Abstract
Purpose:
Stereotactic body radiotherapy (SBRT) in the post-prostatectomy setting is investigational. A major concern is the deformable prostate bed clinical target volume (CTV) and the closely juxtaposed organs-at-risk (OARs). We report a volumetric and dosimetric analysis of kilovoltage cone-beam CT (CBCT) data from the first 18 patients enrolled on a phase II trial of post-prostatectomy SBRT. With instructions on bladder filling and rectal preparation, we hypothesized acceptable CTV coverage while minimal overdosing to OARs could be achieved.
Methods:
All patients received 5 fractions of 6–6.8 Gy to the prostate bed. CBCT were taken prior to and halfway through each fraction. CTV and OARs were contoured for each CBCT. Changes in inter- and intra-fraction volume and dose were calculated. Relative changes in CTV V95%, bladder V32.5 Gy, and rectal V32.5 Gy and V27.5 Gy were evaluated.
Results:
Interfraction CTV volume remained stable, with median change +5.69% (IQR −1.73% to +9.84%). CTV V95% exhibited median change −0.74% (IQR −9.15% to −0.07%). Volumetric and dosimetric changes were minor from interfraction rotation and intrafraction motion. CTV V95% was ≥93% in 13 of 18 (72%) patients; in the remaining five, median change was −14.09% (IQR −16.64% to −13.56%). Interfraction CTV volume change was significantly larger among patients with CTV V95% <93% (+25.04% vs. +2.85%, p = 0.002).
Conclusions:
With specific bladder and rectum filling protocols, CTV underdosing and overdosing to bladder and rectum are avoided in majority of patients. Changes in CTV shape may account for the underdosing that may be observed.
Keywords: Stereotactic body radiotherapy, Post-prostatectomy, Prostate cancer
Approximately a quarter to a third of patients with prostate cancer develop biochemical recurrence following radical prostatectomy [1,2]. In this setting, salvage radiotherapy is the only known curative intervention [2-5]. Alternatively, select patients may have certain adverse pathologic features, and adjuvant radiotherapy has shown to improve biochemical recurrence-free survival in these patients [2-4,6]. Traditionally, adjuvant and salvage radiotherapy have been delivered with conventional fractionated radiotherapy (fraction sizes of 1.8–2 Gy).
A body of preclinical and clinical data suggest that prostate cancer may exhibit an increased sensitivity to higher doses per fraction by virtue of a low α/β ratio [7]. For patients who have not had surgery, moderately hypofractionated radiotherapy (fraction sizes of 2.5–3.5 Gy) has been non-inferior to conventionally fractionated radiotherapy in three large randomized trials [8-10]. More recently, a randomized trial confirmed non-inferiority of extremely hypofractionated radiotherapy (fraction sizes >5 Gy) in definitive treatment setting as well [11]. Extreme hypofractionation delivered using modern techniques in five or fewer fractions, known as stereotactic body radiotherapy (SBRT), has shown to be safe and effective in a pooled consortium of over 2000 patients as well as in a systematic meta-analysis [12,13]. Another recent randomized trial found equivalence of SBRT and longer fractionation schemes with respect to acute toxicity [14].
While hypofractionation is increasingly used in the definitive treatment setting, there is less experience with its use in addressing microscopic or gross residual prostate cancer cells in the post-operative setting. Several single institution prospective and retrospective series delivering hypofractionated radiotherapy ranging from 54 to 66 Gy in 2.3–3.0 Gy per fractions postoperatively have shown favorable acute toxicity profiles while providing comparable biochemical control rates as conventionally fractionated regimens [15-19]. These data provided precedence for a large randomized trial evaluating moderately hypofractionated therapy which just completed accrual (NCT 03274687). Extreme hypofractionation in this setting is much less studied with only one published report of acute toxicity to date [20,21].
One major technical challenge with delivering extremely hypofractionated radiotherapy in the post-prostatectomy setting is the deformable nature of the target, which is a “potential space” that is itself delimited by several deformable organs-at-risk (OARs) such as the bladder and rectum [22]. Thus, accurate dose delivery can be complicated by significant target deformation in addition to inter-and intrafraction motion. Altogether, these can potentially lead to underdosing the target and overdosing normal surrounding tissues [23-25].
We recently initiated a prospective phase II trial evaluating SBRT in patients who have undergone radical prostatectomy. Here, we present a detailed retrospective volumetric and dosimetric study for the first 18 patients enrolled on this trial to evaluate how prostate bed and OAR deformability between and during treatment fractions impact dose delivery.
