Abstract
Oxygen deprivation is one of the main causes of morbidity and mortality in newborns, occurring with a higher prevalence in preterm infants, reaching 20 % to 50 % mortality in newborns in the perinatal period. When they survive, 25 % exhibit neuropsychological pathologies, such as learning difficulties, epilepsy, and cerebral palsy. White matter injury is one of the main features found in oxygen deprivation injury, which can lead to long-term functional impairments, including cognitive delay and motor deficits. The myelin sheath accounts for much of the white matter in the brain by surrounding axons and enabling the efficient conduction of action potentials. Mature oligodendrocytes, which synthesize and maintain myelination, also comprise a significant proportion of the brain's white matter. In recent years, oligodendrocytes and the myelination process have become potential therapeutic targets to minimize the effects of oxygen deprivation on the central nervous system. Moreover, evidence indicate that neuroinflammation and apoptotic pathways activated during oxygen deprivation may be influenced by sexual dimorphism. To summarize the most recent research about the impact of sexual dimorphism on the neuroinflammatory state and white matter injury after oxygen deprivation, this review presents an overview of the oligodendrocyte lineage development and myelination, the impact of oxygen deprivation and neuroinflammation on oligodendrocytes in neurodevelopmental disorders, and recent reports about sexual dimorphism regarding the neuroinflammation and white matter injury after neonatal oxygen deprivation.
Keywords: Oxygen deprivation, White matter injury, Myelination, Oligodendrocytes, Neuroinflammation, Sexual dimorphism
1. Introduction
There is a critical window of neurodevelopment in the perinatal period, when multiple important events take place in brain that are sensitive to the brain tissue oxygenation (Cisneros-Franco et al., 2020; Ujhazy et al., 2013; Failor et al., 2010). Oxygen deprivation (OD) is among the main causes of neonatal morbidity, and affects 0.1 to 0.8 % of term-born babies and 60 % of preterm babies (MacDonald et al., 1980; Kurinczuk et al., 2010). Individuals who survive OD often develop permanent neurological sequelae, such as cognitive and motor deficits (Back and Miller, 2014; Vannucci, 2000; Wilson-Costello et al., 2005), and psychiatric disorders, such as autism spectrum disorder and schizophrenia (Giannopoulou et al., 2018; van Handel et al., 2007).
Among the processes disrupted by perinatal OD, myelination is one of the most prevalent, and white matter injury is a common finding in human post-mortem brains, as well as in live magnetic resonance images. Myelination is a prolonged neurodevelopmental process that ensures the efficiency and speed of action potentials by insulating axons with a specialized membrane compounded of 70 % lipids and 30 % proteins (van Tilborg et al., 2017). The myelination of axons is essential for the maturation of the central nervous system (CNS) and depends on glial cells called oligodendrocytes (OLs). Moreover, OLs support the maturation of neurons by the production of trophic factors, such as glial cell-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), or insulin-like growth factor-1 (IGF-1) (Jang et al., 2019; Schmidt and Knosche, 2019; Wilkins, 2003; Zhao et al., 2001).
The development of the OL lineage is modulated by delicate interactions between CNS cells, the temporal production of trophic factors, signaling molecules, vesicular transport, energy input, as well as the control of intracellular transport, which shows a complex interaction between the axon, environment, and myelinating cell (Nishiyama et al., 2021; Santos et al., 2019). The impact of sexual dimorphism on the production and maturation of OLs has received less attention. However, some studies have observed sex-dependent differences in white matter as well as in the volume of white matter in specific structures, such as the corpus callosum (Cerghet et al., 2009; Fitch et al., 1990).
This review summarizes the latest research about the interaction between the neuroinflammatory status caused by OD in neonates and the associated white matter injury, with a special foccus on the sex-dependent consequences on myelination. To achieve this aim, we discuss (1) the physiological development of the oligodendrocyte lineage, (2) the myelination process, (3) how neonatal OD and neuroinflammation can interact at different stages of myelination, (4) the impact of neuroinflammation on myelination, (5) the common features of the neurodevelopmental disorders due to white matter injury and neonatal OD, and, lastly, (6) the recent reports about the sexual dimorphism in the myelination and neuroinflammation processes after neonatal OD events.
2. Development of oligodendrocytes
OLs arise from the oligodendrocyte progenitor cells (OPCs) that exhibit short processes emanating from opposite poles of the soma and bipolar morphological characteristics of neural precursor cells (Baldassarro et al., 2019; Sharma et al., 2020). OPCs emerge from three restricted periventricular germinative regions: the medial ganglionic eminence and entopeduncular area, the lateral ganglionic eminence, and the dorsal ventricular zone. The development of the OLs in the forebrain is characterized by three waves of OPC production. The first occurs at embryonic day (E) 12.5 in the ventricular zone of the ventral medial ganglionic eminence and entopeduncular area. The second wave occurs through precursors in the lateral and/or caudal ganglionic eminences at around E14.5. Finally, the third wave starts on postnatal day 0 (P0) with the migration of progenitors from the dorsal ventricular zone. In adulthood, OLs are evenly distributed throughout the adult CNS, so OPCs exhibit multidirectional migration from ventricular zones to distant locations (Kessaris et al., 2006).
Signaling molecules such as sonic hedgehog, bone morphogenic protein, and Notch orchestrate the migration of the OPCs to their final locations (Hashimoto et al., 2018; Winkler and Franco, 2019). Once allocated, OPCs undergo local proliferation and differentiation, or apoptosis, in response to diffusible growth and transcription factors, such as Olig1, Olig2, SOX10, and Nkx2.2 (Liu et al., 2007; Qi et al., 2001; Zhou and Anderson, 2002; Zhou et al., 2001; Zhu et al., 2014). Also, the expression of tenascin-C - an extracellular matrix glycoprotein - by the OPCs may be associated to the PDGF signaling to regulate the maturation and survival of these cells (Garwood et al., 2004).
The OPCs can be identified by the expression of markers such as the platelet-derived growth factor-alpha receptors (PDGFRα), the GD3 ganglioside, the NG2, and the Nkx2.2 (Calver et al., 1998; Fruttiger et al., 1999; Gotoh et al., 2018; Liu et al., 2007). These markers play a continued role in the generation of OL throughout life, until these cells differentiate and mature into myelin-forming OL. Also, a recent study showed that Rnd3, a member of the subfamily of Rho GTPases, is expressed in a subset of OPCs and of mature OLs both in vivo and in vitro and is directly required for the differentiation of OLs (Madrigal et al., 2022). However, it is not clear if all OPCs undergo this same sequence of events. As OPCs differentiate they reduce their mitotic ability and gain the expression of sulfatides recognized by the O4 antibody and lose PDGFRα, GD3, and NG2 as they start expressing GalC (Pituch et al., 2015; Scott-Hewitt et al., 2017).
As OPCs mature and establish contact with target axons, OPCs exit the cell cycle and develop into pre-myelinating OLs, which begin to express myelin-related gene products, before axon ensheathment and myelination. CNPase or CNP is one of the earliest OL-specific proteins expressed, and its two isoforms are temporally regulated, with CNP2 being expressed by OPCs, and both CNP1 and CNP2 being expressed at the time of OL differentiation (Lee et al., 2006; Scherer et al., 1994).
This is followed by the expression of the major constituents of CNS myelin, the myelin basic protein (MBP) and proteolipid protein (PLP), which determines that pre-myelinating OLs reach the mature stage (Ray et al., 2003). Myelin-associated/oligodendrocyte basic proteins are expressed at the late stages of myelination, significantly later than the expression of MBP. Myelin-oligodendrocyte glycoprotein (MOG) is one of the last myelin proteins to be expressed and is often used as a marker for mature OLs (Scolding et al., 1989).
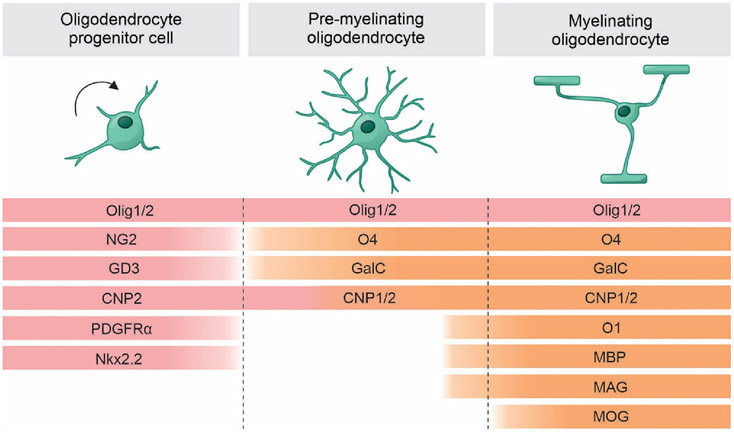
These multiple markers determine OLs functions at different stages of differentiation (Fig. 1). PDGFRα mediates the effects of platelet-derived growth factor with two A subunits (PDGF-AA), which is a potent mitogen and survival factor for OPCs and stimulates migration. The loss of PDGFRα culminates in a decrease in the migratory and proliferative capacity of OPCs and is dependent upon axon-derived factors for survival and differentiation (Deng et al., 2014). Fibroblast growth factor (FGF) signaling and the expression of FGF receptors (FGF-R) are developmentally regulated in OLs: both FGF-R1 and FGF-R2 are required for OPCs ventral generation in the forebrain; FGF-R2 acts as a late-stage regulatory mechanism of myelin growth and myelin sheath thickness; FGF-R3 is expressed transiently by pre-myelinating OLs and is important in the initiation of myelination (Furusho et al., 2012; Furusho et al., 2020; Furusho et al., 2011). Moreover, IGF-I is a survival factor for OLs and coordinates cell cycle progression of OPCs (Frederick and Wood, 2004; Masters et al., 1991; Palacios et al., 2007). However, the precise interactions of these trophic factors in vivo are unclear. When OPCs reach their final destinations, the contact with axons is sufficient for survival and differentiation, under the influence of neuregulin-1 (NRG-1) and Notch/Jagged signaling (Fernandez et al., 2000; Linying et al., 2014; Santhosh et al., 2017).
