Abstract
Mozambique introduced guidelines for integrated gender-based violence (GBV) services in 2012. In 2017, we trained providers on empathetic and supportive services to GBV survivors and introduced home-based services for survivors who are loss-to-follow up. Rate ratios of clinic visits were compared before and after intervention initiation, using exact significance tests. Data of 1,806 GBV survivors were reviewed, with a total of 2005 events. The median age was 23 years (IQR 17–30) and 89% were women. Among those reporting violence, 69% reported physical violence, 18% reported sexual violence (SV), and 12% reported psychological violence. Rates of care-seeking behavior were higher in the intervention period (rate ratio 1.31 [95%CI: 1.18–1.46]); p < 0.01. Among those eligible for post-exposure prophylaxis (PEP), 94% initiated PEP. Uptake of HIV retesting improved in percentage points by 34% (14% to 48%), 34% (8% to 42%) and 26% (5% to 31%) at 1-, 3- and 6-months, respectively. The intervention led to an increase in the rate of GBV survivors seeking health care services, and improved rates of follow-up care among SV survivors initiating PEP. Strengthening of PEP adherence counseling remains crucial for improving GBV services.
Keywords: Gender-based violence (GBV), post-exposure prophylaxis (PEP), Mozambique, adherence, access
Introduction
Gender-based violence (GBV) refers to any act that is perpetrated against a person’s will and is based on gender norms and unequal power relationships. Three major types of violence (physical sexual, psychological) have been described (Breiding et al., 2015; Jansen, 2016; WHO, 2017). GBV can lead to physical, mental and/or emotional challenges among survivors (Iyanda et al., 2021, nov), but much attention has been focused on the physical implications of sexual violence (SV) involving physical contact (including rape), given the risk of unplanned pregnancies (Shamu et al., 2018), psychological morbidities, and sexually transmitted infections (STI) including, human immunodeficiency virus (HIV) (Campbell et al., 2008; Grose et al., 2021; Li et al., 2014).
A meta-analysis in sub-Saharan Africa (SSA) estimated prevalence of emotional intimate partner violence (IPV) at 29%, physical IPV at 26%, and SV at 19% (Muluneh et al., 2020). A 2015 population-based survey revealed that 6% of Mozambican women between 18 and 49 years of age reported being forced to have sexual relations against their will during their lifetime (MOH et al., 2015). Uptake of clinical services is hindered by a lack of disclosure among survivors: 53% of Mozambican women who suffered SV and 41% of women who suffered physical violence never asked for help or told anyone (MOH et al., 2015).
Post-exposure prophylaxis (PEP) is provided to all individuals with an exposure risk for HIV transmission, initiated within 72 h of the exposure, and taken for 28 days (WHO, 2014). Low rates of uptake and adherence to PEP after SV is a concern. A multi-country systematic review indicates an overall low PEP completion rate (57%) among SV survivors, with considerable variation in adherence across settings(Ford et al., 2014). Factors associated with poor access or adherence to PEP include: fear of being blamed for the rape, lack of knowledge, lack of support, HIV stigma, medication side effects, costs, and low perceived HIV risk (Abrahams et al., 2010; Ford et al., 2014; Lacombe et al., 2006; Luque et al., 2007; Shubber & Ford, 2021).
In Mozambique, the Ministry of Health (MOH) introduced a “one-stop” care model in 2012, in which personnel from health services, police, social welfare and/or justice agencies provide care/services to GBV patients in one private space in the health facility to ensure comprehensive, confidential services (MOH, 2012). For survivors of SV involving physical contact (including rape), clinical care including the provision of PEP is initiated for those eligible, as well as follow-up visits and HIV re-testing at one, three and six-months (MOH, 2012). This model of care was implemented in Zambézia in 2014. Friends in Global Health (FGH), a wholly-owned subsidiary of Vanderbilt University Medical Center (VUMC), is a non-governmental organization providing technical assistance in Mozambique through support from the U.S. Government Centers for Disease Control and Prevention (CDC)/President’s Emergency Plan for AIDS Relief (PEPFAR). In coordination with the Provincial Health Authorities, in 2016 FGH began to implement community-based awareness and community leader training activities to promote GBV health services uptake. Between February 2017 and April 2019, a GBV care enhancement intervention at 15 selected health facilities was initiated, including training and mentoring for health professionals and introduction of a home-based care intervention. Consenting SV survivors who had started PEP but did not return to the health facility for follow-up care could receive a home-based follow-up health care visit by trained health counselors who provided counseling and repeat HIV testing. The evaluation sought to determine if this package of enhanced interventions increased the number of GBV survivors seeking post-incident care, increased the proportion of SV survivors seeking post-incident care within 72 h, and/or improved uptake of and retention in PEP treatment.
Methods
Design and study sites
Zambézia province, located in central Mozambique, is home to 5.1 million people. Only 51% of adults over 15 years of age are literate (INE, 2017). Health facilities are government-run, and HIV testing, medication for chronic diseases such as antiretroviral therapy (ART) medications (including PEP) are provided at no cost.
