Abstract
We previously reported that human cytomegalovirus (CMV) glycoprotein B (gB) is transported to apical membranes in CMV-infected polarized retinal pigment epithelial (ARPE-19) cells and in Madin-Darby canine kidney (MDCK) epithelial cells constitutively expressing gB. The cytosolic domain of gB contains a cluster of acidic amino acids, a motif that plays a pivotal role in vectorial trafficking in polarized epithelial cells and may also function as a signal for entry into the endocytic pathway. Here we compared gB internalization and recycling to the plasma membrane in CMV-infected human fibroblasts (HF) and ARPE-19 cells by using antibody-internalization experiments. Immunofluorescence and quantitative assays showed that gB was internalized from the cell surface into clathrin-coated transport vesicles and then recycled to the plasma membrane. gB colocalized with clathrin-coated vesicles containing the transferrin receptor in the early endocytic/recycling pathway, indicating that gB traffics in this pathway. The specific role of the acidic cluster in regulating the sorting of gB-containing vesicles in the early endocytic/recycling pathway was examined in MDCK cells expressing mutated gB derivatives. Immunofluorescence assays showed that derivatives lacking the acidic cluster were impaired in internalization and failed to recycle. These findings, together with our earlier observation that the acidic cluster is a key determinant for targeting gB molecules to apical membranes in epithelial cells, establish that this signal is recognized by cellular proteins that participate in polarized sorting and transport in the early endocytic/recycling pathway.
Human cytomegalovirus (CMV) is a ubiquitous human pathogen that causes a range of clinical illnesses in immunocompetent individuals, congenitally infected infants, and immunocompromised patients (55). Following primary infection, CMV remains latent in a common precursor of dendritic and myeloid cells, periodically reactivates, and persists (11, 15, 50). A major CMV reservoir is also found in persistently infected endothelial cells lining arteries (12, 16, 18, 24). Reactivation results in intermittent shedding in saliva, urine, or other bodily secretions in tissues composed of epithelial cells and thereby disseminates CMV in the population. In patients with AIDS, CMV is an opportunistic pathogen that causes severe morbidity and mortality, infecting cells in the lungs, gastrointestinal tract, and neuronal retina (9).
Even though potent neutralizing antibodies to CMV glycoprotein B (gB), the major component of the virion envelope, are present in relatively high titers following infection (6, 26, 37, 41), antibodies fail to prevent the spread of infection within tissues (reviewed in references 36 and 40). CMV gB is a type I transmembrane (TM) glycoprotein that is cleaved by the endoproteinase furin (5, 7, 34, 42, 51, 52, 58). gB is highly conserved among the human herpesviruses and is essential for virion infectivity (reviewed in reference 33). CMV gB is a multifunctional envelope protein that triggers penetration of cells and enhances the spread of infection in nonpolarized human fibroblasts (HF) (27). In U373 cells, gB promotes syncytium formation, which is modulated by cytosolic sequences in the carboxyl terminus (56, 57).
Polarized epithelial cells, which compose body tissues that are targets of CMV infection, differ considerably from nonpolarized cells with a uniform plasma membrane. These cells perform regulated secretory functions and have a plasma membrane that is divided into different domains by a “fence” that prevents mixing of proteins and lipids (47). Epithelial cells have specialized pathways for protein trafficking in vesicles of the secretory, endocytic, and transcytotic pathways, which maintain the asymmetric membrane domains (8, 31, 43, 46, 49).
To better understand CMV replication in specialized cell types, we used human retinal pigment epithelial (ARPE-19) cells to examine cell-cell transmission of infection and vectorial egress of virions and to study the role of glycoprotein targeting in vesicular pathways in epithelial cells with distinct membrane domains (10, 54). In ARPE-19 cells, CMV virions infect apical membranes and their progeny are released predominantly from this domain (54). CMV gB is transported vectorially to apical membranes, which suggests that it directs virion release to the susceptible membrane domain of polarized cells and thus enhances infection. On the other hand, an accessory CMV glycoprotein, gpUS9, promotes the cell-cell spread of infection across lateral membranes, directly increasing pathogenesis (19, 35).
We found that in polarized Madin-Darby canine kidney (MDCK) cells, which we used as a model system to examine signals for trafficking of CMV glycoproteins, vectorial transport of gB to apical membranes is directed by sorting determinants in the TM anchor and cytosolic domain of the molecule (55). This finding indicates that specific sorting motifs are recognized by proteins in the transport machinery of epithelial cells that regulate vesicle trafficking. Coimmunolocalization experiments and domain-selective biotinylation of polarized cell surface membranes showed that gB was apically targeted independently of other viral envelope glycoproteins and that it traffics in biosynthetic and early endocytic vesicles. Derivatives of gB that lacked the TM anchor or cytosolic sequences, or were specifically mutated in a cluster of acidic amino acids, DSDEEE, were substantially missorted to basolateral membranes. Interestingly, the endoproteinase furin, which cleaves gB in a post-Golgi compartment (58), contains an acidic cluster that directs its trafficking in the endocytic/recycling pathway (44, 59). It was recently reported that the acidic cluster binds a novel class of cytosolic sorting proteins that interface with adapter complexes, which interact with clathrin (25, 60).
In the present study, we examined gB internalization and recycling in CMV-infected HF and ARPE-19 epithelial cells and in MDCK cells constitutively expressing gB derivatives that lacked part of the cytosolic domain or were mutated specifically in the acidic cluster. Immunofluorescence assays and quantitative internalization studies showed that gB was internalized and recycled to the cell surface in infected human cells and in MDCK cells expressing only gB. Colocalization studies with clathrin and the transferrin receptor showed that gB is transported in the early endocytic/recycling pathway of infected HF and ARPE-19 cells. Together, our studies show that the acidic cluster serves as a signal for gB internalization, recycling, and vectorial sorting in epithelial cells.
MATERIALS AND METHODS
Cells, CMV gB constructs, and culture media.
HF were grown in Dulbecco’s minimal essential medium-high glucose containing 10% fetal calf serum (GIBCO) and antibiotics. MDCK (strain II) cells, a gift from Keith Mostov (University of California San Francisco, San Francisco, Calif.), were grown in Dulbecco’s minimal essential medium containing 10% fetal calf serum and antibiotics. ARPE-19 cells (10) were grown in Dulbecco’s modified Eagle’s medium nutrient mixture F12 with HEPES buffer (GIBCO) containing 10% fetal bovine serum (HyClone), 200 mM l-glutamine, 0.1 mg of streptomycin per ml, and 100 U of penicillin per ml. Mutated CMV gB constructs (Fig. 1) and selection of MDCK cells expressing gB and mutated derivatives have been published previously (55).
