Abstract
Background:
Atherosclerosis (AS) is an inflammatory disease linked to vascular events, with dysregulation of microRNA (miR)-125b, contributing to cardiovascular disease pathogenesis. Moreover, there is evidence of the involvement of signal transducer and activator of transcription 3 (STAT3) and sirtuin 6 (SIRT6) in AS. This study aimed to survey the expression levels of miR-125b, STAT3, and SIRT6 in the peripheral blood mononuclear cells (PBMCs) of AS patients and controls, and to find their correlations with biochemical parameters and risk factors.
Methods:
This study included blood samples from 45 controls and 45 AS patients, with PBMCs isolated using Ficoll solution. Expression levels of miR-125b, STAT3, and SIRT6 were determined via quantitative Real Time-PCR.
Results:
The findings revealed a significant increase in miR-125b levels in patients compared to controls (P = 0.017). However, alterations in STAT3 and SIRT6 expression were not significant (P> 0.05). There was no substantial relationship between miR-125b and STAT3 (P = 0.522) or SIRT6 (P = 0.88). miR-125b showed a significant relationship with atherogenic indexes and creatinine (P<0.05), while the association of SIRT6 with HDL and creatinine was significant (P<0.05). STAT3 exhibited high diagnostic power for identifying individuals at risk of heart disease and hypertension (P<0.05).
Conclusion:
STAT3 can serve as a valuable biomarker for detecting AS and AS-related risk factors. miR-125b and SIRT6 may be associated with AS lipid metabolism. However, further studies with larger sample sizes are recommended to mechanistically elucidate the association of these genes.
Key Words: Atherosclerosis, microRNA-125b, STAT3, SIRT6, Leukocyte
Introduction
Atherosclerosis (AS), a common form of cardiovascular disease (CVD), is characterized by the accumulation of lipids on the luminal surface of blood vessels, which can ultimately result in clinical complications such as myocardial infarction (MI) and stroke (1). Despite a declining incidence in some countries, the disease remains the leading cause of death worldwide due to its slow progression. In atherosclerotic lesions, inflammatory cells, lipids, vascular smooth muscle cells (VSMCs), and necrotic cells accumulate and transform over a lifetime within the intimal space (2, 3). The development of atherosclerotic plaques can be influenced by interactions between genetic and environmental factors including dyslipidemia, diabetes mellitus, family history of CVD, hypertension, smoking, physical inactivity, etc. Furthermore, epigenetic changes have become increasingly important in vascular pathology, both from a therapeutic and biomarker perspective (4, 5). In recent years, evidence has shown implication of microRNAs (miRNAs; miRs) in various pathophysiological processes and molecular signaling pathways associated with AS (6,7). Numerous studies have highlighted the crucial role of miRNAs as momentous mediators for regulating the VSMCs phenotype by targeting transcription factors (7–9). For instance, miR-21, miR-145, miR-221, and miR-222 have been identified to have a close association with aberrant VSMC proliferation in AS (10). Moreover, evidence has suggested that miR-125b contributes extensively to the pathogenesis and clinical diagnosis of CVD. Up‐regulation of miR‐125b could enhance the expression of inflammatory cytokines, such as interleukin‐6 (IL‐6) and monocyte chemoattractant protein‐1 (MCP‐1), and correlate with endothelial cells (ECs) senescence (11). Conversely, documents have displayed that miR-125b can target sirtuin 6 (SIRT6) gene, leading to apoptosis and cellular senescence in cancers (12,13). SIRT6 effectively prevents the infiltration of inflammatory cells, the transformation of fibroblasts, and the formation of atherosclerotic plaques. Moreover, it plays a role in inhibiting cardiomyocyte hypertrophy, thereby preventing ECs dysfunction (14). SIRT6 has also shown cardioprotective effects via blocking the activation of signal transducer and activator of transcription 3 (STAT3) in cardiomyocyte hypertrophy (15). Through ECs dysfunction, macrophage polarization, immunity, and inflammation, STAT3 has been reported to be linked to the development of CVDs, including AS. STAT3 phosphorylation could provoke the expression of adhesion molecules and inflammation that promote monocyte migration (16). Additionally, the janus kinase 2 (JAK2)/STAT3 pathway directs numerous cytokine signaling pathways and participates ubiquitously in cell proliferation, differentiation, vascular calcification, and myocardial fibrosis (17,18). Therefore, there may be a hypothesis regarding the possible relationship between SIRT6 and STAT3 with miR-125b and even biochemical parameters and risk factors in atherosclerotic process for therapeutic purposes. For this reason, the present research was aimed to evaluate these correlations.
