Abstract
HIV self-testing (HIVST) is a WHO-recommended strategy to increase testing, especially among key populations, men, and young adults. Between May and December 2019, a pilot was implemented in Zambézia province, Mozambique, allowing clients to purchase HIV self-tests in 14 public/private pharmacies. The study assessed the strategy’s acceptability and uptake. Pharmacy-based exit surveys were conducted among a random sample of clients, during the first three months of the pilot, independent of HIVST purchase. Another random sample of clients who bought an HIVST completed a survey 1–12 weeks after purchase. Chi-square and Mann–Whitney tests were used for the analysis, comparing clients who purchased an HIVST versus not. A total of 1,139 adults purchased 1,344 tests. Buyers were predominantly male (70%) and younger (52% between 15 and 34 years of age). Surveys were completed by 280 exiting pharmacy clients and 82 clients who purchased an HIVST. Main advantages were confidentiality and lack of need of a health provider visit, with main disadvantages being absence of nearby counseling and fear of results. No differences were seen between buyers and non-buyers for these factors. Among all undergoing HIVST, 71 (92%) perceived the instructions to be clear, however, 29 (38%) stated they would have benefitted from additional pre-test information or counseling. Ten (13%) reported following up at a nearby health facility to confirm results and/or receive care. Offering HIVST at public/private pharmacies was acceptable among people who traditionally tend to have a lower HIV testing coverage, such as men and young adults. However, additional resources and/or enhanced educational materials to address the lack of counseling, and linkage-to-care systems need to be put into place before scaling up this strategy.
Keywords: HIV self-testing, Mozambique, pharmacy, HIV/AIDS, acceptability
Introduction
The greatest burden of the HIV/AIDS epidemic continues to be shared across the sub-Saharan African (SSA) region, where ~60% of all incident cases occurred in 2020.1 Mozambique continues to rank among the top five countries most severely affected by the disease with an estimated 2.1 million Mozambicans living with HIV in 2020.1 The national HIV survey data revealed that 39% of women and 60% of men had never been tested for HIV.2
The global community is working toward achievement of the recently announced UNAIDS 95–95-95 goals. One of the strategies to attain the first goal—95% of people living with HIV knowing their HIV status by 2023—is HIV self-testing (HIVST) as recommended by the World Health Organization.3,4 Despite being a promising strategy, various components need to be addressed to achieve effective implementation and outcomes, including counseling, linkage to care, privacy/human rights considerations, and high-quality service.5 In addition, consensus about the optimal location for HIVST has not been reached. The majority of HIVST evaluations or pilot studies to date in SSA have been performed through partner mobile clinics or health facilities.6 In Kenya, the majority of participants preferred the government health facility as a setting to receive HIVST.7 However, public pharmacies have been explored as a means in which to identify clients and refer for HIV screening at the clinic.8,9
At the time of the study, Mozambique did not have formal national guidelines on HIVST. Through means of a public-private partnership, a pharmacy-based HIVST strategy was piloted in Zambézia province, a heavily populated (~5.2m people) province in central Mozambique that has an HIV prevalence of 15.1%2,10 and low HIV testing coverage rates; only 30% of men and 51% of women report ever being tested for HIV.2 Zambézia province also has a young population (81% are under 35 years of age), residing mainly in rural areas.10 The overall objective of the study was to assess the acceptability, feasibility, and linkage to care through the availability of HIV self-tests within participating pharmacies.
Materials and Methods
Study setting
The evaluation was conducted in Zambézia province, central Mozambique, where the non-governmental organization Friends in Global Health (FGH), an affiliate of Vanderbilt University Medical Center (VUMC), supports comprehensive HIV services. The HIVST pilot was implemented between May and December 2019. Public [government-owned free-standing (outside of the health facility)] or private pharmacies were offered the opportunity to participate when they satisfied the following criteria: they (1) were registered by the provincial health authorities, (2) were located in a district supported by FGH at the time of protocol writing, (3) provided services to a minimum of 20 clients per day; (4) confirmed their willingness to participate, and (5) had a functional climate-controlled storage area for the HIVST kits.
Out of the 27 active pharmacies, 19 met eligibility criteria and 14 (74%) of those agreed to participate and signed a Memorandum of Understanding (MoU). The participating pharmacies were located within two urban (eight pharmacies) and five rural (six pharmacies) districts. The average number of technical staff at each participating pharmacy was three, providing care to an average of 88 clients per day. Any person (≥15 years of age) could purchase up to two HIV self-tests (Oraquick™; Orasure Technologies, Inc.) at a subsidized price of 50 Mozambican Meticais (Mzn) (~USD 0.80), an amount based on existing prices of other available self-tests (e.g., urine pregnancy tests and malaria rapid diagnostic tests). Information regarding HIVST was provided through distribution of leaflets, posters at the pharmacies, and local radio messages. Materials were printed in Portuguese, and radio messages were broadcasted in Portuguese and the local language.