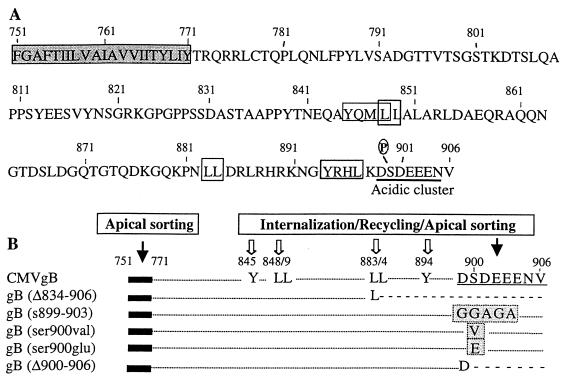
FIG. 1.
Amino acid sequence of the transmembrane (TM) anchor and carboxyl terminus of CMV gB, site-specific mutations, and potential sorting signals. (A) Hydrophobic sequence of the TM anchor (aa 751 to 771) (shaded sequence) and cytosolic domain (aa 772 to 906). Dileucine and Tyr-containing sorting motifs are boxed; the acidic cluster is underlined. P, CKII phosphorylation site. (B) Deletion and substitution mutations. Established internalization/recycling motifs that function as apical sorting signals are indicated by solid arrows; other sorting motifs for trafficking in the endocytic pathway are indicated by open arrows. Deleted sequences are indicated by dashed lines, and substitutions are indicated by shaded boxes. Modified from reference 55.
Antibodies and immunofluorescence assays.
For assays evaluating gB internalization and recycling, we used a pool of monoclonal antibodies (MAbs) reported previously (38). A goat antiserum to clathrin (ICN Pharmaceuticals, Inc.) and a sheep antiserum to transferrin receptor (Harlan Bioproducts) were used for coimmunolocalization experiments. Fluorescein isothiocyanate (FITC)- and Texas red-conjugated anti-mouse, anti-goat, and anti-sheep reagents were purchased from Jackson ImmunoResearch. For immunofluorescence assays of total gB, permeabilized cells were fixed with fresh 3% paraformaldehyde at 4°C for 15 min, permeabilized with 0.1% Triton X-100, and then incubated with antibodies to gB followed by secondary antibodies conjugated to FITC or Texas red.
Internalization assessed by immunofluorescence.
Antibody internalization and immunofluorescence assays reported by Olson and Grose (30) were modified for CMV gB. Briefly, HF and ARPE-19 cells grown on coverslips were infected with strain AD169 at 0.1 and 1.0 PFU/cell, respectively, and were assessed for gB internalization and transport at 3 days (HF) and 5 days (ARPE-19 cells) postinfection. MDCK cells expressing gB derivatives were grown for 2 days prior to evaluation, washed with cold phosphate-buffered saline (pH 7.4), and cooled on ice for 10 min. Then the cells were incubated for 30 min at 4°C in medium without serum, to which the pool of MAbs to gB was added to a dilution of 1:100. Uninfected HF, ARPE-19 cells, and untransfected MDCK cells were used as controls. To allow internalization of CMV gB-MAb complexes, cells were incubated at 37°C for specified intervals from 0 to 60 min. They were then fixed with fresh 3% paraformaldehyde in the cold (15 min), permeabilized with 0.1% Triton X-100, and incubated with goat anti-mouse antibody conjugated to FITC (1 h).
(i) Internalization inhibition.
A modification of the sucrose inhibition assay previously described by Ashworth et al. (4) was used. Briefly, before gB was allowed to internalize, cells were incubated for 30 min with medium containing 10% fetal bovine serum and 0.3 M sucrose. Next, the cells were incubated with MAbs to gB for 30 min at 4°C, washed, and incubated for different times at 37°C in medium containing 0.3 M sucrose. Internalization was monitored as described above.
(ii) Colocalization assays.
To assess colocalization with clathrin, gB was allowed to internalize and then the cells were fixed, permeabilized, and incubated with goat antiserum to clathrin (1 h). Next, the cells were washed with PBS and incubated with goat anti-mouse antibody conjugated to FITC and then with donkey anti-goat antibody conjugated to Texas red. To assess cointernalization of gB and the transferrin receptor (TR), the internalization assays were performed with antibodies to gB and TR at same time. Internalization of gB and TR was visualized by adding goat anti-mouse antibody conjugated to FITC to detect gB and donkey anti-sheep antibody conjugated to Texas red to detect TR. The cells were analyzed with a krypton-argon laser coupled with a Bio-Rad MRC1024 confocal head attached to an Optiphot II Nikon microscope with a Plane Apo 60 ×1.4 objective lens. The cells were scanned simultaneously for FITC and Texas red emission by using the K1 and K2 filter blocks. The data were analyzed with Comos software.
Quantitative internalization assay.
We used a modified quantitative internalization assay which was published previously (3, 32). CMV-infected HF (1.0 PFU/cell) and ARPE-19 cells (10 PFU/cell) and MDCK cells expressing gB derivatives were grown as described above. Uninfected HF and ARPE-19 cells and untransfected MDCK cells were used as controls. The cells were washed twice with cold medium without serum, to which were added 20 mM HEPES and 0.6% bovine serum albumin, and then they were incubated for 30 min at 4°C in medium containing MAbs to gB (1:100). The cells were washed twice with cold medium and incubated for 30 min at 4°C with goat anti-mouse antibody iodinated with 125I (Amersham). Next, they were washed five times with cold medium to remove unbound ligand and then shifted from 4 to 37°C for different time intervals to allow internalization. Two sets of wells with cells were not warmed but kept at 4°C for the zero time point. One set of these cells was not stripped, in order to measure the total amount of bound ligand at the cell surface. The other set was treated with 150 mM glycine in phosphate-buffered saline–0.6% bovine serum albumin (pH 2.5) for 1 h at 4°C to remove membrane-bound but not internalized radioactive antibody. After internalization, the cells were rapidly cooled at 4°C and the uninternalized ligand was stripped from the cell surface with the acidic buffer. The amount of internalized ligand was counted with a Beckman counter and is expressed as a percentage of total ligand initially bound.
Analysis of gB recycling to the plasma membrane.
The assay of recycling to the plasma membrane, described by Olson and Grose (30), was modified for CMV gB. gB was allowed to internalize for 30 min as described above, and then the cells were treated with 1 mg of trypsin (Sigma) per ml at 4°C for 30 min. Cells stripped of surface gB were then shifted for specified intervals to 37°C in medium containing 0.5 mg of trypsin inhibitor (Sigma) per ml and fixed with 2% paraformaldehyde in 0.1 M Na2HPO4. Finally, cells were incubated for 1 h with goat anti-mouse antibody conjugated to FITC and analyzed by immunofluorescence confocal microscopy.
RESULTS
CMV gB is endocytosed in infected fibroblasts and epithelial cells.
