Abstract
Purpose
To compare plan quality among photon volumetric arc therapy (VMAT), Gamma Knife, and three different proton beam modalities.
Methods
Fifty-five brain lesions from 20 patients were planned with three different proton spot size ranges of cyclotron-generated proton beams, CPBs (spot size σ: 2.7–7.0 mm), linear accelerator proton beams, LPBs (σ: 2.9–5.5 mm), and linear accelerator proton minibeams, LPMBs (σ: 0.9–3.9 mm), with and without apertures and compared against photon VMAT and Gamma Knife plans. Dose coverage to each lesion for each proton and photon plan was set to 99% of the GTV receiving the prescription (Rx) dose. All proton plans used ±2 mm setup uncertainty and ±2% range uncertainty in robust evaluation to achieve V100%Rx > 95% of the GTV. Apertures were applied to proton beams irradiating tumors <1 cm3 volume and located <2.5 cm depth. Conformity index (CI), gradient index (GI), V12 Gy, V4.5 Gy, and mean brain dose were compared across all plan types. The Wilcoxon signed rank test was utilized to determine statistical significance of dosimetric results compared between photon and proton plans.
Results
When compared to CPB generated plans, average CI and GI were significantly better for the LPB and LPMB plans. Aperture-based IMPT plans showed improvement from Gamma Knife for all dosimetric metrics. Aperture-based IMPT plans also showed improvement in all dosimetric metrics for shallow tumors (d < 2.5 cm) when compared with non-aperture-based plans.
Conclusion
The LPB and LPMB stand as excellent alternatives to CPB or photon therapy and significantly increase the preservation of normal tissue.
Keywords: Stereotactic radiosurgery, minibeams, linear accelerator proton beams, apertures
INTRODUCTION
Radiation therapy constitutes an important treatment modality for managing brain tumors, for which local control is in high demand. Recent advances in radiation therapy techniques include the use of intensity-modulated radiation therapy (IMRT), volumetric-modulated radiation therapy (VMAT), stereotactic radiosurgery (SRS), and particle therapy.1 Optimal dose conformity provided by particle therapy allows dose escalation and better sparing of critical structures and OARs. Protons may decrease the rate of acute2 and late toxicity3, usually seen with photon therapy, increasing the therapeutic ratio.4 This is also shown in multiple studies establishing guidelines for and comparing photon vs. proton therapy.5
With the progression of technology and treatment techniques for brain metastases and the recent increase in popularity of proton therapy, it is a viable question to ask how exactly proton therapy for treating brain metastases compares with photon-based SRS. One of several areas currently being investigated towards proton SRS is the availability of new treatment technologies that enable sharper dose fall-off6 and better sparing of normal tissues. In prior decades, a foundation for greater adoption of intracranial SRS-based photon therapy has been built, with new improvements, such as on-board cone-beam CT (CBCT)7, and volumetric modulated arc therapy (VMAT)8. However, developments in proton therapy still need improvement in this aspect. Few reports currently exist on the use of proton SRS for brain metastases.9 With the current pencil-beam scanning (PBS) system, proton beam therapy (PBT) may not be comparable to photon radiotherapy in terms of lateral penumbra10, target conformity11 and uncertainties.12
Some newer technologies for proton therapy include the development of linear accelerator-based proton therapy.13-15 Compared with standard cyclotron-generated proton beams, where protons are accelerated in a spiral pattern, a linear accelerator-based proton beam accelerates protons in a straight line, produces a stable spot size with energy past 150 MeV, has superior beam quality, and utilizes electronic energy control, where energy can be changed pulse by pulse.15,16 A proton linac also shows small emittance, resulting in smaller spot sizes and low beam losses, requiring less shielding than cyclotron-generated proton beams. With the added feature of producing proton mini beams with sub-millimeter spot sizes, the proton linac becomes an attractive alternative to photon therapy and cyclotron-generated proton beam therapy.
The purpose of this study was to perform a comprehensive assessment of photon SRS plans using a standard linac and Gamma Knife vs. three proton beam models using IMPT. Gamma Knife photon plans were also compared against standard cyclotron-generated proton plans and linear accelerator proton plans using apertures for small and shallow lesions. Dosimetric parameters, including the RTOG conformity index (RTOG CI), gradient index (GI), V12Gy (volume of the brain receiving 12 Gy dose) for each lesion, and V4.5Gy (volume of the brain receiving 4.5 Gy dose) and mean brain dose, are reported for each patient case studied.17
METHODS AND MATERIALS
Twenty patients treated on GK with single-fraction SRS for multiple brain metastases between 2018 and 2019, with two to three targets each, were retrospectively selected for this study. The study is approved by our institutional IRB. Individual target volumes ranged from 0.02 cm3 to 17.3 cm3. Targets were prescribed with doses ranging from 11 to 24 Gy. Target volumes and prescription doses for each patient are listed in Table 1. All patients received magnetic resonance imaging (MRI) with a 1 mm slice thickness for treatment planning. Based on MR images, the gross target volumes (GTVs) and normal tissue (NT) structures were contoured by a neurosurgeon and transferred to the planning CT after rigid image fusion. We compared CI, GI, V4.5Gy, V12Gy and mean brain dose among five different plan types – VMAT SRS, Gamma Knife, CPB, LPB and LPMB. Aperture-based proton plans were compared with Gamma Knife plans, and non-aperture-based proton plans were compared with photon VMAT SRS plans. To better compare proton plans with robustness vs photon plans planned on a PTV, a separate conformity index, CIPTV, was defined for proton plans, as the 100% isodose volume covering the GTV divided by the corresponding PTV volume from VMAT plans. GTV to PTV margin was 2 mm, so the percent isodose volume covering the GTV was divided by the volume corresponding to the GTV + 2mm. The GI was defined as the volume of the 50% isodose line divided by PTV volume. V4.5Gy and V12Gy are the volume of the brain receiving 4.5 Gy and 12 Gy, respectively. Mean brain dose is defined as the mean dose to the brain, not excluding GTV or PTV.
Table 1.
