Abstract
Background:
A U.S. case-control study (2010–2014) demonstrated vaccine effectiveness (VE) for ≥ 1 dose of the thirteen-valent pneumococcal conjugate vaccine (PCV13) against vaccine-type (VT) invasive pneumococcal disease (IPD) at 86 %; however, it lacked statistical power to examine VE by number of doses and against individual serotypes.
Methods:
We used the indirect cohort method to estimate PCV13 VE against VT-IPD among children aged < 5 years in the United States from May 1, 2010 through December 31, 2019 using cases from CDC’s Active Bacterial Core surveillance, including cases enrolled in a matched case-control study (2010–2014). Cases and controls were defined as individuals with VT-IPD and non-PCV13-type-IPD (NVT-IPD), respectively. We estimated absolute VE using the adjusted odds ratio of prior PCV13 receipt (1-aOR × 100 %).
Results:
Among 1,161 IPD cases, 223 (19.2 %) were VT cases and 938 (80.8 %) were NVT controls. Of those, 108 cases (48.4 %; 108/223) and 600 controls (64.0 %; 600/938) had received ≥ 3 PCV13 doses; 23 cases (17.6 %) and 15 controls (2.4 %) had received no PCV doses. VE ≥ 3 PCV13 doses against VT-IPD was 90.2 % (95 % Confidence Interval75.4–96.1 %), respectively. Among the most commonly circulating VT-IPD serotypes, VE of ≥ 3 PCV13 doses was 86.8 % (73.7–93.3 %), 50.2 % (28.4–80.5 %), and 93.8 % (69.8–98.8 %) against serotypes 19A, 3, and 19F, respectively.
Conclusions:
At least three doses of PCV13 continue to be effective in preventing VT-IPD among children aged < 5 years in the US. PCV13 was protective against serotypes 19A and 19F IPD; protection against serotype 3 IPD did not reach statistical significance.
Keywords: 13-valent pneumococcal conjugate vaccine, Invasive pneumococcal disease, Pneumococcal infections, Streptococcus pneumoniae, Vaccine effectiveness, Indirect cohort
1. Background
Streptococcus pneumoniae (pneumococcus) is a major cause of morbidity and mortality among children globally [1]. Prior to the introduction of the seven-valent pneumococcal conjugate vaccine (PCV7) in 2000 in the United States, the incidence of invasive pneumococcal disease (IPD) among U.S. children aged < 5 years was 98.7 per 100,000 cases [2]. Implementation of PCV7 was associated with reductions in the burden of vaccine-type IPD (VT-IPD) followed by increases in disease caused by serotype 19A and other non-PCV7 serotypes [3,4]. In 2010, the 13-valent pneumococcal conjugate vaccine (PCV13) replaced PCV7 in the routine childhood immunization schedule, and a supplemental dose of PCV13 was recommended for children aged 14–59 months who had received four doses of PCV7 [5]. In 2018–2019, the incidence of IPD among children aged < 5 years in the United States was 7.2 cases per 100,000 [6].
PCV13 was licensed using safety and immunogenicity endpoints only; thus, post-licensure vaccine effectiveness (VE) studies are critical to generate estimates of protection conferred by PCV13 against IPD and monitor vaccine protection under real-world conditions of use [7]. A post-licensure matched case-control study conducted in the United States that enrolled IPD cases and population-based healthy controls, estimated that the VE of ≥ 1 doses of PCV13 against IPD caused by PCV13-type serotypes was 85 % [8]. However, this study was conducted shortly after PCV13 introduction when many children only received one catch-up PCV13 dose as opposed to the recommended four-dose series of PCV13 (3 + 1): three primary doses before 12 months of age followed by a booster dose at 12–15 months [9]. Additionally, protection conferred from PCV13 differs by serotype, and may be lower for serotypes 3 and 19A, although the reported estimates have varied [8,10–12]. Serotype 3 has a high invasive capacity and propensity to cause severe infections; therefore, real-world serotype 3 vaccine effectiveness data remains important to monitor for establishing PCV13 impact on invasive disease [13].
Using additional years of data available post-PCV13 introduction in U.S. children, we sought to estimate effectiveness of the protection conferred by ≥ 3 doses of PCV13 against IPD caused by VT serotypes among children aged < 5 years in the United States. We additionally assessed VE by PCV13 dosing schedule and individual serotype among the most common circulating VT serotypes.
2. Methods
2.1. Data sources
We assessed PCV13 effectiveness against IPD utilizing serotyped IPD cases from Active Bacterial Core surveillance (ABCs) at the Centers for Disease Control and Prevention (CDC). ABCs is an active laboratory- and population-based surveillance system of 10 sites in the United States, either entire states or metropolitan regions [14]. Approximately 45 million residents (14 % of the U.S. population) are under surveillance. We defined cases of IPD as any isolate of S. pneumoniae from a normally sterile site (e.g., blood, cerebrospinal fluid) among residents of the catchment area. During May 1, 2010 – May 31, 2014, surveillance was expanded beyond the ABCs catchment area to include additional sites for the purpose of achieving adequate sample size for the previously published PCV13 case-control evaluation [8]. These areas included New York City, Los Angeles, the state of Utah, and expanded areas in New York State (outside of New York City, beyond the routine ABCs catchment), and Tennessee. During June 1, 2014 through December 31, 2019, all IPD cases were identified via routine IPD surveillance through ABCs.
2.2. Study design and inclusion criteria
We estimated VE using the indirect-cohort method, in which we compare vaccination status of IPD caused by PCV13 (VT) serotypes (cases) to that of IPD caused by non-PCV13 vaccine type (NVT) serotypes (controls). [15]. Children with IPD residing within ABCs surveillance areas aged 2–59 months on the date of pneumococcal culture, with a serotyped pneumococcal isolate and verified pneumococcal vaccination history were eligible for inclusion in the analysis. Pneumococcal isolates were serotyped at the Minnesota Department of Health or CDC Streptococcus laboratory by Quellung reaction, polymerase chain reaction, or whole genome sequencing (2015–2019) [16]. The primary advantage of the indirect-cohort method over other observational study designs (eg. case-control) is by eliminating the need to enroll a separate control population. Instead, the study can be conducted entirely using IPD cases ascertained through routine surveillance, so long as vaccination history is routinely collected and all IPD cases are serotyped. The indirect cohort method provides reliable estimates of VE under the assumption that implementation of the vaccine product does not influence the risk of NVT-IPD [10,15,17].
2.3. Outcomes
The primary outcome was IPD caused by serotypes included in PCV13 (4, 6B, 9 V, 14, 18C, 19F, 23F, 1, 3, 5, 6A, 7F, 19A, and 6C). Due to known cross-protection with 6A antigen, serotype 6C was considered a PCV13 serotype [18]. All other serotypes, including non-typeable pneumococcal cases, were defined as NVT controls. Secondary analyses estimated serotype-specific VE among the most common VT serotypes.