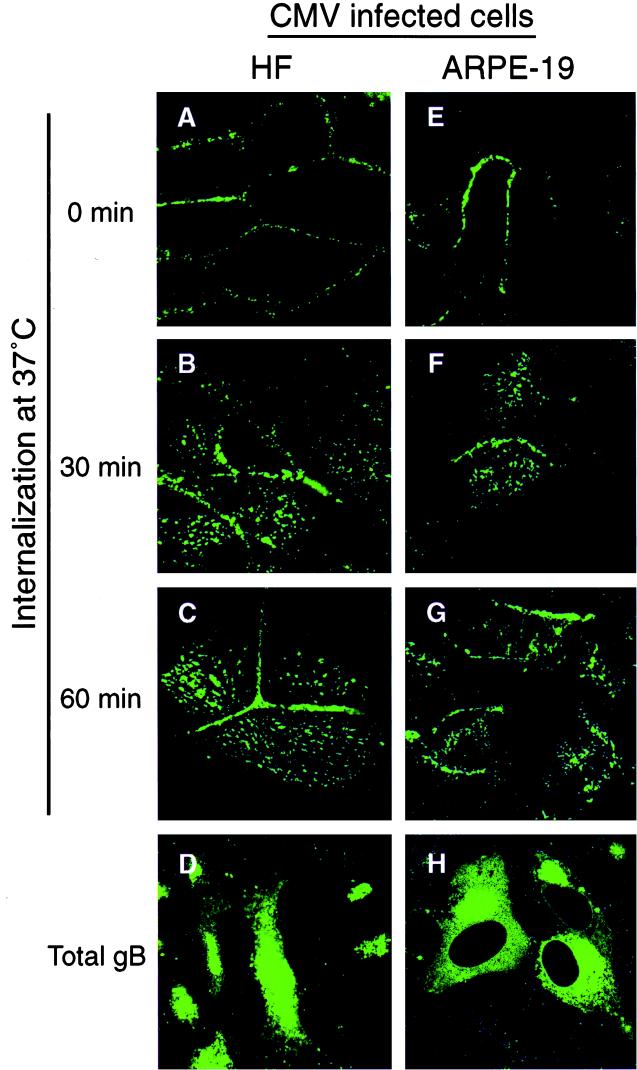
We and others reported that CMV gB is transported in endocytic vesicles in CMV-infected HF (39), U373 cells (13), and polarized MDCK cells expressing gB alone (55). In the first series of experiments, we assessed internalization of the gB-antibody complex from the surface of CMV-infected HF and ARPE-19 cells by immunofluorescence as described in Materials and Methods. Cells were reacted with antibodies to gB at 0°C and then shifted to 37°C for 0, 30, and 60 min to allow internalization of gB-antibody complexes from the plasma membrane. To determine the nonspecific antibody uptake, the MAb pool was added to uninfected HF and ARPE-19 cells and internalization assays were done in parallel with CMV-infected cells. Initially (0 min), gB stained in a ring-like pattern indicative of cell surface staining (Fig. 2A and E). After the shift to 37°C for 30 and 60 min, a fraction of gB was internalized, as indicated by the pattern of intracellular gB-containing vesicles in HF (Fig. 2B and C) and ARPE-19 cells (Fig. 2F and G). Ringlike or vesicular staining was not observed in uninfected HF and ARPE-19 cells at any time, indicating the absence of nonspecific antibody uptake (data not shown). Some peripheral ringlike staining of the plasma membrane was observed, which suggested that a fraction of gB either had not internalized or had recycled back to the cell surface. Total intracellular gB in HF and ARPE-19 cells is shown (Fig. 2D and H). These results suggested that the gB-antibody complex was internalized from the plasma membrane into cytoplasmic vesicles both in fibroblasts and in epithelial cells infected with CMV.
FIG. 2.
CMV gB is internalized in infected HF and ARPE-19 cells. The cells were incubated with a pool of MAbs to gB at 4°C to allow internalization of gB-MAb complexes and then were shifted to 37°C for 0, 30, and 60 min. At the given times, the cells were fixed, permeabilized, and incubated with FITC-conjugated anti-mouse antibody. Internalization of gB was analyzed by laser-scanning confocal microscopy.
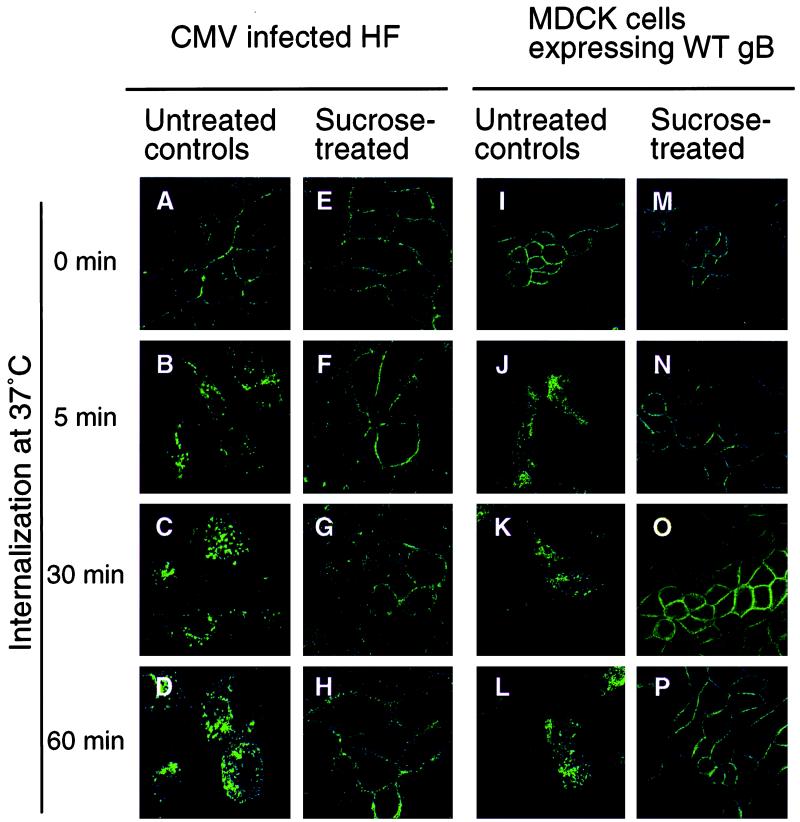
We next ascertained whether gB was internalized into the endocytic pathway by treating cells, as described in Materials and Methods, with hypertonic medium containing sucrose, which precludes the entry of proteins from the cell surface into clathrin-coated vesicles. A comparison of sucrose-treated CMV-infected HF and MDCK cells constitutively expressing wild-type (WT) gB with untreated controls showed that in the controls, plasma membrane-associated gB (0 min) (Fig. 3A and I) was internalized into vesicles at 5 min (Fig. 3B and J). These had dispersed into the cytoplasm by 30 min (Fig. 3C and K) and 60 min (Fig. 3D and L). In contrast, sucrose treatment blocked gB internalization, since the ringlike staining pattern at the plasma membrane at 0 min in infected HF cells (Fig. 3E) remained unchanged after increasingly longer intervals after the temperature shift (Fig. 3F to H). Similarly, sucrose-treated MDCK cells expressing gB at 0 min stained in a ringlike pattern (Fig. 3M) that failed to change after the temperature shift (Fig. 3N to P). These results supported the hypothesis that the gB-antibody complex entered the endocytic pathway by internalizing from the plasma membrane.
