Abstract
Purpose:
We assessed the association of cardiac radiation dose with cardiac events and survival post-chemoradiation therapy (CRT) in patients with locally advanced non-small cell lung cancer (LA-NSCLC) after adoption of modern radiation therapy (RT) techniques, stricter cardiac dose constraints, and immune checkpoint inhibitor (ICI) consolidation.
Methods and Materials:
This single-institution, multi-site retrospective study included 335 patients with LA-NSCLC treated with definitive, concurrent CRT between October 2017 and December 2021. All patients were evaluated for ICI consolidation. Planning dose constraints included heart mean dose < 20 Gy (<10 Gy if feasible) and heart volume receiving ≥ 50 Gy (V50Gy) < 25 %. Twenty-one dosimetric parameters for three different cardiac structures (heart, left anterior descending coronary artery [LAD], and left ventricle) were extracted. Primary endpoint was any major adverse cardiac event (MACE) post-CRT, defined as acute coronary syndrome, heart failure, coronary revascularization, or cardiac-related death. Secondary endpoints were: grade ≥ 3 cardiac events (per CTCAE v5.0), overall survival (OS), lung cancer-specific mortality (LCSM), and other-cause mortality (OCM).
Results:
Median age was 68 years, 139 (41 %) had baseline coronary heart disease, and 225 (67 %) received ICI consolidation. Proton therapy was used in 117 (35 %) and intensity-modulated RT in 199 (59 %). Median LAD V15Gy was 1.4 % (IQR 0–22) and median heart mean dose was 8.7 Gy (IQR 4.6–14.4). Median follow-up was 3.3 years. Two-year cumulative incidence of MACE was 9.5 % for all patients and 14.3 % for those with baseline coronary heart disease. Two-year cumulative incidence of grade ≥ 3 cardiac events was 20.4 %. No cardiac dosimetric parameter was associated with an increased risk of MACE or grade ≥ 3 cardiac events. On multivariable analysis, cardiac dose (LAD V15Gy and heart mean dose) was associated with worse OS, driven by an association with LCSM but not OCM.
Conclusions:
With modern RT techniques, stricter cardiac dose constraints, and ICI consolidation, cardiac dose was associated with LCSM but not OCM or cardiac events in patients with LA-NSCLC.
Keywords: NSCLC, Cardiotoxicity, Cardiac dosimetry, Immunotherapy
Introduction
Patients with lung cancer often have pre-existing cardiovascular comorbidity and receive potentially cardiotoxic therapies, including radiation therapy (RT). In RTOG 0617, higher cardiac radiation dose was associated with worse overall survival (OS) in patients with unresectable, locally advanced non-small cell lung cancer (LA-NSCLC) treated with concurrent chemoradiation therapy (cCRT) [1]. One hypothesis is that higher cardiac dose was associated with cardiac events, and these cardiac events led to worse OS. Subsequent studies found that 15–32 % of patients experienced a grade ≥ 3 cardiac event after CRT [2,3], a higher percentage than previously appreciated. Moreover, studies observed an association between higher cardiac dose and cardiac events [2–5], with left anterior descending coronary artery (LAD) volume receiving ≥ 15 Gy (V15Gy) > 10 % emerging as an important predictor of major adverse cardiac events (MACE) [6].
However, these studies describing cardiotoxicity predated the widespread adoption of intensity-modulated radiation therapy (IMRT) and proton therapy, data-driven cardiac dose constraints, and immunotherapy consolidation. Most patients in these prior studies (ranging from 78 % to 100 %) were treated with 3-dimensional conformal radiotherapy (3D-CRT) which delivers higher cardiac radiation dose [2–5]. Post-RT cardiac event rates and dosimetric predictors of cardiotoxicity, including LAD V15Gy > 10 % and heart mean dose > 10 Gy [3,6], may not extrapolate well to a modern cohort. Advanced RT techniques and stricter cardiac constraints may decrease the risk of RT-associated cardiac events by reducing cardiac dose below critical thresholds [7]. Conversely, immune checkpoint inhibitor (ICI) consolidation may increase the risk of cardiac events either by decreasing the competing risk of lung cancer-specific mortality or through additive, potentially immune-mediated cardiotoxicity [8–11].
In this study, we assessed the association of cardiac dose with post-CRT MACE and OS among a modern cohort of patients treated with cCRT after routine adoption of IMRT and proton therapy, data-driven cardiac constraints (heart mean dose < 20 Gy [optimization target < 10 Gy] and heart V50Gy < 25 %), and ICI consolidation.
Methods
Patients
This single-institution, multi-site retrospective study included consecutive patients with unresectable LA-NSCLC treated with definitive cCRT from October 2017 to December 2021. We excluded patients receiving thoracic reirradiation or < 50 Gy. All patients during this period were evaluated for ICI consolidation following RT completion; patients did not receive consolidation therapy most commonly due to progressive disease, comorbidity/intercurrent illness, and/or unresolved cCRT toxicity [12].
Treatment
RT was delivered with IMRT, proton therapy (either pencil beam scanning or passive scatter), or uncommonly 3D-CRT to a prescription dose of 60–70 Gy (per National Comprehensive Cancer Network guidelines) in 1.8–2 Gy per fraction. Four-dimensional CT (4D-CT) simulation and target volume delineation with positron emission tomography and intravenous contrast were standard. Image guidance consisted of either daily cone beam CT (for photon therapy) or daily 2D kilovoltage imaging with weekly cone beam CT (for proton therapy). Concurrent chemotherapy consisted of a platinum doublet as per physician preference.
In late 2017, our institution began planning LA-NSCLC cases with more careful attention to cardiac dose based on emerging data suggesting its importance [1,2,4,13]. Two cardiac dose constraints were introduced as institutional standards: heart mean dose < 20 Gy (optimization target heart mean dose < 10 Gy without sacrificing tumor coverage or exceeding lung constraints) and heart V50Gy < 25 %. Other dose constraints included lung mean dose < 20 Gy (optimization target < 18 Gy), lung V20Gy < 35 % (optimization target < 25 %), esophagus mean dose < 34 Gy, and esophagus V60Gy < 17 %. For lung dosimetry, lung minus internal gross tumor volume (iGTV) was used.
During this period, there was increased physician awareness and patient counseling of the potential for post-cCRT cardiac events. Patients were considered for cardio-oncology referral for pre-RT cardiovascular risk assessment and co-management if the heart mean dose constraint was exceeded or if they had significant baseline cardiovascular disease.
Study endpoints
The primary endpoint was post-cCRT MACE, defined as acute coronary syndrome, heart failure hospitalization or urgent visit, coronary revascularization, or cardiac-related death, consistent with the definition used by Atkins et al. [3]. Secondary endpoints were grade ≥ 3 cardiac events (graded per CTCAE v5.0), overall survival (OS), freedom from lung cancer-specific mortality (LCSM; defined as death from active or progressive lung cancer), and freedom from other-cause mortality (OCM; including death from NSCLC treatment-related toxicity). All endpoints were measured from the end of RT to the event of interest.