Interested pharmacy clients were asked to watch a demonstration video, explaining how to conduct the self-test before they could purchase a test. Clients who purchased a test received the following: (1) an additional educational booklet with general information on HIV, instructions on HIVST, information on linkage to care, frequently asked questions; (2) a leaflet with summary information; and (3) a referral voucher to the health facility. They could also receive a demonstration video on WhatsApp, and this video was available in Portuguese or the local language, if desired. Clients were asked permission to provide their contact details for an interview after purchase. Counseling and testing staff at six health facilities, located in close proximity to participating pharmacies, were trained on the protocol and HIVST procedures for the purposes of this pilot study.
Study design
The study utilized a cross-sectional design. Exit interviews were conducted at two pre-specified timepoints (at time of study initiation and then three months later) among pharmacy clients (independent of HIVST purchase). Post-purchase interviews were conducted with any pharmacy client who purchased an HIVST kit, had agreed to be contacted, and consented for the interview. Eligibility criteria for both interviews included being 18 years of age or older and providing written informed consent for the respective interview(s). For the pharmacy clients invited for an exit-interview, seeking any pharmacy service at one of the participating pharmacies was an additional eligibility criterion; for the pharmacy clients invited for the post-purchase interviews, the specific inclusion criterion was having purchased an HIVST kit at one of the participating pharmacies.
Study procedures
Systematic random sampling was utilized for the exit interviews, where every third person exiting the pharmacy was approached and invited to participate in the exit interview (Supplementary Data S1), independent of HIVST purchase. Surveys were completed after confirming eligibility criteria and obtaining informed consent. Pharmacy clients who purchased an HIVST kit between study initiation and August 30, 2019 and agreed to be contacted were randomly selected to participate in the post-purchase interview. It was decided to allow a minimum of 7 days to lapse between participant purchase and the interview to account for any delays from time of purchase to actual performance of the test.
The in-person structured interview included questions on prior HIV testing, HIVST knowledge, willingness to purchase an HIVST kit, perceived advantages and disadvantages of HIVST, preferred testing modality, perceptions regarding HIVST cost, and preferred setting to undergo the self-test (Supplementary Data S2). Clients who purchased a test and completed interviews were additionally asked about their individual experiences with HIVST, their test result (if they decided to disclose), and linkage to a nearby health facility. Participants’ responses to the interviews were recorded using mobile cellular devices and stored in a secure cloud-based repository (REDCap™).
Statistical considerations
We aimed to conduct exit interviews with 20 clients at each of the 14 participating pharmacies, for a total of 280 participants. This sample was expected to provide us with the requisite baseline preliminary data, consistent with routine practice for exploratory analysis and to consider the scale-up of this intervention. A simple random sample of up to 10 clients per pharmacy who purchased a test and agreed to be contacted were selected from each of the participating pharmacies to complete the post-purchase interview, using random.org software. In cases where the person did not accept or was not reachable, the next person on the list was contacted.
Descriptive statistics were conducted and presented as medians [with inter-quartile ranges (IQR)] for continuous variables and frequency breakdown (percentages) for categorical variables. Univariate analysis utilizing the chi-square test for categorical variables and Mann–Whitney test for continuous variables was done for covariates comparing clients who purchased an HIVST kit versus those who did not. The software STATA.SE Version 15.0 (StataCorp LLC, College Station, TX) supported the quantitative analysis.
Ethical considerations
The protocol and instruments were approved by the local Institutional Bio-Ethics Committee of the Instituto Nacional de Saúde [Comité Institutional de Bioética em Saúde — Instituto Nacional de Saúde ] (Reference 080/CIBS-INS/2018) and the VUMC Institutional Review Board (IRB, No. 181834) and was reviewed in accordance with the Centers for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. All participants provided written informed consent before data collection.
Results
During the pilot period (May 20–December 20, 2019), 1,344 HIV self-tests were sold to 1,139 people. The majority (636, 70%) of buyers were male, and younger than 35 years of age (613, 69%). Almost half (481, 42%) the people bought from an urban location.
