Abstract
Although the Malawi Lil20/1 (MAL) strain of African swine fever virus (ASFV) was isolated from Ornithodoros sp. ticks, our attempts to experimentally infect ticks by feeding them this strain failed. Ten different collections of Ornithodorus porcinus porcinus ticks and one collection of O. porcinus domesticus ticks were orally exposed to a high titer of MAL. At 3 weeks postinoculation (p.i.), <25% of the ticks contained detectable virus, with viral titers of <4 log10 50% hemadsorbing doses/ml. Viral titers declined to undetectability in >90% of the ticks by 5 weeks p.i. To further study the growth defect, O. porcinus porcinus ticks were orally exposed to MAL and assayed at regular intervals p.i. Whole-tick viral titers dramatically declined (>1,000-fold) between 2 and 6 days p.i., and by 18 days p.i., viral titers were below the detection limit. In contrast, viral titers of ticks orally exposed to a tick-competent ASFV isolate, Pretoriuskop/96/4/1 (Pr4), increased 10-fold by 10 days p.i. and 50-fold by 14 days p.i. Early viral gene expression, but not extensive late gene expression or viral DNA synthesis, was detected in the midguts of ticks orally exposed to MAL. Ultrastructural analysis demonstrated that progeny virus was rarely present in ticks orally exposed to MAL and, when present, was associated with extensive cytopathology of phagocytic midgut epithelial cells. To determine if viral replication was restricted only in the midgut epithelium, parenteral inoculations into the hemocoel were performed. With inoculation by this route, a persistent infection was established although a delay in generalization of MAL was detected and viral titers in most tissues were typically 10- to 1,000-fold lower than those of ticks injected with Pr4. MAL was detected in both the salivary secretion and coxal fluid following feeding but less frequently and at a lower titer compared to Pr4. Transovarial transmission of MAL was not detected after two gonotrophic cycles. Ultrastructural analysis demonstrated that, when injected, MAL replicated in a number of cell types but failed to replicate in midgut epithelial cells. In contrast, ticks injected with Pr4 had replicating virus in midgut epithelial cells. Together, these results indicate that MAL replication is restricted in midgut epithelial cells. This finding demonstrates the importance of viral replication in the midgut for successful ASFV infection of the arthropod host.
African swine fever (ASF) is a lethal, hemorrhagic disease of domestic pigs for which animal slaughter and area quarantine are the only methods of control. ASF virus (ASFV), the causative agent of ASF, is a large, double-stranded DNA virus which is the only member of the Asfarviridae family and the only known DNA arbovirus (5, 6, 9). The genome of ASFV is relatively large, consisting of approximately 180 kbp encoding at least 165 genes. Under a variety of experimental and natural conditions, ASFV infectivity has been shown to be very resistant to inactivation (26). For example, ASFV remained viable for up to 140 days in defibrinated blood held at room temperature (28). In nature, ASFV infects both warthogs (Phacochoerus aethiopicus) and bushpigs (Potamochoerus spp.), as well as ticks of the genus Ornithodoros (34). The natural arthropod host of ASFV is Ornithodoros porcinus porcinus (Walton) (31), a long-lived and nidicolous (burrow-dwelling) argasid tick. Both the vertebrate and arthropod hosts are likely to be required for maintenance of ASFV in the sylvatic cycle, and persistently infected ticks serve as a natural reservoir of the virus. The mechanism of ASFV transmission from the sylvatic cycle to domestic pigs is most likely through infected Ornithodoros ticks feeding on pigs (32, 38), since direct contact with infected warthogs rarely results in transmission to pigs (7, 21, 28, 38). The virus is transmitted between domestic pigs by either direct or indirect contact (26).
Previous studies have described experimental infection of O. porcinus porcinus ticks with a number of different ASFV isolates (15, 20, 33). Although details of the pathogenesis vary in these reports, ASFV infection of O. porcinus porcinus ticks is characterized by establishment of a long-term, persistent infection with relatively high levels of viral replication in a number of different tissues and organs. The initial site of viral replication is the midgut, suggesting a critical role for this tissue in the establishment of infection. The infectious dose of ASFV has been reported to be less than 1 log10 50% hemadsorbing dose (HAD50)/ml for larger ticks (33, 36). ASFV infection of O. porcinus porcinus ticks has been associated with very low mortality (15, 22, 31, 33), except during the gonotrophic cycle (20, 36). These data suggest that ASFV infection of the natural arthropod host represents a well-adapted and possibly coevolved biological system. However, differences in infection rate, infectious dose, or the proportion of ticks which became persistently infected were observed when ticks from the same collection were exposed to different ASFV isolates (15, 33), results which suggest that virus-host adaptation plays a role in the infection of a natural arthropod host with a given ASFV isolate.
Here we describe the results of oral exposure and intrahemocoelic inoculation of O. porcinus porcinus ticks with Malawi Li 20/1 (MAL), an ASFV isolate made from Ornithodoros sp. ticks (8, 18). MAL did not infect ticks exposed orally, although virus entry into midgut cells, early gene expression, and limited late gene expression and viral DNA synthesis occurred. In contrast, when MAL was inoculated intrahemocoelically, a persistent viral infection was established although a slight generalized replication defect was observed. These results indicate that midgut infection and escape constitute important barriers to generalization of ASFV infection of ticks.
MATERIALS AND METHODS
Virus isolates.
Pretoriuskop/96/4/1 (Pr4) was isolated from O. porcinus porcinus ticks collected as previously described (22). MAL was isolated in 1983 from Ornithodoros sp. ticks collected from domestic pig structures during an ASFV epizootic (8, 18). Chiredzi/83/1 (Ch1) was isolated from Ornithodoros sp. ticks collected from warthog burrows near Chiredzi, Zimbabwe, in 1983 (20).
Ticks.
Eight collections of ticks were made from warthog burrows at various locations in Kruger National Park and the Northern Transvaal region of the Republic of South Africa in 1996. A single collection of ticks was made from warthog burrows in the Masai Mara Reserve in Kenya in 1996. All of these ticks were classified as O. porcinus porcinus in accordance with the criteria of Walton (39). A single collection of ticks was made from domestic pig structures in Chalaswa (Mchinji district), Malawi, in 1997. This collection has been identified as O. porcinus domesticus in accordance with the criteria of Walton (39). Ticks used for intrahemocoelic inoculations were from an O. porcinus porcinus colony established from uninfected ticks in the original collection which yielded the Pr4 isolate. Ticks used for reverse transcription (RT)-PCR experiments were from an O. porcinus porcinus colony maintained for an indeterminate period of time at Plum Island Animal Disease Center. All ticks were held at 26°C and a relative humidity of 76% with 12 h of light per 24-h cycle.
RT-PCR analysis.