Fig. 1.
Markers of oligodendrocyte developmental stages. Oligodendrocyte progenitor cells mark the initial stages of differentiation. In this stage, cells can be identified by the expression of NG2, PDGFRα, NKx2.2, CNP2 and GD3. Pre-myelinating period is characterized by the initial expression of mature markers, such as MBP, MAG, CNP1, O4, O1 and GalC. Myelinating period is characterized by the expression of MOG. Olig 1 and 2 are expressed throughout the lineage and support the maturation process. NG2: neuron-glial antigen 2; PDGFαR: platelet-derived growth factor receptor A; Nkx2.2: NK2 homeobox 2; CNP2: CNPase 2; CNP1: CNPase 1; Olig 1: oligodendrocyte transcription factor 1; Olig 2: oligodendrocyte transcription factor 2; GD3: GD3 ganglioside; MBP: myelin basic protein; MAG: myelin associated glycoprotein; MOG: myelin-oligodendrocyte glycoprotein; GalC: galactocerebroside.
3. Myelination
Over the years, many models have been proposed to explain how OLs drastically change their plasma membranes to form internodes and myelinated axons. The “jelly roll or carpet crawler” model proposed for Bunge was inherited from the behavior of Schwann cells in the peripheral nervous system (PNS), According to this model, OLs project their membrane as an extension conformed with the shape of paranode of the axon to be enveloped and repeatedly encircle it (Bunge et al., 1961; Bunge et al., 1989). However, CNS myelination differs from the PNS by the multiple axons that an OL myelinates when compared to a single axon myelinated by a Schwann cell, and by the development of the internodes, of variable length conflicting with this model (Knobler et al., 1976). Subsequent models were proposed to reconcile these divergences, such as a “liquid croissant” (Sobottka et al., 2011).
However, with the advance of in vivo live-imaging techniques, a more suitable and current model of myelination in the CNS was proposed using zebrafish. This model argues that OLs project a growth cone toward the axon in a triangle-like shape, and the inner tongue repeatedly encircles the axon underneath the previous layer. In parallel, a process of lateral extension and compaction of the layers in the direction of the paranodes is coordinated by the interaction of the cytoplasmic channels and PI(3,4,5)P3 lipid levels (Snaidero et al., 2014).
Histological human post-mortem studies and recent in vivo MRI show that myelination follows a hierarchical sequence, in which brain regions dedicated to homeostatic and less complex functions are myelinated first, whereas those involved in higher cognitive functions, such as prefrontal cortex, are myelinated last (Brody et al., 1987; Deoni et al., 2011; Kinney et al., 1988). Moreover, myelination in the CNS is heterogeneous, not only between different areas but also in the same brain region. For example, pyramidal neurons of the somatosensory and visual cortices present different profiles of longitudinal myelinations along cortical layers (Tomassy et al., 2014). Until recently, it has been unclear how axons are selected to be enveloped, but physical, biophysical, and molecular cues seem to be involved in this complex process.
Considering physical aspects, CNS myelination is explained by axon geometries. The caliber of the axons seems to be determinant, since OLs do not wrap axons with a diameter smaller than 0.3 μm, and the thickness of the myelin sheath seems to be correlated to the axon caliber and internode length (Bechler et al., 2015). Cell surface molecules are also involved in myelination guidance. In neurons that should not be myelinated, inhibitory molecules as PSA-NCAM (Charles et al., 2000), JAM2 (Redmond et al., 2016), Lsamp, and Sema3 (Sharma et al., 2015; Syed et al., 2011) are expressed to repulse the neuron-OL interaction.
Myelination is enhanced by neuronal activity, which influences OPCs proliferation, differentiation, and myelin production in an adenosine-dependent mechanism (Gibson et al., 2014; Mitew et al., 2018; Stevens et al., 2002). Recently, a review about the activity-dependent myelination in the central nervous system showed how the advances in novel toolboxes, such as genetic, electrophysiological and the previously cited imaging approaches, led to a better understanding of how this neuron-oligodendrocyte cell interaction and myelination presents both precise temporal and spatial patterns, which can be regulated by extrinsic and intrinsic factors (Heflin and Sun, 2021).
Aligned to neuronal activity, it was demonstrated that dynorphin, released under high neuronal activity, also promotes developmental OPC differentiation and myelination, and this effect requires the expression of the dynorphin endogenous receptor, kappa opioid receptor, in OPCs (Osso et al., 2021). Also, Zonouzi and colleagues showed that certain OLs show a preference to myelinate axons from inhibitory or excitatory neurons and different classes of interneurons have different patterns of myelination in the neocortex (Zonouzi et al., 2019). This finding supports the hypothesis that different neuronal identities and electrophysiological features can influence the myelination process (Micheva et al., 2016).
Besides neurons, astrocytes and microglia are also important players in the myelination process. During myelination and early postnatal development, the astrocytic secretion of BDNF and ciliary neurotrophic factor (CTNF) potentiates myelination (Miyamoto et al., 2015; Stankoff et al., 2002). Also, the direct interaction between OLs and astrocyte connexins is important for the correct ensheathment of axons, since their deletion leads to myelination deficits, as vacuolization and a reduction in both the sheath thickness and OLs number (Tress et al., 2012). The importance of astrocytes in the adhesion of the OLs to the axons was shown using in vitro assays that manipulated PSA-NCAM in co-cultures of retinal ganglion cells and optic nerve (Meyer-Franke et al., 1999). Additionally, astrocytes support the activity of the oligodendrocytes and myelination in low-glucose conditions (Rinholm et al., 2011) as well as provide extracellular lipids that contribute to myelin membrane formation in normal development (Camargo et al., 2017). The contribution of the microglia to myelination, especially during development, is pivotal. Indeed, with the advance of transcriptome analyses, a novel population of microglia CD11c+ localized in the corpus callosum and cerebellum was described as the major source of IGF-1 – an important factor in the expression of myelin proteins, including PLP, MBP, MAG and MOG, and myelination (Wlodarczyk et al., 2017).
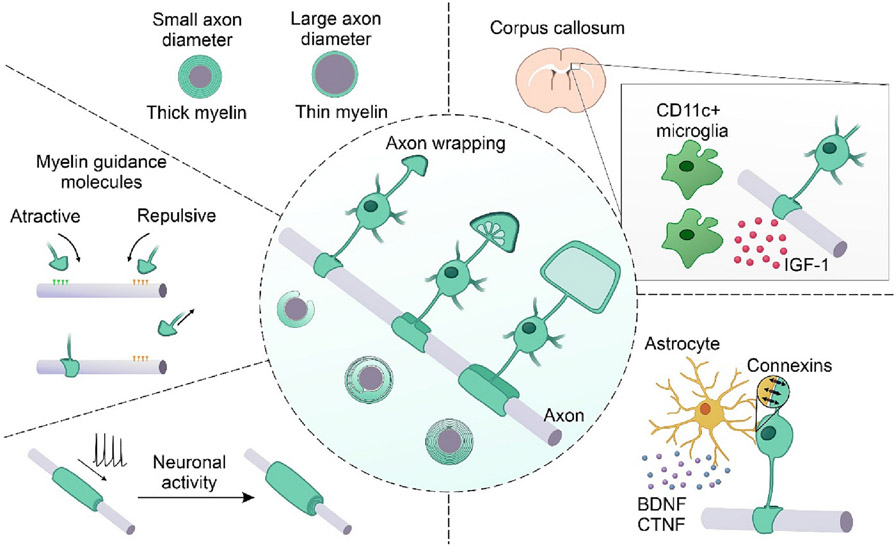
Fig. 2 illustrates the proposed myelination model for the CNS and the guidance factors involved in the myelination process.
Fig. 2.
CNS model of myelination and guidance factors of the myelination process. In the center, the current proposed model of myelination to the CNS is illustrated by an unmyelinated axon in parallel to an oligodendrocyte in three sequential steps of myelination and the respective cross-section illustration. In the first step, the triangle shape inner tongue is projected toward the axon, followed by the repeated encircle of the axon, lateral extension, and compaction of the myelin layers. Factors that guide the oligodendrocytes' decision to myelinate or not an axon are illustrated around the main image. Such factors include axon diameter, attractive or repulsive molecular cues, and neuronal activity. Moreover, the contribution of other glial cells is highlighted: astrocytes secrete BDNF and CTNF couple with oligodendrocytes by connexins; microglia CD11c + support the myelination process by the secretion of IGF-1, especially in the corpus callosum. IGF-1: insulin-like growth factor 1; BDNF: Brain-Derived Neurotrophic Factor; CTNF: ciliary neurotrophic factor.
In summary, the successful ensheathment of the axons by the membrane of OL projections is a multifactorial event, dependent on the axons' geometry, neuronal identity, adjacent glial cells, and molecules derived from the OLs and the surrounding tissue. However, there are still some gaps in the knowledge of the mechanisms underlying the process of oligodendrocyte development and myelination, which require further studies. In the next section, we discuss the impact of OD on the myelination process.
4. OD and myelination
Adequate myelination during brain development is essential for the appropriate performance of motor, cognitive, and sensory functions (Back and Miller, 2014; Branson, 2013; Volpe, 2012). Myelination begins during the gestational period and continues throughout life (Semple et al., 2013; Swire and Ffrench-Constant, 2018). Perinatal insults can result in myelin damage, with subsequent functional impairment.