Characteristics of study population completing exit and post-purchase interviews
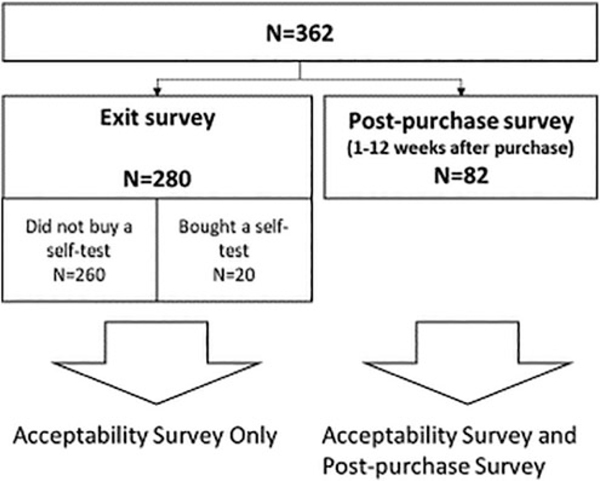
A total of 280 clients were interviewed at the time they exited the pharmacy (exit interviews). Additionally, 82 people completed interviews 1–12 weeks after purchasing an HIVST (post-purchase interviews). From the 280 exit-interviews, 20 people reported that they had purchased an HIV self-test (Fig. 1).
FIG. 1.
Flow chart of survey participants completing exit-interviews and post-purchase interviews (n=362).
Fifty-eight percent (n=211) of the participants visited one of the urban pharmacies. Two-thirds (n=240) of the participants were male, and the majority (70%, n=253) were <35 years of age, with 104 (29%) being 15–24 years of age and 149 (41%) being between 25 and 34 years of age (Table 1). Pharmacy clients who purchased an HIVST were more educated, completing secondary (12th grade) and/or advanced/university level training (p<.001; Table 1). There were no significant differences among participants who purchased an HIVST by religion, occupation, and/or marital status. However, consistent with educational level, participants who purchased an HIVST had higher levels of self-reported Portuguese language skills (87% of those purchasing HIVST reporting good/very good Portuguese language skills compared to 76% of those not purchasing an HIVST; p= .03). Prior HIV testing was reported by 305 (84%) of 362 participants, with 56% (n =170) of these prior HIV tests being performed within the past 5 months. Excluding HIVST results, the majority (88%) reported the result of their HIV tests were negative.
TABLE 1.
Characteristics of Study Participants Completing Exit and Post-Purchase Interviews (N=362)
| Pharmacy clients who did not buy an HIVST (n = 260) n (%) | Pharmacy clients who bought an HIVST (n = 102) n (%) | Total (n = 362) n (%) | p | |
|---|---|---|---|---|
| Sex | ||||
| Male | 168 (65%) | 72 (71%) | 240 (66%) | .28 |
| Female | 92 (35%) | 30 (29%) | 122 (34%) | |
| Age (years), median (IQR) | 29 (22–38) | 29 (26–35) | 29 (23–37) | .65a |
| Age (years) (categories) | ||||
| 18–24 | 87 (33%) | 17 (17%) | 104 (29%) | <.001 |
| 25–34 | 90 (35%) | 59 (58%) | 149 (41%) | |
| 35–44 | 50 (19%) | 18 (17%) | 68 (19%) | |
| 45+ | 33 (13%) | 8 (8%) | 41 (11%) | |
| Level of education | ||||
| Never went to school/alphabetization | 20 (8%) | 3 (3%) | 23 (6%) | <.001 |
| Primary (7th grade) | 49 (19%) | 4 (4%) | 53 (15%) | |
| Basic (10th grade) | 43 (17%) | 12 (12%) | 55 (15%) | |
| Secondary (12th grade) | 116 (45%) | 56 (55%) | 172 (48%) | |
| Superior/University | 32 (12%) | 27 (26%) | 59 (16%) | |
| Religion | ||||
| Muslim | 34 (13%) | 13 (13%) | 47 (13%) | .11 |
| Catholic | 132 (51%) | 58 (56%) | 190 (52%) | |
| Protestant | 80 (31%) | 31 (30%) | 111 (31%) | |
| Other | 14 (5%) | 0 (0%) | 14 (4%) | |