2.4. Exposures
The primary exposure was receipt of ≥ 3 doses of PCV13 given at or after 2 months of age regardless of prior PCV7 receipt compared to receipt of no pneumococcal vaccines among children age-eligible to have received three doses (≥12 months). Persons who received only PCV7 doses were excluded from the primary analysis (Table S1). Exposure to ≥ 1 PCV13 doses, regardless of prior PCV7 receipt, which was assessed in the previous case-control study, was also evaluated. Secondary exposures included PCV13 receipt as categorized by various dosing schedules. Pneumococcal doses given at least 4 weeks after the previous dose of PCV7 or PCV13, and at least 2 weeks before the culture date to allow sufficient time for an immunologic response, were considered valid. For analyses of VE by pneumococcal dosing schedule, doses received before 12 months of age were considered primary series, and any doses received at 12–59 months of age were considered booster doses.
During May 1, 2010 – May 31, 2014, pneumococcal vaccination history was ascertained as part of the previously published case-control study described elsewhere [8]. In brief, study personnel determined pneumococcal vaccination history by referencing written vaccination records and state immunization registries and contacting providers from whom the child may have received vaccinations.
For June 1, 2014 onward, ABCs personnel obtained pneumococcal vaccination history through medical chart review. If vaccination data were unavailable from medical chart review, sites were instructed to attempt up to 10 calls per case to verify pneumococcal vaccination records. If the caregiver or guardian of the case-patient did not answer the phone or could not provide vaccination data, study personnel attempted to contact the primary care provider. Study personnel indicated which source of vaccination data was used to obtain vaccination history (i.e.., medical chart review, registry, or primary care provider). Participants were excluded from the analysis if no source was available to obtain vaccination history.
Following methods established in the previously published case-control study, any PCV dose given prior to March 15, 2010 was assumed to be PCV7. Doses received on or after January 1, 2011 were considered to be PCV13. For doses received during March 15, 2010 through December 31, 2010, lot numbers were referenced to differentiate between doses of PCV7 and PCV13 [8].
2.5. Statistical analysis
We used a logistic regression model to estimate the adjusted odds ratio (aOR) for receipt of ≥ 1 or ≥ 3 doses of PCV13 versus no PCV doses comparing VT-cases and NVT-controls. VE was estimated as (1 – aOR) × 100 %. We adjusted models for potential confounders identified using a directed acyclic graph including age group, race/ethnicity, sex, state, calendar year, report of any immunocompromising conditions, and report of any chronic conditions including asthma. Models were stratified by time period (May 1, 2010 – May 31, 2014 [Early PCV13 implementation period]; June 1, 2014 – December 31, 2019 [Late PCV13 implementation period]; and May 1, 2010 – Dec 31, 2019 [Full study period]).
We conducted a sensitivity analysis in which IPD cases caused by vaccine-related (VR) serotypes were excluded from the primary analysis; VR serotypes included 23B, 9 N, 23A, 6C, 7A, 7B, 7C, 9A, 9L, 18F [11]. To address the possibility of bias arising from inclusion of immunocompromised children, for whom pneumococcal vaccination recommendations differ, we also conducted a sensitivity analysis excluding immunocompromised children [9].
For the full study period (May 1, 2010 – Dec 31, 2019) we estimated VE by PCV13 administration schedule: 1 + 0 (one PCV13 dose received prior to 12 months of age), 2 + 0 (two PCV13 doses received prior to 12 months of age), 3 + 0 (three PCV13 doses received prior to 12 months of age), 2 + 1 (two PCV13 doses received prior to 12 months of age and one PCV13 dose after 12 months of age), and 3 + 1 (three PCV13 doses received prior to 12 months of age and one PCV13 dose after 12 months of age). To estimate VE by schedule of PCV13 receipt, each model was restricted to the sub-set of the study participants who were age-eligible to receive a given schedule. Therefore, for the assessment of the 1 + 0, 2 + 0, and 3 + 0 schedule, models were restricted to participants, 2, 4, and 6 months of age, respectively, corresponding to U.S. dosing schedules. For the assessment of the 2 + 1 and 3 + 1 schedules, models were restricted to participants 12 months or older.
For the assessment of serotype-specific VE among serotypes unique to PCV13 (19A, 3, 7F), participants were considered vaccinated with PCV13 if they had received ≥ 1 or ≥ 3 doses of PCV13, regardless of prior PCV7 receipt. Given that PCV7 should not provide protection against PCV13 unique serotypes, for this secondary analysis we allowed non-PCV13 unique serotype cases who had received PCV7 to be included in the reference group for the analysis of serotype 19A, 3, and 7F VE.
All analyses were conducted in R Statistical Software (version 4.2.1). This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy.1
3. Results
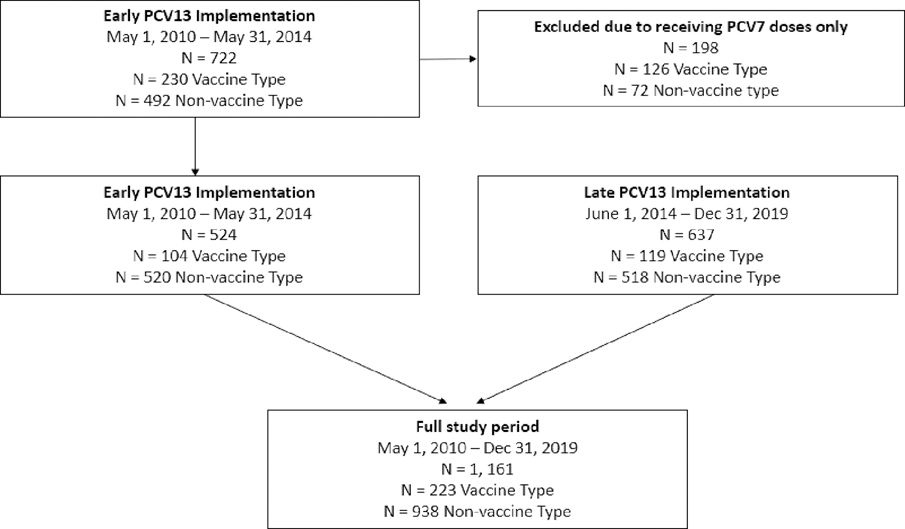
The study included 1,161 children aged 2–59 months with IPD, including 524 children enrolled from a previous post-licensure case-control study of PCV13 VE conducted during the early PCV13 implementation period and 637 children identified through routine surveillance during the late PCV13 implementation period; 61 were excluded because vaccination history could not be verified (Fig. 1; Figure S1). For the full study period, there were 223 cases of IPD caused by PCV13 VT serotypes (VT-cases), and 938 cases of IPD caused by NVT serotypes (NVT-controls) (Table 1; Figure S2).
Fig. 1.
Flow diagram of children included in the analytic dataset1. 1Cases for the late PCV13 Implementation period were enrolled from Active Bacterial Core surveillance (ABCs). Inclusion/exclusion of these cases are outlined in Figure S1. Cases from the early PCV13 Implementation period were included from a previously published case-control study.