OD during the perinatal period represents one of the major causes of mortality and morbidity in newborns. Among the risk factors for this condition are placental insufficiency, pre-eclampsia, anemia, cardiovascular disease, and maternal smoking. Neonates who survive OD can present permanent sequelae, such as seizures, cerebral palsy, and long-lasting cognitive and behavioral impairments (Rees and Inder, 2005). The OD causes extracellular accumulation of glutamate and other excitatory amino acids. The activation of excitatory receptors leads to a calcium (Ca2+) influx, which causes augmented levels of nitric oxide synthase, damage to the mitochondria, and subsequent cell death (Berger et al., 2016; Nyakas et al., 1996; Pearce, 1995). Thus, in the effort to understand the mechanisms underlying OD and to develop potential therapeutic approaches, there are different models developed in primates, sheep, piglets, and especially rodents that attempt to simulate the OD effects. Each model has particularities that are beyond the scope of this article, therefore, we will specify the OD models used throughout this section.
Although several regions of the developing brain are susceptible to hypoxic damage, OD in full-term newborns primarily affects the gray matter. On the other hand, the white matter is more affected in premature infants, and the resulting lesion is known as perinatal white matter injury (PWMI), which includes the periventricular leukomalacia (PVL) and diffuse non-cystic injury (Back, 2006).
It has already been reported the death of OPCs in the developing brain after OD in rodents and humans (Barradas et al., 2016; Kaur et al., 2013; Levison et al., 2001; Volpe, 2001). In addition to cell death, delayed differentiation in humans (Dunst et al., 2014; Wellmann et al., 2014), the interruption of OL maturation in the cortex, striatum, and hippocampus was also documented after OD (Back et al., 2001; Ness et al., 2001; Ziemka-Nalecz et al., 2018). These findings possibly corroborate with damages to myelination changes with MBP labeling, such as deformed myelin sheaths and a few myelinated axons observed in rats (Kessaris et al., 2006) and mice (Cai et al., 2011; Skoff et al., 2001),.
Among the factors involved in the aforementioned damages, the downregulation of several genes involved in OL differentiation after OD plays a fundamental role. IGF-1 is one of the main factors which stimulate the OLs proliferation, differentiation, and survival in rat brain cultures (Masters et al., 1991; McMorris and Dubois-Dalcq, 1988; McMorris et al., 1986; Mozell and McMorris, 1991) by the interaction with the PI3K/mTOR/Akt, MEK/ERK, and JAK/STAT pathways as observed in rat brains in vitro and in situ (Bibollet-Bahena and Almazan, 2009; Ness et al., 2001; Palacios et al., 2007; Subramaniam et al., 2005; Zheng et al., 2018). Changes in the expression of these genes impair myelinogenesis and result in the development of white matter diseases. For example, the reduction of IGF-1 mRNA levels observed after neonatal OD (Lee et al., 1996) and in newborns with hypoxic-ischemic encephalopathy (HIE) (Umran et al., 2016) may contribute to the vulnerability of the abnormal and deficient myelinogenesis and myelination (Ziemka-Nalecz et al., 2018). Recently, a decrease in IGF-1 levels was demonstrated in isolated cultures of OLs 72 h after oxygen and glucose deprivation. A significant decline in the number of dividing cells was seen, suggesting an autocrine effect of IGF-1 on cell division, which was impaired by hypoxia-ischemia (Janowska et al., 2020).
Furthermore, newborn brain's transcriptome analysis showed that hypoxia down-regulated OL genes encoding PLP, PDGFRα, cyclic nucleotide phosphodiesterase, and MAG, among these, MAG was the most altered. Thus, the loss of MAG expression after OD may be indicative of the loss of mature OLs (Curristin et al., 2002).
The development of OLs is also vulnerable to oxidative stress and glutamate accumulation triggered by the reperfusion after OD episodes. Nitrative and oxidative damage may be associated with a direct insult to pre-myelinating OLs (Haynes et al., 2003) by the release of free radicals induced by OD, which results in both decreased glutathione (cellular antioxidant) and promotes the accumulation of reactive oxygen species (ROS) (Haynes et al., 2003; Rathnasamy et al., 2011). Concomitantly, excess extracellular glutamate leads to an overactivation of AMPA and NMDA receptors, present in OLs, and leads to the death of these cells in the developing brain (Jensen, 2002; Salter and Fern, 2005; Sanchez-Gomez et al., 2003).
5. Neuroinflammation and myelination
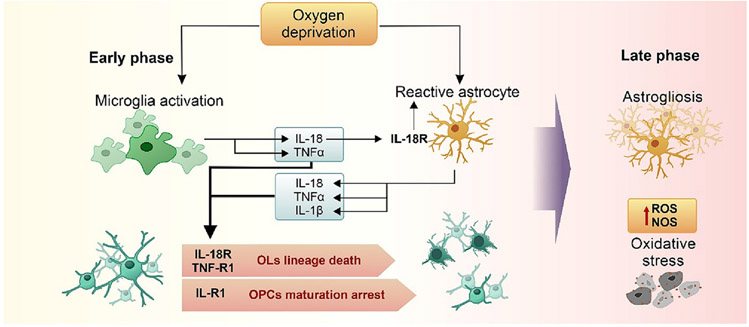
Another reason for changes in the myelination process is the inflammatory response mediated by the release of cytokines, such as TNFα, IL-1β, IL-2, IL-6, IFNγ (Deguchi et al., 1997; Deng et al., 2008; Folkerth et al., 2004; Kadhim and Sebire, 2002; Kadhim et al., 2001; Kaur et al., 2013; Rathnasamy et al., 2011). Neuroinflammation presents two distinct phases (Fig. 3). The early phase is characterized by the direct activation of the microglia leading to the production of pro-inflammatory cytokines, which can also lead to reactive astrogliosis, thus contributing to the late inflammatory phase (Deguchi et al., 1997; Paschon et al., 2019).
Fig. 3.
Neuroinflammatory phases after neonatal oxygen deprivation. The schematic illustration of the early neuroinflammatory phase evidence the activation of the microglia and astrocytes especially by the interaction of the IL-18 secreted by the microglia with the IL-18R expressed by the astrocytes. Next, the interaction of IL-18, TNFα, and IL-1β released by microglia and astrocytes with their respective receptors expressed mainly in pre-myelinating OLs promotes death of the lineage or OPC maturation arrest, culminating in myelination deficits. In the latter phase, astrogliosis promotes the elevation of ROS and NOS levels; as immature OLs are susceptible to oxidative stress, later impairment of oligodendrocyte lineage continues.
The release of pro-inflammatory cytokines is known to reduce the differentiation and maturation of OPCs (Back et al., 2002; Ness et al., 2001; Paneth et al., 1990; Vela et al., 2002; Wellmann et al., 2014; Xie et al., 2016), to increase OL death (Li and Wong, 1998; Baerwald and Popko, 1998), and to suppress axon development (Han et al., 2017). All these changes result in decreased myelination, which was observed in studies by the reduction of MBP expression and the disruption of myelin sheaths in the white matter after hypoxia, with long-term functional deficits as a consequence (Kaur et al., 2006; Kurumatani et al., 1998; van Tilborg et al., 2018a; van Tilborg et al., 2018b; Wang et al., 2016).
Corroborating these findings, the increased expression of IL-18 by microglial and astrocytic cells have been described in the subcortical white matter in mice (Hedtjarn et al., 2005). Moreover, the expression of IL-18R in astrocytes, but not microglial cells, was reported in mice that were exposed to hypoxia, as well, patients with multiple sclerosis showed IL-18R expression in OLs and astrocytes (Cannella and Raine, 2004; Hedtjarn et al., 2005). Therefore, these results suggest that a hypoxic environment can cause neuroinflammation through the direct activation of microglial cells producing inflammatory mediators, such as IL-18, that amplify the reactive astrogliosis and damage to OLs.
Interleukin-specific receptors, such as IL-18R and IL-1R, and membrane receptors, including toll-like receptors (TLRs), mediate the upstream activation of JNK that enhances the transcription of genes involved in proliferation, cell survival and death, metabolism, and inflammation by the induction of the Activator protein 1 (AP-1) transcription factor. In rats subjected to OD, AP-1 showed to be elevated, and the inhibition of JNK promoted the reduction of brain damage and sensory-motor and cognitive behavioral improvements (Nijboer et al., 2010).
One of the inflammatory cytokines transcribed after the JNK activation is the TNFα that interacts with its receptors, TNFR1 and TNFR2. In human brains from early infantile deaths with PVL, an overexpression of TNFα and its receptors was identified in the affected areas, mainly in microglia, astrocytes, and OLs (Kadhim et al., 2006). These findings support the neurotoxic and pro-inflammatory actions, indicated by TNFα/TNRF1/JNK signaling in mice injected with LPS and exposed to hypoxia and ischemia. TNFR1-JNK, but not TNFR2 signaling, is a common pathway leading to apoptosis of pre-myelinating OLs (Wang et al., 2014).
In astrocyte cultures mRNA expression and protein levels of TNFα and IL-1β mRNA, proteins, their respective receptors TNF-R1 and IL-R1, and caspase-3 expression were upregulated significantly after hypoxic exposure (Deng et al., 2014). In addition, IL-1β may inhibit OL proliferation through its receptor (Vela et al., 2002). These results suggest that the release of TNFα and IL-1β by reactive astrogliosis causes the death of OLs and inhibition of OL proliferation.
Together, these data indicate that OD could activate astrocytes to produce inflammatory mediators, which would damage the OLs through their corresponding receptors. Therefore, not only microglia but also astrocytes may play an important role in white matter injury in the hypoxic neonatal brain. Astrogliosis boosts the potential for the production and release of pro-inflammatory cytokines and the overproduction of free radicals that are toxic to neurons and OLs (Kirkley et al., 2017). Cytokines can also be harmful to white matter, for example inhibiting the differentiation of OLs precursors (Favrais et al., 2011).