We conducted a quasi-experimental pre–post study to evaluate the effect of these enhancement activities on the uptake of GBV care, initiation of PEP, completion of follow-up visits and retesting for HIV among those initiating PEP. The study was implemented between December 2016 and April 2019 in 15 health facilities (ten rural and five urban) of 11 districts in Zambézia (Table 1). The rural and urban interventions were not conducted concurrently. At rural sites, GBV clinical care and home-based care visit training for GBV focal points and clinical leaders took place between December 2016 and January 2017. The seven months prior (July 2016–January 2017) was defined as the pre-intervention period, and from February 2017–April 2019 as the intervention period. For urban sites, training took place in January 2018. The pre-intervention period was defined as July 2017–January 2018, with an intervention period from February 2018–April 2019. Rural sites were district-level health facilities; urban sites included the five largest health facilities serving patients in Quelimane City (provincial capital). This resulted in temporal differences in pre-intervention and intervention periods for rural vs. urban sites, and differences in the intervention period lengths for rural vs. urban, though duration of pre-intervention period was consistent across all sites. We captured baseline GBV service delivery indicators for a minimum of six months and intervention outcomes for at least six months after enhanced services were implemented (Table 1). Due to unforeseen changes which led to a transition in FGH’s technical support provision in October 2017, we were unable to continue the intervention/evaluation at three of the rural sites.
Table 1.
Inclusion periods of the 15 participating health facilities.
| Health facilities | Pre-intervention period | Intervention period |
|---|---|---|
| Rural (n = 7) Alto Molócuè, Gilé, Ile, Inhassunge, Maganja da Costa, Namacurra, Pebane | July 2016–January 2017 | February 2017–April 2018 |
| Rural (n = 3) Chinde, Morrumbala, Mopeia | July 2016–January 2017 | February 2017–September 2017 |
| Urban (n = 5) 17 de Setembro, 24 de Julho, Chabeco, Coalane, General Hospital Quelimane | July 2017–January 2018 | February 2018–April 2019 |
Study population
All patients seeking care for physical, psychological, and/or sexual violence at selected health facilities during the evaluation period were included in the evaluation. Sexual violence survivors of any age who started PEP were eligible for home-based follow-up care if consent/assent was provided.
Study procedures
Routinely collected data from July 2016 to April 2019 were extracted from patient medical records and GBV registers. Documented SV cases, defined for this evaluation as any forced sexual contact (including rape), were those which had been confirmed via clinical examination by a trained medical provider. We captured patient uptake of facility-based GBV-related clinical services, including uptake of, and adherence to PEP, follow-up visits and repeated HIV testing. In the intervention period, SV survivors or their caregivers (if they were under 15 years of age) were informed about the option to receive home-based follow-up (counseling and repeat HIV testing at 1, 3 and 6 months) if they did not return to the health facility for their next scheduled appointment. Consent/assent was obtained (at time of PEP initiation) for home-based services.
Data analysis
Socio-demographic and clinical data available in the routine forms were captured, as well as study-specific data on re-testing for HIV during home visits. Double data entry was done for quality assurance, using Epi-Data Version 3.1. Descriptive statistics were used and presented as medians (with interquartile ranges) for continuous variables and frequency breakdown (percentages) for categorical variables. Rate ratios of clinic visits by period were compared using exact significance tests. A multivariable negative binomial regression was used to estimate the effect the intervention had on the number of visits per day, adjusting for possible confounders of location (rural, urban) and day of week of the visit. Statistical significance was evaluated at 0.05. A zero-inflated negative binomial was used to model the excess zeroes, with day of week and location as predictors. Both negative binomial and zero-inflated negative binomial models led to very similar estimates for the intervention effect and so only results based on the first are presented. Analysis was done using STATA Version 15.1 (StataCorp LLC, College Station, Texas, US).
Ethical considerations
The study was approved by the Zambézia Institutional Committee for Health Bioethics (References 03/CIBS-Z/16; 04/CIBS-Z/17) and VUMC Instutional Review Board (Reference #160885). This project was also reviewed in accordance with the CDC human research protection procedures. A waiver of consent was obtained for extraction of routine data. Written informed consent was obtained from all patients accepting home-based services, with parental consent for minors, and additional assent from adolescents.
Results
A total of 1,806 patients sought GBV services (464 pre, 1,342 post) during the evaluation period. Of those, 353 (20%) were cases of SV: 118 (25%) in the pre-intervention period; 235 (18%) in the intervention period (Figure 1). Eighty-nine percent of patients were women/girls,11% were men/boys; the median age was 23 (IQR 17–30) years (Table 2). Among the survivors, 195 (12%) had more than one type of GBV documented in their clinical record; there were no duplicate cases of sexual violence for any of the survivors. Of all types, 1380 (69%) were physical violence, 353 (18%) sexual violence, 265 (13%) psychological, 7 (0%) other. By sex, 1% of the SV, 13% of the physical violence and 12% of the psychological violence cases were reported by male clients. All SV cases in the pre-intervention period were reported by women/girls, while in the intervention period, women/girls reported 98% and men/boys reported 2% of SV cases (Table 3).