| Occupation | ||||
| No income | 22 (8%) | 6 (6%) | 28 (8%) | .29 |
| Agriculture | 19 (7%) | 3 (3%) | 22 (6%) | |
| Sales | 32 (12%) | 14 (14%) | 46 (13%) | |
| Health care professional | 14 (5%) | 12 (12%) | 26 (7%) | |
| Teacher | 42 (16%) | 23 (22%) | 65 (18%) | |
| Fishing | 1 (0%) | 0 | 1 (0%) | |
| Domestic worker | 4 (2%) | 1 (1%) | 5 (1%) | |
| Security guard | 2 (1%) | 0 | 2 (1%) | |
| Police | 7 (3%) | 2 (2%) | 9 (2%) | |
| Other | 117 (45%) | 41 (40%) | 158 (44%) | |
| Marital status | ||||
| Married/officially living together | 156 (60%) | 59 (58%) | 215 (59%) | .42 |
| Divorced | 5 (2%) | 1 (1%) | 6 (2%) | |
| Widow | 5 (2%) | 0 | 5 (1%) | |
| Single (not living with partner) | 94 (36%) | 42 (41%) | 136 (38%) | |
| Type of pharmacy visited | ||||
| Rural | 107 (41%) | 44 (43%) | 151 (42%) | .73 |
| Urban | 153 (59%) | 58 (57%) | 211 (58%) | |
| Mother language | ||||
| Portuguese | 21 (9%) | 13 (13%) | 34 (9%9 | .17 |
| Other | 239 (92%) | 89 (87%) | 328 (91%) | |
| Self-reported Portuguese literacy | ||||
| Can’t read Portuguese | 6 (2%) | 0 | 6 (2%) | .03 |
| Not good | 20 (8%) | 5 (5%) | 25 (7%) | |
| Moderate | 38 (15%) | 8 (8%) | 46 (13%) | |
| Good | 77 (30%) | 25 (25%) | 102 (28%) | |
| Very good | 119 (46%) | 64 (62%) | 183 (50%) | |
| Ever had a prior HIV test | ||||
| No | 43 (17%) | 14 (14%) | 57 (16%) | .51 |
| Yes | 217 (83%) | 88 (86%) | 305 (84%) | |
| Place of last HIV test (not including self-test) (n = 305) | .21 | |||
| HF where I live | 173 (80%) | 71 (81%) | 244 (80%) | |
| HF in a different district | 22 (10%) | 9 (10%) | 31 (10%) | |
| Private clinic | 4 (2%) | 0 | 4 (1%) | |
| Community testing | 10 (3%) | 1 (1%) | 11 (4%) | |
| Other | 7 (0%) | 7 (8%) | 14 (5%) | |
| Time of last HIV test (not including self-test) | ||||
| <3 months ago | 96 (44%) | 22 (25%) | 118 (39%) | .04 |
| 3–5 months ago | 34 (16%) | 18 (20%) | 52 (17%) | |
| 6–11 months ago | 24 (11%) | 15 (17%) | 39 (13%) | |
| 12–23 months ago | 37 (17%) | 16 (18%) | 53 (17%) | |
| >2 years ago | 23 (11%) | 16 (18%) | 39 (13%) | |
| Don’t remember | 3 (1%) | 1 (1%) | 4 (1%) | |
| Result of last HIV test (not including self-test) (n=305) | ||||
| HIV negative | 182 (84%) | 85 (97%) | 267 (88%) | .01 |
| HIV positive | 26 (12%) | 2 (2%) | 28 (9%) | |
| Prefer not to say | 9 (4%) | 1 (1%) | 10 (3%) |
Bold text represents the significant p value <0.05.
Mann–Whitney test.
HIVST, HIV self-testing; HF, health facility.
HIVST acceptability among study participants completing exit and post-purchase interviews (n= 362)
Results of the general acceptability are shown in Table 2. A low proportion (28% overall) of participants had ever heard about HIVST, with a significantly higher proportion (51%) having heard about the HIVST strategy (before the pharmacy visit) among those pharmacy clients purchasing an HIVST (p<.001).
TABLE 2.