Methods and materials
The study population consisted of the first 18 patients who developed biochemical recurrence after prostatectomy for localized prostate cancer and enrolled onto a phase II single-institution trial of postoperative SBRT opened since March 1, 2018 with informed and written consent (NCT03541850). This trial was designed to show improvement in biochemical recurrence free survival based on dose-escalation with SBRT. All patients were required to have a technetium-99 bone scintigraphy scan, fluciclovine PET/CT scan, or prostate-specific membrane antigen (PSMA) PET/CT scan. Additionally, all patients underwent magnetic resonance imaging (MRI) of the pelvis and planning CT simulation scans obtained on Siemens SOMATOM Definition AS Scanner (Siemens Healthcare Diagnostics, Los Angeles, CA) using 1.5 mm slice thickness. The prostate bed and, when applicable, pelvic lymph nodes were contoured to generate the clinical target volume (CTV) per guidelines based on the RTOG consensus guidelines and outlined in the clinical trial protocol [26]. A 5 mm isotropic expansion of the CTV was used to generate the planning target volume (PTV). Radiation plans were designed to deliver 30–34 Gy in 5 fractions to the prostate bed such that 95% of each PTV received prescription dose. 25 Gy in 5 fractions was delivered to pelvic lymph nodes to patients at the physician’s discretion. A table comparing equivalent dose in 2 Gy fractions (EQD2) for this trial to the GU-003 trial can be found in the Appendix. If gross tumor was visible on imaging, a gross tumor volume (GTV) was defined and expanded isotropically by 3–5 mm to form a PTVGross. This target could receive up to 40 Gy in five fractions.
Volumetric modulated arc therapy plans were generated using two to four arcs. All patients were treated on a Novalis Tx (Brainlab, Inc, Westchester, IL 60154) or a Truebeam (Varian Medical Systems, Palo Alto, CA 94304). Every patient was instructed to void their bladder one hour prior to each treatment, and to drink 16–24 ounces of water to maintain a reproducible and comfortably full bladder. They were also instructed to take an enema the night before and morning of each treatment to ensure an empty rectum. All patients had two kilovoltage cone beam CTs (CBCTs) during each treatment; planar imaging with ExacTrac was used for bony alignment prior to the first CBCT. The first CBCT was taken prior to treatment initiation for anatomic verification, while the second was taken halfway through treatment to assess intrafraction motions. If warranted, patients were repositioned based on rigid-body registration of the planning CT and the CBCT acquired prior to treatment delivery to match to bony anatomy. Matching to bony anatomy was preferred to facilitate tracking and ensuring treatment to pelvic lymph nodes as needed while providing global positioning adjustments that balanced all anatomic sites involved in treatment.
Institutional board review approval was in place for the present retrospective analysis. CBCTs acquired during treatment, along with any shifts applied for online registration were imported and fused to the planning CTs in MIMVista (MIM Software, Cleveland, OH). Prostate bed CTVs and OARs were re-drawn onto both CBCTs from each treatment fraction. The percent change in inter- and intra-fractional CTV and OAR volumes were calculated. Inter- and intra-fractional shifts (translational and rotational) were extracted and summarized. The planning dose distribution was transferred onto the CBCTs. Our pre-specified criteria for adequate CTV coverage was CTV V95% >93%. Pre-specified dosimetric criteria for OARs were bladder V32.5 Gy <40%, rectal V32.5 Gy <35%, and rectal V27.5 Gy <50%. These criteria were derived from institutional constraints and previous post-prostatectomy radiotherapy trials (Appendix). The following population dosimetric parameters were extracted and summarized with univariate statistics: mean dose to CTV, bladder, and rectum; CTV V95%; bladder V32.5 Gy; rectum V32.5 Gy; and rectum V27.5 Gy. Unequal variance T-test evaluated target/OAR volume and dosimetric changes between patients with acceptable and unacceptable CTV V95% coverage. Associations between CTV V95% and interfractional CTV/OAR volume changes was assessed by regression analysis. Estimates for achieving greater than 90% tumor control was calculated with tumor control probability (TCP) modeling using the Niemierko method outlined in the Appendix.
Results
Patient demographic and treatment characteristics are found in Supplemental Table A. A total of 175 CBCTs from 18 patients were assessed. One patient did not tolerate the extension of treatment time for the second CBCT per fraction due to difficulty holding his bladder, thus contributed only 5 CBCTs to the present analysis. Interfractional volumetric changes are presented in Table 1. The median change in CTV volume from the simulation to pre-treatment CBCT was +5.69% (IQR −1.73% to +9.84%). Median changes in bladder and rectal volumes were +2.17% (IQR −28.19% to +10.55%) and +20.97% (IQR +5.10% to +34.64%), respectively.
Table 1.