FIG. 3.
CMV gB internalization can be inhibited in infected HF and MDCK cells expressing gB. Infected cells were treated with sucrose and then reacted with MAbs, after which gB was allowed to internalize.
gB is internalized and transported in endocytic vesicles in CMV-infected cells.
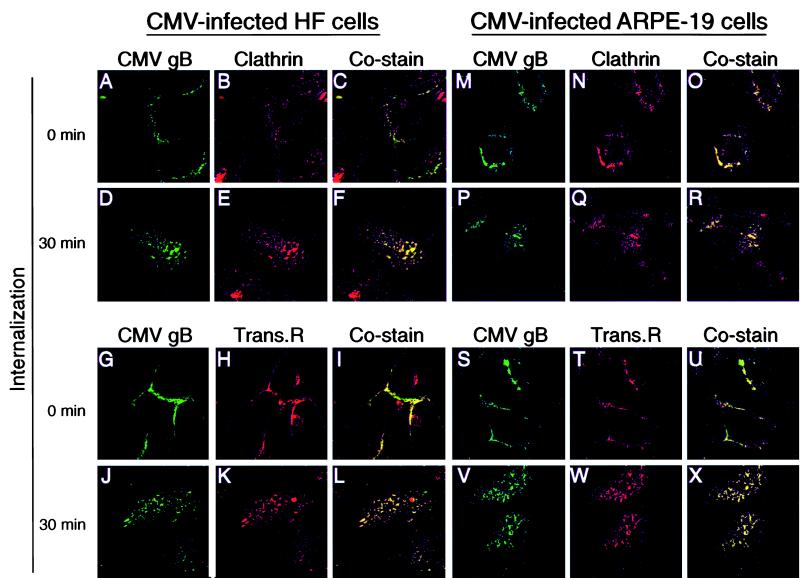
The TR is internalized and transported in clathrin-coated vesicles of the endocytic pathway (reviewed in reference 23). To determine whether CMV gB is transported in endocytic vesicles, we assessed its colocalization with clathrin and the transferrin receptor in CMV-infected HF and ARPE-19 cells. In the first series of costaining experiments with clathrin, gB-antibody complexes formed at the surface of CMV-infected HF and ARPE-19 cells were allowed to internalize. The cells were then permeabilized and reacted with anti-clathrin antibody followed by secondary antibodies conjugated with FITC (gB) and Texas red (clathrin). Immunofluorescence analysis showed that gB and clathrin colocalized at the cell surface at 0 min in HF and ARPE-19 cells (Fig. 4C and O). After 5- and 15-min intervals, a small fraction of cell surface gB was internalized (data not shown). After 30 min, the two proteins were detected in cytoplasmic vesicles in HF (Fig. 4D to F) and ARPE-19 cells (Fig. 4P to R) and gB costained with clathrin. In the next series of costaining experiments, CMV-infected HF and ARPE-19 cells were reacted with antibodies to gB and the TR and both complexes were allowed to internalize together. At 0 min, gB costained with TR at the plasma membrane in HF and ARPE-19 cells (Fig. 4I and U). After 5- and 15-min intervals, a small fraction of cell surface gB was internalized (data not shown). After 30 min, the two proteins were detected in cytoplasmic vesicles in HF (Fig. 4J to L) and in ARPE-19 cells (Fig. 4V to X) and gB colocalized with TR. These results indicated that CMV gB colocalizes with TR at the cell surface and both proteins are transported in endocytic vesicles. The results of colocalization studies with clathrin and TR established that in both fibroblasts and epithelial cells infected with CMV, gB-antibody complexes are internalized in clathrin-coated pits at the plasma membrane and transported in endocytic vesicles that carry TR.
FIG. 4.
CMV gB colocalizes with clathrin in infected HF and ARPE-19 cells (A to F and M to R) and cointernalizes with TR (Trans.R) in endocytic vesicles (G to L and S to X). The cells were incubated with antibodies to gB and to the TR at the same time, whereas anti-clathrin antibodies were added after gB internalization. At the given times, cells were fixed, permeabilized, stained with anti-species antibodies, and analyzed by laser-scanning confocal microscopy.
Internalization of CMV gB in MDCK cells.
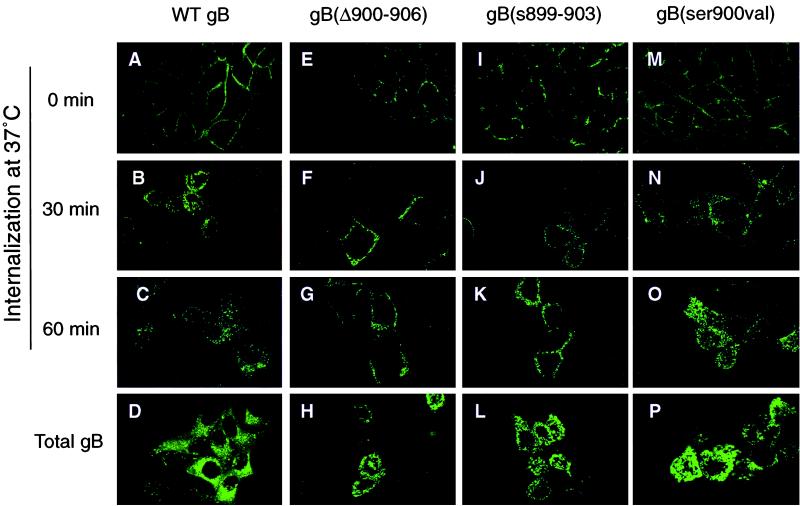
To determine whether the acidic cluster in the cytosolic domain of CMV gB directs gB internalization, we examined MDCK cells expressing WT gB and derivatives with site-specific mutations in the acidic cluster (Fig. 1). Immunofluorescence analysis at 0 min showed WT gB staining in a ringlike pattern at the cell periphery (Fig. 5A). At 5 and 15 min after the temperature shift to 37°C, a small fraction of cell surface gB was internalized (data not shown). By 30 and 60 min, cytoplasmic vesicles stained strongly, indicating that most gB on the cell surface was internalized (Fig. 5B and C). MDCK cells exposed to MAbs did not show specific staining patterns (data not shown). Analysis of derivatives with deletion or substitution mutations in the acidic cluster, gB(Δ900–906) and gB(s899–903), showed that they were impaired in internalization. The ringlike pattern observed for gB(Δ900–906) at 0 min (Fig. 5E) had not changed appreciably at 30 and 60 min after the temperature shift (Fig. 5F and G). Likewise, gB(s899–903) stained at 0, 30 and 60 min in a similar pattern (Fig. 5I to K). On close examination, the broader ringlike pattern observed with the mutated derivatives differed from the tight membrane-restricted pattern found in sucrose-treated MDCK cells (Fig. 3N to P), suggesting that some internalization had occurred. Even though vesicles containing gB appeared at or near the plasma membrane, they failed to disperse into the cytoplasm (compare Fig. 5E and I with Fig. 5F, G, J, and K). Total WT gB and derivatives expressed in the MDCK cells are shown (Fig. 5D, H, L, and P).