Materials and Methods
Study design and population
A total of 90 subjects, including 45 atherosclerotic patients and 45 controls, who were referred to the Cardiology and Angiography Department of Shahid Madani Hospital, Khorramabad, Iran, between 2021 and 2022, were selected. The patients with atherosclerotic lesions were confirmed by standard diagnostic angiography. The inclusion criteria were patients with coronary angiography diagnostic or therapeutic indicators and matched age and sex. The individuals without atherosclerotic lesions were considered controls. In addition, the medical information, including age, weight, height, exercise, history of heart disease, hypertension, smoking, and alcohol utilization was recorded for all participants. Patients with congenital heart disease, pulmonary obstruction, chronic kidney disease, or malignancy were regarded as exclusion criteria. Written informed consent was received from all participants in this study. The research was confirmed by the Ethics Committee of Lorestan University of Medical Sciences and the Research Committee of Shahid Madani Hospital, and administered following the Declaration of Helsinki (19).
Blood samples collection and peripheral blood mononuclear cells (PBMCs) isolation
Two types of blood samples were obtained from all participants following overnight fasting into tubes without anticoagulant for biochemical evaluations and with ethylenediaminetetraacetic acid (EDTA) for molecular assays. Ficoll solution (Pharmacia, Freiburg, Germany) was used to isolate PBMCs. Firstly, 5 ml phosphate buffer saline was added to a 5 ml whole blood sample and mixed. In the next step, the diluted blood was added to a 7.5-ml Ficoll solution in a canonical tube and centrifuged at 2,800 RCF for 20 min. The cloudy layer, including lymphocyte, monocyte, and thrombocyte between the plasma and Ficoll solution was isolated with a pipette, and cells were washed twice with PBS. After the washing, 5 ml of PBS was added, and samples were stored at -70 °C until further analysis.
Biochemical analysis
The serum of collected blood samples was separated, and lipid profile levels consisted of total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglyceride (TG) as well as fasting blood sugar (FBS), serum creatinine (Cr), and blood uremia nitrogen (BUN) levels were measured by an auto-analyzer (Beckman CX4, Fullerton, CA, USA) and Bionic Diagnostic Kits, Iran.
Atherogenic indices and body mass index (BMI) evaluation
The atherogenic indices, such as atherogenic index of plasma (AIP), Castelli's Risk Index-I (CRI-I), and Castelli's Risk Index-II (CRI-II), and cut-off levels were calculated according to the previous study (20). Moreover, BMI was computed with the following formula (21): BMI=Weight (kg)/ (Height (m))2.
RNA isolation and quantitative real-time PCR analysis (qRT-PCR)
Total RNA was separated from the PBMCs samples using the FavorPrep Blood/Cultured Cell Total RNA Purification Kit (Favorgen® Biotech Corp., Pingtung, Taiwan) according to protocol of the kit’s instructions. The purity and integrity of isolated RNA were established via a spectrophotometer (NanoDrop Lite, Thermo Scientific, USA) and 2% agarose gel electrophoresis, respectively. Following RNA quantification, cDNAs were synthesized for SIRT6 and STAT3 genes using a cDNA synthesis kit (Yekta Tajhiz Azma, Iran) and for miR-125b using a cDNA synthesis kit (Zist Pooyesh, Iran) according to the manufacturer’s instructions. The genes expression of SIRT6, STAT3, mirR-125b, and reference genes (Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and Snord-48) were investigated by qRT-PCR applying SYBR Green qPCR Master Mix (Ampliqon, Copenhagen, Denmark). Reactions were conducted in triplicate on Rotor-Gene 6000 (Corbett Life Science, Australia). To analyze the results of qRT-PCR, the 2-∆∆CT standard method was applied. The sequences of sense and antisense primers for each gene are presented in Table 1.
Table 1.
Sequence of primers used for qRT-PCR.
| Gene | Accession numbers | Forward | Reverse | Product Size | Reference |
|---|---|---|---|---|---|
| SIRT6 | NM_016539.4 | 5'-AGTCTTCCAGTGTGGTGTTCC-3' | 5'-TCCATGGTCCAGACTCCGT-3' | 94 | (22) |
| STAT3 | NM_139276.3 | 5'-GCTGCCCCATACCTGAAGAC-3' | 5'-GGTGAGGGACTCAAACTGCC-3' | 165 | (23) |
| GAPDH | NM_002046.7 | 5'-GGTCGGAGTCAACGGATTTGG-3' | 5'-TGATGACAAGCTTCCCGTTCT-3' | 194 | (23) |
SIRT6, Sirtuin 6; STAT3, Signal transducer and activator of transcription 3; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
Data were analyzed using SPSS software version 22 (SPSS Inc., Chicago, IL, USA) and expressed as mean ± standard deviation (SD). The difference between the two groups was characterized by an independent T-test. To evaluate significant differences in the expression of miRNAs between patients and controls, the nonparametric Mann–Whitney U-test was applied. Accordingly, spearman correlation was applied to measure the strength and direction of associations between pairs of continuous variables. P values <0.05 were considered statistically considerable.
