Abstract
Acute infection of cattle with bovine herpesvirus 1 (BHV-1) represses cell-mediated immunity, which can lead to secondary bacterial infections. Since BHV-1 can induce apoptosis of cultured lymphocytes, we hypothesized that these virus-host interactions occur in cattle. To test this hypothesis, we analyzed lymph nodes and peripheral blood mononuclear cells (PBMC) after calves were infected with BHV-1. In situ terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) staining of lymphoid tissues (pharyngeal tonsil, cervical, retropharyngeal, and inguinal) was used to detect apoptotic cells. Calves infected with BHV-1 for 7 days revealed increased apoptotic cells near the corticomedullary junction in lymphoid follicles and in the subcapsular region. Increased frequency of apoptotic cells was also observed in the mucosa-associated lymphoid tissue lining the trachea and turbinate. Immunohistochemistry of consecutive sections from pharyngeal tonsil revealed that CD2+ T lymphocytes were positive for the BHV-1 envelope glycoprotein gD. The location of these CD2+ T lymphocytes in the germinal center suggested that they were CD4+ T cells. Electron microscopy and TUNEL also revealed apoptotic and herpesvirus-infected lymphocytes from this area. Fluorescence-activated cell sorting analyses demonstrated that CD4+ and CD8+ T cells decreased in lymph nodes and PBMC after infection. The decrease in CD4+ T cells correlated with an increase in apoptosis. CD4+ but not CD8+ lymphocytes were infected by BHV-1 as judged by in situ hybridization and PCR, respectively. Immediate-early (bovine ICP0) and early (ribonucleotide reductase) transcripts were detected in PBMC and CD4+ lymphocytes prepared from infected calves. In contrast, a late transcript (glycoprotein C) was not consistently detected suggesting productive infection was not efficient. Taken together, these results indicate that BHV-1 can infect CD4+ T cells in cattle, leading to apoptosis and suppression of cell-mediated immunity.
Bovine herpesvirus 1 (BHV-1) is an important viral pathogen of cattle that can cause severe respiratory infection, conjunctivitis, abortion, vulvovaginitis, balanopostitis, and systemic infection in neonate calves. Secondary bacterial infections resulting in pneumonia and death are common (reviewed in reference 58). BHV-1 belongs to the Alphaherpesvirinae subfamily and shares a number of biological properties with herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) (54). BHV-1 establishes lifelong latency in ganglionic neurons of the peripheral nervous system after initial replication in the mucosal epithelia (reviewed in reference 27). Virus reactivation and spread to other susceptible animals occur after natural or corticosteroid-induced stress.
The mechanism of BHV-1-induced immunosuppression has been studied following exposure of animals to virulent or vaccine virus. Increased susceptibility to secondary infection correlates with depressed cell-mediated immunity after infection. Infection decreases interleukin-2 (IL-2) receptor expression (30), impairs IL-2 production, decreases mitogenic stimulation of peripheral blood mononuclear cells (PBMC) (5), impairs cytotoxic responses (2), and decreases circulating T lymphocytes (14, 15). Inhibition of lymphocyte proliferative responses may be the result of nonproductive infection (5). Compromised CD8+ T-cell recognition of infected cells may, in part, result from major histocompatibility complex class I repression by HSV-1 (1, 40, 56, 60) and BHV-1 (12). Down-regulation of the transporter associated with antigen presentation by BHV-1 also occurred (20). Finally, alphaherpesviruses may induce immune dysfunction by enhancing suppressor T-cell activity (reviewed in reference 44).
In humans and mice, the cellular immune response is predominantly CD4+ and Th1-like after HSV-2 infection (37). CD4+ T cells clear virus from cutaneous sites (35) and reduce establishment of latency because HSV replication at the primary site of infection is lower (38). CD4+ T cells are also the limiting cell type for antigen-induced proliferation in BHV-1 infection (7). CD4-induced cytotoxicity is directed against infected cells expressing late HSV-1 glycoproteins, whereas CD8-induced cytotoxicity is directed against immediate-early (IE) and early (E) proteins (36). CD4+ T lymphocytes are crucial for generating primary cytolytic CD8+ T cells against some HSV-1 antigens (26). CD8+ T lymphocytes limit infection in the peripheral nervous system, maintain the integrity of neurons during primary HSV infection (53), and resolve HSV lesions (42). Finally, gamma/delta T cells may be the first line of defense against HSV infection (34) and protect mice from HSV-1 induced encephalitis (50).
Apoptosis, or programmed cell death, leads to chromatin condensation, nuclear fragmentation, and formation of apoptotic bodies. Apoptosis occurs during development and after virus infection (reviewed in references 19 and 52). Several viruses have evolved mechanisms that block the host apoptotic pathway to maximize production of viral progeny and establishment of latency. HSV-1 antigen-expressing cells and noninfected cord blood T lymphocytes stimulated with phytohemagglutinin undergo apoptosis (23). HSV-1 infection of activated T lymphocytes prepared from peripheral blood leads to apoptosis of CD4+ and HLA-DR-positive T lymphocytes but not CD8+ T cells (22). Live and inactivated BHV-1 can induce apoptosis of cultured PBMC following mitogen stimulation (16–18). Cultured CD4+ T lymphocytes that are activated by standard procedures can be infected resulting in apoptosis (9). Apoptosis of T lymphocytes may account for transient lymphocytopenia and immunosupression following BHV-1 infection. However, it is not known if lymphocytes are infected by BHV-1 or if apoptosis occurs following infection of cattle.
In this study, we demonstrate that reduction of CD4+ T lymphocytes occurs in PBMC and lymph nodes during acute infection of cattle. Relative to mock-infected calves, higher levels of apoptosis were detected in lymphoid tissues. BHV-1 DNA, bovine ICP0 (bICP0) RNA (IE), and ribonucleotide reductase (RR) RNA (E) were consistently detected in CD4+ T lymphocytes. These studies demonstrate that BHV-1 infection of CD4+ T lymphocytes leads to apoptosis during acute infection of cattle. We hypothesize that this novel virus host interaction plays an important role in virus-induced immunosuppression.
MATERIALS AND METHODS
Virus and cells.
MDBK cells (American Type Culture Collection, Rockville, Md.) were maintained in Earle’s modified Eagle’s medium (EMEM) with 10% fetal calf serum (FCS). The Cooper strain of BHV-1, supplied by the National Veterinary Services Laboratory, Animal and Plant Health Inspection Services, Ames, Iowa, was propagated in MDBK cells with a multiplicity of infection of 0.05. When cytopathic effect was evident, virus was harvested, titrated (43) in MDBK cells, aliquoted, and stored at −70°C.
Animals.
Weanling dairy calves (5 to 6 months old) that were not vaccinated against BHV-1 and were seronegative for BHV-1 were used for these studies. Calves were inoculated in the right and left conjunctival sacs and intranasally, 1 ml per site, with 107 50% tissue culture infective doses of BHV-1 Cooper strain per ml. Trachea, turbinate membrane, pharyngeal tonsil, cervical, parotid, retropharyngeal, and inguinal lymph nodes were obtained at 7 days postinfection (dpi) from BHV-1-infected or mock-infected calves (7 dpi is the time of the peak of clinical symptoms and maximal viral gene expression in trigeminal ganglia [47]). The tissue was fixed in 10% buffered formalin and processed by routine histological methods. Experiments using animals were done in accordance with the American Association of Laboratory Animal Care guidelines. Calves were housed under strict isolation containment and given antibiotics before and after infection to prevent secondary bacterial infection.
Preparation of CD4+ and CD8+ lymphocytes from lymphoid organs and PBMC.
Peripheral blood lymphocytes (PBL) and single-cell suspensions from lymphoid tissue were prepared from mock-infected and BHV-1-infected calves at 5, 7, 9, 12, and 16 dpi by density gradient centrifugation on Histopaque 1083 (Sigma Chemical Co., St. Louis, Mo.). Cells located at the interface were collected, washed in CMF-PBS (phosphate-buffered saline) (150 mM NaCl, 5 mM KCl, 100 mM NaHCO3, 14 mM glucose [pH 7.4]), and adjusted to a concentration of 2 × 107 cells/ml before primary antibody incubation.
To prepare single-cell suspensions from pharyngeal tonsil and retropharyngeal lymph nodes, tissue fragments were minced with sterilized razor blades in glass petri dishes. Minced tissue was predigested in 1% trypsin (Gibco) in EMEM (Sigma) supplemented with 25 μg of gentamicin (Gibco BRL, Grand Island, N.Y.) per ml for 30 min at 37°C. Trypsin was inactivated by adding 5% FCS, and samples were centrifuged for 10 min at room temperature (500 × g). The supernatant was discarded, and the pellet was suspended in EMEM containing 0.25% of type IV clostridial collagenase (Worthington Biochemical Corp., Freehold, N.J.) and digested at 37°C. The mixture was pipetted every 30 min for 2 to 3 h until a single-cell suspension was obtained.