Tumor target volumes and prescription doses for all 20 patients studied
| Patient | Target volume and Rx | Target 1 | Target 2 | Target 3 |
|---|---|---|---|---|
| 1 | Target volume (cc) | 6.21 | 1.55 | 2.57 |
| Prescription (Gy) | 15 | 12 | 12 | |
| 2 | Target volume (cc) | 6.41 | 0.73 | N/A |
| Prescription (Gy) | 13 | 13 | N/A | |
| 3 | Target volume (cc) | 7.71 | 0.75 | 0.14 |
| Prescription (Gy) | 18 | 18 | 18 | |
| 4 | Target volume (cc) | 0.6 | 2.12 | 0.66 |
| Prescription (Gy) | 11 | 11 | 11 | |
| 5 | Target volume (cc) | 0.16 | 0.03 | 0.02 |
| Prescription (Gy) | 24 | 18 | 24 | |
| 6 | Target volume (cc) | 4.24 | 2.35 | 0.18 |
| Prescription (Gy) | 11 | 11 | 11 | |
| 7 | Target volume (cc) | 0.19 | 0.42 | 0.07 |
| Prescription (Gy) | 24 | 24 | 18 | |
| 8 | Target volume (cc) | 0.39 | 0.43 | 0.12 |
| Prescription (Gy) | 24 | 24 | 24 | |
| 9 | Target volume (cc) | 5.28 | 3.2 | 0.15 |
| Prescription (Gy) | 15 | 12 | 24 | |
| 10 | Target volume (cc) | 4.28 | 1.53 | 0.09 |
| Prescription (Gy) | 18 | 22 | 22 | |
| 11 | Target volume (cc) | 0.26 | 17.3 | 0.54 |
| Prescription (Gy) | 24 | 15 | 24 | |
| 12 | Target volume (cc) | 1.72 | 0.15 | 1.55 |
| Prescription (Gy) | 24 | 24 | 24 | |
| 13 | Target volume (cc) | 2.13 | 0.13 | N/A |
| Prescription (Gy) | 14 | 14 | N/A | |
| 14 | Target volume (cc) | 9.62 | 8.65 | 1.12 |
| Prescription (Gy) | 15 | 15 | 24 | |
| 15 | Target volume (cc) | 4.74 | 0.81 | 6.9 |
| Prescription (Gy) | 18 | 24 | 18 | |
| 16 | Target volume (cc) | 4.16 | 0.74 | 0.27 |
| Prescription (Gy) | 18 | 24 | 24 | |
| 17 | Target volume (cc) | 0.18 | 6.76 | 3.29 |
| Prescription (Gy) | 18 | 18 | 13 | |
| 18 | Target volume (cc) | 0.31 | 1.9 | 2.92 |
| Prescription (Gy) | 24 | 18 | 18 | |
| 19 | Target volume (cc) | 0.59 | 0.7 | 4.48 |
| Prescription (Gy) | 16 | 16 | 16 | |
| 20 | Target volume (cc) | 0.15 | 0.04 | 0.91 |
| Prescription (Gy) | 24 | 24 | 12 |
Gamma Knife plans
GK plans were created using the Elekta GammaPlan treatment planning system, version 10 (Elekta Inc., Stockholm, Sweden), with the TMR10 dose algorithm, for patients to be treated on the Leksell Gamma Knife Icon, which contains 192 60Co sources, with three available collimator sizes – 4, 8, and 16 mm. Experienced neurosurgeons or medical physicists are responsible for generating GK plans. Multiple GK shots using varying collimator sizes were manually placed inside each target to deliver a prescription dose to > 99% of each target volume. Dose grid sizes varying from 0.5 to 1 mm, depending on target sizes, were used in the dose calculation.
VMAT SRS plans
All patient plans for GK were retrospectively planned using the Raystation treatment planning system (Raysearch Laboratories). One isocenter, placed as the geometric center between all targets for each patient, was utilized for each treatment plan. Plans were optimized using 3-4 arcs, with one arc being coplanar and the other being noncoplanar. Arc configurations were determined based on the planning guideline published by Clark et al.18 and modified accordingly to reduce normal brain dose further. Three ring structures were created and were assigned maximum dose constraints to improve dose fall-off. All plans utilized 6 MV FFF beam energy with high-definition multi-leaf collimation (HD-120 MLC) on a Varian EDGE linear accelerator (Varian, Palo Alto, CA). All plans were normalized such that > 99% of each GTV tumor volume received the prescription dose and the PTV volume received >95% of each prescription dose. Depending on the target volume, a grid resolution of 1 mm was used for the final dose calculation using the collapsed cone convolution algorithm. The final dose was calculated using a 1 mm dose grid.
Proton-based plans
VMAT SRS plans using Raystation treatment planning system (Raysearch Laboratories) were developed and compared against standard cyclotron-generated proton plans and linear accelerator proton plans using a model for the Linac for Image-Guided Hadron Therapy (LiGHT), developed by Application of Detectors and Accelerators to Medicine (ADAM), a spin-off company of CERN. Three different types of proton plans (CPBs (σ: 2.7-7.0 mm), LPBs (σ: 2.9-5.5 mm), and LPMBs (σ: 0.9-3.9 mm) with and without apertures were generated for each patient. Plans were optimized using multi-field optimization (MFO), and a single isocenter, placed towards the center of all targets so that at least two beams irradiate each target. Both coplanar and non-coplanar beam configurations were used and were determined based on the positions of the targets per patient. Two to seven beams were used per plan. Figure 1 demonstrates the proton beam arrangement for a sample patient plan. The dose grid resolution was set to 1 mm for all proton plan types. Variable spot spacing and energy layer spacing with a scale of 0.5 were used in each proton plan type. With this option, spot spacing is determined as 1.06 times the average spot size (1σ) in the patient at the Bragg peak depth, multiplied by the user-defined scaling constant, which was defined as 0.5 in this case, which helped to improve plan quality. In terms of the automatic setting for energy layer spacing, energy layer spacing is determined based on the 80% widths of the Bragg peaks in the machine model, scaled by a user-defined factor, which was 0.5 in this case. This also improved plan quality, due to the small targets present in many of the patient plans.19 With robustness, proton plans were planned on the GTV, using 2 mm positional uncertainty and 2% range uncertainty. The final dose calculation was performed using the Monte Carlo algorithm and 0.3% uncertainty. All plans were normalized such that >99% of each tumor GTV volume received the prescription dose for nominal plans. In order to compare with photon-based plans, V100%Rx > 95% criteria was met for the worst-case scenario in robust planning. Apertures covering the GTV volume plus a margin of 0.3 cm diameter were added to beams for all shallow (distance from patient surface to the deepest part of the edge of the tumor (d < 2.5 cm) and small volume (V < 1 cm3) and compared with Gamma Knife photon plans. All proton doses are reported as Gy[RBE] using RBE=1.1.
Figure 1.
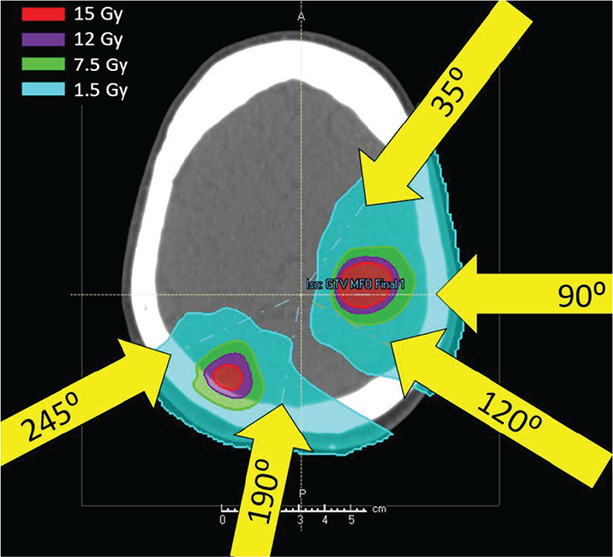
Sample IMPT patient plan showing beam arrangement. Beams were arranged according to tumor positions.