Results
Baseline and biochemical characteristics
As displayed in Table 2, there was no substantial difference between the patient and control groups regarding baseline characteristics (P> 0.05). In addition, levels of TG, FBS, Cr, CRI-II, and AIP in the patient group were meaningfully higher than in the control group (P<0.05).
Table 2.
Baseline and biochemical characteristics.
| Variables | Control (N=45)(mean ± SD) | Patient (N=45)(mean ± SD) | P value |
|---|---|---|---|
| Age (years) | 57.96±12.63 | 61.67±13.33 | 0.712 |
| Gender (women/men), n (%) | 20 (44.4) /25 (55.6) | 15 (33.4) /30 (66.6) | 0.067 |
| Height (cm) | 164.48±9.39 | 165.21±19.94 | 0.144 |
| Weight (kg) | 72.63 ±2.32 | 76.4±3.27 | 0.455 |
| BMI (kg/m2) | 26.63± 4.67 | 37.84±5.26 | 0.818 |
| TC (mg/dl) | 163.2 ±42.4 | 166.94± 87.09 | 0.830 |
| HDL (mg/dl) | 45.2±6.6 | 44.87±10.46 | 0.25 |
| LDL (mg/dl) | 95.73± 27.29 | 99.23± 36.3 | 0.944 |
| TG (mg/dl) | 138.29± 42.4 | 166.94± 87.09 | 0.005* |
| FBS (mg/dl) | 105.21±37.3 | 120.26± 50.8 | 0.045* |
| BUN (mg/dl) | 32.6± 10.92 | 38.66± 50.8 | 0.157 |
| Cr (mg/dl) | 1.03± 0.37 | 1.26±0.40 | 0.037* |
| BUN/Cr | 39.67± 14.72 | 34.83± 10.79 | 0.274 |
| CRI-I | 3.7± 0.83 | 4.06±1.5 | 0.736 |
| CRI-II | 2.11± 0.49 | 2.41± 0.93 | 0.009** |
| AIP | 0.47± 0.119 | 0.52± 0.305 | 0.001** |
P<0.05,
P<0.01,
P<0.0001.
FBS, Fasting blood sugar; BMI, Body mass index; TC, Total cholesterol; TG, Triglycerides; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; BUN, Blood urea nitrogen; Cr, Creatinine; AIP, Atherogenic index of plasma; CRI-I, Castelli’s Risk Index-I; CRI-II, Castelli's Risk Index-II.
Expression levels of miR-125b, STAT3, and SIRT6 in PBMC of patient and control groups
The miR-125b expression was significantly higher in AS patients compared to the control individuals (P< 0.05; Fig. 1a). Additionally, no meaningful difference was found for genes expression of SIRT6 and STAT3 between the two groups (P> 0.05; Figs. 1b and 1c).
Fig. 1.
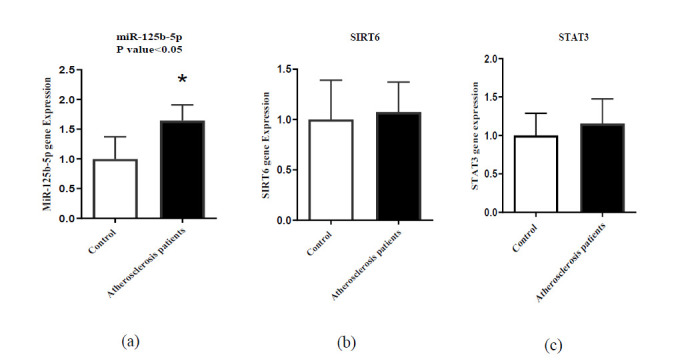
Expression levels of (a) miR-125b; (b) SIRT6, and (c) STAT3 in patient and control groups. *P<0.05.
Receiving operating characteristic (ROC) curves analysis
ROC curve can be used to determine whether a variable can be used as a biomarker for a specific disease. This curve is an appropriate and well-established method for evaluating a laboratory test according to sensitivity and specificity. The area under the ROC characterizes the test power to diagnose disease. The findings of ROC curve analysis demonstrated that STAT3 has high diagnostic power to detect individuals at risk of heart disease and hypertension (Figs. 2a and 2b).
Fig. 2.