Ticks were exposed by feeding on an artificial membrane feeder placed over a pool of heparinized pig blood containing either Ch1 or MAL at 7.0 log10 HAD50/ml. Dissected tick midguts or whole ticks were frozen in liquid nitrogen and then ground to a powder before thawing. Total cellular RNA was prepared by using Tri Reagent (Sigma Chemical Company). Samples were harvested at 2, 4, 10, and 24 days postfeeding. Total cellular RNA (∼5 μg) was treated with 100 U of RNase-free DNase I (Boehringer Mannheim) for 4 h at 37°C. Samples were then extracted with the RNeasy Mini Kit (Qiagen) and then denatured in the presence of 2 μg of random hexamers (Gibco BRL Life Technologies) at 94°C for 5 min. RT was performed with 200 U of SuperScript RNase H− reverse transcriptase (Gibco BRL Life Technologies) for 1 h at 45°C. The negative control for each time point was an aliquot of each sample without addition of reverse transcriptase. Resulting cDNAs were amplified by PCR for 30 cycles (94°C, 10 s; 58°C, 30 s; 72°C, 30 s) with a final 10-min incubation at 72°C. A second PCR amplification was performed by using the same protocol with nested primer sets. The primers used for first-round amplification were p72f1, (5′-GCGTTGTGACATCCGAACTA-3′), p72r1 (5′-CAAGATTATATTGGCCCAAG-3′), p30f1 (5′-CCATGAGTCTTACCACCTCT-3′), and p30r1 (5′-GGAGGTCATCTTCAAAACGG-3′). The primers used for second-round amplification were p72f2 (5′-CTCTAAAGGTGTTTGGTTGTC-3′), p72r2 (5′-ATTTTAAGCCTTATGTTCCAG-3′), p30f2 (5′-GAGGGGTTCCATGAATGGTT-3′), and p30r2 (5′-GTAGAATTGTTACGACCGCT-3′).
Tick inoculations.
Ticks were exposed by feeding on an artificial membrane feeder placed in heparinized pig blood. For the 18-day time course (Fig. 1), immunohistochemistry (IHC) and in situ hybridization (ISH) experiments (see Fig. 2), and ultrastructural experiments (see Fig. 3), the titer of the bloodmeal for both MAL- and Pr4-exposed ticks was 7.3 log10 HAD50/ml. For the experiment which compared orally exposed Pr4 to injected Pr4 (see Fig. 4), orally exposed ticks were fed on a viremic pig. The viremic titer of Pr4 on the day of tick feeding was 8.3 log10 HAD50/ml.
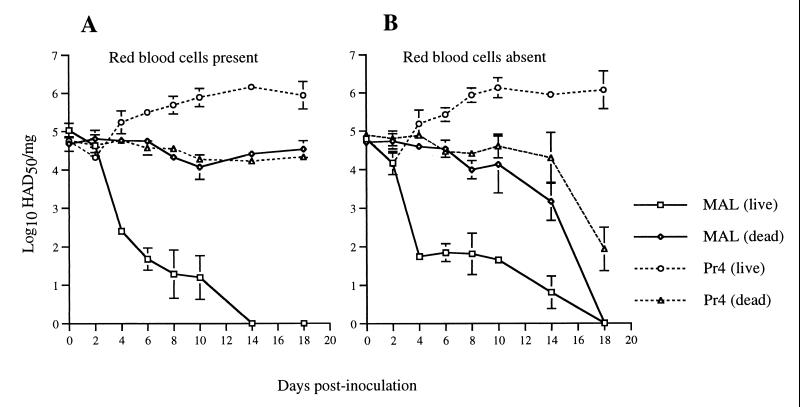
FIG. 1.
Viral replication in O. porcinus porcinus ticks following oral exposure. Ticks were exposed by membrane feeding. The inoculum contained MAL or Pr4 diluted in either heparinized pig blood (A) or fetal bovine serum in place of pig blood (B). Immediately postfeeding, a portion of each group was killed by momentary freezing in liquid nitrogen. At the indicated times p.i., individual ticks (n = 4) from each group were ground in cell culture medium and titrations were performed. The values are mean titers ± the standard errors of the means.
FIG. 2.
IHC and ISH analysis of midgut epithelial cells. Ticks were exposed by membrane feeding on an inoculum containing either MAL (A, C, and E) or Pr4 (B, D, and E). Analysis for ASFV protein p30 (A and B) or p72 (C and D) or ASFV DNA (E and F) was performed at 6 (A and B) or 11 (C, D, E, and F) days p.i. H, hemocoel; L, midgut lumen.
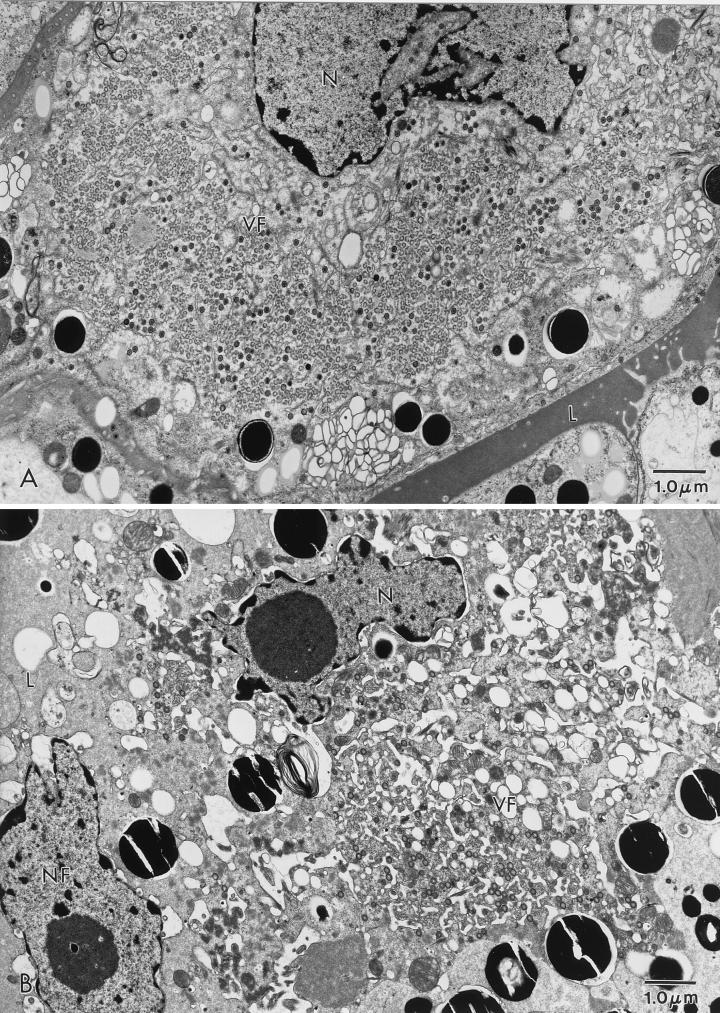
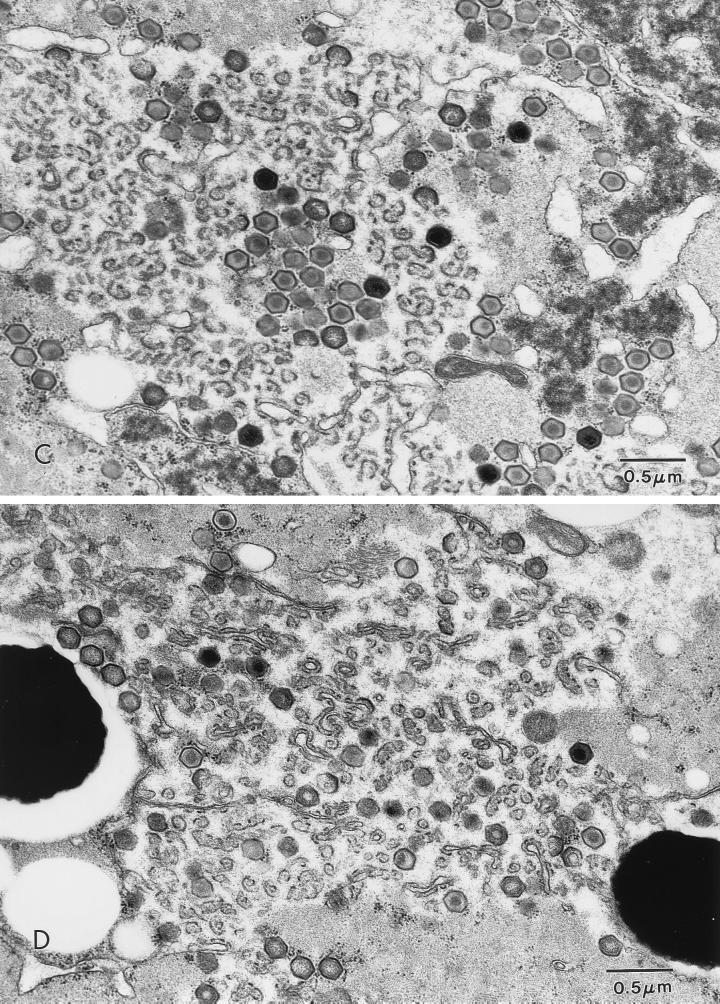
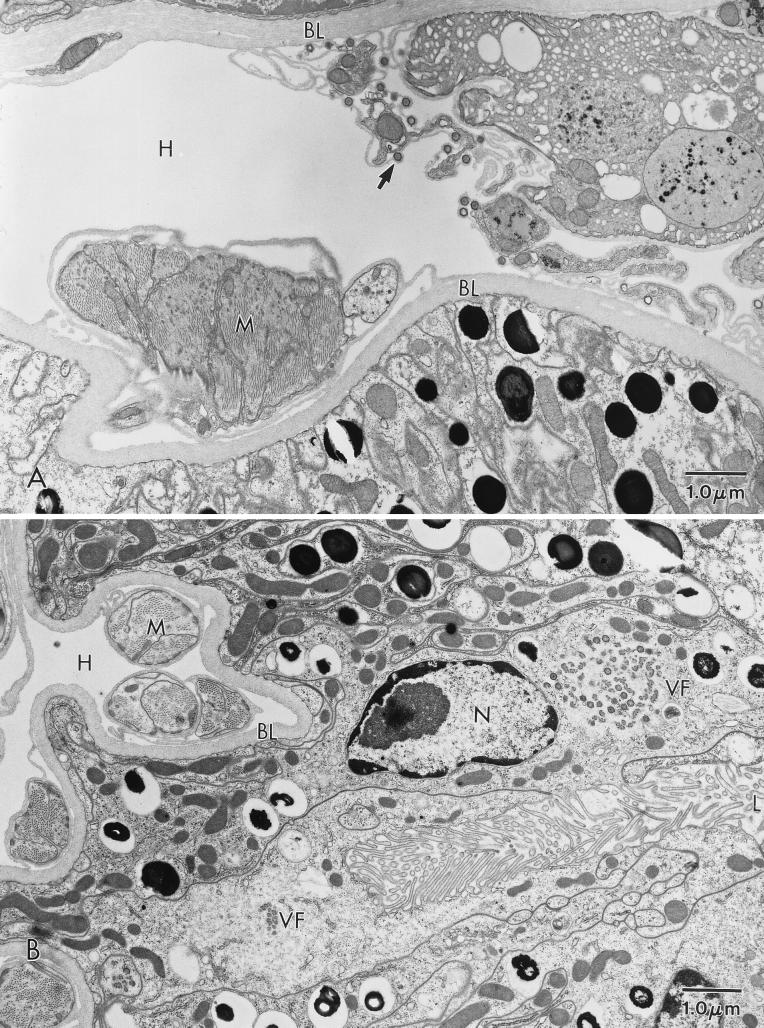
