have also been linked to several distinct features of ciliary biology and ciliary proteins, and these functional interactions between Wnt-signaling and ciliary biology is the focus of this article.
Canonical Wnt/β-catenin signaling is the most critical of the Wnt-pathways in both development and disease (Clevers, 2006; Clevers & Nusse, 2012; MacDonald et al., 2009; Niehrs, 2012). Although quite a bit is understood about the pathway architecture, its relationship to cilia and ciliary function has remained unclear, with sometimes contradictory conclusions. Recent new insights have provided clarification to this and these new data are a main focus of this article (see below).
1.1. The canonical Wnt-signaling pathway
Canonical Wnt-signaling is highly conserved with critical roles in the regulation of a vast catalog of developmental processes, including but not restricted to tissue patterning as a morphogen across the animal kingdom, cell fate specification, cell proliferation, cell survival, and cell migration (Cadigan & Waterman, 2012; Clevers, 2006; Logan & Nusse, 2004; MacDonald et al., 2009). Dysregulated pathway components and or mis/overexpression of pathway proteins are in most cases associated with diseases, including several cancer types (Clevers, 2006; Coombs, Covey, & Virshup, 2008; Clevers & Nusse, 2012; Prestwich & Macdougald, 2007).
At the center of active canonical Wnt signaling is the stabilization of β-catenin (Armadillo/Arm in Drosophila) (Cadigan & Waterman, 2012; MacDonald et al., 2009; Valenta, Hausmann, & Basler, 2012) (Fig. 1 shows a simplified schematic). β-catenin/Arm is a multi-functional protein that is critical both, as a component of adherens junctions (AJs) and their link to cytoskeletal elements (reviewed in Bienz, 2005; McEwen, Escobar, & Gottardi, 2012; Tian et al., 2011) and as the key component in Wnt/Wg signaling to transduce pathway activation into a nuclear response. In the canonical Wnt pathway, β-catenin/Arm is the essential transcriptional co-activator for Wnt-target gene expression (Cadigan & Waterman, 2012; MacDonald et al., 2009; Valenta et al., 2012). Wnt/Wg proteins bind to Frizzled (Fz/Fzd in mammals) receptors and LRP5/6 (Arrow in Drosophila) co-receptors, resulting in the disassembly of the so-called “destruction complex” (DC), composed of Axin, APC (Adenomatous Polyposis Coli), and the kinases GSK3β and Casein kinase 1 (CK1). Both kinases, GSK3β and CK1, phosphorylate the cytoplasmic pool of β-catenin/Arm and thus target it for proteasomal degradation (Cadigan & Waterman, 2012; MacDonald et al., 2009; Niehrs, 2012; Valenta et al., 2012). Note that there are two pools of β-catenin/Arm, one being stably associated with cadherins within adherens junction (AJ) complexes and the other free, cytoplasmic pool that is targeted for degradation in the absence of Wnt-signaling. Wnt pathway activation results in the break-up of the destruction complex and re-localization of Axin to the plasma membrane, where it associates with Dsh/Dvl and the Fz/Fzd receptors and LRP5/6 co-receptors, forming large complexes of Axin–Dsh–Fz–LRP5/6 aggregates in endocytosed vesicles, generally referred to as signalosomes (Cadigan & Waterman, 2012; Cliffe, Hamada, & Bienz, 2003; MacDonald et al., 2009). Generally, the Dsh/Dvl and Axin proteins behave like protein scaffolds that hold together either the destruction complex (Axin) or the signalosome (Dsh/Dvl) (Cadigan & Waterman, 2012; MacDonald et al., 2009; Valenta et al., 2012). Axin removal from the destruction complex leads not only to complex disassembly, but importantly releases cytoplasmic β-catenin/Arm from GSK3β and CK1 phosphorylation, which is then stable in the cytoplasm and can translocate into the nucleus to act as a co-activator of the TCF/Lef transcription factors (Cadigan & Waterman, 2012; MacDonald et al., 2009). Again, in the absence of Wnt/Wg presence, β-catenin/Arm is phosphorylated by the “destruction complex” and targeted to the proteasome (Clevers & Nusse, 2012; Niehrs, 2012). While the membrane proximal and cytoplasmic aspects of the canonical Wnt-pathway are quite well defined, the mechanistic aspects of β-catenin/Arm nuclear translocation remain poorly understood. Its primary sequence does not contain a bona fide NLS (nuclear localization signal). Although its molecular function and interaction partners in the nucleus are again well known, the steps in between remain unclear. Recent insight into the potential mechanism(s) of how β-catenin/Arm is translocated in the nucleus has emerged (Balmer et al., 2015; Vuong et al., 2018) and is linked to ciliary proteins. These aspects will be discussed in a dedicated section below.
Fig. 1.
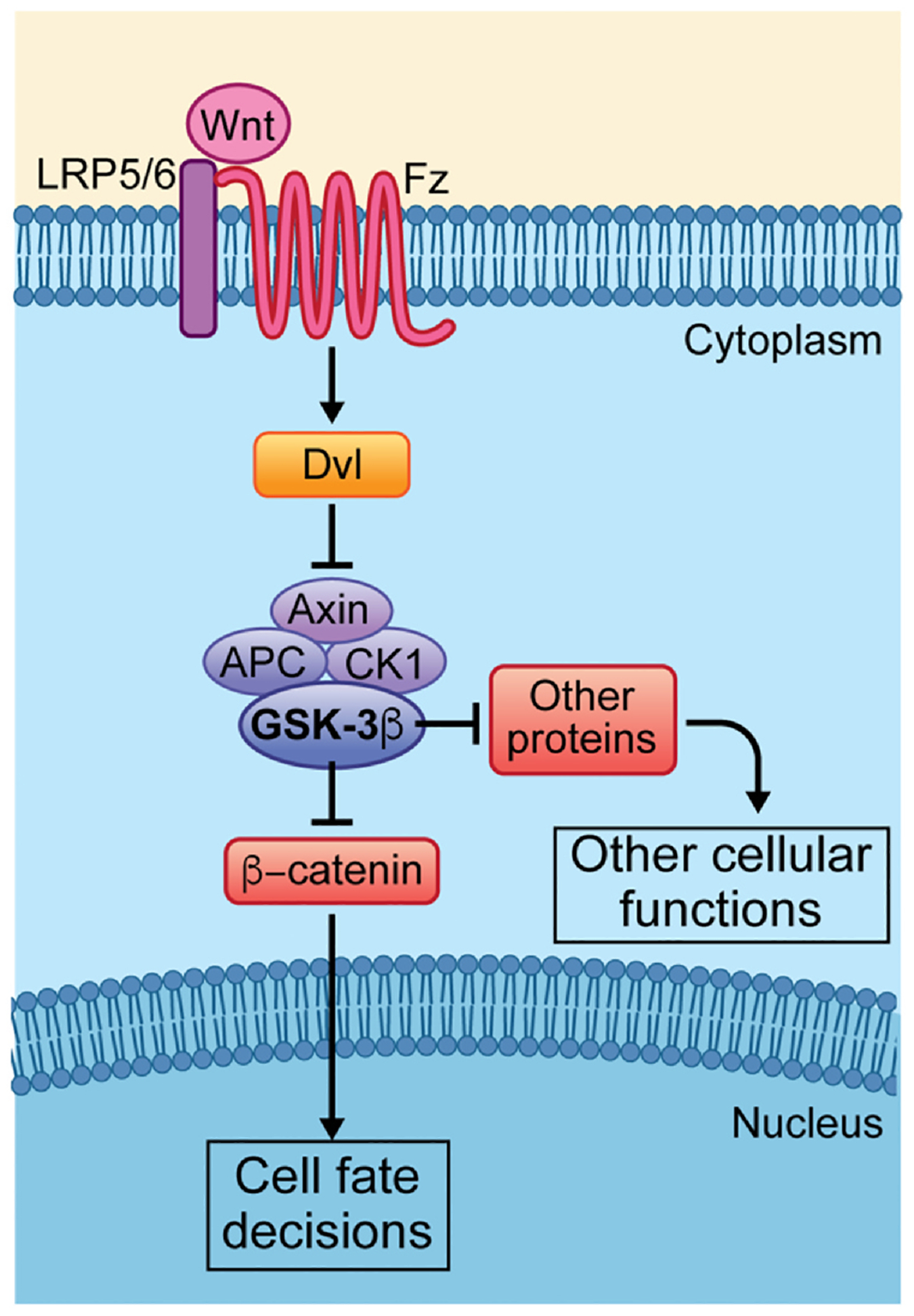
Overview of canonical Wnt/β-catenin and related “destruction complex” associated Wnt-signaling pathways. When Wnt is activated, Wnt binding causes dimerization of Fz (Fzd in vertebrates) and LRP5/6 co-receptors. The receptors recruit Dvl to a signaling complex that leads to its interaction with Axin and results in the disassembly of the “destruction complex”/DC (the DC includes Axin, APC, CK1 and GSK-3β). The functions of the DC are to phosphorylate β-catenin and other proteins and thus target these to proteasomal degradation. Upon Wnt signal pathway stimulation the DC target proteins are freed from phosphorylation and can accumulate in the cytoplasm to activate/perform the respective cellular functions. Thus, for example, upon the disassembly of the DC β-catenin in the cytoplasm can translocate to the nucleus, where it associates with transcription factors to activate Wnt target genes regulating cell fate decisions, proliferation and cell survival. Other targets of the DC serve distinct cellular functions in different contexts.
Besides the canonical Wnt/Wg-β-catenin signaling pathway, several alternate canonical branches that are independent of β-catenin stabilization have emerged recently (Acebron, Karaulanov, Berger, Huang, & Niehrs, 2014; Albrecht et al., 2020; Huang, Anvarian, Doderlein, Acebron, & Niehrs, 2015; Koch, Acebron, Herbst, Hatiboglu, & Niehrs, 2015). In this case, the notion of being “canonical” refers to the usage of the Fzd–LRP5/6 co-receptor pair and its activation functioning to antagonize the destruction complex activity (Axin, APC, CK1, and GSK3β) and associated GSK3β mediated phosphorylation to target other proteins for degradation (reviewed in Acebron & Niehrs, 2016) (see also Fig. 1 scheme). This pathway has been named Wnt–STOP signaling and shown to act on a variety of cellular processes (see review above), including sperm maturation and motility (Koch et al., 2015). A novel aspect of this signaling branch has recently been implicated in the biogenesis and function of cilia (Seidl et al., 2023; Zhang et al., 2023), and this new observation is the focus of one of the chapters below.
1.2. Wnt/Fz–PCP signaling
Like the canonical Wnt/Wg-pathway, Wnt/Planar Cell Polarity (PCP) signaling was first discovered in insects and is most studied and best characterized in Drosophila tissues like the wing or the eye (Adler, 2002; Seifert & Mlodzik, 2007). In flies, PCP is evident in all adult cuticular structures and the compound eye. The simple ease of observation of PCP features on the Drosophila cuticle has been instrumental in identifying PCP pathway components genetically and assembling these into a Wnt/PCP signaling pathway (Axelrod, Miller, Shulman, Moon, & Perrimon, 1998; Boutros, Paricio, Strutt, & Mlodzik, 1998; Das, Jenny, Klein, Eaton, & Mlodzik, 2004; Jenny, Reynolds-Kenneally, Das, Burnett, & Mlodzik, 2005; Tree, Ma, & Axelrod, 2002; Tree, Shulman, et al., 2002; Usui et al., 1999; Vinson & Adler, 1987; Vinson, Conover, & Adler, 1989; Wolff & Rubin, 1998) (Fig. 2 shows a simple schematic overview).