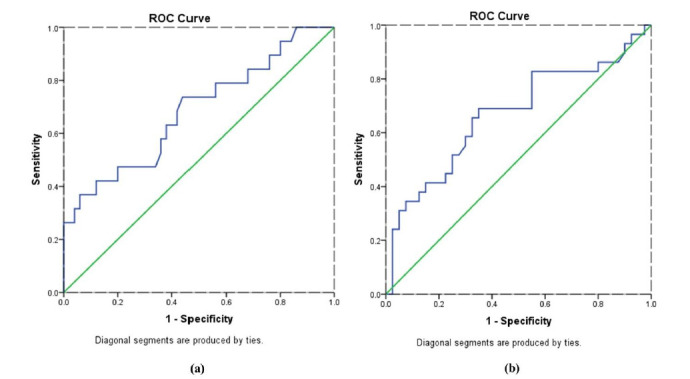
ROC curves of STAT3 diagnostic value to detect individuals at risk (a) Heart disease; (b) Hypertension.
Correlation of miR-125b with STAT3 and SIRT6 genes and their relationship with biochemical parameters
In this study, no substantial association was found between miR-125b and genes of STAT3 and SIRT6 (P> 0.05). The correlation between miR- 125b, STAT3, and SIRT6 with biochemical parameters was analyzed in the patient group (Fig. 3).
Fig. 3.
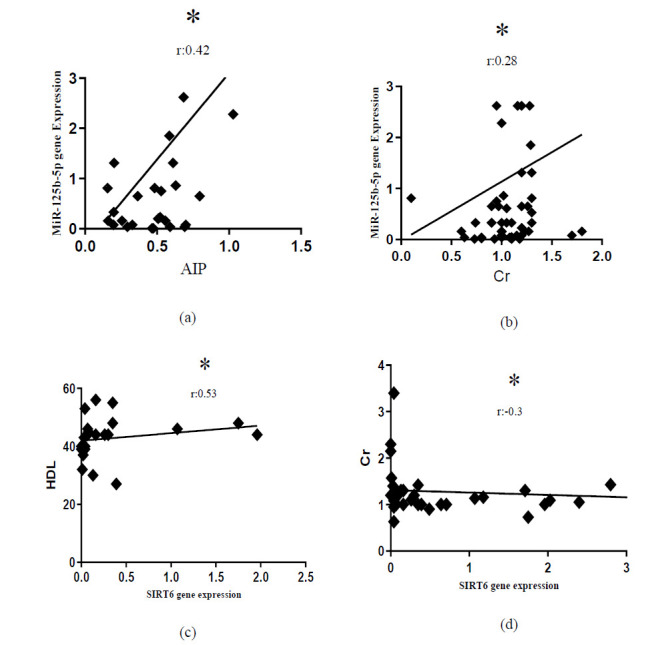
Correlation between miR-125b and SIRT6 gene with biochemical parameters. (a) Correlation of miR-125b with AIP; (b) Correlation of miR-125b with Cr; (c) Correlation of SIRT6 with HDL; (d) Correlation of SIRT6 with Cr. AIP, Atherogenic index of plasma; Cr, Creatinine; HDL, High-density lipoprotein. *P<0.05.
Comparison of STAT3 gene expression in groups with and without history of heart disease and hypertension
Based on Fig. 4, the expression of the STAT3 gene in groups with a history of heart disease and hypertension was substantially different compared to the group without a history of heart disease and hypertension (P<0.05).
Fig. 4.
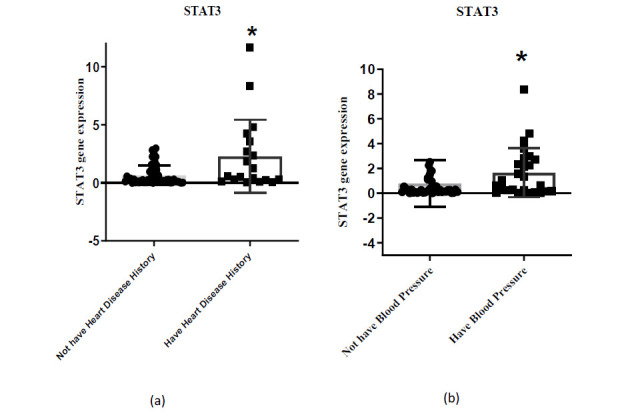
The expression levels of STAT3 in PBMC cells. (a) Groups with a history of heart disease and without a history of heart disease; (b) Groups with hypertension and without hypertension. * P<0.05.
Discussion
The findings of this study showed a significant increase in the expression of miR-125b in AS patients compared to control subjects. In this regard, Hueso et al. showed that miR-125b overexpresses in human atherosclerotic plaque, confirming the involvement of CD40/NF-kB signaling in the expression of miR-125b (24). Additionally, increased plasma levels of miR-125b are related to functional outcomes and stroke severity in acute ischemic stroke patients receiving thrombolysis (25). The previous document also illustrated that lower values of miR-125b lead to ameliorating long-term survival in patients with multivessel disease (MVD) and acute coronary syndrome (ACS) (26). Based on several studies, miR-125b augments activated nature of macrophages and downregulates scavenger receptor B1 (SR-B1) in macrophages and VSMCs (27,28). Therefore, it seems that miR-125b can be a potential diagnostic biomarker in clinical settings and be implemented as a promising therapeutic target to provide better management of AS (29).
