ABSTRACT
Acute encephalitis syndrome (AES) causes significant morbidity and mortality worldwide. In Nepal, Japanese encephalitis virus (JEV) accounts for ~5-20% of AES cases, but ~75% of AES cases are of unknown etiology. We identified a gemykibivirus in CSF collected in 2020 from an 8-year-old male patient with AES using metagenomic next-generation sequencing. Gemykibiviruses are single stranded, circular DNA viruses in the family Genomoviridae. The complete genome of 2,211 nucleotides was sequenced, which shared 98.69% nucleotide identity to its closest relative, Human associated gemykibivirus 2 isolate SAfia-449D. Two real-time PCR assays were designed, and screening of 337 cerebrospinal fluid (CSF) and 164 serum samples from AES patients in Nepal collected in 2020 and 2022 yielded 11 CSF and 1 serum sample that were positive in both PCR assays. Complete genomes of seven of the positives were sequenced. These results identify a potential candidate etiologic agent of encephalitis in Nepal.
IMPORTANCE
Viral encephalitis is a devastating disease, but unfortunately, worldwide, the causative virus in many cases is unknown. Therefore, it is important to identify viruses that could be responsible for cases of human encephalitis. Here, using metagenomic sequencing of CSF, we identified a gemykibivirus in a male child from Nepal with acute encephalitis syndrome (AES). We subsequently detected gemykibivirus DNA in CSF or serum of 12 more encephalitis patients by real-time PCR. The virus genomes we identified are highly similar to gemykibiviruses previously detected in CSF of three encephalitis patients from Sri Lanka. These results raise the possibility that gemykibivirus could be an underrecognized human pathogen.
KEYWORDS: acute encephalitis syndrome, gemykibivirus, metagenomic analysis, emerging infection
INTRODUCTION
Encephalitis is a neurological disorder associated with a high mortality rate on a global scale (1). It is the inflammation of the brain parenchyma with clinical features of fever, altered mental state, and/or new onset of seizures. At present, low sociodemographic index regions in Asia and Africa carry the highest burden of encephalitis (2). In the year 2021, a total of 512 cases of acute encephalitis syndrome (AES) were reported in Nepal (3). More than 100 different infectious agents that cause encephalitis are known including bacteria, viruses, fungi, and parasites (4, 5). The major known etiologic agents of encephalitis as reported in Nepal and internationally are Japanese encephalitis virus (JEV), enteroviruses, herpes simplex, and varicella zoster viruses (6, 7). In Nepal, there has been a national surveillance program for JEV since 2004 wherein CSF and serum samples of suspected viral cases of encephalitis are collected from sentinel sites throughout Nepal with technical support from WHO and analyzed at the National Public Health Laboratory (NPHL) by serology for anti-JEV IgM. In numerous studies in Nepal assessing cases since 2000, ~ 70-95% of the AES cases per year have no diagnosis (3, 8–11). A significant fraction of encephalitis in other countries similarly lacks diagnosis, despite extensive testing (5, 12, 13). In recent years, the application of metagenomic analysis to patients with encephalitis has begun to identify a range of emerging viruses linked to encephalitis (14–19).
The Genomoviridae family of viruses has single stranded DNA genomes of ~2.1–2.2 kb (20) that encode a capsid protein (CP) and a replication associated protein (REP). They have been identified from a wide range of hosts including plants, insects, animals, and humans (20–22). There are ten genera in the Genomoviridae family (20). Viruses in the genus Gemykibivirus have been identified in multiple human cases and in multiple specimen types including blood of febrile Tanzanian children (23), the respiratory tract of an elderly woman with respiratory distress in China (24), feces from diarrhea patients in Brazil (25), blood of healthy blood donors of Brazil (21), blood of HIV-positive Cameroonian males (26), and in cervical swab of HIV/HPV-infected pregnant females (27). Specific to encephalitis, there are reports of gemykibiviruses in CSF from an encephalitic child from China (15) and CSF from three patients with encephalitis from Sri Lanka (25). Furthermore, analysis of Nepalese sewage yielded the complete genome of a gemykibivirus (25). Here, we used metagenomic next generation sequencing (NGS) to identify the presence of a gemykibivirus in CSF from a patient with encephalitis in Nepal. Further PCR screening identified 12 additional positive cases.