Interfraction and intrafraction relative percent volume changes.
| Interfraction |
Intrafraction |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Q1 | Q3 | Mean | Median | Q1 | Q3 | |
| CTV volume (%) | 2.95 | 5.69 | −1.73 | 9.84 | 6.19 | −0.27 | −0.88 | 0.43 |
| Bladder volume (%) | 0.85 | 2.17 | −28.19 | 10.55 | 18.42 | 16.80 | 9.07 | 22.70 |
| Rectum volume (%) | 25.38 | 20.97 | 5.10 | 34.64 | 3.03 | 1.46 | 0.55 | 4.95 |
CTV: clinical target volume; Q1: 1st quartile; Q3: 3rd quartile
Overall, intrafractional volume changes of the CTV were relatively small and also summarized in Table 1. The median intrafractional change in CTV volume was −0.27% (IQR −0.88% to +0.43%). Median volume change from intrafraction motion for the bladder was +16.80% (IQR +9.07% to +22.70%) and +1.46% (IQR +0.55% to +4.95%) for the rectum.
Translational and rotational shift data are presented in Table 2. The median time a patient was on the treatment table, defined as the time between first treatment arc to the end of last treatment arc was approximately 15.5 min (IQR 14–18.7). Interfractional shifts were required in 18 of 90 (20%) treatment fractions, while shifts accounting for intrafraction motion in the prostate bed were required in 10 of 90 (11%) fractions.
Table 2.
Variations in prostate bed motion.
| Interfraction |
Intrafraction |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Q1 | Q3 | Mean | Median | Q1 | Q3 | |
| Vert. (AP; mm) | −1.8 | −2.0 | −0.6 | −2.0 | −1.1 | −2.0 | −2.0 | −0.3 |
| Long. (SI; mm) | 0.0 | 2.0 | −0.5 | 4.8 | 2.3 | 1.5 | 0.0 | 4.5 |
| Lat.(RL; mm) | 1.5 | −1.0 | −2.2 | 3.0 | 2.0 | 0.0 | −3.5 | 2.3 |
| Rot. (°) | 0.3 | 0.1 | −0.3 | 0.3 | 0.6 | 0.5 | 0.4 | 0.7 |
Vert.: vertical; AP: anterior-posterior; Long.: longitudinal; SI: superior-inferior; Lat.: latitudinal; RL: right-left; Rot.: rotational; Q1: 1st quartile; Q3: 3rd quartile
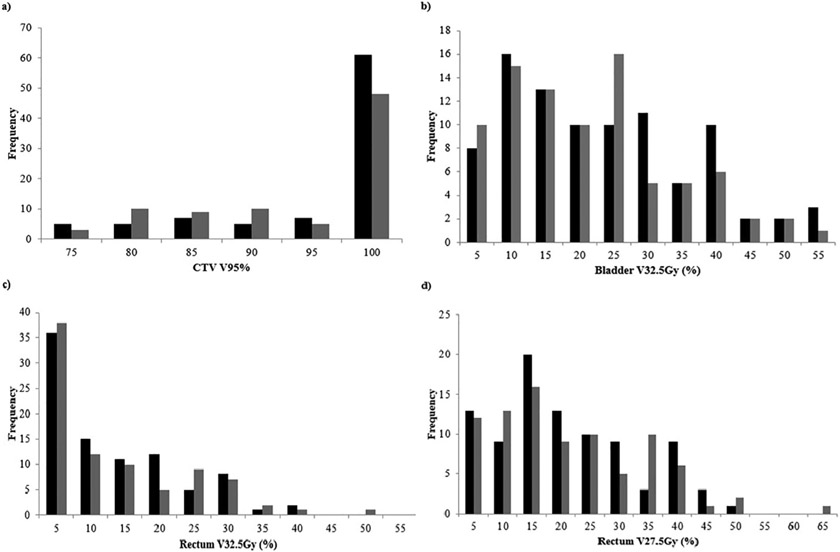
Interfractional dosimetric changes for the CTV, bladder, and rectum are presented in Table 3. The absolute median change in CTV V95% was −0.74% (IQR −9.15% to −0.07%). After interfractional shifts were applied, CTV V95% coverage goal of ≥93% was met for 63 of 90 (70%) of fractions delivered, corresponding to acceptable coverage in 13 of 18 (72%) patients (Fig. 1a). Median changes in bladder and rectal doses were also relatively small. Fig. 1b-c summarizes interfractional OAR dosimetry. Eighty-three of 90 (92%) total treatment fractions had a bladder V32.5 Gy <40%, with median bladder V32.5 Gy equal to 18.79% (IQR +9.50% to +28.18%). The median absolute change in bladder V32.5 Gy was −2.00% (IQR −6.29% to +3.30%). Similarly, 88 of 90 (98%) treatment fractions had rectal V32.5 Gy <35%, and median rectal V32.5 Gy was +8.05% (IQR +1.94% to +16.5%). Absolute median change in rectal V32.5 Gy was −1.21% (IQR −4.91% to +0.29%). All fractions had a rectal V27.5 Gy <50%. Median rectal V27.5 Gy was +16.02% (IQR +10.14% to +25.30%), and the absolute median interfraction change was −4.88% (IQR, −9.02% to +0.31%).