Fig. 2.
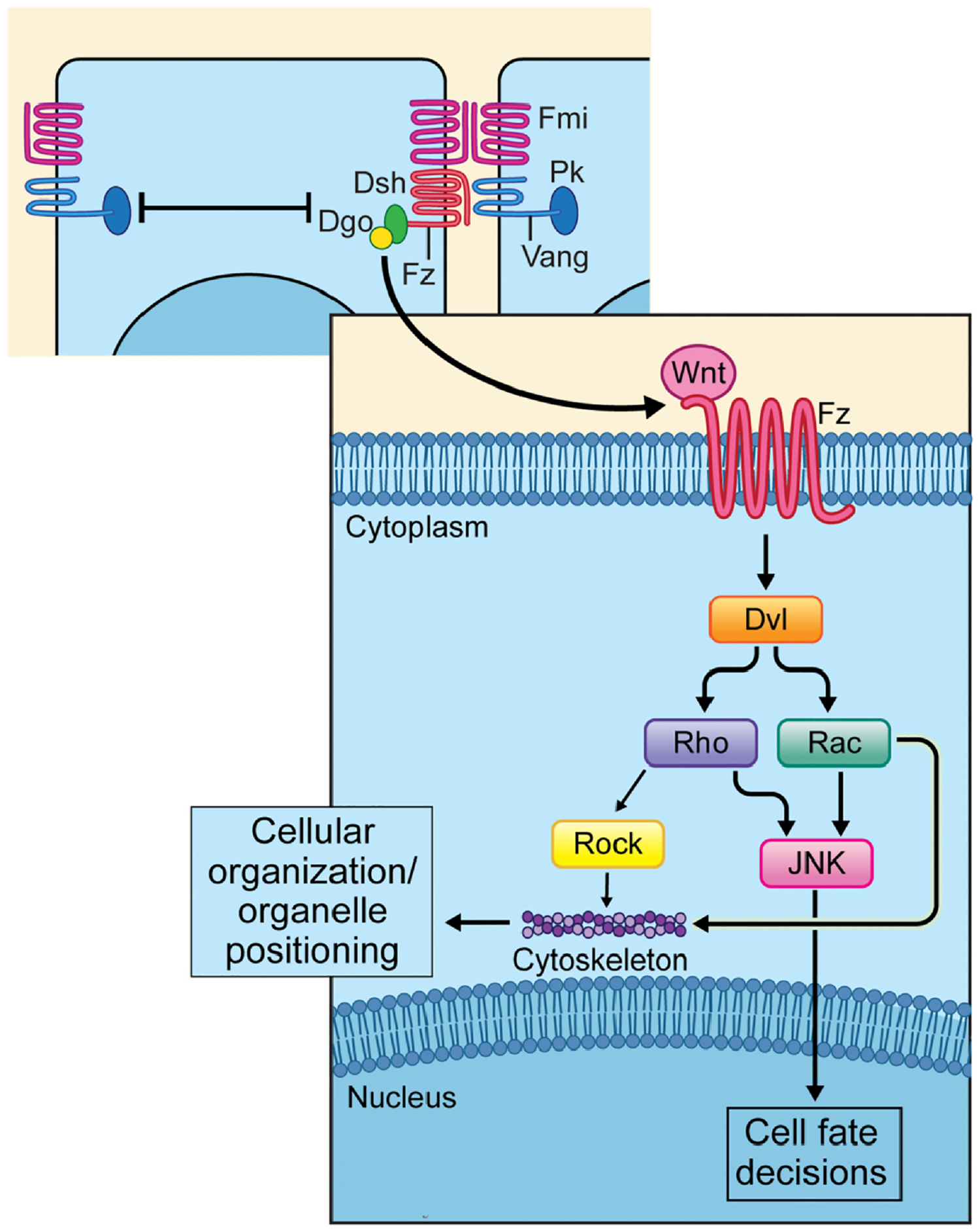
Overview of the non-canonical Wnt/PCP pathway. Upper part: a simplified diagram of PCP core components, which results in the establishment of two stable complexes, the Fz–Fmi–Dsh–Dgo complex and the Vang–Fmi–Pk complex. Lower part: Wnt/PCP signaling pathway downstream of the Fz–Dsh–Dgo complex. The PCP pathway is activated when a Wnt ligand binds to a Frizzled (Fz) receptor and induces a clustering of the core PCP protein to either proximal (Vang/Vangl [Van Gogh-like] and Prickle [Pk]) or distal (Dishellved [Dvl], Fz and Diego [Dgo]) cellular domains. Activation of PCP signaling is associated with Dvl phosphorylation. Effectors of Dvl are for example members of the Rho-subfamily of GTPases, e.g. Rho itself and Rac, stimulating Rock and JNK activity, with the respective kinases regulating either the cytoskeleton, that controls cellular organization or organelle positioning, or triggering the expression of target genes in the nucleus (JNK) thus regulating cell fate decisions.
Cellular patterning in tissues often requires directional information, either at the level of general cellular orientation among equivalent cells or distinct cell fate specification between neighboring cells. Both, mesenchymal cells and most epithelia are polarized within the planar axis of the given tissue, which is referred to as PCP. PCP-type cellular arrangements provide cells and tissues with positional information, allowing them to organize polarized structures that are oriented with respect to tissue axes, or embed specialized cells, for example sensory neurons, with a specific orientation in any given organ. PCP-signaling also regulates cellular movement in a directed manner, or can instruct the direction/axis of cell division, often in contexts of asymmetric cell division and associated differentiation, including in the context of progenitor or stem cell divisions. All these PCP signaling and read-out aspects are conserved across species and critical for development and homeostasis (Aw & Devenport, 2017; Butler & Wallingford, 2017; Davey & Moens, 2017; Goodrich & Strutt, 2011; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012; Simons & Mlodzik, 2008). They are also often linked to human disease (Aw & Devenport, 2017; Butler & Wallingford, 2017; Humphries & Mlodzik, 2018; Simons & Mlodzik, 2008). The feature of asymmetrically polarized cytoskeletal elements and organelle positioning involves the specific localization of centrioles, centrosomes, and importantly also cilia (via their basal bodies) as a conserved downstream effect of Wnt/Fz–PCP signaling (see dedicated section below).
Drosophila wing development, arguably the best understood tissue organized by PCP signaling, serves as an excellent system to dissect Wnt/PCP mediated regulation of cytoskeletal orientation and assembly, and the establishment and maintenance of the asymmetric distribution of core PCP factors from late larval to pupal stages of development. All wing cells form a single actin-based cellular hair that is oriented in the proximodistal axis, positioned in the distal vertex, and this uniform orientation pattern, when disrupted in mutants of PCP components is easily appreciated as such. Similarly, in eyes, the very regular arrangement of ommatidia (or unit eyes, a.k.a. facets) with respect to the anterior–posterior (AP) and dorsal–ventral (DV) axes is altered in PCP mutants (Adler, 2002; Goodrich & Strutt, 2011; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007). Based on the associated phenotypes, a core group of evolutionarily conserved Wnt–PCP genes have been identified, which are generally referred to as the ‘core Frizzled (Fz)/PCP factors’. The core Fz/PCP genes include three transmembrane proteins: Fz, Flamingo (Fmi, a.k.a. Starry night/Stan; Celsr1–3 in vertebrates), and Van Gogh (Vang; a.k.a. Strabismus/Stbm; Vangl1/2 in vertebrates), and three cytoplasmic factors Dishevelled (Dsh, Dvl1–3 in mammals), Prickle (Pk, with three Pk genes in mammals), and Diego (Dgo, with the presumed homogues Inversin and Diversin in vertebrates) (Aw & Devenport, 2017; Butler & Wallingford, 2017; Davey & Moens, 2017; Goodrich & Strutt, 2011; Harrison et al., 2020; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012). The core PCP factors interact with each other, resolving into two stable complexes: one composed of Fmi–Fz–Dsh–Dgo and the other of Fmi–Vang–Pk. These complexes are stabilizing each other between cells across cell membranes, with a Fmi–Fz and Fmi–Vang intercellular adhesive interaction, and they antagonize each other within a given cell, via negative interactions between the Fz–Dsh–Dgo complex and the Vang–Pk complex. As a result, these complexes become stably and asymmetrically localized along the polarity axis of any given tissue (Aw & Devenport, 2017; Aw, Heck, Joyce, & Devenport, 2016; Butler & Wallingford, 2017; Davey & Moens, 2017; Goodrich & Strutt, 2011; Harrison et al., 2020; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012; Yang & Mlodzik, 2015). In addition to the core PCP factors, several kinases that phosphorylate the core factors are critical in the process of establishing stable and correctly localized PCP complexes (Chuykin, Itoh, Kim, & Sokol, 2021; Gao et al., 2011; Humphries et al., 2023; Kelly, Wu, Yanfeng, & Mlodzik, 2016; Klein, Jenny, Djiane, & Mlodzik, 2006; Singh, Aaronson, & Mlodzik, 2010; Strutt, Price, & Strutt, 2006; Strutt & Strutt, 2020). In addition, it has been demonstrated that the polarization axes are generally associated with a localized expression of Wnt factors, with cells orienting towards the Wnt source (Carvajal-Gonzalez, Mulero-Navarro, & Mlodzik, 2016; Gao et al., 2011; Minegishi et al., 2017; Wu, Roman, Carvajal-Gonzalez, & Mlodzik, 2013).
Once stable polarization is achieved, largely by the interactions between the core PCP components, the asymmetric localization of the core complexes regulates downstream effects and effectors to elicit responses, which are often tissue and cell type specific, affecting both cytoskeletal orientation (including the alignment of the mitotic spindle and localization of centrioles and cilia) and nuclear signaling (to induce cell fate) (Aw et al., 2016; Butler & Wallingford, 2017; Carvajal-Gonzalez, Mulero-Navarro, et al., 2016; Goodrich & Strutt, 2011; Humphries & Mlodzik, 2018; Peng & Axelrod, 2012; Segalen & Bellaiche, 2009; Wallingford, 2010). Importantly, not only are the core Wnt–Fz/PCP factors conserved across the animal kingdom, but so are the downstream cellular responses (see review articles above). Two of the most obvious and common read-outs are (i) polarization of acto-myosin cytoskeletal elements and (ii) alignment of mitotic spindles and related positioning of centrioles and cilia. The latter is the focus of a section in this article (see below).