RESULTS
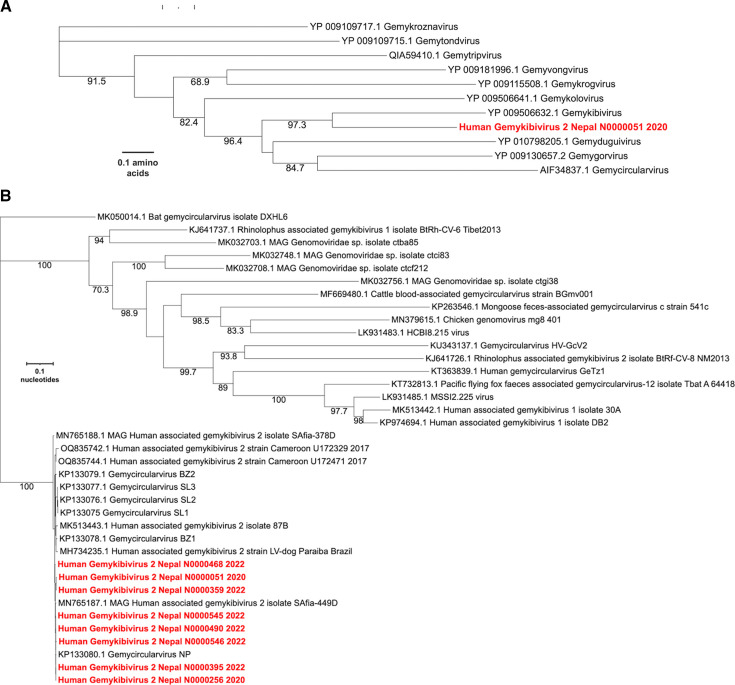
Detection of a gemykibivirus by metagenomic NGS
NGS of nucleic acids extracted from the CSF of an 8-year-old boy yielded reads that could be assembled into two contigs that shared 97 and 99% nucleotide similarity with Human associated gemykibivirus 2 SAfia-449D (accession# MN765187.1). Using PCR, gaps between the two contigs were spanned to generate a complete circular genome of 2211 nt. To formally assess the taxonomic relationship of this virus to viruses in the family Genomoviridae, we generated a maximum likelihood phylogenetic tree of the REP protein with the type species of each genus, in accordance with the ICTV guidelines (20, 22) (Fig. 1A). The virus was most similar to the prototype virus from the genus Gemykibivirus. To further assess its relationship within the genus Gemykibivirus, we generated a maximum likelihood tree using the whole genome sequence (Fig. 1B), which demonstrated that it is most closely related to Human associated gemykibivirus 2 SAfia-449D, a virus detected in the blood of Tanzanian children (23), with 98.69% nucleotide identity. Based on these criteria, the virus genome from the index case was designated Human gemykibivirus 2 Nepal/N0000051/2020. Human gemykibivirus 2 Nepal/N0000051/2020 was also closely related to Gemycircularvirus-SL1 (accession# KP133075), a previously reported gemykibivirus detected in CSF from an encephalitis patient in Sri Lanka (25) sharing 97.96% nucleotide identity (33 single nucleotide polymorphisms [SNPs] across the genome and a 12-bp insertion located in a region of tandem repeat hexamers), and it shared 98.64% identity to another gemykibivirus, Gemycircularvirus NP (accession# KP133080), detected in sewage from Nepal (25).
Fig 1.
Phylogenetic trees of (A) amino acid sequences of the index case REP protein and representatives of each genus in the family Genomoviridae; (B) whole genome nucleotide sequences of the positive samples from Nepal compared with other genomes within the genus Gemykibivirus.
Gene predictions identified three open reading frames (ORFs), characteristic of Gemykibiviruses (Fig. 2). The CP ORF is 969 bp, the REP ORF, generated by splicing is 1114 bp, and the unknown ORF3, which overlaps with the REP ORF, is 702 bp. A large intergenic region (LIR) of 127 bp is present, and the putative viral origin of replication nona-nucleotide motif 5′-TAAAATTTA-3′ described in Gemycircularvirus NP (accession# KP133080) is conserved. A predicted stem loop in the LIR is observed from nucleotides 23 to 57. The stem loop structure is present in genomoviruses and geminiviruses where it is necessary for rolling circle replication (28, 29).
