Abstract
The fatal disease induced by SIVsmmPBj4 clinically resembles endotoxic shock, with the development of severe gastrointestinal disease. While the exact mechanism of disease induction has not been fully elucidated, aspects of virus biology suggest that immune activation contributes to pathogenesis. These biological characteristics include induction of peripheral blood mononuclear cell (PBMC) proliferation, upregulation of activation markers and Fas ligand expression, and increased levels of apoptosis. To investigate the role of immune activation and viral replication on disease induction, animals infected with SIVsmmPBj14 were treated with one of two drugs: FK-506, a potent immunosuppressive agent, or PMPA, a potent antiretroviral agent. While PBMC proliferation was blocked in vitro with FK-506, pig-tailed macaques treated preinoculation with FK-506 were not protected from acutely lethal disease. However, these animals did show some evidence of modulation of immune activation, including reduced levels of CD25 antigen and FasL expression, as well as lower tissue viral loads. In contrast, macaques treated postinoculation with PMPA were completely protected from the development of acutely lethal disease. Treatment with PMPA beginning as late as 5 days postinfection was able to prevent the PBj syndrome. Plasma and cellular viral loads in PMPA-treated animals were significantly lower than those in untreated controls. Although PMPA-treated animals showed acute lymphopenia due to SIVsmmPBj14 infection, cell subset levels subsequently recovered and returned to normal. Based upon subsequent CD4+ cell counts, the results suggest that very early treatment following retroviral infection can have a significant effect on modifying the subsequent course of disease. These results also suggest that viral replication is an important factor involved in PBJ-induced disease. These studies reinforce the idea that the SIVsmmPBj model system is useful for therapy and vaccine testing.
Since it was first isolated in 1985 and 1986 (1, 5, 10, 26), the simian immunodeficiency viruses (SIV) have become an important tool for investigating numerous aspects of lentivirus-host interactions. These viruses have been instrumental in our understanding of the disease process induced by human immunodeficiency virus type 1 (HIV-1) infection in humans. In addition, the SIV-macaque model has been important for investigating new methods for vaccine and therapy development. The SIV isolates from sooty mangabeys (Cercocebus atys) (SIVsmm) and rhesus macaques (Macaca mulatta) (SIVmac) induce a disease in Asian macaques that is remarkably similar to HIV-1 infection in humans (20, 24). The usefulness of the SIV-macaque model system is that it recapitulates HIV-1 pathogenesis in a shorter time. However, the pathogenic nature of SIV varies between isolates. Some isolates, such as the SIVmac1A11 isolate (23), have not been shown to cause any disease. However, most isolates are at least moderately pathogenic and induce AIDS-like disease within a time frame of 5 to 18 months.
We have been investigating a highly pathogenic variant of SIV, termed SIVsmmPBj14 (PBj). This isolate induces an acutely lethal disease that is characterized by diarrhea, dehydration, and anorexia, culminating in death in 5 to 14 days postinfection (11). A major focus of replicating virus is found in the lymphoid system of the intestinal tract. This is also where significant pathology occurs, including blunted intestinal villi and hyperproliferative tissue (9, 11). While the disease appears to be an exaggerated form of acute retroviral disease, clinical signs of disease are similar to those of animals experiencing endotoxic shock. The highly pathogenic nature of this virus suggested that changes in both the genotype and phenotype of SIV contributed to the new disease syndrome. Initial studies showed that this virus had unique characteristics, including the ability to replicate in unstimulated peripheral blood mononuclear cells (PBMC) and induce PBMC to proliferate (8). The latter characteristic appears to correlate with the observation that animals dying of PBj-induced acute disease have hyperproliferative lymphoid tissue in the gut. Contributing to this hyperproliferative state could be the redirection of lymphocytes from the periphery to the intestinal area through induction of specific integrins (12). We have been evaluating the basis for disease development at the molecular, biological, and pathologic levels. It is now clear that multiple viral genetic elements are required for the acutely lethal nature of PBj (27, 28), including a single amino acid change in Nef (7, 33). Furthermore, we have demonstrated that the in vitro phenomenon of PBMC proliferation induction by this virus is linked to its ability to induce acute disease (27). Inoculation of cells in vitro, or of animals, with PBj has been shown to induce the upregulation of activation markers (CD25 and CD45RO) (33). These results, coupled with the additional finding of significantly increased levels of apoptosis in the hyperplastic gut lymphoid areas, strongly suggest that immune activation is intimately associated with the acute disease syndrome (13, 40).
To further investigate the roles of immune activation and viral replication in disease development, we used two methods to try to prevent disease development: immunosuppression and antiretroviral therapy. The results presented here suggest that while immune activation may play a significant role in the pathogenesis of PBj-induced disease, viral replication also appears to contribute importantly to disease development.
MATERIALS AND METHODS
Animals, cells, and virus stocks and compounds.
Juvenile pig-tailed macaques were used for all animal studies described here. These monkeys were determined to be seronegative for SIV, simian T-lymphotropic virus, and simian retrovirus type D before use. All animals were housed according to established National Institutes of Health (NIH) guidelines (26a). The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. PBMC were obtained from EDTA-treated blood collected from anesthetized macaques by separation of blood on LSM (lymphocyte separation medium; ICN, Costa Mesa, Calif.). Cells were stimulated with concanavalin A (ConA) and interleukin 2 (IL-2) for 3 to 5 days before use. The cell line CEMx174 was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES, and antibiotics. The infectious molecular clone of SIVsmmPBj, termed PBj6.6, has been described earlier (27). Virus stocks of PBj6.6 were prepared by transfection of DNA into CEMx174 cells using the DEAE-dextran method. At peak reverse transcriptase (RT) activity, cell-free supernatants were harvested, aliquoted, and stored under liquid nitrogen. The concentration of p27 in virus stock was determined by using a commercially available antigen capture kit (SIV core antigen kit; Coulter, Hialeah, Fla.). Titration of virus stock was determined by using end point RT detection and calculated by the method of Reed and Muench. FK-506 (Prograf; Fujisawa) was purchased from the Emory University Medical School pharmacy as a sterile, injectable solution for use in these studies. The FK-506 was diluted in phosphate-buffered saline (PBS) for in vitro and in vivo use. PMPA [(R)-9-(2-phosphonomethoxypropyl)adenine] was generously supplied by Gilead Sciences. The PMPA was prepared by adding 3 g to 20 ml of sterile, distilled H2O. Ten normal NaOH was added dropwise to the PMPA suspension until the powder dissolved completely. The volume was brought to 30 ml with sterile H2O and was passed through a 0.22-μm-pore-size filter. The pH of the PMPA solution was always between 7.0 and 7.5.
Proliferation assays.
In vitro proliferation assays were conducted as described previously (27). Briefly, PBMC isolated from pig-tailed macaques were resuspended to a final concentration of 2 × 105 cells/ml in RPMI 1640 containing 10% FBS. Cells (100 μl) were added to the wells of a 96-well microtiter plate, followed by virus (PBj6.6; 10 ng of p27). Controls included phytohemagglutinin (PHA) (5 μg) and medium. For testing of inhibition, compounds were always added at the same time as virus. FK-506 (Prograf) and PMPA were diluted in medium prior to addition. At 6 days after the addition of virus, 1 μCi of [3H]thymidine was added to the wells. Following an 18-h incubation, cells were harvested onto glass fiber filters that were subsequently washed. The incorporated radioactivity was determined by scintillation counting. Results are presented as stimulation indices.