6. Myelination alterations and neurodevelopmental disorders (NDD)
Changes in myelination are found in different clinical conditions in which OD has occurred, just as in distinct animal models used to study the underlying mechanisms of the anoxic or hypoxic episodes (van Tilborg et al., 2017; Ziemka-Nalecz et al., 2018). The employment of these animal models recapitulates the OD episodes that occur in humans and allows the evaluation of lesions in the white matter, which are associated with the occurrence of cerebral palsy, epilepsy, schizophrenia, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), learning and memory deficits, and cognitive impairments observed in humans (Cainelli et al., 2020; Juliano et al., 2015; van Tilborg et al., 2017).
Moreover, myelination disruption is not limited to the neonatal period. In a double-hit model in which neonatal hypoxia is performed after perinatal inflammation, van Tilborg and colleagues showed that Wistar rats that had experienced hypoxia after perinatal inflammation expressed lower levels of MBP 21 or 28 days after the stimulus and a delay in myelination up to the age of P30. These animals also presented impairment of OL maturation which was related to the increase in IBA1+ and GFAP+ cells, and also exhibited behavior similar to ASD, with decreased sociability, stereotyped behavior and increased anxiety behavior (van Tilborg et al., 2017). In another study, reduced myelination was accompanied by sensory and motor deficits in C57Bl/6 J mice after exposure to intermittent hypoxia in 7-day-old pups (Juliano et al., 2015). It might indicate that different models used to mimic neonatal OD may have different ways to impact the cellular mechanisms of OLs, influencing somatosensory development and cognitive impairment, evident in neurodevelopmental disorders.
These results agree with human clinical epidemiology where 25–50 % of individuals born prematurely and with extremely low birth weight present moderate cognitive, behavioral, and sensorimotor deficits throughout their lives (Volpe et al., 2011) whereas 5–10 % of surviving babies develop cerebral palsy (Doyle et al., 2010; Janowska et al., 2020).
Due to the loss of white matter integrity, pathologies such as schizophrenia and epilepsy are also associated with OD insults. In a study, 10-day-old C57/Bl6 mice exposed to hypoxia-ischemia show a pattern of neuronal activity similar to that seen in the EEG of human neonates with hypoxic-ischemic injury; the animals exhibited burst suppression interrupted by wave peaks during and after the hypoxia period in the hippocampus and cerebral cortex. Interestingly, in the context of the model used, HI with unilateral ischemia, the ischemic hemisphere showed greater activity than the contralateral hemisphere, with asymmetric activation occurring in the somatosensory cortex, hippocampal structures, and the olfactory system. Conversely, the anterior regions were activated bilaterally, especially in the motor cortex (Burnsed et al., 2019). Moreover, the expression of MBP was more prevalent in the anterior region of the corpus callosum, unlike the posterior region, which did not show MBP labelling and whose structures showed an asymmetric activation pattern (Burnsed et al., 2019). It is important to note that the corpus callosum is composed of axons that cross the midline to connect the cerebral hemispheres; however, the propagation of convulsive activity was shown bilaterally only in the frontal lobe, coinciding with the higher expression of MBP in the anterior part of the corpus callosum (Burnsed et al., 2019). These results suggests that, although the immaturity of myelination is important in limiting the propagation of seizure activity, the occurrence of the hypoxic-ischemic event triggers abnormalities in the activity of the neuronal circuitry and the present myelin is crucial in propagating such activity (Aberg et al., 2007; Nyakas et al., 1996).
Corroborating the hypothesis of the existence of myelination-associated long-term deficits, Wang and colleagues showed that, after neonatal hypoxia, 28-day-old C57Bl mice present neurons with disorganized sheaths and low-density of myelin (Wang et al., 2020). This is in line with van Tilborg's findings that showed disorganization of myelinated axons with fewer intersections and fiber length reduction in animal models of neonatal OD (Kurumatani et al., 1998). These results indicate a strong relationship between neonatal hypoxia events and the sequelae seen throughout life (Juliano et al., 2015), such as those related to attention, sociability, and task execution, which seem to be linked to disturbances in connectivity caused by white matter damage (Aberg et al., 2007). In schizophrenic patients, changes in myelin are common and include a reduced number of OLs, swollen processes, and axonal atrophy (Burnsed et al., 2019; Wang et al., 2018). Also, obstetric complications leading to preterm birth and neonatal hypoxia/anoxia are strong risk factors for the white matter damage observed in these patients (Berger et al., 2016; Takahashi et al., 2011). In an animal model using 1-day-old Wistar rats exposed to hypoxia-ischemia, the rats exhibited behaviors similar to those found in schizophrenia, such as impaired prepulse startle reflex inhibition and locomotor hyperactivity, 38 days after injury (Tejkalova et al., 2007). In another study in Sprague-Dawley rats, hyperactivity accompanied by memory deficits was associated with white matter and axonal damage (Thaker, 2008).
In conclusion, neurodevelopmental disorders share a close relationship with prematurity and neonatal OD. Moreover, OL development is vulnerable to inflammatory cytokines and oxidative stress, and the damage to this cell population ends up in impaired myelination and alterations of cell metabolism, which significantly impact the establishment of neuronal circuits and other important cellular processes through the different stages of neurodevelopment.
7. Myelination and sexual dimorphism in NDD
Studies have already noted that the incidence of many neurological disorders differs between the sexes and elucidating the underlying biological basis has become a high-priority challenge (Fabres et al., 2022; Netto et al., 2017; Sanches et al., 2013a). The observed physiological processes of myelination also appear to show differences depending on sex. Both the volume of white matter, the volume of myelinated nerve fibers and the volume of myelin sheaths in the white matter were significantly higher in male rats aged 6 to 8 months compared to female rats of the same age. However, these three evaluated parameters are shown to be higher in females than males when they reach around 18 months of age (Yang et al., 2008; Lai and Yang, 2011).
Previous studies suggest that female mice have smaller myelin volume and number of OLs compared to males (Cerghet et al., 2009; Schumacher et al., 2020; Swamydas et al., 2009). The difference in the number of OLs was most notable in younger rodents, with males showing a number of OLs 40 % higher than females. In rats, the proliferation of glial cells can be 2-fold higher in corpus callosum of females; however, cell death of glial cells (evaluated by active caspase-3, an apoptosis marker) was 50 % higher in female mice compared to males. Similar data were observed by Cerghet et al. when they observed that calpain increased 2-fold higher in females in the spinal cord and brain (Cerghet et al., 2009). Calpain, calcium-activated neutral proteinase, can be found in the myelin sheath and participates in the degradation of intracellular myelin protein with an increase in cytoplasmic calcium at pathophysiological levels (Shields et al., 1999). Therefore, as proliferation and cell death increase in females, OLs in females are likely to have a shorter life and faster turnover than males (Cerghet et al., 2009).
These developmental differences may be due to sexual steroids. The effects of sex steroids on myelin have been observed not only in the development of the white matter but also in the injured white matter. For example, estradiol has a dose-dependent protective effect against oxygen-deprivation-induced apoptotic cell death in primary OLs. However, estradiol and dihydrotestosterone (DHT) were shown to have similar effects in male and female mice. Estrogen had minor effects on OL numbers; and DHT reduced OL numbers in both sexes, but more so in females (Swamydas et al., 2009).
Also progesterone, one of the most widely studied neurosteroids, restored myelination and increased the density of OPC in a spinal cord injury model, and in combination with estradiol restored myelin protein expression in experimental autoimmune encephalitis (EAE), a multiple sclerosis model (Cerghet et al., 2009; Garay et al., 2008). However, administration of progesterone in a culture model of OLs can be seen that both sexes are protected, but females are slightly more protected than males. In other study, Marin-Hussteg and collaborators observed a higher number of oligodendrocytes in females compared to males using primary cultures of oligodendrocyte progenitors isolated from male and female neonatal rodent brains. This study also observed that estrogen receptor β are located mainly in the cytoplasm of oligodendrocytes, suggesting that estradiol can interact with this receptor and have some effect. Furthermore, they observed that treatment with progesterone increased cellular branching and the use of estradiol increased membrane sheet formation (Marin-Husstege et al., 2004). In these studies females always showed greater differences than males regarding changes in OLs numbers and signaling molecules.
While analyzing the adult brain model, progesterone therapy also promoted the repair of severe chronic demyelinating lesions induced by the OLs toxin cuprizone in female mice. Progesterone increased the density of OPC and mature OLs in the cuprizone-demyelinated corpus callosum and stimulated the formation of new myelin sheaths. Regarding myelination and remyelination during brain development, the proremyelinating effect of progesterone in adults was progesterone receptor (PR)-dependent (Schumacher et al., 2020).
Among the effects of progesterone, it was observed that in OL cultures the pAkt was more up-regulated in females than males, correlating with the effects of progesterone on OL numbers. The PI3K/Akt pathway is an important anti-apoptotic signaling and cell survival pathway. Akt activation stimulates substrates involved in cell survival and inhibits pro-apoptotic substrates such as caspase-3, inhibiting apoptosis pathways and contributing to cell survival by downstream mTOR activation via transcriptional regulation (Chong et al., 2007; Hay, 2005).
The administration of progesterone for treatment increases the expression of the mTOR protein which plays an important role in the Akt pathway which, in turn, acts on the survival of OLs. Using the PI3 kinase inhibitor whose effect is to block the Akt pathway, caused the reduction of the effects of progesterone, and thus reduced its influence on the regulation of OL survival, confirming that the Akt pathway is one of the keys to the action of progesterone in the central nervous system (Swamydas et al., 2009).
Additionally, some studies show that the striatum and white matter of females are more severely affected when exposed to a hypoxic environment (Arteni et al., 2010; Sanches et al., 2013a; Sanches et al., 2013b; Uluc et al., 2013), whereas others indicate that males are more severely affected (Zhu et al., 2003; Mayoral et al., 2009). Hypomyelination, resulting from the interruption of myelin synthesis after an injury, is commonly found in white matter injury (WMI) survivors, especially in diffuse WMI cases. Degeneration and dematuration of OLs may contribute to the pathogenesis of hypomyelination. In perinatal WMI, decreased expression of MBP was associated with long-term cognitive dysfunction (Liu et al., 2019).