In this study, the expression of STAT3 and SIRT6 genes altered between two control and patient groups, which was not statistically significant. Multiple studies have focused on exploring the role of STAT3 and SIRT6 in CVDs (14,30). The findings of Luo and colleagues revealed that increased IL-6 levels and subsequent expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), are accompanied by activation of STAT3 pathway (31). It has been shown that blocking the NF-KB and STAT3, as therapeutic interventions, suppress AS by inhibiting inflammation (32). Besides, SIRT6 represses the initiation of AS and plaque instability via regulating inflammation, macrophage polarization, and foam cell formation (33). Based on available data, SIRT6 plays a crucial role in regulating STAT3 expression, thereby protecting cardiomyocyte hypertrophy (15). There is evidence that STAT3 and SIRT6 could be novel targets for miR-125b in various diseases such as cancers (34–36).
We investigated the association of miR-125b with the expression of STAT3 and SIRT6 genes in patients with AS. The results showed a negative relationship that was not significant. However, further studies are needed to improve the understanding of miR-125b/ SIRT6 / STAT3 signaling in vascular diseases, especially AS.
In addition to the mentioned results, the correlation of miR-125b with AIP and Cr was significantly positive. Generally, the interaction between miRNAs expression with classical risk factors, including Cr, has been reported in some studies, suggesting their important role in susceptibility to coronary heart diseases (37).
Additionally, different miRNAs deregulation are well identified to be involved in TG and cholesterol metabolism and to be related to the development of metabolic disorders (38). The results of a vivo study reported that miR-125a dysregulation enhances the development of insulin resistance induced by obesity, involving lipid metabolism (39).
AIP in our study also indicated a significant rise in AS patients compared to controls, suggesting a diagnostic and screening tool for AS risk. In agreement with our results, several studies have indicated that AIP is correlated with disorders of lipoprotein and lipid metabolism, including dyslipidemia, which is considered the major reason for obesity-induced CVDs (40–42). Hence, it seems that the correlation of miR-125b with AIP can present an emerging mechanism in AS pathophysiology.
Importantly, SIRT6 in the present study had remarkable positive and negative correlations with HDL and Cr in patients, respectively. The results of a study in 2018 indicated that SIRT6 overexpression ameliorates elevation of renal dysfunction markers (BUN and serum Cr) as well as inflammation and apoptosis by suppressing ERK1/2 expression (43). Moreover, the findings of an in vivo study indicated that SIRT6 and forkhead box O3 (FoxO3) have a regulatory role in LDL homeostasis, and overexpression of SIRT6 could lower LDL levels by suppressing the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene (44). Also, studies have indicated that SIRT6 positively is associated with HDL, which supports the link between SIRT6 and lipid metabolism (45). Hence, it is believed that modulating SIRT6 in lipid metabolism can represent a beneficial strategy to prevent AS.
The findings of this research showed the expression of the STAT3 gene in the group with a history of heart disease and hypertension was substantially higher than group without a history of heart disease and hypertension. Interestingly, ROC curves indicated that STAT3 can be regarded as a valuable diagnostic biomarker for individuals at risk of heart disease and hypertension. So far, numerous studies have surveyed the association of STAT3 with risk factors of CVDs. A strong correlation has been observed between mRNAs (STAT3/JAK2) and stroke-related risk factors involving hypertension, dyslipidemia, smoking, and AS (46). According to ROC curve results, higher mRNA levels of STAT3/JAK2 could be associated with ischemic stroke risk. The findings of a study in 2021 have also proposed that STAT3/JAK2 may serve as complementary biomarkers for improving stroke diagnostic accuracy (46). Thus, understanding the mechanisms of STAT3 in pathological cardiac conditions can facilitate the development of therapeutic interventions against AS.
This study had some limitations which should be acknowledged. The statistical population in this study was small owing to sampling constraints and limited access to suitable participants. It is necessary to have a larger sample size in future research to establish the obtained results conclusively. Another limitation of this study was the lack of measurement of miR-125b and SIRT6 serum levels and inflammatory cytokines. Because it could provide more accurate results from the relationship between miR-125b and SIRT6 gene from the perspective of inflammation. In addition, quantitative information related to vascular risk factors of AS, such as the effects of different drugs, including statin, can be considered as inclusion criteria to achieve more precise findings.