FIG. 5.
CMV gB endocytosis is altered in mutated derivatives. MDCK cells were reacted with MAbs to gB, the complexes were allowed to internalize, and cells were incubated with anti-species antibody. Internalization was examined by laser-scanning confocal microscopy.
Next, we examined the role of gB phosphorylation-dephosphorylation in internalization by evaluating the trafficking of derivatives with mutations in the casein kinase II (CKII) site, gB(ser900val) and gB(ser900glu), which mimic uncharged and charged sites, respectively. We found that gB(ser900val), which was concentrated in the plasma membrane at 0 min (Fig. 5M), was internalized and transported in cytoplasmic vesicles after 30 and 60 min (Fig. 5N and O). A similar staining pattern was noted with gB(ser900glu) (data not shown). Together, the results of these studies indicate that the gB-antibody complex is internalized from the plasma membrane of epithelial cells into endocytic vesicles and that mutations altering the charged acidic cluster, but not the CKII site (ser900), impair internalization of gB vesicles from the cell surface.
Quantitative analysis of gB internalization in infected HF and ARPE-19 cells and MDCK cells expressing gB and mutated derivatives.
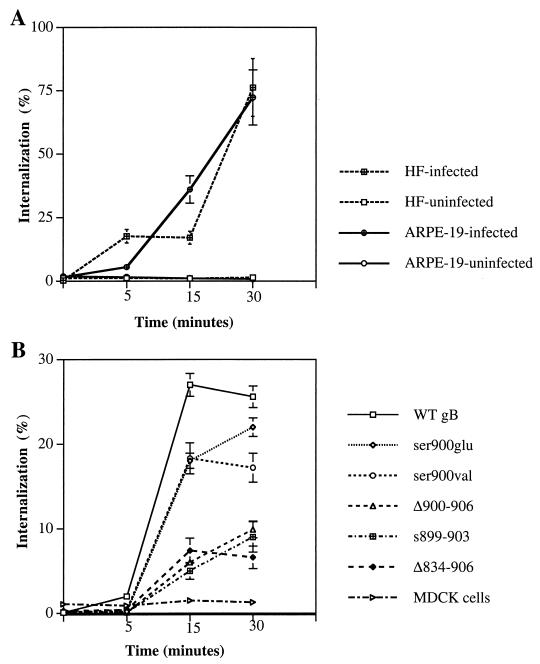
In the next experiments, we quantitated the amount of internalized gB in CMV-infected HF and ARPE-19 cells (Fig. 6A) and MDCK cells expressing WT gB and mutated derivatives (Fig. 6B). Uninfected HF and ARPE-19 cells and untransfected MDCK cells were used as controls. As described in Materials and Methods, cells were reacted with a pool of MAbs to gB at 4°C and then with 125I-labeled goat anti-mouse immunoglobulin G. To allow gB internalization, cells were shifted to 37°C for 5, 15, and 30 min. After each period, radiolabeled protein complexes at the cell surface were removed and the amount of internalized radiolabel was quantitated. The percentage of gB internalized was calculated relative to the amount of label bound (0 min), which represents total gB on the cell surface before internalization. Infected HF and ARPE-19 cells had internalized small amounts of gB at 5 min, after which the amounts increased (Fig. 6A). After 30 min, approximately equal amounts of gB (78%) had been internalized in both HF and ARPE-19 cells. Uninfected HF and ARPE-19 cells treated with MAbs showed negligible internalization.
FIG. 6.
Quantitative analysis of CMV gB internalization in CMV-infected HF and ARPE-19 cells (A) and in MDCK cells constitutively expressing gB and its mutated derivatives (B). The cells were reacted with antibodies to gB and then with iodinated anti-species antibody. The amount of internalized ligand is expressed as percentage of total ligand initially bound. The results shown are means of three separate experiments done in triplicate; bars show standard deviation.
Comparison of MDCK cells constitutively expressing WT gB (Fig. 6B) showed that the total amount of gB internalized (25%) was considerably smaller than in CMV-infected HF and ARPE-19 cells (76%) (Fig. 6A). Internalization of derivatives mutated in the CKII site, gB(ser900val) and gB(ser900glu) was 10% lower than for WT gB at 15 and 30 min (Fig. 6B), in particular that of gB(ser900val) at 30 min. The rate of internalization of mutated gB derivatives with a deletion or a substitution in the acidic cluster, gB(s899–903), gB(Δ900–906), and gB(Δ834–906), was considerably lower than that of WT gB. These mutated forms were internalized about equally, reaching a maximum (6 to 10%) at 30 min. MDCK cells treated with MAbs alone showed approximately 1% internalization. These quantitative analyses support the results of the immunofluorescence assays and show that gB is efficiently internalized in CMV-infected HF and ARPE-19 cells. The finding that WT gB internalization in MDCK cells was less efficient than in infected cells suggests that other viral gene products may participate in endocytic vesicle trafficking. Mutated forms lacking the acidic cluster were poorly internalized compared with WT gB, indicating the importance of this motif in directing gB trafficking in the endocytic pathway. Lastly, mutations in the CKII site did not substantially alter gB internalization in epithelial cells.
CMV gB recycles to the plasma membrane following internalization.
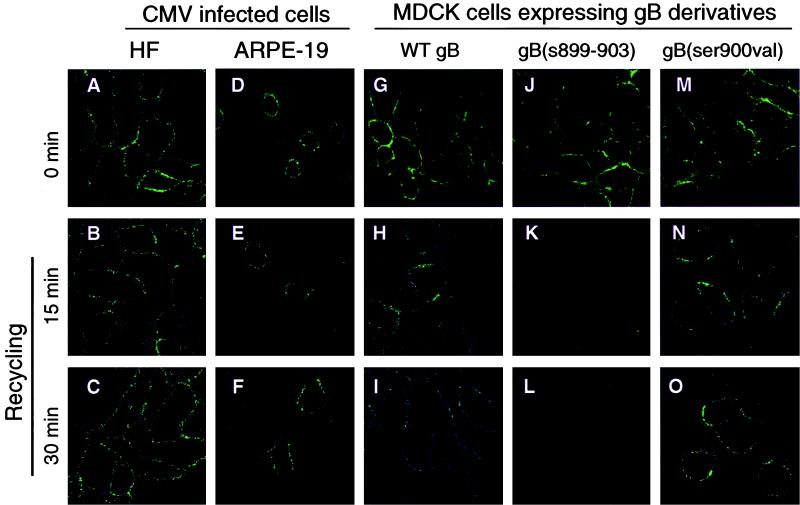
Signals that mediate the internalization of membrane proteins from the cell surface can be used for recycling endocytic vesicles to the plasma membrane (23). We next used an antibody-recycling assay to establish whether endocytic vesicles containing the internalized gB-antibody complex were returned to the plasma membrane. CMV-infected HF and ARPE-19 cells as well as MDCK cells expressing gB were exposed to gB MAbs at 4°C as described in Materials and Methods. Next, the cells were warmed to 37°C for 30 min, returned to 4°C, and treated with trypsin to remove gB from the cell surface. Then the cells were warmed to 37°C again to allow internalized gB to recycle to the plasma membrane. We found that gB was recycled to the plasma membrane, staining the surface of CMV-infected HF cells at 0 min (before internalization) (Fig. 7A) and after 15 and 30 min at 37°C (after recycling) (Fig. 7B and C). In ARPE-19 cells, gB transport vesicles that were internalized recycled to the plasma membrane in the same time intervals, as indicated by a ringlike staining pattern comparable to that in infected HF cells (Fig. 7D to F).