Figure 1.
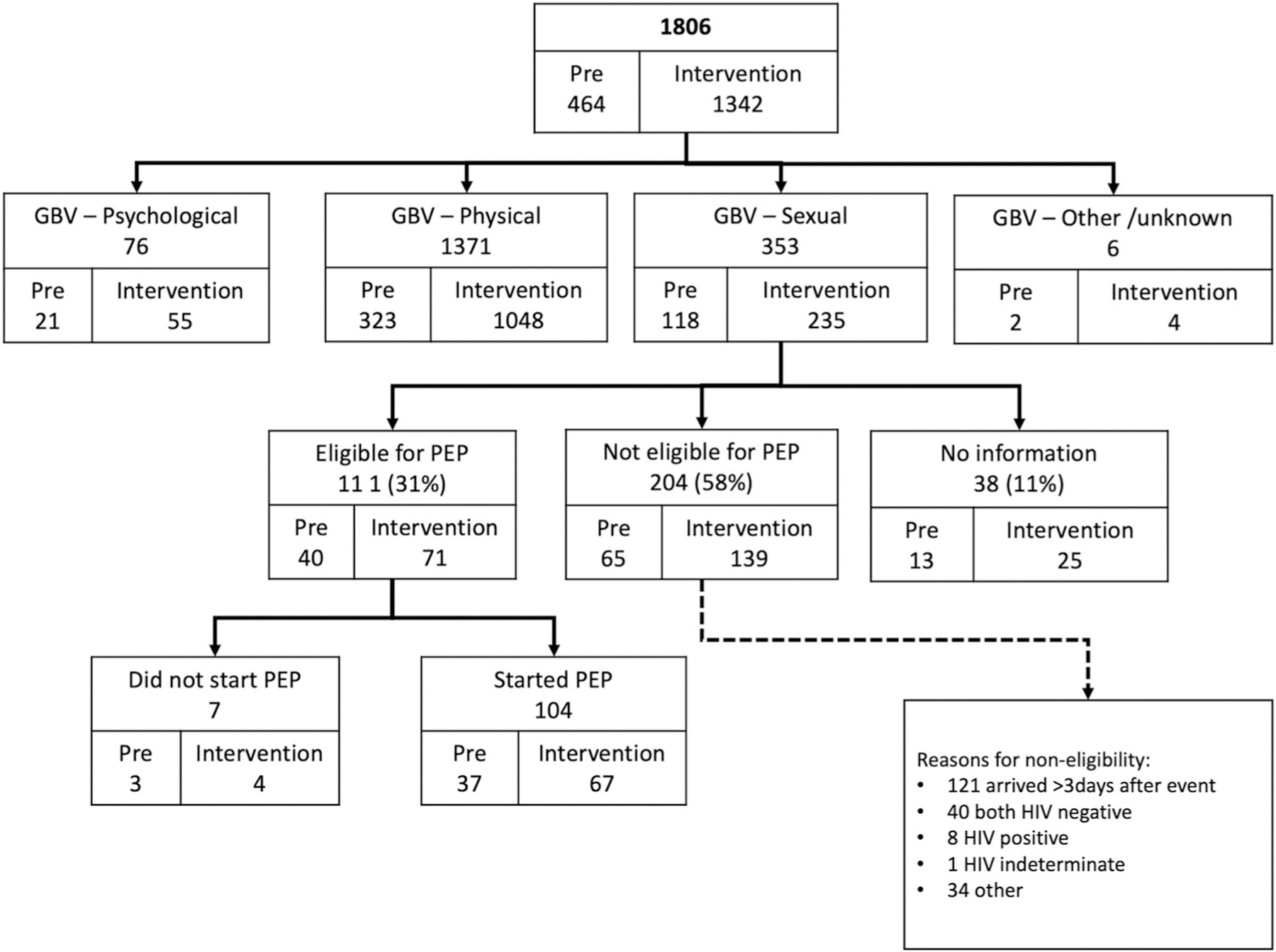
Flow chart of access to GBV services, pre-intervention and intervention period. GBV – Gender based violence; PEP – Post-exposure prophylaxis.
Table 2.
Characteristics of the GBV survivors (n = 1806).
| Female |
Male |
Overall |
||||
|---|---|---|---|---|---|---|
| pre (N = 416) |
post (N = 1196) |
pre (N = 48) |
post (N = 146) |
pre (N = 464) |
post (N = 1342) |
|
| Age (years) | ||||||
| Median [IQR] | 22 [15, 30] | 23 [17, 30] | 25 [19, 30] | 25 [19, 35] | 22 [15, 30] | 23 [17, 30] |
| Mean (SD) | 23 (13) | 24 (12) | 27 (15) | 27 (13) | 23 (13) | 24 (12) |
| Missing | 2 | 0 | 1 | 1 | 3 | 1 |
IQR – Inter quartile range; SD – Standard Deviation.