As a consequence of this study, high miR-125b level in PBMCs of patients was strongly correlated with the progression of AS and could serve as a valuable biomarker for the recognition of AS. In addition, the expression of STAT3 and SIRT6 genes did not alter substantially in the two groups, recommending more detailed research on these genes and their relationship with AS. However, STAT3 indicated high diagnostic power to detect individuals at risk of heart disease and hypertension. Notably, miR-125b and SIRT6 had positive correlations with AIP and HDL, respectively, indicating the possible association of these genes with lipid metabolism. Also, the levels of atherogenic indices including AIP and CRI-II increased in individuals with AS, which seems to be a more important index compared to other lipid profile indices in screening AS. Nevertheless, we suggest conducting clinical trials as well as further detailed studies with larger-size samples to mechanistically elucidate the association of miR-125b with STAT3 and SIRT6 in AS and even other CVDs.
Funding
The work described in this article was the thesis of Atefeh Mirderikvand for the MSc in clinical biochemistry. This work was supported by a grant from the Lorestan University of Medical Sciences (grant number 1397-1-99-1894).
Conflict of interest
The authors declare that they have no conflict of interest
Acknowledgements
The authors would like to thank all staff of Shahid Madani Hospital and all the individuals participating in this study.
References
- 1.Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. . Cell. 2022;185(10):1630–45. doi: 10.1016/j.cell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. . Curr Atheroscler Rep. 2017;19(11):42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 3.Mohammed HSE, Kamal MM, ElBadre HM, Hosni A, Elfadl AA, Mostafa MA, El-Mahdy RI. Lectin-Like OLR1 3′ UTR Rs1050286 gene polymorphism and plasma Oxidized-LDL in coronary artery disease and their relation to cardiovascular risk and outcomes. . Rep Biochem Mol Biol. . 2022;10(4):537–53. doi: 10.52547/rbmb.10.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khyzha N, Alizada A, Wilson MD, Fish JE. Epigenetics of atherosclerosis: emerging mechanisms and methods. . Trends Mol Med. . 2017;23(4):332–347. doi: 10.1016/j.molmed.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Alizadeh S, Mirshafiey A, Djalali M, Alvandi E, Mohammadzadeh Honarvar N, Javanbakht MH. Vitamin D3 induces gene expression of Ox-LDL scavenger receptors in streptozotocin-induced diabetic rat aortas: new insight into the role of vitamin D in diabetic atherosclerosis. Rep Biochem Mol Biol. . 2018;6(2):170–177. [PMC free article] [PubMed] [Google Scholar]
- 6.Feinberg MW, Moore KJ. MicroRNA regulation of atherosclerosis. . Circ Res. 2016;118(4):703–20. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao Y, Li S, Ding Y, Du X, Zhang M, Tang W, Zhou S. MiRNA: a potential target for gene diagnosis and treatment of atherosclerotic stroke. Int J Neurosci. 2021;131(3):283–8. doi: 10.1080/00207454.2020.1738428. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Atanasov AG. The microRNAs regulating vascular smooth muscle cell proliferation: a minireview. Int J Mol Sci. 2019;20(2):324. doi: 10.3390/ijms20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Cao Y, Liu D, Zhang J, Guo Y. The etiology and molecular mechanism underlying smooth muscle phenotype switching in intimal hyperplasia of vein graft and the regulatory role of microRNAs. Front Cardiovasc Med. . 2022;9:935054. doi: 10.3389/fcvm.2022.935054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goettsch C, Rauner M, Pacyna N, Hempel U, Bornstein SR, Hofbauer LC. miR-125b regulates calcification of vascular smooth muscle cells. . Am J Pathol. . 2011;179(4):1594–600. doi: 10.1016/j.ajpath.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao CT, Yeh HY, Yuan TH, Chiang CK, Chen HW. MicroRNA-125b in vascular diseases: An updated systematic review of pathogenetic implications and clinical applications. J Cell Mol Med. 2019;23(9):5884–5894. doi: 10.1111/jcmm.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han L, Jia L, Zan Y. Long intergenic noncoding RNA smad7 (Linc-smad7) promotes the epithelial-mesenchymal transition of HCC by targeting the miR-125b/SIRT6 axis. Cancer Med. 2020;9(23):9123–9137. doi: 10.1002/cam4.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song S, Yang Y, Liu M, Liu B, Yang X, Yu M, et al. MiR-125b attenuates human hepatocellular carcinoma malignancy through targeting SIRT6. Am J Cancer Res. 2018;8(6):993–1007. [PMC free article] [PubMed] [Google Scholar]