Fig 2.
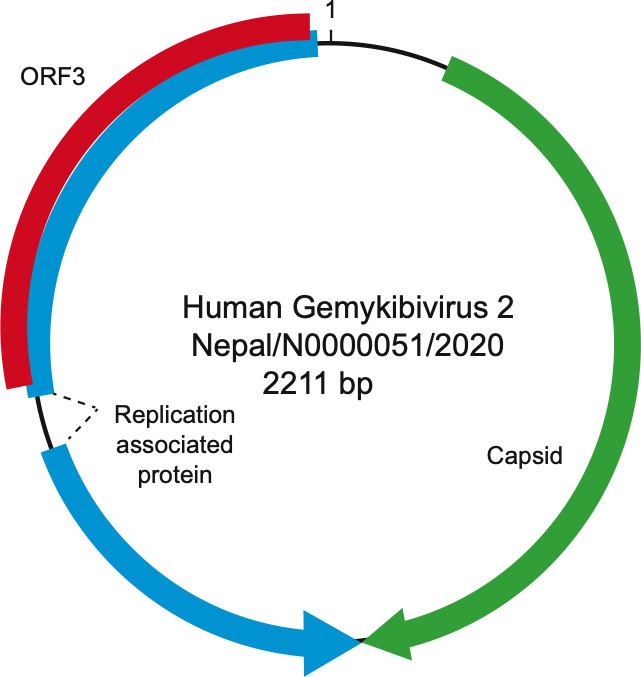
Schematic of the genome of Human gemykibivirus 2 Nepal/N0000051/2020.
Identification of additional positive specimens from encephalitis patients
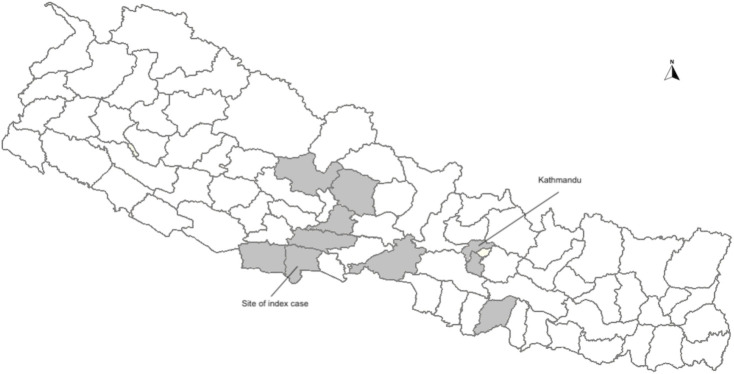
To define the prevalence of this virus, two Taqman real-time PCR assays were designed and validated, one targeting the REP gene and one targeting the CP gene. A total of 337 CSF samples and 164 serum samples were tested using both assays. Sample with Ct. values <33 for both assays were considered positive. There were three gemykibivirus positive CSF specimens from 2020 and nine positive specimens (eight CSF and one serum) from 2022 (Table 1). The prevalence rate in CSF was 3.3% (11/337) and in serum was 0.6% (1/164). Positive patients ranged from 4 months to 72 years of age. Including the index case, there were seven male and six female patients. Geographically, most positive patients were from districts in south-central Nepal (Fig. 3). Unfortunately, no additional clinical details are available for the patients in this study besides meeting the acute case definition of encephalitis.
TABLE 1.