Cells located at the interface after density gradient centrifugation on Histopaque 1083 were collected, washed in CMF-PBS, and suspended in EMEM supplemented with 10% FCS. Cell preparations from pharyngeal tonsil and lymph nodes (but not PBL) were plated on tissue culture dishes for 2 h in at 37°C in a humidified CO2 incubator to remove adherent cells and debris. Nonadherent cells were removed by pipetting, and dishes were rinsed with medium. Cells were then washed in CMF-PBS and adjusted to a concentration of 2 × 107 cells/ml. Mononuclear cells were incubated with primary monoclonal antibodies, mouse immunoglobulin G1 (IgG1) anti-bovine CD4 (MCA834; Serotec, Oxford, United Kingdom) and mouse IgG2a anti-bovine CD8 (MCA837). CD4+ and CD8+ lymphocytes were purified by positive selection using goat anti-mouse IgG-coated Dynabeads (M-450; Dynal, Lake Success, N.Y.) according to the manufacturers’ recommendation. For fluorescence-activated cell sorting (FACS) analysis, the same secondary antibodies as described for flow cytometry were used.
Flow cytometry.
Indirect immunofluorescence analysis was performed according to standard techniques. PBMC were incubated with primary monoclonal antibodies, mouse IgG1 anti-bovine CD4 and mouse IgG2a anti-bovine CD8, for 1 h at 4°C. Antibodies were diluted according to the manufacturers’ recommendation in CMF-PBS with 1% bovine serum albumin (BSA). PBL were washed for three times in CMF-PBS at 500 × g at 4°C for 8 min. Single-color or double-color immunofluorescence was carried out by incubating with goat anti-mouse IgG1-phycoerythrin (PE) conjugate (Southern Biotechnology Associates, Inc., Birmingham, Ala.) and/or goat anti-mouse IgG2a-fluorescein isothiocyanate (FITC) conjugate (Southern Biotechnology Associates) for 1 h at 4°C. PBL were washed three times and suspended in CMF-PBS. Nonspecific staining was determined by incubating cells with PE and FITC alone or with isotype-matched control antibodies. Initially, the monoclonal antibody mouse IgG2a anti-bovine CD2 (IL-A42; gift from S. Srikumaran, Department of Veterinary and Biomedical Sciences, University of Nebraska, Lincoln) was used to determine the gate settings for lymphocytes. Lymphocyte populations were gated by standard forward and side scatter properties, which excluded debris and monocytes. Flow cytometry analysis and cell sorting for CD4+ and CD8+ lymphocytes were performed with a FACSVantage (Becton Dickinson Immunocytometry Systems, San Jose, Calif.); 2 × 104 events were acquired and analyzed with the CellQuest program.
The frequency of apoptosis was determined by identifying the number of propidium iodide (PI)-stained cells containing hypodiploid DNA. CD4+ and CD8+ T lymphocytes in PBL were labeled with goat anti-mouse IgG-FITC secondary antibody (Sigma) and fixed in 70% ethanol for 30 min at 4°C. These cells were subsequently stained overnight at 4°C in the dark with Telford reagent (0.01 mM EDTA, 26.8 mg of RNase A per liter [93 U/mg], and 50 mg of PI per liter, 0.1% Triton X-100 in PBS). The PI fluorescence of individual cells was measured with a flow cytometer (FACScan; Becton-Dickinson).
Cytospin preparation of CD4+ and CD8+ lymphocytes.
CD4+ and CD8+ lymphocytes were prepared from mock-infected and BHV-1-infected calves (2, 5, 7, and 9 dpi). These cells were applied to Superfrost Plus slides (Fisher Scientific, Pittsburgh, Pa.) by centrifugation at 200 × g for 5 min in a cytocentrifuge at a density of 5 × 104 per spot. Fixation was performed in 4% paraformaldehyde in PBS (pH 7.4) for 5 min at room temperature. The slides were then washed in CMF-PBS and dehydrated with graded ethanol. Dehydrated slides were stored at 4°C. Cells were stained with hematoxylin and eosin for morphological evaluation.
In situ detection of apoptosis.
Tissue sections, 4 to 5 μm thick on Superfrost Plus slides, were deparaffinized in xylene for 10 min, rehydrated in a graded ethanol series, and treated with proteinase K (20 μg/ml; Gibco BRL) in Tris buffer (100 mM Tris-HCl, 150 mM NaCl [pH 7.6]) for 20 min at 37°C. Nick end labeling of DNA strand breaks in the tissue was performed by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, which utilizes alkaline phosphatase (Boehringer Mannheim Corp., Indianapolis, Ind.). Slides were counterstained with methyl green (Vector Laboratories) and then coverslipped with Permount (Fisher).
In situ hybridization (ISH).
The DNA probe specific for BHV-1 glycoprotein C (gC) (229 bp) was contained in plasmid pKS92-2 and was obtained from S. I. Chowdhury (Kansas State University). The gC fragment was labeled by PCR with digoxigenin-dUTP (DIG; Boehringer Mannheim). PCR conditions using gC upstream and downstream primers have been previously described (47). Deproteinization of deparaffinized and rehydrated tissue sections of lymphocytes on slides was carried out in 0.2 N HCl for 20 min at room temperature. Permeabilization with proteinase K (4 μg/ml for cells and 20 μg/ml for tissue; Boehringer Mannheim) was performed as described for tissue in PBS. After rinsing with PBS (three times for 5 min each), cells and tissues were fixed in 4% paraformaldehyde in PBS for 5 min at room temperature. Acetylation was performed to reduce nonspecific binding of the probe to other reactive groups in 0.1 M triethanolamine-HCl buffer (pH 8.0) with 0.25% acetic anhydride. After 5 min of incubation at room temperature, 0.25% acetic anhydride was added for an additional 5 min. Slides were rinsed in 2× SSC (1× SSC is 150 mM NaCl plus 15 mM sodium citrate [pH 7.0]) and then incubated for 1 h at 60°C in 200 μl of prehybridization mixture, consisting of 50% deionized formamide, 4× SSC, 10% dextran sulfate, 1× Denhardt’s solution (0.02% Ficoll 400, 0.02% polyvinylpyrrolidone, 0.02% BSA), 2 mM EDTA, and 500 μg of salmon testis DNA (Sigma) per ml. Labeled probe (0.1 ng/μl) was added to the prehybridization mixture, and hybridization was performed overnight at 56°C.
After hybridization, slides were washed twice in 4× SSC for 5 min at room temperature, once in 2× SSC for 5 min at 45°C, once in 0.2× SSC containing 60% formamide for 5 min at 45°C, twice in 2× SSC for 5 min at room temperature, twice in 0.2× SSC for 5 min at room temperature, and once in buffer I (100 mM maleic acid, 150 mM NaCl [pH 7.5]) for 5 min at room temperature. The anti-DIG-alkaline phosphatase conjugate (Boehringer Mannheim) was diluted according to the manufacturer’s recommendation in buffer II (1% blocking reagent in buffer I; Boehringer Mannheim), and incubation was carried out for 1 h at room temperature. Slides were washed twice in buffer I for 5 min each and then once in buffer III (100 mM Tris-HCl, 100 mM NaCl, 50 mM MgCl2 [pH 9.5]). Slides were next incubated with color substrate solution consisting of 4-nitroblue tetrazolium chloride (Boehringer Mannheim) and 5-bromo-4-chloro-3-indolylphosphate (X-phosphate; Boehringer Mannheim) in buffer III. The color reaction was stopped with TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). Slides were counterstained in methyl green and coverslipped as described above.
Immunohistochemistry (IHC).
Tissues sections, deparaffinized and rehydrated in graded ethanol series as described above, were treated with 0.05% protease (type XIV; Sigma) in Tris buffer (0.05 M Tris [pH 7.6]) for 7 min at room temperature. Nonspecific binding was blocked by incubation with 5% normal swine serum (Sigma) for 45 min at room temperature. The monoclonal antibodies used for this study were IgG2a directed against BHV-1 gD (MM113), IgG2a anti-bovine CD2 (IL-A42), IgG1 anti-bovine CD4 (MCA834; Serotec), IgG2a anti-bovine CD8 (MCA837; Serotec), and IgG anti-bovine macrophage (MCA920; Serotec). Each antibody was diluted to a final concentration of 1 to 5 μg/ml in PBS with 1% BSA, applied to the slides, and then incubated overnight at 4°C. Primary antibodies were detected by using large-volume DAKO LSAB 2 kit alkaline phosphatase (Dako Corp., Carpinteria, Calif.) according to the manufacturer’s directions. Finally, the slides were incubated with freshly prepared substrate (Vector Red Alkaline Phosphatase Substrate Kit I; Vector Laboratories) for 5 to 10 min, rinsed with distilled water, counterstained in methyl green (Vector Laboratories), and coverslipped before microscopic examination. The specificity of the assay was assessed by the lack of positive signal when the gD monoclonal antibody was incubated with tissue from mock-infected calves. A negative reaction using mouse nonimmune serum incubated with mock-infected and BHV-1-infected tissues was also used as a control.