HIV Self-Testing Acceptability Among Study Participants Completing Exit and Post-Purchase Interviews (N=362)
| Pharmacy clients who did not buy an HIVST (n = 260) n (%) | Pharmacy clients who bought an HIVST (n = 102) n (%) | Total (n = 362) n (%) | p | |
|---|---|---|---|---|
| Ever heard about HIVST (before the pharmacy visit)? | ||||
| No | 211 (81%) | 50 (49%) | 261 (72%) | <.001 |
| Yes | 49 (19%) | 52 (51%) | 101 (28%) | |
| Advantages of HIVSTa | ||||
| Maintains confidentiality | 204 (78%) | 86 (84%) | 290 (80%) | .21 |
| Simple/no need for health provider | 118 (45%) | 66 (64%) | 184 (51%) | .001 |
| Result is fast | 89 (34%) | 44 (43%) | 133 (37%) | .11 |
| To be able to test with my partner | 35 (13%) | 12 (12%) | 47 (13%) | .67 |
| Disadvantages of HIVSTa | ||||
| No counseling nearby | 75 (29%) | 35 (34%) | 110 (30%) | .31 |
| Fear of test result | 65 (25%) | 22 (22%) | 87 (24%) | .49 |
| Doubts on the quality of the test | 28 (11%) | 9 (9%) | 37 (10%) | .58 |
| Fear of somebody discovering | 28 (11%) | 3 (3%) | 31 (9%) | .02 |
| Don’t know how to use it | 17 (7%) | 5 (5%) | 22 (6%) | .56 |
| Too expensive | 18 (7%) | 2 (2%) | 20 (6%) | .06 |
| Not able to read the instructions | 4 (2%) | 2 (2%) | 6 (2%) | .78 |
| Opinion on price of the test | ||||
| Very cheap | 22 (8%) | 7 (7%) | 29 (8%) | .02 |
| Cheap | 22 (8%) | 9 (9%) | 31 (9%) | |
| Acceptable price | 142 (55%) | 72 (71%) | 214 (59%) | |
| Expensive | 73 (28%) | 14 (14%) | 87 (24%) | |
| Price willing to pay for HIVST | ||||
| Don’t want to pay | 14 (5%) | 0 (0%) | 14 (4%) | <.001 |
| Up to 10 Mzn | 77 (30%) | 16 (16%) | 93 (26%) | |
| Up to 50 Mzn | 131 (50%) | 47 (46%) | 178 (49%) | |
| Up to 100 Mzn | 20 (8%) | 25 (25%) | 45 (12%) | |
| Up to 200 Mzn | 8 (3%) | 8 (8%) | 16 (4%) | |
| Up to 500 Mzn | 4 (2%) | 3 (3%) | 7 (2%) | |
| More than 500 Mzn | 4 (2%) | 2 (2%) | 6 (2%) | |
| No response | 2 (1%) | 1 (1%) | 3 (1%) | |
| Preferred place to get an HIVST | ||||
| Private/public pharmacy | 145 (59%) | 69 (68%) | 214 (59%) | .07 |
| Public health facility | 97 (37%) | 24 (23%) | 121 (33%) | |
| Private clinic | 9 (3%) | 6 (6%) | 15 (4%) | |
| Other | 8 (3%) | 3 (3%) | 11 (3%) | |
| Preferred HIVST modality (oral vs. finger-prick) | ||||
| Oral | 141 (54%) | 66 (65%) | 207 (57%) | .17 |
| Finger-prick | 103 (40%) | 30 (29%) | 133 (37%) | |
| Either | 15 (6%) | 5 (5%) | 20 (6%) |
Bold text represents the significant p value <0.05.
Participants were instructed to mark all that apply for these survey questions.
Mzn, Meticais.
The most frequently reported advantage of HIVST was that it maintained confidentiality, stated by 290 (80%) of 362 participants overall and among a slightly higher proportion (84%) of those who purchased an HIVST. Another advantage of HIVST highlighted by 51% of participants overall and a significantly higher proportion (64%; p=.001) of those purchasing an HIVST was that the test was simple with no need to interface with a health provider. Other reported advantages included HIVST being able to be performed quickly (i.e., a rapid turnaround time) and providing the opportunity for people to test with their partners, which were reported by 37% and 13% of respondents, respectively, and were not found to significantly differ between both groups. In terms of disadvantages, 30% of respondents reported the lack of nearby counseling, which was not significant (p=.31) by HIVST purchase status, despite a slightly higher proportion (34% vs. 29%) of those purchasing an HIVST reporting lack of counseling as a disadvantage.
The only disadvantage significantly associated with purchasing an HIVST was the fear of someone discovering their test results, which was reported among 9% of overall participants, 11% of those not purchasing a test compared to 3% of those purchasing an HIVST (p=.02). Other disadvantages not significant by HIVST purchase status included fear of test results, doubts about the quality of the test itself, and the test being too expensive, reported by 24%, 10%, and 6% of overall respondents, respectively.
Approximately one quarter (24%) of overall respondents perceived the HIVST as being too expensive, with a significantly higher proportion of clients not purchasing an HIVST having this perception (29%), compared to 14% among those purchasing the test (p=.02). Overall, only 4% of all interviewed pharmacy clients were not willing to pay for an HIVST. Clients who purchased an HIVST were also willing to pay more for the test compared to those who did not buy an HIVST (p<.001). While the majority (59%) of respondents stated that the pharmacy was a preferred setting to undergo HIVST, one-third (33%) of people interviewed would have preferred to receive a self-test at a public health facility. No significant difference in preference of modality of self-testing was seen (oral vs. finger-prick) among respondents, with more than one-third (37%) favoring finger-prick over saliva-based testing.