2. Relationship of cilia and canonical Wnt/β-catenin signaling
2.1. Cilia formation is not required for canonical Wnt/β-catenin read-outs
Canonical Wnt/β-catenin signaling is one of the most important developmental signaling pathways that control cell fate decisions and tissue patterning (Cadigan, 2012; MacDonald et al., 2009; Cadigan & Waterman, 2012; Logan & Nusse, 2004; Nusse & Clevers, 2017). To simplify, a secreted Wnt ligand binds to a Frizzled (Fz) receptor and an LRP5/6 co-receptor and the resulting pathway activation leads to stabilization and nuclear localization of β-catenin and subsequent transcriptional activation of Wnt target genes (Behrens et al., 1996; Bernatik et al., 2011; Gonzalez-Sancho et al., 2013; Molenaar et al., 1996; Pinson, Brennan, Monkley, Avery, & Skarnes, 2000; Tamai et al., 2000; Wehrli et al., 2000) (see also Section 1.1).
Primary cilia have emerged as a potential “center” of signal transduction in vertebrate cells with defects of primary cilia often resulting in a group of developmental and degenerative diseases (Thompson et al., 2017; Volta & Gerdes, 2017; Ware, Aygun, & Hildebrandt, 2011). Ciliary signaling has been suggested to play a role in the modulation of the canonical Wnt signaling pathway (Lancaster, Gopal, et al., 2011; Lancaster, Schroth, & Gleeson, 2011; McDermott, Liu, Tlsty, & Pazour, 2010; Takemaru et al., 2003; Voronina et al., 2009; Willemarck et al., 2010). Indeed, numerous Wnt molecules were suggested to be involved in cilium formation (Otto et al., 2003; Simons et al., 2005; Watanabe et al., 2003). However, the role of cilia in canonical Wnt signaling transduction activation (Clevers & Nusse, 2012) has remained controversial, with some studies showing data supporting the hypothesis of a link between the structure of the cilium and Wnt signaling, and others disputing this (Berbari, O’Connor, Haycraft, & Yoder, 2009; Rosso, Sussman, Wynshaw-Boris, & Salinas, 2005; Simons et al., 2005). Furthermore, there are reports suggesting that primary cilia, or their disruption leads to upregulation of the Wnt pathway activity (Corbit et al., 2008; McDermott et al., 2010; Patnaik et al., 2019; Tan, Zhou, Zhou, & Liu, 2014; Wiens et al., 2010). The first direct look at a link between cilia and Wnt signaling arose from the observation that knockdown of certain genes associated with the well-defined ciliopathy Bardet–Biedl syndrome (BBS1, BB4, and MKKS) resulted in a hyperactive Wnt response in cultured cells (Gerdes et al., 2007). Moreover, the depletion Kif3a, the kinesin motor essential for ciliogenesis, also caused an upregulation of a cellular response to exogenous Wnt3A (Gerdes et al., 2007). These studies were shortly followed by a report revealing that disruption of primary cilia in mice carrying mutations in kif3a, Ift88, or ofd1 similarly resulted in a marked increase in cellular responses to the activation of canonical Wnt pathway (Corbit et al., 2008). Furthermore, a recent report described a direct involvement of Wnt/β-catenin signaling in the promotion of primary cilia biogenesis through β-catenin driven stabilization of centriolar satellites in RPE-1 cells (Kyun et al., 2020).
On the other hand, several other studies contradict these ideas, suggesting that canonical Wnt/β-catenin signaling does not require primary cilia for pathway activation and/or signal transduction. For instance, IFT88 mutant zebrafish fails to make cilia, but it retains normal canonical Wnt signaling (Huang & Schier, 2009). Likewise, it was shown that the Wnt target gene Axin2 and a transgenic Wnt-reporter were both normally activated in mouse embryos lacking ift88, ift172 or kif3a (Ocbina, Tuson, & Anderson, 2009). Moreover, this study also examined MEFs generated from wild-type and cilia-defective mice using quantitative Wnt-reporter analyses, detecting no difference in the activation response to Wnt ligands (Ocbina et al., 2009). Similarly, canonical Wnt signaling displayed no defects in mice lacking Inversin (Invs), the mouse homolog of the NPHP2 protein, which is mutated in nephronophthisis, a degenerative renal ciliopathy (Sugiyama, Tsukiyama, Yamaguchi, & Yokoyama, 2011). Similarly, using cell lines that commonly serve as ciliogenesis model systems (RPE-1, NIH3T3, and HEK293), recent work has suggested that there is no evidence for the activation of Wnt/β-catenin signaling to facilitate ciliogenesis. Using either Wnt3A to activate the pathway or LGK974 to block signaling, ciliogenesis, cilium length, and percentage of ciliated cells were examined. These studies revealed that Wnt3a treatment had no effect on cilia formation (Sugiyama et al., 2011). Importantly, the inhibition of secretion of endogenous Wnt ligands, using LGK974 which thus blocks Wnt signaling, also had no detectable effect on ciliogenesis. Finally, using the equivalent cell lines where key Wnt pathway components, namely DVL1/2/3, LRP5/6, or Axin1/2, were knocked-out, no effects on ciliogenesis were observed, indicating that neither activation nor blocking of the Wnt/β-catenin pathway affects the process of ciliogenesis (Bernatik, Paclikova, Kotrbova, Bryja, & Cajanek, 2021). Moreover, studies in lung cancer cells, with Wnt/β-catenin signaling inhibited by Kif3a bound to β-arrestin, have shown no effect on primary cilia (Kim et al., 2016).
A genetic screen to test for potential non-ciliary roles of all ciliary proteins was recently performed in Drosophila, using the non-ciliated epithelial imaginal disks as experimental model to allow the separation of ciliary and non-ciliary functions of proteins associated with ciliogenesis and ciliary function(s). Strikingly, this screen identified proteins of the Intraflagellar Transport complex A (IFT-A) to modulate canonical Wnt/Wg signaling, independently of the ciliary role of IFTs (Balmer et al., 2015). Subsequent mechanistic dissection demonstrated that the IFT-A protein complex associates with Kinesin2 and also binds cytoplasmic β-catenin, thus bridging it to Kinesin2 via Kap3, and this protein complex is required for nuclear translocation of β-catenin upon Wnt/Wg pathway activation (Vuong et al., 2018). Importantly, this non-ciliary IFT-A requirement is conserved in mammalian cells, and thus not require the presence of a primary cilium (Vuong et al., 2018). See section below for details.
2.2. Canonical Wnt/β-catenin signaling requires a non-ciliary function of the IFT-A complex for nuclear translocation of β-catenin
In canonical Wnt/β-catenin signaling, β-catenin is the main effector of pathway activation in the nucleus. However, the mechanisms underlying the translocation and retention of β-catenin inside the nucleus upon Wnt activation remain unclear. In the classic nuclear transport model, nuclear localization sequence (NLS)-containing proteins bind to Importin-α, which facilitates transport through the nuclear pore. With the assistance of Ran GTPase, the cargo is moved through the nuclear envelope/pore and released inside the nucleus (Fagotto, Gluck, & Gumbiner, 1998; Hieda et al., 1999; Ito, Muto, Green, & Yokoyama, 1999). Given that β-catenin lacks an intrinsic NLS, a proposed model for the nuclear translocation of β-catenin has been that it is using association with other proteins to translocate into the nucleus in a complex with such proteins, for example FOXM1 or BCL9 have been proposed (Townsley, Cliffe, & Bienz, 2004). It has also been suggested that the maintenance of β-catenin levels in the nucleus is regulated by TCF and BCL9/Lgs via multiple mechanisms, and that TCF/Lef can enrich β-catenin in the nucleus by retention (Behrens et al., 1996; Huber et al., 1996) and that BCL9 and its nuclear interactor Pygopus can also strongly bind to β-catenin and maintain its levels high in nuclei (Kramps et al., 2002; Townsley et al., 2004) (Fig. 3).
Fig. 3.
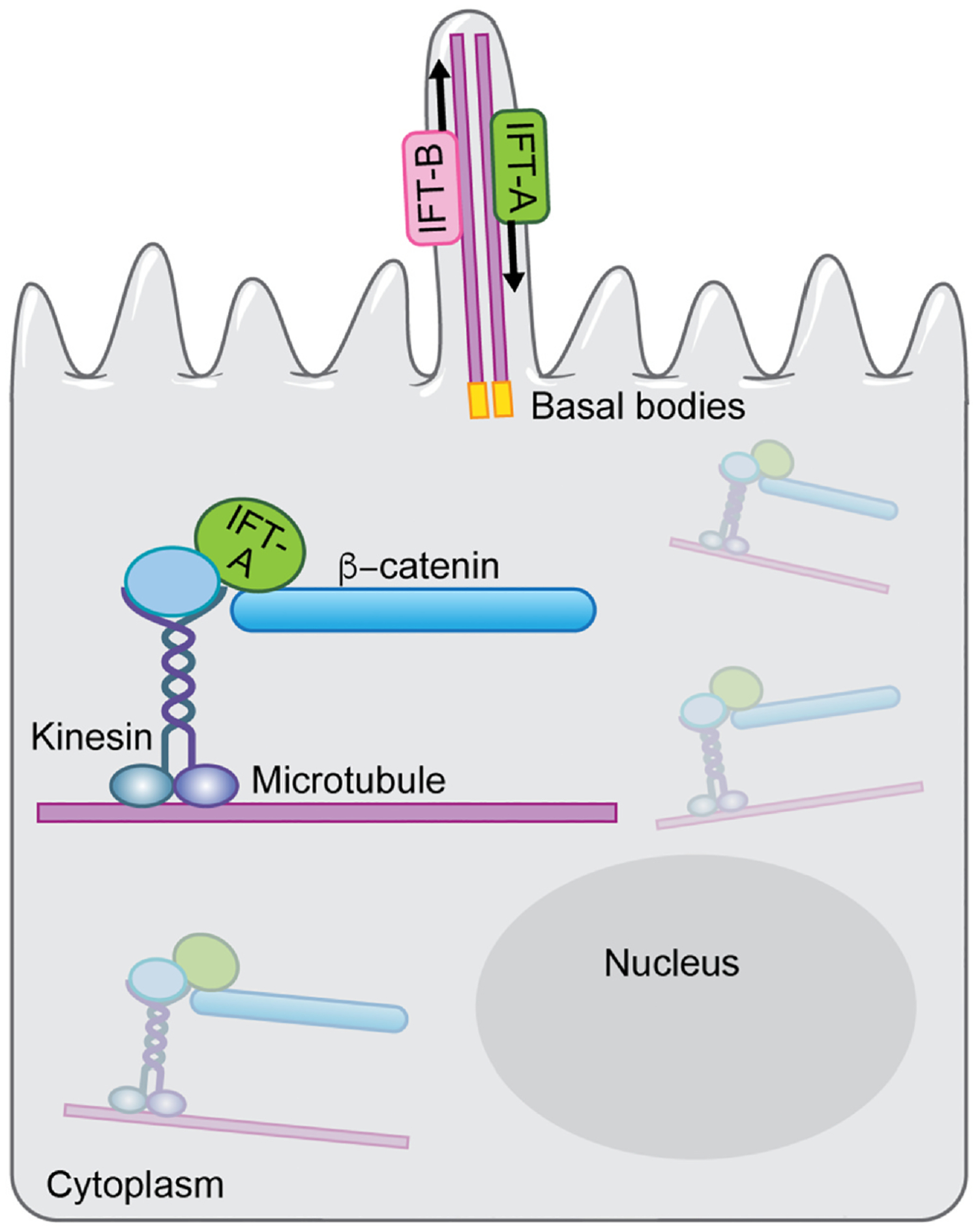
Wnt/β-catenin signaling requires the ciliary IFT-A complex for nuclear translocation of β-catenin. Upper part: The primary cilium is formed on the apical surface of the cells, with microtubules (called axonemes in the cilium) emanating from the basal bodies, which are specialized centrioles. The basal body is located in a depression of membrane known as the ciliary pocket, and is connected to membrane by transition fibers. IFT-A proteins complexes are required for retrograde transport from the cilium tip to the base, and IFT-B protein complexes are required for antegrade transport into the cilium. Lower part/inside the cell: Upon Wnt/β-catenin signaling activation, kinesin associates with IFT-A on cytoplasmic microtubules. IFT-A then binds to cytoplasmic β-catenin, forms a kinesin/IFT-A/β-catenin complex, which could allow its movement along microtubules toward the nucleus, independently of the IFT-A function in cilia.