Viral replication assays.
Replication inhibition assays were performed with ConA-stimulated PBMC isolated from uninfected pig-tailed macaques. Cells (1 × 107 to 2 × 107) were mixed with virus (10 ng of p27) and incubated overnight to allow virus adsorption. The following day, cells were washed and resuspended in 10 ml of RPMI 1640 medium containing 10% FBS, 5% IL-2, 10 mM HEPES, and antibiotics. Compounds to be tested were added at the start of the absorption period or at various times afterward. At the indicated times postinfection, cell-free supernatants were harvested and stored at −80°C until use. After all samples were harvested, viral activity in the supernatants was quantified by an RT assay.
Infection of macaques, treatments, and subsequent monitoring.
Following anesthetization, juvenile macaques were inoculated intravenously with a high dose (104 50% tissue culture infective doses [TCID50]) or a minimum lethal dose (1 TCID50) of virus derived from the PBj6.6 molecular clone. Animals were monitored on a daily basis for development of disease. On days 3, 7, 10, 14, and 21, and monthly thereafter, animals were anesthetized and blood was collected for use in in vitro assays (complete blood count, lymphocyte subset analysis, viral assays, and serological assays). For treatment with FK-506, animals were pretreated on days −2 and −1 (relative to the virus inoculation day), and every other day thereafter, with a dose of 0.75 mg/kg of body weight given subcutaneously. In our hands, this dose had provided excellent levels of FK-506 in plasma when tested in uninfected animals. Trough levels of FK-506 were determined for infected animals at the indicated times after infection by the clinical laboratories at the Emory University Hospital. For treatment with PMPA, infected animals were treated beginning either on day 3 or on day 5 postinfection. The macaques received a subcutaneous injection of PMPA (dose, 30 mg/kg) once per day until day 14 postinfection, after which the animals were withdrawn from therapy. Control animals for both treatments received inoculations of saline.
Virus isolations, plasma p27 level determinations, and SIV bDNA assays.
Virus isolations from infected macaques were performed by using 107 ConA-stimulated PBMC cocultured with CEMx174 cells. Cultures were monitored on a weekly basis for development of RT activity. If no RT activity developed within 60 days of coculture, the cells were determined to be negative for virus isolation. Plasma p27 antigen levels were determined with a commercially available enzyme-linked immunosorbent assay (ELISA) kit (SIV Core Antigen Assay; Coulter). Measurements of SIV RNA were performed at the Bayer Reference Testing Laboratory (Bayer Diagnostics, Emeryville, Calif.) by using a branched-DNA (bDNA) assay specific for SIV (4). This assay is similar to the Quantiplex HIV RNA assay (30) except that target probes were designed to hybridize with the pol region of the SIVmac group of strains. It has been demonstrated that the SIV bDNA assay can equivalently quantify SIVmac and SIVsmm strains (SIVmac32H and SIVsmmPBj6.6, respectively) (3a). SIV pol RNA in plasma samples was quantified by comparison with a standard curve produced by using serial dilutions of cell-free SIV-infected tissue culture supernatant. The quantification of this standard curve was determined by comparison with purified, quantified, in vitro-transcribed SIVmac239 pol RNA. The lower quantification limit of the assay was 1,500 copies per plasma sample.
Tissue samples and immunohistochemistry.
Macaques that developed acute disease were euthanized. Tissues were fixed in 10% formalin and trimmed into paraffin-embedded blocks or were frozen in OCT medium. Paraffin-embedded intestinal tissues were cut in 5-μm-thick sections on a rotary microtome and mounted on slides coated with Vectabond (Vector Corp.). Tissue sections were heated in a 56°C oven for 30 min, then deparaffinized by immersion in Propar (Anatech) for 15 min. Samples were rehydrated in a series of graded alcohols (100, 95, and 70% ethanol) prior to immersion in PBS (pH 7.4). For antigen retrieval, tissue sections were processed by heating in a microwave oven on high power for 10 min in 5% urea in a covered plastic coplin jar. Jars were uncovered, and the solution was allowed to cool for 15 min. Sections were then rinsed in PBS, and tissue antigens were further exposed by digestion of sections in 0.1% trypsin for 10 min at room temperature. Tissue sections were then reacted with 3% H2O2 in methanol in order to inactivate any endogenous peroxidase. Nonspecific binding was decreased by incubating tissue sections with 10% normal horse serum or 10% normal goat serum (Vector Laboratories) for 1 h at room temperature.
The following mouse monoclonal antibodies were used in this study: (i) anti-CD45RO, clone UCHL1 (ready-to-use quick-staining horseradish peroxidase reagent; Dako); (ii) anti-SIVmac/smm gp41, clone KK41 (19) (1:200 dilution; NIH AIDS Research and Reference Reagent Program); and (iii) anti-CD25, clone M-A251 (Pharmingen) (1:500 dilution; reacts with the alpha chain of the IL-2 receptor and is specific for activated T cells). CD45RO represents a marker for both resting memory T cells and mature activated T lymphocytes, while the anti-SIV antibody is specific for cells that are productively infected by SIV. Detection of bound antibodies was performed by using a horseradish peroxidase-conjugated avidin-biotin complex (Vector ABC; Vector Laboratories). Chromagens used to visualize bound peroxidase were nickel-enhanced diaminobenzidine or VIP (Vector Laboratories). Staining for CD25 was performed on frozen tissue sections.
IL-6 ELISA.
To determine the levels of IL-6 in the plasma of SIVsmmPBj14-infected animals, a capture ELISA was used (39). An anti-IL-6 capture antibody (M-620-E) was diluted to 20 μg/ml in carbonate-bicarbonate buffer and applied to the wells of a MaxiSorp ELISA plate (Nunc, Rochester, N.Y.) (100 μl/well) overnight at 4°C. The following day, the coating material was removed and 200 μl of blocking buffer (PBS containing 4% bovine serum albumin [BSA]) was added. Plates were blocked for 1 h at 37°C. The blocking buffer was removed, and 50 μl of the secondary, biotinylated detection antibody (M-621-B, 1:2,000 dilution; Endogen) was added to the wells. Fifty microliters of the plasma sample or diluted IL-6 standard (IL-6, PHC0065; dilutions of 1,000 to 15.6 pg/ml; Biosource International) was added to the wells and incubated for 2 h at 37°C. The sample-antibody preparation was removed from the wells, and the plate was washed six times with wash buffer (PBS–0.05% Tween 20). To each of the wells was added 100 μl of a 1:20,000 dilution of streptavidin-horseradish peroxidase conjugate (Kirkegaard & Perry Laboratories). The plate was incubated at 37°C for 45 min. Following a washing step, 100 μl of substrate (3,3′,5,5′-tetramethylbenzidine [TMB]; Kirkegaard & Perry) was added, and the reaction mixture was incubated for 30 min. The color development was stopped by adding 50 μl of 0.18 M H2SO4. The optical densities were read by using a microplate reader set to 450 nm. To determine the concentration of IL-6 in plasma samples, a standard curve was constructed based upon results obtained with IL-6 standard dilutions. A linear curve fit was generated by using the Prism graphing program (Graph Pad Software), and unknown concentrations were calculated from the resulting equation.
RESULTS
Inhibition of SIVsmmPBj-induced immune activation (PBMC proliferation).