FIG. 7.
Internalized CMV gB is recycled in infected HF and ARPE-19 cells and in MDCK cells expressing gB derivatives. Internalized MAb-gB complexes were allowed to return to the plasma membrane for different intervals. Immunofluorescence staining patterns were analyzed by laser-scanning confocal microscopy.
Next, we compared the recycling of gB and mutated gB derivatives in the endocytic/recycling pathway in MDCK cells. WT gB and gB(ser900val), which were at the cell surface at 0 min (Fig. 7G and M), were recycled during 15- and 30-min intervals (Fig. 7H and I and Fig. 7N and O, respectively). In contrast, gB(s899–903), mutated in the acidic cluster, was in the plasma membrane at 0 min (Fig. 7J) but was not internalized; it was stripped from the surface during trypsin treatment and therefore was not recycled to the cell surface (Fig. 7K and L). Together with the results of antibody internalization studies, the recycling experiments establish that gB-containing vesicles in the early endocytic pathway recycle to the plasma membrane in CMV-infected HF and ARPE-19 cells. The results of gB recycling in MDCK cells confirm that the acidic cluster is a signal for internalization and that endocytic vesicles transporting gB recycle to the plasma membrane. Our results also suggest that phosphorylation may not play a central role in recycling gB-containing vesicles to the surface of MDCK cells.
DISCUSSION
CMV gB internalizes and recycles to the plasma membrane in epithelial cells and fibroblasts.
In this study, we established that gB-antibody complexes are internalized from the plasma membrane in CMV-infected HF and ARPE-19 cells and in MDCK cells constitutively expressing gB in the absence of other viral glycoproteins. Immunofluorescence studies and quantitative internalization assays showed that gB is efficiently endocytosed in both HF and ARPE-19 cells infected with CMV. Like the TR, gB traffics in clathrin-coated vesicles that are internalized and routed back to the cell surface in the early endocytic pathway. Mutations that alter the overall hydrophilic charge of the acidic cluster (amino acids [aa] 899 to 904) in the cytosolic domain of gB impair internalization and preclude recycling. Since the extreme carboxyl terminus of gB contains sequential epitopes, this mutation did not alter gB conformation (38). In contrast, mutations in the CKII site of the acidic cluster have a negligible effect on gB sorting in MDCK cells. Interestingly, gB internalization is more efficient in infected cells than in cells constitutively expressing gB, which suggests that other CMV glycoproteins may modulate vesicle transport in the early endosomes. Together with our earlier finding that the acidic cluster and the TM anchor domain are apical sorting determinants in epithelial cells, the present results indicate that the acidic cluster also directs gB internalization from the plasma membrane. Our studies establish that a selected population of sorting/recycling vesicles in the early endosomal pathway may be targeted to the vectorial transport pathway in polarized epithelial cells.
Intracellular targeting of herpesvirus envelope glycoproteins.
Envelope glycoproteins of several herpesvirus family members are transported in vesicles from the trans-Golgi network (TGN) and early endocytic pathway in different cell types. The gE homologues of pseudorabies virus, varicella-zoster virus (VZV), and herpes simplex virus are internalized from the cell surface into the endocytic pathway (1, 2, 14, 29, 30, 53, 62, 63). CMV gB trafficking may resemble that of VZV gE, which contains signals that recruit adapter protein 1 (AP-1) to endocytic vesicles budding from the TGN; it is routed to the cell surface and then internalizes into early endosomes that recycle to the TGN. The subcellular cycling of VZV gE depends on bipartite endocytic determinants in the cytosolic domain, i.e., a Tyr-based signal and an acidic cluster with a CKII site (1). In the present study, we did not examine TGN localization of gB in CMV-infected cells, but we previously reported a strong colocalization of gB with both AP-1 and furin in vesicles budding from the TGN in epithelial cells (55). Together, these results strongly suggest that gB may also recycle from the plasma membrane into endocytic vesicles that are targeted to the TGN.
Sorting signals that target gB-containing vesicles in the endocytic pathway may modulate its functions in different cell types.
Cell-specific recognition of cytosolic signals may regulate the sorting of gB vesicles and may also modulate its membrane-associated functions. In U373 cells, CMV gB promotes syncytium formation with neighboring cells expressing gB in their membranes (56). Mutagenesis studies established that determinants modulating syncytium activity reside in the cytosolic domain (57). Thus, syncytium formation may be related to the capacity for rapid concentration into endocytic vesicles formed at the plasma membrane of U373 cells. This possibility is bolstered by studies of truncated gB forms lacking cytosolic sequences, which fail to form syncytia (57). One mutated derivative, gB(Δ834–906), which lacks the acidic cluster as well as other endocytic motifs (Fig. 1), is considerably impaired in syncytium formation in U373 cells. In the present study, we found that this derivative was poorly internalized. By extension, internalization of gB(Δ834–906) could be impaired in U373 cells because it may fail to cluster into clathrin-coated vesicles or may sequester in glycosphingolipid rafts whose sorting is regulated by different cellular proteins (45, 48, 55). Consequently, the failure of gB(Δ834–906) to cluster into clathrin-coated vesicles may cause reduced syncytium formation in U373 cells. With regard to another cytosolic signal, we and others have shown that CMV gB is phosphorylated on a CKII site in the cytosolic acidic cluster (13, 28, 57). Dephosphorylation of this site by a tautomycin-sensitive phosphatase promotes internalization of gB into clathrin-coated vesicles in U373 cells but is not required for internalization from the plasma membrane in HF cells expressing gBs mutated in the CKII site (13). In the present study we extended these findings to epithelial cells, showing that dephosphorylation may alter the rate of gB internalization from the surface of MDCK cells.