Table 3.
Interfraction and intrafraction percent dose changes.
| Interfraction |
Intrafraction |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Q1 | Q3 | Mean | Median | Q1 | Q3 | |
| CTV mean dose (%)* | −1.63 | −0.40 | −2.43 | −0.17 | −0.42 | 0.00 | −0.03 | 0.00 |
| CTV V95% | −5.05 | −0.74 | −9.15 | −0.07 | −7.57 | −1.76 | −16.50 | −1.29 |
| Bladder mean dose (%)* | 0.30 | −1.33 | −11.77 | 6.55 | −4.73 | −3.73 | −6.46 | −2.47 |
| Bladder V32.5 (%) | −2.59 | −2.00 | −6.29 | 3.30 | −5.01 | −4.29 | −10.65 | 0.04 |
| Rectum mean dose (%)* | −6.98 | −8.53 | −15.34 | −2.86 | −1.23 | −0.57 | −2.50 | 0.00 |
| Rectum V32.5 (%) | −0.85 | −1.21 | −4.91 | 0.29 | −0.66 | −0.58 | −3.12 | 0.40 |
| Rectum V27.5 (%) | −4.09 | −4.88 | −9.02 | −0.31 | −4.55 | −3.17 | −11.42 | −0.49 |
CTV: clinical target volume; CTV V95%: proportion of CTV volume receiving 95% of prescribed dose or more. Coverage goal was CTV95% to be >98% ±5%; V32.5: proportion of volume receiving 32.5 Gy or more; V27.5: proportion of volume receiving 27.5 Gy or more; Q1:1st quartile; Q3: 3rd quartile;
Expressed in relative percentage. Non-asterisk values are expressed in absolute percentages
Fig. 1.
Frequency distribution of interfractional (black) and intrafractional (grey) dosimetry for each treatment session. (a) CTV V95%, with coverage goal CTV V95% >98% ±5% was considered acceptable, (b) Bladder V32.5 with ideal constraint <35% +5%, (c) Rectum V32.5 with ideal constraint <30% +5% and (d) Rectum 27.5 with ideal constraint <45% +5%.
The impact of interfraction rotational changes alone on dosimetry was overall minimal and summarized in Table 4. There was zero median change to CTV dose, and an absolute median change of 0.01% (IQR −0.25% to +1.42%) in CTV V95%. Absolute median change in bladder V32.5 Gy, rectum V32.5 Gy, and rectum V27.5 Gy from rotational shifts alone were −0.05% (IQR −0.13% to +0.11%), −0.07% (IQR −0.25% to −0.01%), and −0.18%, (IQR −0.39% to +0.06%) respectively.
Table 4.
Interfraction percent dose changes due to rotational shifts.
| Mean | Median | Q1 | Q3 | |
|---|---|---|---|---|
| CTV dose (%)* | 0.28 | 0.00 | 0.00 | 0.00 |
| CTV V95% | 0.61 | 0.01 | −0.25 | 1.42 |
| Bladder dose (%)* | 0.38 | 0.02 | −0.30 | 0.53 |
| Bladder V32.5 (%) | 0.32 | −0.05 | −0.13 | 0.11 |
| Rectum dose (%)* | −0.08 | 0.10 | −0.70 | 1.15 |
| Rectum V32.5 (%) | −0.42 | −0.07 | −0.25 | −0.01 |
| Rectum V27.5 (%) | −0.50 | −0.18 | −0.39 | 0.06 |
CTV: clinical target volume; CTV V95%: proportion of CTV volume receiving 95% of prescribed dose or more. Coverage goal was CTV95% to be >98% ±5%; V32.5: proportion of volume receiving 32.5 Gy or more; V27.5: proportion of volume receiving 27.5 Gy or more; Q1: 1st quartile; Q3 = 3rd quartile;
Expressed in relative percentage. Non-asterisk values are expressed in absolute percentages.
Dosimetric impact from intrafraction motion was also minor. We found zero median change to CTV dose overall, and an absolute median change in CTV V95% of −1.76% (IQR −16.50% to −1.29%). Of 85 analyzed intrafractional CBCTs, CTV V95% ≥93% was not achieved in 36 fractions (compared with 27 based on the pre-treatment CBCT). Thus, intrafraction motion led to an additional 9 of 54 (16.67%) fractions having target under-coverage despite acceptable dosimetry on the pre-treatment CBCT (Fig. 1). The beam-on times for seven of these nine fractions were in or above the 3rd quartile of beam-on times for all patients. Median intrafraction changes of bladder and rectal doses were small and are summarized in Table 3.