Table 1.
Characteristics of study participants by VT (IPD caused by serotypes included in PCV13) and NVT IPD (IPD caused by non-PCV13 serotype) stratified by study period.
| Full study period | Early PCV13 implementation | Late PCV13 implementation | ||||
|---|---|---|---|---|---|---|
|
|
|
|
|
|||
| May 1, 2010 - Dec 31 2019 | May 1, 2010 - May 31 2014 | June 1, 2014 - Dec 31 2019 | ||||
|
|
|
|
|
|||
| VT | NVT | VT | NVT | VT | NVT | |
|
|
|
|
|
|
|
|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
|
|
|
|
|
|
|
|
| N = 223 | N = 938 | N = 104 | N = 420 | N = 119 | N = 518 | |
|
| ||||||
| Age group | ||||||
| 2–6 months | 44 (19.7) | 153 (16.3) | 26 (25.0) | 68 (16.2) | 18 (15.1) | 85 (16.4) |
| 7–11 months | 26 (11.7) | 166 (17.7) | 15 (14.4) | 80 (19.0) | 11 (9.2) | 86 (16.6) |
| 12–23 months | 49 (22.0) | 292 (31.1) | 23 (22.1) | 139 (33.1) | 26 (21.8) | 153 (29.5) |
| 24–59 months | 104 (46.6) | 327 (34.9) | 40 (38.5) | 133 (31.7) | 64 (53.8) | 194 (37.5) |
| Sex | ||||||
| Male | 133 (59.6) | 544 (58.0) | 56 (53.8) | 233 (55.5) | 77 (64.7) | 311 (60.0) |
| Female | 90 (40.4) | 394 (42.0) | 48 (46.2) | 187 (44.5) | 42 (35.3) | 207 (40.0) |
| Race and ethnicity | ||||||
| White, non-Hispanic | 89 (39.9) | 418 (44.6) | 35 (33.7) | 175 (41.7) | 54 (45.4) | 243 (46.9) |
| Black, non-Hispanic | 58 (26.0) | 274 (29.2) | 21 (20.2) | 118 (28.1) | 37 (31.1) | 156 (30.1) |
| American Indian/ Alaska Native, non-Hispanic | 7 (3.1) | 16 (1.7) | 3 (2.9) | 9 (2.1) | 4 (3.4) | 7 (1.4) |
| Asian/Pacific Islander, non-Hispanic | 11 (4.9) | 53 (5.7) | 5 (4.8) | 25 (6.0) | 6 (5.0) | 28 (5.4) |
| Unknown race, non-Hispanic | 2 (0.9) | 4 (0.4) | 2 (1.9) | 4 (1.0) | 3 (2.5) | 11 (2.1) |
| Hispanic | 4 (1.8) | 14 (1.5) | 1 (1.0) | 3 (0.7) | 15 (12.6) | 73 (14.1) |
| Calendar year | ||||||
| 2010 | 16 (7.2) | 28 (3.0) | 16 (15.4) | 28 (6.7) | - - | - - |
| 2011 | 23 (10.3) | 111 (11.8) | 23 (22.1) | 111 (26.4) | - - | - - |
| 2012 | 22 (9.9) | 99 (10.6) | 22 (21.2) | 99 (23.6) | - - | - - |
| 2013 | 26 (11.7) | 128 (13.6) | 26 (25.0) | 128 (30.5) | - - | - - |
| 2014 | 29 (13.0) | 113 (12.0) | 17 (16.3) | 54 (12.9) | 12 (10.1) | 59 (11.4) |
| 2015 | 25 (11.2) | 102 (10.9) | - - | - - | 25 (21.0) | 102 (19.7) |
| 2016 | 23 (10.3) | 103 (11.0) | - - | - - | 23 (19.3) | 103 (19.9) |
| 2017 | 23 (10.3) | 79 (8.4) | - - | - - | 23 (19.3) | 79 (15.3) |
| 2018 | 21 (9.4) | 89 (9.5) | - - | - - | 21 (17.6) | 89 (17.2) |
| 2019 | 15 (6.7) | 86 (9.2) | - - | - - | 15 (12.6) | 86 (16.6) |
| Underlying conditions | ||||||
| Chronic conditions1 | 17 (8.5) | 85 (10.1) | 8 (7.7) | 38 (9.0) | 9 (9.5) | 47 (11.2) |
| Immunocompromising conditions 2 | 7 (3.5) | 64 (7.7) | 2 (1.9) | 19 (4.5) | 5 (5.3) | 45 (10.9) |
| Asthma | 28 (13.5) | 121 (14.2) | 20 (19.2) | 81 (19.3) | 8 (7.7) | 40 (9.2) |
| PCV history3 | ||||||
| No doses of any PCV4 | 47 (21.1) | 53 (5.7) | 18 (17.3) | 21 (5.0) | 29 (24.4) | 32 (6.2) |
| ≥1 dose PCV13 | 176 (78.9) | 885 (94.3) | 86 (82.7) | 399 (95.0) | 90 (75.6) | 486 (93.8) |
| ≥1 dose PCV13, 0 PCV7 doses | 145 (82.4) | 768 (86.8) | 56 (65.1) | 283 (70.9) | 89 (98.9) | 485 (99.8) |
| ≥1 dose PCV13 + ≥1 PCV7 doses | 31 (17.6) | 117 (13.2) | 30 (34.9) | 116 (29.1) | 1 (1.1) | 1 (0.2) |
| ≥3 doses PCV13 | 108 (48.4) | 600 (64.0) | 35 (33.7) | 223 (53.1) | 73 (61.3) | 377 (72.8) |
| ≥3 doses PCV13, 0 PCV7 doses | 104 (96.3) | 589 (98.2) | 31 (88.6) | 212 (95.1) | 73 (100.0) | 377 (100.0) |
| ≥3 doses PCV13 + ≥1 PCV7 doses | 4 (3.7) | 11 (1.8) | 4 (11.4) | 11 (4.9) | 0 (0) | 0 (0) |
| PCV13 vaccination schedule Infant schedule5 | ||||||
| 1 + 0: One dose < 12 mo | 27 (12.1) | 86 (9.2) | 18 (17.3) | 30 (7.1) | 9 (7.6) | 56 (10.8) |
| 2 + 0: Two doses < 12 mo | 13 (5.8) | 77 (8.2) | 7 (6.7) | 36 (8.6) | 6 (5.0) | 41 (7.9) |
| 3 + 0: Three doses < 12 mo | 24 (10.8) | 217 (23.1) | 13 (12.5) | 100 (23.8) | 11 (9.2) | 117 (22.6) |
| 2 + 1: Two dose < 12 + 1 dose ≥ 12 mo | 4 (1.8) | 19 (2.0) | 0 (0.0) | 2 (0.5) | 4 (3.4) | 17 (3.3) |
| 3 + 1: Three doses < 12 + 1 dose ≥ 12 mo | 73 (32.7) | 346 (36.9) | 18 (17.3) | 108 (25.7) | 55 (46.2) | 238 (45.9) |
| Toddler doses (≥12 mo)6 | 0 (0.0) | 12 (1.2) | 0 (0.0) | 4 (1.0) | 0 (0.0) | 8 (1.8) |
Abbreviations: VE = Vaccine Effectiveness; PCV = pneumococcal conjugate vaccine; PCV13- 13-valent pneumococcal conjugate vaccine; VT = PCV13 vaccine type serotypes, including serotype 6C due to cross protection with 6A; NVT = non-vaccine type, serotypes not included in PCV123.