- 14.Saiyang X, Deng W, Qizhu T. Sirtuin 6: A potential therapeutic target for cardiovascular diseases. Pharmacol Res. . 2021;163:105214. doi: 10.1016/j.phrs.2020.105214. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Li W, Shen P, Feng X, Yue Z, Lu J, et al. STAT3 Suppression Is Involved in the Protective Effect of SIRT6 Against Cardiomyocyte Hypertrophy. J Cardiovasc Pharmacol. 2016;68(3):204–14. doi: 10.1097/FJC.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Lv J, Yang W, Xu B, Wang Z, Yu Z, et al. Targeted inhibition of STAT3 as a potential treatment strategy for atherosclerosis. . Theranostics. 2019;9(22):6424–6442. doi: 10.7150/thno.35528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An X, Liu J, Li Y, Dou Z, Li N, Suo Y, et al. Chemerin/CMKLR1 ameliorates nonalcoholic steatohepatitis by promoting autophagy and alleviating oxidative stress through the JAK2-STAT3 pathway. Peptides. . 2021;135:170422. doi: 10.1016/j.peptides.2020.170422. [DOI] [PubMed] [Google Scholar]
- 18.Yandong Z, Yaning B, Xinqiao C, Lei H, Cheng S, Yu L, et al. Protective effect of Chushizi (Fructus Broussonetiae) on acetaminophen-induced rat hepatitis by inhibiting the Toll-like receptor 3/c-Jun N-terminal kinase/c-jun/c-fos/janus protein tyrosine kinase/activators of transcription 3 pathway. J Tradit Chin Med. 2020;40(6):965–973. doi: 10.19852/j.cnki.jtcm.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57(6):695–713. doi: 10.1111/j.1365-2125.2004.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahsavari G, Ahmadpour F, Yalameha B. Association between myeloperoxidase polymorphism (MPO-129G/A), oxidized low-density lipoprotein level, and atherogenic indices in patients with atherosclerosis; a case-control study. . Gene Reports. 2023;33:101816. [Google Scholar]
- 21.el Brutto OH, Mera RM, Atahualpa Project Investigators Inverse relationship between the body mass index and severity of carotid siphon calcifications (another obesity paradox): Results from the Atahualpa Project. . Atherosclerosis. 2017;259:1–4. doi: 10.1016/j.atherosclerosis.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Luo H, Zhou M, Ji K, Zhuang J, Dang W, Fu S, et al. Expression of Sirtuins in the Retinal Neurons of Mice, Rats, and Humans. . Front Aging Neurosci. . 2017;9:366. doi: 10.3389/fnagi.2017.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davari N, Ahmadpour F, Kiani AA, Azadpour M, Asadi ZT. Evaluation of microRNA-223 and microRNA-125a expression association with STAT3 and Bcl2 genes in blood leukocytes of CLL patients: a case–control study. . BMC Res Notes. 2021;14(1):21. doi: 10.1186/s13104-020-05428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hueso M, De Ramon L, Navarro E, Ripoll E, Cruzado JM, Grinyo JM, Torras J. Silencing of CD40 in vivo reduces progression of experimental atherogenesis through an NF-κB/miR-125b axis and reveals new potential mediators in the pathogenesis of atherosclerosis. . Atherosclerosis. 2016;255:80–89. doi: 10.1016/j.atherosclerosis.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 25.He XW, Shi YH, Liu YS, Li GF, Zhao R, Hu Y, et al. Increased plasma levels of miR-124-3p, miR-125b-5p and miR-192-5p are associated with outcomes in acute ischaemic stroke patients receiving thrombolysis. . Atherosclerosis. . 2019;289:36–43. doi: 10.1016/j.atherosclerosis.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Gager GM, Eyileten C, Postula M, Gasecka A, Jarosz-Popek J, Gelbenegger G, et al. Association between the expression of microRNA-125b and survival in patients with acute coronary syndrome and coronary multivessel disease. Front Cardiovasc Med. 2022;9:948006. doi: 10.3389/fcvm.2022.948006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Yao N, Zhang J, Liu Z. MicroRNA-125b is involved in atherosclerosis obliterans in vitro by targeting podocalyxin. . Mol Med Rep. . 2015;12(1):561–8. doi: 10.3892/mmr.2015.3384. [DOI] [PubMed] [Google Scholar]
- 28.Hueso M, Griñán R, Mallen A, Navarro E, Purqueras E, Gomá M, et al. MiR-125b downregulates macrophage scavenger receptor type B1 and reverse cholesterol transport. . Biomed Pharmacother. . 2022;146:112596. doi: 10.1016/j.biopha.2021.112596. [DOI] [PubMed] [Google Scholar]