As previously mentioned, 13 of 18 (72%) patients achieved treatment adequacy of CTV V95% ≥93%, while 5 (28%) patients did not. Among patients who met with interfraction CTV V95% ≥93%, the median change in interfraction CTV V95% was −0.45% (IQR −1.6% to −0.23%), while for patients with interfraction CTV V95% <93%, the median change was −14.09% (IQR −16.64% to −13.56%) (p = 0.001). The median interfraction change in CTV volume was significantly larger in patients with unacceptable versus acceptable coverage (+25.04% [IQR +21.08% to +26.40%] versus +2.85% [IQR −4.41% to +8.05%], p = 0.002). Changes in OAR volumes or dose were not significantly different between patients with acceptable versus unacceptable CTV V95% (Table 5). Regression analysis also found no significant associations between changes in CTV, bladder, or rectal volume changes with treatment adequacy in both patients who had acceptable or unacceptable CTV V95% coverage. Recognizing that patients may have intrinsic differences in pelvic anatomy from one other, we identified 4 patients who displayed both acceptable and unacceptable CTV V95% coverages over their treatment courses to evaluate for any associations between bladder and rectal volume changes with treatment adequacy. There remained no significant associations between OAR volume changes with treatment adequacy on regression analysis for each patient.
Table 5.
Comparative percent changes in interfraction volume and dosimetry among patient with acceptable and unacceptable CTV V95% coverage.
| Acceptable CTV V95% |
Unacceptable CTV V95% |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Q1 | Q3 | Mean | SD | Median | Q1 | Q3 | p-value | |
| CTV V95% | −1.53 | 2.65 | −0.45 | −1.60 | −0.23 | −15.69 | 4.21 | −14.09 | −16.64 | −13.56 | p = 0.001 |
| CTV volume (%) | −1.96 | 19.76 | 2.85 | −4.41 | 8.05 | 23.32 | 9.36 | 25.04 | 21.08 | 26.40 | p = 0.002 |
| Bladder volume (%) | 18.23 | 52.08 | −2.93 | −14.56 | 47.18 | 0.59 | 33.22 | −0.60 | −5.44 | 21.33 | p = 0.411 |
| Rectal volume (%) | 20.97 | 57.43 | 22.63 | 10.08 | 28.18 | 35.42 | 33.91 | 32.58 | 16.61 | 36.67 | p = 0.522 |
| Bladder V32.5 (%) | −2.28 | 4.93 | 0.25 | −6.56 | 1.28 | −2.94 | 8.47 | −1.84 | −2.49 | 0.30 | p = 0.877 |
| Rectal V32.5 (%) | −2.32 | 3.04 | −0.71 | −4.65 | −0.36 | 3.57 | 10.10 | −3.14 | −3.78 | 14.19 | p = 0.270 |
| Rectum 27.5 (%) | −6.34 | 5.72 | −4.60 | −7.92 | −3.22 | 0.74 | 13.20 | −7.46 | −8.16 | 15.11 | p = 0.299 |
CTV: clinical target volume; CTV V95%: proportion of CTV volume receiving 95% of prescribed dose or more. Coverage goal was CTV95% to be >98% ±5%; V32.5: proportion of volume receiving 32.5 Gy or more; V27.5: proportion of volume receiving 27.5 Gy or more.
To investigate the efficacy of post-prostatectomy SBRT, tumor control probability (TCP) modeling was performed (Supplemental Table B). For TCP to be greater than 90%, a median CTV V100% of 85% (IQR 79–88%) with range of 69–95% was predicted to be required. Of five patients who did not meet CTV V95% >93% criteria, four patients had CTV V100% from all treatment fractions within the range predicted to have 90% tumor control, while one patient had two of his treatment fractions fall below this range.
Discussion
In this volumetric and dosimetric analysis of 18 patients who prospectively received SBRT to the prostate bed with specific bladder and rectal filling instructions, we found there was relative interfractional stability of CTV volume and median dose. 72% of patients met pre-specified coverage goal of CTV V95% >93%, with a median decline in coverage of approximately 15% in those patients with CTV V95% <93%. The CTV volume appeared to be significantly larger among those patients with underdosed CTVs, but rectal and bladder volumes were not significantly different. Intrafractional volumetric changes and shifts were generally minor, but in 16.67% (9 of 54) of evaluable instances, the CTV V95% coverage changed from acceptable to unacceptable on the basis of intrafractional motion. On the other hand, interfractional changes were notable with nearly 20% of treatments requiring shifts due to interfractional motion related to suboptimal bladder and/or rectal preparation. Median shifts were anisotropic, and were similar to the shifts reported for conventionally fractionated regimens [23,25,27].