Nucleic acid extractions.
DNA and RNA were extracted from bovine tissues from mock-infected and BHV-1-infected calves as described previously (47). Total RNA was extracted from previously purified CD4+ and CD8+ lymphocytes by using RNAgents (Promega Co., Madison, Wis.) according to the manufacturer’s instructions. DNA extraction from purified CD4+ and CD8+ lymphocytes was performed as described before (47).
DNase I treatment, RT, and PCR.
DNase I treatment, reverse transcription (RT), and PCR were done as described before (47), using primers specific for BHV-1 genes. The IE gene tested was bICP0, and the primers are TTCTCTGGGCTCGGGGCTGC (sense) and AGAGGTCGACAAACACCCGCGGT (antisense). The E gene tested was ribonucleotide RR, and the primers are GACCGCCTGCTCGCTGCTATCC (sense) and GCCTGTGTAGTTGGTGCTGCGGC (antisense). The late (L) gene tested was gC, and the primers are GAGCAAAGCCCCGCCGAAGGA (sense) and TACGAACAGCAGCACGGGCGG (antisense). All oligonucleotides are listed 5′ to 3′.
Electron microscopy.
Lymphoid tissue from mock-infected and BHV-1-infected calves (7 dpi) were cut into 1-mm3 cubes and fixed in 2% buffered glutaraldehyde. Samples were postfixed in 1% osmium tetroxide in phosphate buffer and stained en bloc with uranyl acetate. After dehydration, samples were embedded in Epon araldite, cut into thin sections, stained with lead citrate and uranyl acetate, and then examined with a Philips 410 microscope.
Statistical analyses.
Differences in CD4+ and CD8+ lymphocyte percentages were analyzed by independent t test using a two-tailed P value and P < 0.05 as the criterion for statistical significance.
RESULTS
BHV-1 infection induces apoptosis in lymphoid tissues.
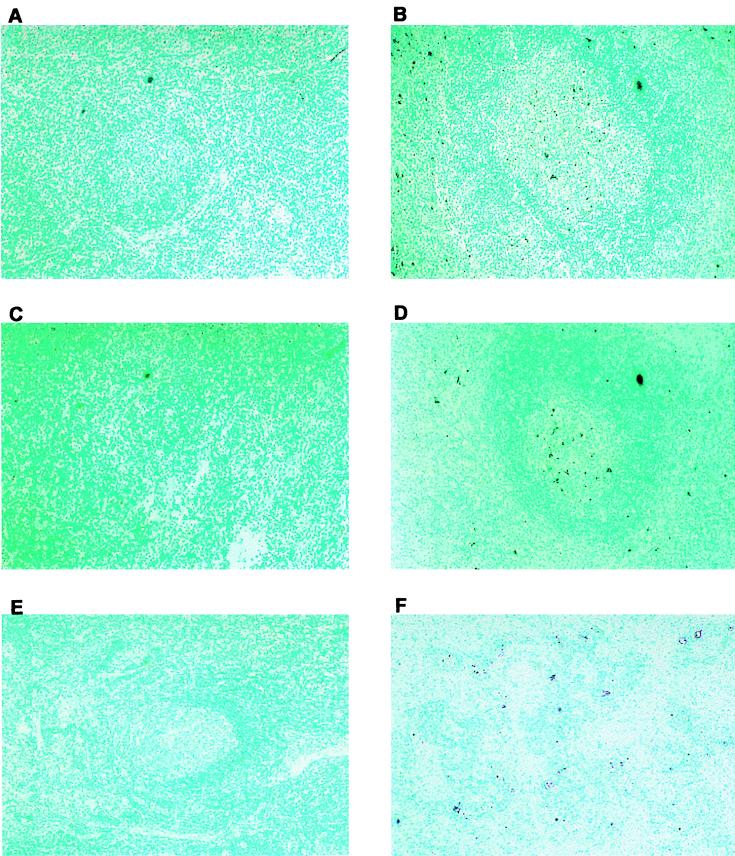
To determine if apoptosis occurred in lymphoid tissue after BHV-1 infection, calves were infected for 7 days and apoptosis was examined in lymph nodes. Previous studies established that cultured lymphocytes undergo apoptosis when infected (9, 16–18), but it is not known if apoptosis occurs when calves are infected. Pharyngeal tonsil contained many TUNEL-positive (TUNEL+) cells at 7 dpi (Fig. 1E and F) relative to mock-infected calves (Fig. 1D). TUNEL+ cells were localized in the corticomedullary junction in germinal centers of secondary lymphoid follicles and subcapsular regions (Fig. 1E and F; Fig. 2B and D). TUNEL+ cells were also detected in lymphoid areas located within the tracheal submucosa, which corresponds to mucosa-associated lymphoid tissue (Fig. 1B and C), and turbinate membrane (Fig. 1H and I).
FIG. 1.
In situ detection of cell death in tissues from mock-infected and BHV-1-infected calves. Thin sections were prepared from various tissues. Free 3′-OH termini were detected by the addition of terminal deoxynucleotidyltransferase and revealed by the anti-DIG-alkaline phosphatase conjugate. Trachea (A to C), pharyngeal tonsil (D to F), and turbinate mucosa (G to I) were tested. Original magnifications: samples from mock-infected calves (A, D, and G), ×100; samples from BHV-1-infected calves at 7 dpi, ×40 (E) and ×100 (B, H, and F); samples from infected calves at 7 dpi (C and I), ×250. Methyl green was used as counterstaining. Dark purple color indicates the TUNEL+ cells.
FIG. 2.
In situ detection of cell death in lymph nodes from mock-infected and BHV-1-infected calves. Thin sections were prepared as described for Fig. 1 from cervical lymph nodes from a mock-infected (A) and a BHV-1-infected calf (B), retropharyngeal lymph nodes from a mock-infected (C) and a BHV-1 infected calf (D), and inguinal lymph nodes from a mock-infected (E) and a BHV-1 infected calf (F). All samples are at a magnification of ×100. Methyl green was used to counterstain. Dark purple color indicates the TUNEL+ cells.
To determine if lymph nodes near the site of infection contained increased TUNEL+ cells, cervical and retropharyngeal lymph nodes were examined at 7 dpi. Relative to mock-infected calves, cervical and retropharyngeal lymph nodes contained more TUNEL+ cells and staining was located in or near the follicle (Fig. 2B and D). Inguinal lymph node from infected calves also had TUNEL+ cells (Fig. 2F), but fewer than in pharyngeal tonsil. As expected, few or no TUNEL+ cells were detected in the corresponding tissues of mock-infected animals (Fig. 1A, D, and G; Fig. 2A, C, and E). In summary, following infection, higher levels of TUNEL+ cells were detected in pharyngeal tonsil and other lymph nodes.