Hypomyelination in the corpus callosum and cortex result in insufficient information processing and conduction speed between the cortical regions or between the cortex and the deep gray nuclei, such as the connections between the thalamus and the cortex. Liu and colleagues reported a reduction in numerical density and an increase in the ultrastructural defects of the synapses in the thalamus of rats with WMI, induced by HI. In addition, studies show that male mice would have greater expression of total MBP, summing the 4 isoforms, compared to females; however, the 21.5 kDa isoform was found to be more abundant in females by approximately 30–35 % (Benjamins and Morell, 1978; Cerghet et al., 2009). Therefore, if only female or male animals are used for white matter research under various experimental conditions, the conclusion may be affected by white matter sex differences.
In addition, sex differences in the corpus callosum were also identified in post-mortem studies in human brains, in this study it was observed that the width and surface area of the splenium were less in males when compared to females (DeLacoste-Utamsing and Holloway, 1982). In addition, increased fiber density was also observed in females compared to males in subregions of the corpus callosum (Highley et al., 1999).
An important question is whether the sex differences observed in myelin formation, particularly in the cells involved in myelination during the critical period of neurodevelopment, persist into adulthood. Daniel et al. 2015 observed an increase in the expression of MBP and PLP in adult female rats (around 85 days old) compared to male rats of the same age in the orbital frontal cortex (OFC) (Bayless and Daniel, 2015). In 2019, Daniel and collaborators verified whether the sexual difference previously observed in the OFC could be attributed to variations in neurodevelopment. For this, male and female animals were gonadectomized at two different ages, simulating adolescence and adulthood. Animals submitted to surgery in prior to the onset of puberty (adolescence) showed a reduction in MBP in OFC compared to animals without surgery and animals that underwent gonadectomization in adulthood. These results suggest a possible relationship between sex hormones and the neurodevelopment of myelinating cells and that this difference can persist into adulthood (Darling and Daniel, 2019).
Surprisingly, despite the evidence showing the importance of studying both sexual dimorphisms in NDDs, many studies still do not report the sex of animals used or use only males. Previous studies have observed that both inflammation and cell death in vitro and in vivo models of OD are sex-dependent. Therefore, our review aims to encourage further work that tries to understand the consequences of NDDs on myelination, death, proliferation, and differentiation of OLs and their sex-dependent effects.
Acknowledgements
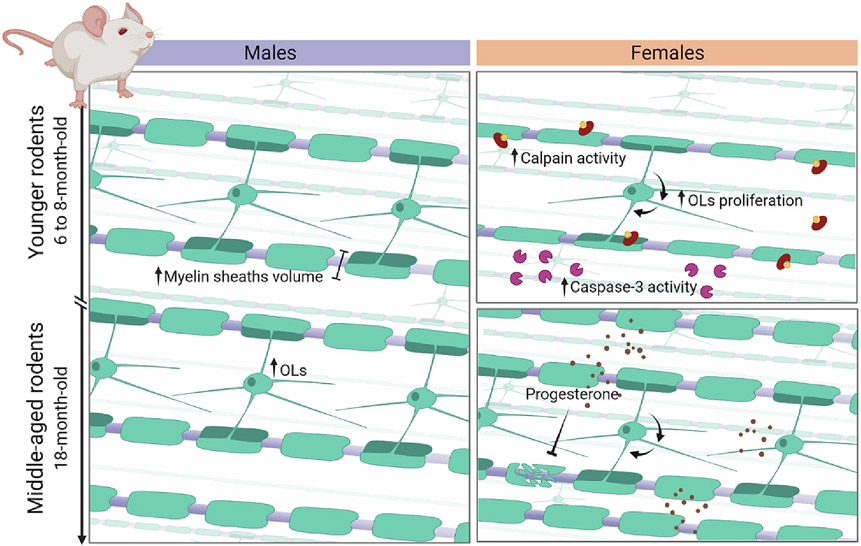
We acknowledge BioRender and Alexandre Hiroaki Kihara for the high-quality images in Fig. 4.
Fig. 4.
Myelin differences among males and females throughout development. Males' myelination, in the left, is constant from younger through adulthood rodents, if the myelin sheath, the myelinated fibers volume, and the number of OLs are considered. In the right, if compared to younger males, the schematic illustration shows the higher calpain and caspase-3 activity, and faster turnover of OLs in females of the same age, which is reflected in the reduced myelin sheath and myelinate fibers volume, and number of OLs. In adulthood, females and males have comparable myelination, despite faster turnover in females. In this age, progesterone administration in females is a neuroprotector that restore demyelination lesions.
Funding
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, grant numbers 88887.510579/2020-00 DSC; 88887.466666/2019-00 BPA), Financiadora de Estudos e Projetos (FINEP) and by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant number 20/16268-67 SHT).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mcn.2023.103864.
Data availability
No data was used for the research described in the article.
References
- Aberg ND, Johansson UE, Aberg MA, Hellstrom NA, Lind J, Bull C, Isgaard J, Anderson MF, Oscarsson J, Eriksson PS, 2007. Peripheral infusion of insulin-like growth factor-I increases the number of newborn oligodendrocytes in the cerebral cortex of adult hypophysectomized rats. Endocrinology 148, 3765–3772. [DOI] [PubMed] [Google Scholar]
- Arteni NS, Pereira LO, Rodrigues AL, Lavinsky D, Achaval ME, Netto CA, 2010. Lateralized and sex-dependent behavioral and morphological effects of unilateral neonatal cerebral hypoxia-ischemia in the rat. Behav. Brain Res 210, 92–98. [DOI] [PubMed] [Google Scholar]
- Back SA, 2006. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment. Retard. Dev. Disabil. Res. Rev 12, 129–140. [DOI] [PubMed] [Google Scholar]
- Back SA, Miller SP, 2014. Brain injury in premature neonates: a primary cerebral dysmaturation disorder? Ann. Neurol 75, 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC, 2001. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci 21, 1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM, 2002. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci 22, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald KD, Popko B, 1998. Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J. Neurosci. Res 52, 230–239. [DOI] [PubMed] [Google Scholar]
- Baldassarro VA, Krezel W, Fernandez M, Schuhbaur B, Giardino L, Calza L, 2019. The role of nuclear receptors in the differentiation of oligodendrocyte precursor cells derived from fetal and adult neural stem cells. Stem Cell Res. 37, 101443. [DOI] [PubMed] [Google Scholar]
- Barradas PC, Savignon T, Manhaes AC, Tenorio F, da Costa AP, Cunha-Rodrigues MC, Vaillant J, 2016. Prenatal systemic hypoxia-ischemia and oligodendroglia loss in cerebellum. Adv. Exp. Med. Biol 949, 333–345. [DOI] [PubMed] [Google Scholar]
- Bayless DW, Daniel JM, 2015. Sex differences in myelin-associated protein levels within and density of projections between the orbital frontal cortex and dorsal striatum of adult rats: implications for inhibitory control. Neuroscience 300, 286–296. [DOI] [PubMed] [Google Scholar]
- Bechler ME, Byrne L, Ffrench-Constant C, 2015. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol 25, 2411–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins JA, Morell P, 1978. Proteins of myelin and their metabolism. Neurochem. Res 3, 137–174. [DOI] [PubMed] [Google Scholar]
- Berger HR, Morken TS, Vettukattil R, Brubakk AM, Sonnewald U, Wideroe M, 2016. No improvement of neuronal metabolism in the reperfusion phase with melatonin treatment after hypoxic-ischemic brain injury in the neonatal rat. J. Neurochem 136, 339–350. [DOI] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G, 2009. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J. Neurochem 109, 1440–1451. [DOI] [PubMed] [Google Scholar]
- Branson HM, 2013. Normal myelination: a practical pictorial review. Neuroimaging Clin. N. Am 23, 183–195. [DOI] [PubMed] [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH, 1987. Sequence of central nervous system myelination in human infancy. I. an autopsy study of myelination. J. Neuropathol. Exp. Neurol 46, 283–301. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Bunge RP, Ris H, 1961. Ultrastructural study of remyelination in an experimental lesion in adult cat spinal cord. J. Biophys. Biochem. Cytol 10, 67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge RP, Bunge MB, Bates M, 1989. Movements of the Schwann cell nucleus implicate progression of the inner (axon-related) Schwann cell process during myelination. J. Cell Biol 109, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnsed J, Skwarzynska D, Wagley PK, Isbell L, Kapur J, 2019. Neuronal circuit activity during neonatal hypoxic-ischemic seizures in mice. Ann. Neurol 86, 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Tuong CM, Gozal D, 2011. A neonatal mouse model of intermittent hypoxia associated with features of apnea in premature infants. Respir. Physiol. Neurobiol 178, 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cainelli E, Arrigoni F, Vedovelli L, 2020. White matter injury and neurodevelopmental disabilities: a cross-disease (dis)connection. Prog. Neurobiol 193, 101845. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD, 1998. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron 20, 869–882. [DOI] [PubMed] [Google Scholar]
- Camargo N, Goudriaan A, van Deijk AF, Otte WM, Brouwers JF, Lodder H, Gutmann DH, Nave KA, Dijkhuizen RM, Mansvelder HD, Chrast R, Smit AB, Verheijen MHG, 2017. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 15, e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella B, Raine CS, 2004. Multiple sclerosis: cytokine receptors on oligodendrocytes predict innate regulation. Ann. Neurol 55, 46–57.14705111 [Google Scholar]
- Cerghet M, Skoff RP, Swamydas M, Bessert D, 2009. Sexual dimorphism in the white matter of rodents. J. Neurol. Sci 286, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P, Hernandez MP, Stankoff B, Aigrot MS, Colin C, Rougon G, Zalc B, Lubetzki C, 2000. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc. Natl. Acad. Sci. U. S. A 97, 7585–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K, 2007. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int. J. Mol. Med 19, 263–272. [PMC free article] [PubMed] [Google Scholar]
- Cisneros-Franco JM, Voss P, Thomas ME, de Villers-Sidani E, 2020. Critical periods of brain development. Handb. Clin. Neurol 173, 75–88. [DOI] [PubMed] [Google Scholar]