- 29.Churov A, Summerhill V, Grechko A, Orekhova V, Orekhov A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int J Mol Sci. 2019;20(22):5547. doi: 10.3390/ijms20225547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harhous Z, Booz GW, Ovize M, Bidaux G, Kurdi M. An update on the multifaceted roles of STAT3 in the heart. . Front Cardiovasc Med. 2019;6:150. doi: 10.3389/fcvm.2019.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo P, Shi W, Wang Y, Ma H, Liu T, Yan D, et al. Raloxifene inhibits IL-6/STAT3 signaling pathway and protects against high-fat-induced atherosclerosis in ApoE-/- mice. . Life Sci. 2020;261:118304. doi: 10.1016/j.lfs.2020.118304. [DOI] [PubMed] [Google Scholar]
- 32.An HJ, Gwon MG, Gu H, Bae S, Leem J, Lee JB, et al. STAT3/NF-kB decoy oligodeoxynucleotides inhibit atherosclerosis through regulation of the STAT/NF-kB signaling pathway in a mouse model of atherosclerosis. Int J Mol Med. 2023;51(5):37. doi: 10.3892/ijmm.2023.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren SC, Chen X, Gong H, Wang H, Wu C, Li PH, et al. SIRT6 in Vascular Diseases, from Bench to Bedside. Aging Dis. 2022;13(4):1015–1029. doi: 10.14336/AD.2021.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Xing Q, Mao J, Sun H, Teng W, Shan Z. miRNA-125b-5p suppresses hypothyroidism development by targeting signal transducer and activator of transcription 3. . Med Sci Monit. . 2018;24:5041–5049. doi: 10.12659/MSM.907510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng J, Fan Y, Ayiheng Q, Zhang H, Yong J, Hu B. MicroRNA-125b targeted STAT3 to inhibit laryngeal squamous cell carcinoma cell growth and motility. Oncol Lett. 2017;14(1):480–486. doi: 10.3892/ol.2017.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Yang W, Xiao Y, Shan L. MiR-125b inhibits cell proliferation and induces apoptosis in human colon cancer SW480 cells via targeting STAT3. . Recent Pat Anticancer Drug Discov. 2022;17(2):187–194. doi: 10.2174/1574892816666210708165037. [DOI] [PubMed] [Google Scholar]
- 37.Ding XQ, Ge PC, Liu Z, Jia H, Chen X, An FH, et al. Interaction between microRNA expression and classical risk factors in the risk of coronary heart disease. Sci Rep. 2015;5:14925. doi: 10.1038/srep14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoda ME, Abo-almatty D, Same MS, Amr TT. Ameliorative effect of grape seed extract on metabolic disorders caused by high fat diet induced obesity in rats by reversing the increase in hepatic miR-33a and miR-122. . Afr J Pharm Pharmacol. . 2016;10(34):699–708. [Google Scholar]
- 39.Liu R, Wang M, Li E, Yang Y, Li J, Chen S, et al. Dysregulation of microRNA-125a contributes to obesity-associated insulin resistance and dysregulates lipid metabolism in mice. . Biochim Biophys Acta Mol Cell Biol Lipids. . 2020;1865(5):158640. doi: 10.1016/j.bbalip.2020.158640. [DOI] [PubMed] [Google Scholar]
- 40.Li YW, Kao TW, Chang PK, Chen WL, Wu LW. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. . Sci Rep. . 2021;11(1):9900. doi: 10.1038/s41598-021-89307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA, Pérez-Maldonado IN. Atherogenic index of plasma: novel predictive biomarker for cardiovascular illnesses. . Arch Med Res. 2019;50(5):285-294;50(5):285–294. doi: 10.1016/j.arcmed.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Shen SW, Lu Y, Li F, Yang CJ, Feng YB, Li HW, et al. Atherogenic index of plasma is an effective index for estimating abdominal obesity. Lipids Health Dis. . 2018;17(1):11. doi: 10.1186/s12944-018-0656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z, Xu K, Zhang N, Amador G, Wang Y, Zhao S, et al. Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. . Kidney Int. 2018;93(4):881–892. doi: 10.1016/j.kint.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J Biol Chem. 2013;288(41):29252–9. doi: 10.1074/jbc.M113.481473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Z, Wang X, Liu YS, Xing XW, Zhang XG, Lu QH. Decreased serum SIRT6 as a novel predictor of coronary artery disease. . Eur Rev Med Pharmacol Sci. . 2021;25(21):6660–6669. doi: 10.26355/eurrev_202111_27110. [DOI] [PubMed] [Google Scholar]
- 46.Adly Sadik N, Ahmed Rashed L, Ahmed Abd-El Mawla M. Circulating miR-155 and JAK2/STAT3 axis in acute ischemic stroke patients and its relation to post-ischemic inflammation and associated ischemic stroke risk factors. Int J Gen Med. 2021;14(1469):1484. doi: 10.2147/IJGM.S295939. [DOI] [PMC free article] [PubMed] [Google Scholar]