Cardiovascular assessment
We undertook a two-fold approach to assess baseline cardiovascular comorbidity and post-cCRT cardiac events. First, we performed an electronic-health-record (EHR)-based automated screen of a variety of cardiovascular diagnosis codes to assess conditions present prior to the start of RT (baseline conditions) and those present only after the end of RT (post-cCRT events) (Supplementary Table 1). Second, we performed manual chart review to verify results of the EHR screen and finalize assessment of baseline conditions and post-cCRT cardiac events. Baseline coronary heart disease (CHD) was defined as per Atkins et al. and included any of the following: heart failure, coronary artery disease, peripheral arterial disease, and cerebrovascular accident; arrhythmic events were excluded from this definition but included in the definition of baseline cardiovascular disease [3]. Framingham 10-year cardiovascular disease risk scores were calculated for those without baseline CHD; patients were grouped based on low (<10 %), moderate (10–20 %), and high (>20 %) Framingham risk [14].
Cardiac dosimetric parameters
RT plans were exported from the Varian Eclipse treatment planning system (Varian, Palo Alto, CA) to MIM (version 7.1.4, MIM Software, Cleveland, OH). The heart, left ventricle (LV), and LAD were autosegmented using previously validated deep learning models [15–17]. Structures were then manually reviewed and corrected based on a validated cardiac contouring atlas (Feng et al.) [18]. Twenty-one dosimetric parameters (mean dose [Gy], minimum dose to the hottest x% volume [Dx%(Gy); x from 5 to 95 in 5 % intervals], and maximum dose [D0.03 cc(Gy)]) for each structure were extracted, as well as LAD V15Gy (%) [6,13]. Dx parameters were primarily used instead of Vx parameters based on preferred statistical properties [19].
Statistical analysis
The cumulative incidence method was used to model MACE and grade ≥ 3 cardiac events; non-cardiac death was considered a competing event. Gray’s test was used to compare the cumulative incidence of MACE and grade ≥ 3 cardiac events stratified by baseline CHD, LAD V15Gy ≥ 10 % (for MACE), and heart mean dose ≥ 10 Gy (for grade ≥ 3 cardiac events) [6]. Fine-Gray regression was used to assess associations of cardiac dosimetric parameters with either MACE or grade ≥ 3 cardiac events. We primarily sought to test whether LAD V15Gy or heart mean dose was associated with MACE; however, we tested numerous other dosimetric associations in exploratory analyses described below.
Dosimetric associations were assessed among all patients and separately when stratified by baseline CHD (yes vs. no), RT modality (proton vs. proton therapy), and consolidation ICI receipt (yes vs. no) (7 total groups). For MACE, a total of 448 dosimetric associations were tested (21 dosimetric parameters per structure, 3 structures [whole heart, LV, and LAD], 7 groups [21 * 3 * 7 = 441; plus 7 for LAD V15Gy; total 448]). For grade ≥ 3 cardiac events, 147 dosimetric associations were tested (21 dosimetric parameters for whole heart, 7 groups [21 * 7 = 147]). Only whole heart metrics, and not LV or LAD metrics, were tested for grade ≥ 3 cardiac events because of the heterogenous event types and to limit the overall number of statistical tests. The following baseline factors were also assessed: age, sex, ECOG performance status, smoking history (number of pack-years), baseline cardiovascular disease, baseline CHD, Framingham risk (among those without baseline CHD), pre-existing atrial fibrillation/flutter, hypertension, hyperlipidemia, diabetes, body mass index (BMI), baseline statin use, laterality of primary tumor, proton (vs. photon) therapy, chemotherapy regimen, and consolidation ICI receipt.
OS, freedom from LCSM, and freedom from OCM were assessed with the Kaplan-Meier method and compared between various strata with the log-rank test. For OS, Cox regression was performed. Because numerous cardiac dosimetric parameters were associated with OS, we focused on LAD V15Gy and heart mean dose. Additional variables tested were: histology, PD-L1 expression, overall stage (IIIB/IIIC), T4 disease, N3 disease, primary gross tumor volume (GTVp), nodal GTV (GTVn), lung mean dose, esophagus mean dose, and effective radiation dose to immune circulating cells (EDIC), calculated per Jin et al. as a function of heart mean dose, lung mean dose, integral total dose volume, and number of RT fractions [20].
Variables with p < 0.1 on univariable analysis or thought to be clinically relevant (despite p > 0.1) were considered for multivariable analysis. Given collinearity between different cardiac dosimetric parameters, only one such parameter (either LAD V15Gy or heart mean dose, but not both together) was included in each multivariable model. Additionally, because EDIC is a function of heart, lung, and body dose, multivariable models that included EDIC did not include heart or lung dose.
Hypothesis tests were two-sided. When testing associations of LAD V15Gy and heart mean dose with the primary endpoint of MACE, p < 0.025 was considered statistically significant (Bonferroni correction, 0.05/2). All other tests were considered exploratory and p < 0.05 was considered significant. Analyses were performed with SAS OnDemand for Academics and SAS version 9.4 (Cary, NC).
Results
A total of 335 patients were included (Supplementary Figure 1). Table 1 shows baseline characteristics among all patients and stratified by baseline CHD. Median age was 68 years (interquartile range [IQR] 62–74), 139 patients (41 %) had baseline CHD, and 225 (67 %) received ICI consolidation. IMRT was used for 199 patients (59 %), proton therapy for 117 (35 %), and 3D-CRT for 19 (6 %). Median heart mean dose was 8.7 Gy (IQR 4.6–14.4), median heart V50Gy was 4.2 % (IQR 1.2–8.1), and median LAD V15Gy was 1.4 % (IQR 0–22). Supplementary Table 2 shows summary statistics for all other cardiac dosimetric parameters. Median follow-up was 3.3 years (95 % confidence interval [CI] 3.1–3.5).
Table 1.
Baseline characteristics.