- Curristin SM, Cao A, Stewart WB, Zhang H, Madri JA, Morrow JS, Ment LR, 2002. Disrupted synaptic development in the hypoxic newborn brain. Proc. Natl. Acad. Sci. U. S. A 99, 15729–15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling JS, Daniel JM, 2019. Pubertal hormones mediate sex differences in levels of myelin basic protein in the orbitofrontal cortex of adult rats. Neuroscience 406, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi K, Oguchi K, Takashima S, 1997. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr. Neurol 16, 296–300. [DOI] [PubMed] [Google Scholar]
- DeLacoste-Utamsing C, Holloway RL, 1982. Sexual dimorphism in the human corpus callosum. Science 216 (4553), 1431–1432. [DOI] [PubMed] [Google Scholar]
- Deng Y, Lu J, Sivakumar V, Ling EA, Kaur C, 2008. Amoeboid microglia in the periventricular white matter induce oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway in hypoxic neonatal rats. Brain Pathol. 18, 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Xie D, Fang M, Zhu G, Chen C, Zeng H, Lu J, Charanjit K, 2014. Astrocyte-derived proinflammatory cytokines induce hypomyelination in the periventricular white matter in the hypoxic neonatal brain. PLoS One 9, e87420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG, 2011. Mapping infant brain myelination with magnetic resonance imaging. J. Neurosci 31, 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Roberts G, Anderson PJ, Group Victorian Infant Collaborative Study, 2010. Outcomes at age 2 years of infants < 28 weeks’ gestational age born in Victoria in 2005. J. Pediatr 156, 49–53 e1. [DOI] [PubMed] [Google Scholar]
- Dunst B, Benedek M, Koschutnig K, Jauk E, Neubauer AC, 2014. Sex differences in the IQ-white matter microstructure relationship: a DTI study. Brain Cogn. 91, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabres RB, Nunes RR, de Medeiros M, de Mattos MKG, Andrade APR, Martini ID, Tassinari EF, Sanches LS de Fraga, Netto CA, 2022. Therapeutic hypothermia for the treatment of neonatal hypoxia-ischemia: sex-dependent modulation of reactive astrogliosis. Metab. Brain Dis 37, 2315–2329. [DOI] [PubMed] [Google Scholar]
- Failor S, Nguyen V, Darcy DP, Cang J, Wendland MF, Stryker MP, McQuillen PS, 2010. Neonatal cerebral hypoxia-ischemia impairs plasticity in rat visual cortex. J. Neurosci 30, 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, Lacaud A, Saliba E, Dammann O, Gallego J, Sizonenko S, Hagberg H, Lelièvre V, Gressens P, 2011. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol 70, 550–565. [DOI] [PubMed] [Google Scholar]
- Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC, 2000. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron 28, 81–90. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Berrebi AS, Cowell PE, Schrott LM, Denenberg VH, 1990. Corpus callosum: effects of neonatal hormones on sexual dimorphism in the rat. Brain Res. 515, 111–116. [DOI] [PubMed] [Google Scholar]
- Folkerth RD, Keefe RJ, Haynes RL, Trachtenberg FL, Volpe JJ, Kinney HC, 2004. Interferon-gamma expression in periventricular leukomalacia in the human brain. Brain Pathol. 14, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick TJ, Wood TL, 2004. IGF-I and FGF-2 coordinately enhance cyclin D1 and cyclin E-cdk2 association and activity to promote G1 progression in oligodendrocyte progenitor cells. Mol. Cell. Neurosci 25, 480–492. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, Richardson WD, 1999. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development 126, 457–467. [DOI] [PubMed] [Google Scholar]
- Furusho M, Kaga Y, Ishii A, Hebert JM, Bansal R, 2011. Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. J. Neurosci 31, 5055–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Dupree JL, Nave KA, Bansal R, 2012. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J. Neurosci 32, 6631–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Ishii A, Hebert JM, Bansal R, 2020. Developmental stage-specific role of Frs adapters as mediators of FGF receptor signaling in the oligodendrocyte lineage cells. Glia 68, 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay L, Gonzalez Deniselle MC, Gierman L, Meyer M, Lima A, Roig P, De Nicola AF, 2008. Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation 15, 76–83. [DOI] [PubMed] [Google Scholar]
- Garwood J, Garcion E, Dobbertin A, Heck N, Calco V, ffrench-Constant C, Faissner A, 2004. The extracellular matrix glycoprotein tenascin-C is expressed by oligodendrocyte precursor cells and required for the regulation of maturation rate, survival and responsiveness to platelet-derived growth factor. Eur. J. Neurosci 20, 2524–2540. [DOI] [PubMed] [Google Scholar]
- Giannopoulou I, Pagida MA, Briana DD, Panayotacopoulou MT, 2018. Perinatal hypoxia as a risk factor for psychopathology later in life: the role of dopamine and neurotrophins. Hormones (Athens) 17, 25–32. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M, 2014. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh H, Wood WM, Patel KD, Factor DC, Boshans LL, Nomura T, Tesar PJ, Ono K, Nishiyama A, 2018. NG2 expression in NG2 glia is regulated by binding of SoxE and bHLH transcription factors to a Cspg4 intronic enhancer. Glia 66, 2684–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Qianpeng, Lin Qiongyu, Huang Peixian, Chen Mengmeng, Xin Hu, Hui Fu, He Shaoru, Shen Fengcai, Zeng Hongke, Deng Yiyu, 2017. Microglia-derived IL-1β contributes to axon development disorders and synaptic deficit through p38-MAPK signal pathway in septic neonatal rats. J. Neuroinflammation 14, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Jiang W, Yoshimura T, Moon KH, Bok J, Ikenaka K, 2018. Strong sonic hedgehog signaling in the mouse ventral spinal cord is not required for oligodendrocyte precursor cell (OPC) generation but is necessary for correct timing of its generation. Neurochem. Int 119, 178–183. [DOI] [PubMed] [Google Scholar]
- Hay N, 2005. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8, 179–183. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC, 2003. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J. Neuropathol. Exp. Neurol 62, 441–450. [DOI] [PubMed] [Google Scholar]
- Hedtjarn M, Mallard C, Arvidsson P, Hagberg H, 2005. White matter injury in the immature brain: role of interleukin-18. Neurosci. Lett 373, 16–20. [DOI] [PubMed] [Google Scholar]
- Heflin JK, Sun W, 2021. Novel toolboxes for the investigation of activity-dependent myelination in the central nervous system. Front. Cell. Neurosci 15, 769809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ, 1999. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain 122 (Pt 1), 99–110. [DOI] [PubMed] [Google Scholar]
- Jang M, Gould E, Xu J, Kim EJ, Kim JH, 2019. Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowska J, Gargas J, Ziemka-Nalecz M, Zalewska T, Sypecka J, 2020. Oligodendrocyte response to pathophysiological conditions triggered by episode of perinatal hypoxia-ischemia: role of IGF-1 secretion by glial cells. Mol. Neurobiol 57, 4250–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BS, 2002. BMS-204352: a potassium channel opener developed for the treatment of stroke. CNS Drug Rev. 8, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C, Sosunov S, Niatsetskaya Z, Isler JA, Utkina-Sosunova I, Jang I, Ratner V, Ten V, 2015. Mild intermittent hypoxemia in neonatal mice causes permanent neurofunctional deficit and white matter hypomyelination. Exp. Neurol 264, 33–42. [DOI] [PubMed] [Google Scholar]
- Kadhim H, Sebire G, 2002. Immune mechanisms in the pathogenesis of cerebral palsy: implication of proinflammatory cytokines and T lymphocytes. Eur. J. Paediatr. Neurol 6, 139–142. [DOI] [PubMed] [Google Scholar]
- Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G, 2001. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology 56, 1278–1284. [DOI] [PubMed] [Google Scholar]
- Kadhim H, Khalifa M, Deltenre P, Casimir G, Sebire G, 2006. Molecular mechanisms of cell death in periventricular leukomalacia. Neurology 67, 293–299. [DOI] [PubMed] [Google Scholar]
- Kaur C, Sivakumar V, Ang LS, Sundaresan A, 2006. Hypoxic damage to the periventricular white matter in neonatal brain: role of vascular endothelial growth factor, nitric oxide and excitotoxicity. J. Neurochem 98, 1200–1216. [DOI] [PubMed] [Google Scholar]
- Kaur C, Rathnasamy G, Ling EA, 2013. Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J. NeuroImmune Pharmacol 8, 66–78. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD, 2006. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci 9, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH, 1988. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J. Neuropathol. Exp. Neurol 47, 217–234. [DOI] [PubMed] [Google Scholar]
- Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB, 2017. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflammation 14, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler RL, Stempak JG, Laurencin M, 1976. Nonuniformity of the oligodendroglial ensheathment of axons during myelination in the developing rat central nervous system. A serial section electron microscopical study. J. Ultrastruct. Res 55, 417–432. [DOI] [PubMed] [Google Scholar]
- Kurinczuk JJ, White-Koning M, Badawi N, 2010. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev 86, 329–338. [DOI] [PubMed] [Google Scholar]
- Kurumatani T, Kudo T, Ikura Y, Takeda M, 1998. White matter changes in the gerbil brain under chronic cerebral hypoperfusion. Stroke 29, 1058–1062. [DOI] [PubMed] [Google Scholar]
- Lai MC, Yang SN, 2011. Perinatal hypoxic-ischemic encephalopathy. J. Biomed. Biotechnol 2011, 609813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Wang GM, Seaman LB, Vannucci SJ, 1996. Coordinate IGF-I and IGFBP5 gene expression in perinatal rat brain after hypoxia-ischemia. J. Cereb. Blood Flow Metab 16, 227–236. [DOI] [PubMed] [Google Scholar]