RESULTS
Non-aperture-based planning
All types of proton plans performed well for all dosimetric metrics (conformity, gradient index, V4.5Gy, V12Gy and Mean Brain dose) when compared with VMAT SRS plans. Figure 2 a-e show the trend for all dosimetric metrics, while Figure 2f shows the dose distributions for VMAT vs CPB, LPB and LPMB plans with no aperture. Mean brain dose shows the greatest improvement from VMAT to minibeam proton plans, with the average mean brain dose for LPMB plans being 81% less than VMAT plans. V12Gy shows a 30% improvement, and V4.5Gy shows an 80% improvement for LPMB plans when compared with VMAT. Conformity index and gradient index also show a 20% and 33% improvement, respectively. Significant differences are evident between VMAT SRS plans and proton plans, except for V12Gy for CPB and LPB plans, where this particular metric was only slightly greater for CPB and LPB when compared with VMAT SRS plans. P-values, computed using the Wilcoxon signed rank test, to predict statistical significance of plan metrics for photon-based vs. proton-based plans are reported above data for each proton plan type.
Figure 2.
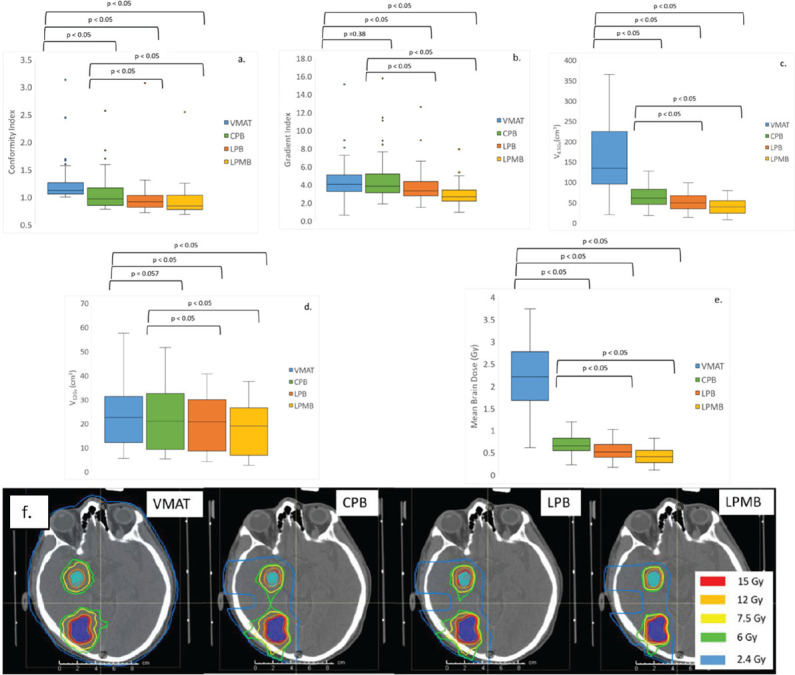
(a) Conformity index, (b) Gradient index, (c) V4.5Gy, (d) V12Gy, (e) Mean Brain Dose for VMAT SRS vs nonaperture-based IMPT plans (CPB, LPB and LPMB) and (f) dose distributions between VMAT, CPB, LPB and LPMB plans without apertures. Low dose regions are reduced greatly by proton plans.
Aperture-based planning
All aperture-based proton plans performed better than gamma knife plans for all dosimetric metrics, except for V12Gy. Mean brain dose showed the greatest improvement in these cases, with an 81% improvement between gamma knife and LPMB plans. Minibeam plans showed a 52% improvement in conformity, a 12% improvement in gradient index, a 7.8% improvement for V4.5Gy and a 72% improvement in mean brain dose. V12Gy showed the lowest improvement between Gamma Knife plans and minibeam plans with apertures, showing only a 5% improvement between these two modalities. Figure 3 a-e, display the trend in dosimetric metrics for Gamma Knife, CPB, LPB, and LPMB plans. Figure 3f compares the dose distribution between all proton plan types. All proton plan types showed significant differences compared with GK plans for all metrics, except for Gradient index and V12Gy. V12Gy for CPB and LPB plans were also slightly greater than V12Gy for Gamma Knife plans. P-values, computed using the Wilcoxon signed rank test, to predict statistical significance of plan metrics for photon-based vs. proton-based plans with apertures are reported above data for each proton plan type.
Figure 3.
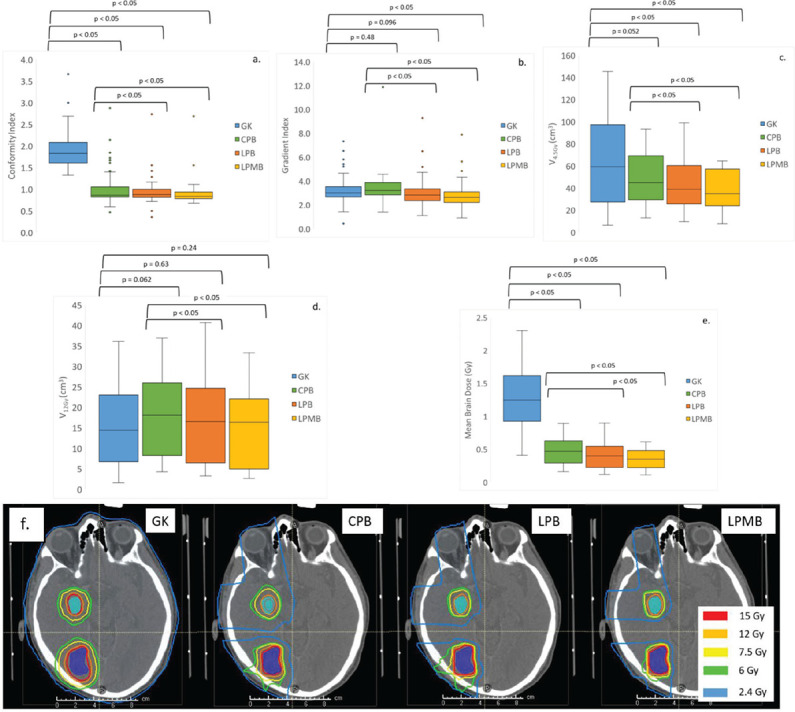
(a) Conformity index, (b) Gradient index, (c) V4.5Gy, (d) V12Gy, (e) Mean Brain Dose for GK SRS vs aperturebased IMPT plans (CPB, LPB and LPMB), and (f) trend in dose distribution for GK vs. proton plans with apertures. Low dose reduction and improvement in conformity is most prevalent for CPB, LPB and LPMB plans.