To our knowledge, this is the first study evaluating the dosimetric implications of target and pelvic OAR deformation when delivering SBRT postoperatively for prostate cancer. Despite target and OAR deformation, the proportion of patients with acceptable coverage in this study were similar to those reported in prior studies evaluating the impact of interfractional motion on target dosimetry in the context of conventionally-fractionated post-prostatectomy radiotherapy [25,28]. Zhu et al. reported inter- and intra-fractional target motions of the prostate bed from 16 patients, and their associations with treatment adequacy defined as the minimum dose to the CTV and the dose received by 95% of the CTV [25]. Five of 16 (31%) patients were considered to have inadequate treatments, but the under-dosed volume was small and the authors did not find significant associations between CTV volume and treatment adequacy which agrees with findings from our study [25]. Albeit for a different pre-specified acceptability criteria of V95% >93%, our study reports 28% of patients not meeting coverage goal. Interestingly, Zhu et al. found a significant association between rotational changes with treatment adequacy. In the present study, dosimetric changes from rotational shifts alone were minor, and likely were not associated with treatment adequacy. Our mean inter- and intrafractional rotational changes were smaller (0.3 and 5.7 degrees, respectively) compared to a range of maximal rotations of 4.1–12.2 degrees in their study. The absolute median change in CTV V95% from interfraction rotation alone was 0.01% (IQR −0.25% to 1.42%), and similarly dosimetric changes to bladder and rectum were also minimal. This difference may be in part due to more stringent bladder filling and rectal emptying preparations implemented in our trial. Notably, similar rates of target undercoverage were achieved despite tighter PTV margins of 5 mm.
In another analysis of 10 patients with 102 pre-treatment CTs, Liu et al. reported ranges of daily CTV, bladder, and rectal volumes of 75–116%, 50–270%, and 30–180%, respectively [28]. 53% of treatments met their acceptable coverage criteria of CTV V100 >95%, which was lower than the 72% reported in our study. It is unclear whether Liu et al. used specific bladder filling and rectum emptying instructions, which is important, particularly in hypofractionated regimens [29,30]. Overall, perhaps implementing more rigorous OAR preparations may have lessened the degree of OAR interfraction volume changes observed in our study, thereby improving target coverage. Thus, delivering high dose per fraction in the post-prostatectomy setting necessitates internal pelvic anatomy to be as close to those from planning simulation scans as possible. Additional methods that ensure optimal OAR anatomy could be use of bladder ultrasounds or rectal balloons prior to treatment.
The dosimetric changes between and during treatments were minor despite the deformable nature of the CTV, which is delimited by deformable OARs such as the bladder and rectum. Notably, the rectum experienced a relatively large median interfractional volume change (+20.97%, compared to 2.17% for bladder and 5.69% for the CTV) though relatively minor dosimetric changes were noted and OAR dosing was never unacceptably high. The same holds true for OAR dosimetry as a result of intrafractional changes. In this scenario, the bladder experienced a relatively large median intrafractional volume change (+16.80%, compared to +1.46% for rectum and −0.27% for CTV), but the dosimetric impacts were small. No significant associations were found between interfractional OAR volume changes and CTV V95% in patients with acceptable or unacceptable treatment coverage. Moreover, almost all interfractional CTV volumes from our study were within the predicted range to achieve high probability for tumor control. Of note, reported dosimetric measurements were calculated from re-contoured structures and imported planning dose distributions onto CBCTs instead from a new dose re-calculation. Thus, reported dosimetric measurements may underestimate true dosimetric changes, especially for patients whose external pelvic anatomy evolves during the course of treatment. So far, we have experienced stable external pelvic anatomy in our patients and have observed ~1–2% dosimetric differences when comparing dose-volume histograms derived from re-contoured structures on CBCTs and superimposed targets/OARs on planning CT.