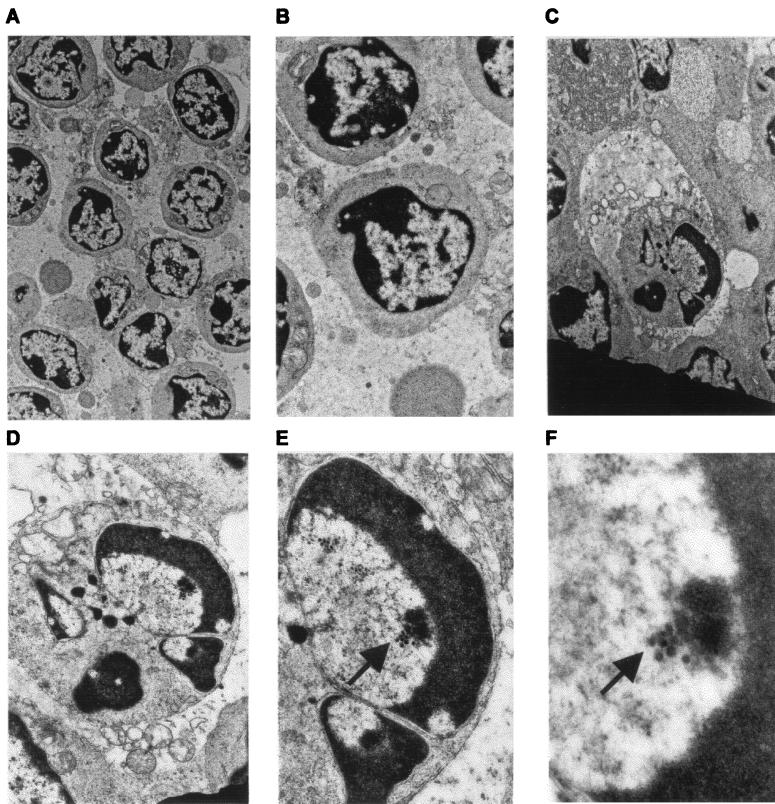
As expected, electron microscopy revealed that lymphocytes from mock-infected calves had no marginally condensed chromatin (Fig. 3A and B). In contrast, lymphocytes in lymph nodes of infected cattle contained condensed chromatin and other morphological characteristics seen during apoptosis. One apoptotic lymphocyte from a lymphoid follicle in pharyngeal tonsil of BHV-1-infected calves is shown in Fig. 3C to F. This lymphocyte has condensed chromatin shaped like a horseshoe or half-moon (Fig. 3C to F). The cell adjacent to the lymphocyte appears to be a dendritic cell or macrophage. The close proximity of the lymphocyte to the large cell suggested that it was phagocytosed. However, this was unlikely because we found no evidence of two double membranes surrounding the lymphocyte, which is indicative of phagocytosis. Herpesvirus nucleocapsid structures measuring 105 nm in diameter were detected inside the apoptotic nucleus (Fig. 3E and F). All apoptotic lymphocytes had morphological changes consistent to that shown in Fig. 3C, and many contained herpesvirus nucleocapsids within apoptotic nuclei.
FIG. 3.
Electron microscopy of pharyngeal tonsil from mock-infected and BHV-1-infected calves. Mock-infected and BHV-1-infected calves were euthanized, and pharyngeal tonsil tissue was collected, fixed in 2% buffered glutaraldehyde, and embedded in Epon araldite. Thin sections were stained with lead citrate and uranyl acetate and then examined with a Philips 410 microscope. (A and B) Sections from the germinal center in pharyngeal tonsil prepared from a mock-infected calf (magnification of ×6,000 [A] and 14,000 [B]); (C to F) micrographs of a BHV-1-infected calf (7 dpi). In panels C and D, a phagocytic cell (×9,000 and ×20,000) is in the process of engulfing the apoptotic lymphocyte; in panels E and F, the same apoptotic lymphocyte contains herpesvirus nucleocapsids (arrows) measuring 105 nm in diameter (×40,000 and ×90,000).
To test whether infection led to decreased levels of CD4+ and CD8+ T-lymphocyte populations in pharyngeal tonsil and retropharyngeal lymph nodes, mononuclear cells were prepared by density gradient centrifugation. Adherent cells were separated from the other mononuclear cells by incubating on a plastic culture dish for 2 h at 37°C in a humidified CO2 incubator. T cells were not readily detected in the adherent population based on cell morphology and hematoxylin-eosin staining (57). At 7 dpi, threefold fewer CD4+ T cells were detected in the pharyngeal tonsil (n = 2, P = 0.013). CD8+ T-cell numbers were also decreased in the pharyngeal tonsil at 7 dpi (n = 2, P = 0.022) (Fig. 4). The retropharyngeal lymph node also had decreased levels of CD4+ T cells (n = 2, P = 0.02) and CD8+ T cells (n = 2, P = 0.028) at 7 dpi. In summary, these results demonstrated that lymph nodes located close to the initial site of infection contained high levels of apoptotic lymphocytes, herpesvirus nucleocapsids were detected in these lymphocytes, and the numbers of T cells decreased.
FIG. 4.
Analysis of T-lymphocyte populations in pharyngeal tonsil and retropharyngeal lymph nodes. CD4+ and CD8+ lymphocytes from mock-infected and BHV-1-infected calves (7 dpi) were prepared from pharyngeal tonsil and retropharyngeal lymph node as described in Materials and Methods. Mononuclear cells were incubated with monoclonal antibodies directed against bovine CD4+ and CD8+ T cells. FITC- or PE-conjugated IgG that was directed against the monoclonal antibody was used as a secondary antibody. FITC- or PE-positive cells were identified by flow cytometry.
Detection of gD in lymphoid tissue of infected calves.
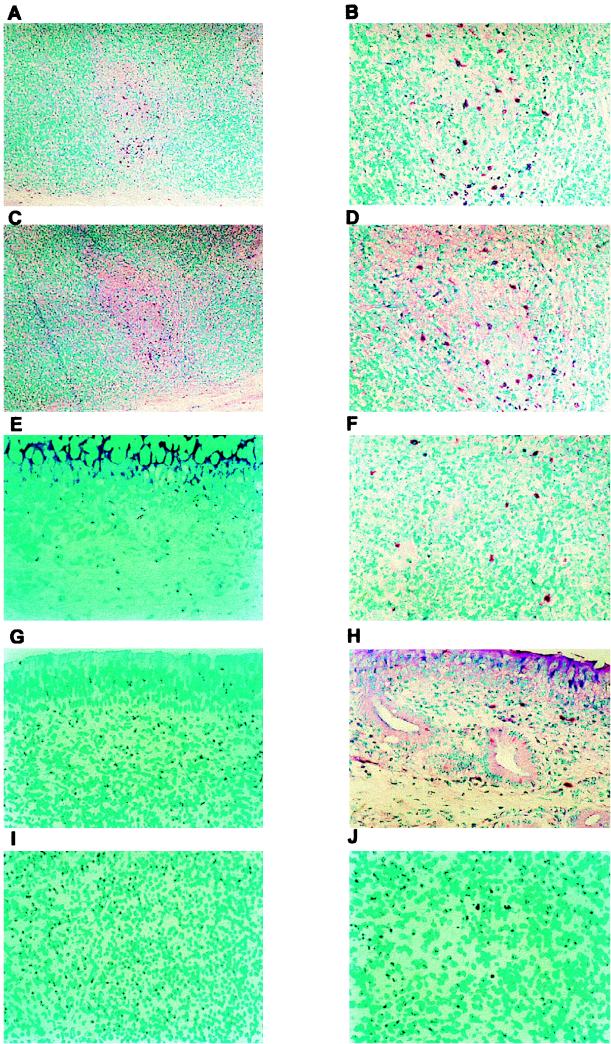
The results in Fig. 1 to 3 suggested that BHV-1 could infect lymphocytes of calves, leading to a reduction in the number of T cells in the tonsil and retropharyngeal lymph nodes. To test this hypothesis, we examined the relevant tissues for viral glycoprotein (gD) by using a monoclonal antibody and IHC. gD was consistently detected in lymphoid and nonlymphoid tissues from two infected calves but not from two uninfected calves (Fig. 5 and data not shown). In lymphoid tissues, gD was detected in the germinal centers, the corticomedullary junction of lymphoid follicles, the subcapsular region, and randomly throughout individual lymphoid tissues (Fig. 5A and B). gD was also detected in the submucosa of turbinate and trachea (Fig. 5F and H). Consecutive sections of pharyngeal tonsil revealed that some of the infected cells also expressed the lymphocyte surface protein CD2 present in the germinal center (Fig. 5A to D). We observed overlapping of gD and CD2+ cells in 13 individual cells from a single germinal center. The location of these CD2+ T lymphocytes suggested they are CD4+ T cells, which are normally present in germinal centers to activate B lymphocytes. In other lymph nodes (cervical, retropharyngeal, and parotid), gD was also detected (data not shown). A few gD-positive cells were present in the inguinal lymph node (Fig. 5J). Although this study suggested that gD was expressed in these tissues as a result of productive infection, it was also possible that virus was delivered to lymph nodes via the lymphatic system but infection did not occur.
FIG. 5.
gD and CD2 detection by IHC on tissue sections of mock-infected and BHV-1-infected calves. Tissue sections were incubated with gD or CD2 monoclonal antibodies overnight at 4°C. After the slide was washed, the biotinylated secondary antibody was added. The alkaline phosphatase reaction was performed as described in Materials and Methods. (A and B) Pharyngeal tonsil stained for BHV-1 gD in BHV-1-infected calves (7 dpi) (magnifications of ×100 and ×250, respectively); (C and D) pharyngeal tonsil stained for the lymphocyte surface protein CD2 (magnification of ×100 and ×250, respectively) in consecutive sections from panels A and B; (E to I) gD staining of tissue from the turbinate membrane (F), tracheal submucosa (H), and inguinal lymph nodes (J) of calves infected for 7 days and gD staining of turbinate mucosa (E), tracheal mucosa (G), and inguinal lymph node (I) of an infected calf (all at a magnification of ×250). The results are representative of data obtained from three different calves.