Table 3.
Characteristics of the GBV survivors, per type of violence (n = 2005 events).
| Sexual Violence (n = 353) | ||||||
|---|---|---|---|---|---|---|
| Female |
Male |
Overall |
||||
| pre (N = 118) |
post (N = 230) |
pre (N = 0) |
post (N = 5) |
pre (N = 118) |
post (N = 235) |
|
| Age (years) | ||||||
| Median [IQR] | 10 [4, 14] | 12 [6, 15] | – | 6 [3, 7] | 10 [4, 14] | 12 [6, 15] |
| Mean (SD) | 10 (8) | 12 (9) | – | 6 (4) | 11 (8) | 12 (9) |
| Psychological Violence (n = 265) | ||||||
| Female |
Male |
Overall |
||||
| pre (N = 83) |
post (N = 150) |
pre (N = 12) |
post (N = 20) |
pre (N = 95) |
post (N = 170) |
|
|
| ||||||
| Age (years) | ||||||
| Median [IQR] | 25 [20, 33] | 21 [17, 28] | 25 [18, 30] | 19 [16, 26] | 25 [20, 32] | 21 [16, 28] |
| Mean (SD) | 27 (10) | 23 (10) | 27 (17) | 21 (10) | 27 (11) | 23 (10) |
| Physical Violence (n = 1380) | ||||||
| Female |
Male |
Overall |
||||
| pre (N = 280) |
post (N = 918) |
pre (N = 47) |
post (N = 135) |
pre (N = 327) |
post (N = 1053) |
|
|
| ||||||
| Age (years) | ||||||
| Median [IQR] | 26 [21, 32] | 25 [20, 31] | 25 [19, 30] | 26 [20, 36] | 26 [21, 32] | 25 [20, 32] |
| Mean (SD) | 28 (11) | 27 (11) | 27 (15) | 28 (13) | 28 (12) | 27 (12) |
| Missing | 2 (1%) | 0 (0%) | 1 (2%) | 1 (1%) | 3 (1%) | 1 (0%) |
| Other Violence (n = 7) | ||||||
| Female |
Male |
Overall |
||||
| pre (N = 3) |
post (N = 3) |
pre (N = 0) |
post (N = 1) |
pre (N = 3) |
post (N = 4) |
|
|
| ||||||
| Age (years) | ||||||
| Median [IQR] | 28 [16, 30] | 21 [20, 33] | – | 20 [20, 20] | 28 [16, 30] | 21 [20, 27] |
| Mean (SD) | 21 (15) | 28 (14) | – | NA | 21 (15) | 26 (12) |
IQR – Inter quartile range; SD – standard deviation.
Access to care for GBV survivors (all types)
In the pre-intervention period, 466 visits were registered, with an average of 0.14 daily visits. There were 1,346 visits, an average of 0.18 visits daily, in the intervention period. Overall, we found a significant increase in the rate of GBV care-seeking visits (rate ratio (RR) of 1.31 [1.18–1.46]) in the intervention period when compared to the pre-intervention period (p < 0.001). There was, however, a substantial difference between care-seeking visit rates seen at urban and rural facilities. In urban facilities, the rate of registry decreased (RR: 0.69 [0.57–0.83]) during the intervention period (p < 0.001), while in rural facilities the rate increased (RR: 1.74 [1.52–2.00]) (Table 4). Adjusted for day of the week and district location, the incidence rate ratio (IRR) of clinic registry for the post period was 1.35 times that of the pre-intervention period (IRR: 1.35 [1.19–1.53], p < 0.001).
Table 4.
Effect of intervention on access to care rate for HIV prevention services for GBV survivors (all [n = 1806]; and SV survivors [n = 353]).
| Number of visits |
Visits per day |
Unadjusted analysis |
Adjusted analysis* |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Intervention | Pre | Intervention | Rate ratio (95% CI) | p-value | IRR (95% CI) | p-value | |
| All GBV Survivors (n = 1806) | ||||||||
| Total | 464 | 1342 | 0.14 | 0.19 | 1.31 (1.18–1.46) | <0.001 | 1.35 (1.19–1.53) | <0.001 |
| Rural | 266 | 1074 | 0.12 | 0.22 | 1.74 (1.52–2.00) | <0.001 | ||
| Urban | 198 | 268 | 0.18 | 0.13 | 0.69 (0.57–0.83) | <0.001 | ||
|
Sexual Violence Survivors (n = 353) | ||||||||
| Total | 118 | 235 | 0.04 | 0.03 | 0.91 (0.72–1.14) | 0.377 | 0.95 (0.75–1.20) | 0.662 |
| Rural | 49 | 164 | 0.02 | 0.03 | 1.45 (1.18–1.82) | 0.020 | ||
| Urban | 69 | 71 | 0.06 | 0.03 | 0.52 (0.37–0.74) | <0.001 | ||
Generalized negative binomial regression adjusted for day of week (weekend/weekday) and health facility; IRR – Incident rate ratio.