Shallow vs. deep seated tumors for aperture-based planning
When apertures were implemented in the treatment planning for shallow and small lesions, shallow brain tumors showed the most remarkable improvement in conformity and gradient index, as shown in Figure 4a. A 6.7% difference in average conformity is calculated for non-aperture vs. aperture-based plans for mini beams. Improvement in gradient index for non-aperture vs. aperture-based plans was significant for deep seated tumors for CPB and LPB plan types, as seen in Figure 4b. Figure 4c shows non-aperture vs aperture-based plans for small lesions only. Conformity indices were not improved for these types of lesions, but gradient indices were greatly improved for CPB and LPB plans, by 38% and 27%, respectively. P-values, computed using the Wilcoxon signed rank test, to predict statistical significance of plan metrics for proton-based plans with and without apertures are reported above data for each proton plan type.
Figure 4.
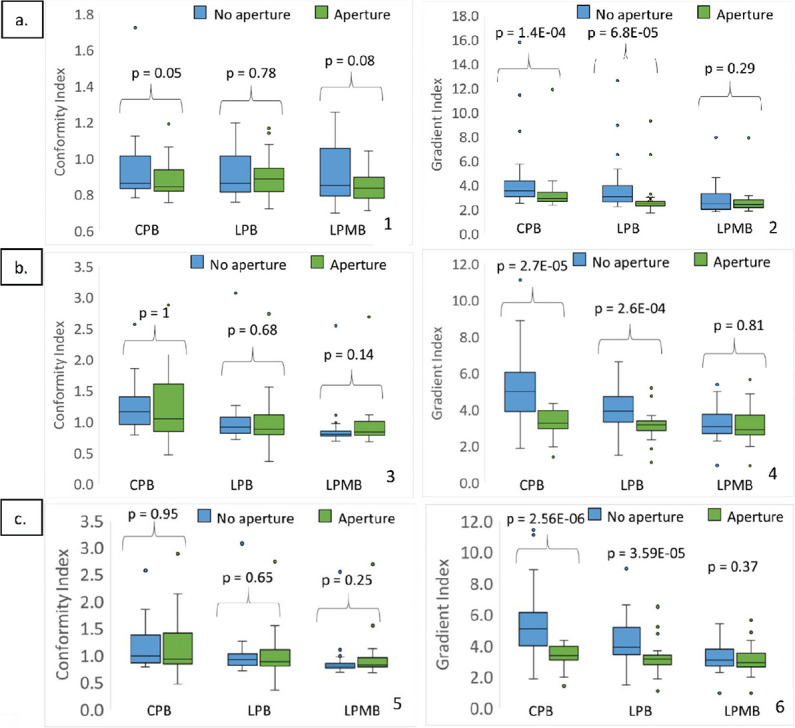
(a) Conformity index (1) and Gradient Index (2) for non-aperture-based plans vs aperture-based plans for shallow brain tumors (d<2.5 cm), (b) Conformity index (3) and Gradient index (4) for non-aperture-based plans vs aperture-based plans for deep seated brain tumors (d>2.5 cm), and (c) Conformity index (5) and Gradient Index (6) for non-aperture-based plans vs. aperture-based plans for small lesions, <1 cc.
Figure 5 depicts the impact apertures have on proton plans. We also see a comparison between CPB, LPB and LPMB plans with and without apertures in 6b (1 and 2). Improvements in low dose coverage as well as conformity are visible. The impact of using an aperture for LPB plans is also shown in Figure 5. The DVH in Figure 5b shows the improved dose homogeneity created from aperture use.
Figure 5.
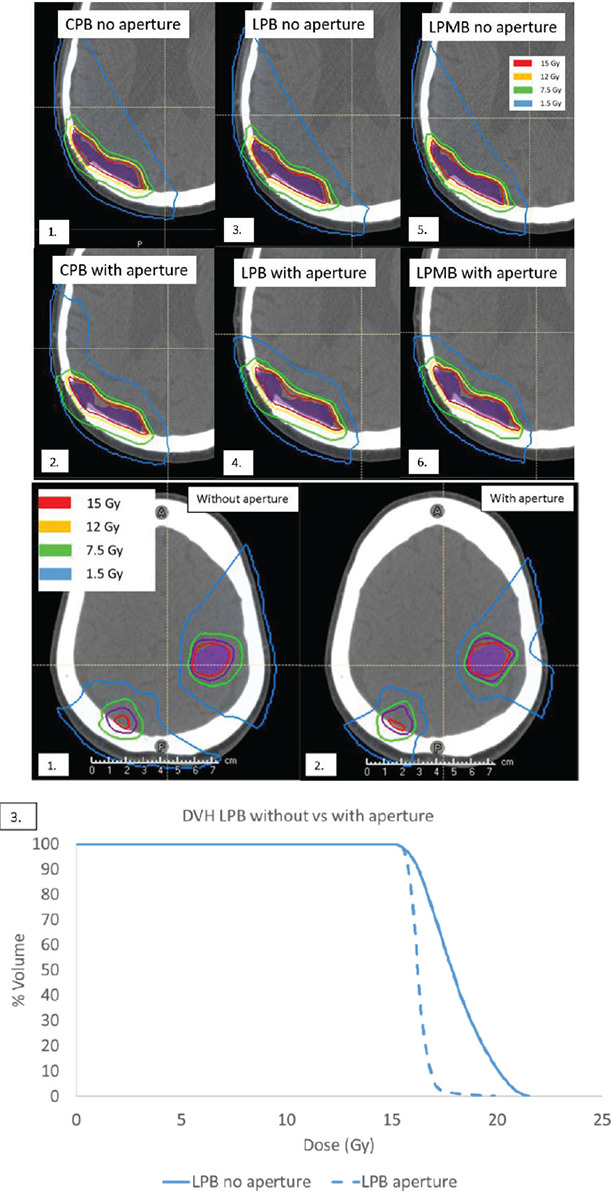
(a) Non-aperture-based vs. aperture-based proton plans for a large, shallow tumor. (1 and 2) CPB plan without and with an aperture, (3 and 4) LPB plan without and with an aperture, and (5 and 6) LPMB plans without and with an aperture, and (b) DVH of GTV for (1) LPB plan with no aperture (2) LPB plan with aperture and (3) corresponding DVH showing non-aperture-based plan (solid line) vs aperture-based plan (dotted line).
Organ-at-Risk (OAR) analysis
Tables 2 and 3 below show average doses for selected OARs across all plan types (VMAT and Gamma Knife vs. CPB, LPB, and LPMB). The lowest doses are shown for LPMB plans, except for the chiasm for aperture-based plans.