As mentioned above, an unbiased genetic screen to test for non-ciliary functions and requirements of all ciliary proteins, the entire “ciliome”, was performed in Drosophila. To this end, the fact that the imaginal disc epithelia do not have primary cilia and thus are bonafide non-ciliated epithelial cells was used as an ideal experimental model system to address this question. Strikingly, this screen identified proteins of the Intraflagellar Transport complex A (IFT-A) to be positively required for canonical Wnt/Wg Armadillo (Arm)/β-catenin signaling, independently of the ciliary role of IFTs (Balmer et al., 2015). Subsequent functional and mechanistic studies demonstrated that the IFT-A protein complex associates with Kinesin2 and also binds cytoplasmic β-catenin, with IFT140 within IFT-A at the center of these interactions, binding directly to β-catenin and bridging it to Kinesin2 via Kap3. This protein complex is subsequently required for nuclear translocation of β-catenin upon Wnt/Wg pathway activation (Vuong et al., 2018).
IFTs are known as large protein complexes that are required to move cargo along axonemal microtubules in and out of cilia, and such transport is essential for the formation and maintenance of eukaryotic cilia and flagella in general. IFT particles are composed of about 20 proteins, organized into IFT-A and IFT-B complexes (Cole et al., 1998; Piperno & Mead, 1997). Among the five conserved core IFT-A proteins in Drosophila, four of these, IFT122, IFT121, IFT140 and IFT43, displayed identical phenotypic defects, resembling loss-of-function Wg-signaling phenotypes in Drosophila wing development, with growth defects and wing margin loss. Wg-signaling target gene activation was also severely reduced in mutants background of these IFT-A genes, indicating that they play a positive and critical role in canonical Wg/Wnt signaling (Balmer et al., 2015). It remained unclear whether the cytoplasmic IFT-A proteins associate with microtubular structures and whether such association is required for their function in Wnt/Wg-signaling. Functional studies demonstrated that IFT-A proteins, and IFT140 in particular, associate with Kinesin2 and promote nuclear translocation of β-catenin upon Wnt/Wg pathway activation (Vuong et al., 2018; Vuong, Mukhopadhyay, & Choi, 2014). This non-ciliary IFT-A requirement, acting downstream of β-catenin stabilization in canonical Wnt-signaling, has also been demonstrated to be conserved from Drosophila to mammalian cells (Vuong et al., 2018) (see also below).
How does this novel cytoplasmic complex work in more mechanistic detail? Loss of function of either Kinesin2 or IFT-A proteins in Drosophila wing tissues displayed indistinguishable effects on Wg/Wnt target gene expression and wing development. Kinesin2 and IFT140 interact genetically and physically with each other. Furthermore, Kap3, a non-motor component of Kinesin2, directly interacts with IFT140, and acts as the adapter to transport IFT140/IFT-A as cargo along microtubules. Both single mutant clones and double mutant clones for kinesin2 and ift140 or kap3 look indistinguishable and fail to activate Wg/Wnt signaling targets in Drosophila wings (Vuong et al., 2018). Strikingly, double mutant clones for either kinesin2 or IFT140 with axin (they key scaffold protein holding the destruction complex together) displayed high levels of stabilized cytoplasmic Arm/β-catenin (an axin loss-of-function phenotype) in both wing imaginal disc cells and salivary glands, but the nuclear translocation of Arm/β-catenin was almost completely lost, as nicely visualized in the large salivary gland cells and their large nuclei. Similarly, mammalian cells, specifically mouse embryonic fibroblast (MEFs) mutant for ift140−/−, showed a striking reduction of nuclear β-catenin upon Wnt3a mediated pathway stimulation (Vuong et al., 2018). It is important to note here again that this effect was independent of the presence or absence of cilia, as nuclear β-catenin localization was not affected in control mutant MEFs that lacked cilia eliminated by a distinct genetic background. Here, ift88 mutant MEFs, with IFT88 a key component of IFT-B complexes, lacked cilia but maintained IFT-A and Wnt3 induced canonical nuclear β-catenin translocation was not affected at all (Vuong et al., 2018). These data confirm the non-ciliary role of IFT-A in canonical Wnt/β-catenin signaling in mammalian contexts. Taken together, these findings allowed to propose a new function of IFT-A proteins, fully independent of their well-documented ciliary role. Upon Wg/Wnt signaling activation, the complex of Kinesin 2/Kap3/IFT-A binds cytoplasmic Arm/β-catenin (via IFT140) and this complex might then transport β-catenin along the microtubules towards the nucleus (Vuong et al., 2018).
In summary, the role of cilia in the regulation of canonical Wnt signaling remains still unclear, and requires further investigation. The key to understanding of the role of ciliary protein function in Wnt/β-catenin signaling depends on well controlled experiments in non-ciliated cells. It may further also depend on characteristics of cilia in different cell types and tissues, and in the past, it might have been further confused through cross-talk between different signaling pathways (e.g. Shh/Hedgehog signaling) and Wnt signaling. However, the data summarized above, clearly demonstrate that the physical presence of cilia is not required for canonical Wnt/β-catenin signaling and nuclear β-catenin signaling read-outs.
3. Wnt–LRP6 signaling via the destruction complex/GSK3β promotes ciliary biogenesis
Previous studies have established that novel branches of “canonical” Wnt signaling via the destruction complex and GSK3 kinase activity, but independent of β-catenin, are required in several contexts (see above and reviewed in Acebron & Niehrs, 2016). Recent follow up work provides exciting new insight that such related Wnt-signaling mechanisms are required for ciliogenesis and ciliary function in several contexts (see below this chapter).
Based on the observation that Cyclin Y (ccny) mouse mutants display an exencephaly phenotype and ciliogenesis defects, the resulting mechanistic analyses demonstrated that “downstream” of Cyclin Y, its function here being priming phosphorylation of LRP6, Wnt-LRP signaling defects are the cause of these phenotypes (Zhang et al., 2023). Elegant subsequent dissection of the signaling requirements revealed that the biogenesis of cilia requires a Wnt–Fzd5/LRP6–GSK3 signaling axis. While there are several identical requirements to Wnt/β-catenin signaling, this new canonical Wnt-branch also displays some unique features. Zhang et al. (2023) demonstrate that the key target of GSK3 in the ciliogenesis context is a regulatory component of PP1, specifically PPP1R2, which is required to inhibit PP1. Inhibition of PP1 is critical, as it itself inhibits ciliogenesis. As such, Wnt-signaling activation via Fzd5/LRP6 engagement protects PPP1R2 from GSK3-mediated phosphorylation and associated proteasomal degradation (Zhang et al., 2023).
Which signaling aspects make this “canonical” branch unique? Importantly, it is proposed that the required Wnt-signaling components physically localize to the cilium. These include Fzd5 and Dvl3, which have been previously suggested to display ciliary localization (Gupta et al., 2015). Significantly, only Dvl3 appears to be required for ciliogenesis, as mutants and siRNA assays with Dvl1 and Dvl2 show no significant effects on ciliogenesis. The authors (Zhang et al., 2023) define LRP6 as the key ciliary co-receptor, with LRP6 is being both necessary and sufficient for the ciliary Wnt-signal transduction. It is further corroborated by the fact that only LRP6—and not LRP5—contains a ciliary targeting sequence (CTS). Strikingly, the presence of the CTS in LRP6 is critical for ciliogenesis and its localization to the cilium (Zhang et al., 2023). Subsequent mechanistic studies defined the cilium as a Wnt-transducing organelle with the relevant Wnt-signaling associated features, like for example the monitoring of acute Wnt-signaling activation being detected in the cilium: upon Wnt3a stimulation phospho-LRP6 is detected inside the cilium (Zhang et al., 2023). The definition of the target of Wnt–Fzd5/LRP6–GSK3 for ciliogenesis was aided by previous studies implicating PP1 as a regulator of ciliary biology (Habermacher & Sale, 1996; Vijayaraghavan et al., 1996). As PP1 needs to be inhibited for normal ciliogenesis to occur, an inhibitory regulator was a good candidate with PPP1R2 standing out as the best candidate, as it was shown in the past to be a GSK3-phosphorylation target (Cohen, 1989). Indeed, Zhang et al. (2023) elegantly demonstrate the requirement of PPP1R2 as the Wnt–LRP6 target, and thus stabilization of PPP1R2 in cilia is the key Wnt-signaling outcome to promote ciliogenesis (Fig. 4).
Fig. 4.