Patient demographics and quantitative real-time PCR (qPCR) Ct. values of gemykibivirus positive casesd
| Sample ID | Specimen | Age | Gender | Ct. Gemy_1a |
Ct. Gemy_2b |
Specimen collection date | Districtc | Province |
|---|---|---|---|---|---|---|---|---|
| N0000218 | CSF | 14 yrs | M | 32.42 | 30.68 | 2020 Feb | Kaski | Gandaki |
| N0000256 | CSF | 9 mos | F | 29.41 | 29.34 | 2020 July | Myagdi | Gandaki |
| N0000300 | CSF | 8 yrs | F | 32.95 | 31.46 | 2020 Sep | Palpa | Lumbini |
| N0000358 | Serum | 6 mos | M | 29.27 | 29.94 | 2022 Feb | Sarlahi | Madhesh |
| N0000359 | CSF | 5 yrs | F | 27.4 | 27.08 | 2022 Feb | Kapilvastu | Lumbini |
| N0000395 | CSF | 4 mos | M | 30.74 | 30.73 | 2022 April | Kaski | Gandaki |
| N0000434 | CSF | 57 yrs | M | 29.02 | 30.06 | 2022 June | Lalitpur | Bagmati |
| N0000468 | CSF | 35 yrs | F | 30.84 | 31.47 | 2022 July | Chitwan | Bagmati |
| N0000490 | CSF | Unknown | M | 29.86 | 32.08 | 2022 July | Syangja | Gandaki |
| N0000545 | CSF | 72 yrs | F | 26.84 | 25.35 | 2022 Aug | Kathmandu | Bagmati |
| N0000546 | CSF | 21 yrs | M | 24.65 | 23.53 | 2022 Aug | Kathmandu | Bagmati |
| N0000722 | CSF | 25 yrs | F | 28.74 | 29.72 | 2022 Nov | Chitwan | Bagmati |
Gemy_1: qPCR targeting capsid protein gene.
Gemy_2: qPCR targeting replication associated protein (REP) gene.
Districts involved are highlighted in Fig. 3 as shade.
Ct., cycle threshold; yrs, years; mos, months.
Fig 3.
Map of Nepal and locations of the gemykibivirus positive cases (shaded). The map was adapted from https://github.com/itsbohara/nepal-gis.git under Apache License 2.0.
Whole genome sequencing and phylogenetic analysis of additional positive cases
From the 10 positive specimens with the highest Gemykibivirus copy number, we tried to amplify the whole genome using PCR. For seven of the samples, we obtained amplicons that corresponded to the whole genome. These were cloned and sequenced to 3× coverage to determine their consensus sequences. These 7 genomes varied from the index genome by 4 to 21 SNPs. The intron of the Rep gene contains a region with seven tandemly repeated hexamers, and deletions of one or more of the hexamers are observed in some of the genomes. Phylogenetic analysis of the complete genomes demonstrated that they formed a clade that included known Gemykibiviruses previously detected in human CSF, stool, and blood.
DISCUSSION
We used metagenomic NGS to detect Human gemykibivirus 2 Nepal/N0000051/2020 in CSF of an 8-year-old encephalitic child from Rupandehi district (Fig. 3) of Nepal, which is alongside the border of India. Further screening through qPCR identified an additional 12 positive specimens from patient samples collected in 2020 and 2022. We found that these cases were mostly concentrated in and around the south-central region of Nepal (Fig. 3).
The identified virus genomes were very closely related (97.96% nt. identity) to a gemykibivirus previously detected in CSF from three encephalitis patients from Sri Lanka (25). In addition, a distinct gemykibivirus (Human gemykibivirus 4) has also been reported in CSF of an encephalitis patient from China (15). Together with our study, these data implicate viruses in the genus Gemykibivirus as potential candidate agents of encephalitis. There is one report of Gemykibivirus in sewage from Nepal, which is also highly similar to the sequences we detected in Nepalese patients from 2020 and 2022. This suggests that gemykibivirus has been circulating in Nepal for at least the past decade. Furthermore, detection in sewage raises the possibility that gemykibivirus may be transmitted fecal-orally, similar to some neurotropic viruses such as polio and enteroviruses. Detection of highly similar viruses in patients in Tanzania (23) and Brazil (21) suggests that gemykibiviruses are globally widespread.