Table 2.
OAR analysis for VMAT SRS and proton plans without aperture (CPB, LPB and LPMB)
| Modality | Chiasm (Gy) | Brainstem (Gy) | Cochlea_ Left (Gy) | Cochlea_ Right (Gy) |
|---|---|---|---|---|
| VMAT | 2.2 | 4.2 | 1.5 | 2.0 |
| CPB | 0.6 | 2.0 | 0.4 | 0.5 |
| LPB | 0.3 | 1.5 | 0.4 | 0.2 |
| LPMB | 0.3 | 1.1 | 0.3 | 0.2 |
Table 3.
OAR analysis for Gamma Knife and proton plans with apertures (CPB, LPB and LPMB)
| Modality | Chiasm (Gy) | Brainstem (Gy) | Cochlea_ Left (Gy) | Cochlea_ Right (Gy) |
|---|---|---|---|---|
| GK | 1.1 | 2.2 | 0.9 | 1.1 |
| CPB | 0.1 | 1.4 | 0.4 | 0.2 |
| LPB | 0.1 | 1.0 | 0.4 | 0.1 |
| LPMB | 0.2 | 1.0 | 0.1 | 0.0 |
DISCUSSION
In this study, we compared three different types of proton machine models on multiple brain metastases plans and compared them against two types of photon plans, VMAT SRS and Gamma Knife plan types. Cyclotron-generated proton plans and two linear accelerator-generated proton beams, LPB and LPMB, were used for proton planning. Results of this study show that treatment of multiple brain metastases using cyclotron-generated and linear accelerator-generated protons offers a potentially superior alternative to photon-based therapies for multiple brain metastases. Prior studies have shown that proton therapy achieves reasonable local control for some high-grade tumors with acceptable toxicity.20,21 Other studies have suggested that proton beam therapy may not be comparable to photon radiotherapy in terms of lateral penumbra, target conformity, and uncertainties that are critical for SRS.10-12 However, proton beam therapy has many advantages, including utilizing its Bragg peak, for example, which is not fully explored for intracranial SRS.22 In terms of new treatment technologies for proton SRS, two research directions showing dynamic collimation system, and proton arc therapy have recently been investigated. The dynamic collimation system demonstrates a sharper lateral penumbra, while proton arc therapy demonstrated better dosimetric quality in terms of target conformity and V12Gy to brain tissue when compared with conventional VMAT plans.6,11 However, proton arc therapy may not be suited for single fractionation SRS due to toxicity.23 One of the newest technologies for proton therapy, the LiGHT system15, has been investigated for use in the present study and shows several advantages over photon-based and cyclotron-generated protons for brain SRS.
For all proton plans, all dosimetric metrics were improved when compared with photon-based plans, except for V12Gy in some cases. We have found that both cyclotron generated protons (CPB) and linear accelerator protons (LPB) are superior to photon-based treatment techniques when treatment plans are compared using the TPS. At this time, however, no clinical trials comparing photon-based SRS modalities with proton SRS exist.22 Feasibility and outcome of patients treated using proton SRS compared with photon-based SRS, however, was noted in Atkins et al.9 Authors of this study retrospectively studied 815 metastases from 370 patients treated with proton SRS. Cumulative incidence estimates of local failure, distance brain failure, pathologically confirmed radionecrosis and overall survival were calculated. Authors of this study concluded that moderate-dose proton SRS is well tolerated and can achieve good local control outcomes compared with photon-based SRS. Boczkoski et al.24 determined optimum beam configurations and settings for proton SRS treatment plans and used these settings to perform a planning comparison between a variety of different SRS cases. Proton-based plans were compared against Hyperarc VMAT SRS plans. It was demonstrated in this study that Hyperarc plans are superior to proton plans, except as it related to integral dose, where in most cases, proton therapy gives less integral dose. Our findings in the present study correlate with findings in this study, with the exception that proton plan metrics were, on average, better than those for most VMAT SRS plans with the exception of V12Gy for CPB and LPB plans. Only one mestastases case was included in Boczkoski et al., however, for which plan metrics for the proton plan were superior to those for VMAT plans. This study also utilized the Pinnacle treatment planning system for proton plans; whereas in our study, the Raystation treatment planning system was used. Both Pinnacle and Raystation position spots outside of the defined target volume (target plus a margin), but only Raystation has the option to vary spot spacing according to variable spot size with depth. This could be a possible difference in planning results between studies. Byskov et al.25 compared proton treatment plans with photon VMAT plans for lower-grade gliomas and found a significant reduction in mean dose to uninvolved brain and contralateral hippocampus for proton plans. They also predicted secondary cancer risk was reduced for patients treated using protons. Overall, doses to OARs were much less for protons when compared to VMAT plans. Results of the present study are similar to this particular study, showing a large reduction in OAR doses with proton plan types. Use of apertures for proton plans in the present study further improved target conformity and reduced OAR doses by up to 79%. The use of apertures also lowered mean brain dose by 51%, which can also potentially improve patient treatment outcome in future clinical studies. Both coplanar and non-coplanar beam placement was utilized in the present study as well, depending on the tumor location in each patient. Coplanar fields were used in cases where tumors were in the same plane or close to being in the same plane, and where using non-coplanar fields did not show any benefit. Non-coplanar fields were only used when very necessary, as using coplanar fields generally shows plan quality and robustness similar to that of using non-coplanar fields in many cases. Coplanar plans are also of more clinical benefit, due to treatment time being more efficient with comparable plan quality to plans with non-coplanar fields.26 In addition, intracranial targets typically require proton energies from the low MeV range for targets located near the cranium to about 180 MeV.27 In a study by Sengbusch et al., the total radiological path length through bone and brain, not simply water density transit, was utilized to calculate the proton kinetic energy necessary to traverse this radiological path length. A range shifter was not included in this study, which would add to the required energy in some cases. In the present study, spot sizes (defined in air) varied from 7 mm to 2.7 mm for CPB, decreasing as the energy increased from 70 to 230 MV. For LPB beamline, the spot sizes varied from 5.5 mm to 2.9 mm, decreased from 70 MeV to 150 Mev and remained constant after 150 MeV. For LPB beamline, the spot sized (defined in air) varied from 3.9 mm to 0.9 mm, decreasing as the energy increased from 70 to 150 MeV and remained constant after 150 MeV.