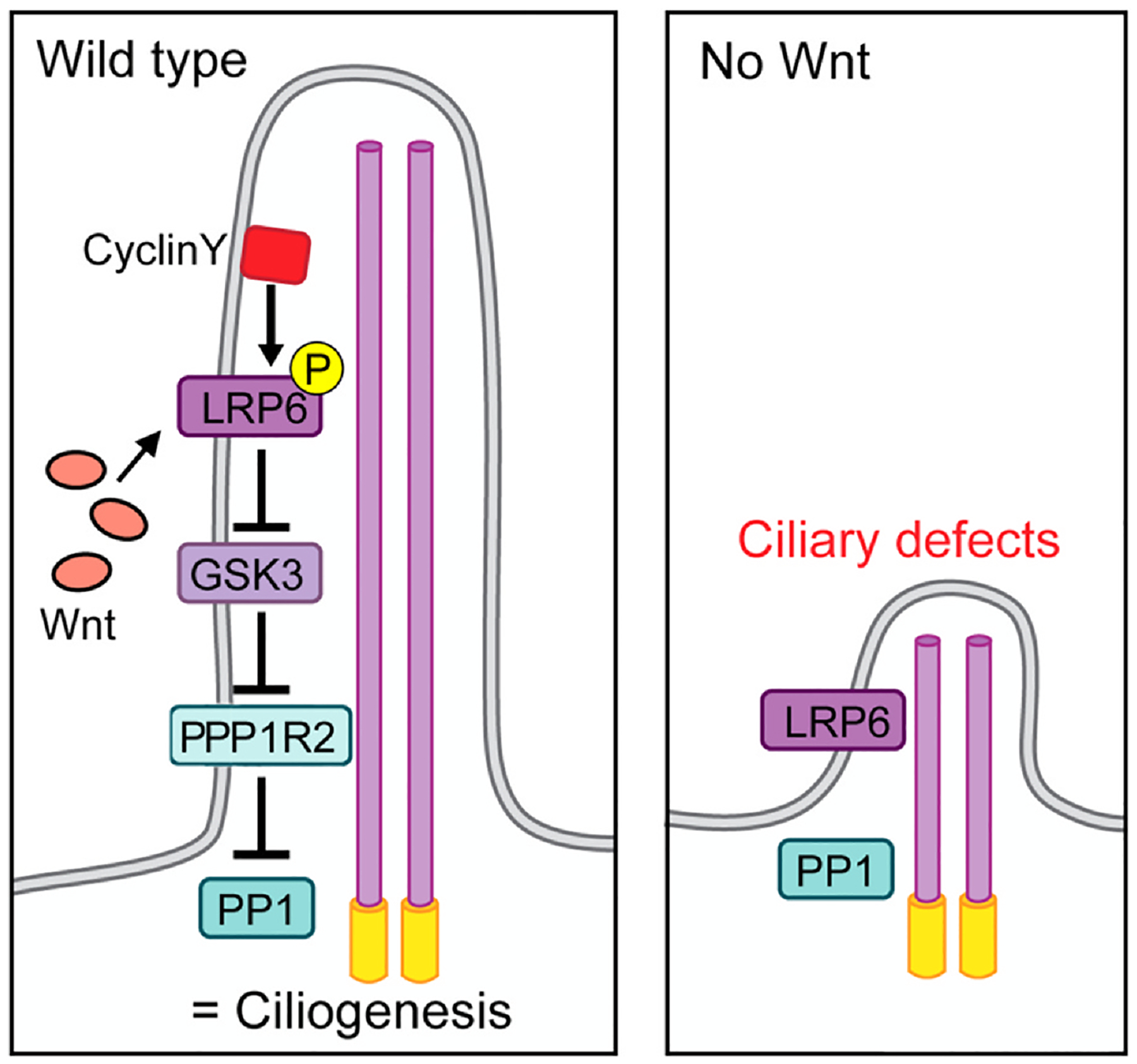
Wnt/GSK3 signaling promotes ciliogenesis in primary cilia. In wild-type primary cilia, CyclinY (CCNY) phosphorylates and primes the Wnt co-receptor LRP6, which upon Wnt-binding inhibits GSK3 activity. The inhibition of GSK3 prevents PPP1R2 from becoming phosphorylated and inactivated, allowing it to repress PP1 activity to allow ciliogenesis to proceed. When PP1 is active, either in the absence of Wnt signals or in CCNY mutants, it suppresses ciliogenesis and causes ciliary defects. A such, the role of Wnt-signaling here is to allow ciliary biogenesis via “activating” PPP1R2 so that it can inhibit PP1. The signaling logic is very similar to canonical Wnt/β-catenin signaling with PPP1R2 being here equivalent to β-catenin and the direct target of GSK3, which is inhibited upon Wnt signaling stimulation. In contrast, when Wnt signaling is not activated, GSK3 is active, leading to PP1 being active, which prevents normal ciliogenesis causing ciliary defects.
The study discussed above defines a few novel and exciting facts, (i) primary cilia require (at least in certain cell types and contexts) a Wnt-signal for their biogenesis, (ii) primary cilia appear to be Wnt-signaling organelles with Wnt stimulation being transduced inside them via a subset of the protein family members that are required to form the signalosome (Fzd5–LRP6–Dvl3), (iii) the Wnt-signal leads to the inhibition of PP1 and this promotes ciliogenesis, and (iv) this Wnt-signaling branch can be correlated with certain ciliopathies (Zhang et al., 2023). The work also opens up a few questions. First, previous work by Bernatik and co-workers (Bernatik et al., 2021) has demonstrated that in RPE-1 and HEK293 cells ciliary formation does not require a Wnt signal. How can this be reconciled with the study discussed above? Zhang et al. (2023) confirm this finding, providing evidence that Wnt–LRP6 ciliary signaling is indeed not required in these cell lines. This implies that the requirement for the Wnt–Fzd5/LRP6–GSK3–PP1 signaling axis is not absolute and might be cell type specific. The authors note that it appears to be required in a subset of cell types (although likely the majority of cell types) and also in the cells where it is essential, the cilia are not completely lost but they are malformed, often stunted (Zhang et al., 2023). More analyses will be needed to define the full extent of the requirement of this new Wnt-signaling branch for ciliogenesis (see also below). Furthermore, it remains unknown which Wnts are critical in vivo for this signaling to occur and where these Wnts are secreted from.
As a follow up to the Zhang et al. (2023) study, the Niehrs lab has also addressed the role of this novel Wnt-pathway branch in the context of motile cilia in multiciliated cells (MCCs). While Zhang et al. (2023) establish the requirement for Wnt–LRP6 signaling in primary cilia for their biogenesis, motile cilia are presumed to be nano-motors and largely signaling inert. Using the skin of Xenopus tropicalis, which has long served as an excellent model for the development and function of MCCs in a mucociliary epithelial context, the Niehrs lab demonstrates very similar requirements for Wnt-signaling in the establishment and motile cilia and their activity in the MCCs (Seidl et al., 2023). Importantly, the MCCs are postmitotic and highly differentiated cells and thus these cilia are very different from primary cilia, which are reabsorbed during cell division and need to be re-established subsequently. The authors demonstrate nicely that the same β-catenin independent “canonical” Wnt-signaling branch is critical for the biogenesis and function of motile cilia in MCCs. They also show that the motile cilia serve as a Wnt-transducing organelle, with the signal itself and its effect being transduced and ‘activated’ inside the cilia, like they have shown for primary cilia. Furthermore, they define the signaling branch to be the same Wnt–LRP6 -| PP1 pathway, as was defined in primary cilia (Seidl et al., 2023). Using an elegant biosensor assay they confirm that the pathway is indeed activated and active inside the individual motile cilia on the apical surface of the MCCs. And again, these observations were further extended to demonstrate that they are conserved in mammalian cells, analyzing airway epithelial MCCs, and a link to several ciliopathies was also confirmed. The only, minor, difference in the pathways between the primary and motile cilia is that a different inhibitory subunit of PP1 is the direct target of GSK3 phosphorylation, it is PPP1R11 in the motile cilia, as compared to PPP1R2 in primary cilia (Seidl et al., 2023).
In the context of the MCCs it is worth noting that several distinct Wnt-signaling branches are required for their correct cell fate induction, epithelial intercalation, and cell polarity. Canonical Wnt/β-catenin signaling is required in earlier stages for the specification of MCCs via a standard β-catenin mediated transcriptional activation program (Caron, Xu, & Lin, 2012; Walentek, Beyer, Thumberger, Schweickert, & Blum, 2012). Furthermore, the positioning and orientation of the motile cilia within the MCC context requires Wnt–PCP signaling (Park, Mitchell, Abitua, Kintner, & Wallingford, 2008)(see also below in chapter 4), and the biogenesis of the motile cilia then uses the Wnt–LRP6 -| PP1 pathway. As such, the requirements for Wnt-signaling branches in these complex and highly specialized cells is truly multi-fold (see also below).
4. Wnt/Frizzled–PCP signaling regulates ciliary positioning and also centriole positioning in non-ciliated cells
The Wnt–PCP pathway was discovered because of defects in cytoskeletal organization, as visible in the insect cuticle (Gubb & Garcia-Bellido, 1982). In Drosophila epithelial cells, once PCP components are fully asymmetrically localized (for example at 30–32 h after puparium formation in developing wings), the actin-based hairs are positioned at the distal vertex of their apical membrane, pointing towards the distal side of the wing. Loss or gain-of-function of genes of the Fz–PCP core group display a randomization of the positioning of the single actin-based hair within the apical membrane apex. See Section 1.2 for more details and conservation features of the Wnt/Fz–PCP pathway.
The earliest observation that Wnt–PCP signaling might affect ciliary positioning in mammalian context came from experiments in the mouse cochlea, where a connection between asymmetrically localized PCP components and the kinocilium was discovered (Montcouquiol & Kelley, 2003; Montcouquiol et al., 2003). Here the kinocilium is a specialized primary cilium in these sensory cells and it then affects the organization of the actin based ‘stereocilia’ (the name “stereocilia” being misleading here, as a cilium is a microtubules-based structure, and the so-called “sterocilia” are actin-based filaments). In the cochlear hair cells, a core PCP mutant VanglLp/Lp affected cellular poisoning of the kinocilium and the orientation of the stereociliary actin hair bundles. Subsequently, mutations in several core PCP proteins, including loss-of-function alleles of Vangl2, Celsr1, mutants in Dvl1 and 2 proteins, and Fzd3 and Fzd6 double mutants, were shown to display random positioning of the basal body of the kinocilium within the apical membrane, while maintaining the relative relationship between the actin-based stereocilia and the kinocilium (Curtin et al., 2003; Montcouquiol et al., 2003; Wang et al., 2006) (Fig. 5).
Fig. 5.