Chronic conditions were defined a priori and include: Congestive heart failure (chronic heart disease), Cardiomyopathies (chronic heart disease), Chronic liver disease, Chronic obstructive pulmonary disease (chronic lung disease), Emphysema (chronic lung disease). Proportions are calculated out of the total number of individuals who are not missing data for chronic, immunocompromising or asthma.
Immunocompromising conditions were defined a priori and include: Congenital or acquired asplenia, Sickle cell disease or other hemoglobinopathies, Chronic renal failure, Congenital or acquired immunodeficiencies, Generalized malignancy, HIV infection, Hodgkin disease, iatrogenic immunosuppression, Leukemia, Lymphoma, Multiple myeloma, Nephrotic syndrome, Solid organ transplant.
The primary exposure is receipt of at least one dose of PCV13 given after 6 weeks of age, at least 4 weeks after the previous dose of PCV7 or PCV13 (if ≥ 1 dose given), and at least 2 weeks before the culture date, versus receipt of no pneumococcal vaccines. Participants who received PCV7 doses are excluded from the primary analysis. Characteristics of the study population including participants who received PCV7 only are outlined in Table S2.
The distribution of age among children receiving zero PCV doses (N = 100) was N = 46, 2–6 months; N = 16, 7–11 months; N = 22, 12–23 months; N = 16; 25–59 months. N = 63 of the children were male. N = 51 children were white, non-Hispanic; N = 26 black, non-Hispanic; N = 16 % Hispanic; N = 7 other race/ethnicity. The proportion of participants with no doses by study year are summarized in Table S5.
Counts may not sum to total (N = 1161) because some children did not receive their doses of PCV13 in any of these schedules and are excluded (N = 263). Participants who received PCV7 were excluded from this analysis (Table S1). Of note, the median days between a participant’s fourth dose of PCV13 and their pneumococcal culture during the early and late PCV13 implementation period was 245 (range: 15–1132) and 605 (range: 14–1436), respectively.
Represents children who received one or more doses of PCV13 at 12 months of age or older and no prior doses of PCV7. Among the N = 12 participants, 4 received one toddler dose and 8 received 2 toddler doses.
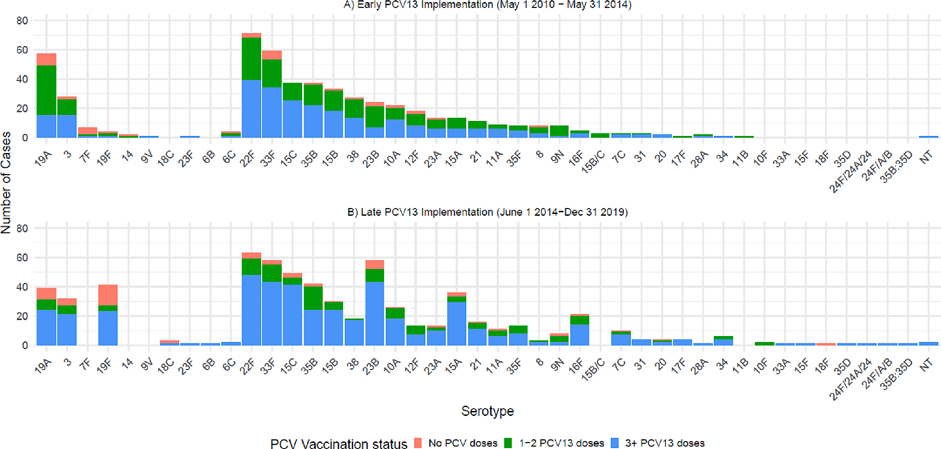
During the full study period, three VT serotypes (19A, 3, and 19F) were responsible for 90.1 % (201/223) of VT-IPD; serotype 19A was the most common VT-serotype causing IPD, responsible for 43.0 % (96/223) of VT-IPD throughout the study period, followed by serotype 3 and serotype 19F (Figure S2, Table S2). Whereas serotype 19F was responsible for 3.8 % during the early implementation period, 19F was responsible for 34.4 % of cases of VT-IPD during the late PCV13 implementation period (Fig. 2). The majority of NVT disease was caused by serotypes 22F and 33F (Figure S2; Table S3).
Fig. 2.
Cases included in analysis stratified by serotype and receipt of PCV13.
Among the 1,161 participants included, 176 VT-cases (78.9 %; 176/223) and 885 NVT-controls (94.3 %; 885/938) had received ≥ 1 dose of PCV13 regardless of prior PCV7 receipt; 47 VT-cases (21.1 %; 47/223) and 53 NVT-controls (5.7 %; 53/938) had received no PCV doses (Table 2; Table S4). For the primary analysis assessing VE of ≥ 3 PCV13 doses, we excluded 353 participants (68 VT cases and 285 NVT-controls) who received 1 or 2 doses of PCV13. Therefore, among the 808 participants included in the primary analysis (297 during the early PCV13 implementation period and 511 during the late PCV13 implementation period), 108 VT-cases and 600 NVT-controls had received ≥ 3 doses of PCV13 (Table 2).
Table 2.
Vaccine effectiveness by study period for the outcome vaccine-type IPD.