Specific planning strategies were utilized in order to maximize the plan quality specifically for multiple brain metastases. One of these was the use of a smaller range uncertainty. Uncertainties in the exact position of the distal dose gradient arises from organ motion, setup and anatomical variations, dose calculation approximations and biological considerations. Targets in the brain generally do not have a lot of motion with very little anatomical variations in this region of the body, so a smaller range uncertainty is sufficient. For intracranial tumor patients, a fixation mask is used and the treatment is only one fraction. Also, it was demonstrated in a recent study that reduction in range uncertainty may make greater use of the sharper dose fall-off at the distal beam edge feasible, which can improve OAR sparing. In this case, having less range uncertainty played a role in making dose to OARs as low as reasonably achievable, especially for targets close to OARs.28 MFO allows for superior dose distributions compared with SFO technique. MFO can also achieve a more homogenous dose distribution within diverse geometric targets while limiting the radiation dose to normal structures. MFO is generally more flexible with more degrees of freedom and produces more conformal dose distributions, which are important for SRS. MFO techniques also allow us to take full advantage of IMPT capabilities by optimizing multiple beams simultaneously to treat tumors that are hard to reach. MFO could be more sensitive to variations in the range of a proton beam, daily set-up errors and anatomical changes in tumor volume and/or shape, but given the fact that the tumors being treated in this manuscript are intracranial tumors, these factors are not of grave concern.29-32 In clinical proton beams, a major source of neutron contamination is a brass patient specific aperture, shaped to match the target. This may cause unwanted dose, which should be considered for tissues outside of the target volume and is important for the long term health of cancer patients. Since the aperture shapes the beam to a smaller size, it is necessarily bombarded with a large amount of protons. In the case that apertures are used in IMPT, neutron dose to the patient can be limited by making sure that the smallest nozzle to match the aperture be used. Moving the scattering foils further from the patient is another technique used to reduce neutron dose and is used in newer systems. It is unclear as to what the actual clinical relevance of the neutron dose is exactly; however, it is generally agreed upon that whole body neutron doses are too small to cause early or late radiation effects.33 The concern lies mostly with the issue of the risk of secondary malignancy for the patient. In a study by Hall et al., it has been suggested that lifetime risk for developing a malignancy for a 15-year old treated with proton therapy with a passive scatter beam is 4.7% and 11.1% for a boy and a girl, respectively.34 This risk decreases with age and the use of active scanning proton beams, which were used in the present study. Furthermore, in a study by Chung et al., a total of 503 patients treated with protons were matched to one to three patients treated with radiation from the SEER database by age at treatment, sex, year of treatment, cancer histology and site. The major outcome measure was the incidence of second malignancies after radiation. Results of this study demonstrated that 32 patients (6.4%) treated with protons developed secondary malignancies as compared to 66 patients (13.1%), who received photons. This difference was statistically significant.35
The LiGHT system, when compared with conventional cyclotron-generated protons, has several attractive properties, including superior transverse beam properties that improve dose conformity for patient targets and normal tissue sparing.13,15 To our knowledge, the present study is the first study to compare linear accelerator generated protons against cyclotron generated protons and photon-based planning for multiple brain metastases. We have shown that linear accelerator based proton plans demonstrate superior conformity, gradient index, V4.5Gy and mean brain dose when compared with CPB plans. LPMB plans showed the greatest improvement for all dosimetric metrics for both aperture-based and non-aperture-based proton plans. Another advantage of the LiGHT system, is it’s ability to utilize apertures in the treatment process. Aperture-based plans demonstrated a 26% improvement in gradient index and up to a 7% improvement for conformity index. A study by Baumer et al.36 implemented proton therapy using PBS delivery combined with static apertures for craniopharyngiomas, re-irradiations and ocular tumors. Authors of this study found that supplementary apertures improved the conformity index by an average of 15.3% and the volume of the dose gradient surrounding the PTV (evaluated between the 80 and 20% dose levels) was decreased on average by 17.6%. Use of apertures in this particular study also facilitated the sparing of OARs, particularly those near the high dose region. An additional study by Righetto et al.37 studied dosimetric advantages of apertures in intracranial single fraction proton radiosurgery. Six neuroma and ten meningioma patients were investigated. Authors of this particular study concluded that the impact of apertures could be more significant for higher prescription doses, as in the treatment of brain metastases and arteriovenous malformations, where doses higher than 20 Gy in a single fraction are normally prescribed. In these cases, use of an aperture significantly reduced dose to normal brain tissue. A similar phenomenon was seen in the present study. Additionally, we have observed that Gradient index improves with apertures, but conformity index is only improved for shallow tumors (d<2.5cm). Figure 5 demonstrates the effect of using apertures for proton plans. Conformity and gradient index are both improved by using an aperture to treat the lesion.
The metric V12Gy is commonly used as an indicator of propensity to develop radiation necrosis. Knowing that CPB and LPB plans demonstrated higher V12Gy than photon-based plans (both VMAT SRS and GK), indicates that use of these modalities may be prone to producing radionecrosis in patients. In a study by Sud et al.38 comparing passively scattered protons vs photons for pituitary adenomas, authors reported no significant difference in V12Gy between proton and photon plans, although one finding indicated a higher whole brain V12Gy for protons. In the present study, average V12Gy was only higher by 1% and 0.3% for aperture-based plans and comparable for non-aperture-based plans and no significant difference is reported in the data between photon and proton-based plans.
In the present time, there is much uncertainty existing still for the radiobiological aspect of proton beams, however, particularly due to LET uncertainty at the end of the Bragg peak. There is potentially large LET variation along a proton pencil beam track, causing RBE at the distal end of the Bragg peak to be higher than 1.1 with large uncertainty.22 This is especially true for low energy beams with small spread out Bragg peak width.39 It has also been shown that actual RBE varies depending on tissue type, dose levels and LET.40 Currently, no commercially available TPS is capable of accurately estimating RBE for small fields and the possibility of having an uncertain but elevated dose distal to a very small target is high. This represents a possible limitation to treating small brain tumors with protons. However, future studies will involve the exploration of LET-based optimization techniques and accurate estimation of RBE.
CONCLUSION
We successfully quantified plan quality and evaluated the robustness of CPBs, LPBs, and LPMBs for brain metastases. We have demonstrated that the LPB and LPMB stand as excellent alternatives to CPB or photon therapy and photon-based therapies, such as VMAT SRS and Gamma Knife, primarily due to the significantly increased preservation of normal tissue. Aperture-based IMPT plans can achieve better quality than standard IMPT, VMAT SRS, and Gamma Knife. Further studies involving radiobiological effects can demonstrate further how LPBs and LPMBs compare with CPBs and photon-based plans.
ACKNOWLEDGMENTS
Authors’ disclosure of potential conflicts of interest
Dr Farr reports collaborative research grant with Advanced Oncotherapy (AVO) — IRB number 19-1180, DATA1119: Using proton mini-beams to improve SBRT/SRS plan conformity, Board member at large for the AAPM Journal of Medical Physics, stock in Advanced Oncotherapy, plc and he is the Chief Clinical Officer of A.D.A.M SA Director General. Dr Xia reports collaborative research grant with Advanced Oncotherapy (AVO) — IRB number 19-1180, DATA1119: Using proton mini-beams to improve SBRT/SRS plan conformity. Other authors have nothing to disclose.