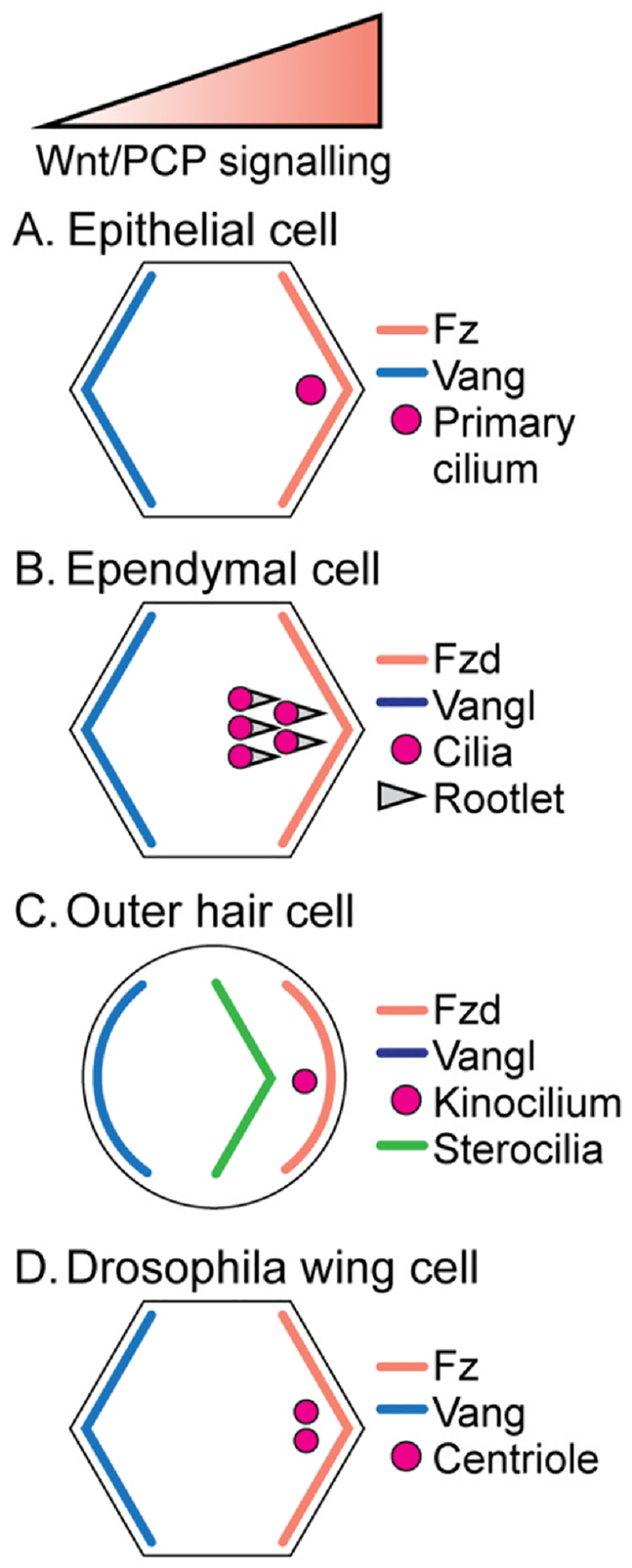
Wnt/PCP signaling regulates the positioning of centrioles and cilia. (A) In epithelial cells, the primary cilium localizes to the side of the cell that is enriched in Fz–Dsh complexes and devoid of Vang–Pk complexes. (B) In apical membrane regions of ependymal cells, the cilia as a multi-ciliary arrangement are localized near the Fzd expression domain. The rootlets (the basal foot of the cilia) are all aligned in the same direction, towards the Fzd–Dvl complexes. (C) In the inner ear, outer hair cells position the basal bodies of the kinocilium near the Fzd localization domain and stereociliary bundles are arranged in a chevron shape (V-shaped) more centrally from the kinocilum. (D) In Drosophila wing cells, centrioles are localized near the Fz–Dsh localization domain on the distal vertex, similar to vertebrate primary cilia or mouse ependymal cells and outer hair cells.
Within the apical membranes of ependymal cells, cilia have an additional form of polarity, called rotational polarity. It refers to the orientation of a single cilium in a multiciliated context and can be visualized by the orientation of the ciliary rootlet of each cilium. PCP mutants in the core Fz–PCP system, for example in the Celsr1, Fzd3 and Vangl2 genes, which coordinate also translational polarity in ependymal cells, show defects in both, ciliary positioning and also rotational polarity (Boutin et al., 2014). Importantly, Celsr2, Celsr3, Fzd3 and Vangl2 also organize multiciliated arrangements in individual cells with cilia located on the opposite side of the Vangl2 PCP complex (Boutin et al., 2014; Tissir & Goffinet, 2010). In the mouse oviduct, knock-out of Celsr1 showed ciliary orientation defect (Shi et al., 2014). Furthermore, Dvl1–3 triple knock-out mice displayed consistently a hydrocephalus phenotype due to abnormal positioning of cilia in the ependymal cells (Ohata & Alvarez-Buylla, 2016). Additionally, knock-downs of individual Dvl genes or combined Dvl1, Dvl2 and Dvl3 mutants in the Xenopus epidermis showed ciliary polarity defects with the phenotype also observed in PCP effector protein knock-downs, e.g. Inturned or Fuzzy (Park et al., 2008; Wallingford, 2006). Upon closer examination at the subcellular level, individual mutants revealed that the effectors, acting downstream of the core PCP factors, do not share the same defects as the core factors. For example, the inner ear sensory defects examined in detail at the level of the positioning of stereocilia and the kinocilium are distinct. Importantly, loss of function mutants in Vangl show that the stereociliary actin bundle is still arranged in a V-shape like in wild-type, but the kinocilium is randomly positioned; while in mutants affecting ciliogenesis (downstream of the core PCP factors) the stereociliary bundles appear abnormal often forming a circular or flat form arrangement. These data indicate that core Wnt–PCP signaling regulates the positioning of the kinocilium/primary cilium and not ciliogenesis, but that subsequently the cilium regulates the arrangement of the actin-bundles of the stereocilia. For example, in Gαi3-KO and mPins-KO, stereociliary hair bundles display flattened shapes and the kinocilium is more centrally positioned in the outer hair cells (Ezan et al., 2013). Interestingly, in the mutants affecting ciliogenesis and stereocilia arrangement, asymmetric localization of core PCP factors, e.g. Vangl2, is not affected and is indistinguishable from wild-type. Similarly, in mice mutant for Ift88, which is required and critical for ciliogenesis, localization of all core PCP proteins remains normal with a wild-type appearance (Jones & Chen, 2008). These data indicate that core PCP factor signaling and resulting asymmetric localization is upstream and independent of ciliogenesis, and that ciliary positioning is a downstream read-out of core PCP signaling. A very similar phenotypic relationship was also observed in asymmetric SOP cell division in Drosophila, where the centrosome alignment axis follows the core PCP asymmetric localization axis, without affecting the asymmetry of the core PCP complexes (Bellaiche, Beaudoin-Massiani, Stuttem, & Schweisguth, 2004; Besson et al., 2015).
Among vertebrate cells with single cilia, often displaying the primary cilium in any given cell, all analyses thus far revealed a Wnt–PCP signaling dependent localization of basal bodies and hence cilia (reviewed in Carvajal-Gonzalez, Mulero-Navarro, et al., 2016). Obvious examples include lens epithelial cells and corneal endothelial cells in the mouse eye (Blitzer et al., 2011; Sugiyama et al., 2010), epithelial/neuroepithelial cells in the Zebrafish embryo with PCP and cilia aligned in the antero-posterior axis (Borovina, Superina, Voskas, & Ciruna, 2010), and mouse node cells with polarized PCP signaling and cilia to establish the left–right axis asymmetry of the body (Hashimoto et al., 2010; Minegishi et al., 2017), all showed PCP signaling dependent polarization of basal bodies (Wu & Mlodzik, 2017). Taken together, all the above data and observations are consistent with the model that core Wnt/PCP signaling factors act upstream in positioning of cilia/basal bodies within the apical apex of any given cell and are independent of ciliogenesis per se.
As epithelial cells in Drosophila tissues are not ciliated it was initially not a concern to analyze localization of related structure (basal bodies are specialized centrioles) in the Drosophila system. However, upon careful analysis of centriole positioning in Drosophila wings it became apparent that core PCP signaling acts upstream of centriole positioning in Drosophila wing cells as well (Carvajal-Gonzalez, Roman, & Mlodzik, 2016). Here the centrioles localize to the side of the cell that is enriched in the Fz–Dsh core PCP complexes and opposite to the Vang–Pk complexes. This is fully consistent with all data and observed regulation of ciliary positioning in vertebrates (see above). Moreover, in Drosophila, ablation of centrioles, as observed in sas4 mutant cells, does not cause any PCP phenotypes with cellular hairs in this mutation positioned at the distal vertex like in wild-type, and localization of core PCP factors, for example Fmi/Celsr displaying a perfect asymmetric localization in acentriolar cells (Carvajal-Gonzalez, Roman, et al., 2016), which is again consistent with the notion that Wnt–PCP signaling is upstream of centriole and basal body/cilia positioning. In addition, these data are again consistent with the observation that PCP regulated mitotic spindle orientation in the dorsal thorax of the Drosophila pupa is also downstream of core PCP signaling. Here, during the division of SOPs (sensory organ precursor) cells, the centrosomes are aligned with the PCP axis via interactions with the Fz–Dsh and Vang complexes (Bellaiche, Gho, Kaltschmidt, Brand, & Schweisguth, 2001; Segalen & Bellaiche, 2009).
And while it might seem ironic that Drosophila was “late to the party” establishing a firm connection between Wnt/PCP signaling and general centriole positioning, it provided evidence that the most conserved cell biological effect or read-out of Wnt–PCP signaling is the positioning of centrioles/centrosomes/basal bodies (and hence cilia). Taken together, all these studies suggest that the core PCP factors localize centrioles/basal bodies within the apical cellular apex and the centrioles positioned to the side of the cells that enriched in Fz–Dvl proteins. The data in Drosophila wing cells truly emphasize the intimate relationship between core Wnt–PCP signaling and centriole localization/polarization and establish the notion that “centriole positioning” as the universal read-out of Wnt/PCP signaling (Carvajal-Gonzalez, Roman, et al., 2016).
5. Conclusions
As discussed here in this article the relationships between the Wnt-signaling branches and cilia are indeed complex. For clarity, we would like to repeat and emphasize the aspects which have recently emerged and help to clarify some of the confusing literature. For details and a more comprehensive set of references please see above in the individual chapters.
First, it now appears safe to say that the canonical Wnt/β-catenin pathway, resulting in the transcriptional activation of Wnt-target genes, does not require the physical presence of the cilium as a structure. What canonical Wnt/β-catenin signaling does require, is a defined subset of ciliary proteins, the IFT-A complex and associated kinesin motors, which promote nuclear β-catenin translocation upon Wnt-signaling activation. They do so however in the cytoplasm, associating with and actin on cytoplasmic microtubules (Vuong et al., 2018). Second, does ciliogenesis require a new branch of the canonical Wnt-pathway? The answer should be yes, based on recent papers addressing this both in the context of primary cilia as well as motile cilia in MCCs (Seidl et al., 2023; Zhang et al., 2023). The branch of canonical signaling used here is however more specialized (using for example exclusively LRP6 and Dvl3) and β-catenin independent, but rather converging on the inhibition of PP1 to allow ciliogenesis to occur. The general requirement of this signaling branch for the biogenesis of cilia is nonetheless still to be questioned and/or addressed further, as work in widely used and common cell lines, e.g. RPE-1 and HEK293 cells, argues against a general canonical Wnt-signaling requirement for ciliogenesis (Bernatik et al., 2021).
In parallel, work on the Wnt/PCP pathway has established two clear conclusions. First, ciliogenesis or the physical presence of cilia is not required for normal Wnt/PCP signaling. This has been documented in several independent tissues and model systems. Second, the Wnt/PCP signaling axis appears to act upstream of centrioles/centrosomes/cilia in all contexts analyzed, with the centrioles or cilia being positioned on the side of the cells that enrich in the Fz–Dvl complexes and away from the Vang–Pk complexes (e.g. Carvajal-Gonzalez, Roman, et al., 2016; Minegishi et al., 2017; Montcouquiol et al., 2003). Similarly, the centrosomes during cell division are generally captured by the Fz–Dvl complexes, thus aligning the spindle within the axis of PCP polarization. Furthermore, in multiciliated cells with many motile cilia, all such cilia are aligned in the PCP axis and oriented towards the Fz–Dvl complexes to allow coordinate ciliary beating and thus coordinated motion across the ciliary field (e.g. Park et al., 2008). For obvious reasons this makes sense and is critical in the context of mucociliary movement on either the frog skin epithelia or the human airway epithelia. Analyses in distinct multiciliated cellular models will help to confirm the general applicability of such polarity arrangements.