Previously, we had described the association of SIVsmmPBj-induced proliferation with acutely lethal disease development (27), the induction of apoptosis (13), mucosal integrin expression (12), and Fas ligand upregulation (17) in infected animals. This evidence suggested that immune activation was intimately associated with the acute disease induced by this unique virus. In conjunction with upregulation of Fas ligand expression, we had also demonstrated that cyclosporine (CsA) could prevent PBj-induced PBMC proliferation in vitro (40). We hypothesized that treatment of infected animals with an immunosuppressant might be able to prevent acute disease induction.
Because CsA is normally administered either intravenously or orally, we searched for an alternative compound that could be easily given to infected animals without the need for anesthetization. We thus chose to investigate the use of FK-506, an immunosuppressive drug that is also used for transplantation. FK-506 has a mechanism of action similar to that of CsA but binds to different proteins in mediating its effects on cells (14, 16). Once bound to these proteins, the complexes block the phosphatase activity of calcineurin, an essential component of the T-cell activation pathway. To test the ability of FK-506 to inhibit SIVsmmPBj-induced proliferation, we conducted inhibition assays, performed essentially as previously described for CsA (40). FK-506 was able to block SIVsmmPBj-induced PBMC proliferation at several concentrations (Table 1). Inhibition ranged from 99 to 84%, over a 2-log-unit difference in concentration. The intermediate concentration used in these studies (10 ng/ml) is the cutoff concentration in plasma for FK-506 effectiveness in transplantation procedures. These results suggested that FK-506 might be able to inhibit SIVsmmPBj-induced disease.
TABLE 1.
Inhibition of PBMC proliferation by FK-506
| Material added | FK-506 concn (μg/ml) | Mean cpm (±SD)a | SIb | % Inhibition |
|---|---|---|---|---|
| Medium | None | 701 (217) | 1.0 | |
| PHA (5 μg) | None | 15,280 (819) | 21.8 | |
| 0.1 | 1,590 (206) | 2.3 | 89.5 | |
| PBj6.6 | None | 27,494 (4,336) | 39.2 | |
| 0.1 | 137 (44) | 0.2 | 99.8 | |
| 0.01 | 375 (147) | 0.5 | 99 | |
| 0.001 | 4,335 (636) | 6.2 | 84 |
Mean of triplicate samples.
SI, stimulation index.
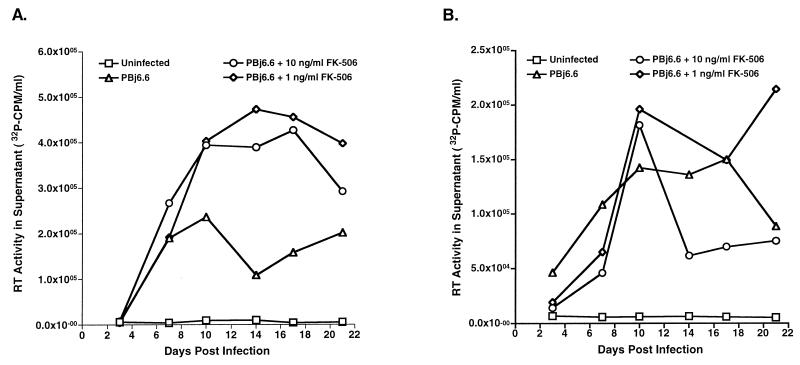
Parallel experiments were performed to assess any effects of FK-506 on replication of SIVsmmPBj. For these studies, FK-506 was added to unstimulated PBMC at the same time as virus. Unstimulated cells were used to determine if the inhibition of proliferation had any effects on the unique feature of SIVsmmPBj replication in unstimulated cells. FK-506 was kept in the medium throughout the replication study. Supernatants obtained at various times after infection were used for quantitation of RT activity. In stimulated cells, FK-506 treatment did not inhibit SIVsmmPBj replication (Fig. 1A). However, at later times after infection, the presence of the immunosuppressive drug appeared to be beneficial to replicating virus—cultures containing FK-506 showed higher levels of RT activity than did untreated cultures. In unstimulated cells, FK-506 apparently had a minor effect on the replication of SIVsmmPBj. Data for early time points (3 and 7 days postinfection) show that RT activity in treated cultures was lower than that observed in untreated cultures (Fig. 1B). However, FK-506-treated cultures had higher levels of RT activity later after infection, similar to levels observed in ConA-stimulated PBMC cultures, demonstrating that the inhibition occurred only during early stages. Additionally, FK-506 did not result in elevated levels of cell cytotoxicity as determined in these assays (data not shown).
FIG. 1.
Replication of SIVsmmPBj in the presence of FK-506. Pig-tailed macaque PBMC were stimulated with ConA (A) or used unstimulated (B) for virus replication studies. Cells (1 × 107 to 2 × 107) were inoculated with virus (in amounts equivalent to 10 ng of p27) derived from the molecular clone PBj6.6 either in the presence or in the absence of the indicated concentrations of FK-506. Cell-free supernatants harvested at the indicated times postinfection were tested for the presence of RT activity. The results are representative of two separate experiments.
FK-506 treatment of pig-tailed macaques.
Because FK-506 was effective at blocking PBJ-induced proliferation in vitro, we sought to investigate the effects of FK-506 on SIVsmmPBj infection in vivo. For these experiments, treatment was initiated prior to inoculation, to optimize results. Two pig-tailed macaques were administered FK-506 (Prograf) subcutaneously, at a dosage of 0.75 mg/kg. Animals were treated on days −2 and −1 (relative to virus inoculation on day 0), and every other day thereafter. The treatment regimen was designed to continue through day 13. A control group of two animals was administered an equal volume of sterile saline. At day 0, animals were inoculated with 104 TCID50 of virus derived from molecular clone PBj6.6. Treatment of PBj6.6-infected macaques with FK-506 was unable to prevent the development of acutely lethal disease induced by SIVsmmPBj (Table 2). Control animals did succumb to disease more rapidly (4 to 5 days postinfection) than treated animals (6 to 7 days postinfection), and this suggested possible protective effects; however, the results are not statistically significant. Interestingly, the untreated controls also showed higher levels of plasma p27 antigen at earlier times. This was similar to in vitro results obtained with FK-506, discussed above.
TABLE 2.
Results of treatment with FK-506
| Animal code | Dose of virus (TCID50) | Daya of death | FK-506 level (ng/ml)b
|
Plasma p27 level (ng/ml)
|
CD4+ cell count
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 10 | Day 3 | Day 6/7c | Day 10 | Day 0 | Day 3 | Day 6/7 | Day 9/10 | |||
| PKf | 104 | 6 | ND | 47.3 | NA | 0 | 1.70 | NA | 1,398 | 616 | 451 | NA |
| PZf | 104 | 7 | ND | 49.7 | NA | 0 | 1.20 | NA | 643 | 287 | 44 | NA |
| POd | 104 | 4 | —d | — | — | 0.34 | NAe | NA | 938 | 136 | NA | NA |
| PUe | 104 | 5 | — | — | — | 0.05 | 18.00 | NA | 1,769 | 284 | 386 | NA |
| PGj | 1 | 10 | 49.2 | 22.0 | 18.1 | 0 | 0 | 0.26 | 839 | 1,490 | 793 | 421 |
| PLj | 1 | 10 | 38.8 | 19.3 | 10.7 | 0 | 0 | 2.83 | 1,496 | 2,388 | 1,069 | 390 |
| PHj | 1 | 10 | — | — | — | 0 | 0.38 | 9.19 | 1,247 | 1,886 | 223 | 219 |
| PIj | 1 | 10 | — | — | — | 0 | 0 | 23.17 | 3,591 | 2,663 | 966 | 529 |
Day, day postinfection.