Author contributions
Conception and design: Tara Gray, Ping Xia, Jonathan Farr
Data collection: Tara Gray, Chieh-Wen Liu, Young-Bin Cho, Jeremy Donaghue, Samuel Chao, John Suh
Data analysis and interpretation: Tara Gray, Anna Maria Kolano, Ping Xia, Jonathan Farr
Manuscript writing: Tara Gray, Ping Xia
Final approval of manuscript: Tara Gray, Chieh-Wen Liu, Young-Bin Cho, Jeremy Donaghue, Samuel Chao, John Suh, Anna Maria Kolano, Ping Xia, Jonathan Farr
REFERENCES
- 1.Scaringi C, Agolli L, Minniti G. Technical advances in radiation therapy for brain tumors. Anticancer Res. 2018;38(11):6041–6045. doi:10.21873/anticanres.12954 [DOI] [PubMed] [Google Scholar]
- 2.Indelicato DJ, Rotondo RL, Uezono H, Sandler ES, Aldana PR, Ranalli NJ, Beier AD, Morris CG, Bradley JA. Outcomes following proton therapy for pediatric low-grade glioma. Int J Radiat Oncol Biol Phys. May 1 2019;104(1):149–156. doi:10.1016/j.ijrobp.2019.01.078 [DOI] [PubMed] [Google Scholar]
- 3.Sherman JC, Colvin MK, Mancuso SM, Batchelor TT, Oh KS, Loeffler JS, Yeap BY, Shih HA. Neurocognitive effects of proton radiation therapy in adults with low-grade glioma. J Neurooncol. Jan 2016;126(1):157–164. doi:10.1007/s11060-015-1952-5 [DOI] [PubMed] [Google Scholar]
- 4.Weber DC, Lim PS, Tran S, Walser M, Bolsi A, Kliebsch U, Beer J, Bachtiary B, Lomax T, Pica A. Proton therapy for brain tumours in the area of evidence-based medicine. Br J Radiol. Mar 2020;93(1107):20190237. doi:10.1259/bjr.20190237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penfold SN, Dell’Oro M, Gorayski P, Hwang E, Le H, Penfold M, Sykes J, Vu H, Yeo A. Proton-to-photon comparative treatment planning guidelines for the Australian context. J Med Imaging Radiat Oncol. Feb 1 2023;doi:10.1111/1754-9485.13510 [DOI] [PubMed] [Google Scholar]
- 6.Hyer DE, Hill PM, Wang D, Smith BR, Flynn RT. A dynamic collimation system for penumbra reduction in spot-scanning proton therapy: proof of concept. Med Phys. Sep 2014;41(9):091701. doi:10.1118/1.4837155 [DOI] [PubMed] [Google Scholar]
- 7.Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. Aug 1 2002;53(5):1337–1349. doi:10.1016/s0360-3016(02)02884-5 [DOI] [PubMed] [Google Scholar]
- 8.Yu CX, Li XA, Ma L, Chen D, Naqvi S, Shepard D, Sarfaraz M, Holmes TW, Suntharalingam M, Mansfield CM. Clinical implementation of intensity-modulated arc therapy. Int J Radiat Oncol Biol Phys. Jun 1 2002;53(2):453–463. doi:10.1016/s0360-3016(02)02777-3 [DOI] [PubMed] [Google Scholar]
- 9.Atkins KM, Pashtan IM, Bussière MR, Kang KH, Niemierko A, Daly JE, Botticello TM, Hurd MC, Chapman PH, Oh K, Loeffler JS, Shih HA. Proton stereotactic radiosurgery for brain metastases: A single-institution analysis of 370 patients. Int J Radiat Oncol Biol Phys. Jul 15 2018;101(4):820–829. doi:10.1016/j.ijrobp.2018.03.056 [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Dirksen B, Hyer DE, Buatti JM, Sheybani A, Dinges E, Felderman N, TenNapel M, Bayouth JE, Flynn RT. Impact of spot size on plan quality of spot scanning proton radiosurgery for peripheral brain lesions. Med Phys. Dec 2014;41(12):121705. doi:10.1118/1.4901260 [DOI] [PubMed] [Google Scholar]
- 11.Ding X, Li X, Qin A, Zhou J, Yan D, Stevens C, Krauss D, Kabolizdeh P. Have we reached proton beam therapy dosimetric limitations? – A novel robust, delivery-efficient and continuous spot-scanning proton arc (SPArc) therapy is to improve the dosimetric outcome in treating prostate cancer. Acta Oncol. Mar 2018;57(3):435–437. doi:10.1080/0284186x.2017.1358463 [DOI] [PubMed] [Google Scholar]
- 12.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions. Phys Med Biol. Feb 21 2008;53(4):1043–1056. doi:10.1088/0031-9155/53/4/015 [DOI] [PubMed] [Google Scholar]
- 13.Farr JB, Flanz JB, Gerbershagen A, Moyers MF. New horizons in particle therapy systems. Med Phys. 2018;45(11):e953–e983. doi: 10.1002/mp.13193 [DOI] [PubMed] [Google Scholar]
- 14.Myers S, Degiovanni A, Farr JB. Future prospects for particle therapy accelerators. Rev Accel Sci Technol. 2019;10(01):49–92. doi:10.1142/s1793626819300056 [Google Scholar]
- 15.Farr JB, Kolano AM, Aumon S, Degiovanni A, Caldara M, Valloni A. Proton LINAC Transverse beam scanning performance implications for therapeutic quality improvement. Int J Radiat Oncol Biol Phys. 2020;108(3):e351. doi:10.1016/j.ijrobp.2020.07.2334 [Google Scholar]
- 16.Farr J, Grilj V, Malka V, Sudharsan S, Schippers M. Ultra-high dose rate radiation production and delivery systems intended for FLASH. Med Phys. Jul 2022;49(7):4875–4911. doi:10.1002/mp.15659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CW, Ahmed S, Gray T, Ma T, Cho YB, Neyman G, Chao S, Suh J, Xia P. Is there a volume threshold of brain metastases for Linac-based stereotactic radiotherapy? J Radiosurg SBRT. 2021;7(4):309–319. [PMC free article] [PubMed] [Google Scholar]
- 18.Clark GM, Popple RA, Prendergast BM, Spencer SA, Thomas EM, Stewart JG, Guthrie BL, Markert JM, Fiveash JB. Plan quality and treatment planning technique for single isocenter cranial radiosurgery with volumetric modulated arc therapy. Pract Radiat Oncol. Oct–Dec 2012;2(4):306–313. doi:10.1016/j.prro.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 19.Janson M, Glimelius L, Fredriksson A, Traneus E, Engwall E. Treatment planning of scanned proton beams in RayStation. Medical Dosimetry. 2023/November/22/ 2023;doi: 10.1016/j.meddos.2023.10.009 [DOI] [PubMed] [Google Scholar]