Experience of HIVST among study participants completing post-purchase interviews (n= 82)
Between May 20, 2019 and September 31, 2019, 809 individuals purchased an HIVST, with 386 (48%) agreeing to provide their phone contact details. Among those who provided contact information, 143 (37%) were successfully contacted. Slightly more than half (57%; n=82) of study participants provided consent to complete post-purchase interviews. Among these 82 participants completing post-purchase interviews, the vast majority (94%; 77) stated that they had utilized the purchased kit and had performed the HIVST. A description of their reported experiences is shown in Table 3.
TABLE 3.
Acceptability and Reported Experience Among Participants Purchasing HIV Self-Tests (N=82)
| n (%) | |
|---|---|
| Did you do the HIV self-test (at time of interview) | |
| Yes | 77 (94%) |
| No | 5 (94%) |
| (Following questions only for participants who performed the test) | |
| Did the test alone or with somebody | |
| Alone | 53 (69%) |
| With family member or friend | 17 (22%) |
| In the pharmacy | 3 (4%) |
| With other | 4 (5%) |
| Clearness of the instructions | |
| Very easy | 27 (35%) |
| Easy | 44 (57%) |
| Difficult | 1 (1%) |
| Very difficult | 0 (0%) |
| No info | 5 (8%) |
| Difficulties in reading and understanding instructions | |
| Very easy | 24 (31%) |
| Easy | 45 (58%) |
| Difficult | 2 (3%) |
| Very difficult | 0 (0%) |
| No info | 6 (8%) |
| Difficulties in performing HIV self-test | |
| Very easy | 32 (42%) |
| Easy | 38 (49%) |
| Difficult | 5 (6%) |
| Very difficult | 2 (3%) |
| Most difficult step | |
| Step 1—preparing the kit | 13 (17%) |
| Step 2—taking the sample | 6 (8%) |
| Step 3—doing the test | 2 (3%) |
| Step 4—reading the results | 11 (14%) |
| No step was difficult | 44 (57%) |
| Feeling if test was correctly done | |
| Yes | 77 (100%) |
| No | 0 (0%) |
| Belief in the test result | |
| Yes | 75 (97%) |
| No | 2 (3%) |
| Did you feel you needed additional information or counseling before the test | |
| Yes | 29 (38%) |
| No | 48 (62%) |
| Preference of doing test alone or with help of somebody | |
| Unassisted, alone | 66 (86%) |
| Assisted at home | 3 (4%) |
| Assisted at pharmacy | 3 (4%) |
| Assisted at Health Facility | 3 (6%) |
| Would you recommend HIVST to someone else? | |
| Yes | 75 (97%) |
| No | 1 (1%) |
| Don’t know | 1 (1%) |
| HIV testing preference (HIVST vs. conventional health facility-based testing at a designated VCT site) | |
| HIVST | 64 (83%) |
| VCT | 13 (17%) |
| HIVST result (among those willing to reveal) | |
| HIV neg | 43 (56%) |
| HIV pos | 2 (3%) |
| Invalid | 0 (0%) |
| Prefer not to say or no response | 32 (42%) |
| Did you go to the HF after doing the HIVST | |
| Yes | 10 (13%) |
| No | 67 (87%) |
| HIV test result among those who went to health facility after HIVST | |
| HIV neg | 9 (90%) |
| HIV pos | 1 (10%) |
| Indeterminate/invalid | 0 (0%) |
| Prefer not to say or no response | 0 (0%) |
VCT, voluntary counseling and testing.
The majority (92%) of participants perceived the instructions to be clear, stating that the procedures were very easy/easy to understand and follow. Participants felt that the preparation of the HIVST kit (17%) and self-interpretation of the results (14%) were the most difficult steps of the procedures; however, 58% reported that none of the HIVST steps were difficult. Almost all (97%) of the respondents stated that they would recommend HIVST to someone else. Belief in the test results was high (97%), and all participants (100%) felt that their individual self-tests were performed correctly. However, approximately one-third (38%) of participants did feel that they would have benefitted from additional information or counseling before performing the test.
Follow-up rates to nearby health facility among those performing HIVST
Among participants who performed the self-test, 45 (59%) revealed their test result: 2 (3%) reported being HIV positive and 43 (56%) reported being HIV negative. The two participants reporting a positive HIV test result stated that they had tested HIV positive previously, one of whom reported going to the health facility for follow-up. Follow-up to a nearby health facility was reported by 10 (22%) of those revealing their test result stating they went to a nearby health facility to confirm their result (Table 3).