| Characteristic | All patients (N = 335) N (%) | Baseline CHD (N = 139) N (%) | No baseline CHD (N = 196) N (%) |
|---|---|---|---|
| Age (median, IQR) | 68 (62–74) | 70 (65–75) | 66 (60–72) |
| Female | 180 (54) | 63 (45) | 117 (60) |
| ECOG PS | |||
| 0 | 115 (34) | 36 (26) | 79 (40) |
| 1 | 185 (55) | 79 (57) | 106 (54) |
| 2 | 35 (10) | 24 (17) | 11 (6) |
| Smoking, pack-years (median, IQR) | 37.5 (17–50) | 45 (25–60) | 30 (10–50) |
| Current/former smoker | 303 (90) | 135 (97) | 168 (86) |
| Baseline CVD | 161 (48) | 139 (100) | 22 (11) |
| Baseline CHD | 139 (41) | 139 (100) | 0 |
| Heart failure | 34 (10) | 34 (24) | 0 |
| Coronary artery disease | 100 (30) | 100 (72) | 0 |
| Prior myocardial infarction | 39 (12) | 39 (28) | 0 |
| Peripheral arterial disease | 67 (20) | 68 (48) | 0 |
| Cerebrovascular accident | 31 (9) | 31 (22) | 0 |
| Atrial fibrillation/flutter | 51 (15) | 32 (23) | 19 (10) |
| Other arrythmia | 9 (3) | 6 (4) | 3 (2) |
| Symptomatic valvulopathy | 15 (4) | 13 (9) | 2 (1) |
| Framingham risk, % (median, IQR) | N/A | N/A | 21 (13–32) |
| Low (<10 %) | N/A | N/A | 31 (16) |
| Moderate (10 %−20 %) | N/A | N/A | 63 (32) |
| High (>20 %) | N/A | N/A | 102 (52) |
| Hypertension | 215 (64) | 110 (79) | 105 (54) |
| Hyperlipidemia | 162 (48) | 95 (68) | 67 (34) |
| Diabetes mellitus | 71 (21) | 46 (33) | 25 (13) |
| BMI (kg/m2) (median, IQR) | 27.3 (23.8–30.9) | 27.7 (24.4–31.1) | 26.4 (23.1–30.8) |
| Baseline statin use | 184 (55) | 110 (79) | 74 (38) |
| Histology | |||
| Adenocarcinoma | 170 (51) | 65 (47) | 105 (54) |
| Squamous cell carcinoma | 137 (41) | 62 (45) | 75 (38) |
| Other | 28 (8) | 12 (9) | 16 (8) |
| AJCC Stage (8th edition) | |||
| II | 23 (7) | 12 (9) | 11 (6) |
| IIIA | 156 (47) | 67 (48) | 89 (45) |
| IIIB | 124 (37) | 49 (35) | 75 (38) |
| IIIC | 32 (10) | 11 (8) | 21 (11) |
| T stage | |||
| 0–1 | 104 (31) | 52 (37) | 52 (27) |
| 2 | 68 (20) | 28 (20) | 40 (20) |
| 3 | 67 (20) | 25 (18) | 42 (21) |
| 4 | 96 (29) | 34 (24) | 62 (32) |
| N stage | |||
| 0 | 35 (10) | 15 (11) | 20 (10) |
| 1 | 27 (8) | 14 (10) | 13 (7) |
| 2 | 199 (59) | 78 (56) | 121 (62) |
| 3 | 74 (22) | 32 (23) | 42 (21) |
| Laterality of primary tumor | |||
| Right | 205 (61) | 72 (52) | 133 (68) |
| Left | 114 (34) | 58 (42) | 56 (29) |
| Mediastinum | 16 (5) | 9 (6) | 7 (4) |
| PD-L1 expression | |||
| < 1% | 111 (33) | 40 (29) | 71 (36) |
| ≥ 1% | 162 (48) | 67 (48) | 95 (48) |
| Unknown | 62 (19) | 32 (23) | 30 (15) |
| RT dose, Gy (median, IQR) | 66.6 (66.6–70) | 66.6 (66–70) | 66 (66–70) |
| RT technique | |||
| Proton therapy | 117 (35) | 51 (37) | 66 (34) |
| Pencil beam scanning | 74 (22) | 28 (20) | 46 (23) |
| Passive scatter | 43 (13) | 23 (17) | 20 (10) |
| Photon therapy | 218 (65) | 88 (63) | 130 (66) |
| Intensity-modulated RT | 199 (59) | 79 (57) | 120 (61) |
| 3D-conformal RT | 19 (6) | 9 (6) | 10 (5) |
| Concurrent chemotherapy regimen | |||
| Carboplatin/paclitaxel | 242 (72) | 109 (78) | 133 (68) |
| Cisplatin/etoposide | 39 (12) | 13 (9) | 26 (13) |
| Carboplatin/pemetrexed | 24 (7) | 9 (6) | 15 (8) |
| Other | 30 (9) | 8 (6) | 22 (11) |
| Consolidation ICI receipt | 225 (67) | 92 (66) | 133 (68) |
| Durvalumab | 215 (64) | 88 (63) | 127 (65) |
| Other ICI | 10 (3) | 4 (3) | 6 (3) |
| Weeks of treatment (median, IQR) | 34 (12–50) | 33.5 (11–48) | 36 (12–51) |
| GTVp, cc (median, IQR) | 41.8 (12.1–124.1) | 34.3 (6.6–119.6) | 51.1 (16.9–133.4) |
| GTVn, cc (median, IQR) | 23.2 (6.0–47.0) | 23.2 (6.5–44.1) | 23.3 (5.9–49.9) |
| LAD V15Gy, % (median, IQR) | 1.4 (0–21.9) | 1.4 (0–21.0) | 1.0 (0–22.1) |
| Heart mean dose, Gy (median, IQR) | 8.7 (4.6–14.4) | 8.2 (4.1–14.4) | 9.0 (4.8–14.5) |
| Heart V25Gy, % (median, IQR) | 11.3 (5.1–21.3) | 9.9 (4.0–20.3) | 12.2 (5.8–22.8) |
| Heart V50Gy, % (median, IQR) | 4.2 (1.2–8.1) | 3.3 (1.0–7.8) | 4.6 (1.6–8.3) |
| Lung mean dose, Gy (median, IQR) | 15.3 (12.1–17.7) | 14.6 (11.7–17.5) | 15.8 (12.5–17.9) |
| Esophagus mean dose, Gy (median, IQR) | 20.4 (12.8–26.9) | 19.1 (11.7–27.3) | 20.9 (14.7–26.7) |
| EDIC (median, IQR) | 4.4 (3.4–5.5) | 4.2 (3.3–5.4) | 4.5 (3.4–5.6) |
CVD, cardiovascular disease; CHD, coronary heart disease; IQR, interquartile range; ECOG PS, Eastern Cooperative Oncology Group performance status; N/A, not assessed; BMI, body mass index; AJCC, American Joint Committee on Cancer; PD-L1, programmed death-ligand 1; RT, radiation therapy; ICI, immune checkpoint inhibitor; GTVp, primary gross tumor volume; GTVn, nodal gross tumor volume; Vx Gy, volume receiving ≥ x Gy; EDIC, effective radiation dose to immune circulating cells.
A total of 35 patients (10.4 %) experienced at least one MACE (Supplementary Table 3); 1- and 2-year cumulative incidences were 4.2 % (95 % CI 2.4–6.7) and 9.5 % (95 % CI 6.6–13.1), respectively. One- and 2-year cumulative incidences were 5.8 % (95 % CI 2.7–10.6) and 14.3 % (95 % CI 8.9–20.8) for those with baseline CHD and 3.1 % (95 % CI 1.3–6.2) and 6.0 % (95 % CI 3.2–10.0) for those without baseline CHD (p = 0.011; Fig. 1A). Cardiac dose, including LAD V15Gy and heart mean dose, was not associated with an increased risk of MACE on univariable (Supplementary Table 4) or multivariable (Table 2) analysis. LAD V15Gy ≥ 10 % was not associated with MACE among all patients or when stratified by baseline CHD (Fig. 1B–D).
Fig. 1.