It is exciting times in both Wnt-signaling studies and ciliary cell biology, and ongoing and future studies will further clarify these relationships and shed more light on the once confusing scenarios.
Acknowledgments
We are grateful to all Mlodzik lab members and Carlo Iomini for their helpful discussions and support. We wish to thank Jill Gregory at the Academic Medical Illustration Department of the Icahn School of Medicine at Mount Sinai for excellent assistance with figure preparation. Related research in the Mlodzik laboratory is supported by a National Institutes of Health grants from the NIGMS R35 GM127103 and the NEI R01 EY13256.
References
- Acebron SP, Karaulanov E, Berger BS, Huang YL, & Niehrs C (2014). Mitotic wnt signaling promotes protein stabilization and regulates cell size. Molecular Cell, 54, 663–674. [DOI] [PubMed] [Google Scholar]
- Acebron SP, & Niehrs C (2016). beta-Catenin-independent roles of Wnt/LRP6 signaling. Trends in Cell Biology, 26, 956–967. [DOI] [PubMed] [Google Scholar]
- Adler PN (2002). Planar signaling and morphogenesis in Drosophila. Developmental Cell, 2, 525–535. [DOI] [PubMed] [Google Scholar]
- Albrecht LV, Tejeda-Munoz N, Bui MH, Cicchetto AC, Di Biagio D, Colozza G, … De Robertis EM (2020). GSK3 inhibits macropinocytosis and lysosomal activity through the Wnt destruction complex machinery. Cell Reports, 32, 107973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw WY, & Devenport D (2017). Planar cell polarity: Global inputs establishing cellular asymmetry. Current Opinion in Cell Biology, 44, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw WY, Heck BW, Joyce B, & Devenport D (2016). Transient tissue-scale deformation coordinates alignment of planar cell polarity junctions in the mammalian skin. Current Biology: CB, 26, 2090–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, & Perrimon N (1998). Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes & Development, 12, 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer S, Dussert A, Collu GM, Benitez E, Iomini C, & Mlodzik M (2015). Components of intraflagellar transport complex a function independently of the cilium to regulate canonical Wnt signaling in drosophila. Developmental Cell, 34, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, & Birchmeier W (1996). Functional interaction of beta-catenin with the transcription factor LEF-1. Nature, 382, 638–642. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, Beaudoin-Massiani O, Stuttem I, & Schweisguth F (2004). The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development (Cambridge, England), 131, 469–478. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, Gho M, Kaltschmidt JA, Brand AH, & Schweisguth F (2001). Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nature Cell Biology, 3, 50–57. [DOI] [PubMed] [Google Scholar]
- Berbari NF, O’Connor AK, Haycraft CJ, & Yoder BK (2009). The primary cilium as a complex signaling center. Current Biology: CB, 19, R526–R535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatik O, Ganji RS, Dijksterhuis JP, Konik P, Cervenka I, Polonio T, … Bryja V (2011). Sequential activation and inactivation of Dishevelled in the Wnt/beta-catenin pathway by casein kinases. The Journal of Biological Chemistry, 286, 10396–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatik O, Paclikova P, Kotrbova A, Bryja V, & Cajanek L (2021). Primary cilia formation does not rely on WNT/beta-catenin signaling. Frontiers in Cell and Developmental Biology, 9, 623753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson C, Bernard F, Corson F, Rouault H, Reynaud E, Keder A, … Schweisguth F (2015). Planar cell polarity breaks the symmetry of PAR protein distribution prior to mitosis in drosophila sensory organ precursor cells. Current Biology: CB, 25, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Bienz M (2005). beta-Catenin: A pivot between cell adhesion and Wnt signalling. Current Biology: CB, 15, R64–R67. [DOI] [PubMed] [Google Scholar]
- Blitzer AL, Panagis L, Gusella GL, Danias J, Mlodzik M, & Iomini C (2011). Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proceedings of the National Academy of Sciences of the United States of America, 108, 2819–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, & Ciruna B (2010). Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nature Cell Biology, 12, 407–412. [DOI] [PubMed] [Google Scholar]
- Boutin C, Labedan P, Dimidschstein J, Richard F, Cremer H, Andre P, … Tissir F (2014). A dual role for planar cell polarity genes in ciliated cells. Proceedings of the National Academy of Sciences of the United States of America, 111, E3129–E3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, & Mlodzik M (1998). Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell, 94, 109–118. [DOI] [PubMed] [Google Scholar]
- Butler MT, & Wallingford JB (2017). Planar cell polarity in development and disease. Nature Reviews. Molecular Cell Biology, 18, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM (2012). TCFs and Wnt/beta-catenin signaling: More than one way to throw the switch. Current Topics in Developmental Biology, 98, 1–34. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, & Waterman ML (2012). TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harbor Perspectives in Biology, 4, a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron A, Xu X, & Lin X (2012). Wnt/beta-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer’s vesicle. Development (Cambridge, England), 139, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Gonzalez JM, & Mlodzik M (2014). Mechanisms of planar cell polarity establishment in Drosophila. F1000Prime Reports, 6, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Gonzalez JM, Mulero-Navarro S, & Mlodzik M (2016). Centriole positioning in epithelial cells and its intimate relationship with planar cell polarity. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology, 38, 1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Gonzalez JM, Roman AC, & Mlodzik M (2016). Positioning of centrioles is a conserved readout of Frizzled planar cell polarity signalling. Nature Communications, 7, 11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuykin I, Itoh K, Kim K, & Sokol SY (2021). Frizzled3 inhibits Vangl2-Prickle3 association to establish planar cell polarity in the vertebrate neural plate. Journal of Cell Science, 134, jcs258864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006). Wnt/beta-catenin signaling in development and disease. Cell, 127, 469–480. [DOI] [PubMed] [Google Scholar]
- Clevers H, & Nusse R (2012). Wnt/beta-catenin signaling and disease. Cell, 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, & Bienz M (2003). A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Current Biology: CB, 13, 960–966. [DOI] [PubMed] [Google Scholar]
- Cohen P (1989). The structure and regulation of protein phosphatases. Annual Review of Biochemistry, 58, 453–508. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, & Rosenbaum JL (1998). Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. The Journal of Cell Biology, 141, 993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs GS, Covey TM, & Virshup DM (2008). Wnt signaling in development, disease and translational medicine. Current Drug Targets, 9, 513–531. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, … Reiter JF (2008). Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nature Cell Biology, 10, 70–76. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, … Murdoch JN (2003). Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Current Biology: CB, 13, 1129–1133. [DOI] [PubMed] [Google Scholar]
- Das G, Jenny A, Klein TJ, Eaton S, & Mlodzik M (2004). Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development (Cambridge, England), 131, 4467–4476. [DOI] [PubMed] [Google Scholar]
- Davey CF, & Moens CB (2017). Planar cell polarity in moving cells: Think globally, act locally. Development (Cambridge, England), 144, 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D (2014). The cell biology of planar cell polarity. The Journal of Cell Biology, 207, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann DM (2005). Wnt signaling. WormBook: The online review of C. Elegans biology 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezan J, Lasvaux L, Gezer A, Novakovic A, May-Simera H, Belotti E, … Montcouquiol M (2013). Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nature Cell Biology, 15, 1107–1115. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Gluck U, & Gumbiner BM (1998). Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Current Biology: CB, 8, 181–190. [DOI] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, … Yang Y (2011). Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Developmental Cell, 20, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, … Katsanis N (2007). Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nature Genetics, 39, 1350–1360. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Greer YE, Abrahams CL, Takigawa Y, Baljinnyam B, Lee KH, … Brown AM (2013). Functional consequences of Wnt-induced dishevelled 2 phosphorylation in canonical and noncanonical Wnt signaling. The Journal of Biological Chemistry, 288, 9428–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, & Strutt D (2011). Principles of planar polarity in animal development. Development (Cambridge, England), 138, 1877–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D, & Garcia-Bellido A (1982). A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. Journal of Embryology and Experimental Morphology, 68, 37–57. [PubMed] [Google Scholar]
- Gupta GD, Coyaud E, Goncalves J, Mojarad BA, Liu Y, Wu Q, … Pelletier L (2015). A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell, 163, 1484–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermacher G, & Sale WS (1996). Regulation of flagellar dynein by an axonemal type-1 phosphatase in Chlamydomonas. Journal of Cell Science, 109(Pt 7), 1899–1907. [DOI] [PubMed] [Google Scholar]
- Harrison C, Shao H, Strutt H, & Strutt D (2020). Molecular mechanisms mediating asymmetric subcellular localisation of the core planar polarity pathway proteins. Biochemical Society Transactions, 48, 1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, … Hamada H (2010). Planar polarization of node cells determines the rotational axis of node cilia. Nature Cell Biology, 12, 170–176. [DOI] [PubMed] [Google Scholar]
- Hieda M, Tachibana T, Yokoya F, Kose S, Imamoto N, & Yoneda Y (1999). A monoclonal antibody to the COOH-terminal acidic portion of Ran inhibits both the recycling of Ran and nuclear protein import in living cells. The Journal of Cell Biology, 144, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, & Schier AF (2009). Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development (Cambridge, England), 136, 3089–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Anvarian Z, Doderlein G, Acebron SP, & Niehrs C (2015). Maternal Wnt/STOP signaling promotes cell division during early Xenopus embryogenesis. Proceedings of the National Academy of Sciences of the United States of America, 112, 5732–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, & Kemler R (1996). Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mechanisms of Development, 59, 3–10. [DOI] [PubMed] [Google Scholar]
- Humphries AC, & Mlodzik M (2018). From instruction to output: Wnt/PCP signaling in development and cancer. Current Opinion in Cell Biology, 51, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries AC, Molina-Pelayo C, Sil P, Hazelett CC, Devenport D, & Mlodzik M (2023). A Van Gogh/Vangl tyrosine phosphorylation switch regulates its interaction with core planar cell polarity factors Prickle and Dishevelled. PLoS Genetics, 19, e1010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Muto Y, Green MR, & Yokoyama S (1999). Solution structures of the first and second RNA-binding domains of human U2 small nuclear ribonucleoprotein particle auxiliary factor (U2AF(65)). The EMBO Journal, 18, 4523–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, & Mlodzik M (2005). Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nature Cell Biology, 7, 691–697. [DOI] [PubMed] [Google Scholar]
- Jones C, & Chen P (2008). Primary cilia in planar cell polarity regulation of the inner ear. Current Topics in Developmental Biology, 85, 197–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LK, Wu J, Yanfeng WA, & Mlodzik M (2016). Frizzled-induced Van Gogh phosphorylation by CK1epsilon promotes asymmetric localization of core PCP factors in Drosophila. Cell Reports, 16, 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Suh YA, Oh JH, Lee BR, Kim J, & Jang SJ (2016). KIF3A binds to beta-arrestin for suppressing Wnt/beta-catenin signalling independently of primary cilia in lung cancer. Scientific Reports, 6, 32770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Jenny A, Djiane A, & Mlodzik M (2006). CKIepsilon/discs overgrown promotes both Wnt-Fz/beta-catenin and Fz/PCP signaling in Drosophila. Current Biology: CB, 16, 1337–1343. [DOI] [PubMed] [Google Scholar]