FIG. 3.
ASFV in an O. porcinus porcinus midgut. Ticks were exposed by membrane feeding. The inoculum contained Pr4 (A and C) or MAL (B and D). Analysis was performed 6 days p.i. (A) Virus factory (VF) in a midgut epithelial cell from a Pr4-exposed tick. (B) Virus factory in a midgut epithelial cell from an MAL-exposed tick. (C) Higher magnification of a virus factory from a Pr4-exposed tick. (D) Higher magnification of a virus factory from an MAL-exposed tick. N, nucleus; L, midgut lumen; NF, nucleus free in the midgut lumen.
FIG. 4.
Viral replication in O. porcinus porcinus ticks following intrahemocoelic (intrahemo.) or oral inoculation (inoc.). Ticks were infected by intrahemocoelic injection of either Pr4 or MAL or by feeding on a pig infected with Pr4. Individual ticks (n = 4) from each group were dissected at the indicated times p.i., and tissue viral titers were determined. Total-tick viral titers represent the sum of all dissected and undissected tissues. The values are mean titers ± the standard errors of the means.
Intrahemocoelic inoculations were performed by injecting 3.9 log10 HAD50 of either MAL or Pr4 in a volume of 2 μl with a finely pulled, calibrated glass capillary pipet. Injections were made in the membranous region located between the coxa and trochanter of the second leg of either N4, N5, or adult ticks weighing 10 to 25 mg. Viral stocks were prepared from dissected midguts of 10 MAL-injected ticks 42 to 98 days postinoculation (p.i.) by inoculation of primary porcine peripheral blood mononuclear cell cultures (14, 30). Naive ticks were then fed on artificial membrane feeders placed in heparinized pig blood which contained the resulting viral stocks at approximately 6.75 log10 HAD50/ml.
Virus titrations.
Individual whole ticks were ground in 0.5 ml of cell culture medium in sterile tubes with plastic pestles (Pellet pestle; Kontes). The samples were stored at −70°C. Immediately prior to titration, samples were thawed at 37°C and sonicated for 1 min. Samples were serially diluted and then added to porcine peripheral blood mononuclear cells as previously described (22) and endpoint titers were calculated (35).
To determine the virus titers in isolated organs, ticks were dissected by using a binocular microscope and tissues were titrated as previously described (22). The titer of hemolymph was obtained by clipping the distal tarsus of the second leg and collecting 1 to 2 μl of hemolymph in a sterile tube. All samples were diluted to 0.5 ml and titrated as described above.
For data presented in Table 3, individual ticks were fed on a membrane feeding apparatus as previously described (22, 25). Following feeding, coxal fluid (if any was produced during a 2-h postfeeding observation period) and blood (containing salivary secretions) from beneath the membrane were collected, diluted to 1 ml with cell culture medium containing 20% fetal bovine serum, and then held at −70°C until assayed by virus titration. Values were normalized to the volume of the diluted sample. The undiluted sample and the first serial dilution were blind passaged for any samples that were negative for ASFV on the initial titration.
TABLE 3.