| Exposure1 | PCV13-type Case | Non-PCV13-type Control | Unadjusted | Adjusted2 | |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| N (%) | N (%) | VE (95 % CI) | VE (95 % CI) | ||
|
| |||||
| Early PCV13 Implementation3 (May 2010 - May 2014) | No doses of PCV | 18 (17.3) | 21 (5.0) | ref. | ref. |
| ≥1 dose PCV13 | 86 (82.7) | 399 (95.0) | 74.9 (51.0,87.2) | 69.7 (33.0, 86.2) | |
| No doses of PCV | 7 (16.7) | 3 (1.3) | ref. | ref. | |
| ≥3 doses PCV13 | 35 (83.3) | 223 (98.7) | 93.3 (72.9,98.40) | 86.5 (28.0,97.3) | |
| Late PCV13 Implementation (June 2014 - Dec 2019) | No doses of PCV | 29 (24.4) | 32 (6.2) | ref. | ref. |
| ≥1 dose PCV13 | 90 (75.6) | 486 (93.8) | 79.6 (64.7,88.3) | 89.6 (77.4, 95.3) | |
| No doses of PCV | 16 (18.0) | 12 (3.1) | ref. | ref. | |
| ≥3 doses PCV13 | 73 (82.0) | 377 (96.9) | 85.5 (68.2,93.4) | 90.5 (68.1, 97.2) | |
| Full Study Period (May 2010- Dec 2019)4 | No doses of PCV | 47 (21.1) | 53 (5.7) | ref. | ref. |
| ≥1 dose PCV13 | 176 (78.9) | 885 (94.3) | 77.6 (65.8,85.4) | 81.7 (69.1, 89.1) | |
| No doses of PCV | 23 (17.6) | 15 (2.4) | ref. | ref. | |
| ≥3 doses PCV13 | 108 (82.4) | 600 (97.6) | 88.3 (76.9, 94.1) | 90.2 (75.4, 96.1) | |
| 1 + 0: One dose before 12 mo | No doses of PCV | 47 (63.5) | 53 (38.1) | ref. | ref. |
| One dose < 12 mo | 27 (36.5) | 86 (61.9) | 64.6 (37.0,80.4) | 58.3 (9.7, 80.3) | |
| 2 + 0: Two doses before 12 mo | No doses of PCV | 32 (71.1) | 34 (30.6) | ref. | ref. |
| Two doses < 12 mo | 13 (28.9) | 77 (69.4) | 82.1 (61.9,91.7) | 58.2 (-35.1, 86.8) | |
| 3 + 0: Three doses before 12 mo | No doses of PCV | 29 (54.7) | 28 (11.5) | ref. | ref. |
| Three doses < 12 mo | 24 (45.3) | 216 (88.5) | 89.3 (79.2,94.5) | 87.6 (67.9, 95.2) | |
| 2 + 1: Two doses before 12 mo + 1 dose ≥12 mo | No doses of PCV | 23 (85.2) | 15 (44.1) | ref. | ref. |
| 2 + 1 doses | 4 (14.8) | 19 (55.9) | 86.3 (52.1,96.1 | 99.9 (78.2, 100.0) | |
| 3 + 1: Three doses before 12 mo + 1 dose ≥12 mo | No doses of PCV | 23 (24.0) | 15 (4.2) | ||
| 3 + 1 doses | 73 (76.0) | 346 (95.8) | 86.2 (72.5,93.2) | 92.4 (78.2, 97.2) | |
| 1 + 1: One dose before 12 mo + 1 dose ≥12 mo | No doses of PCV | 23 (95.8) | 15 (78.9) | DNC | DNC |
| 1 + 1 doses | 1 (4.2) | 4 (21.1) | |||
Abbreviations: VE = Vaccine Effectiveness; PCV = pneumococcal conjugate vaccine; PCV13- 13-valent pneumococcal conjugate vaccine; CI = confidence interval; ref. = reference level; DNC = Model did not converge.
For the period specific analyses, participants were categorized as having received PCV13, regardless of prior history of PCV7. Therefore, the “exposed” may include receipt of one or more doses of PCV13, regardless of prior history of PCV7. For the schedule specific estimates, participants who received PCV7 were excluded to estimate PCV13-schedule specific VE. We outline inclusion and exclusion criteria of PCV recipients in Table S1.
Logistic regression model adjusted for age group (categorical), race/ethnicity (combined variable), sex, state, calendar year, immunocompromising conditions, chronic conditions or asthma. VE for the full study period excluding children with at least one immunocompromising condition (as opposed to adjusting for immunocompromising conditions) was 81.6 % (68.3–89.1 %) for ≥ 1 doses and 88.0 (74.9–94.3 %) for ≥ 3 doses.
Exclusion of N = 68 vaccine-related serotypes (23B [N = 29], 9 N [N = 9], 23A [N = 15], 6C [N = 11], 7C [N = 4]) during this time period increased adjusted VE estimates for ≥ 1 dose(s) to 72.9 % (95 % CI: 37.5 – 88.3 %) and ≥ 3 dose(s) to 86.0 % (46.7–96.3 %).
Schedule specific estimates are conducted for the full study period (May 1, 2010 - Dec 31, 2019); for each schedule specific assessment stratified data sets are restricted to cases and controls who were age-eligible to have received each vaccination schedule. We conducted a sensitivity analysis limiting the population to children 2.5 months of age or older (N = 349 VT-cases; N = 1010 NVT-controls) and for the full study period estimated an unadjusted VE of ≥ 1 dose to be 76.4 % (61.6,85.6) and adjusted VE of ≥ 1 dose to be 79.6 % (63.2,88.7).
For the full study period, effectiveness against VT-IPD afforded by ≥ 3 doses of PCV13 versus no doses of any pneumococcal vaccine was estimated to be 90.2 % (95 % confidence interval: 75.4–96.1 %) (Table 2). Point estimates of PCV13 VE for ≥ 3 doses were higher in the late PCV13 implementation period (90.5 % [68.1–97.2 %]) as compared to the early PCV13 implementation period (86.5 % [28.0–97.3 %]); however, the confidence intervals overlapped. For the early and late PCV13 implementation period, upon exclusion of VR serotypes from the analysis, VE of ≥ 3 doses of PCV13 was estimated to be 90.6 % (32.5–98.6 %) and 88.0 (57.6–96.5 %), respectively. VE of ≥ 3 doses for the full study period excluding children with at least one immunocompromising condition was 88.0 % (74.9–94.3 %). VE for ≥ 1 dose for the full study period was 81.7 % (69.1–89.1 %). In a sensitivity analysis including participants who received 1–2 doses in a separate exposure category, VE estimates were similar (90.2 % [75.4–96.1 %]).
VE for a single primary dose of PCV13 (1 + 0), two primary doses of PCV13 (2 + 0), and three primary does of PCV13 (3 + 0) were 58.3 % (9.7–80.3 %), 58.2 % (−35.1–86.8 %), and 87.6 % (67.9–95.2 %), respectively (Table 2). VE among participants who received three primary doses and a booster dose (3 + 1 schedule) or two primary doses and a booster (2 + 1) was 92.4 % (78.2–97.2 %) and 99.9 % (78.2–100.0 %), respectively.
For the full study period, ≥3 doses of PCV13 were highly effective for serotype 19A (86.8 % [73.7–93.3 %]), serotype 7F (94.5 % [13.5–99.6 %]), and serotype 19F (93.8 % [69.8–98.8 %]) (Table 3). However, VE for ≥ 3 doses of PCV13 against serotype 3 had a lower point estimate and was not statistically significant: 50.2 % (− 28.4–80.5 %). Owing to limited number of cases during the early PCV13 implementation period VE of ≥ 1 PCV13 dose against serotype 19F could not be estimated; however, VE of ≥ 1 PCV13 dose against 19F during the late PCV13 implementation period was 94.1 % (80.1–98.2 %) (Table 4).
Table 3.