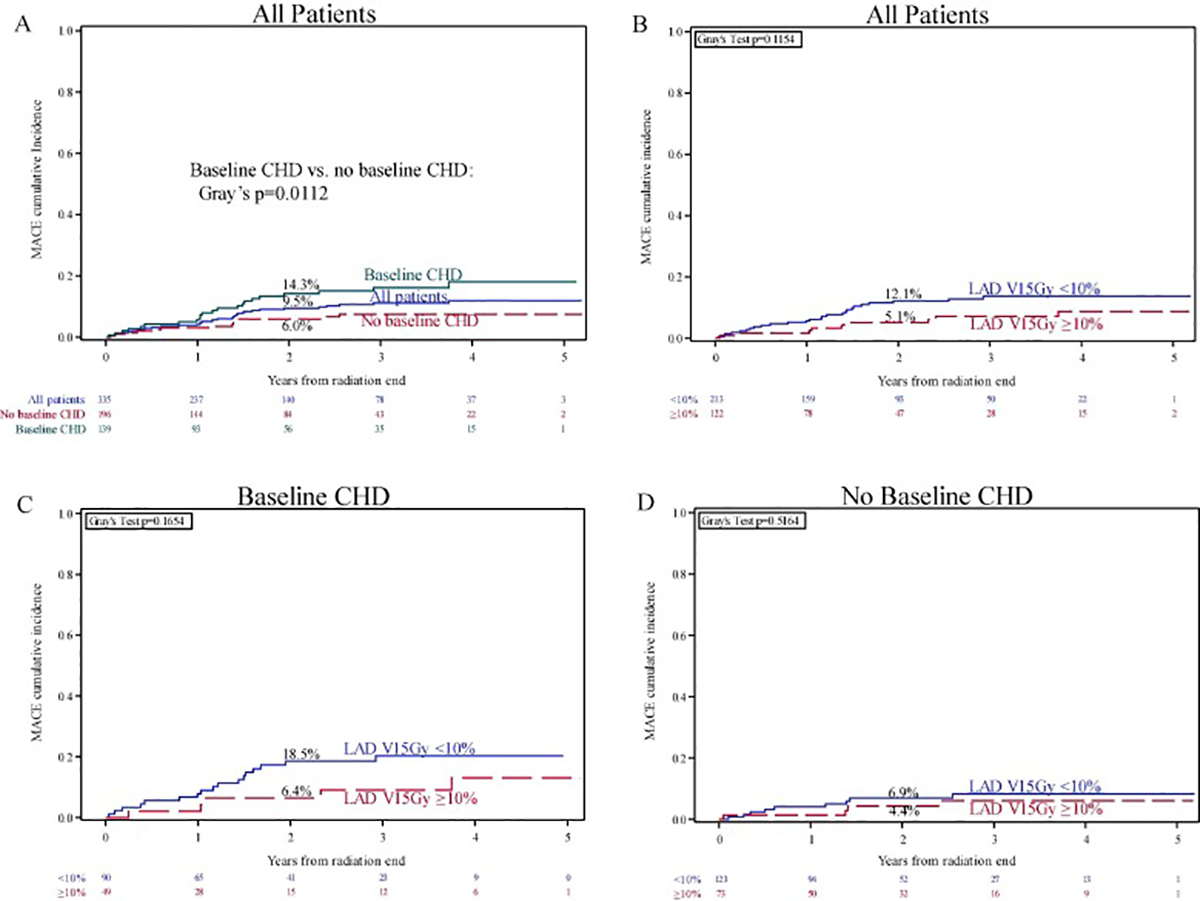
Cumulative incidence of major adverse cardiac events, (A) among all patients and stratified by baseline coronary heart disease (CHD); (B-D) stratified by LAD V15Gy ≥ 10 % (B) among all patients, (C) among patients with baseline CHD, and (D) among patients without baseline CHD.
Table 2.
Fine-Gray regression for major adverse cardiac events among all patients (N = 335).
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| sHR (95 % CI) | P | sHR (95 % CI) | P | |
| Age (y) | 1.04 (0.99–1.08) | 0.089 | ||
| Female | 1.71 (0.86–3.44) | 0.13 | ||
| ECOG PS (ref: 0) | ||||
| 1 | 2.99 (1.14–7.81) | 0.026 | 2.89 (1.03–7.87)1 | 0.042 |
| 2 | 6.11 (1.98–18.9) | 0.0016 | 4.96 (1.57–15.6)1 | 0.0063 |
| Smoking pack-years (per 10) | 1.02 (0.93–1.10) | 0.74 | ||
| Baseline CVD | 1.86 (0.94–3.69) | 0.075 | ||
| Baseline CHD | 2.41 (1.22–4.77) | 0.012 | 1.96 (0.98–3.92)1 | 0.056 |
| Framingham risk (ref: low)2 | ||||
| Moderate | 2.47 (0.29–21.3) | 0.41 | ||
| High | 2.06 (0.26–16.6) | 0.50 | ||
| Baseline atrial fibrillation/flutter | 0.72 (0.25–2.01) | 0.52 | ||
| Baseline hypertension | 1.56 (0.73–3.33) | 0.25 | ||
| Baseline hyperlipidemia | 1.12 (0.58–2.17) | 0.73 | ||
| Baseline diabetes | 1.99 (0.99–4.00) | 0.052 | ||
| BMI (kg/m2) | 1.03 (0.97–1.08) | 0.36 | ||
| Baseline statin use | 2.06 (0.99–4.29) | 0.052 | ||
| Laterality of primary tumor (ref: right) | ||||
| Left | 0.54 (0.24–1.18) | 0.12 | ||
| Mediastinum | 0.48 (0.066–3.55) | 0.47 | ||
| Proton therapy | 1.24 (0.63–2.43) | 0.53 | ||
| Carboplatin/paclitaxel (ref: all else) | 1.54 (0.69–3.43) | 0.30 | ||
| Consolidation ICI receipt | 0.76 (0.38–1.50) | 0.43 | ||
| LAD V15Gy (%)3 | 0.99 (0.97–1.01) | 0.37 | 0.99 (0.97–1.01)1 | 0.34 |
| Heart mean dose (Gy)3 | 0.97 (0.92–1.02) | 0.27 | 0.97 (0.92–1.03)4 | 0.30 |
sHR, subdistribution hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; ref, reference; CVD, cardiovascular disease; CHD, coronary heart disease; ICI, immune checkpoint inhibitor; LAD, left anterior descending coronary artery; Vx Gy, volume receiving ≥ x Gy.
Model 1 with ECOG PS, baseline CHD, and LAD V15Gy.
Among the 196 patients without baseline CHD.
Only LAD V15Gy and heart mean dose are included in this Table as they are representative dosimetric parameters; Supplementary Table 4 shows all univariable tests performed.
Model 2 with ECOG PS, baseline CHD, and heart mean dose.