One limitation of this study is that the samples analyzed were residual specimens from a surveillance repository without additional available clinical metadata, thus limiting our knowledge of the precise symptoms, disease severity, and outcomes of these patients. It is also possible that there are additional microbes present in the index case that were not detected at the current sequencing depth. While detection of gemykibiviruses in presumptively sterile CSF supports the hypothesis that they could be causal agents of encephalitis, additional research to culture the virus and establish animal models to fulfill Koch’s postulates is needed to definitively establish causality. As the only gemykibivirus with a proven host to date infects a fungus (28, 30), one possibility is that gemykibivirus may infect another eukaryotic microbe present in these clinical samples, as has been reported for Redondoviruses that likely infect the protozoan Entamoeba gigivalis in the oral cavity (31). In addition, more prevalence studies in encephalitis and other diseases are also needed as are serological studies to define the extent of human infection by gemykibiviruses. Finally, additional, unbiased approaches are needed to define the etiologies of encephalitis in Nepal, and worldwide.
MATERIALS AND METHODS
Study population and collection of biospecimens
The study population focused on AES patients who were negative for JE IgM. Residual samples from national JE surveillance sentinel sites in Nepal were utilized. The index case CSF sample in the NPHL repository was collected in 2020 from Rupandehi district. For prevalence studies, 122 repository specimens (82 CSF, 40 sera) were collected in 2020 and 379 repository specimens (255 CSF, 124 sera) from 2022 were tested by PCR for gemykibivirus.
Total nucleic acid extraction
Total nucleic acid extraction was performed using the Invitrogen Pure Link Viral RNA/DNA mini kit (Thermo Fisher Scientific) and eluted in 50 µL volume following the instruction manual of the kit. Samples were stored at −80°C.
Metagenomic NGS analysis
Extracted total nucleic acid was randomly amplified as described previously (32) and used for library construction with NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs). The sample library was sequenced on the Illumina MiSeq instrument using the 2 × 250 bp paired-end protocol. NGS data were analyzed for the presence of viruses using CZID (33). NGS data is available at ENA: PRJEB72279.
Genome sequencing of index case and additional cases
The NGS contigs were confirmed using PCR, cloning, and sanger sequencing with primers (Table S1). Using a pair of primers that amplified the entire circular genome, Gemy1xgenomeF (5’TTAATCGATCTAGAGGATCCTTGTTAGATATCCATATGGCGG-3′) and Gemy1xgenomeR (5′-TTAGTAATGGGCCCGGATCCACGAGAGGAACACG-3′), three independent PCR reactions were performed, and the resulting fragments were cloned into pCR4.0 and sequenced using the Oxford Nanopore technologies (Plasmidsauraus). Additional positive cases were similarly amplified and whole genomes sequenced to 3× coverage.
Gemykibivirus qPCR development
Two sets of Taqman real-time PCR primers and probes were designed using express software (Applied Biosystems), one targeting the CP gene and the other targeting the REP gene. The primers and the probes were supplied by IDT (Integrated DNA Technologies, USA). The first assay, Gemy_1, targets the CP gene using primers GemykibiTM_8917F (5′-ACCTCTTATCCGGTTTGGCA-3′) and GemykibiTM_8917R (5′-AGCGCGAAATTCCTCTTGAC-3′) and the probe GemykibiTM_8917Probe(5’- [6-FAM]CGGACCTGA[ZEN]CCGGATGCCCGG[3IABkFQ]−3’) that uses FAM and the dual quencher Zen and Iowa Black. The second assay, Gemy_2, targets the REP gene with GemykibiTM_9967F (5′-GGTCAGAGCCTAGTGTTGTATG-3′) GemykibiTM_9967R (5′-CGACGTTGTCTGTGTCTTCT-3′) GemykibiTM_9967Probe (5’-[6-FAM]AAGACACTC[ZEN]TGGGCAAGAAGCC TT[IABkFQ]−3’) using the same fluor and quencher.
For both assays, standard curves were generated using serial 10-fold dilution ranging from 2 × 108 to 2 × 101 copies of positive control plasmid (plasmid PCR4 containing the respective target sequence). A 20-µL PCR mixture was made comprising 2 µL of extracted nucleic acid sample, 10 µL of 2× TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific), and 5 pmol of each primer and probe. The PCR reactions were performed in 96-well plates on a CFX Opus 96 thermocycler (Bio-Rad) with one negative control nuclease free water in each row and one positive control of 2 × 103 copies per plate. The cycling conditions were 50°C for 2 mins, 95°C for 30 secs, and 40 cycles of 95°C for 5 secs followed by 60°C for 30 secs. The threshold of all plates was set at standard value, and data were analyzed using Bio-Rad CFX Maestro 2.3 software. Samples were counted as positive if their threshold cycle (Ct.) value was less than 33.