Discussion
This pilot study evaluated the acceptability and individual utilization practices of a pharmacy-based HIVST strategy in central Mozambique. Our results showed that a public or private pharmacy-based HIVST approach was successfully implemented, predominantly reaching men and younger adults, who are important drivers of the HIV epidemic.11–13 In addition, 13% of those purchasing HIVST were first-time testers, thus indicating an opportunity to reach populations that may be less likely to test, at least via the existing health facility-based modalities (voluntary or provider-initiated counseling and testing). The overall acceptability of HIVST was favorable, with data regarding advantages and disadvantages being reported by clients that will aid in the design of possible revisions to this approach before being scaled up.
We found that maintaining patient confidentiality was the main reported advantage, and this is in line with what has been reported in other SSA settings.9,14 Health literacy needs to be an essential consideration when expanding HIVST strategies. Mozambique has a low reported literacy rate of 52%,10 and knowledge regarding HIV transmission remains suboptimal, with only 30% of young adults (15–24 years of age) possessing comprehensive HIV prevention knowledge.2 This could explain why the lack of nearby/additional counseling was highlighted as a disadvantage for HIVST by a significant proportion of respondents. In our context, additional strategies offering more in-depth and easier to understand information and education regarding HIVST is needed and could be offered within the pharmacy itself (e.g., additional educational pamphlets, continuous loop videos showing on wall-mounted televisions, etc.) or via community-based initiatives such as community radio messaging or the mass provision of HIVST information, education, and communication materials.
This pilot program in Mozambique was a pharmacy-based strategy, designed as a means to not place additional burdens on an already overburdened and fragile health system. The cost per test may be a barrier for some individuals, as seen in our study. In Tanzania, where health facility-based HIV testing is free of charge, costs associated with the purchase of an oral HIV self-test outweighed the disadvantages of time spent traveling to and from the health facility, and the transportation costs themselves.9 In Zimbabwe, HIVST demand was price sensitive where a variety of approaches were suggested depending on setting. The demand was lower especially among people living in rural areas and men. In urban areas, demand was higher when distributed through pharmacies, compared to clinic-distribution.15
Mozambique is one of the world’s poorest countries, and our finding that a quarter of the pharmacy clients believe the HIVST test too expensive was not unexpected, especially given the fact that comprehensive HIV testing (and treatment) services are free-of-charge within the national health system. Moreover, clients expressing greater price-sensitivity were significantly less likely to have purchased an HIVST.
Our study population was pharmacy clients, who typically are more likely to be employed and therefore have a higher purchasing power, which might explain why approximately half of them were still willing to pay 50 Mzn or ~USD 0.80 for individual HIVST. However, nationally there are only 793 registered private pharmacies, which is insufficient to cover demand if only a pharmacy-based HIVST strategy was adopted.16 In addition, low-income countries do not have the capacity to subsidize the HIVST. This creates a significant challenge in overcoming the cost barrier, emphasizing the need for Ministry of Health (MOH) representatives involved in commodity procurement decisions to speak with manufacturers about making HIVST affordable.
The success of a pharmacy-based approach hinges on the buy-in of the private sector and their engagement to ensure that sufficient HIVST stocks are maintained, the requisite security and tracking issues are instituted, and linkage to care issues are prioritized in collaboration with public sector stakeholders. It is important, therefore, to include pharmacy staff in the decision-making process to understand their needs and perceptions. In addition, if pharmacies are to be utilized, a major challenge is human resources, and lack of time reported by pharmacy technicians to provide high quality, in-depth counseling required for HIVST.
The study has several limitations. Data are not representative for the country, as the study was only done in one province in Mozambique, and only pharmacy clients (having a higher purchasing capacity) were included in the surveys. Data on test confirmation were collected through self-reporting and this could lead to an under- or overestimation of the linkage. In addition, a significant proportion of clients (42%) purchasing HIVST did not reveal their status, potentially contributing to low reporting rates. Finally, among those who purchased HIVST, only a minority could be contacted and of those, only 58% agreed to be surveyed. This attrition could have introduced bias if, for instance, those refusing to be surveyed were more likely to be HIV positive. Among respondents, the proportion reporting newly diagnosed HIV positivity was low, but this estimate is based on only two HIV positive results. A larger-scale implementation would be necessary to better estimate the yield of this testing modality.