A total of 87 patients (26 %) experienced at least one grade ≥ 3 cardiac event (Supplementary Table 5); 1- and 2- year cumulative incidences were 12.6 % (95 % CI 9.3–16.4) and 20.4 % (95 % CI 16.1–25.0), respectively. One- and 2-year cumulative incidences were 13.1 % (95 % CI 8.1–19.3) and 23.9 % (95 % CI 17.0–31.4) for those with baseline CHD and 12.3 % (95 % CI 8.1–17.3) and 17.7 % (95 % CI 12.6–23.6) for those without baseline CHD (p = 0.13; Supplementary Figure 2A). Cardiac dose was not associated with an increased risk of grade ≥ 3 cardiac events on univariable (Supplementary Table 6) or multivariable (Supplementary Table 7) analysis. Heart mean dose ≥ 10 Gy was not associated with grade ≥ 3 cardiac events among all patients or when stratified by baseline CHD (Supplementary Figure 2B–D).
There were 183 deaths, including 125 from lung cancer progression and 58 from other causes (Supplementary Table 8). Median OS was 2.6 years (95 % CI 2.1–3.0). Fig. 2 shows the risk of death, MACE and grade ≥ 3 cardiac events among all patients and stratified by baseline CHD.
Fig. 2.
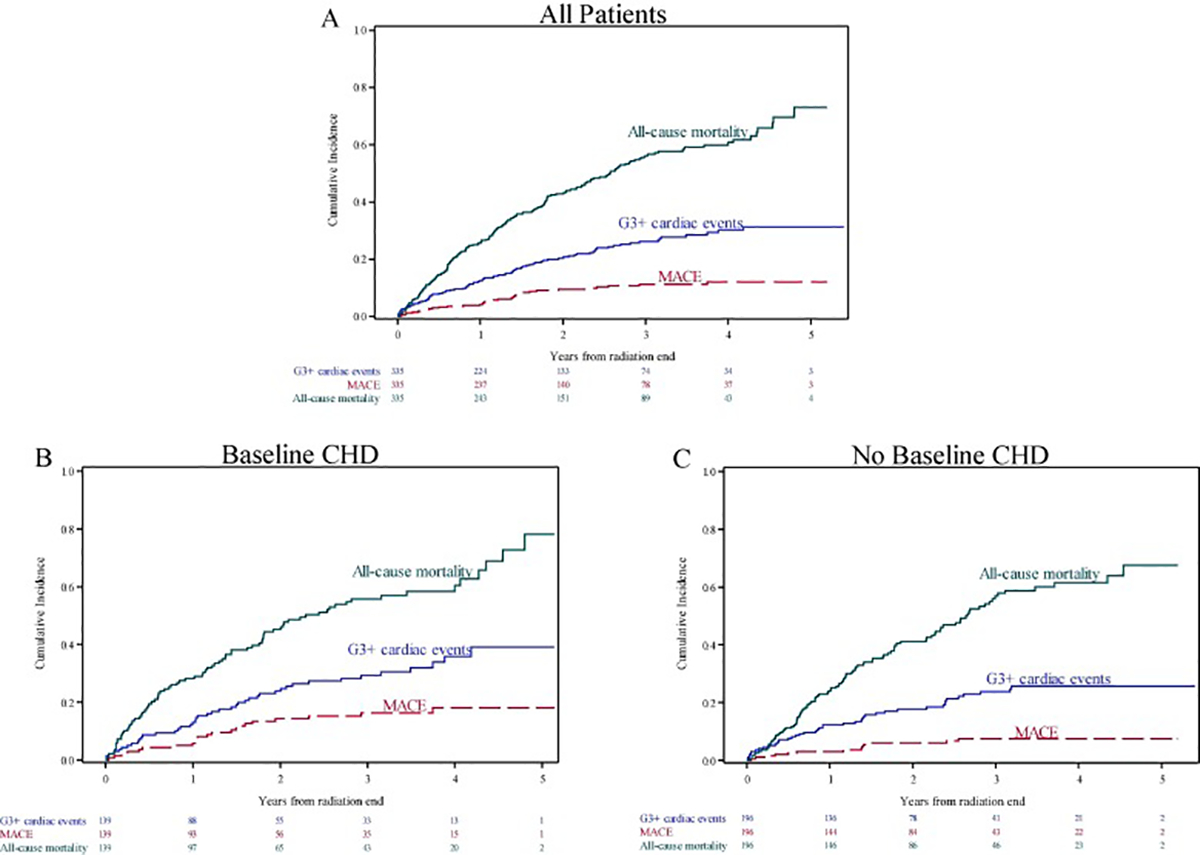
Cumulative incidence of all-cause mortality, major adverse cardiac events (MACE), and grade ≥ 3 cardiac events (A) among all patients, (B) among patients with baseline coronary heart disease (CHD), and (C) among patients without baseline CHD. MACE and grade ≥ 3 cardiac events are adjusted for the competing risk of death.
LAD V15Gy, heart mean dose, and EDIC were associated with worse OS in separate multivariable models (Table 3). There was no interaction between LAD V15Gy and baseline CHD, use of proton therapy, or receipt of ICI consolidation (interaction p = 0.13, 0.77, and 0.71, respectively). LAD V15Gy ≥ 10 % (Fig. 3A) and heart mean dose ≥ 10 Gy (Supplementary Figure 3A) were associated with worse OS; these OS detriments were driven by associations with LCSM and not OCM (Fig. 3B–C and Supplementary Figure 3B–C).
Table 3.
Cox regression for overall survival among all patients (N = 335).