Access to post-exposure prophylaxis services for SV survivors
In total, 353 SV events were registered in the participating facilities, 118 in the pre-intervention period (daily rate of 0.04 visits) and 235 in the intervention period (daily rate of 0.03 visits). The unadjusted rate ratios for access to services for SV survivors are presented in Table 4. There was no significant increase in the rate of SV patients seeking care (RR: 0.91 [0.72–1.14], p = 0.377). Interestingly, rural sites showed a significant increase in SV care seeking (RR: 1.45 [1.18–1.82], p = 0.020) while urban sites showed a significant decrease (RR: 0.52 [0.37–0.74], p < 0.001). After adjusting for day of the week and district location, the intervention period compared to the pre-intervention period failed to show an improvement in the frequency of SV patients seeking care (IRR: 0.95 [0.75–1.20], p = 0.662). Access to services within 72 h was not significantly higher in the intervention period (54%) compared to the pre-intervention period (62%) (p = 0.214) (data not shown).
Of all SV events, 111 (31%) were reported as eligible for PEP. Reasons for PEP non-eligibility were late access to care (i.e., >72 h), known HIV-negative serostatus of both assailant and survivor, known HIV-positive or indeterminate serostatus of survivor, or other (Figure 1). Among the 111 survivors eligible for PEP, 104 (94%) initiated PEP; 39 (38%) completed a follow-up visit at 1 month and 36 (35%) re-tested for HIV. As shown in Table 5, uptake to visits was higher in the rural areas, and there was a notable absence of follow-up visits during the pre-intervention period in urban areas. Follow-up visits at 3 and 6 months showed 31 (35%) and 23 (28%) completed visits, respectively. Among all survivors who completed a follow-up visit, 92% re-tested for HIV at 1-month, and all (100%) re-tested at 3- and 6-months visit (Table 5). Over the entire evaluation period, there were no reported cases of HIV seroconversion among SV survivors initiating PEP.
Table 5.
Follow-up visits and retesting among SV survivors eligible for PEP and initiating PEP, by area and period (n = 104).
| Rural (N = 67) |
Urban (n = 37) |
Total (n = 104) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre (n = 17) |
Post (n = 50) |
Total (n = 67) |
Pre (n = 20) |
Post (n = 17) |
Total (n = 37) |
Pre (n = 37) |
Post (n = 67) |
Total (n = 104) |
|
| SV survivors who initiated PEP and completed the 1-month follow-up visit (11 missing for rural; 3 missing for urban) | 5 (16%) | 27 (84%) | 32 | 2 (29%) | 5 (71%) | 7 | 7 (18%) | 32 (82%) | 39 |
| SV survivors who initiated PEP and retested at 1-month follow-up visit (proportion of those with visit done) | 4 (80%) | 26 (96%) | 30 (94%) | 1 (50%) | 5 (100%) | 6 (86%) | 5 (29%) | 31 (97%) | 36 (92%) |
| SV survivors who initiated PEP and completed the 3-month follow-up visit (14 missing rural; 1 missing urban) | 3 (10%) | 26 (90%) | 29 | 0 | 2 (100%) | 2 | 3 (10%) | 28 (90%) | 31 |
| SV survivors who initiated PEP and retested at 3-month follow-up visit (proportion of those with visit done) | 3 (100%) | 26 (100%) | 29 (100%) | 0 | 2 (100%) | 2 (100%) | 3 (100%) | 28 (100%) | 31 (100%) |
| SV survivors who initiated PEP and completed the 6-month follow-up visit (18 missing rural; 3 missing urban) | 2 (9%) | 20 (91%) | 22 | 0 | 1 (100%) | 1 | 2 (9%) | 21 (91%) | 23 |
| SV survivors who initiated PEP and retested at 6-month follow-up visit (proportion of those with visit done) | 2 (100%) | 20 (100%) | 22 (100%) | 0 | 1 (100%) | 1 (100%) | 2 (100%) | 21 (100%) | 23 (100%) |
Of the SV survivors who sought care during the intervention period (n = 235), 71 were eligible for PEP; 67 initiated PEP. All survivors were scheduled to return to the health facility at 1, 3, and 6 months for repeat HIV counseling and testing. Among eligible survivors, 49 (73%) consented for a home visit if they missed their follow-up appointments. Those who consented for home visits (n = 49) completed their follow-up visit more frequently than those who declined to consent for home visits (n = 19), with 28 (57%), 55 (55%) and 20 (41%) returning for services at the scheduled 1-, 3- and 6-month visit, versus 4 (25%), 1 (6%) and 1 (6%), for the same respective visits (Table 6). Among those who consented for home visits and had a follow-up visit, 1 out of the 28 (4%) follow-up visits at 1-month, 3 out of the 27 (11%) visits at 3-months, and 2 out of the 20 (10%) visits at 6-months took place at survivors’ homes. All survivors visited at home were retested for HIV. For 46%, no information was available in the clinical record on location of the follow-up visit.