Serotype specific vaccine effectiveness against IPD for ≥ 3 doses of PCV13, May 1, 2010 - December 31, 2019.
| Serotype | Exposure1 | Serotype specific Cases | Non-PCV13-type Controls | Unadjusted | Adjusted2 |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| N (%) | N (%) | VE (95 % CI) | VE (95 % CI) | ||
|
| |||||
| Serotype 19A | |||||
| No doses of PCV13 | 80 (67.2) | 85 (12.4) | ref. | ref. | |
| ≥3 doses PCV13 | 39 (32.8) | 600 (87.6) | 93.1 (89.3,95.6) | 86.8 (73.7,93.3) | |
| Serotype 3 | |||||
| No doses of PCV13 | 18 (33.3) | 85 (12.4) | ref. | ref. | |
| ≥3 doses PCV13 | 36 (66.7) | 600 (87.6) | 71.7 (48.3,84.7) | 50.2 (−28.4,80.5) | |
| Serotype 7F | |||||
| No doses of PCV13 | 24 (96.0) | 85 (12.4) | ref. | ref. | |
| ≥3 doses PCV13 | 1 (4.0) | 600 (87.6) | 99.4 (95.6,99.9) | 94.5 (13.5,99.6) | |
| Serotype 19F | |||||
| No doses of PCV | 8 (25.0) | 15 (2.4) | ref. | ref. | |
| ≥3 doses PCV13 | 24 (75.0) | 600 (97.6) | 92.5 (80.8,97.1) | 93.8 (69.8,98.8) | |
Abbreviations: VE = Vaccine Effectiveness; PCV = pneumococcal conjugate vaccine; PCV13- 13-valent pneumococcal conjugate vaccine; CI = confidence interval; ref. = reference level.
For the assessment of Serotype 19A, 3 and 7F VE, participants who received either 7-valent pneumococcal conjugate vaccine (PCV7) or no doses of any pneumococcal vaccination were included in the reference group (No doses of PCV13). For the assessment of Serotype 19F VE, participants were included in the reference group if they received no doses of any PCV products. Persons who received PCV7 only were excluded from this analysis.
Logistic regression model adjusted for age (categorical), race/ethnicity (combined variable), sex, state, calendar year, immunocompromising conditions, chronic conditions or asthma.
Table 4.
Serotype specific vaccine effectiveness against IPD for ≥ 1 doses of PCV13, by study period.
| Exposure1 | Serotype specific Cases | Non-PCV13-type Controls | Unadjusted | Adjusted2 | ||
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
| N (%) | N (%) | VE (95 % CI) | VE (95 % CI) | |||
|
| ||||||
| Early PCV13 Implementation (May 1, 2010 - May 31, 2014) | ||||||
| Serotype 19A | No doses of PCV13 | 81 (62.3) | 93 (18.9) | ref. | ref. | |
| ≥1 dose PCV13 | 49 (37.7) | 399 (81.1) | 85.9 (78.7,90.8) | 76.5 (58.9, 86.5) | ||
| Serotype 3 | No doses of PCV13 | 17 (39.5) | 93 (18.9) | ref. | ref. | |
| ≥1 dose PCV13 | 26 (60.5) | 399 (81.1) | 64.4 (32.2,81.5) | 68.2 (19.6, 87.1) | ||
| Serotype 7F | No doses of PCV13 | 30 (93.8) | 93 (18.9) | ref. | ref. | |
| ≥1 dose PCV13 | 2 (6.2) | 399 (81.1) | 98.5 (93.4,99.6) | 98.2 (89.9, 99.7) | ||
| Late PCV13 Implementation (June 1, 2014 - December 31, 2019) | ||||||
| Serotype 19A | No doses of PCV13 | 8 (20.5) | 32 (6.2) | ref. | ref. | |
| ≥1 dose PCV13 | 31 (79.5) | 486 (93.8) | 74.5 (40.4,89.3) | 88.0 (58.2, 96.5) | ||
| Serotype 3 | No doses of PCV13 | 5 (15.6) | 32 (6.2) | ref. | ref. | |
| ≥1 dose PCV13 | 27 (84.4) | 486 (93.8) | 64.5 (2.4,87.3) | 83.5 (11.3, 96.9) | ||
| Serotype 19F | No doses of PCV | 14 (34.1) | 32 (6.2) | ref. | ref. | |
| ≥1 dose PCV13 | 27 (65.9) | 486 (93.8) | 87.3 (73.7,94.0) | 94.1 (80.1, 98.2) | ||
| Full study period (May 1, 2010 - December 31, 2019) | ||||||
| Serotype 19A | No doses of PCV13 | 89 (52.7) | 125 (12.4) | ref. | ref. | |
| ≥1 dose PCV13 | 80 (47.3) | 885 (87.6) | 87.3 (82.0,91.1) | 77.9 (65.6, 85.7) | ||
| Serotype 3 | No doses of PCV13 | 22 (29.3) | 125 (12.4) | ref. | ref. | |
| ≥1 dose PCV13 | 53 (70.7) | 885 (87.6) | 66.0 (42.6,80.1) | 58.4 (19.0, 79.0) | ||
| Serotype 7F | No doses of PCV13 | 30 (93.8) | 125 (12.4) | ref. | ref. | |
| ≥1 dose PCV13 | 2 (6.2) | 885 (87.6) | 99.1 (96.0,99.8) | 97.2 (86.6, 99.4) | ||
| Serotype 19F | No doses of PCV | 15 (33.3) | 53 (5.7) | ref. | ref. | |
| ≥1 dose PCV13 | 30 (66.7) | 885 (94.3) | 88.0 (76.6,94.0) | 92.7 (79.1, 97.3) | ||
Abbreviations: VE = Vaccine Effectiveness; PCV = pneumococcal conjugate vaccine; PCV13- 13-valent pneumococcal conjugate vaccine; CI = confidence interval; ref. = reference level.
For the assessment of Serotype 19A, 3 and 7F VE, participants who received either 7-valent pneumococcal conjugate vaccine (PCV7) or no doses of any pneumococcal vaccination were included in the reference group (No doses of PCV13). For the assessment of Serotype 19F VE, participants were included in the reference group if they received no doses of any PCV products. Persons who received PCV7 only were excluded from this analysis.
Logistic regression model adjusted for age (categorical), race/ethnicity (combined variable), sex, state, calendar year, immunocompromising conditions, chronic conditions or asthma.
4. Discussion
Receipt of ≥ 3 doses of PCV13 conferred strong protection against VT-IPD among U.S. children aged 2–59 months. This aligns with recent estimates of PCV13 effectiveness from a multi-center indirect cohort study conducted in Europe during a similar time period, 2012–2018 [11] and the previously published case-control study in the United States [8]. VE increased with increasing number of primary doses among schedules without a booster although the confidence intervals overlapped, and the effectiveness of schedules with a booster dose (2 + 1 and 3 + 1) were both > 90 %. During the full study period, three VT serotypes (19A, 3, and 19F) were responsible for the majority of VT-IPD; we estimated significant VE for serotypes 19A, 19F, and 7F, but VE was not significant for serotype 3. Non-PCV13 types 22F and 33F were the most common NVTs contributing to IPD, both of which are included in higher valency PCVs [6,19].