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95 % CI) | P | HR (95 % CI) | P | |
| Age (y) | 1.02 (1.00–1.04) | 0.039 | 1.02 (1.00–1.04)1 | 0.039 |
| Female | 1.05 (0.78–1.40) | 0.75 | ||
| ECOG PS (ref: 0) | ||||
| 1 | 2.22 (1.56–3.14) | < 0.001 | 1.97 (1.37–2.83)1 | < 0.001 |
| 2 | 3.25 (1.99–5.29) | < 0.001 | 2.17 (1.27–3.70)1 | 0.0047 |
| Smoking pack-years (per 10) | 1.04 (0.99–1.09) | 0.096 | ||
| Baseline CVD | 1.21 (0.91–1.62) | 0.19 | ||
| Baseline CHD | 1.10 (0.83–1.48) | 0.50 | 1.09 (0.80–1.49)1 | 0.59 |
| Baseline atrial fibrillation/flutter | 1.34 (0.91−1.97) | 0.14 | ||
| Baseline hypertension | 1.08 (0.79–1.47) | 0.62 | ||
| Baseline hyperlipidemia | 0.98 (0.73–1.31) | 0.88 | ||
| Baseline diabetes | 1.13 (0.80–1.60) | 0.50 | ||
| BMI (kg/m2) | 0.99 (0.97–1.02) | 0.68 | ||
| Baseline statin use | 0.98 (0.73–1.32) | 0.91 | ||
| Histology (ref: squamous) | ||||
| Adenocarcinoma | 0.57 (0.42–0.78) | < 0.001 | 0.81 (0.58–1.13)1 | 0.22 |
| Other | 0.86 (0.51–1.45) | 0.57 | 0.64 (0.45–1.39)1 | 0.11 |
| PD-L1 expression (ref: < 1%) | ||||
| ≥ 1% | 0.65 (0.47–0.88) | 0.0066 | 0.66 (0.47–0.93)1 | 0.016 |
| Unknown | 0.60 (0.39–0.91) | 0.018 | 0.71 (0.45–1.12)1 | 0.14 |
| Stage IIIB/IIIC (ref: II/IIIA) | 1.11 (0.83–1.48) | 0.50 | ||
| T4 (ref: T0–3) | 1.53 (1.12–2.08) | 0.0072 | 1.07 (0.74–1.56)1 | 0.72 |
| N3 (ref: N0–2) | 1.01 (0.72–1.41) | 0.97 | ||
| Laterality of primary tumor (ref: right) | ||||
| Left | 1.30 (0.96–1.76) | 0.085 | 1.04 (0.71–1.54)1 | 0.83 |
| Mediastinum | 0.40 (0.15–1.07) | 0.069 | 0.42 (0.15–1.16)1 | 0.095 |
| Proton therapy | 0.91 (0.67–1.24) | 0.55 | ||
| Carboplatin/paclitaxel (ref: all else) | 1.61 (1.13–2.28) | 0.0076 | 1.43 (0.98–2.08)1 | 0.061 |
| Consolidation ICI receipt | 0.56 (0.41–0.75) | < 0.001 | 0.63 (0.46–0.87)1 | 0.0045 |
| GTVp (per 10 cc) | 1.01 (1.01–1.02) | < 0.001 | 1.01 (1.00–1.02)1 | 0.092 |
| GTVn (per 10 cc) | 1.02 (1.00–1.05) | 0.069 | 1.03 (1.01–1.06)1 | 0.018 |
| LAD V15Gy (%) | 1.01 (1.01–1.02) | < 0.001 | 1.01 (1.00–1.02)1 | 0.010 |
| Heart mean dose (Gy) | 1.03 (1.01 −1.05) | < 0.001 | 1.03 (1.00–1.06)2 | 0.033 |
| Lung mean dose (Gy) | 1.04 (1.00–1.08) | 0.061 | 1.03 (0.98–1.08)1 | 0.29 |
| Esophagus mean dose (Gy) | 1.01 (1.00–1.02) | 0.23 | 1.00 (0.98–1.02)1 | 0.86 |
| EDIC (Gy) | 1.15 (1.05–1.25) | 0.0023 | 1.16 (1.03–1.32)3 | 0.018 |
HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; ref, reference; CVD, cardiovascular disease; CHD, coronary heart disease; BMI, body mass index; PD-L1, programmed death-ligand 1; ICI, immune checkpoint inhibitor; GTVp, primary gross tumor volume; GTVn, nodal gross tumor volume; LAD, left anterior descending coronary artery; Vx Gy, volume receiving ≥ x Gy; EDIC, effective radiation dose to immune circulating cells.
Model 1: includes LAD V15Gy.
Model 2: includes heart mean dose instead of LAD V15Gy.
Model 3: Includes EDIC instead of heart mean dose, LAD V15Gy, and lung mean dose.
Fig. 3.
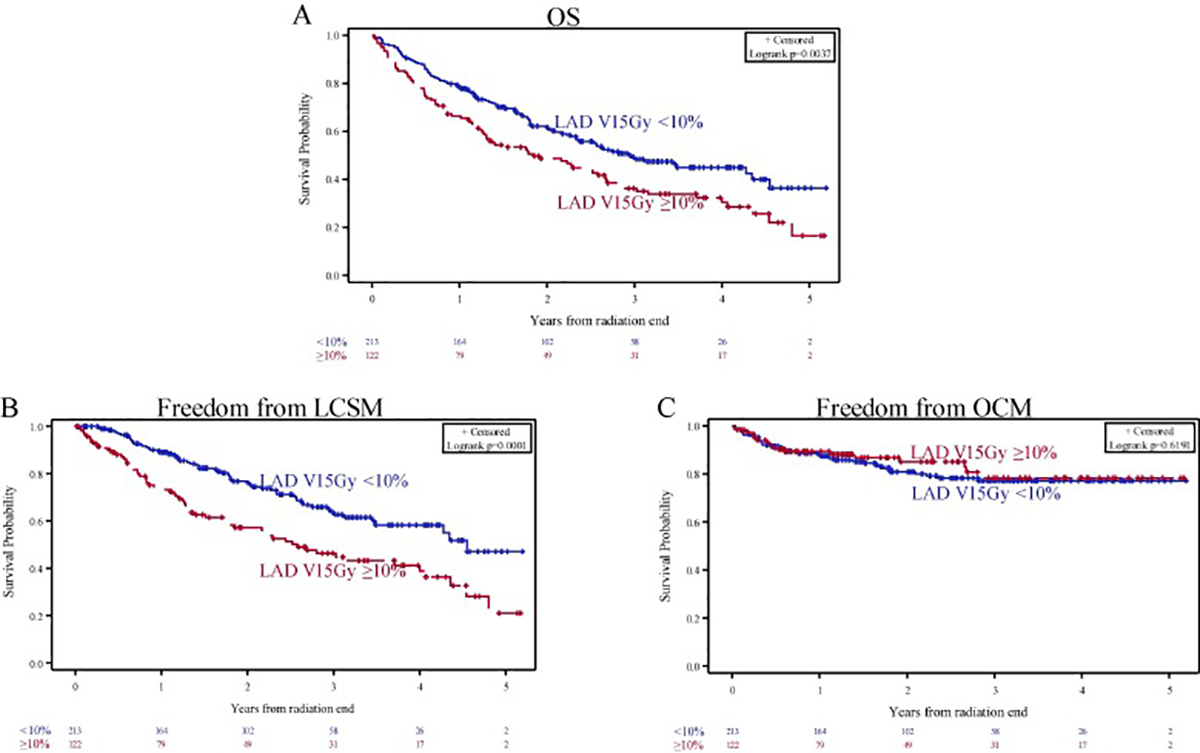
(A) Overall survival (OS), (B) freedom from lung cancer-specific mortality (LCSM), and (C) freedom from other-cause mortality (OCM) stratified by LAD V15Gy ≥ 10 %.
Discussion
Among 335 patients with LA-NSCLC treated with cCRT in the era of modern RT techniques, updated cardiac dose constraints, and immunotherapy consolidation, we report three main findings. First, post-cCRT cardiac events were not uncommon (2-year rates were 9.5 % for MACE and 20.4 % for grade ≥ 3 events), particularly among those with baseline CHD (14.3 % and 23.9 %, respectively). Second, cardiac radiation dose was not associated with an increased risk of either MACE or grade ≥ 3 cardiac events. Third, cardiac dose was associated with worse OS, driven by an association with LCSM but not OCM. Our findings describe the risk of cardiac events after contemporary cCRT and ICI consolidation and add to a growing body of literature associating cardiac dose with OS [1,13,21,22].