ASFV transmission by Ornithodoros ticks following parenteral inoculation
| Days p.i. | Mean titera ± SEM (no. of ticks positive/no. tested)
|
|||
|---|---|---|---|---|
| Salivary secretion
|
Coxal fluid
|
|||
| Pr4 | MAL | Pr4 | MAL | |
| 21–50 | 3.2 ± 0.3 (12/18) | 0 (0/14) | 3.7 ± 0.3 (15/15) | 0.8 (1/10) |
| 65–101 | 3.6 ± 0.4 (11/11) | 0.8 (1/11) | 4.1 ± 0.1 (10/10) | 2.3 ± 0.4 (6/10) |
| 117–167 | 3.4 ± 0.2 (12/12) | 1.4 ± 0.2 (5/13) | 4.0 ± 0.3 (8/8) | 2.5 ± 0.2 (10/11) |
Log10 HAD50 per milliliter.
Ultrastructural procedures.
At various times p.i., ticks were fixed and embedded as previously described (22). For analysis, 70- to 90-nm sections were collected on single-slot grids coated with Formvar and stabilized with carbon (Electron Microscopy Sciences) and then observed and photographed with a Philips 410 electron microscope operated at 80 kV.
IHC and ISH.
At the indicated times p.i., ticks were cut along the sagittal plane to allow infiltration of the fixative solutions (10% neutral buffered formalin), embedded in paraffin, and sectioned. Sections were allowed to adhere to Superfrost/plus slides (Fisher Scientific, Pittsburgh, Pa.), heated for 20 min at 65°C, and then deparaffinized by using xylene. Sections were rehydrated through graded alcohol and washed with phosphate-buffered saline (PBS) (pH 7.4) for IHC and ISH. Four-micrometer-thick sections were used for IHC and ISH.
IHC was performed essentially as described previously (37). Briefly, sections were first treated with 3% hydrogen peroxide in PBS for 20 min, followed by washes in PBS and digestion with 0.05% Protease XIV (Sigma Chemical Co., St. Louis, Mo.) for 2 min at 37°C. After several washes in PBS, sections were incubated in a blocking solution (5% normal goat serum in PBS) for 30 min at room temperature and then incubated for 2 h at 4°C with specific polyclonal antiserum (diluted 1:200 in PBS) directed against ASFV structural protein p30 or p72. Following washes with PBS, slides were incubated with alkaline phosphatase-conjugated, goat anti-rabbit antibody for 20 min at room temperature.
ISH was performed as described previously (37), with minor modifications. Probes containing both the p30 and p72 genes were labeled by a random priming reaction with digoxigenin-dUTP (DIG; Boehringer Mannheim Corp., Indianapolis, Ind.). Labeled DNA probes were diluted in the prehybridization mixture and then heated for 10 min at 95°C and placed on ice before being applied to the sections. Coverslips were applied, and the target DNA was denatured by placing the slides for 5 min on a hot plate preheated to 96°C. The slides were then placed on ice for 3 min. Hybridization was performed overnight at 37°C. An anti-DIG–alkaline phosphatase conjugate (diluted to 1:500) was then added to the tissue sections and incubated for 2 h at room temperature. Sections were then incubated with color substrate solution for 2 to 3 h in the dark, and then the reaction was stopped with distilled water. Sections were counterstained with 0.5% methyl green.
RESULTS
MAL does not infect ticks following oral inoculation.
Ten different collections of O. porcinus porcinus ticks and one collection of O. porcinus domesticus ticks were orally exposed to high titers of MAL (Table 1). Based on the size of the bloodmeal, a minimum of 6 to 7 log10 HAD50 was inoculated into each tick. By 3 weeks postfeeding, less than 25% of the ticks contained detectable virus and in the ticks in which virus could be detected, titers had fallen by at least 100-fold. By five weeks p.i., virus could be detected in less than 10% of the ticks and the titers had fallen by 10,000-fold.
TABLE 1.
Oral exposure of Ornithodoros sp. ticks to MAL
| Tick collection | Source | Titer of inoculuma | Mean titerb ± SEM (no. positive/no. tested)
|
|
|---|---|---|---|---|
| 21 days p.i. | 35 days p.i. | |||
| O. porcinus porcinus | Colonized tick | 8.0 | NA (0/20) | NA (0/14) |
| Cr1 | Krugerc | 8.3 | 4.0 ± 0.5 (3/6) | NA (0/6) |
| Cr3 | Kruger | 8.3 | 3.4 ± 0.5 (6/6) | 3.0 ± 0.8 (3/6) |
| Pr4 | Kruger | 8.3 | 4.4 (1/27) | NA (0/12) |
| Pr5 | Kruger | 8.3 | 3.0 (1/6) | NA (0/6) |
| Skuk6 | Kruger | 8.3 | 3.6 ± 0.9 (3/6) | 1.9 ± 0.1 (2/6) |
| Nat1 | Kruger | 8.3 | 3.3 ± 0.3 (3/6) | NA (0/6) |
| No6 | N. Transvaald | 8.3 | 3.2 ± 0.3 (6/6) | 3.0 ± 0.3 (2/6) |
| G3 | N. Transvaal | 8.3 | NA (0/6) | NA (0/6) |
| Masai Marae | Kenya | 6.9 | NA (0/6) | NA (0/12) |
| Malawif | Malawi | 7.0 | 2.0 (1/14) | 1.8 (1/14) |
| Total | 24/109 | 8/88 | ||
Log10 HAD50 per milliliter.
Log10 HAD50 per milligram. NA, not applicable.
Kruger National Park, Republic of South Africa.
Northern Transvaal, Republic of South Africa.
Masai Mara Reserve, Kenya.
Tentatively identified as O. porcinus domesticus.
In a second experiment, ticks from the Pr4 colony were orally exposed to either MAL or Pr4 and sampled regularly between 0 and 18 days p.i. (Fig. 1). One group was fed an inoculum containing swine blood, and the second group was fed with fetal bovine serum in place of the blood. In both groups, MAL titers declined 1,000-fold between 2 and 6 days p.i. By 14 to 18 days p.i., no virus was detected in MAL-exposed ticks. In contrast, titers of Pr4 increased by 10-fold at 10 days p.i. and 50-fold by 14 days p.i. In an independent replicate, MAL titers were not detected at 14, 21, 28, or 60 days following oral exposure (data not shown). As a control for the stability of the virus remaining in the midgut lumen, a portion of each group was killed by momentary freezing in liquid nitrogen and then returned to the same holding conditions as the live ticks (Fig. 1). For ticks exposed to virus in blood and then killed immediately p.i., viral titers did not decline appreciably over the sampling period. For ticks exposed to virus in serum and then killed, viral titers were stable until 14 days p.i., after which they declined 100- to 1,000-fold.
Limited MAL gene expression and viral DNA synthesis occur following oral inoculation.
ASFV gene expression in ticks orally exposed to MAL was assessed by RT-PCR at various times p.i. (Table 2). Assays were performed for expression of genes encoding structural proteins p30 and p72, which are encoded by an early and a late gene in ASFV infection of swine macrophages, respectively (1, 4). Following oral inoculation with MAL, p30 expression was detected in the midguts of 90% of the ticks at 2 days p.i., 60% of the ticks at 4 days p.i., and none of the ticks at 10 days p.i. In contrast, p30 expression in ticks orally exposed to Ch1, an ASFV isolate which has been previously shown to replicate in ticks (22), was detected in a majority of the ticks sampled at each time point. Expression of the late gene encoding p72 was detected at low frequency (22% of dissected midguts) only at 10 days p.i. following oral exposure to MAL but was detected at a considerably higher frequency in Ch1-exposed ticks.