While this study was not adequately powered to address serotype-specific VE for all VT serotypes, we did not find evidence of significant PCV13 protection against serotype 3 disease, adding to the real-world evidence suggesting PCV13 may not provide adequate protection against disease caused by this serotype [20]. In clinical trials, the serotype 3 immune response was lower than the other additional five PCV13 serotypes (i.e., serotypes included in PCV13 but not PCV7) [21]. Serotype 3 has unique capsular properties compared to other pneumococcal serotypes including a thick capsule, high invasive capacity, and resistance to opsonic killing, all of which may contribute to lower VE [22–25]. As higher valency and PCVs using new or different technology are implemented in the United States, it will be important to continue to monitor and evaluate VE against serotype 3 [19].
Cases of serotype 19F, a serotype contained in both PCV7 and PCV13, were observed at a higher frequency during the later study period as compared to the early study period among both recipients and nonrecipients of PCV13. Comparative estimates of 19F VE by study period are not available owing to the small number of 19F cases during the early PCV13 implementation period; however, VE of ≥ 3 doses against 19F estimated from the current study for the full study period (93 %) was similar to estimates from a U.S.-based case-control study conducted during the PCV7 era (87 %), and a recent multi-center indirect cohort study in Europe (84 %) [3,11]. However, evidence of persistent 19F carriage among children throughout the PCV13 era has been well documented across various settings despite early reports from a trial indicating PCV13 reduced 19F carriage [26–28]. Additionally, some immunogenicity studies have demonstrated lower 19F immunogenicity following PCV13 than PCV7 after the primary infant doses [29,30]. These data indicate that PCV13 provides direct protection against serotype 19F disease. However, protection against carriage is less complete. Thus, indirect effects against serotype 19F IPD could have decreased post-PCV13 introduction compared with post-PCV7 introduction. If so, this could explain the increase in 19F disease during the late PCV13 implementation period, particularly among the unvaccinated.
The majority of children enrolled in this study received four doses of PCV13 in a 3 + 1 schedule, in accordance with U.S. recommendations [31]. As such, we were not able to evaluate VE for reduced dosing schedules (e.g., 1 + 1), which have garnered global interest particularly in countries with mature PCV programs [32]. VE increased in schedules without booster doses with increasing number of primary doses, although the confidence intervals overlapped likely due to the small number of children who received PCV13 based on 1 + 0 or 2 + 0 schedules. Larger studies are needed to evaluate the impact of VE by number of primary doses. However, growing evidence regarding the two World Health Organization-recommended PCV schedules (2 + 1 or 3 + 0) suggests that administration of the booster dose is what provides durable protection leading to stronger protection among the unvaccinated population [33]. Point estimates from our schedule-specific analysis suggest VE was > 90 % for both schedules with a booster dose (2 + 1, 3 + 1). Given that the United States is one of the few countries in the world that continues to recommend a 3 + 1 PCV schedule, additional evidence that directly compares VE across vaccine schedules would help inform the discussion on a possible transition to a reduced-dose PCV schedule in United States in the future.
VE estimates of ≥ 1 PCV13 dose produced for the early PCV13 implementation period from our study (69.7 % [33.0–86.2 %]) were not statistically different from estimates produced in a previous case-control study (86 % [75.5–92.3 %]) that enrolled matched, population-based controls [8]. Point estimates of VE from the indirect cohort study were lower than the case control study; this is consistent with the expectation that case-control studies may bias VE estimates due to differential healthcare seeking behavior between cases and population-based controls [34]. In other words, vaccination coverage among controls may not represent the source population if enrolled controls are more likely to seek vaccination than the source population, resulting in biased overestimates of VE. The indirect cohort method mitigates potential selection biases that may arise due to differences in the characteristics between cases and controls as both are identified using the same case definition [15,35].
This study has limitations. First, while we attempted to adjust for confounders that may bias the causal pathway between pneumococcal vaccination status and IPD using a multivariate model, the possibility of unmeasured confounding persists. Second, inclusion of cases with VR serotypes as NVT, for which PCVs may confer some protection, may result in underestimates of VE [11,36]. Indeed, sensitivity analyses which excluded VR serotypes resulted in higher point estimates of VE, though differences were not meaningfully different from models that included the VR serotypes. Additionally, the indirect cohort method relies on the assumption that implementation of vaccination does not influence the risk of NVT-IPD. However, given that the US has not observed significant serotype replacement following PCV13 introduction, this assumption is likely not violated [37]. Third, exposure misclassification is possible despite robust efforts by surveillance sites to obtain detailed vaccination history. Fourth, while this is one of the largest published studies of pediatric PCV13 vaccine effectiveness in the United States as of writing to our knowledge, owing to the success of PCV implementation, IPD case numbers were too small to detect significant effects for some stratified analyses. Lastly, we were unable to estimate effectiveness of alternative dosing schedules such as one primary dose with a booster dose (1 + 1) as the majority of vaccinated children had received a four-dose schedule, in accordance with U.S. recommendations [9].
In summary, ≥3 doses of PCV13 continues to be effective in preventing VT-IPD among children aged < 5 years in the United States, although protection against serotype 3 IPD was non-significant. Indirect cohort studies are an efficient platform in a setting with robust IPD surveillance to both estimate VE and mitigate potential bias from healthcare seeking behaviors influencing estimates from traditional case-control studies. As new PCVs become available for use in children, additional data are needed to monitor effectiveness including serotype-specific protection and differences by dosing schedule.
Supplementary Material
Acknowledgments
This work was funded by the Centers for Disease Control and Prevention.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Lee Harrison provides service on advisory boards (Pfizer, Sanofi, GSK) and a DSMB (Merck); while LH does not receive any fees for this work he is reimbursed for occasional travel. Tamara Pilishvili reports a relationship with GSK that includes: employment. During data collection, analysis, and write-up, TP was employed by CDC. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.].
CRediT authorship contribution statement
Kristin L. Andrejko: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Formal analysis, Data curation. Ryan Gierke: Writing – review & editing, Data curation. Jemma V. Rowlands: Writing – review & editing, Investigation. Jennifer B. Rosen: Writing – review & editing, Investigation. Ann Thomas: Writing – review & editing, Investigation. Zachary Q. Landis: Writing – review & editing, Investigation. Maria Rosales: Writing – review & editing, Investigation. Sue Petit: Writing – review & editing, Investigation. William Schaffner: Writing – review & editing, Investigation. Corinne Holtzman: Writing – review & editing, Investigation. Meghan Barnes: Writing – review & editing, Investigation. Monica M. Farley: Writing – review & editing, Investigation. Lee H. Harrison: Writing – review & editing, Investigation. Lesley McGee: Writing – review & editing, Data curation. Sopio Chochua: Writing – review & editing, Data curation. Jennifer R. Verani: Writing – review & editing, Methodology. Adam L. Cohen: Writing – review & editing, Methodology. Tamara Pilishvili: Writing – review & editing, Supervision, Methodology, Conceptualization. Miwako Kobayashi: Writing – review & editing, Supervision, Project administration, Methodology, Conceptualization.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2024.04.061.