- 20.Amichetti M, Amelio D, Minniti G. Radiosurgery with photons or protons for benign and malignant tumours of the skull base: a review. Radiat Oncol. Dec 14 2012;7:210. doi:10.1186/1748-717x-7-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohan R, Grosshans D. Proton therapy – Present and future. Adv Drug Deliv Rev. Jan 15 2017;109:26–44. doi:10.1016/j.addr.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deraniyagala R, Ding X, Alonso-Basanta M, Li T, Rong Y. It is beneficial to invest resources to implement proton intracranial SRS. J Appl Clin Med Phys. Jul 2022;23(7):e13701. doi:10.1002/acm2.13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber DC, Chan AW, Bussiere MR, Harsh GRt, Ancukiewicz M, Barker FG, Thornton AT, Martuza RL, Nadol JB, Jr., Chapman PH, Loeffler JS. Proton beam radiosurgery for vestibular schwannoma: tumor control and cranial nerve toxicity. Neurosurgery. Sep 2003;53(3):577–586; discussion 586-8. doi:10.1227/01.neu.0000079369.59219.c0 [DOI] [PubMed] [Google Scholar]
- 24.Boczkowski A, Kelly P, Meeks SL, Erhart K, Bova FJ, Willoughby TR. Proton vs Hyperarc™ radiosurgery: A planning comparison. J Appl Clin Med Phys. 2020;21(12):96–108. doi: 10.1002/acm2.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byskov CS, Hansen CR, Dahlrot RH, Haldbo-Classen L, Haslund CA, Kjær-Kristoffersen F, Kristensen TO, Lassen-Ramshad Y, Lukacova S, Muhic A, Nyström PW, Weber B, Kallehauge JF. Treatment plan comparison of proton vs photon radiotherapy for lower-grade gliomas. Phys Imaging Radiat Oncol. Oct 2021;20:98–104. doi:10.1016/j.phro.2021.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi B, Jatczak J, Deng W, Poirier YP, Yao W, Witek ME, Molitoris JK, Zakhary MJ, Zhang B, Biswal NC, Ferris MJ, Mossahebi S. Is noncoplanar plan more robust to inter-fractional variations than coplanar plan in treating bilateral HN tumors with pencil-beam scanning proton beams? J Appl Clin Med Phys. 2024;25(2):e14186. doi: 10.1002/acm2.14186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sengbusch E, Pérez-Andújar A, DeLuca PM, Jr., Mackie TR. Maximum proton kinetic energy and patient-generated neutron fluence considerations in proton beam arc delivery radiation therapy. Med Phys. Feb 2009;36(2):364–372. doi:10.1118/1.3049787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tattenberg S, Madden TM, Bortfeld T, Parodi K, Verburg J. Range uncertainty reductions in proton therapy may lead to the feasibility of novel beam arrangements which improve organ-at-risk sparing. Med Phys. Jul 2022;49(7):4693–4704. doi:10.1002/mp.15644 [DOI] [PubMed] [Google Scholar]
- 29.Butkus MP, Brovold N, Diwanji T, Xu Y, De Ornelas M, Dal Pra A, Abramowitz M, Pollack A, Dogan N. Assessment of IMPT versus VMAT plans using different uncertainty scenarios for prostate cancer. Radiat Oncol. 2022/September/29 2022;17(1):162. doi:10.1186/s13014-022-02126-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugh TJ, Amos RA, John Baptiste S, Choi S, Nhu Nguyen Q, Ronald Zhu X, Palmer MB, Lee AK. Multifield optimization intensity-modulated proton therapy (MFO-IMPT) for prostate cancer: Robustness analysis through simulation of rotational and translational alignment errors. Med Dosim. Autumn 2013;38(3):344–350. doi:10.1016/j.meddos.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan Grover M. Utilizing multi-field optimization with IMPT for head and neck cancer treatment. https://www.varian.com/utilizing-multi-field-optimization-impt-head-and-neck-cancer
- 32.Zhu XR, Poenisch F, Li H, Zhang X, Sahoo N, Wu RY, Li X, Lee AK, Chang EL, Choi S, Pugh T, Frank SJ, Gillin MT, Mahajan A, Grosshans DR. A single-field integrated boost treatment planning technique for spot scanning proton therapy. Radiat Oncol. Sep 11 2014;9:202. doi:10.1186/1748-717x-9-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eric Shinohara M. MSCI. Proton therapy professional education. OncoLink. https://www.oncolink.org/healthcare- professionals/oncolink-university/proton-therapy-professional-education/oncolink-proton-education-modules
- 34.Hall EJ, Novak JK, Kellerer AM, Rossi HH, Marino S, Goodman LJ. RBE as a function of neutron energy. I. Experimental observations. Radiat Res. Nov 1975;64(2):245–255. [PubMed] [Google Scholar]
- 35.Chung CS, Yock TI, Nelson K, Xu Y, Keating NL, Tarbell NJ. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. Sep 1 2013;87(1):46–52. doi:10.1016/j.ijrobp.2013.04.030 [DOI] [PubMed] [Google Scholar]
- 36.Bäumer C, Plaude S, Khalil DA, Geismar D, Kramer PH, Kröninger K, Nitsch C, Wulff J, Timmermann B. Clinical implementation of proton therapy using pencil-beam scanning delivery combined with static apertures. Front Oncol. 2021;11:599018. doi:10.3389/fonc.2021.599018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Righetto R, Fellin F, Scartoni D, Amichetti M, Schwarz M, Amelio D, Farace P. Is it beneficial to use apertures in proton radiosurgery with a scanning beam? A dosimetric comparison in neurinoma and meningioma patients. J Appl Clin Med Phys. 2022;23(2):e13459. doi: 10.1002/acm2.13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sud S, Botticello T, Niemierko A, Daly J, Bussiere M, Shih HA. Dosimetric comparison of proton versus photon radiosurgery for treatment of pituitary adenoma. Adv Radiat Oncol. Nov-Dec 2021;6(6):100806. doi:10.1016/j.adro.2021.100806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paganetti H, Blakely E, Carabe-Fernandez A, Carlson DJ, Das IJ, Dong L, Grosshans D, Held KD, Mohan R, Moiseenko V, Niemierko A, Stewart RD, Willers H. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys. Mar 2019;46(3):e53–e78. doi:10.1002/mp.13390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paganetti H, van Luijk P. Biological considerations when comparing proton therapy with photon therapy. Semin Radiat Oncol. Apr 2013;23(2):77–87. doi:10.1016/j.semradonc.2012.11.002 [DOI] [PubMed] [Google Scholar]