Levels above 8 ng/ml are considered normal for transplantation procedures. ND, not done; NA, not applicable.
Day 6/7, day 6 or 7 postinfection.
—, no treatment.
Animal died on day 4; no sample available.
Because of these observations, we postulated that the input virus dose might have been too overwhelming to allow observation of any clinical effect of treatment with FK-506. Therefore, the experiment was repeated, this time with the minimum lethal dose of SIVsmmPBj (1 TCID50), determined by in vivo titration. Results of this experiment were similar to those of the first experiment—FK-506 treatment could not prevent acutely lethal disease, and all animals died at 10 days postinfection. The extended period of disease development was due to the lower virus dose used for inoculation. Based upon in vitro results (Table 2), plasma FK-506 levels in both sets of animals (the low and high virus dose groups) were high enough to suggest that PBj-induced proliferation in vivo would be inhibited. It should be noted that these were trough levels, taken just prior to the next administration of FK-506, and represented the lowest concentrations present in plasma. Again, as with the first set of animals, the second set of treated animals had lower levels of p27 in plasma, suggesting that these animals had lower viral loads. Unfortunately, adequate samples for additional viral load testing were not available from this cohort.
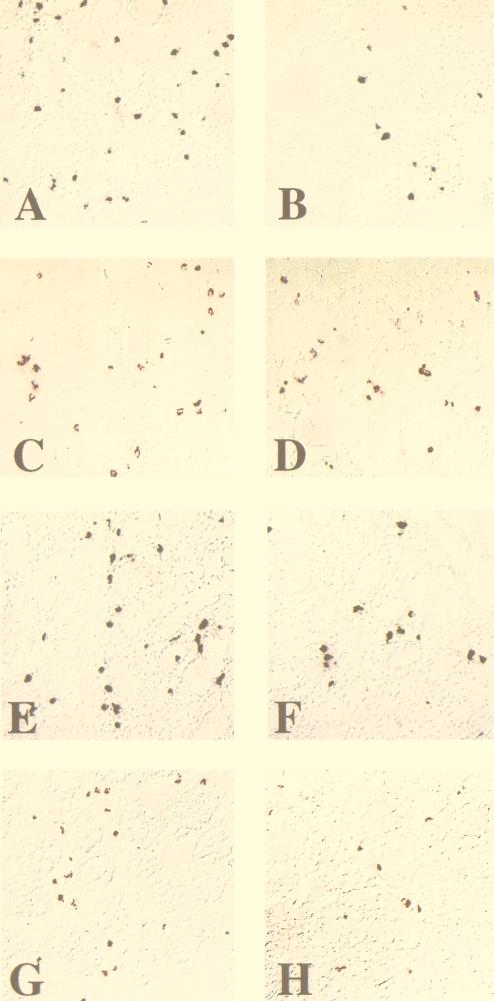
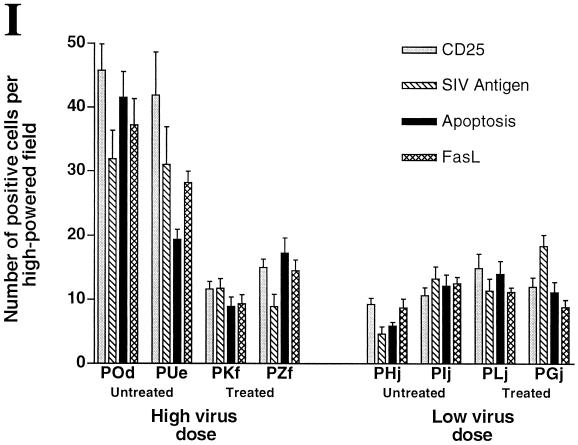
Because of the differences in time to death in the first set of animals and because of the differences in plasma p27 loads, we hypothesized that FK-506 might have had a minor in vivo effect. To determine if such an effect occurred, tissue samples from both treated and untreated animals were examined for gross histopathology, virus levels, and expression of activation markers. As in our previous studies (13, 17, 33), the tissue section selected for analyses was from the ileum, where the majority of PBj-induced pathology occurs. Blinded analysis of intestinal tissues showed that no differences could be detected in gross and microscopic histopathology between treated and untreated animals. All animals showed the classical lesions observed as a result of PBj infection, including villus blunting, foci of hemorrhage, and lymphoid hyperplasia. In contrast, macaques receiving the higher dose of virus and treated with FK-506 had apparently lower levels of virus, CD25+ cells, and FasL-expressing cells in the intestinal tract (ileum and colon) than untreated animals as measured by immunohistochemistry (Fig. 2). These data suggested that FK-506 might have had a partial effect on SIVsmmPBj-induced activation. Quantitative analysis showed that when the results from the treated animals (POd and PUe) were compared with those from the untreated animals (PKf and PZf) (Fig. 2I), statistically significant differences were detected in SIV antigen load (P < 0.01), numbers of CD25+ cells (P < 0.005), and numbers of FasL-positive cells (P < 0.001), as measured by the student t test. Results for the animals receiving the lower dose of virus did not show similar differences between treated and untreated animals (Fig. 2I).
FIG. 2.
Immunocytochemical localization of virus, apoptosis, and activation markers in intestinal tissue from PBj6.6-infected macaques. (A through H) Ileum sections from high-dose PBj6.6-infected pig-tailed macaques that were either left untreated (POd) (A, C, E, and G) or treated with FK-506 (PKf) (B, D, F, and H) were used to investigate the local effects of immunosuppressive therapy on SIVsmmPBj14 infection. Apoptotic nuclei were visualized by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling method (A and B). SIV antigen-positive cells (C and D), CD25-positive cells (E and F), and Fas ligand (CD95L)-positive cells (G and H) were identified by immunohistochemistry using specific antisera (see Materials and Methods). (I) Quantitation of the mean number of positive cells for each item pictured in panels A through H for all animals in the FK-506 cohort. Nine random fields of ileum tissue were examined to determine the mean number of positive cells for each evaluation. In the high-virus-dose group, statistically significant differences were observed between treated and untreated animals in the numbers of CD25+ cells (P < 0.005), SIV antigen levels (P < 0.01), and FasL expression (P < 0.001).
PMPA inhibition of SIVsmmPBj-induced proliferation and virus replication.