See e.g., 45C.F.R. part 46.102(1)(2), 21C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Data availability
The authors do not have permission to share data.
References
- [1].O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009;374:893–902. [DOI] [PubMed] [Google Scholar]
- [2].Invasive Pneumococcal Disease in Children 5 Years After Conjugate Vaccine Introduction — Eight States, 1998–2005. MMWR 2008; 57:144–148. [PubMed] [Google Scholar]
- [3].Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006;368:1495–502. [DOI] [PubMed] [Google Scholar]
- [4].Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201:32–41. [DOI] [PubMed] [Google Scholar]
- [5].Licensure of a 13-Valent Pneumococcal Conjugate Vaccine (PCV13) and Recommendations for Use Among Children — Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morbidity and Mortality Weekly Report 2010; 59:258–261. [PubMed] [Google Scholar]
- [6].Kobayashi M Use of 15-Valent Pneumococcal Conjugate Vaccine Among U.S. Children: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep 2022; 71. Available at: https://www.cdc.gov/mmwr/volumes/71/wr/mm7137a3.htm. Accessed 23 January 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].US Food and Drug Administration. Prevnar 13: clinical review of new product license application. 2010. Available at: https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-13.
- [8].Moore MR, Link-Gelles R, Schaffner W, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med 2016;4:399–406. [DOI] [PubMed] [Google Scholar]
- [9].Nuorti JP, Whitney CG. Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010;59:1–18. [PubMed] [Google Scholar]
- [10].Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014;14:839–46. [DOI] [PubMed] [Google Scholar]
- [11].Savulescu C, Krizova P, Valentiner-Branth P, et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine 2022;40:3963–74. [DOI] [PubMed] [Google Scholar]
- [12].Ricketson LJ, Bettinger JA, Sadarangani M, Halperin SA, Kellner JD. Vaccine effectiveness of the 7-valent and 13-valent pneumococcal conjugate vaccines in Canada: An IMPACT study. Vaccine 2022;40:2733–40. [DOI] [PubMed] [Google Scholar]
- [13].Yildirim I, Hanage WP, Lipsitch M, et al. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine 2010;29:283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schuchat A, Hilger T, Zell E, et al. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis 2001;7:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med 1980;303:549–52. [DOI] [PubMed] [Google Scholar]
- [16].Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect 2020;26:512.e1–512.e10. [DOI] [PubMed] [Google Scholar]
- [17].De Serres G, Pilishvili T, Link-Gelles R, et al. Use of surveillance data to estimate the effectiveness of the 7-valent conjugate pneumococcal vaccine in children less than 5 years of age over a 9 year period. Vaccine 2012;30:4067–72. [DOI] [PubMed] [Google Scholar]
- [18].Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MCC, Nahm MH. Discovery of a New Capsular Serotype (6C) within Serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol 2007;45:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Senders S, Klein NP, Lamberth E, et al. Safety and Immunogenicity of a 20-valent Pneumococcal Conjugate Vaccine in Healthy Infants in the United States. Pediatr Infect Dis J 2021;40:944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sings HL, De Wals P, Gessner BD, et al. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Invasive Disease Caused by Serotype 3 in Children: A Systematic Review and Meta-analysis of Observational Studies. Clin Infect Dis 2019;68:2135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nuorti JP, Whitney C. Prevention of Pneumococcal Disease Among Infants and Children – Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine. MMWR 2010;49:1–24. [PubMed] [Google Scholar]
- [22].Luck JN, Tettelin H, Orihuela CJ. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Frontiers in Cellular and Infection Microbiology 2020; 10. Available at: https://www.frontiersin.org/articles/10.3389/fcimb.2020.613287. Accessed 27 July 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sleeman KL, Griffiths D, Shackley F, et al. Capsular Serotype-Specific Attack Rates and Duration of Carriage of Streptococcus pneumoniae in a Population of Children. J Infect Dis 2006;194:682–8. [DOI] [PubMed] [Google Scholar]
- [24].Kim JO, Romero-Steiner S, Sørensen UB, et al. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun 1999;67:2327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Weinberger DM, Trzciński K, Lu Y-J, et al. Pneumococcal Capsular Polysaccharide Structure Predicts Serotype Prevalence. PLoS Pathog 2009;5:e1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gounder PP, Bruce MG, Bruden DJT, et al. Effect of the 13-Valent Pneumococcal Conjugate Vaccine on Nasopharyngeal Colonization by Streptococcus pneumoniae—Alaska, 2008–2012. J Infect Dis 2014;209:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dagan R, Patterson S, Juergens C, et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis 2013;57:952–62. [DOI] [PubMed] [Google Scholar]
- [28].McFarland M, Szasz TP, Zhou JY, et al. Colonization with 19F and other pneumococcal conjugate vaccine serotypes in children in St. Louis, Missouri, USA. Vaccine 2017; 35:4389–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weckx LY, Thompson A, Berezin EN, et al. A phase 3, randomized, double-blind trial comparing the safety and immunogenicity of the 7-valent and 13-valent pneumococcal conjugate vaccines, given with routine pediatric vaccinations, in healthy infants in Brazil. Vaccine 2012;30:7566–72. [DOI] [PubMed] [Google Scholar]
- [30].Yeh SH, Gurtman A, Hurley DC, et al. Immunogenicity and Safety of 13-Valent Pneumococcal Conjugate Vaccine in Infants and Toddlers. Pediatrics 2010;126:e493–505. [DOI] [PubMed] [Google Scholar]
- [31].Advisory Committee on Immunization Practices. Licensure of a 13-Valent Pneumococcal Conjugate Vaccine (PCV13) and Recommendations for Use Among Children — Advisory Committee on Immunization Practices (ACIP), 2010. 2010. [PubMed] [Google Scholar]
- [32].O’Brien KL. When less is more: how many doses of PCV are enough? Lancet Infect Dis 2018;18:127–8. [DOI] [PubMed] [Google Scholar]
- [33].Whitney CG. Examining Duration of Protection: Should a Booster Dose Be Part of All Infant Pneumococcal Conjugate Vaccine Programs? Clin Infect Dis 2018;67:375–7. [DOI] [PubMed] [Google Scholar]
- [34].Chua H, Feng S, Lewnard JA, et al. The use of test-negative controls to monitor vaccine effectiveness: a systematic review of methodology. Epidemiology 2020;31:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Verani JR, Baqui AH, Broome CV, et al. Case-control vaccine effectiveness studies: Preparation, design, and enrollment of cases and controls. Vaccine 2017;35:3295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Andrews N, Kent A, Amin-Chowdhury Z, et al. Effectiveness of the seven-valent and thirteen-valent pneumococcal conjugate vaccines in England: The indirect cohort design, 2006–2018. Vaccine 2019;37:4491–8. [DOI] [PubMed] [Google Scholar]
- [37].Gierke R Current Epidemiology of Pediatric Pneumococcal Disease, United States. 2023; Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-22/Pneumococcal-02-Gierke-508.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.