TABLE 2.
ASFV gene expression in midguts of orally exposed ticks
| Virus | Primer set | No. of ticks positive/no. exposed
|
|||
|---|---|---|---|---|---|
| 2 days p.i. | 4 days p.i. | 10 days p.i. | 24 days p.i. | ||
| Ch1 | p30 | 10/10 | 9/10 | 6/10 | 4/4 |
| MAL | p30 | 9/10 | 6/10 | 0/9 | NDa |
| Ch1 | p72 | 2/10 | 6/10 | 4/10 | 4/4 |
| MAL | p72 | 0/10 | 0/10 | 2/9 | ND |
ND, not determined.
IHC performed at 3, 6, and 11 days p.i. using polyclonal antibodies against p30 and p72 corroborated the RT-PCR results. At 6 days p.i., p30, but not p72, could be readily detected in midgut epithelial cells following oral inoculation of MAL (Fig. 2A). At 11 days p.i., p72 expression could be detected, but only in an occasional section (Fig. 2C). In contrast, both p30 and p72 were expressed in a majority of midgut cells at both 6 and 11 days p.i. following oral inoculation with Pr4 (Fig. 2B and D). When midgut sections were analyzed by ISH for ASFV DNA at 3, 6, and 11 days p.i., a small percentage of midgut cells were found to be weakly positive in MAL-exposed ticks (Fig. 2E) at 11 days p.i. However, for Pr4-exposed ticks, many more midgut cells were positive for ASFV DNA by this technique and the intensity of staining was greater in Pr4-exposed ticks than in MAL-exposed ticks (Fig. 2F).
Ultrastructural analysis of orally exposed ticks was performed at 4, 6, and 10 days p.i. In MAL-exposed ticks, viral replication factories were rarely observed in midgut epithelial cells. When present, MAL factories were comparatively smaller and contained few fully formed viral particles (Fig. 3B). These particles often had an atypical morphology characterized by either an acentric or absent condensed viral nucleoid structure (Fig. 3D). When MAL factories were observed, the cells which contained them had a fragmented cellular membrane, mitochondrial condensation, pulling away of the nuclear membrane, and extensive vacuolization (Fig. 3B). Sloughed digestive midgut epithelial cells and free nuclei (Fig. 3B) were commonly observed in the midgut lumen. These observations indicated considerable cytopathology in phagocytic midgut epithelial cells following oral inoculation of MAL. In Pr4-exposed ticks, virus factories were commonly observed at 6 days p.i. Numerous large virus factories with a large number of fully formed particles were present in the midgut epithelial cells of these ticks (Fig. 3A and C). In Pr4-exposed ticks, viral factories were similar, if not identical, to those observed following oral exposure to the Ch1 isolate (22). The phagocytic midgut epithelial cells which contained Pr4 viral factories showed minimal evidence of cellular pathology, and sloughed midgut epithelial cells were rarely observed in the lumen of Pr4-infected ticks.
A persistent infection is established by intrahemocoelic inoculation of MAL.
To determine if MAL replication was restricted in tissues other than the midgut, parenteral inoculations into the hemocoel were performed. In contrast with oral exposure to MAL, intrahemocoelic inoculation resulted in a generalized, persistent infection. Although total tick MAL titers did not increase through 27 days p.i., by 50 days p.i., the total-tick viral titer and most tissue viral titers had increased 10- to 100-fold (Fig. 4). Maximum MAL tissue titers, which occurred at 98 days p.i., were at least 100-fold higher than titers measured immediately p.i. However, even at their maximum, most MAL tissue titers were 10- to 100-fold lower than Pr4 titers. The only exception was the hemolymph titer, which was 50-fold higher in MAL-injected ticks than in Pr4-injected ticks at 98 days p.i. After 98 days p.i., MAL tissue including hemolymph, titers declined approximately 10- to 100-fold but did not decrease further during the period of study (364 days). The infection rate for MAL-injected ticks was 100% (40 of 40 ticks sampled). Intrahemocoelic injection of Pr4 resulted in rapid dissemination of infection to all of the tissues assayed. By the first sampling at 12 days p.i., total tick Pr4 titers had increased 1,000-fold to 6.1 log10 HAD50/mg and did not decrease appreciably for the duration of the study. Most tissue viral titers peaked by 12 days p.i. In contrast to all of the other tissues assayed, viral titers of the hemolymph declined dramatically in Pr4-injected ticks after the initial sampling at 12 days p.i. The infection rate of Pr4-injected ticks was 100% (40 of 40 ticks sampled). As a control for the intrahemocoelic route of infection, tissue viral titers from ticks exposed to Pr4 by this route were compared to the titers of ticks exposed orally to Pr4 (Fig. 4). Although intrahemocoelic inoculation is an unnatural route of infection, once generalization of infection occurred, viral titers in injected ticks were not different than those in orally exposed ticks.
At 98 days p.i., MAL midgut titers were approximately 3.2 log10 HAD50/mg and midgut viral titers in Pr4-injected ticks were nearly equivalent to those in ticks orally exposed to Pr4 (Fig. 4). These data suggested that following injection, virus may have crossed the basal lamina of the midgut and was replicating in midgut epithelial cells. To determine if this had occurred, ultrastructural analysis of injected ticks was performed. Analysis of 1,195 midgut epithelial cells (in 24 cecae from three ticks) of MAL-injected ticks failed to identify a single infected midgut epithelial cell. However, mature MAL particles and small viral factories were observed in connective tissue cells and hemocytes which adhered to (or were closely associated with) the hemocoelic side of the midgut (Fig. 5A) and thus likely contributed to the titers observed in dissected midguts (Fig. 4). In contrast, ultrastructural analysis of Pr4-injected ticks demonstrated that Pr4 had crossed the basal lamina from the hemocoel and was replicating in midgut epithelial cells (Fig. 5B). Approximately 7% (93 of 1,223 cells in 24 cecae from three ticks) of midgut epithelial cells from Pr4-injected ticks were observed to have virus factories.
FIG. 5.
ASFV in an O. porcinus porcinus midgut. Analysis was performed 14 weeks after intrahemocoelic inoculation. (A) Mature virions (arrow) budding from a connective tissue cell adjacent to the basal lamina (BL) of the midgut in an MAL-injected tick. (B) Virus factory (VF) in a midgut epithelial cell from a Pr4-injected tick. H, hemocoel; L, lumen; M, muscle; N, nucleus.
Virus isolated from midguts of MAL-injected ticks was refed to naive ticks, but this failed to result in an infection. Viral stocks were prepared from the midguts of 10 ticks (dissected from 42 to 98 p.i.) and then refed to ticks from the same colony. At 29 days p.i., 12 ticks per midgut sample were assayed individually for virus. All of the ticks from 8 of the 10 groups contained no detectable virus while two groups had a single positive tick with a titer of ≤2.5 log10 HAD50. A subsequent passage of virus isolated from these two ticks failed to infect any naive ticks. Thus, even after a period which would allow adaptation to midgut epithelial cells, MAL could not establish an infection when administered by the oral route.