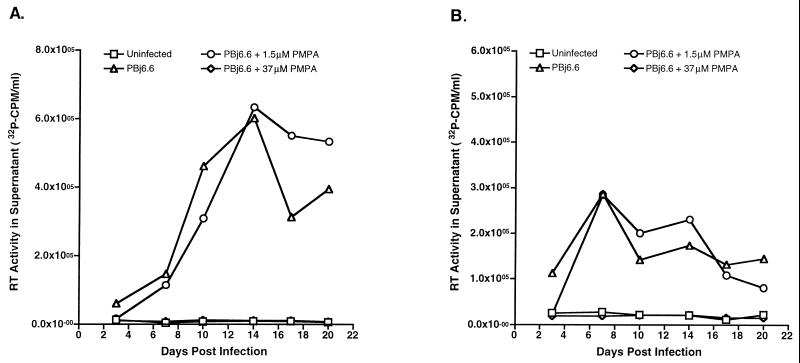
Although FK-506 appeared to have a small effect on immune activation induced by SIVsmmPBj, treatment did not prevent disease. These results suggested that viral replication could play an important role in the development of the acute syndrome. To investigate this hypothesis, we used an antiretroviral drug. PMPA was chosen for two reasons: the ease of administration (subcutaneous injection once per day) and the previously demonstrated active nature of this compound on SIV (35–37), especially at early times after infection (38). Before using this compound in vivo, we first tested the effectiveness of PMPA at inhibiting PBj-induced PBMC proliferation in vitro. Like FK-506, PMPA was able to inhibit proliferation at several concentrations (Table 3). We postulated that, unlike that with FK-506, proliferation inhibition due to PMPA was related to the ability of this compound to fully inhibit SIV replication. Replication studies showed this to be true (Fig. 3). PMPA was able to prevent PBj replication in both unstimulated and stimulated PBMC at a concentration of 37 μM, but not at a concentration of 1.5 μM.
TABLE 3.
Inhibition of PBMC proliferation by PMPA
| Material added | PMPA concn (ng/ml) | Mean cpm (±SD)a | SIb | % Inhibition |
|---|---|---|---|---|
| Medium | None | 2,355 (166) | 1.0 | |
| PHA (5 μg) | None | 14,701 (1,054) | 6.2 | |
| 10 | 13,108 (816) | 5.6 | 9.7 | |
| PBj6.6 | None | 42,314 (359) | 18.0 | |
| 10 | 3,448 (747) | 1.5 | 92 | |
| 5 | 6,121 (548) | 2.6 | 86 | |
| 1 | 10,534 (1,366) | 4.5 | 75 | |
| 0.5 | 19,973 (4,910) | 8.5 | 53 |
Mean of triplicate samples.
SI, stimulation index.
FIG. 3.
Replication of SIVsmmPBj in the presence of PMPA. Pig-tailed macaque PBMC were stimulated with ConA (A) or were used unstimulated (B) for virus replication studies. Cells (1 × 107 to 2 × 107) were inoculated with virus (equivalent to 10 ng of p27) derived from the molecular clone PBj6.6 either in the presence or in the absence of the indicated concentrations of PMPA. Cell-free supernatants harvested at the indicated times postinfection were tested for the presence of RT activity. The results are representative of two separate experiments.
PMPA treatment of PBj-infected macaques.
As indicated above, we sought to examine if PMPA could inhibit PBj disease. Previous results using another antiretroviral drug had shown that zidovudine (AZT) could inhibit PBj disease (22). However, we were interested in examining the effects of postinoculation therapy to investigate how quickly and efficiently the effects of PMPA could be observed in this rapid model of disease. For this experiment, six juvenile pig-tailed macaques were inoculated intravenously with 1 TCID50 of virus derived from PBj6.6 (day 0). On day 3 postinfection, two animals were started on PMPA treatment. On day 5, two additional animals were started on PMPA treatment. The last two animals remained as controls and received saline injections beginning on day 3. All animals receiving PMPA treatment survived the acute phase of disease (Table 4). Animals beginning treatment on day 3 postinfection did not show any signs of acute disease. One animal that began treatment on day 5 postinfection developed some diarrhea and anorexia on day 7 but quickly recovered and did not exhibit any additional signs of SIVsmmPBj-induced acute disease. The second day-5-treated animal showed no signs of disease. Both sham-treated animals developed disease and were euthanatized on the 9th day postinfection.
TABLE 4.
Results of treatment with PMPA
| Animal code | Daya of death | Day of PMPA start | Signs of disease | CD4+ cell count
|
|||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | Day 10 | ||||
| PIe | 9 | Diarrhea, wasting, anorexia, dehydration | 1,865 | 1,342 | 283 | 61 | |
| PKh | 9 | Diarrhea, wasting, anorexia, dehydration | 2,654 | 2,527 | 1,285 | 446 | |
| PAi | 3 | None | 2,146 | 1,334 | 1,621 | 1,357 | |
| PNi | 3 | None | 2,424 | 1,811 | 1,389 | 1,352 | |
| PEe | 5 | Diarrhea, mild anorexia | 559 | 593 | 168 | 773 | |
| PZh | 5 | None | 834 | 1,033 | 383 | 775 | |
Day, day postinfection.
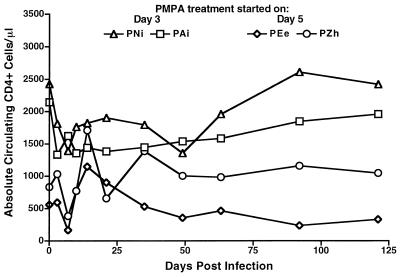
Treatment of macaques with PMPA continued through day 14 postinfection, at which time animals were removed from treatment and observed for any signs of disease. No signs of acute disease were observed in animals that were discontinued from treatment. A closer clinical examination of the four treated animals showed that all experienced the typical severe lymphopenia associated with acute SIVsmmPBj infection, exemplified by CD4+ cell counts (Fig. 4). Lymphopenia stabilized during PMPA treatment, and cell counts returned to near-baseline levels within the next 2 months.
FIG. 4.
Longitudinal analysis of circulating CD4+ cells in SIVsmmPBj14-infected macaques treated with PMPA. Blood samples from pig-tailed macaques infected with the PBj6.6 virus and treated with PMPA beginning at either 3 days (PAi and PNi) or 5 days (PEe and PZh) postinfection were used for the enumeration of absolute circulating CD4+ cells at the indicated time points by fluorescence-activated cell sorter analysis.
Early viral loads in these animals were monitored by plasma p27 levels, plasma virus RNA (bDNA) levels, and the ability to isolate virus from PBMC. In untreated animals, virus was isolated from PBMC at days 7 and 9 postinfection (day 9 was the day of necropsy) (Table 5). Additionally, these two animals showed detectable plasma p27 levels on both days. Plasma viral-RNA loads were extremely high in these macaques, at both day 7 and day 9, reaching levels of 2 × 108 to 4 × 108 copies/ml. For the macaques receiving PMPA beginning on day 3, virus could not be isolated from PBMC or detected by p27 antigen on days 7, 10, and 14. Viral RNA was undetectable in the plasma of both PAi and PNi on all days tested (days 3 to 14). These results suggested that PMPA had an early and sustained effect on viral replication. However, animals receiving PMPA treatment beginning on day 5 after infection had a less dramatic response. Virus could be isolated from animal PEe on days 3 and 10 after infection. This animal also had measurable levels of p27 antigen in the plasma on day 7. Virus could be isolated from the second animal, PZh, only on day 10 after infection. However, this macaque had measurable plasma p27 levels on day 10. In both PZh and PEe, plasma viral-RNA levels were detectable on days 7, 10, and 14 postinfection. PEe showed higher viral-RNA levels at day 7 postinfection, equal to those of the untreated animals. In both animals, response to PMPA treatment was evident; their day-7 plasma viral-RNA levels were 2 log units below those of the untreated animals. Viral-RNA levels continued to decline to day 14 after infection, with PZh having undetectable virus at this point.
TABLE 5.