The lack of association between cardiac dose and cardiac events might be explained by risk mitigation with modern RT techniques, stricter cardiac dose constraints, and/or heightened awareness of cardiotoxicity. In our study, 94 % of patients were treated with IMRT or proton therapy, whereas in prior studies that found an association between cardiac dose and cardiac events most patients (ranging from 78 % to 100 %) were treated with 3D-CRT [2–5]. We also used strict cardiac dose constraints in the setting of modern RT techniques, leading to low cardiac dose; by contrast, cardiac dose constraints were inconsistent in prior studies. Consequently, median heart mean dose/LAD V15Gy were 8.7 Gy/1.4 % in our cohort compared to 15 Gy/38 % in RTOG 0617 and 12.3 Gy/13.8 % in Atkins et al. (Supplementary Figure 4) [3,6,23,24]. Although 122/335 patients (36 %) in our cohort received LAD V15Gy ≥ 10 %, these patients had a higher risk of LCSM but not OCM, suggesting these were the patients with the most unfavorable lung cancers (e.g., bulky, unfavorable location, multifocal nodal disease) and that further efforts to reduce LAD dose in this group may not be clearly cardioprotective. Moreover, since the publication of RTOG 0617, Dess et al. and Wang et al. [1,2,4], at our institution we have more closely counseled patients on the risks of post-cCRT cardiac events, encouraged patients to follow up with their cardiologist, and considered referring high-risk patients (specifically, those receiving heart mean dose > 20 Gy or with significant baseline cardiovascular disease) to cardio-oncology for cardiovascular risk assessment, optimization, and co-management of cardiac comorbidities. Conceivably, these measures may have mitigated the risk of RT-associated cardiotoxicity.
It remains unclear how ICI consolidation modifies a patient’s risk of post-CRT cardiac events. ICI consolidation decreases the competing risk of LCSM, thereby theoretically increasing the relative impact of other comorbidities (e.g., cardiopulmonary disease) on survival, and has the potential for additive cardiotoxicity [9–11,25]. A comparison of cardiac events between patients who did and did not receive ICI consolidation in our cohort is fraught with bias given the poor prognosis of the latter group. Nevertheless, only one cardiac event was clearly related to ICI; this patient was hospitalized 3.6 months post-cCRT with grade 3 myocarditis and successfully treated with steroids. This patient had baseline CHD and previously received a heart mean dose of 5.4 Gy, LV mean dose of 1.6 Gy, and 4 weeks of durvalumab consolidation.
Cardiac dose was associated with LCSM but not OCM which may reflect factors apart from cardiotoxicity. Cardiac dose is associated with other toxicities (e.g., immunosuppression) and is a surrogate for multiple poor prognostic factors (e.g., mediastinal lymph node burden, central location of tumor, tumor size, lung dose, esophagus dose) that are independently associated with worse lung cancer-specific outcomes [20,26]. Prior studies found that EDIC, which is a function of heart, lung, and body dose, was associated with grade ≥ 3 lymphopenia, worse tumor control, and inferior survival [20,27,28]. In our study, we also found an association between EDIC and worse OS, and we have a forthcoming study suggesting EDIC is also associated with treatment-related lymphopenia [30]. Additionally, residual confounding remains a concern; for example, despite controlling for GTVp, GTVn, lung mean dose, and esophagus mean dose in our multivariable model for OS, we could not control for nodal multifocality or location, which may be associated with both heart dose and worse OS. We would urge cautious interpretation of any association between cardiac dose and OS for two reasons: first, cardiac dose is associated with multiple poor prognostic factors that are unlikely to be fully controlled for in a regression model; and second, OS is dominated by cancer progression events rather than toxicity.
The cardiac dose constraints used herein, including heart mean dose < 20 Gy (optimization target < 10 Gy) and heart V50Gy < 25 % may be a reasonable starting point to reduce the risk of RT-associated cardiotoxicity. At our institution, we do not employ cardiac substructure constraints (e.g., LAD V15Gy < 10 %), as the added value remains unclear, particularly if the association between cardiac dose and OS largely reflects mechanisms apart from cardiotoxicity (e.g., immunosuppression). Nevertheless, we attempt to minimize heart dose, while also minimizing lung dose (optimization target mean dose < 18 Gy and V20 < 25 %) without sacrificing tumor coverage.
This study shares the limitations of prior cardiotoxicity studies. First, baseline cardiovascular disease and post-cCRT cardiac events were retrospectively assessed, which may lead to misestimations in both. It is possible that even with reduced cardiac dose, subclinical cardiac changes occur following cCRT. Such subclinical changes (i.e., CTCAE grade 1–2 toxicities) are challenging to capture retrospectively. At our institution, we have partnered with cardio-oncology to conduct serial prospective observational studies to better understand potential subclinical cardiac perturbations following thoracic RT (e.g., NCT04305613 and [29]). Second, the relatively limited sample size restricts the ability to report on a lack of association between cardiac dose and cardiac events; nevertheless, no association was observed despite testing numerous dosimetric parameters in several subgroups (Supplementary Tables 4 and 6).
Conclusion
Cardiac radiation dose was associated with worse survival but not cardiac events for patients with LA-NSCLC treated in the era of modern RT techniques (predominantly IMRT or proton therapy), data-driven cardiac dose constraints (heart mean dose < 20 Gy [<10 Gy if feasible] and heart V50Gy < 25 %), heightened awareness of the potential for RT-associated cardiotoxicity, and immunotherapy consolidation. The survival detriment was limited to LCSM and not OCM which may reflect factors apart from cardiotoxicity.
Supplementary Material
Disclosures (unrelated to current work)
RBC: Consulting or Advisory Role: AstraZeneca Pharmaceuticals, Cantargia.
Research Funding: Fstar, Cantargia.
CJL: Consulting or Advisory Role: Amgen, AstraZeneca Pharmaceuticals, Takeda Pharmaceuticals, Genentech, Novocure, Regeneron Pharmaceuticals, Pfizer Inc., Sanofi Genzyme.
Research Funding: Merck & Co, Janssen Pharmaceuticals (J&J), Incyte Corporation.
BK: Grants: NIH, American Heart Association.
Other funding: Pfizer, Roche, American College of Cardiology.
Footnotes
CRediT authorship contribution statement
Nikhil Yegya-Raman: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. Sang Ho Lee: Data curation, Methodology, Writing – review & editing. Cole Friedes: Conceptualization, Data curation, Methodology, Visualization, Writing – review & editing. Xingmei Wang: Formal analysis, Methodology, Visualization. Michelle Iocolano: Data curation, Writing – review & editing. Timothy P. Kegelman: Methodology, Writing – review & editing. Lian Duan: Data curation, Writing – review & editing. Bolin Li: Data curation, Writing – review & editing. Eva Berlin: Writing – review & editing. Kristine N. Kim: Writing – review & editing. Abigail Doucette: Data curation, Writing – review & editing. Srinivas Denduluri: Data curation, Methodology, Writing – review & editing. William P. Levin: Conceptualization, Writing – review & editing. Keith A. Cengel: Conceptualization, Writing – review & editing. Roger B. Cohen: Writing – review & editing. Corey J. Langer: Writing – review & editing. Boon-Keng Kevin Teo: Methodology, Writing – review & editing. Wei Zou: Writing – review & editing. Rupal P. O’Quinn: Writing – review & editing. Joseph O. Deasy: Methodology, Writing – review & editing. Jeffrey D. Bradley: Writing – review & editing. Lova Sun: Methodology, Writing – review & editing. Bonnie Ky: Methodology, Investigation, Writing – review & editing. Ying Xiao: Methodology, Investigation, Writing – review & editing. Steven J. Feigenberg: Conceptualization, Investigation, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radonc.2023.110005.