- Lee J, O’Neill RC, Park MW, Gravel M, Braun PE, 2006. Mitochondrial localization of CNP2 is regulated by phosphorylation of the N-terminal targeting signal by PKC: implications of a mitochondrial function for CNP2 in glial and non-glial cells. Mol. Cell. Neurosci 31, 446–462. [DOI] [PubMed] [Google Scholar]
- Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ, 2001. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev. Neurosci 23, 234–247. [DOI] [PubMed] [Google Scholar]
- Li YQ, Wong CS, 1998. Apoptosis and its relationship with cell proliferation in the irradiated rat spinal cord. Int. J. Radiat. Biol 74, 405–417. [DOI] [PubMed] [Google Scholar]
- Linying Z, Wei W, Minxia W, Wenmin Z, Liangcheng Z, 2014. Neuroprotective effects of neuregulin-1 ss on oligodendrocyte type 2 astrocyte progenitors following oxygen and glucose deprivation. Pediatr. Neurol 50, 357–362. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hu X, Cai J, Liu B, Peng X, Wegner M, Qiu M, 2007. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev. Biol 302, 683–693. [DOI] [PubMed] [Google Scholar]
- Liu N, Tong X, Huang W, Fu J, Xue X, 2019. Synaptic injury in the thalamus accompanies white matter injury in hypoxia/ischemia-mediated brain injury in neonatal rats. Biomed. Res. Int 2019, 5249675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald HM, Mulligan JC, Allen AC, Taylor PM, 1980. Neonatal asphyxia. I. Relationship of obstetric and neonatal complications to neonatal mortality in 38,405 consecutive deliveries. J. Pediatr 96, 898–902. [DOI] [PubMed] [Google Scholar]
- Madrigal MP, Ballester-Lurbe B, Gomez O, Moreno-Bravo JA, Puelles E, Jurado S, Garcia-Verdugo JM, Perez-Roger I, Terrado J, 2022. Rnd3 is necessary for the correct oligodendrocyte differentiation and myelination in the central nervous system. Brain Struct. Funct 227, 829–841. [DOI] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P, 2004. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev. Neurosci 26, 245–254. [DOI] [PubMed] [Google Scholar]
- Masters BA, Werner H, Roberts CT Jr., LeRoith D, Raizada MK, 1991. Insulin-like growth factor I (IGF-I) receptors and IGF-I action in oligodendrocytes from rat brains. Regul. Pept 33, 117–131. [DOI] [PubMed] [Google Scholar]
- Mayoral SR, Omar G, Penn AA, 2009. Sex differences in a hypoxia model of preterm brain damage. Pediatr. Res 66, 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris FA, Dubois-Dalcq M, 1988. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J. Neurosci. Res 21, 199–209. [DOI] [PubMed] [Google Scholar]
- McMorris FA, Smith TM, DeSalvo S, Furlanetto RW, 1986. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc. Natl. Acad. Sci. U. S. A 83, 822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Franke A, Shen S, Barres BA, 1999. Astrocytes induce oligodendrocyte processes to align with and adhere to axons. Mol. Cell. Neurosci 14, 385–397. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, Bock DD, 2016. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, Emery B, 2018. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun 9, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K, 2015. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J. Neurosci 35, 14002–14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozell RL, McMorris FA, 1991. Insulin-like growth factor I stimulates oligodendrocyte development and myelination in rat brain aggregate cultures. J. Neurosci. Res 30, 382–390. [DOI] [PubMed] [Google Scholar]
- Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW, 2001. Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev. Neurosci 23, 203–208. [DOI] [PubMed] [Google Scholar]
- Netto CA, Sanches E, Odorcyk FK, Duran-Carabali LE, Weis SN, 2017. Sex-dependent consequences of neonatal brain hypoxia-ischemia in the rat. J. Neurosci. Res 95, 409–421. [DOI] [PubMed] [Google Scholar]
- Nijboer CH, van der Kooij MA, van Bel F, Ohl F, Heijnen CJ, Kavelaars A, 2010. Inhibition of the JNK/AP-1 pathway reduces neuronal death and improves behavioral outcome after neonatal hypoxic-ischemic brain injury. Brain Behav. Immun 24, 812–821. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Shimizu T, Sherafat A, Richardson WD, 2021. Life-long oligodendrocyte development and plasticity. Semin. Cell Dev. Biol 116, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakas C, Buwalda B, Luiten PG, 1996. Hypoxia and brain development. Prog. Neurobiol 49, 1–51. [DOI] [PubMed] [Google Scholar]
- Osso LA, Rankin KA, Chan JR, 2021. Experience-dependent myelination following stress is mediated by the neuropeptide dynorphin. Neuron 109, 3619–32 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios N, Sanchez-Franco F, Fernandez M, Sanchez I, Villuendas G, Cacicedo L, 2007. Opposite effects of two PKA inhibitors on cAMP inhibition of IGF-I-induced oligodendrocyte development: a problem of unspecificity? Brain Res. 1178, 1–11. [DOI] [PubMed] [Google Scholar]
- Paneth N, Rudelli R, Monte W, Rodriguez E, Pinto J, Kairam R, Kazam E, 1990. White matter necrosis in very low birth weight infants: neuropathologic and ultrasonographic findings in infants surviving six days or longer. J. Pediatr 116, 975–984. [DOI] [PubMed] [Google Scholar]
- Paschon V, Morena BC, Correia FF, Beltrame GR, Dos Santos GB, Cristante AF, Kihara AH, 2019. VDAC1 is essential for neurite maintenance and the inhibition of its oligomerization protects spinal cord from demyelination and facilitates locomotor function recovery after spinal cord injury. Sci. Rep 9, 14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce WJ, 1995. Mechanisms of hypoxic cerebral vasodilatation. Pharmacol. Ther 65, 75–91. [DOI] [PubMed] [Google Scholar]
- Pituch KC, Moyano AL, Lopez-Rosas A, Marottoli FM, Li G, Hu C, van Breemen R, Mansson JE, Givogri MI, 2015. Dysfunction of platelet-derived growth factor receptor alpha (PDGFRalpha) represses the production of oligodendrocytes from arylsulfatase A-deficient multipotential neural precursor cells. J. Biol. Chem 290, 7040–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M, 2001. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 128, 2723–2733. [DOI] [PubMed] [Google Scholar]
- Rathnasamy G, Ling EA, Kaur C, 2011. Iron and iron regulatory proteins in amoeboid microglial cells are linked to oligodendrocyte death in hypoxic neonatal rat periventricular white matter through production of proinflammatory cytokines and reactive oxygen/nitrogen species. J. Neurosci 31, 17982–17995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Matzelle DD, Sribnick EA, Guyton MK, Wingrave JM, Banik NL, 2003. Calpain inhibitor prevented apoptosis and maintained transcription of proteolipid protein and myelin basic protein genes in rat spinal cord injury. J. Chem. Neuroanat 26, 119–124. [DOI] [PubMed] [Google Scholar]
- Redmond SA, Mei F, Eshed-Eisenbach Y, Osso LA, Leshkowitz D, Shen YA, Kay JN, Aurrand-Lions M, Lyons DA, Peles E, Chan JR, 2016. Somatodendritic expression of JAM2 inhibits oligodendrocyte myelination. Neuron 91, 824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, Inder T, 2005. Fetal and neonatal origins of altered brain development. Early Hum. Dev 81, 753–761. [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D, 2011. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci 31, 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Fern R, 2005. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 438, 1167–1171. [DOI] [PubMed] [Google Scholar]
- Sanches EF, Arteni NS, Nicola F, Boisserand L, Willborn S, Netto CA, 2013a. Early hypoxia-ischemia causes hemisphere and sex-dependent cognitive impairment and histological damage. Neuroscience 237, 208–215. [DOI] [PubMed] [Google Scholar]
- Sanches EF, Arteni NS, Scherer EB, Kolling J, Nicola F, Willborn S, Wyse AT, Netto CA, 2013b. Are the consequences of neonatal hypoxia-ischemia dependent on animals’ sex and brain lateralization? Brain Res. 1507, 105–114. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C, 2003. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J. Neurosci 23, 9519–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhosh KT, Alizadeh A, Karimi-Abdolrezaee S, 2017. Design and optimization of PLGA microparticles for controlled and local delivery of Neuregulin-1 in traumatic spinal cord injury. J. Control. Release 261, 147–162. [DOI] [PubMed] [Google Scholar]
- Santos AK, Vieira MS, Vasconcellos R, Goulart VAM, Kihara AH, Resende RR, 2019. Decoding cell signalling and regulation of oligodendrocyte differentiation. Semin. Cell Dev. Biol 95, 54–73. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J, 1994. Differential regulation of the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene during oligodendrocyte development. Neuron 12, 1363–1375. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Knosche TR, 2019. Action potential propagation and synchronisation in myelinated axons. PLoS Comput. Biol 15, e1007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Liere P, Ghoumari A, 2020. Progesterone and fetal-neonatal neuroprotection. Best Pract. Res. Clin. Obstet. Gynaecol 69, 50–61. [DOI] [PubMed] [Google Scholar]
- Scolding NJ, Frith S, Linington C, Morgan BP, Campbell AK, Compston DA, 1989. Myelin-oligodendrocyte glycoprotein (MOG) is a surface marker of oligodendrocyte maturation. J. Neuroimmunol 22, 169–176. [DOI] [PubMed] [Google Scholar]