Viral loads in PMPA-treated animals
| Animal code | Virus isolationa on dayb:
|
Plasma p27 concn (ng/ml) on day:
|
Plasma viral-RNA level (copies/ml) on day:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 10 | 14 | 21 | 35 | 49 | 63 | 3 | 7 | 10 | 14 | 3 | 7 | 10 | 14 | |
| No treatment | ||||||||||||||||
| PKh | − | + | +c | NA | NA | NA | NA | NA | 0 | 0.18 | 14.5 | NA | <45,000d | 1.3 × 106 | 1.9 × 108 | NA |
| PIe | − | + | +c | NA | NA | NA | NA | NA | 0 | 0.05 | 21.2 | NA | <45,000d | 2.4 × 106 | 3.8 × 108 | NA |
| Treatment starting on day 3 | ||||||||||||||||
| PAi | − | − | − | − | + | + | + | + | 0 | 0 | 0 | 0 | <45,000d | <1,500 | 1,500 | <1,500 |
| PNi | − | − | − | − | + | − | − | + | 0 | 0 | 0 | 0 | <45,000d | <1,500 | <45,000d | <1,500 |
| Treatment starting on day 5 | ||||||||||||||||
| PEe | + | − | + | − | + | − | + | + | 0 | 0.05 | 0 | 0 | <45,000d | 3.0 × 106 | 1.2 × 106 | 2.9 × 10d |
| PZh | − | − | + | − | + | − | + | + | 0 | 0 | 0.12 | 0 | <45,000d | 1.8 × 104 | 2.5 × 106 | <1,500 |
+, virus was isolated; −, virus could not be isolated; NA, not applicable.
Day, day postinfection.
PKh and PIe were euthanized on day 9 postinfection; results listed under day 10 are day-9 data.
Not enough sample to quantitate to lower level.
Subsequent to day 10 after infection, virus could be isolated at numerous times from all treated animals, demonstrating that these animals were persistently infected. In addition, all animals developed an immune response to SIV (as measured by ELISA and confirmed by Western blotting) by day 35 postinfection (data not shown).
Cytokine responses.
IL-6 has been implicated as a possible effector in the disease syndrome induced by SIVsmmPBj (2). We chose to examine the induction of this cytokine in both the FK-506-treated and the PMPA-treated animals. For these purposes, we used ELISA methods previously developed for macaque cytokines (31, 38). In the animals treated with FK-506, two responses were observed. Among the animals receiving a high dose of virus, the FK-506-treated macaques showed little or no IL-6 in the plasma at the time of death (Table 6). The untreated control (for which there was a sample) showed very high levels of IL-6 (636 pg/ml). In contrast, the animals receiving the low dose of virus inoculum showed similarly high levels of IL-6, irrespective of treatment status. In the PMPA treatment group, a dramatic difference between treated and untreated animals was observed. Animals receiving PMPA showed no detectable IL-6 in the plasma at day 10 postinfection. Additionally, IL-6 was not detected in the plasma of PMPA-treated animals at any time postinfection, through day 10.
TABLE 6.
Plasma IL-6 levels in SIVsmmPBj14-infected macaques
| Animal code | Virus dose (TCID50) | Treatment (day)a | Plasma IL-6 concn (pg/ml) on day of deathb |
|---|---|---|---|
| PKf | 104 | FK-506 | <15.6c |
| PZf | 104 | FK-506 | 15.6 |
| POd | 104 | None | NAd |
| PUe | 104 | None | 636.8 |
| PGj | 1 | FK-506 | 450 |
| PLj | 1 | FK-506 | >1,000 |
| PHj | 1 | None | 105 |
| PIj | 1 | None | >1,000 |
| PIe | 1 | None | 142 |
| PKh | 1 | None | >1,000 |
| PAi | 1 | PMPA (3) | <15.6 |
| PNi | 1 | PMPA (3) | <15.6 |
| PEe | 1 | PMPA (5) | <15.6 |
| PZh | 1 | PMPA (5) | <15.6 |
Day postinfection on which treatment was started.
Or on day 10 postinfection for animals surviving the acute phase of infection.
Range of detection was 15.6 to 1,000 pg/ml.
NA, not applicable. Animal died on day 4 postinfection; no plasma sample was available.
DISCUSSION
The syndrome induced by SIVsmmPBj in pig-tailed macaques is a rapidly fatal disease that is associated with high viral loads (particularly in the gut) and hyperproliferative expansion of the gut lymphoid tissue. This disease state is atypical with regards to lentiviral pathogenesis and is highly associated with immune activation. Because of this immune activation, we hypothesized that immunosuppressive therapy might be able to prevent or prolong the disease course in infected animals. Clearly, in vitro analysis demonstrated that immunostimulation induced by SIVsmmPBj (i.e., PBMC proliferation) was preventable by CsA (40) and FK-506 (this paper), both highly active immunosuppressants. This provided support for testing our hypothesis. When tested in replication assays, CsA (data not shown) and FK-506 both showed minor inhibition of virus growth. This was probably due to the lack of expansion of infectible cells induced by virus-associated PBMC proliferation and not to direct inhibition of virus growth. Previous studies have shown that CsA and related compounds can inhibit HIV-1 replication, but not SIV replication, due to selective incorporation of cyclophilin A by HIV-1 (3). The fact that unstimulated PBMC cultures could replicate SIVsmmPBj in the presence of immunosuppressive drugs suggests that this unique ability of SIVsmmPBj is not dependent upon expansion and activation of susceptible cells. Several concentrations of FK-506 were able to significantly suppress PBMC proliferation by PBj. Concentrations of 10 ng/ml were able to suppress PBMC proliferation by 99%. This was significant, since plasma FK-506 levels of 8 ng/ml or greater are considered normal, active levels in patients treated with these immunosuppressive agents prior to transplantation.
Because of these results, we expected that FK-506 treatment of PBj-infected macaques would prevent disease development. However, this was not the case. Results of two separate experiments, using both high and minimal doses of SIVsmmPBj14, showed that this compound could not prevent disease. Indeed, FK-506-treated animals inoculated with the lower dose of virus exhibited a disease course similar to that of untreated animals. A closer examination of animals that received the high-dose inoculum of virus indicated that FK-506 may have had some effect upon activation. Treated animals in this group showed slower progression to disease, lower viral loads in plasma and tissue, and lower levels of cellular activation as determined by levels of CD25, apoptosis, and FasL expression. An additional indication of the partial effectiveness of FK-506 is the difference in plasma IL-6 levels between treated and untreated animals in the high-virus-dose group. However, the fact that the treated animals still died of acute disease suggests that while FK-506 levels in the plasma were adequate, drug levels in the intestinal tissue may not have been sufficiently high. Similarly, FK-506 may not penetrate intestinal tissue, and lymphocytes may be directed to the gut area prior to activation. While induction of homing integrins has not been well investigated in PBj-induced disease, this could occur prior to cellular activation. In vitro analysis of PBj-induced activation shows day 3 after the addition of virus as the start of detectable proliferation and day 6 as the maximum (our unpublished data). Clearly, this area of SIVsmmPBj biology needs additional investigation.
Another alternative explanation is that extensive viral replication may overcome the effects of high-dose immunosuppressive treatment. As discussed above, although FK-506 could prevent PBj-induced PBMC activation in vitro, viral replication was not prevented, even in unstimulated cells. To investigate the role of continued high-level viral replication in PBj disease, the ability of antiretroviral therapy to prevent disease was examined. PMPA was chosen for use in these studies because of its documented high effectiveness against SIV (35–38).