Phylogenetic analysis
Representative protein sequences of the REP gene of prototypes of each genus in the Genomoviridae were downloaded from GenBank. Alignments were generated using Clustal Omega (34). The alignment converted to fasta via http://sequenceconversion.bugaco.com/converter/biology/sequences/clustal_to_fasta.php. Maximum likelihood trees were generated with bootstrapping, using W-IQ-TREE (35). Trees were visualized using iTOL (36). All available complete genomes in the species Gemykibivirus humas2 were downloaded from Genbank along with representative genomes from the species Gemykibivirus humas 1, 3, 4, and 5, and the top 10 additional complete genomes with highest BLASTn scores. Alignments were generated using Clustal Omega (34) Maximum likelihood trees were generated with bootstrapping, using W-IQ-TREE (35). Trees were visualized using iTOL (36).
ACKNOWLEDGMENTS
This project was supported by National Institutes of Health grant U01AI151810.
Contributor Information
Krishna Das Manandhar, Email: krishna.manandhar@gmail.com.
Benhur Lee, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
ETHICS APPROVAL
This study was approved by the Nepal Health Research Council (approval# 274-2020) and the Human Research Protection Office of Washington University in Saint Louis (202004087).
DATA AVAILABILITY
Complete genome sequences are available at GenBank (Accession# PP270194-PP270201).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00219-24.
Primers used for PCR.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Why encephalitis matters? report of the virtual meeting, 28-29 June 2022. 2023. Geneva: World Health Organization. https://www.who.int/publications/i/item/9789240069176. [Google Scholar]
- 2. Wang H, Zhao S, Wang S, Zheng Y, Wang S, Chen H, Pang J, Ma J, Yang X, Chen Y. 2022. Global magnitude of encephalitis burden and its evolving pattern over the past 30 years. J Infect 84:777–787. doi: 10.1016/j.jinf.2022.04.026 [DOI] [PubMed] [Google Scholar]
- 3. Department of health services annual report 2078/79. 2021. Kathmandu: Government of Nepal Ministry of Health and Population. http://dohs.gov.np/wp-content/uploads/Annual%20Report%202078-79.pdf. [Google Scholar]
- 4. Granerod J, Cunningham R, Zuckerman M, Mutton K, Davies NWS, Walsh AL, Ward KN, Hilton DA, Ambrose HE, Clewley JP, Morgan D, Lunn MP, Solomon T, Brown DWG, Crowcroft NS. 2010. Causality in acute encephalitis: defining aetiologies. Epidemiol Infect 138:783–800. doi: 10.1017/S0950268810000725 [DOI] [PubMed] [Google Scholar]
- 5. Glaser CA, Honarmand S, Anderson LJ, Schnurr DP, Forghani B, Cossen CK, Schuster FL, Christie LJ, Tureen JH. 2006. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis 43:1565–1577. doi: 10.1086/509330 [DOI] [PubMed] [Google Scholar]
- 6. Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS, UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group . 2010. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 10:835–844. doi: 10.1016/S1473-3099(10)70222-X [DOI] [PubMed] [Google Scholar]
- 7. Rayamajhi P, Nepal G, Ojha R, Rajbhandari R, Gajurel BP, Karn R. 2022. Evaluating cognitive outcomes in adult patients with acute encephalitis syndrome: a prospective study from a tertiary care center in Nepal. Encephalitis 2:36–44. doi: 10.47936/encephalitis.2021.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khanal B, Singh RR, Bhattacharya SK. 2012. Japanese encephalitis in eastern Nepal: access to the laboratory diagnosis. Int J Infect Dis 16:e88. doi: 10.1016/j.ijid.2012.05.206 [DOI] [Google Scholar]
- 9. Dhital B, Bhat N. 2022. Clinico-epidemiological presentation of acute encephalitis syndrome in patients visiting a tertiary hospital in Kathmandu, Nepal: a descriptive cross-sectional study. JGMC Nepal 15:168–172. doi: 10.3126/jgmcn.v15i2.44719 [DOI] [Google Scholar]