Analysis of T lymphocytes in PBMC during acute infection.
Figures 1 to 5 demonstrated that virus infection of lymphocytes occurred in lymphoid tissue near the site of infection. However, it was not clear whether circulating lymphocytes become infected or undergo apoptosis as a consequence of infection. Six-month-old calves that were seronegative for BHV-1 were bled three times before BHV-1 infection, and the normal levels of CD4+ and CD8+ T lymphocytes were compared to those in infected calves. After BHV-1 infection, calves were bled at regular intervals (5, 7, 9, 12, and 16 dpi) and the distribution of CD4+ and CD8+ T lymphocytes was determined. It was not necessary to incubate PBMC with plastic to remove adherent cells and debris because T cells in PBMC were readily separated from other cell populations during FACS (57). BHV-1 infection reduced the CD4+ T-cell population in PBMC at days 5 (n = 4, P = 0.014), 7 (n = 4, P = 0.002), 9 (n = 3, P = 0.009), and 12 (n = 4, P = 0.008) (Table 1). CD8+ T-cell populations also decreased slightly at days 7 (n = 4, P = 0.001) and 9 (n = 3, P = 0.010).
TABLE 1.
Flow cytometric analysis of CD4+ and CD8+ T lymphocytes from PBMCa
| T lymphocytes | % of total T lymphocytes (no. of calves tested)
|
|||||
|---|---|---|---|---|---|---|
| Mock | 5 dpi | 7 dpi | 9 dpi | 12 dpi | 16 dpi | |
| CD4+ | 20.5 (3) | 15.5 (4)b | 14.5 (4)b | 15.1 (3)b | 15.8 (4)b | 18.8 (2) |
| CD8+ | 13.6 (3) | 13.4 (4) | 9.4 (4)b | 9.1 (3)b | 15.2 (4) | 14.7 (2) |
Calves that were seronegative for BHV-1 were inoculated with 107 50% tissue culture infective doses of BHV-1 per ml. At the indicated times, CD4+ and CD8+ T lymphocytes were prepared from PBMC after density gradient centrifugation on Histopaque 1083. Mononuclear cells were incubated with a monoclonal antibody directed against bovine CD4 or anti-bovine CD8. A secondary antibody conjugated to FITC or PE was then incubated with the samples, and flow cytometry was performed.
Significant difference (P < 0.05, independent t test) compared with mock-infected calves.
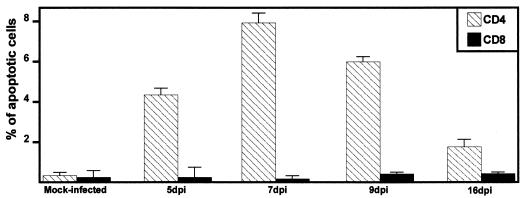
Two-color immunofluorescence of PBMC to measure apoptosis in CD4+ and CD8+ T lymphocytes was performed with mouse anti-bovine CD4 and CD8 antibodies, FITC-labeled secondary antibodies, and PI. The percentage of apoptotic cells was determined by measuring the subgenomic DNA peak in mock-infected and infected calves at different times postinfection. Figure 6 shows representative data from flow cytometric analysis stained with PI. Increased apoptosis was detected in CD4+ T lymphocytes from PBMC at days 5 (n = 2, P = 0.006), 7 (n = 2, P = 0.002), 9 (n = 2, P = 0.002), and 16 (n = 2, P = 0.022) after infection. At 7 dpi, numbers of CD4+ T cells undergoing apoptosis were 30-fold higher than in the same animal prior to infection. In contrast, the number of apoptotic CD8+ T lymphocytes was not significantly different from the number in BHV-1-infected calves (P > 0.05). In summary, the results demonstrated that infection increased the number of apoptotic CD4+ T cells but not CD8+ cells in PBMC, which resulted in a transient decrease in T cells during acute infection.
FIG. 6.
Analysis of apoptosis in T-lymphocyte populations prepared from PBMC. CD4+ and CD8+ lymphocytes were prepared from PBMC of mock-infected and infected calves as described in Materials and Methods. Ethanol-fixed lymphocytes were stained with Telford reagent. The PI fluorescence of individual cells with hypodiploid DNA content was measured with a flow cytometer.
Detection of BHV-1 DNA in lymphocytes.
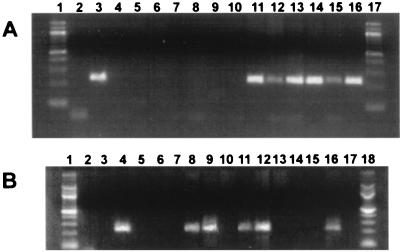
PCR was performed with gC-specific primers to determine if BHV-1 DNA was present in pharyngeal tonsil, cervical, retropharyngeal, parotid, and inguinal lymph nodes and in PBMC. The expected product of 229 bp was amplified in the tissue from an infected calf at 7 dpi (Fig. 7A, lanes 11 to 15) but not in the tissue from a mock-infected calf (lanes 5 to 10). No PCR product was amplified from the nontemplate control (lane 2) or from uninfected MDBK cells (lane 4). The two positive controls, MDBK cells infected for 18 h with BHV-1 (lane 3) and trigeminal ganglia from a BHV-1-infected calf (lane 16), contained the 229-bp PCR product.
FIG. 7.
Detection of BHV-1 DNA in lymphoid tissue and lymphocytes after infection. (A) Pharyngeal tonsil, cervical, retropharyngeal, parotid, and inguinal lymph nodes and trigeminal ganglia were collected from euthanized calves. DNA extraction and PCR conditions were as described in Materials and Methods. Lanes: 1 and 17, molecular weight marker (100-bp DNA ladder); 2, no-template control; 3, MDBK cells infected with BHV-1 for 16 h; 4, mock infected MDBK cells; 5 to 10, pharyngeal tonsil, cervical, retropharyngeal, parotid, and inguinal lymph nodes and trigeminal ganglia, respectively, from a mock-infected calf; 11 to 16, pharyngeal tonsil, cervical, retropharyngeal, parotid, and inguinal lymph nodes and trigeminal ganglia, respectively, from a BHV-1-infected calf (7 dpi). The results are representative of four different infected calves. (B) PBMC were prepared by equilibrium centrifugation. CD4+ and CD8+ lymphocytes were prepared from PBMC and pharyngeal tonsil as described in Materials and Methods. DNA extraction and PCR conditions were as described in Materials and Methods. Lanes: 1 and 18, molecular weight marker (100-bp DNA ladder); 2, no-template control; 3, mock-infected MDBK cells; 4, MDBK cells infected with BHV-1 for 16 h; 5, PBMC from a mock-infected calf; 6 and 7, CD4+ (lane 6) and CD8+ T cells (lane 7) prepared from peripheral blood of a mock-infected calf; 8 to 10, PBMC, CD4+, and CD8+ cells (peripheral blood), respectively, from a BHV-1-infected calf (5 dpi); 11 to 13, PBMC, CD4+, and CD8+ cells (peripheral blood), respectively, from a BHV-1-infected calf (7 dpi); 14 and 15, CD4+ (lane 14) and CD8+ (lane 15) cells (pharyngeal tonsil) from a mock-infected calf; 16 and 17, CD4+ (lane 16) and CD8+ (lane 17) cells (pharyngeal tonsil) from a BHV-1-infected calf (7 dpi). Two micrograms of DNA was used for each PCR. The results are representative of four different infected calves.
As expected, no PCR product was amplified from the nontemplate control (Fig. 7B, lane 2) or uninfected MDBK cells (lane 3). The amplified product was also not detected in PBMC (lanes 5 to 7) or T lymphocytes prepared from pharyngeal tonsil (lanes 14 and 15) of a mock-infected calf. The amplified 229-bp product was detected when DNA was prepared from PBMC and from sorted CD4+ lymphocytes at 5 and 7 dpi (lanes 8, 9, 11, and 12). CD4+ lymphocytes from pharyngeal tonsil at 7 dpi also contained the amplified product (lane 16). BHV-1 DNA was not detected in CD8+ lymphocytes at 5 and 7 dpi from PBMC and from pharyngeal tonsil (lanes 10, 13, and 17).