- Koch S, Acebron SP, Herbst J, Hatiboglu G, & Niehrs C (2015). Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell, 163, 1225–1236. [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, … Basler K (2002). Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell, 109, 47–60. [DOI] [PubMed] [Google Scholar]
- Kyun ML, Kim SO, Lee HG, Hwang JA, Hwang J, Soung NK, … Lee KH (2020). Wnt3a stimulation promotes primary ciliogenesis through beta-Catenin phosphorylation-induced reorganization of centriolar satellites. Cell Reports, 30, 1447–1462.e1445. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Gopal DJ, Kim J, Saleem SN, Silhavy JL, Louie CM, … Gleeson JG (2011). Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nature Medicine, 17, 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Schroth J, & Gleeson JG (2011). Subcellular spatial regulation of canonical Wnt signaling at the primary cilium. Nature Cell Biology, 13, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, & Nusse R (2004). The Wnt signaling pathway in development and disease. Annual Review of Cell and Developmental Biology, 20, 781–810. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, & He X (2009). Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Developmental Cell, 17, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KM, Liu BY, Tlsty TD, & Pazour GJ (2010). Primary cilia regulate branching morphogenesis during mammary gland development. Current Biology: CB, 20, 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen AE, Escobar DE, & Gottardi CJ (2012). Signaling from the adherens junction. Sub-cellular Biochemistry, 60, 171–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi K, Hashimoto M, Ajima R, Takaoka K, Shinohara K, Ikawa Y, … Hamada H (2017). A Wnt5 activity asymmetry and intercellular signaling via PCP proteins polarize node cells for left-right symmetry breaking. Developmental Cell, 40, 439–452.e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, … Clevers H (1996). XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell, 86, 391–399. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, & Kelley MW (2003). Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. The Journal of Neuroscience, 23, 9469–9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, & Kelley MW (2003). Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature, 423, 173–177. [DOI] [PubMed] [Google Scholar]
- Niehrs C (2012). The complex world of WNT receptor signalling. Nature Reviews. Molecular Cell Biology, 13, 767–779. [DOI] [PubMed] [Google Scholar]
- Nusse R, & Clevers H (2017). Wnt/beta-Catenin signaling, disease, and emerging therapeutic modalities. Cell, 169, 985–999. [DOI] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, & Varmus H (1984). Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature, 307, 131–136. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, & Wieschaus E (1980). Mutations affecting segment number and polarity in Drosophila. Nature, 287, 795–801. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, Tuson M, & Anderson KV (2009). Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One, 4, e6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata S, & Alvarez-Buylla A (2016). Planar organization of multiciliated ependymal (E1) cells in the brain ventricular epithelium. Trends in Neurosciences, 39, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, … Hildebrandt F (2003). Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nature Genetics, 34, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, & Wallingford JB (2008). Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nature Genetics, 40, 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik SR, Kretschmer V, Brucker L, Schneider S, Volz AK, Oancea-Castillo LDR, & May-Simera HL (2019). Bardet-Biedl syndrome proteins regulate cilia disassembly during tissue maturation. Cellular and Molecular Life Sciences: CMLS, 76, 757–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, & Axelrod JD (2012). Asymmetric protein localization in planar cell polarity: mechanisms, puzzles, and challenges. Current Topics in Developmental Biology, 101, 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, & Skarnes WC (2000). An LDL-receptor-related protein mediates Wnt signalling in mice. Nature, 407, 535–538. [DOI] [PubMed] [Google Scholar]
- Piperno G, & Mead K (1997). Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proceedings of the National Academy of Sciences of the United States of America, 94, 4457–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich TC, & Macdougald OA (2007). Wnt/beta-catenin signaling in adipogenesis and metabolism. Current Opinion in Cell Biology, 19, 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, & Salinas PC (2005). Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature Neuroscience, 8, 34–42. [DOI] [PubMed] [Google Scholar]
- Segalen M, & Bellaiche Y (2009). Cell division orientation and planar cell polarity pathways. Seminars in Cell & Developmental Biology, 20, 972–977. [DOI] [PubMed] [Google Scholar]
- Seidl C, Da Silva F, Zhang K, Wohlgemuth K, Omran H, & Niehrs C (2023). Mucociliary Wnt signaling promotes cilia biogenesis and beating. Nature Communications, 14, 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, & Mlodzik M (2007). Frizzled/PCP signalling: A conserved mechanism regulating cell polarity and directed motility. Nature reviews, 8, 126–138. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, & He X (2007). SnapShot: Noncanonical Wnt signaling pathways. Cell, 131, 1378. [DOI] [PubMed] [Google Scholar]
- Sharma RP, & Chopra VL (1976). Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Developmental Biology, 48, 461–465. [DOI] [PubMed] [Google Scholar]
- Shi D, Komatsu K, Hirao M, Toyooka Y, Koyama H, Tissir F, … Fujimori T (2014). Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development (Cambridge, England), 141, 4558–4568. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, … Walz G (2005). Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genetics, 37, 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, & Mlodzik M (2008). Planar cell polarity signaling: From fly development to human disease. Annual Review of Genetics, 42, 517–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Aaronson SA, & Mlodzik M (2010). Drosophila Abelson kinase mediates cell invasion and proliferation through two distinct MAPK pathways. Oncogene, 29, 4033–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Price MA, & Strutt D (2006). Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Current Biology: CB, 16, 1329–1336. [DOI] [PubMed] [Google Scholar]
- Strutt H, & Strutt D (2020). DAnkrd49 and Bdbt act via Casein kinase Iepsilon to regulate planar polarity in Drosophila. PLoS Genetics, 16, e1008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N, Tsukiyama T, Yamaguchi TP, & Yokoyama T (2011). The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney International, 79, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Stump RJ, Nguyen A, Wen L, Chen Y, Wang Y, … McAvoy JW (2010). Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Developmental Biology, 338, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, & Moon RT (2003). Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature, 422, 905–909. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, … He X (2000). LDL-receptor-related proteins in Wnt signal transduction. Nature, 407, 530–535. [DOI] [PubMed] [Google Scholar]
- Tan RJ, Zhou D, Zhou L, & Liu Y (2014). Wnt/beta-catenin signaling and kidney fibrosis. Kidney International. Supplement 2011, 4(1), 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Plant JC, Wann AK, Bishop CL, Novak P, Mitchison HM, … Knight MM (2017). Chondrocyte expansion is associated with loss of primary cilia and disrupted hedgehog signalling. European Cells & Materials, 34, 128–141. [DOI] [PubMed] [Google Scholar]
- Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR, … Zheng G (2011). E-cadherin/beta-catenin complex and the epithelial barrier. Journal of Biomedicine & Biotechnology, 2011, 567305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, & Goffinet AM (2010). Planar cell polarity signaling in neural development. Current Opinion in Neurobiology, 20, 572–577. [DOI] [PubMed] [Google Scholar]
- Townsley FM, Cliffe A, & Bienz M (2004). Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nature Cell Biology, 6, 626–633. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, & Axelrod JD (2002). A three-tiered mechanism for regulation of planar cell polarity. Seminars in Cell & Developmental Biology, 13, 217–224. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, & Axelrod JD (2002). Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell, 109, 371–381. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, … Uemura T (1999). Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell, 98, 585–595. [DOI] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, & Basler K (2012). The many faces and functions of betacatenin. The EMBO Journal, 31, 2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, & Greengard P (1996). Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biology of Reproduction, 54, 709–718. [DOI] [PubMed] [Google Scholar]
- Vinson CR, & Adler PN (1987). Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature, 329, 549–551. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, & Adler PN (1989). A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature, 338, 263–264. [DOI] [PubMed] [Google Scholar]
- Volta F, & Gerdes JM (2017). The role of primary cilia in obesity and diabetes. Annals of the New York Academy of Sciences, 1391, 71–84. [DOI] [PubMed] [Google Scholar]
- Voronina VA, Takemaru K, Treuting P, Love D, Grubb BR, Hajjar AM, … Moon RT (2009). Inactivation of Chibby affects function of motile airway cilia. The Journal of Cell Biology, 185, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong LT, Iomini C, Balmer S, Esposito D, Aaronson SA, & Mlodzik M (2018). Kinesin-2 and IFT-A act as a complex promoting nuclear localization of beta-catenin during Wnt signalling. Nature Communications, 9, 5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong LT, Mukhopadhyay B, & Choi KW (2014). Kinesin-II recruits Armadillo and Dishevelled for Wingless signaling in Drosophila. Development (Cambridge, England), 141, 3222–3232. [DOI] [PubMed] [Google Scholar]
- Walentek P, Beyer T, Thumberger T, Schweickert A, & Blum M (2012). ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus left-right development. Cell Reports, 1, 516–527. [DOI] [PubMed] [Google Scholar]
- Wallingford JB (2006). Planar cell polarity, ciliogenesis and neural tube defects. Human Molecular Genetics, 15(Spec No 2), R227–R234. [DOI] [PubMed] [Google Scholar]
- Wallingford JB (2010). Planar cell polarity signaling, cilia and polarized ciliary beating. Current Opinion in Cell Biology, 22, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, … Wynshaw-Boris A (2006). Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development (Cambridge, England), 133, 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, & Nathans J (2007). Tissue/planar cell polarity in vertebrates: New insights and new questions. Development (Cambridge, England), 134, 647–658. [DOI] [PubMed] [Google Scholar]
- Ware SM, Aygun MG, & Hildebrandt F (2011). Spectrum of clinical diseases caused by disorders of primary cilia. Proceedings of the American Thoracic Society, 8, 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, & Hamada H (2003). The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development (Cambridge, England), 130, 1725–1734. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, … DiNardo S (2000). arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature, 407, 527–530. [DOI] [PubMed] [Google Scholar]
- Wiens CJ, Tong Y, Esmail MA, Oh E, Gerdes JM, Wang J, … Leroux MR (2010). Bardet-Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signaling. The Journal of Biological Chemistry, 285, 16218–16230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemarck N, Rysman E, Brusselmans K, Van Imschoot G, Vanderhoydonc F, Moerloose K, … Swinnen JV (2010). Aberrant activation of fatty acid synthesis suppresses primary cilium formation and distorts tissue development. Cancer Research, 70, 9453–9462. [DOI] [PubMed] [Google Scholar]
- Wolff T, & Rubin GM (1998). Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development (Cambridge, England), 125, 1149–1159. [DOI] [PubMed] [Google Scholar]
- Wu J, & Mlodzik M (2017). Wnt/PCP instructions for cilia in left-right asymmetry. Developmental Cell, 40, 423–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, & Mlodzik M (2013). Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nature Cell Biology, 15, 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, & Mlodzik M (2015). Wnt-Frizzled/planar cell polarity signaling: Cellular orientation by facing the wind (Wnt). Annual Review of Cell and Developmental Biology, 31, 623–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Da Silva F, Seidl C, Wilsch-Brauninger M, Herbst J, Huttner WB, & Niehrs C (2023). Primary cilia are WNT-transducing organelles whose biogenesis is controlled by a WNT-PP1 axis. Developmental Cell, 58, 139–154.e138. [DOI] [PubMed] [Google Scholar]