Table 6.
Completion of follow-up visits and retesting among SV survivors who initiated PEP in the intervention period (n = 65), by area of residence.
| Rural (n = 48) |
Urban (n = 17) |
Total (n = 65)* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Consent for home visit (n = 39) |
No consent for home visit (n = 9) |
Total (n = 48) |
Consent for home visit (n = 10) |
No consent for home visit (n = 7) |
Total (n = 17) |
Consent for home visit (n = 49) |
No consent for home visit (n = 16) |
Total (n = 65) |
|
| SV survivors initiating PEP who completed 1-month follow-up visit (4 missing - rural) | 24 (89%) | 3 (11%) | 27 | 4 (80%) | 1 (20%) | 5 | 28 (88%) | 4 (12%) | 32 |
| SV survivors who initiated PEP and retested at 1-month follow-up visit (proportion among those who had a visit) | 23 (96%) | 3 (100%) | 26 (96%) | 4 (100%) | 1 (100%) | 5 (100%) | 27 (97%) | 4 (100%) | 31 (97%) |
| SV survivors initiating PEP who completed 3-month follow-up visit (6 missing - rural) | 25 (96%) | 1 (4%) | 26 | 2 (100%) | 0 | 2 | 27 (96%) | 1 (4%) | 28 |
| SV survivors who initiated PEP and retested at 3-month follow-up visit (proportion among those who had a visit) | 25 (100%) | 1 (100%) | 26 (100%) | 2 (100%) | 0 | 2 (100%) | 27 (100%) | 1 (100%) | 28 (100%) |
| SV survivors initiating PEP who completed 6-month follow-up visit (9 missing rural; 1 missing urban) | 19 (95%) | 1 (5%) | 20 | 1 (100%) | 0 | 1 | 20 (95%) | 1 (5%) | 21 |
| SV survivors who initiated PEP and retested at 6-month follow-up visit (proportion among those who had a visit) | 19 (100%) | 1 (100%) | 20 (100%) | 1 (100%) | 0 | 1 (100%) | 20 (100%) | 1 (100%) | 21 (100%) |
No information on follow-up care/visits/consent for home visits for two SV survivors.
Discussion
This study found that care-seeking behavior for GBV survivors increased during the implementation period but did not improve timely access of services for SV survivors. Among SV survivors who were eligible for and initiated PEP, we found an increased adherence to follow-up visits and repeat HIV testing. In Kenya, a reproductive health voucher program aimed to increase the uptake of GBV services by providing education and free access to services, however, no improvements were seen in uptake of GBV services. Health provider respondents indicated that community members may have seen GBV as a “family matter” to be addressed in a way that best protects the family name, potentially limiting the success of such a program (Njuki et al., 2012). In Zambézia province, similar cultural beliefs and norms have been reported and could therefore play a role in impeding timely care-seeking for crucial health care services (Graves, 2015) Our study found a decrease in uptake of GBV services in the urban health facilities during the intervention period. While contextual factors seem to play a role, underlying reasons remained unclear and further investigation is needed.
We found that almost 60% of SV survivors arrived more than 72 h after the exposure, thus were ineligible for PEP. Community-wide and targeted educational initiatives and community sensitization efforts need to improve to increase uptake of services within the 72-hour window. We also found that uptake of retesting at 1, 3 and 6 months was suboptimal, even during the intervention phase. This was worse in the urban health facilities. We believe this can be attributed to the under-capacitated health care system in this region. While we created the system for health care workers to conduct testing in survivors’ homes, time constraints, transportation barriers, and challenges finding survivors limited its impact.
Survivors of SV in the evaluation sites were considerably younger than survivors of other types of violence. However, we do not believe that the median age of 11 found in this analysis represents the entire group of SV survivors from the communities during the study period. The 2019 Mozambique Violence against Children and Youth Survey showed that 14.3% of females and 8.4% of males 18–24 years experienced any SV in childhood, but only 5.5% and 8.3% of respective groups actually sought services (INS et al., 2020). This is similar to other SSA countries where many survivors (adults, adolescents, and children) do not disclose or seek professional help after experiencing SV (Meinck et al., 2017; Ngale et al., 2019; Pereira et al., 2020; Rumble et al., 2015).
It seems that SV remains common among youth in this region, even if underreported (Andersson et al., 2012). We hypothesize that among this rural Zambézian population, if a child is sexually assaulted, members of the family or community more frequently attribute this to coercion or force over the child, and therefore more easily recognize, denounce, and seek care for the child for this type of violence than when perpetrated among adolescents or adults.