To assess the ability of MAL-injected ticks to transmit the virus during feeding, individual ticks were fed on artificial membrane feeders (Table 3). Prior to 50 days p.i., only a single sample from an MAL-injected tick contained the virus and the titer of this coxal fluid sample was low. Sixty-six percent of the salivary secretion samples and all of the coxal fluid samples from Pr4-injected ticks contained considerable levels of the virus. Between 65 and 101 days p.i., 60% of MAL-injected ticks secreted the virus in the coxal fluid; however, the presence of the virus in the salivary secretion was still rare (1 of 11 samples). In contrast, all of the salivary secretion and coxal fluid samples from Pr4-injected ticks contained the virus after 65 days p.i. After 117 days p.i., 38% of the salivary secretion samples and 91% of the coxal fluid samples from MAL-injected ticks were positive. In addition to a delay before transmission and a lower frequency of positive samples from MAL-injected ticks, positive samples had a mean titer that was typically 50- to 100-fold lower than that of Pr4-injected controls.
Assessment of transovarial transmission demonstrated that MAL-injected female ticks were not capable of transmitting the virus to their offspring. Approximately 120 days p.i., MAL- and Pr4-injected females were mated with uninfected males from the same colony and then fed individually on uninfected blood. All of the resulting first-stage nymphs (n = 24 per egg mass) from MAL-injected females were negative when assayed for the virus after both the first (n = 8 egg masses) and second (n = 6 egg masses) gonotrophic cycles. In contrast, all (n = 5) egg masses from the first gonotrophic cycle of Pr4-injected females contained first-stage nymphs that were positive for the virus and the mean infection rate was 49% of the nymphs (n = 24 per egg mass).
DISCUSSION
MAL is an ASFV isolate that is highly pathogenic for domestic pigs. Although the MAL we used had been isolated from a pool of four adult male Ornithodoros sp. ticks collected in a domestic pig structure during an ASF epizootic (8, 18), our attempts to infect ticks by oral exposure to MAL failed. In other studies, oral exposure of O. porcinus porcinus ticks to ASFV resulted in an infection characterized by primary replication in the midgut, followed by dissemination, with relatively high tissue viral titers and persistence of infection for at least several months or, in some studies, for several years (15, 22, 33). While the data presented in Table 1 suggest that a portion of the ticks may have been infected, this infection is likely to have been abortive (nonproductive) since both the virus titers and the number of ticks containing virus (for all collections) declined between 21 and 35 days postfeeding. Based on data collected in all other experiments, the titers detected in this initial experiment were most likely due to the high titer of the inoculum coupled with the stability of the virus in the midgut lumen. This may also be the reason why MAL was originally isolated from ticks, since it is likely that ticks had ample opportunity to feed on pigs with viremic titers in excess of 8 log10 HAD50/ml. Alternatively, we have observed that gut contents will occasionally (<2% of the ticks fed) leak into the hemocoel during or shortly after feeding without tick mortality (36). This occurrence would have the same effect as intrahemocoelic inoculation of MAL and could have resulted in persistent infection of the field-collected ticks from which MAL was originally isolated. Leakage of midgut contents into the hemocoel following blood feeding is known to occur in mosquitos (17). However, the incidence documented in one study (16% of the mosquitos fed) (41) was much higher than what we have observed in Ornithodoros ticks.
Following oral exposure to MAL, there is a rapid decline in viral infectivity and after 18 days p.i., no virus was detected in these ticks. The results of RT-PCR, IHC, and ISH experiments all suggest that MAL enters midgut epithelial cells following oral exposure. The decline in MAL titers is not due to degradation of the virus in the midgut lumen (following failure or delay of virus entry into midgut epithelial cells), since titers in ticks which were killed immediately following feeding declined at a much slower rate than in live ticks. Additionally, since nearly identical declines in MAL titers were obtained for ticks exposed either with or without erythrocytes, the loss of infectivity is not due to virus being internalized via hemadsorption to erythrocytes, which are subsequently phagocytosed and digested within midgut epithelial cells. The results of RT-PCR and IHC experiments also demonstrate that early gene expression occurred at levels similar to that detected after inoculation with a virus that can infect ticks by the oral route. However, both late gene expression and viral DNA synthesis occurred at comparatively lower levels in MAL-exposed ticks. Thus, the restriction of MAL replication in midgut epithelial cells is likely to occur late in the viral replication cycle. As would be predicted from these results, the appearance of progeny virus was quite rare in midgut epithelial cells of MAL-exposed ticks.
When MAL viral factories were observed in midgut epithelial cells, the mature particles often had atypical morphology, suggesting that they are noninfectious. Interestingly, the phagocytic midgut epithelial cells which contained MAL particles exhibited cellular pathology which included condensation of chromatin, pulling away of the nuclear membrane, roughening of the cell border, and sloughing of cells into the midgut lumen. Cytopathology has been rarely observed following arboviral infection of the natural host. In previous studies, pathologic effects were not detected following infection of ixodid ticks with Thogoto virus (2) or Dugbe virus (3) but have been reported following infection of mosquitos with eastern equine encephalomyelitis virus (40) and western equine encephalomyelitis virus (42). Additional work is required to establish a correlation between nonproductive infection with MAL and the death of midgut cells. Early death of midgut cells may be a mechanism which prevents productive infection of MAL in this tissue and could possibly be due to failure of the virus to express specific genes which allow the production of large quantities of progeny virus without damaging the host cell. Alternatively, early cell death may be an active mechanism of host defense and MAL may lack the viral gene(s) required to counter this response. It is interesting that, despite obvious cytopathology, we have observed no increase in the mortality of MAL-exposed ticks over that of Pr4-exposed controls (24).
There are several possible explanations for the failure of MAL to infect ticks following oral exposure. First, the natural arthropod host for MAL may be a species or subspecies of Ornithodoros ticks which was not tested in this study and/or MAL may have a very narrow arthropod host range. However, the 11 tick collections in which MAL infectivity was tested represent at least four geographic regions of sub-Saharan Africa. Most significantly, the O. porcinus domesticus ticks tested were collected in the same village from which MAL was isolated 13 years earlier. Based on the work of others and our unpublished results, it appears that all of the other ASFV isolates studied to date will infect most, but not all, collections of O. porcinus porcinus ticks (24, 33), as well as a large number of distantly related congeners, such as O. savignyi (27), O. coriaceus (16, 19), O. turicata (19), O. puertoricensis (10, 11, 19), and O. marocanus (12, 13). Second, although the MAL virus stock was subjected to a limited number of subpassages, these passages were in either domestic pigs or porcine peripheral blood mononuclear cells. Thus, rearrangements or deletions of genes important for infection of midgut epithelial cells may have occurred since MAL was isolated from ticks. However, it is significant that Pr4 has been subjected to numerous successive rounds of plaque purification on porcine peripheral blood mononuclear cells without any detectable loss of the ability to infect ticks (24), a result which suggests that this is not a likely explanation for the MAL defect. Finally, as suggested above, MAL may be a virus that is not capable of infecting ticks following oral inoculation but was isolated from ticks following feeding on a pig with a high viremic titer.