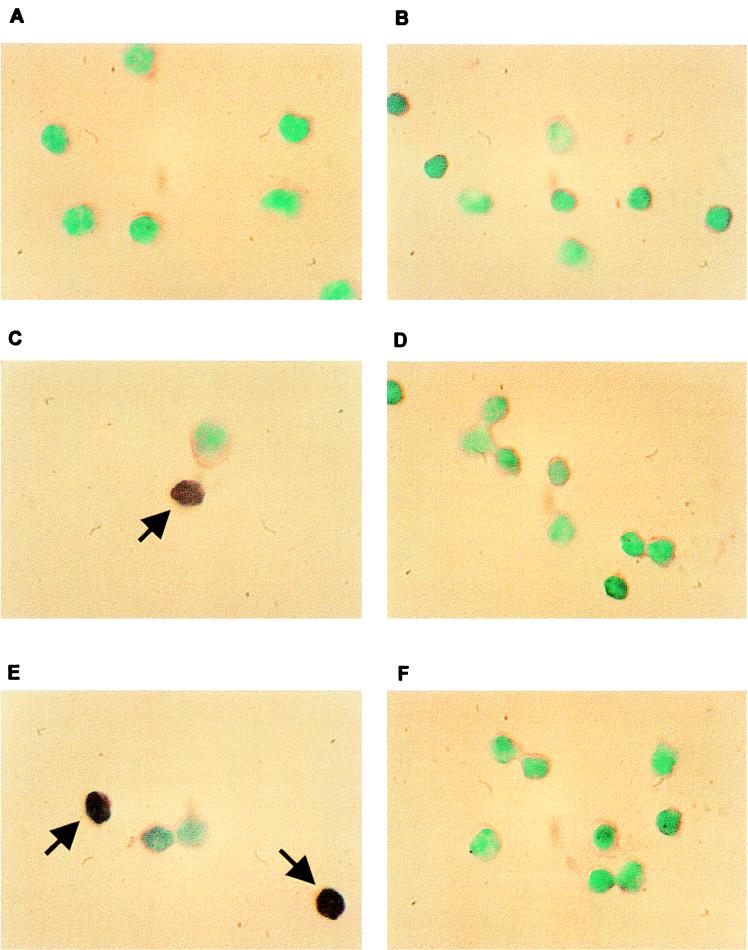
To rule out the possibility that the PCR results was not due to contaminating non-T cells, ISH was performed with sorted CD4+ and CD8+ T lymphocytes from mock-infected and BHV-1-infected calves. The DIG-labeled BHV-1 gC probe (229 bp) used for these studies was detected with anti-DIG-alkaline phosphatase conjugate. BHV-1 DNA was not detected in mock-infected CD4+ and CD8+ lymphocytes prepared from PBMC (Fig. 8A and B). CD4+ T cells from both BHV-1-infected calves showed positive hybridization signal at 7 dpi (Fig. 8C and E). Approximately 13% of the CD4+ T lymphocytes were BHV-1 positive at 7 dpi. BHV-1 DNA was also detected in CD4+ T lymphocytes at 5 and 9 dpi (data not shown). At 7 dpi, less than 1% of the CD8+ T lymphocytes were positive, as judged by ISH (Fig. 8D and F). As judged by hematoxylin-eosin staining by light microscopy, approximately 98% of the lymphocyte populations were small agranular cells showing a relative high nuclear/cytoplasmic ratio. ISH-positive lymphocytes were also detected in germinal centers of pharyngeal tonsil at 7 dpi (data not shown). In summary, these results demonstrated that CD4+ T cells contained BHV-1 DNA sequences.
FIG. 8.
Detection of BHV-1 DNA by ISH in T lymphocytes prepared from PBMC. The gC probe and ISH conditions are described in Materials and Methods. PBMC were prepared from mock-infected and BHV-1-infected calves by equilibrium centrifugation. CD4+ and CD8+ lymphocytes were sorted by FACS and applied to slides by cytospin. Shown are CD4+ (A) and CD8+ (B) lymphocytes prepared from a mock-infected calf, CD4+ (C) and CD8+ (D) lymphocytes from a calf infected for 7 days, and CD4+ (E) and CD8+ (F) lymphocytes from another calf infected for 7 days. Dark purple staining in the nucleus of CD4+ T cells in panels C and E was indicative of a positive ISH signal (denoted by arrows). Methyl green was used for counterstaining; magnification is ×1,000.
Detection of viral gene expression in PBMC.
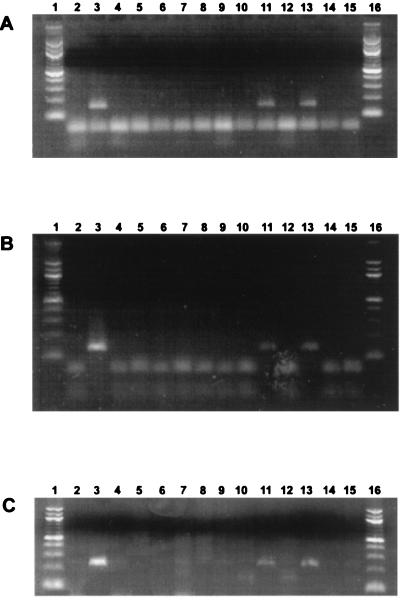
To determine if viral gene expression occurred in CD4+ T cells, RT-PCR was performed with primers that detect IE (bICP0), E (RR), or L (gC) transcripts. Two-color immunofluorescence of PBMC was performed to sort CD4+ and CD8+ T cells, and RNA was prepared from the samples (47). bICP0 cDNA was amplified in PBMC, and CD4+ lymphocytes were prepared from PBMC at 7 dpi (Fig. 9A, lanes 11 and 13). RR transcripts were also detected in PBMC and CD4+ T cells (Fig. 9B, lanes 11 and 13). gC RNA was detected in PBMC and CD4+ lymphocytes at 7 dpi in three of three PCRs from only one of three calves (Fig. 9C, lane 11 and 13). In contrast, RR and bICP0 were detected in all calves tested. The same primers did not amplify cDNAs from isolated CD8+ lymphocytes at 7 dpi (Fig. 9A to C, lane 15). When reverse transcriptase was omitted from RT reactions, amplified products were not detected in the positive samples (Fig. 9A to C, lanes 10, 12, and 14). As expected, the primers (bICP0, RR, and gC) did not amplify cDNA products from mock-infected calves (Fig. 9A to C, lanes 5, 7, and 9). In summary, expression of bICP0 and RR genes was consistently detected in CD4+ T cells but not CD8+ T cells.
FIG. 9.
Productive viral gene expression occurs in PBMC and lymphoid tissue. RNA extraction, RT, and PCR were performed as described in Materials and Methods. cDNAs were PCR amplified with bICP0 (A), RR (B), and gC (C) primers. Lanes: 1 and 16, molecular weight marker (100-bp DNA ladder); 2 and 3, MDBK cells infected with BHV-1 without and with RT, respectively; 4 and 5, PBMC from a mock-infected calf without and with RT, respectively; 6 and 7, CD4+ T cells prepared from a mock-infected calf without and with RT, respectively; 8 and 9, CD8+ T cells from a mock-infected calf without and with RT, respectively; 10 and 11, PBMC from a BHV-1-infected calf without and with RT, respectively; 12 and 13, CD4+ T cells from a BHV-1-infected calf without and with RT, respectively; 14 and 15, CD8+ T cells from a BHV-1-infected calf without and with RT, respectively.
DISCUSSION
In this study, we demonstrated that infection of cattle with BHV-1 leads to infection of CD4+ T cells but not CD8+ T cells. gC RNA expression was not consistently detected in PBMC or CD4+ T cells, suggesting a block in the infection cycle. Infection of CD4+ T cells led to an increase in apoptosis. It is also likely that the stress of infection resulted in higher levels of corticosteroids, which can lead to lymphocyte apoptosis and immunosuppression (19a). Consequently, we hypothesized that BHV-1 infection, directly and indirectly, induced apoptosis of CD4+ T cells, resulting in transient immunosuppression. This novel virus-host interaction is important because it would increase the probability that secondary bacterial infections occur.
Many viruses induce apoptosis when cultured cells are infected (reviewed in references 41, 52, and 59). Previous studies with cultured cells have shown that BHV-1 induces apoptosis in activated T lymphocytes, mitogen-stimulated PBMC, B lymphocytes, monocytes, CD4+ T lymphocytes (9, 13, 16–18), and nonlymphoid cells (8). Prior to this study, it was not known whether apoptosis occurred when cattle were infected. TUNEL+ cells were detected in lymphoid tissues (pharyngeal tonsil, cervical, retropharyngeal, and inguinal lymph nodes), trachea, and turbinate membrane from infected calves at 7 dpi. Viral DNA and proteins were also detected in the same sections that contained TUNEL+ cells, suggesting that virus infection induced apoptosis.