Our evaluation found fewer reported cases of GBV against men/boys as compared to women/girls. A 2019 study looking at burden and pattern of domestic violence in Beira, the second-largest city in Mozambique, found the women:men ratio of post-GBV care visits was 8:1 (Cebola et al., 2019). Uptake of GBV services among men/boys may also be influenced by their lower rates of health care seeking behavior in this region, underscoring the need for more research to better understand the prevalence of GBV and SV, and the post-SV care needs, among men/boys.
Our study has several limitations. Our use of a pre-intervention-intervention design does not allow for control for changes over time unrelated to our intervention, such as community education activities which were implemented throughout the province prior to and during the intervention period. Routinely collected data are sensitive to data entry errors and/or missing data. Lastly, there were temporal differences in pre- and post-intervention periods for rural vs. urban health facilities. Thus, site-specific context variability during these different time frames, including seasonal variations whose measurement was beyond the scope of this evaluation, could have influenced results.
This study provides evidence that clinical mentoring contributed to the improvement of GBV service delivery. Among those who experienced SV, receiving care during the period of GBV initiative enhancement activities was associated with a higher proportion of completed follow-up and HIV re-testing. The role of counseling (including information and support given) for survivors at the initial visit remains fundamental for successful retention in post-GBV care and minimizing risk of HIV incidence.
Acknowledgements
We thank the participants for their time, the health staff – especially all GBV services focal points - for their collaboration and support in recruitment of participants.
Funding
This publication was supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), through the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC, the Department of Health and Human Services, or the U.S. Government.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abrahams N, Jewkes R, Lombard C, Mathews S, Campbell J, & Meel B (2010, Oct). Impact of telephonic psycho-social support on adherence to post-exposure prophylaxis (PEP) after rape. AIDS Care, 22(10), 1173–1181. 10.1080/09540121003692185 [DOI] [PubMed] [Google Scholar]
- Andersson N, Paredes-Solis S, Milne D, Omer K, Marokoane N, Laetsang D, & Cockcroft A (2012). Prevalence and risk factors for forced or coerced sex among school-going youth: National cross-sectional studies in 10 southern African countries in 2003 and 2007. BMJ Open, 2(2), e000754. 10.1136/bmjopen-2011-000754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiding M, Basile K, S, S. G., Black M, & Mahendra R (2015). Intimate partner violence surveillance uniform definitions and recommended data elements. https://www.cdc.gov/violenceprevention/pdf/ipv/intimatepartnerviolence.pdf
- Campbell JC, Baty ML, Ghandour RM, Stockman JK, Francisco L, & Wagman J (2008, Dec). The intersection of intimate partner violence against women and HIV/AIDS: A review. International Journal of Injury Control and Safety Promotion, 15(4), 221–231. 10.1080/17457300802423224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebola B, Menegazzo F, Salmaso L, Facchin P, Isidoris V, Figueredo R, Mazive S, Schiavone M, Boscardin C, Putoto G, & Pizzol D (2019). Pattern of domestic violence from 2011 to 2015 in Beira, Mozambique. African Health Sciences, 19(1), 1499–1506. 10.4314/ahs.v19i1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford N, Irvine C, Shubber Z, Baggaley R, Beanland R, Vitoria M, Doherty M, Mills EJ, & Calmy A (2014, Nov 28). Adherence to HIV postexposure prophylaxis: A systematic review and meta-analysis. Aids (london, England), 28(18), 2721–2727. 10.1097/QAD.0000000000000505 [DOI] [PubMed] [Google Scholar]
- Graves E (2015). Assessing the quality and impact of GBV clinical services in rural Zambézia: Final summary report for initial observational assessments of GBV Evaluation. [Google Scholar]
- Grose RG, Chen JS, Roof KA, Rachel S, & Yount KM (2021, Jan). Sexual and reproductive health outcomes of violence against women and girls in lower-income countries: A review of reviews. The Journal of Sex Research, 58(1), 1–20. 10.1080/00224499.2019.1707466 [DOI] [PubMed] [Google Scholar]
- INE. (2017). IV Recenseamento Geral da População e Habitação (2017). http://www.ine.gov.mz/iv-rgph-2017/mocambique/censo-2017-brochura-dos-resultados-definitivos-do-iv-rgph-nacional.pdf
- INS, MOH, MGCAS, INE, & CDC. (2020). Violence against children and youth survey in Mozambique, 2019 (VACS 2019). https://ins.gov.mz/wp-content/uploads/2021/12/INVIC_EN-Re-edition_2021.pdf