To determine if the inability to replicate was specific to midgut epithelial cells, MAL was injected intrahemocoelically into O. porcinus porcinus ticks. When MAL was inoculated by this route, a generalized and persistent viral infection occurred in 100% of the ticks. MAL-injected ticks secreted the virus during feeding but did not transmit the virus transovarially. While the tissue distribution of the virus was similar to that in Pr4-injected ticks, differences in viral tissue titers and the time course of infection were observed. On average, MAL tissue titers were 10- to 100-fold lower than Pr4 titers and there was a delay of approximately 40 days before MAL titers began to increase. As expected, transmission of MAL during feeding was also delayed and both the frequency and the quantity of the virus excreted were reduced compared to those of Pr4. Therefore, MAL has a slight generalized replication defect in O. porcinus porcinus ticks. However, the midgut is the only tissue in which MAL has an absolute replication defect, suggesting a narrow genetic basis for this phenotype. The midgut replication defect appears to be stable, since even after a period which would allow adaptation (up to 98 days following intrahemocoelic injection), MAL was not detected in midgut epithelial cells and reinoculation of MAL isolated from injected ticks failed to infect ticks by the oral route. These results demonstrate conclusively that midgut infection and escape constitute barriers to dissemination of MAL and, presumably, other ASFV isolates following oral exposure of O. porcinus porcinus ticks. An earlier ASFV pathogenesis study suggested that a midgut barrier did not exist in O. porcinus porcinus ticks, but the supporting data were indirect (15).
In summary, we have characterized the phenotype of an ASFV isolate that does not replicate in the midgut epithelium of the arthropod host, O. porcinus porcinus, and thus cannot establish an infection when these ticks feed on viremic blood. MAL differs from other ASFV isolates previously studied in its inability to infect O. porcinus porcinus ticks following oral exposure. The MAL midgut defect is likely to be due to missing or defective viral genes, and future experiments can now be designed to elucidate the gene(s) required for infection of the midgut epithelium, the first and most important tissue encountered by a blood-borne pathogen infecting a hematophagous arthropod. Identification of the gene(s) important for ASFV infection of the midgut is feasible since the ASFV genome can be manipulated through deletion, addition, or substitution of specific genes and/or regions of the viral genome by either reverse genetics or marker rescue techniques (23, 29, 30, 43, 44). Since Ornithodoros ticks are an important route by which ASFV moves from the sylvatic cycle to domestic pigs in sub-Saharan Africa, knowledge of the viral genes required to infect ticks may suggest novel control methods.
ACKNOWLEDGMENTS
We thank Daniel L. Rock and Douglas M. Moore for valuable discussions and critical reviews of the manuscript. We also thank Gray Matita (Central Veterinary Laboratory, Lilongwe, Malawi) for collecting ticks from the Chalaswa village in Malawi, Stefan Swanepoel (Onderstepoort Institute for Exotic Animal Diseases) for assistance in collecting ticks from South Africa, and Cherise Rohr (Yale University School of Medicine) for collecting ticks from the Masai Mara Reserve, Kenya.
REFERENCES
- 1.Afonso C, Alcaraz C, Brun A, Sussman M D, Rock D L. Characterization of p30, a highly antigenic membrane and secreted protein of African swine fever virus. Virology. 1992;189:368–373. doi: 10.1016/0042-6822(92)90718-5. [DOI] [PubMed] [Google Scholar]
- 2.Booth T F, Davies C R, Jones L D, Staunton D, Nuttall P A. Anatomical basis of Thogoto virus infection in BHK cell culture and in the Ixodid tick vector, Rhipicephalus appendiculatus. J Gen Virol. 1989;70:1093–1104. doi: 10.1099/0022-1317-70-5-1093. [DOI] [PubMed] [Google Scholar]
- 3.Booth T F, Steele G M, Marriott A C, Nuttall P A. Dissemination, replication, and trans-stadial persistence of Dugbe virus (Nairovirus, Bunyaviridae) in the tick vector Amblyomma variegatum. Am J Trop Med Hyg. 1991;45:146–157. doi: 10.4269/ajtmh.1991.45.146. [DOI] [PubMed] [Google Scholar]
- 4.Borca M V, Irusta P, Carrillo C, Afonso C L, Burrage T, Rock D L. African swine fever virus structural protein p72 contains a conformational neutralizing epitope. Virology. 1994;201:413–418. doi: 10.1006/viro.1994.1311. [DOI] [PubMed] [Google Scholar]
- 5.Brown F. The classification and nomenclature of viruses: summary of results of meetings of the International Committee on Taxonomy of Viruses in Sendai, September 1984. Intervirology. 1986;25:141–143. doi: 10.1159/000150091. [DOI] [PubMed] [Google Scholar]
- 6.Costa J V. African swine fever virus. In: Darai G, editor. Molecular biology of iridoviruses. Norwell, Mass: Kluwer Academic Publishers; 1990. pp. 247–270. [Google Scholar]
- 7.DeTray D E. African swine fever. Adv Vet Sci Comp Med. 1963;8:299–333. [PubMed] [Google Scholar]
- 8.Dixon L K. Molecular cloning and restriction enzyme mapping of an African swine fever virus isolate from Malawi. J Gen Virol. 1988;69:1683–1694. doi: 10.1099/0022-1317-69-7-1683. [DOI] [PubMed] [Google Scholar]
- 9.Dixon L K, Rock D L, Vinuela E. African swine fever-like viruses. Arch Virol. 1995;10:92–94. [Google Scholar]
- 10.Endris R G, Haslett T M, Hess W R. Experimental transmission of African swine fever virus by the tick Ornithodoros (Alectorobius) puertoricensis (Acari: Argasidae) J Med Entomol. 1991;28:854–858. doi: 10.1093/jmedent/28.6.854. [DOI] [PubMed] [Google Scholar]
- 11.Endris R G, Haslett T M, Hess W R. African swine fever virus infection in the soft tick, Ornithodoros (Alectorobius) puertoricensis (Acari: Argasidae) J Med Entomol. 1992;29:990–994. doi: 10.1093/jmedent/29.6.990. [DOI] [PubMed] [Google Scholar]
- 12.Endris R G, Hess W R, Caiado J M. African swine fever virus infection in the Iberian soft tick, Ornithodoros (Pavlovskyella) marocanus (Acari: Argasidae) J Med Entomol. 1992;29:874–878. doi: 10.1093/jmedent/29.5.874. [DOI] [PubMed] [Google Scholar]
- 13.Endris R G, Hess W R. Experimental transmission of African swine fever virus by the soft tick, Ornithodoros (Pavlovskyella) marocanus (Acari: Ixodoidea: Argasidae) J Med Entomol. 1992;29:652–656. doi: 10.1093/jmedent/29.4.652. [DOI] [PubMed] [Google Scholar]