References
- [1].Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dess RT, Sun Y, Matuszak MM, Sun G, Soni PD, Bazzi L, et al. Cardiac events after radiation therapy: Combined analysis of prospective multicenter trials for locally advanced non–small-cell lung cancer. J Clin Oncol 2017;35:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Atkins KM, Rawal B, Chaunzwa TL, Lamba N, Bitterman DS, Williams CL, et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol 2019;73:2976–87. [DOI] [PubMed] [Google Scholar]
- [4].Wang K, Eblan MJ, Deal AM, Lipner M, Zagar TM, Wang Y, et al. Cardiac toxicity after radiotherapy for stage III non–small-cell lung cancer: Pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol 2017;35:1387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yegya-Raman N, Wang K, Kim S, Reyhan M, Deek MP, Sayan M, et al. Dosimetric predictors of symptomatic cardiac events after conventional-dose chemoradiation therapy for inoperable NSCLC. J Thorac Oncol 2018;13:1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Atkins KM, Chaunzwa TL, Lamba N, Bitterman DS, Rawal B, Bredfeldt J, et al. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non-small cell lung cancer. JAMA Oncol 2021;7:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yegya-Raman N, Zou W, Nie K, Malhotra J, Jabbour SK. Advanced radiation techniques for locally advanced non-small cell lung cancer: intensity-modulated radiation therapy and proton therapy. J Thorac Dis 2018;10:S2474–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yegya-Raman N, Berlin E, Feigenberg SJ, Ky B, Sun L. Cardiovascular toxicity and risk mitigation with lung cancer treatment. Curr Oncol Rep 2023. [DOI] [PubMed] [Google Scholar]
- [9].Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- [10].Laenens D, Yu Y, Santens B, Jacobs J, Beuselinck B, Bechter O, et al. Incidence of cardiovascular events in patients treated with immune checkpoint inhibitors. J Clin Oncol 2022. [DOI] [PubMed] [Google Scholar]
- [11].Du S, Zhou L, Alexander GS, Park K, Yang L, Wang N, et al. PD-1 modulates radiation-induced cardiac toxicity through cytotoxic T lymphocytes. J Thorac Oncol 2018;13:510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yegya-Raman N, Friedes C, Sun L, Iocolano M, Kim KN, Doucette A, et al. Utilization and factors precluding receipt of checkpoint inhibitor consolidation for stage III NSCLC in a large U.S. academic health system. Clin Lung Cancer 2023. [DOI] [PubMed] [Google Scholar]
- [13].Speirs CK, Dewees TA, Rehman S, Molotievschi A, Velez MA, Mullen D, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol 2017;12:293–301. [DOI] [PubMed] [Google Scholar]
- [14].D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care. Circulation 2008;117: 743–53. [DOI] [PubMed] [Google Scholar]
- [15].Apte AP, Iyer A, Thor M, Pandya R, Haq R, Jiang J, et al. Library of deep-learning image segmentation and outcomes model-implementations. Phys Med 2020;73: 190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Haq R, Hotca A, Apte A, Rimner A, Deasy JO, Thor M. Cardio-pulmonary substructure segmentation of radiotherapy computed tomography images using convolutional neural networks for clinical outcomes analysis. Phys Imaging Radiat Oncol 2020;14:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].SH Lee D W, Natarajan J, Yegya-Raman N, Kegelman T, Feigenberg S, Kao G, Xiao Y. Cardiac Substructure Segmentation Using Self-Configuring NnUNet and NnFormer for Cardiac-Sparing Lung Cancer Radiotherapy. AAPM 2022; Washington DC: 2022. [Google Scholar]
- [18].Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thor M, Deasy JO, Hu C, Gore E, Bar-Ad V, Robinson C, et al. Modeling the impact of cardiopulmonary irradiation on overall survival in NRG oncology trial RTOG 0617. Clin Cancer Res 2020;26:4643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jin J-Y, Hu C, Xiao Y, Zhang H, Paulus R, Ellsworth SG, et al. Higher radiation dose to the immune cells correlates with worse tumor control and overall survival in patients with stage III NSCLC: A secondary analysis of RTOG0617. Cancers 2021;13:6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McWilliam A, Kennedy J, Hodgson C, Vasquez Osorio E, Faivre-Finn C, van Herk M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer 2017;85:106–13. [DOI] [PubMed] [Google Scholar]
- [22].Zhang TW, Snir J, Boldt RG, Rodrigues GB, Louie AV, Gaede S, et al. Is the importance of heart dose overstated in the treatment of non-small cell lung cancer? A systematic review of the literature. Int J Radiat Oncol Biol Phys 2019;104:582–9. [DOI] [PubMed] [Google Scholar]
- [23].Thor M, Apte A, Haq R, Iyer A, LoCastro E, Deasy JO. Using auto-segmentation to reduce contouring and dose inconsistency in clinical trials: The simulated impact on RTOG 0617. Int J Radiat Oncol Biol Phys 2021;109:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McKenzie E, Zhang S, Zakariaee R, Guthier CV, Hakimian B, Mirhadi A, et al. Left anterior descending coronary artery radiation dose association with all-cause mortality in NRG oncology trial RTOG 0617. Int J Radiat Oncol Biol Phys 2022. [DOI] [PubMed] [Google Scholar]
- [25].Rubio-Infante N, Ramírez-Flores YA, Castillo EC, Lozano O, García-Rivas G, Torre-Amione G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail 2021;23:1739–47. [DOI] [PubMed] [Google Scholar]
- [26].McNew LK, Bowen SR, Gopan O, Nyflot MJ, Patel SA, Zeng J, et al. The relationship between cardiac radiation dose and mediastinal lymph node involvement in stage III non-small cell lung cancer patients. Adv Radiat Oncol 2017;2:192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2019;105:346–55. [DOI] [PubMed] [Google Scholar]
- [28].McCall NS, McGinnis HS, Janopaul-Naylor JR, Kesarwala AH, Tian S, Stokes WA, et al. Impact of radiation dose to the immune cells in unresectable or stage III non-small cell lung cancer in the durvalumab era. Radiother Oncol 2022;174:133–40. [DOI] [PubMed] [Google Scholar]
- [29].Demissei BG, Freedman G, Feigenberg SJ, Plastaras JP, Maity A, Smith AM, et al. Early changes in cardiovascular biomarkers with contemporary thoracic radiation therapy for breast cancer, lung cancer, and lymphoma. Int J Radiat Oncol Biol Phys 2019;103:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Friedes C, Iocolano M, Lee SH, Duan L, Li B, Doucette A, et al. The Effective Radiation Dose to Immune Cells Predicts Lymphopenia and Inferior Cancer Control in Locally Advanced NSCLC. Radiother Oncol 2023;24:110030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