In vitro, PMPA treatment was able to prevent both PBj-induced PBMC proliferation and viral replication. Thus, the inhibition of proliferation was apparently due to the inhibition of viral replication. This is in contrast to previously published reports of the ability of inactivated virus to induce PBMC proliferation (8). This aspect requires additional research. Previous studies have shown that antiretroviral therapy can prevent the acute disease syndrome induced by SIVsmmPBj14 (22). However, these investigators used preinoculation therapy. PMPA has been shown to prevent infection of macaques when treatment is begun either before infection or up to 24 h after infection (35). Additionally, early therapy with PMPA has been shown to prolong the time to disease development in SIV-infected macaques (38). Because of this potent ability of PMPA, we chose to use postinoculation therapy beginning at two different times after infection, so that we could ensure infection of the animals and thus test the effectiveness of therapy on infected macaques.
Treatment with PMPA was able to prevent the acutely lethal syndrome in SIVsmmPBj-inoculated animals when initiated at either 3 or 5 days postinfection. Virus loads in animals treated starting on day 3 were extremely low—no virus could be isolated from PBMC and no p27 antigen or viral RNA could be detected in plasma until after treatment was withdrawn on day 14. No incidence of acute infection was observed in these animals. In contrast, animals treated beginning on day 5 postinfection showed detectable levels of virus as measured by PBMC coculture isolation and by levels of viral RNA and p27 antigen in plasma. One animal in this group (PEe) even exhibited some clinical signs of sickness, including diarrhea and anorexia. The high viral load (bDNA) in this animal on day 7 postinfection was similar to that of the untreated controls, suggesting that the clinical signs of disease were due to accelerated viral replication compared to that of its treatment partner, PZh. These results suggest that a difference of just 2 days could have a significant effect in dampening virus replication in certain animals. The observation that no IL-6 was detected in the plasma of PMPA-treated animals supports earlier contentions that this is an important cytokine in the pathogenesis of disease. Additional cytokines may also be involved (34); however, a detailed study will need to be performed on tissue samples to determine the relevance of cytokines to pathogenic events.
The fact that animals treated with PMPA showed acute lymphopenia, like untreated animals, suggests that this is an early effect of SIVsmmPBj14 replication in pig-tailed macaques. Withdrawal of PMPA treatment from infected macaques did not allow a resumption of acute pathogenesis. One could hypothesize that the reduction of early viral load allowed the development of a significant immune response to combat early virus infection. Indeed, this hypothesis could be extended to argue that the acute disease syndrome induced by SIVsmmPBj14 might be partially due to evasion of immune surveillance or a lack of a significant immune response. A similar argument has been presented to explain the pathogenicity of another virus, SIVmacJ5, which induces AIDS in a short time, in the absence of measurable cytotoxic immune responses (32, 41).
The return to normal levels of CD4+ cells (and other cell types) in PMPA-treated animals suggests that therapy may have had a significant effect on the long-term pathogenesis of SIVsmmPBj14. While no untreated animals infected with the virus derived from the PBj6.6 molecular clone have survived acute infection, animals infected with other clones have survived acute disease (6, 18, 25). These acute survivors went on to develop typical AIDS disease, and most did not show a return to normal levels of CD4+ cells. As an example, animals vaccinated with a Semliki Forest virus recombinant expressing SIV envelope, while protected from acute disease when challenged with SIVsmmPBj, developed AIDS and showed no recovery of CD4+ lymphocytes (25, 29a). These combined results would lead one to hypothesize a prolonged course of disease in the PMPA-treated animals. Of course, this will have to be borne out by long-term observation of these macaques. However, this study again begs the question of how important early therapy may be for HIV-infected persons. The results would suggest that the earlier the therapy is administered, the more beneficial the outcome, even if therapy is given only for a relatively short interval.
The SIVsmmPBj isolate, while clearly a unique variant of typical SIVs, provides a useful model system for investigating the effects of antiretroviral therapy strategies and evaluating potential vaccine candidates, due to the very quick readout of acute disease development. The recent use of a version of PBj6.6 with a deletion in nef demonstrated that this virus was effective at protection of animals from heterologous and homologous virus challenges (21). The use of the pig-tailed macaque system, as pointed out in this publication and others (15, 29), provides a model which appears to be more sensitive to the pathogenic nature of SIV. Thus, results obtained from experimentation with these nonhuman primates may be more suggestive of the dramatic effects of therapy or vaccination and may provide important experimental data on which to build.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of Anne Brodie-Hill, Ellen Lockwood, and the primate care technicians at the Yerkes Regional Primate Research Center. We also thank Francois Villinger for assistance with cytokine ELISAs and Peter Dailey and Jennifer Booth for performing the SIV bDNA assays. The monoclonal antibody, KK-41, was obtained through the NIH AIDS Research and Reference Reagent Program and was donated by K. Kent.
This work was supported by grants R01-CA-67364 (to F.J.N.), R01-AI39397 (to S.D.), K04-AI-01240 (to S.D.), and F31-GM17802 (to S.H.), and by grant RR-00165 to the Yerkes Center.
REFERENCES
- 1.Benveniste R E, Arthur L O, Tsai C-C, Sowder R, Copeland T D, Henderson L E, Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986;60:483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birx D L, Lewis M G, Vahey M, Tencer K, Zack P M, Brown C R, Jahrling P B, Tosato G, Burke D, Redfield R. Association of interleukin-6 in the pathogenesis of acutely fatal SIVsmm/PBj14 in pigtailed macaques. AIDS Res Hum Retroviruses. 1993;9:1123–1129. doi: 10.1089/aid.1993.9.1123. [DOI] [PubMed] [Google Scholar]
- 3.Braaten D, Franke E K, Luban J. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIVCPZGAB but not group O HIV-1 or other primate immunodeficiency viruses. J Virol. 1996;70:4220–4227. doi: 10.1128/jvi.70.7.4220-4227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Dailey, P. Personal communication.