- 14.Genovesi E V, Villinger F, Gerstner D J, Whyard T C, Knudson R C. Effect of macrophage-specific colony-stimulating factor (CSF-1) on swine monocyte/macrophage susceptibility to in vitro infection by African swine fever virus. Vet Microbiol. 1990;25:153–176. doi: 10.1016/0378-1135(90)90074-6. [DOI] [PubMed] [Google Scholar]
- 15.Greig A. The localization of African swine fever virus in the tick Ornithodoros moubata porcinus. Archiv Gesamte Virusforsch. 1972;39:240–247. doi: 10.1007/BF01241546. [DOI] [PubMed] [Google Scholar]
- 16.Groocock C M, Hess W R, Gladney W J. Experimental transmission of African swine fever virus by Ornithodoros coriaceus, an argasid tick indigenous to the United States. Am J Vet Res. 1980;41:591–594. [PubMed] [Google Scholar]
- 17.Hardy J L, Houk E J, Kramer L D, Reeves W C. Intrinsic factors affecting vector competence of mosquitos for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 18.Haresnape J M, Wilkinson P J, Mellor P S. Isolation of African swine fever virus from ticks of the Ornithodoros moubata complex (Ixodoidea: Argasidae) collected within the African swine fever enzootic area of Malawi. Epidemiol Infect. 1988;101:173–185. doi: 10.1017/s0950268800029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess W R, Endris R G, Haslett T M, Monahan M J, McCoy J P. Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Vet Parasitol. 1987;26:145–155. doi: 10.1016/0304-4017(87)90084-7. [DOI] [PubMed] [Google Scholar]
- 20.Hess W R, Endris R G, Lousa A, Caiado J M. Clearance of African swine fever virus from infected tick (Acari) colonies. J Med Entomol. 1989;26:314–317. doi: 10.1093/jmedent/26.4.314. [DOI] [PubMed] [Google Scholar]
- 21.Heuschele W P, Coggins L. Epizootiology of African swine fever in warthogs. Bull Epizoot Dis Afr. 1969;17:179–183. [PubMed] [Google Scholar]
- 22.Kleiboeker S B, Burrage T G, Scoles G A, Fish D, Rock D L. African swine fever virus infection in the argasid host, Ornithodoros porcinus porcinus. J Virol. 1997;72:1711–1724. doi: 10.1128/jvi.72.3.1711-1724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleiboeker S B, Kutish G F, Neilan J G, Lu Z, Zsak L, Rock D L. A conserved African swine fever virus right variable region gene, l11l, is nonessential for growth in vitro and virulence in domestic swine. J Gen Virol. 1998;79:1189–1195. doi: 10.1099/0022-1317-79-5-1189. [DOI] [PubMed] [Google Scholar]
- 24.Kleiboeker, S. B., G. A. Scoles, and D. L. Rock. Unpublished data.
- 25.Mango C K A, Galun R. Ornithodoros moubata: breeding in vitro. Exp Parasitol. 1977;42:282–288. doi: 10.1016/0014-4894(77)90085-6. [DOI] [PubMed] [Google Scholar]
- 26.Mebus C A. African swine fever. Adv Virus Res. 1988;35:251–269. doi: 10.1016/s0065-3527(08)60714-9. [DOI] [PubMed] [Google Scholar]
- 27.Mellor P S, Wilkinson P J. Experimental transmission of African swine fever virus by Ornithodoros savignyi (Audouin) Res Vet Sci. 1985;39:353–356. [PubMed] [Google Scholar]
- 28.Montgomery R E. On a form of swine fever occurring in British East Africa (Kenya Colony) J Comp Pathol. 1921;34:159–191. [Google Scholar]
- 29.Moore D M, Zsak L, Neilan J G, Lu Z, Rock D L. The African swine fever virus tymidine kinase gene is required for efficient replication in swine macrophages and for virulence in swine. J Virol. 1998;72:10310–10315. doi: 10.1128/jvi.72.12.10310-10315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neilan J G, Lu Z, Kutish G F, Zsak L, Burrage T G, Borca M V, Carrillo C, Rock D L. A BIR motif containing gene of African swine fever virus, 4CL, is nonessential for growth in vitro and viral virulence. Virology. 1997;230:252–264. doi: 10.1006/viro.1997.8481. [DOI] [PubMed] [Google Scholar]
- 31.Plowright W, Parker J, Pierce M A. African swine fever virus in ticks (Ornithodoros moubata, Murray) collected from animal burrows in Tanzania. Nature. 1969;221:1071–1073. doi: 10.1038/2211071a0. [DOI] [PubMed] [Google Scholar]
- 32.Plowright W, Parker J, Pierce M A. The epizootiology of African swine fever in Africa. Vet Rec. 1969;85:668–674. [PubMed] [Google Scholar]
- 33.Plowright W, Perry C T, Pierce M A, Parker J. Experimental infection of the argasid tick, Ornithodoros moubata porcinus, with African swine fever virus. Archiv Gesamte Virusforsch. 1970;31:33–50. doi: 10.1007/BF01241664. [DOI] [PubMed] [Google Scholar]
- 34.Plowright W, Thomson G R, Neser J A. African swine fever. In: Coetzer J A W, Thomson G R, Tustin R C, editors. Infectious diseases of livestock with special reference to southern Africa. Cape Town, South Africa: Oxford University Press; 1994. pp. 568–599. [Google Scholar]
- 35.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 36.Scoles, G. A., S. B. Kleiboeker, and D. L. Rock. Unpublished data.
- 37.Sur J-H, Cooper V L, Galeota J A, Hesse R A, Doster A R, Osorio F A. In vivo detection of porcine reproductive and respiratory syndrome virus RNA by in situ hybridization at different times postinfection. J Clin Microbiol. 1996;34:2280–2286. doi: 10.1128/jcm.34.9.2280-2286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson G R. The epidemiology of African swine fever: the role of free-living hosts in Africa. Onderstepoort J Vet Res. 1985;52:201–209. [PubMed] [Google Scholar]
- 39.Walton G A. A taxonomic review of the Ornithodoros moubata (Murray) 1877 (sensu Walton, 1962) species group in Africa. Rec Adv Acarol. 1979;11:491–500. [Google Scholar]
- 40.Weaver S C, Scott T W, Lorenz L H, Lerdthusnee K, Romoser W S. Togavirus-associated pathologic changes in the midgut of a natural mosquito vector. J Virol. 1988;62:2083–2090. doi: 10.1128/jvi.62.6.2083-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weaver S C, Scott T W, Lorenz L H, Repik P M. Detection of eastern equine enchephalomyelitis virus deposition in Culiseta melanura following ingestion of radiolabeled virus in blood meals. Am J Trop Med Hyg. 1991;44:250–259. doi: 10.4269/ajtmh.1991.44.250. [DOI] [PubMed] [Google Scholar]
- 42.Weaver S C, Lorenz L H, Scott T W. Pathologic changes in the midgut of Culex tarsalis following infection with western equine encephalomyelitis virus. Am J Trop Med Hyg. 1992;47:691–701. doi: 10.4269/ajtmh.1992.47.691. [DOI] [PubMed] [Google Scholar]
- 43.Zsak L, Lu Z, Kutish G F, Neilan J G, Rock D L. An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34.5 gene. J Virol. 1996;70:8865–8871. doi: 10.1128/jvi.70.12.8865-8871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zsak L, Caler E, Lu Z, Kutish G F, Neilan J G, Rock D L. A nonessential African swine fever virus gene UK is a significant viral virulence determinant in domestic swine. J Virol. 1997;72:1028–1035. doi: 10.1128/jvi.72.2.1028-1035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]