With respect to epithelial cells, gB is targeted to apical membranes of CMV-infected ARPE-19 cells (54) and MDCK cells, where it colocalizes with cellular proteins that promote clustering into clathrin-coated vesicles (55). These proteins include adapter proteins, AP-1 in the TGN/endosomal pathway and AP-2 in the early endosome sorting/recycling pathway at the plasma membrane, and rab4 and rab5 in recycling endocytic vesicles. The present study extends these observations by showing that gB traffics in clathrin-coated vesicles carrying transferrin receptor. TR is internalized into transport vesicles in the early endocytic pathway and recycles to the cell surface, strengthening the proposed homology between this compartment and the recycling endosome of nonpolarized cells (61). Interestingly, gB internalized from the surface membrane of HF was incorporated into the virion envelope (39), which indicates that gB trafficking in the endocytic pathway may be important for virion infectivity. It further suggests either that CMV envelopment occurs in vesicles of the early endocytic pathway or that glycoproteins from the plasma membrane traffic through endosomes and then recycle to the TGN, where they are assembled into the virion envelope in a post-TGN compartment. Much remains to be learned about the role of the early endocytic pathway in virion envelopment and vectorial release of progeny virions from polarized human cells derived from different tissues.
Sorting signals recognized by cellular adapters and proteins that regulate targeting in the cytosolic domain of CMV gB.
Recycling pathways for endosomes from the plasma membrane and TGN may be considered mirror images of one another (25), and they use similar sorting motifs in the cytosolic domain of membrane-anchored glycoproteins. These include a Tyr (Y) within the sequence YXXØ, where X is any amino acid and Ø is one with a bulky hydrophobic group (20, 23); dileucine motifs (17, 21, 22); and a cluster of acidic residues (59). In this study we focused specifically on the role of the acidic cluster (aa 899 to 906) and the CKII site (ser900) in CMV gB internalization and recycling in the endocytic pathway. However, other signals that could be recognized by regulatory proteins modulating vesicle trafficking in recycling endosomes are concentrated in the cytosolic domain of gB (Fig. 1). They include Y-based and overlapping dileucine signals (aa 845 to 849), a Y-based motif (aa 894 to 897) proximal to the acidic cluster, and a dileucine motif directly upstream (aa 883 to 884) (55). Quantitative internalization assays in MDCK cells showed that gB(s899–903), gB(Δ900–906), and gB(Δ834–906), which lacks the Y-based, dileucine, and acidic cluster signals, were all poorly internalized. These results suggest that other sorting signals may not contribute appreciably to gB endocytosis.
Our finding that gB derivatives with site-specific mutations in the acidic cluster fail to internalize from the plasma membrane strongly suggests that the acidic cluster functions as a signal interacting with adapter molecules associated with clathrin-coated endocytic vesicles (23, 25). Studies on the endoprotease furin indicate that the acidic cluster regulates recycling in the TGN/early endosomal pathway and transport to early endosomes by first binding to PACS-1 (phosphofurin acidic cluster sorting) protein and then to the gamma chain of AP-1, which binds the clathrin coat on endocytic vesicles from the TGN (25, 60). PACS-1 represents a new family of cytosolic connector proteins that serve an intermediate binding function between the acidic cluster and adapter protein AP-1 in the TGN. Our finding that mutations in the CMV gB acidic cluster block internalization suggest that this signal may interact with PACS-1 and AP-2 in clathrin-coated transport vesicles formed at the plasma membrane. The acidic cluster may also function in shuttling gB to endosomes recycling between the TGN and the plasma membrane. Whether the acidic cluster modulates gB trafficking, and consequently the site of virion envelopment in human cells, remains to be determined.
ACKNOWLEDGMENTS
These studies were supported in part by Public Health Service grants EY10138 and EY11223 from the National Institutes of Health (L.P.). S.T. was supported in part by a grant from the Academic Senate of the University of California San Francisco. J.X. was supported by fellowship awards from Fight for Sight (PD97038) and the Universitywide AIDS Research Program (P97-SF-106).
We thank Zoya Kharitonov for excellent laboratory assistance. We are especially grateful to Evangeline Leash for editorial assistance and to Keith Mostov for valuable comments.
REFERENCES
- 1.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada A, Bauer U, Sodeik B, Hoflack B. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J Virol. 1999;73:377–387. doi: 10.1128/jvi.73.1.377-387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aroeti B, Mostov K E. Polarized sorting of the polymeric immunoglobulin receptor in the exocytotic and endocytotic pathways is controlled by the same amino acids. EMBO J. 1994;13:2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashworth R, Yu R, Nelson E J, Dermer S, Gershengorn M C, Hinkle P M. Visualization of the thyrotropin-releasing hormone receptor and its ligand during endocytosis and recycling. Proc Natl Acad Sci USA. 1995;92:512–516. doi: 10.1073/pnas.92.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britt W, Auger D. Synthesis and processing of the envelope gp55-116 complex of human cytomegalovirus. J Virol. 1986;58:185–191. doi: 10.1128/jvi.58.1.185-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt W J, Vugler L G. Antiviral antibody responses in mothers and their newborn infants with clinical and subclinical congenital cytomegalovirus infections. J Infect Dis. 1990;161:214–219. doi: 10.1093/infdis/161.2.214. [DOI] [PubMed] [Google Scholar]
- 7.Cranage M P, Kousarides T, Bankier A T, Satchwell S, Weston K, Tomlinson P, Barrell B, Hart H, Bell S E, Minson A C, Smith G L. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986;5:3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Hoop M J, Dotti C G. Membrane traffic in polarized neurons in culture. J Cell Sci Suppl. 1993;17:85–92. doi: 10.1242/jcs.1993.supplement_17.13. [DOI] [PubMed] [Google Scholar]
- 9.Drew L. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988;158:449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- 10.Dunn K C, Aotaki K A, Putkey F R, Hjelmeland L M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 11.Fish K N, Britt W, Nelson J A. A novel mechanism for persistence of human cytomegalovirus in macrophages. J Virol. 1996;70:1855–1862. doi: 10.1128/jvi.70.3.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fish K N, Soderberg-Naucler C, Mills L K, Stenglein S, Nelson J A. Human cytomegalovirus persistently infects aortic endothelial cells. J Virol. 1998;72:5661–5668. doi: 10.1128/jvi.72.7.5661-5668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fish K N, Soderberg-Naucler C, Nelson J A. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J Virol. 1998;72:6657–6664. doi: 10.1128/jvi.72.8.6657-6664.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn G, Jores R, Mocarski E S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix M G, Daemen M, Bruggeman C A. Cytomegalovirus nucleic acid distribution within the human vascular tree. Am J Pathol. 1991;138:563–567. [PMC free article] [PubMed] [Google Scholar]
- 17.Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lathey J L, Wiley C A, Verity M A, Nelson J A. Cultured human brain capillary endothelial cells are permissive for infection by human cytomegalovirus. Virology. 1990;176:266–273. doi: 10.1016/0042-6822(90)90252-m. [DOI] [PubMed] [Google Scholar]