- 10. Rayamajhi A, Ansari I, Ledger E, Bista KP, Impoinvil DE, Nightingale S, Kumar R, Mahaseth C, Solomon T, Griffiths MJ. 2011. Clinical and prognostic features among children with acute encephalitis syndrome in Nepal; a retrospective study. BMC Infect Dis 11:294. doi: 10.1186/1471-2334-11-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar Pant D, Tenzin T, Chand R, Kumar Sharma B, Raj Bist P. 2017. Spatio-temporal epidemiology of Japanese encephalitis in Nepal, 2007-2015. PLoS One 12:e0180591. doi: 10.1371/journal.pone.0180591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huppatz C, Durrheim DN, Levi C, Dalton C, Williams D, Clements MS, Kelly PM. 2009. Etiology of encephalitis in Australia, 1990-2007. Emerg Infect Dis 15:1359–1365. doi: 10.3201/eid1509.081540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mailles A, Stahl JP, Steering C, Investigators G. 2009. Infectious encephalitis in france in 2007: a national prospective study. Clin Infect Dis 49:1838–1847. doi: 10.1086/648419 [DOI] [PubMed] [Google Scholar]
- 14. Edridge AWD, Deijs M, Namazzi R, Cristella C, Jebbink MF, Maurer I, Kootstra NA, Buluma LR, van Woensel JBM, de Jong MD, Idro R, Boele van Hensbroek M, van der Hoek L. 2019. Novel orthobunyavirus identified in the cerebrospinal fluid of a Ugandan child with severe encephalopathy. Clin Infect Dis 68:139–142. doi: 10.1093/cid/ciy486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou C, Zhang S, Gong Q, Hao A. 2015. A novel gemycircularvirus in an unexplained case of child encephalitis. Virol J 12:197. doi: 10.1186/s12985-015-0431-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smits SL, Zijlstra EE, van Hellemond JJ, Schapendonk CME, Bodewes R, Schürch AC, Haagmans BL, Osterhaus ADME. 2013. Novel cyclovirus in human cerebrospinal fluid, Malawi, 2010-2011. Emerg Infect Dis 19:1511–1513. doi: 10.3201/eid1909.130404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Q, Wang X, Xie C, Ding S, Yang H, Guo S, Li J, Qin L, Ban F, Wang D, Wang C, Feng L, Ma H, Wu B, Zhang L, Dong C, Xing L, Zhang J, Chen H, Yan R, Wang X, Li W. 2021. A novel human acute encephalitis caused by pseudorabies virus variant strain. Clin Infect Dis 73:e3690–e3700. doi: 10.1093/cid/ciaa987 [DOI] [PubMed] [Google Scholar]
- 18. Manso CF, Bibby DF, Mohamed H, Brown DWG, Zuckerman M, Mbisa JL. 2020. Enhanced detection of DNA viruses in the cerebrospinal fluid of encephalitis patients using metagenomic next-generation sequencing. Front Microbiol 11:1879. doi: 10.3389/fmicb.2020.01879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quan PL, Wagner TA, Briese T, Torgerson TR, Hornig M, Tashmukhamedova A, Firth C, Palacios G, Baisre-De-Leon A, Paddock CD, Hutchison SK, Egholm M, Zaki SR, Goldman JE, Ochs HD, Lipkin WI. 2010. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg Infect Dis 16:918–925. doi: 10.3201/eid1606.091536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varsani A, Krupovic M. 2021. Family genomoviridae: 2021 taxonomy update. Arch Virol 166:2911–2926. doi: 10.1007/s00705-021-05183-y [DOI] [PubMed] [Google Scholar]
- 21. Zucherato VS, Giovanetti M, Costa LOA, Krause LMF, Alves DCC, Moreira RMA, Pimentel BMS, Haddad R, Bitencourt HT, Ciccozzi M, Alcantara LCJ, Kashima S, Covas DT, Slavov SN. 2023. Molecular identification of the emerging human gemykibivirus-2 (HuGkV-2) among Brazilian blood donors. Transfus Apher Sci 62:103516. doi: 10.1016/j.transci.2022.103516 [DOI] [PubMed] [Google Scholar]
- 22. International Committee on Taxonomy of Viruses (ICTV) . 2023. Current ICTV taxonomy release. Available from: https://ictv.global/taxonomy