Since the TUNEL assay can detect degradation of DNA during necrotic cell death (29), electron microscopy was used to confirm that apoptosis occurred. Apoptotic cells that have the characteristic morphology and size of lymphocytes were detected in the pharyngeal tonsil of infected calves at 7 dpi. Apoptotic cells provide a potent stimulus for phagocytosis through exposure of phospholipids (10), suggesting that this occurs after BHV-1 infection. Viral nucleocapsids (105 nm), which are typical for alphaherpesviruses (6), were frequently detected inside the nuclei of apoptotic cells. Further studies demonstrated that viral DNA (Fig. 7B and 8) and viral gene expression (Fig. 9) occurred in CD4+ T cells. Thus, reduction of CD4+ T cells was, in part, the result of virus infection and apoptosis.
The presence of gD and TUNEL+ cells in germinal centers and mucosa-associated lymphoid tissue suggested this was the route used to infect T lymphocytes and disseminate through the lymphatic system. CD2+ T cells expressing BHV-1 antigens within the germinal center of the pharyngeal tonsil may be CD4+ T helper cells (49). B cells present in germinal centers require second signals, provided by CD4+ T helper cells, for their terminal differentiation to antibody-producing plasma cells (reviewed in reference 46). An earlier study demonstrated that BHV-1 infection can occur within germinal centers of pharyngeal tonsil, but virus replication in leukocytes was not detected (48). In this study, we were unable to demonstrate that CD8+ T cells (Fig. 7 to 9) were infected. It is tempting to speculate that certain populations of CD4+ T cells express a protein that can serve as a receptor for virus infection. Other viruses (e.g., canine distemper virus [24] and human herpesvirus 6 [33, 35]), can also infect CD4+ T cells and induce immunosuppression, suggesting this is a common strategy to enhance virus spread in vivo.
A slight increase in apoptosis was also observed in CD8+ T cells (Fig. 6) and macrophages (data not shown) after infection. TUNEL+ macrophages could be the result of BHV-1 infection (16, 45) or related to phagocytosis of apoptotic bodies. Since few CD8+ T cells were positive for BHV-1 DNA, it is possible that apoptosis observed in CD8+ cells was related to an indirect mechanism of virus infection, the bystander effect. The bystander effect mediates apoptosis in CD8+ T cells and other uninfected cells of HIV-positive individuals (11, 25, 39). With respect to human herpesvirus 6, entry and replication are not required for induction of apoptosis in lymphocytes (21, 59). Following infection of cattle, apoptosis of uninfected cells could also be induced by CTL that have promiscuous cytotoxic activity (31).
CD4+ T cells, but not gamma/delta T cells or CD8+ T cells, were identified as the limiting cell type for antigen-induced proliferation, thus playing a pivotal role in BHV-1 infection (7). CD4+ T cells are necessary to generate a CD8+ CTL response and viral clearance after infection with ectromelia virus (28), vaccinia virus, lymphocytic choriomeningitis virus, HSV (26), and Moloney sarcoma virus (3, 32). In ectromelia virus infection, depletion of CD4+ T lymphocytes reduces CTL responses threefold and inhibits viral clearance. The presence of CD8+ T cells is required for viral clearance of influenza A virus (4), ectromelia virus (28), and HSV (42) infection. CD4 depletion leads to higher titers of HSV-1 challenge virus at the site of infection and increases viral load in trigeminal ganglia (38). We suggest that BHV-1 infection and apoptosis of CD4+ T cells contribute to immunosuppression and enhance establishment of latency.
ACKNOWLEDGMENTS
This research was supported by grants from the USDA (9702394 and 9802064) and the Center for Biotechnology.
We thank F. Osorio and C. Wood for critically reviewing the manuscript. We acknowledge K. Arumuganathan, (Center for Biotechnology, University of Nebraska, Lincoln) for assistance with flow cytometry and S. I. Chowdhury (Department of Diagnostic Medicine and Pathobiology, College of Veterinary Medicine, Kansas State University) for the gC plasmid. At the Department of Veterinary and Biomedical Sciences, University of Nebraska, Lincoln, we are grateful to T. Bargar for assistance with electron microscopy, S. Srikumaran for the monoclonal antibodies directed against BHV-1 gD and CD2, T. Holt for technical assistance, and M. Stone for helpful comments. We thank J.-H. Sur (Plum Island Animal Disease Center, African Swine Fever Virus Research Group) for the ISH protocols and helpful suggestions. We also thank B. Clowser, M. Klintworth, J. Wilkinson and T. Green for assistance with cattle experiments. Finally, we thank R. Olmscheid and V. Johns for assistance with histological preparations.
REFERENCES
- 1.Ahn K, Meyer T H, Uebel S, Sempé P, Djaballah H, Yang Y, Peterson P A, Früh K, Tampé R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 2.Babiuk L A, Bielefeldt Ohmann H. Bovine herpesvirus-1 (BHV-1) infection in cattle as a model for viral induced immunosuppression. In: Gilmore N, Wainberg M A, editors. Viral mechanisms of immune suppression. New York, N.Y: Alan R. Liss; 1985. pp. 99–114. [Google Scholar]
- 3.Battegay M, Bachmann M F, Burhkart C, Viville S, Benoist C, Mathis D, Hengartner H, Zinkernagel R M. Antiviral immune responses of mice lacking MHC class II or its associated invariant chain. Cell Immunol. 1996;167:115–121. doi: 10.1006/cimm.1996.0014. [DOI] [PubMed] [Google Scholar]
- 4.Bender B S, Croghan T, Zhang L, Small P A., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter J J, Weinberg A D, Pollard A, Reeves R, Magnuson J A, Magnuson N S. Inhibition of T-lymphocyte mitogenic responses and effects on cell functions by bovine herpesvirus 1. J Virol. 1989;63:1525–1530. doi: 10.1128/jvi.63.4.1525-1530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dargan D J. Electron microscopy of proteins. 5. Viral structure. London, England: Academic Press, Inc., London Ltd.; 1986. The structure and assembly of herpesviruses; pp. 359–437. [Google Scholar]
- 7.Denis M, Kaashoek M J, van Oirschot J T, Pastoret P P, Thiry E. Quantitative assessment of the specific CD4+ T lymphocyte proliferative response in bovine herpesvirus 1 immune cattle. Vet Immunol Immunopathol. 1994;42:275–286. doi: 10.1016/0165-2427(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 8.Devireddy L R, Jones C J. Activation of caspases and p53 by bovine herpesvirus 1 infection results in programmed cell death and efficient virus release. J Virol. 1999;73:3778–3788. doi: 10.1128/jvi.73.5.3778-3788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskra L, Splitter G A. Bovine herpesvirus-1 infects activated CD4+ lymphocytes. J Gen Virol. 1997;78:2159–2166. doi: 10.1099/0022-1317-78-9-2159. [DOI] [PubMed] [Google Scholar]
- 10.Fadok V A, Voelker D R, Campbell P A, Coehn J J, Braton D L, Henson P M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 11.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks T, Baba T W, Ruprecht R M, Kupper A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith K, Chen W, Johnson D C, Hendricks R L. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griebel P J, Ohmann H B, Lawman M J, Babiuk L A. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. J Gen Virol. 1990;71:369–377. doi: 10.1099/0022-1317-71-2-369. [DOI] [PubMed] [Google Scholar]
- 14.Griebel P J, Qualtiere L, Davis W C, Lawman M J, Babiuk L A. –1988. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol. 1987;1:267–286. doi: 10.1089/vim.1987.1.267. [DOI] [PubMed] [Google Scholar]
- 15.Griebel P J, Qualtiere L, Davis W C, Gee A, Bielefeldt Ohmann H, Lawman M J, Babiuk L A. –1988. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1987;1:287–304. doi: 10.1089/vim.1987.1.287. [DOI] [PubMed] [Google Scholar]
- 16.Hanon E, Lambot M, Hoornaert S, Lyaku J, Pastoret P P. Bovine herpesvirus 1-induced apoptosis: phenotypic characterization of susceptible peripheral blood mononuclear cells. Arch Virol. 1998;143:441–452. doi: 10.1007/s007050050301. [DOI] [PubMed] [Google Scholar]
- 17.Hanon E, Hoornaert S, Dequiedt F, Vanderplasschen A, Lyaku J, Willems L, Pastoret P P. Bovine herpesvirus 1-induced apoptosis occurs at the G0/G1 phase of the cell cycle. Virology. 1997;232:351–358. doi: 10.1006/viro.1997.8562. [DOI] [PubMed] [Google Scholar]