- Iyanda AE, Boakye KA, Olowofeso OH, Lu Y, & Salcido Giles J (2021, Nov). Determinants of gender-based violence and its physiological effects among women in 12 African countries. Journal of Interpersonal Violence, 36(21–22), 11800–11823. 10.1177/0886260519888536 [DOI] [PubMed] [Google Scholar]
- Jansen H (2016). Measuring prevalence of violence against women: Key terminology. https://asiapacific.unfpa.org/en/publications/violence-against-women-key-terminology-knowvawdata
- Lacombe K, Daguenel-Nguyen A, Lebeau V, Fonquernie L, Girard PM, & Meyohas MC (2006). Determinants of adherence to non-occupational post HIV exposure prophylaxis. AIDS, 20(2), 291–294. 10.1097/01.aids.0000199832.10478.83 [DOI] [PubMed] [Google Scholar]
- Li Y, Marshall CM, Rees HC, Nunez A, Ezeanolue EE, & Ehiri JE (2014). Intimate partner violence and HIV infection among women: A systematic review and meta-analysis. Journal of the International AIDS Society, 17(1), 18845. 10.7448/IAS.17.1.18845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque A, Hulse S, Wang D, Shahzad U, Tanzman E, Antenozzi S, & Smith B (2007, Jun). Assessment of adverse events associated with antiretroviral regimens for postexposure prophylaxis for occupational and nonoccupational exposures to prevent transmission of human immunodeficiency virus. Infection Control & Hospital Epidemiology, 28(6), 695–701. 10.1086/518349 [DOI] [PubMed] [Google Scholar]
- Meinck F, Cluver L, Loening-Voysey H, Bray R, Doubt J, Casale M, & Sherr L (2017, Mar). Disclosure of physical, emotional and sexual child abuse, help-seeking and access to abuse response services in two South African provinces. Psychology, Health & Medicine, 22(sup1), 94–106. 10.1080/13548506.2016.1271950 [DOI] [PubMed] [Google Scholar]
- MOH. (2012). Guia para Atendimento Integrado às Vítimas de Violência. https://reprolineplus.org/system/files/resources/GBV_Manual_Pt.pdf
- MOH, INE, & ICF. (2015). Inquérito de Indicadores de Imunização, Malária e HIV/SIDA em Moçambique 2015. Relatório Preliminar de Indicadores de HIV. https://www.misau.gov.mz/index.php/inqueritos-de-saude?download=566:relatorio-final-imasida
- Muluneh MD, Stulz V, Francis L, & Agho K (2020, Feb 1). Gender based violence against women in Sub-Saharan Africa: A systematic review and meta-analysis of cross-sectional studies. International Journal of Environmental Research and Public Health, 17(3), 903. 10.3390/ijerph17030903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngale K, Cummings B, & Horth R (2019, Aug). Unseen, unheard and unprotected: Prevalence and correlates of violence among female sex workers in Mozambique. Culture, Health & Sexuality, 21(8), 898–913. 10.1080/13691058.2018.1524512 [DOI] [PubMed] [Google Scholar]
- Njuki R, Okal J, Warren CE, Obare F, Abuya T, Kanya L, Undie CC, Bellows B, & Askew I (2012, Jun 12). Exploring the effectiveness of the output-based aid voucher program to increase uptake of gender-based violence recovery services in Kenya: A qualitative evaluation. BMC Public Health, 12(1), 426. 10.1186/1471-2458-12-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A, Peterman A, Neijhoft AN, Buluma R, Daban RA, Islam A, Kainja ETV, Kaloga IF, Kheam T, Johnson AK, Maternowska MC, Potts A, Rottanak C, Samnang C, Shawa M, Yoshikawa M, & Palermo T (2020, Jul 2). Disclosure, reporting and help seeking among child survivors of violence: A cross-country analysis. BMC Public Health, 20(1), 1051. 10.1186/s12889-020-09069-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumble L, Mungate T, Chigiji H, Salama P, Nolan A, Sammon E, & Muwoni L (2015, Aug). Childhood sexual violence in Zimbabwe: Evidence for the epidemic against girls. Child Abuse & Neglect, 46, 60–66. 10.1016/j.chiabu.2015.04.015 [DOI] [PubMed] [Google Scholar]
- Shamu S, Munjanja S, Zarowsky C, Shamu P, Temmerman M, & Abrahams N (2018, May 3). Intimate partner violence, forced first sex and adverse pregnancy outcomes in a sample of Zimbabwean women accessing maternal and child health care. BMC Public Health, 18(1), 595. 10.1186/s12889-018-5464-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubber Z, & Ford N (2021, Jun). Adherence to HIV post-exposure prophylaxis for children/adolescents who have been sexually assaulted: A systematic review of barriers, enablers, and interventions. Child Abuse & Neglect, 116(Pt 1), 104143. 10.1016/j.chiabu.2019.104143 [DOI] [PubMed] [Google Scholar]
- WHO. (2014). Post-exposure prophylaxis for HIV. Supplementary section to the 2013 WHO consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. https://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf
- WHO. (2017). Violence against women. https://www.who.int/news-room/fact-sheets/detail/violence-against-women [Google Scholar]