- 23. Cordey S, Laubscher F, Hartley M-A, Junier T, Keitel K, Docquier M, Guex N, Iseli C, Vieille G, Le Mercier P, Gleizes A, Samaka J, Mlaganile T, Kagoro F, Masimba J, Said Z, Temba H, Elbanna GH, Tapparel C, Zanella M-C, Xenarios I, Fellay J, D’Acremont V, Kaiser L. 2021. Blood virosphere in febrile Tanzanian children. Emerg Microbes Infect 10:982–993. doi: 10.1080/22221751.2021.1925161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Li Y, He X, Ma J, Hong W, Hu F, Zhao L, Li Q, Zhang J, Zhang C, Zhang F. 2019. Gemykibivirus genome in lower respiratory tract of elderly woman with unexplained acute respiratory distress syndrome. Clin Infect Dis 69:861–864. doi: 10.1093/cid/ciz072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phan TG, Mori D, Deng X, Rajindrajith S, Ranawaka U, Fan Ng TF, Bucardo-Rivera F, Orlandi P, Ahmed K, Delwart E. 2015. Small circular single stranded DNA viral genomes in unexplained cases of human encephalitis, diarrhea, and in untreated sewage. Virology 482:98–104. doi: 10.1016/j.virol.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orf GS, Olivo A, Harris B, Weiss SL, Achari A, Yu G, Federman S, Mbanya D, James L, Mampunza S, Chiu CY, Rodgers MA, Cloherty GA, Berg MG. 2023. Metagenomic detection of divergent insect- and bat-associated viruses in plasma from two African individuals enrolled in blood-borne surveillance. Viruses 15:1022. doi: 10.3390/v15041022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siqueira JD, Curty G, Xutao D, Hofer CB, Machado ES, Seuánez HN, Soares MA, Delwart E, Soares EA. 2019. Composite analysis of the virome and bacteriome of HIV/HPV co-infected women reveals proxies for immunodeficiency. Viruses 11:422. doi: 10.3390/v11050422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu X, Li B, Fu Y, Jiang D, Ghabrial SA, Li G, Peng Y, Xie J, Cheng J, Huang J, Yi X. 2010. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc Natl Acad Sci U S A 107:8387–8392. doi: 10.1073/pnas.0913535107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heyraud F, Matzeit V, Schaefer S, Schell J, Gronenborn B. 1993. The conserved nonanucleotide motif of the geminivirus stem-loop sequence promotes replicational release of virus molecules from redundant copies. Biochimie 75:605–615. doi: 10.1016/0300-9084(93)90067-3 [DOI] [PubMed] [Google Scholar]
- 30. Kinsella CM, Deijs M, Becker C, Broekhuizen P, van Gool T, Bart A, Schaefer AS, van der Hoek L. 2022. Host prediction for disease-associated gastrointestinal cressdnaviruses. Virus Evol 8:veac087. doi: 10.1093/ve/veac087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keeler EL, Merenstein C, Reddy S, Taylor LJ, Cobián-Güemes AG, Zankharia U, Collman RG, Bushman FD. 2023. Widespread, human-associated redondoviruses infect the commensal protozoan Entamoeba gingivalis. Cell Host Microbe 31:58–68. doi: 10.1016/j.chom.2022.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim ES, Zhou Y, Zhao G, Bauer IK, Droit L, Ndao IM, Warner BB, Tarr PI, Wang D, Holtz LR. 2015. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med 21:1228–1234. doi: 10.1038/nm.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalantar KL, Carvalho T, de Bourcy CFA, Dimitrov B, Dingle G, Egger R, Han J, Holmes OB, Juan Y-F, King R, et al. 2020. IDseq-An open source cloud-based pipeline and analysis service for metagenomic pathogen detection and monitoring. Gigascience 9:giaa111. doi: 10.1093/gigascience/giaa111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235. doi: 10.1093/nar/gkw256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Letunic I, Bork P. 2021. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296. doi: 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for PCR.
Data Availability Statement
Complete genome sequences are available at GenBank (Accession# PP270194-PP270201).