- 18.Hanon E, Vanderplasschen A, Lyaku S, Keil G, Denis M, Pastoret P P. Inactivated bovine herpesvirus 1 induces apoptotic cell death of mitogen-stimulated bovine peripheral blood mononuclear cells. J Virol. 1996;70:4116–4120. doi: 10.1128/jvi.70.6.4116-4120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardwick J M. Viral interference with apoptosis. Cell Dev Biol. 1998;9:339–349. doi: 10.1006/scdb.1998.0243. [DOI] [PubMed] [Google Scholar]
- 19a.Heimberg A, Auphan N, Caelles C, Karin M. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 1995;14:452–460. doi: 10.1002/j.1460-2075.1995.tb07021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinkley S, Hill A B, Srikumaran S. Bovine herpesvirus-1 infection affects the peptide transport activity in bovine cells. Virus Res. 1998;53:91–96. doi: 10.1016/s0168-1702(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Yasukawa M, Fujita S. Induction of T-cell apoptosis by human herpesvirus 6. J Virol. 1997;71:3751–3759. doi: 10.1128/jvi.71.5.3751-3759.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito M, Watanabe M, Kamiya H, Sakurai M. Herpes simplex virus type 1 induces apoptosis in peripheral blood T lymphocytes. J Infect Dis. 1997;175:1220–1224. doi: 10.1086/593672. [DOI] [PubMed] [Google Scholar]
- 23.Ito M, Koide W, Watanabe M, Kamiya H, Sakurai M. Apoptosis of cord blood T lymphocytes by herpes simplex virus type 1. J Gen Virol. 1997;78:1971–1975. doi: 10.1099/0022-1317-78-8-1971. [DOI] [PubMed] [Google Scholar]
- 24.Iwatsuki K, Okita M, Ochikubo F, Gemma T, Shin Y S, Miyashita N, Mikami T, Kai C. Immunohistochemical analysis of the lymphoid organs of dogs naturally infected with canine distemper virus. J Comp Pathol. 1995;113:185–190. doi: 10.1016/s0021-9975(05)80033-7. [DOI] [PubMed] [Google Scholar]
- 25.Jamieson B D, Uittenbogaart C H, Schmid I, Zack J A. High viral burden and rapid CD4+ cell depletion in human immunodeficiency virus type 1-infected SCID-hu mice suggest direct viral killing of thymocytes in vivo. J Virol. 1997;71:8245–8253. doi: 10.1128/jvi.71.11.8245-8253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings S R, Bonneau R H, Smith P M, Wolcott R M, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 27.Jones C. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv Virus Res. 1998;51:47–99. doi: 10.1016/s0065-3527(08)60784-8. [DOI] [PubMed] [Google Scholar]
- 28.Karupiah G, Buller R M L, van Rooijen N, Duarte C J, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kressel M, Groscurth P. Distinction of apoptotic and necrotic cell death by in situ labelling of fragmented DNA. Cell Tissue Res. 1994;278:549–556. doi: 10.1007/BF00331373. [DOI] [PubMed] [Google Scholar]
- 30.Lan H C, Chambers M A, Fergunson J A, Srivastava K K, Reddy P G. Effect of bovine herpesvirus-1 on expression of interleukin-2 receptors and effect of interleukin-12 on lymphocyte proliferation. Vet Microbiol. 1996;49:59–66. doi: 10.1016/0378-1135(95)00175-1. [DOI] [PubMed] [Google Scholar]
- 31.Lawman M J, Griebel P, Hutchings D L, Davis W C, Heise J, Qualtiere L, Babiuk L A. –1988. Generation of IL-2 dependent bovine cytotoxic T lymphocyte clones reactive against BHV-1 infected target cells: loss of genetic restriction and virus specificity. Viral Immunol. 1987;1:163–176. doi: 10.1089/vim.1987.1.163. [DOI] [PubMed] [Google Scholar]
- 32.Leist T P, Kohler M, Zinkernagel R M. Impaired generation of anti-viral cytotoxicity against lymphocytic choriomeningitis and vaccinia virus in mice treated with CD4+-specific monoclonal antibody. Scand J Immunol. 1989;30:679–686. doi: 10.1111/j.1365-3083.1989.tb02476.x. [DOI] [PubMed] [Google Scholar]
- 33.Lusso P, De Maria A, Balotta C, DeRocco S E, Markham P D, Gallo R C. Productive infection of human CD4+ and CD8+ mature human T cell populations and clones by HHV-6. Transcriptional down-regulation of CD3. J Immunol. 1991;147:685–691. [PubMed] [Google Scholar]
- 34.Maccario R, Comoli P, Percivalle E, Montagna D, Locatelli F, Gerna G. Herpes simplex virus-specific human cytotoxic T-cell colonies expressing either gamma delta or alpha beta T-cell receptor: role of accessory molecules on HLA-unrestricted killing of virus-infected targets. Immunology. 1995;85:49–56. [PMC free article] [PubMed] [Google Scholar]
- 35.Manickan E, Rouse R J, Yu Z, Wire W S, Rouse B T. Genetic immunization against herpes simplex virus. Protection is mediated by CD4/+ T lymphocytes. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 36.Mikloska Z, Kesson A M, Penfold M E, Cunningham A L. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J Infect Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 37.Milligan G N, Bernstein D I. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995;212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 38.Morrison L A, Knipe D M. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology. 1997;239:315–326. doi: 10.1006/viro.1997.8884. [DOI] [PubMed] [Google Scholar]
- 39.Muro-Cacho C A, Pantaleo G, Fauci A S. Analysis of apoptosis in lymph nodes in HIV-infected persons. J Immunol. 1995;154:5556–5566. [PubMed] [Google Scholar]
- 40.Nataraj S, Eidmann S, Hariharan M J, Sur J H, Perry G A, Srikumaran S. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 1997;10:21–34. doi: 10.1089/vim.1997.10.21. [DOI] [PubMed] [Google Scholar]
- 41.Pignata C, Fiore M, de Filippo S, Cavalcanti M, Gaetaniello L, Scotese I. Apoptosis as a mechanism of peripheral blood mononuclear cell death after measles and varicella-zoster virus infections in children. Pediatr Res. 1998;43:77–83. doi: 10.1203/00006450-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Posavad C M, Koelle D M, Corey L. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J Virol. 1996;70:8165–8168. doi: 10.1128/jvi.70.11.8165-8168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 44.Rinaldo C R, Jr, Torpey D J. Cell-mediated immunity and immunosuppression in herpes simplex virus infection. Immunodeficiency. 1993;5:33–90. [PubMed] [Google Scholar]
- 45.Rossi C R, Kiesel G K. Susceptibility of bovine macrophages and tracheal ring cultures to bovine viruses. Am J Vet Res. 1977;38:1705–1708. [PubMed] [Google Scholar]
- 46.Scadding, G. K. Immunology of the tonsil: a review. J. R. Soc. Med. 83:104–107. [DOI] [PMC free article] [PubMed]
- 47.Schang L M, Jones C. Analysis of bovine herpesvirus 1 transcripts during a primary infection of trigeminal ganglia of cattle. J Virol. 1997;71:6786–6795. doi: 10.1128/jvi.71.9.6786-6795.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuh J C L, Bielefeldt Ohmann H, Babiuk L A, Doige C E. Bovine herpesvirus-1-induced pharyngeal tonsil lesions in neonatal and weanling calves. J Comp Pathol. 1992;106:243–253. doi: 10.1016/0021-9975(92)90053-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuh J C L, Oliphant L W. Development and immunophe- notyping of the pharyngeal tonsil (adenoid) in cattle. J Comp Pathol. 1992;106:229–241. doi: 10.1016/0021-9975(92)90052-v. [DOI] [PubMed] [Google Scholar]
- 50.Sciammas R, Kodukula P, Tang Q, Hendricks R L, Bluestone J A. T cell receptor-gamma/delta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheffy B E, Rodman S. Activation of latent infectious bovine rhinotracheitis infection. J Am Vet Med Assoc. 1973;163:850–851. [Google Scholar]
- 52.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 53.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spear A. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 55.Takahashi K, Sonoda S, Higashi K, Kondo T, Takahashi H, Takahashi M, Yamanishi K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol. 1989;63:3161–3163. doi: 10.1128/jvi.63.7.3161-3163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tigges M A, Leng S, Johnson D C, Burke R L. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]
- 57.Winkler, M. T., and C. Jones. Unpublished observation.
- 58.Wyler R, Engels M, Schwyzer M. Infectious bovine rhinotracheitis/vulvovaginitis (BHV-1) In: Witman G, editor. Herpesviruses diseases of cattle, horses, and pigs, developments in veterinary medicine. Boston, Mass: Kluver Academic Publishers; 1989. pp. 172–183. [Google Scholar]
- 59.Yasukawa M, Inoue Y, Ohminami H, Terrada K, Fujita S. Apoptosis of CD4+ T lymphocytes in human herpesvirus-6 infection. J Gen Virol. 1998;79:143–147. doi: 10.1099/0022-1317-79-1-143. [DOI] [PubMed] [Google Scholar]
- 60.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]