- Scott-Hewitt NJ, Folts CJ, Hogestyn JM, Piester G, Mayer-Proschel M, Noble MD, 2017. Heterozygote galactocerebrosidase (GALC) mutants have reduced remyelination and impaired myelin debris clearance following demyelinating injury. Hum. Mol. Genet 26, 2825–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ, 2013. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol 106-107, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Schmitt S, Bergner CG, Tyanova S, Kannaiyan N, Manrique-Hoyos N, Kongi K, Cantuti L, Hanisch UK, Philips MA, Rossner MJ, Mann M, Simons M, 2015. Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci 18, 1819–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KD, Pandanaboina SC, Srivatsan M, Xie JY, 2020. Predominant differentiation of rat fetal neural stem cells into functional oligodendrocytes in vitro. Neurosci. Lett 736, 135264. [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL, 1999. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc. Natl. Acad. Sci. U. S. A 96, 11486–11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoff RP, Bessert DA, Barks JD, Song D, Cerghet M, Silverstein FS, 2001. Hypoxic-ischemic injury results in acute disruption of myelin gene expression and death of oligodendroglial precursors in neonatal mice. Int. J. Dev. Neurosci 19, 197–208. [DOI] [PubMed] [Google Scholar]
- Snaidero N, Mobius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave KA, Simons M, 2014. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N, 2011. CNS live imaging reveals a new mechanism of myelination: the liquid croissant model. Glia 59, 1841–1849. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C, 2002. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J. Neurosci 22, 9221–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD, 2002. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Shahani N, Strelau J, Laliberte C, Brandt R, Kaplan D, Unsicker K, 2005. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. J. Neurosci 25, 2838–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamydas M, Bessert D, Skoff R, 2009. Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J. Neurosci. Res 87, 3306–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swire M, Ffrench-Constant C, 2018. Seeing is believing: myelin dynamics in the adult CNS. Neuron 98, 684–686. [DOI] [PubMed] [Google Scholar]
- Syed YA, Hand E, Mobius W, Zhao C, Hofer M, Nave KA, Kotter MR, 2011. Inhibition of CNS remyelination by the presence of semaphorin 3A. J. Neurosci 31, 3719–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T, Davis KL, Buxbaum JD, 2011. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog. Neurobiol 93, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejkalova H, Kaiser M, Klaschka J, Stastny F, 2007. Does neonatal brain ischemia induce schizophrenia-like behavior in young adult rats? Physiol. Res 56, 815–823. [DOI] [PubMed] [Google Scholar]
- Thaker GK, 2008. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr. Bull 34, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P, 2014. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tress O, Maglione M, May D, Pivneva T, Richter N, Seyfarth J, Binder S, Zlomuzica A, Seifert G, Theis M, Dere E, Kettenmann H, Willecke K, 2012. Panglial gap junctional communication is essential for maintenance of myelin in the CNS. J. Neurosci 32, 7499–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujhazy E, Dubovicky M, Navarova J, Sedlackova N, Danihel L, Brucknerova I, Mach M, 2013. Subchronic perinatal asphyxia in rats: embryo-foetal assessment of a new model of oxidative stress during critical period of development. Food Chem. Toxicol 61, 233–239. [DOI] [PubMed] [Google Scholar]
- Uluc K, Kendigelen P, Fidan E, Zhang L, Chanana V, Kintner D, Akture E, Song C, Ye K, Sun D, Ferrazzano P, Cengiz P, 2013. TrkB receptor agonist 7, 8 dihydroxyflavone triggers profound gender- dependent neuroprotection in mice after perinatal hypoxia and ischemia. CNS Neurol. Disord. - Drug Targets 12, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umran RM, Al-Tahir M, Jagdish D, Chouthai N, 2016. Insulin-like growth Factor-1 levels in term newborns with hypoxic-ischemic encephalopathy. Am. J. Perinatol 33, 640–645. [DOI] [PubMed] [Google Scholar]
- van Handel M, Swaab H, de Vries LS, Jongmans MJ, 2007. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur. J. Pediatr 166, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilborg E, van Kammen CM, de Theije CGM, van Meer MPA, Dijkhuizen RM, Nijboer CH, 2017. A quantitative method for microstructural analysis of myelinated axons in the injured rodent brain. Sci. Rep 7, 16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilborg E, Achterberg EJM, van Kammen CM, van der Toorn A, Groenendaal F, Dijkhuizen RM, Heijnen CJ, Vanderschuren Ljmj, Benders Mnjl, Nijboer CHA, 2018a. Combined fetal inflammation and postnatal hypoxia causes myelin deficits and autism-like behavior in a rat model of diffuse white matter injury. Glia 66, 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilborg E, de Theije CGM, van Hal M, Wagenaar N, de Vries LS, Benders MJ, Rowitch DH, Nijboer CH, 2018b. Origin and dynamics of oligodendrocytes in the developing brain: implications for perinatal white matter injury. Glia 66, 221–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC, 2000. Hypoxic-ischemic encephalopathy. Am. J. Perinatol 17, 113–120. [DOI] [PubMed] [Google Scholar]
- Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C, 2002. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol. Cell. Neurosci 20, 489–502. [DOI] [PubMed] [Google Scholar]
- Volpe JJ, 2001. Perinatal brain injury: from pathogenesis to neuroprotection. Ment. Retard. Dev. Disabil. Res. Rev 7, 56–64. [DOI] [PubMed] [Google Scholar]
- Volpe JJ, 2012. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann. Neurol 72, 156–166. [DOI] [PubMed] [Google Scholar]
- Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA, 2011. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci 29, 423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW, Chang YC, Chen SJ, Tseng CH, Tu YF, Liao NS, Huang CC, Ho CJ, 2014. TNFR1-JNK signaling is the shared pathway of neuroinflammation and neurovascular damage after LPS-sensitized hypoxic-ischemic injury in the immature brain. J. Neuroinflammation 11, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Armstrong JS, Reyes M, Kulikowicz E, Lee JH, Spicer D, Bhalala U, Yang ZJ, Koehler RC, Martin LJ, Lee JK, 2016. White matter apoptosis is increased by delayed hypothermia and rewarming in a neonatal piglet model of hypoxic ischemic encephalopathy. Neuroscience 316, 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Yang YJ, Yang N, Chen XJ, Huang NX, Zhang J, Wu Y, Liu Z, Gao X, Li T, Pan GQ, Liu SB, Li HL, Fancy SPJ, Xiao L, Chan JR, Mei F, 2018. Enhancing oligodendrocyte myelination rescues synaptic loss and improves functional recovery after chronic hypoxia. Neuron 99 (689-701), e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Y, Wang L, Shi S, Yang C, Jiang W, Luan Z, Liu L, Yao R, 2020. Oligogenesis in the “oligovascular unit” involves PI3K/AKT/mTOR signaling in hypoxic-ischemic neonatal mice. Brain Res. Bull 155, 81–91. [DOI] [PubMed] [Google Scholar]
- Wellmann S, Buhrer C, Schmitz T, 2014. Focal necrosis and disturbed myelination in the white matter of newborn infants: a tale of too much or too little oxygen. Front. Pediatr 2, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins PA, 2003. Lower respiratory problems of the neonate. Vet. Clin. N. Am., Equine Pract 19 (19–33), v. [DOI] [PubMed] [Google Scholar]
- Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M, 2005. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 115, 997–1003. [DOI] [PubMed] [Google Scholar]
- Winkler CC, Franco SJ, 2019. Loss of Shh signaling in the neocortex reveals heterogeneous cell recovery responses from distinct oligodendrocyte populations. Dev. Biol 452, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, Benmamar-Badel A, de Boer-Bergsma JJ, Martin NA, Karram K, Kramer I, Boddeke EW, Waisman A, Eggen BJ, Owens T, 2017. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D, Shen F, He S, Chen M, Han Q, Fang M, Zeng H, Chen C, Deng Y, 2016. IL-1beta induces hypomyelination in the periventricular white matter through inhibition of oligodendrocyte progenitor cell maturation via FYN/MEK/ERK signaling pathway in septic neonatal rats. Glia 64, 583–602. [DOI] [PubMed] [Google Scholar]
- Yang S, Li C, Zhang W, Wang W, Tang Y, 2008. Sex differences in the white matter and myelinated nerve fibers of Long-Evans rats. Brain Res. 1216, 16–23. [DOI] [PubMed] [Google Scholar]
- Zhao L, Mason NA, Morrell NW, Kojonazarov B, Sadykov A, Maripov A, Mirrakhimov MM, Aldashev A, Wilkins MR, 2001. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation 104, 424–428. [DOI] [PubMed] [Google Scholar]
- Zheng K, Wang C, Yang J, Huang H, Zhao X, Zhang Z, Qiu M, 2018. Molecular and genetic evidence for the PDGFRalpha-independent population of oligodendrocyte progenitor cells in the developing mouse brain. J. Neurosci 38, 9505–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ, 2002. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61–73. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ, 2001. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 31, 791–807. [DOI] [PubMed] [Google Scholar]
- Zhu C, Qiu L, Wang X, Hallin U, Cande C, Kroemer G, Hagberg H, Blomgren K, 2003. Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J. Neurochem 86, 306–317. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zhao X, Zheng K, Li H, Huang H, Zhang Z, Mastracci T, Wegner M, Chen Y, Sussel L, Qiu M, 2014. Genetic evidence that Nkx2.2 and Pdgfra are major determinants of the timing of oligodendrocyte differentiation in the developing CNS. Development 141, 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemka-Nalecz M, Janowska J, Strojek L, Jaworska J, Zalewska T, Frontczak-Baniewicz M, Sypecka J, 2018. Impact of neonatal hypoxia-ischaemia on oligodendrocyte survival, maturation and myelinating potential. J. Cell. Mol. Med 22, 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonouzi M, Berger D, Jokhi V, Kedaigle A, Lichtman J, Arlotta P, 2019. Individual oligodendrocytes show bias for inhibitory axons in the neocortex. Cell Rep. 27, 2799–808 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.