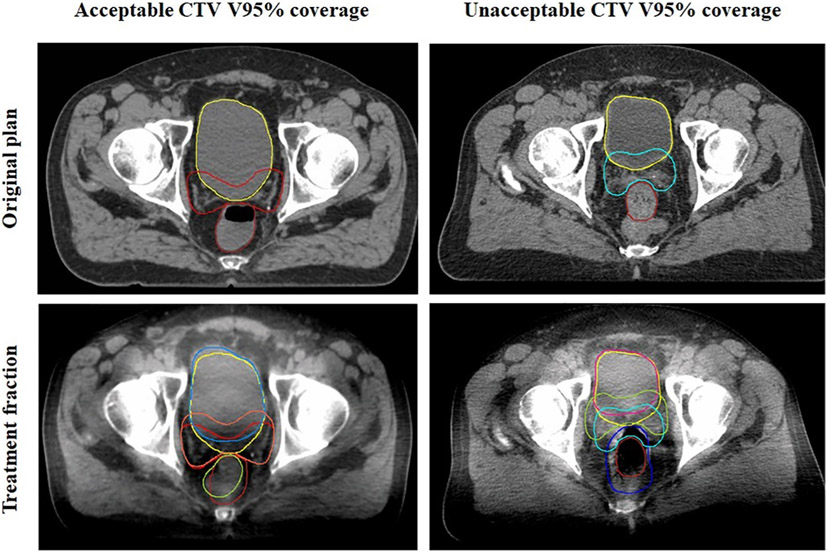
Altogether these data suggest that while OAR volumes change, they are not the sole determining factor for CTV coverage. Instead, the shape of target and OARs may also have dosimetric impact. Fig. 2 shows a side-by-side comparison of two patients with relatively similar changes in bladder and rectal volumes yet different CTV V95% coverage outcomes. Online adaptive radiotherapy protocols may be integral in optimizing the delivered dose distribution for post-prostatectomy radiotherapy [22,25].
Fig. 2.
Comparison of CTV and OAR contours between a patient who had acceptable and unacceptable CT V95% coverage. Both patients had relatively smaller bladder volumes and larger rectal volumes compared to original plan. However, the shape of the OAR, and thus the prostate bed CTV, still appears different at the level of the femoral head.
A possible strategy to further improve acceptable CTV coverage raises into question whether larger PTV margins should be used to properly account for deformation. However, a larger PTV margin would lead to overdosing adjacent OARs. Given that comparatively few studies, even in the setting of conventional fractionation, have truly investigated the optimal dose delivered to the prostatic fossa CTV, an intriguing possibility is that the dose presumed to be necessary for oncologic outcome may be higher than the actual required dose for cancer control. That is, the prostate bed CTV may be adequately covered by a lower isodose line than the prescription dose, and this lower dose may be the true necessary oncologic dose. However, it is too premature to report either efficacy or toxicity data from the current trial, as the primary endpoint is four-year biochemical recurrence-free survival and the median follow-up for the 18 patients in this subgroup analysis is still short.
This study is not without limitations. First, the soft-tissue resolution of kilovoltage CBCTs is not optimal, potentially affecting the accuracy of contours derived from CBCTs. As all contours were done by a single investigator and reviewed by an independent investigator, there was high internal consistency. Improvements in image quality may account for more accurate daily anatomic changes on CBCT images. MRI image guidance has superior soft tissue contrast compared to CT based images, making it a potential tool to accurately assess changes in post-operative prostate bed and open opportunities for adaptive re-planning. Another limitation of this study is that only one CBCT was taken to account for intrafraction motion during any given treatment session. While continuous or frequent monitoring is ideal to assess the true extent of intrafraction motion, one CBCT was thought to suffice in this study to balance the time required to acquire on-board imaging with the increased likelihood of intrafraction motion with time. However, the true volumetric and dosimetric impacts from intrafraction motion may be underestimated.
In conclusion, the dosimetric and volumetric data from this prospective trial indicate that with specific bladder filling and rectal emptying patient instructions, CTV coverage is well maintained without overdosing OARs despite the deformable anatomy involved with post-prostatectomy SBRT. To maintain adequate CTV coverage and OAR safety when delivering high doses per fraction necessitates optimizing anatomic conditions on day of treatment to be as close to those from planning simulation scans. Acceptable CTV coverage may also be influenced by the shapes OARs ultimately assume at the time of treatment.
Supplementary Material
Funding
Dr. Kishan reports research funding from the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence (P50CA09213), the Radiological Society of North America (RSNA) (grant RSD1836), the STOP Cancer organization, the Jonsson Comprehensive Cancer Center, the Rosenson Family Foundation, and has received a Career Development Award from the American Society of Radiation Oncology and the Prostate Cancer Foundation.
Footnotes
Conflict of interest
Dr. Steinberg and Dr. Cao report personal fees and non-financial support from Varian and American Society for Radiation Oncology (ASTRO).
*There are no conflicts of interests
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radonc.2020.04.007.