- 4.Dailey P J, Zamroud M, Kelso R, Kolberg J, Urdea M. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. J Med Primatol. 1995;24:209. [Google Scholar]
- 5.Daniel M D, Letvin N L, King N W, Kannagi M, Sehgal P K, Hunt R D, Kanki P J, Essex M, Desrosiers R C. Isolation of a T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- 6.Dewhurst S, Embretson J E, Anderson D C, Mullins J I, Fultz P N. Sequence analysis and acute pathogenicity of molecularly cloned SIVsmm-PBj14. Nature. 1990;345:636–640. doi: 10.1038/345636a0. [DOI] [PubMed] [Google Scholar]
- 7.Du Z, Lang S M, Sasseville V G, Lackner A A, Illyinskii P O, Daniel M D, Jung J U, Desrosiers R C. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 8.Fultz P N. Replication of an acutely lethal simian immunodeficiency virus activates and induces proliferation of lymphocytes. J Virol. 1991;65:4902–4909. doi: 10.1128/jvi.65.9.4902-4909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fultz P N, Zack P M. Unique lentivirus-host interactions: SIVsmmPBj14 infection of macaques. Virus Res. 1994;32:205–225. doi: 10.1016/0168-1702(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 10.Fultz P N, McClure H M, Anderson D C, Swenson R B, Anand R, Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc Natl Acad Sci USA. 1986;83:5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fultz P N, McClure H M, Anderson D C, Switzer W M. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/smm) AIDS Res Hum Retroviruses. 1989;5:397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- 12.Gummuluru S, Novembre F J, Seshi B, Dewhurst S. SIVsmmPBj14 induces expression of a mucosal integrin on macaque lymphocytes. Virology. 1996;215:97–100. doi: 10.1006/viro.1996.0010. [DOI] [PubMed] [Google Scholar]
- 13.Gummuluru S, Novembre F J, Lewis M, Gelbard H A, Dewhurst S. Apoptosis correlates with immune activation in intestinal lymphoid tissue from macaques acutely infected by a highly enteropathic simian immunodeficiency virus, SIVsmmPBj14. Virology. 1996;225:21–32. doi: 10.1006/viro.1996.0571. [DOI] [PubMed] [Google Scholar]
- 14.Halloran P F, Kung L, Noujaim J. Calcineurin and the biological effect of cyclosporine and tacrolimus. Transplant Proc. 1998;30:2167–2170. doi: 10.1016/s0041-1345(98)00577-6. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch V M, Dapolito G, Johnson P R, Elkins W R, London W T, Montali R J, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree G R. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 17.Hodge S, Novembre F J, Whetter L, Gelbard H A, Dewhurst S. Induction of Fas ligand expression by an acutely lethal simian immunodeficiency virus, SIVsmmPBj14. Virology. 1998;252:354–363. doi: 10.1006/viro.1998.9477. [DOI] [PubMed] [Google Scholar]
- 18.Israel Z, Dean G A, Maul D H, O’Neil S P, Dreitz M J, Mullins J I, Fultz P N, Hoover E A. Early pathogenesis of disease caused by SIVsmmPBj14 molecular clone 1.9 in macaques. AIDS Res Hum Retroviruses. 1993;9:277–285. doi: 10.1089/aid.1993.9.277. [DOI] [PubMed] [Google Scholar]
- 19.Kent K A, Rud E, Corcoran C, Powell C, Thiriart C, Collignon C, Stott E J. Identification of two neutralizing and 8 non-neutralizing epitopes on simian immunodeficiency virus envelope using monclonal antibodies. AIDS Res Hum Retroviruses. 1992;8:1147–1151. doi: 10.1089/aid.1992.8.1147. [DOI] [PubMed] [Google Scholar]
- 20.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Wasdron L M, Mackey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 21.Lewis M G, Yalley-Ogunro J, Greenhouse J J, Brennan T P, Jiang J B, VanCott T C, Lu Y, Eddy G A, Birx D L. Limited protection from a pathogenic chimeric simian-human immunodeficiency virus challenge following immunization with attenuated simian immunodeficiency virus. J Virol. 1999;73:1262–1270. doi: 10.1128/jvi.73.2.1262-1270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lori F, Gallo R C, Malykh A, Cara A, Romano J, Markham P, Franchini G. Didanosine but not high doses of hydroxyurea rescue pigtail macaque from a lethal dose of SIVsmmPBj14. AIDS Res Hum Retroviruses. 1997;13:1083–1088. doi: 10.1089/aid.1997.13.1083. [DOI] [PubMed] [Google Scholar]
- 23.Marthas M L, Sutjipto S, Higgins J, Lohman B, Torten J, Luciw P A, Marx P A, Pedersen N C. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990;64:3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClure H M, Anderson D C, Fultz P N, Ansari A A, Lockwood E, Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet Immunol Immunopathol. 1989;21:13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- 25.Mossman S P, Bex F, Berglund P, Arthos J, O’Neil S P, Riley D, Maul D H, Bruck C, Momin P, Burny A, Fultz P N, Mullins J I, Liljeström P, Hoover E. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J Virol. 1996;70:1953–1960. doi: 10.1128/jvi.70.3.1953-1960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphey-Corb M, Martin L N, Rangan S R S, Baskin G B, Gormus B J, Wolf R H, Andes W A, West M A, Montelaro R C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- 26a.National Research Council . Guide to the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 27.Novembre F J, Johnson P R, Lewis M G, Anderson D C, Klumpp S, McClure H M, Hirsch V M. Multiple viral determinants contribute to pathogenicity of the acutely lethal simian immunodeficiency virus SIVsmmPBj variant. J Virol. 1993;67:2466–2474. doi: 10.1128/jvi.67.5.2466-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novembre F J, Saucier M M, Hirsch V M, Johnson P R, McClure H M. Viral genetic determinants in SIVsmmPBj pathogenesis. J Med Primatol. 1994;23:136–145. doi: 10.1111/j.1600-0684.1994.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 29.Novembre F J, DeRosayro J, O’Neil S P, Anderson D C, Klumpp S A, McClure H M. Isolation and characterization of a neuropathogenic simian immunodeficiency virus derived from a sooty mangabey. J Virol. 1998;72:8841–8851. doi: 10.1128/jvi.72.11.8841-8851.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.O’Neil, S. P. Personal communication.
- 30.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S J, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, et al. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg Y J, Lewis M G, Villinger F, Ansari A A. Cytokines and simian immunodeficiency virus infections. Res Immunol. 1994;145:706–712. doi: 10.1016/s0923-2494(05)80059-8. [DOI] [PubMed] [Google Scholar]
- 32.Rud E W, Cranage M, Yon J, Quirk J, Ogilvie L, Cook N, Webster S, Dennis M, Clarke B E. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J Gen Virol. 1994;75:529–543. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- 33.Saucier M, Hodge S, Dewhurst S, Gibson T, Gibson J P, McClure H M, Novembre F J. The tyrosine-17 residue of Nef in SIVsmmPBj14 is required for acute pathogenesis and contributes to replication in macrophages. Virology. 1998;244:261–272. doi: 10.1006/viro.1998.9133. [DOI] [PubMed] [Google Scholar]
- 34.Schwiebert R, Fultz P N. Immune activation and viral burden in acute disease induced by simian immunodeficiency virus SIVsmmPBj14: correlation between in vitro and in vivo events. J Virol. 1994;68:5538–5547. doi: 10.1128/jvi.68.9.5538-5547.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai C-C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 36.Tsai C-C, Emau P, Follis K E, Beck T W, Benveniste R E, Bischofberger N, Lifson J D, Morton W R. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl)adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rompay K K A, Cherrington J M, Marthas M L, Berardi C J, Mulato A S, Spinner A, Tarara R P, Canfield D R, Telm S, Bischofberger N, Pedersen N C. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rompay K K A, Dailey P J, Tarara R P, Canfield D R, Aguirre N L, Cherrington J M, Lamy P D, Bischofberger N, Pedersen N C, Marthas M L. Early short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine treatment favorably alters the subsequent disease course in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1999;73:2947–2955. doi: 10.1128/jvi.73.4.2947-2955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villinger F, Hunt D, Mayne A, Vuchetich M, Findley H, Ansari A A. Qualitative and quantitative studies of cytokines synthesized and secreted by non-human primate peripheral blood mononuclear cells. Cytokine. 1993;5:469–479. doi: 10.1016/1043-4666(93)90038-7. [DOI] [PubMed] [Google Scholar]
- 40.Whetter L, Novembre F J, Saucier M, Gummuluru S, Dewhurst S. Costimulatory pathways in lymphocyte proliferation induced by the simian immunodeficiency virus SIVsmmPBj14. J Virol. 1998;72:6155–6158. doi: 10.1128/jvi.72.7.6155-6158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X-N, Screaton G R, Gotch F M, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott E J, McMichael A J. Evasion of cytotoxic T lymphocyte (CTL) responses by Nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]