- 19.Maidji E, Tugizov S, Jones T, Zheng Z, Pereira L. Accessory human cytomegalovirus glycoprotein US9 in the unique short component of the viral genome promotes cell-to-cell transmission of virus in polarized epithelial cells. J Virol. 1996;70:8402–8410. doi: 10.1128/jvi.70.12.8402-8410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks M, Ohno H, Kirchhausen T, Bonifacino J D. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 21.Marks M S, Woodruff L, Ohno H, Bonifacino J S. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matter K, Yamamoto E M, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 24.Melnick J L, Hu C, Burek J, Adam E, DeBakey M E. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J Med Virol. 1994;42:170–174. doi: 10.1002/jmv.1890420213. [DOI] [PubMed] [Google Scholar]
- 25.Molloy S, Anderson E, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 26.Navarro D, Lennette E, Tugizov S, Pereira L. Humoral immune response to functional regions of human cytomegalovirus glycoprotein B. J Med Virol. 1997;52:451–459. [PubMed] [Google Scholar]
- 27.Navarro D, Paz P, Tugizov S, Topp K, LaVail J, Pereira L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, the transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 28.Norais N, Hall J A, Gross L, Tang D, Kaur S, Chamberlain S H, Burke R L, Marcus F. Evidence for a phosphorylation site in cytomegalovirus glycoprotein gB. J Virol. 1996;70:5716–5719. doi: 10.1128/jvi.70.8.5716-5719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson J K, Grose C. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parton R G, Simons K, Dotti C G. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelchen-Matthews A, Armes J E, Marsh M. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. EMBO J. 1989;8:3641–3649. doi: 10.1002/j.1460-2075.1989.tb08538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira L. Function of glycoprotein B homologues of the family herpesviridae. Infect Agents Dis. 1994;3:9–28. [PubMed] [Google Scholar]
- 34.Pereira L, Hoffman M, Tatsuno M, Dondero D. Polymorphism of human cytomegalovirus glycoproteins characterized by monoclonal antibodies. Virology. 1984;139:73–86. doi: 10.1016/0042-6822(84)90331-3. [DOI] [PubMed] [Google Scholar]
- 35.Pereira L, Maidji E, Tugizov S, Jones T. Deletion mutants in human cytomegalovirus glycoprotein US9 are impaired in cell-cell transmission and in altering tight junctions of polarized human retinal pigment epithelial cells. Scand J Infect Dis Suppl. 1995;99:82–87. [PubMed] [Google Scholar]
- 36.Pereira L, Navarro D, Jahn G. Proteins of human cytomegalovirus that elicit humoral immunity. In: Becker Y, Darai G, Huang E-S, editors. Molecular aspects of human cytomegalovirus diseases. Vol. 2. Heidelberg, Germany: Springer-Verlag KG; 1993. pp. 437–464. [Google Scholar]
- 37.Pereira L, Stagno S, Hoffman M, Volanakis J. Cytomegalovirus-infected cell polypeptides immune precipitated by children with congenital and perinatal cytomegalovirus infections. Infect Immun. 1983;39:100–108. doi: 10.1128/iai.39.1.100-108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qadri I, Navarro D, Paz P, Pereira L. Assembly of conformation-dependent neutralizing domains on human cytomegalovirus glycoprotein B. J Gen Virol. 1992;73:2913–2921. doi: 10.1099/0022-1317-73-11-2913. [DOI] [PubMed] [Google Scholar]
- 39.Radsak K, Eickmann M, Mockenhaupt T, Bogner E, Kern H, Eis H A, Reschke M. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch Virol. 1996;141:557–572. doi: 10.1007/BF01718317. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen L. Immune response to human cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:221–254. doi: 10.1007/978-3-642-74980-3_9. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen L, Matkin C, Spaete R, Pachl C, Merigan T C. Antibody response to human cytomegalovirus glycoproteins gB and gH after natural infection in humans. J Infect Dis. 1991;164:835–842. doi: 10.1093/infdis/164.5.835. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen L, Nelson M, Neff M, Merigan T C. Characterization of two different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology. 1988;163:309–318. doi: 10.1016/0042-6822(88)90271-1. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Boulan E, Powell S K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- 44.Schafer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse M L, Kern H F, Klenk H D, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheiffele P, Verkade P, Fra A M, Virta H, Simons K, Ikonen E. Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J Cell Biol. 1998;140:795–806. doi: 10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simons K, Dupree P, Fiedler K, Huber L A, Kobayashi T, Kurzchalia T, Olkkonen V, Pimplikar S, Parton R, Dotti C. Biogenesis of cell-surface polarity in epithelial cells and neurons. Cold Spring Harbor Symp Quant Biol. 1992;57:611–619. doi: 10.1101/sqb.1992.057.01.067. [DOI] [PubMed] [Google Scholar]
- 47.Simons K, Fuller S D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- 48.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 49.Simons K, Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993;11:789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- 50.Soderberg-Naucler C, Fish K N, Nelson J A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 51.Spaete R R, Saxena A, Scott P I, Song G J, Probert W S, Britt W J, Gibson W, Rasmussen L, Pachl C. Sequence requirements for proteolytic processing of glycoprotein B of human cytomegalovirus strain Towne. J Virol. 1990;64:2922–2931. doi: 10.1128/jvi.64.6.2922-2931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spaete R R, Thayer R M, Probert W S, Masiarz F R, Chamberlain S H, Rasmussen L, Merigan T C, Pachl C. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology. 1988;167:207–225. doi: 10.1016/0042-6822(88)90071-2. [DOI] [PubMed] [Google Scholar]
- 53.Tirabassi R S, Enquist L W. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J Virol. 1998;72:4571–4579. doi: 10.1128/jvi.72.6.4571-4579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tugizov S, Maidji E, Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol. 1996;77:61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- 55.Tugizov S, Maidji E, Xiao J, Zheng Z, Pereira L. Human cytomegalovirus glycoprotein B contains autonomous determinants for vectorial targeting to apical membranes of polarized epithelial cells. J Virol. 1998;72:7374–7386. doi: 10.1128/jvi.72.9.7374-7386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tugizov S, Navarro D, Paz P, Wang Y, Qadri I, Pereira L. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology. 1994;201:263–276. doi: 10.1006/viro.1994.1291. [DOI] [PubMed] [Google Scholar]
- 57.Tugizov S, Wang Y, Qadri I, Navarro D, Maidji E, Pereira L. Mutated forms of human cytomegalovirus glycoprotein B are impaired in inducing syncytium formation. Virology. 1995;209:580–591. doi: 10.1006/viro.1995.1290. [DOI] [PubMed] [Google Scholar]
- 58.Vey M, Schafer W, Reis B, Ohuchi R, Britt W, Garten W, Klenk H D, Radsak K. Proteolytic processing of human cytomegalovirus glycoprotein B (gpUL55) is mediated by the human endoprotease furin. Virology. 1995;206:746–749. doi: 10.1016/s0042-6822(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 59.Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan L, Molloy S S, Thomas L, Liu G, Xiang Y, Rybak S L, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 61.Zacchi P, Stenmark H, Parton R G, Orioli D, Lim F, Giner A, Mellman I, Zerial M, Murphy C. Rab17 regulates membrane trafficking through apical recycling endosomes in polarized epithelial cells. J Cell Biol. 1998;140:1039–1053. doi: 10.1083/jcb.140.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]