References
- [1].Bianco FJ Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”). Urology 2005;66:83–94. [DOI] [PubMed] [Google Scholar]
- [2].Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet (London, England) 2012;380:2018–27. [DOI] [PubMed] [Google Scholar]
- [3].Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96–02/AUO AP 09/95 trial. Eur Urol 2014;66:243–50. [DOI] [PubMed] [Google Scholar]
- [4].Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 2009;181:956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stish BJ, Pisansky TM, Harmsen WS, et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol 2016;34:3864–71. [DOI] [PubMed] [Google Scholar]
- [6].Hackman G, Taari K, Tammela TL, et al. Randomised trial of adjuvant radiotherapy following radical prostatectomy versus radical prostatectomy alone in prostate cancer patients with positive margins or extracapsular extension. Eur Urol 2019. [DOI] [PubMed] [Google Scholar]
- [7].Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095–101. [DOI] [PubMed] [Google Scholar]
- [8].Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 2016;34:2325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017;35:1884–90. [DOI] [PubMed] [Google Scholar]
- [11].Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet (London, England) 2019;394:385–95. [DOI] [PubMed] [Google Scholar]
- [12].Kishan AU, Dang A, Katz AJ, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Network Open 2019;2:e188006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jackson WC, Silva J, Hartman HE, et al. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys 2019;104:778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Katayama S, Striecker T, Kessel K, et al. Hypofractionated IMRT of the prostate bed after radical prostatectomy: acute toxicity in the PRIAMOS-1 trial. Int J Radiat Oncol Biol Phys 2014;90:926–33. [DOI] [PubMed] [Google Scholar]
- [16].Lewis SL, Patel P, Song H, et al. Image guided hypofractionated postprostatectomy intensity modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2016;94:605–11. [DOI] [PubMed] [Google Scholar]
- [17].Fersino S, Tebano U, Mazzola R, et al. Moderate hypofractionated postprostatectomy volumetric modulated arc therapy with daily image guidance (VMAT-IGRT): a mono-institutional report on feasibility and acute toxicity. Clin Genitourinary Cancer 2017;15:e667–73. [DOI] [PubMed] [Google Scholar]
- [18].Kruser TJ, Jarrard DF, Graf AK, et al. Early hypofractionated salvage radiotherapy for postprostatectomy biochemical recurrence. Cancer 2011;117:2629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barra S, Belgioia L, Marcenaro M, et al. Moderate hypofractionated radiotherapy after prostatectomy for cancer patients: toxicity and clinical outcome. Cancer Manag Res 2018;10:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sampath S, Yuh B, Frankel P, et al. Prostate bed stereotactic body radiation therapy (PB-SBRT) for postprostatectomy biochemical recurrence: first toxicity results of a phase 1 dose-escalation trial. Int J Radiat Oncol Biol Phys 2016;96:E227–8. [Google Scholar]
- [21].Ballas L, Aron M, Jhimlee S, et al. Phase I study of stereotactic body radiotherapy following radical prostatectomy. J Clin Oncol 2018;36. TPS158–TPS158. [Google Scholar]
- [22].Gill S, Pham D, Dang K, et al. Plan of the day selection for online image-guided adaptive post-prostatectomy radiotherapy. Radiother Oncol 2013;107:165–70. [DOI] [PubMed] [Google Scholar]
- [23].Gill S, Isiah R, Adams R, et al. Conventional margins not sufficient for post-prostatectomy prostate bed coverage: an analysis of 477 cone-beam computed tomography scans. Radiother Oncol 2014;110:235–9. [DOI] [PubMed] [Google Scholar]
- [24].Bell LJ, Cox J, Eade T, Rinks M, Kneebone A. The impact of rectal and bladder variability on target coverage during post-prostatectomy intensity modulated radiotherapy. Radiother Oncol 2014;110:245–50. [DOI] [PubMed] [Google Scholar]
- [25].Zhu M, Bharat S, Michalski JM, Gay HA, Hou WH, Parikh PJ. Adaptive radiation therapy for postprostatectomy patients using real-time electromagnetic target motion tracking during external beam radiation therapy. Int J Radiat Oncol Biol Phys 2013;85:1038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010;76:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ost P, De Meerleer G, De Gersem W, Impens A, De Neve W. Analysis of prostate bed motion using daily cone-beam computed tomography during postprostatectomy radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:188–94. [DOI] [PubMed] [Google Scholar]
- [28].Liu F, Ahunbay E, Lawton C, Li XA. Assessment and management of interfractional variations in daily diagnostic-quality-CT guided prostate-bed irradiation after prostatectomy. Med Phys 2014;41:031710. [DOI] [PubMed] [Google Scholar]
- [29].Yahya S, Zarkar A, Southgate E, Nightingale P, Webster G. Which bowel preparation is best? Comparison of a high-fibre diet leaflet, daily microenema and no preparation in prostate cancer patients treated with radical radiotherapy to assess the effect on planned target volume shifts due to rectal distension. Br J Radiol 2013;86. 20130457–20130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Diot Q, Olsen C, Kavanagh B, Raben D, Miften M. Dosimetric effect of online image-guided anatomical interventions for postprostatectomy cancer patients. Int J Radiat Oncol Biol Phys 2011;79:623–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.