Conclusions
This is one of the first studies in Mozambique of a successfully implemented pharmacy-based strategy for HIVST, showing a high degree of acceptability. The HIVST strategy reached an important proportion of first-time testers. Instructions on use were well understood and clear. The subsidized sale of HIV self-tests appears to be an innovative strategy to reach a population that otherwise might not have undergone HIV testing. However, additional studies are required to determine the specific needs on additional counseling and information for users. Tailored strategies designed to improve linkage to nearby health facilities need to be explored further. In summary, offering HIVST at public/private pharmacies was acceptable with excellent uptake among populations having a traditionally low uptake of HIV testing, thus contributing toward progress attaining UNAIDS lofty 95–95-95 goals.
Supplementary Material
Acknowledgments
We want to thank all participants for their involvement in this evaluation. We thank OraSure Technologies, Inc., for kindly donating the HIV self-tests utilized in the study.
Funding Information
This evaluation has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement (CoAg) #U2GGH001943.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS: UNAIDS data 2021. Available at https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf, accessed June 2, 2022.
- 2.INS, INE, ICF: Inquérito de Indicadores de Imunização, Malária e HIV/SIDA em Moçambique 2015. Relatório Preliminar de Indicadores de HIV. Available at www.ins.gov.mz/images/IMASIDA/IMASIDA2015_HIV.pdf, accessed April 28, 2021 [Google Scholar]
- 3.WHO: Guidelines on HIV self-testing and partner notification: supplement to Consolidated guidelines on HIV testing services. Available at https://www.who.int/hiv/pub/vct/hiv-self-testing-guidelines/en, accessed April 28, 2021. [PubMed]
- 4.WHO: WHO Recommends HIV Self-Testing-Evidence Update and Considerations for Success Policy Brief. Available at https://www.who.int/publications-detail/who-recommends-hiv-self-testing-evidence-update, accessed April 28, 2021.
- 5.Makusha T, Knight L, Taegtmeyer M, et al. : HIV self-testing could “Revolutionize testing in South Africa, but it has got to be done properly”: Perceptions of key stakeholders. Graham SM, ed. PLoS One 2015;10:e0122783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony G, Bazant E, Stephan M, Njogu R, Jonesmus WT, Mudany MJ: Provision of oral HIV self-test Kits triples uptake of HIV testing among male partners of antenatal care clients: Results of a randomized trial in Kenya. International AIDS Conference, Durban, 18–22 July 2016. [Google Scholar]
- 7.Okal J, Obare F, Tun W, Matheka J: Possible Channels for Distribution of HIV Oral Self-Test Kits in Kenya Grantee Final Report. Available at www.3ieimpact.org/media/filer_public/2014/08/04/possible_channels_for_distribution-test_study_final_report.pdf, accessed April 28, 2021.
- 8.Mugo PM, Micheni M, Shangala J, et al. : Uptake and acceptability of oral hiv self-testing among community pharmacy clients in Kenya: A feasibility study. Dezzutti CS, ed. PLoS One 2017;12:e0170868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennings L, Conserve DF, Merrill J, et al. : Perceived cost advantages and disadvantages of purchasing HIV self-testing kits among urban Tanzanian men: An inductive content analysis. J AIDS Clin Res 2017;8. [Epub ahead of print]; DOI: 10.4172/2155-6113.1000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.INE: IV Recenseamento Geral da População e Habitação, 2017. Available at www.ine.gov.mz/iv-rgph-2017, accessed April 28, 2021.
- 11.Karim SSA, Baxter C: HIV incidence rates in adolescentgirls and young women in sub-Saharan Africa. Lancet Glob Health 2019;7:e1470–e1471. [DOI] [PubMed] [Google Scholar]
- 12.Birdthistle I, Tanton C, Tomita A, et al. : Recent levels and trends in HIV incidence rates among adolescent girls and young women in ten high-prevalence African countries: A systematic review and meta-analysis. Lancet Glob Health 2019;7:e1521–e1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PEPFAR: Mozambique Country Operational Plan COP20 Strategic Directio Summary. Available at https://www.state.gov/wp-content/uploads/2020/07/COP-2020-Mozambique-SDS-FINAL.pdf, accessed November 7, 2021.
- 14.Njau B, Covin C, Lisasi E, et al. : A systematic review of qualitative evidence on factors enabling and deterring uptake of HIV self-testing in Africa. BMC Public Health 2019;19. [Epub ahead of print]; DOI: 10.1186/s12889-019-7685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang W, Matambanadzo P, Takaruza A, et al.: Effect of prices, distribution strategies, and marketing on demand for HIV self-testing in Zimbabwe: A randomized clinical trial. JAMA Netw open 2019;2:e199818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Inventory of Health Infrastructure, Resources, Equipment and Services (SARA 2018). Available at https://www.misau.gov.mz/index.php/inqueritos-de-saude, accessed November 7, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.