Abstract
Key Clinical Message
Antinuclear antibody‐negative full‐house lupus nephritis though previously reported, is fairly uncommon. Some patients go on to develop antibodies later in the disease course. The presence of RO‐52 antibody in this case suggests an underlying immunological cause. Swift management based on strong clinical suspicion can be life‐saving to the patient.
Abstract
Lupus nephritis (LN) is a serious complication of systemic lupus erythematosus (SLE) and is more likely to progress to end‐stage renal disease (ESRD). With the recent EULAR/ACR criteria mandating antinuclear antibody (ANA) positivity as an entry criterion, clinicians are faced with a diagnostic dilemma in diagnosing cases of seronegative SLE. We present the case of a 25‐year‐old female who presented with photosensitive malar rash, hair loss, oral ulcers, menorrhagia, and kidney dysfunction, suggestive of SLE. Her ANA tests were negative, raising doubts about the diagnosis. Biopsy was delayed owing to anemia and thrombocytopenia, and clinical judgment led to the patient being diagnosed with LN, with prompt treatment resulting in significant improvement. Renal biopsy subsequently confirmed the case as diffuse class IV LN with full‐house nephropathy. This case highlights the limitations of relying solely on ANA positivity in diagnosing LN and underscores the need for a comprehensive diagnostic approach for SLE that incorporates clinical features, immunological markers, and patient demographics. ANA‐negative SLE patients demand heightened clinical suspicion, especially when other diagnostic parameters align with the disease. Swift intervention with immunosuppressive therapy, as seen in this case, can be life‐saving.
Keywords: case reports, glomerulonephritis, lupus erythematosus, lupus nephritis, systemic
1. INTRODUCTION
Lupus nephritis (LN) is the renal manifestation of systemic lupus erythematosus (SLE), which usually develops within the first 6–36 months of SLE diagnosis and might even be present at the very outset of the disease. 1 With elevated risks of transitioning to end‐stage renal disease (ESRD), LN is linked to increased morbidity and mortality in patients with SLE. 1 However, the prompt diagnosis and treatment of LN has significantly increased survival in SLE patients from approximately 44% to 95% in a span of five decades. 2
There is no one‐size‐fits‐all diagnostic criterion for SLE because of its varied clinical presentations, and diagnosis often relies on a combination of clinical judgment, medical history, physical examination, and laboratory tests. However, classification systems have been developed and updated over time to create homogenous groups of SLE patients to aid in research and clinical trials. The 1997 ACR criteria, which require the presence of any 4 out of 11 clinical or laboratory presentations, were widely used classification criteria for SLE. 3 The shortcomings of the ACR criteria led to the development of the Systemic Lupus International Collaborating Clinics (SLICC) criteria in 2012, which necessitated 4 out of 17 criteria to be qualified as SLE. 4 SLICC mandated at least one clinical and immunological criterion and considered biopsy‐confirmed LN, alongside either antinuclear antibody (ANA) or anti‐dsDNA antibodies, as a qualifying criterion when other criteria were not met. 4 The SLICC criteria have been reported to have higher specificity in diagnosing SLE than the ACR criteria. 5
The most recent classification system for SLE was developed as a joint effort between the ACR and the European League Against Rheumatism (EULAR) in 2019; this system is known as the ACR/EULAR criteria. 6 The significant change in the new criterion was the incorporation of positive ANA as an entry criterion, the weighted scoring system, and the streamlined approach. This was done to improve the sensitivity and specificity. 6 However, the absence of ANA does not rule out SLE in clinical practice, and a separate cohort of ANA‐negative SLE patients has previously been described in clinical practice. 7
Here, we present the case of a 25‐year‐old female with ANA‐negative SLE who was subsequently found to have class IV LN with full‐house nephropathy. This case shows that the diagnostic landscape of LN is complex and can defy the conventional expectations associated with positive ANA results.
2. CASE DESCRIPTION
A 25‐year‐old female with no known prior comorbidities presented with complaints of excessive hair loss and photosensitive malar rash for the past 3 months and generalized body swelling and reduced urine output without causing oliguria for the past 2 weeks. She also had mucosal ulceration and menorrhagia.
On examination, she had pallor and bilateral pedal edema. Malar rash was present, her blood pressure was 130/70 mm Hg, her pulse rate was 102 beats/min, her respiratory rate was 26 breaths/min, and her saturation was 94% in room air. On chest auscultation, bilateral crepitations were observed, more prominently on lower lung fields with reduced air entry on both sides, and abdominal examination revealed shifting dullness. There was no organomegaly, and cardiac and neurological examinations were normal.
3. METHODS (INVESTIGATIONS, DIFFERENTIAL DIAGNOSIS, AND TREATMENT)
Laboratory examination revealed the following pertinent findings: anemia (hemoglobin of 7 g/dL), thrombocytopenia (platelet count of 25,000 per cubic millimeter), hypoalbuminemia (1.4 g/dL), acute kidney injury (creatinine of 1.8 mg/dL), and transaminitis (SGOT 411 U/L and SGPT 322 U/L). Alkaline phosphatase activity increased (207 U/L), as did LDH (636 U/L). Peripheral blood smear revealed normocytic normochromic to microcytic hypochromic red cells with less than 1% schistocytes and large platelets. Tests for scrub typhus, dengue, and leptospirosis were also negative. Total cholesterol and LDL were within normal limits, while HDL was low (0.4 mmol/dL) and triglycerides were elevated (5.2 mmol/dL). The iron profile was normal except for elevated ferritin, which was well above 2000 ng/mL. The patient's vitamin D concentration was low (12 ng/mL). Urine analysis revealed 6–8 pus cells, 30–35 red blood cells (RBCs) per high‐power field, and a trace amount of albumin. No casts were noted, and 24‐h urine collection revealed 1.5 g of protein, which was below the nephrotic range. Urine culture was negative for any organism.
The patient tested negative for serological markers of SLE, such as antinuclear antibodies (ANA—by both ELISA and immunofluorescence), anti‐Smith antibodies, anti‐dsDNA, and anti‐cytoplasmic antibodies such as c‐ANCA, p‐ANCA, anti‐GBM, and anti‐cardiolipin. However, an extractable nuclear antigen panel (ENA) revealed that RO 52 was positive despite ANA negativity. C3 was reduced (0.43 g/L), while C4 was within normal limits (0.21 mg/dL) and a direct Coombs test was positive (2+). Her CRP (26.35 mg/L) and procalcitonin (39.55 ng/mL) levels were elevated. Serology for human immunodeficiency virus and hepatitis virus was negative. The patient's chest radiograph revealed bilateral pleural effusion. Ultrasonography revealed right and left kidney sizes of 9.1 and 9.3 cm, respectively, with maintained corticomedullary differentiation and gross ascites. Ascitic fluid analysis revealed a low SAAG and low‐protein ascites. Echocardiography revealed minimum pericardial effusion with a normal ejection fraction (60%).
A strong clinical suspicion of LN was made despite the negative results for antinuclear antibodies. The patient was rescued with 3 doses of methylprednisolone 500 mg for three consecutive days. Following the pulses of methylprednisolone, the patient was kept on mycophenolate mofetil. Moreover, the patient's clinical symptoms started to improve during her hospital stay. Renal biopsy was postponed to buy time for platelet counts and hemoglobin levels to improve.
4. RESULTS (OUTCOME AND FOLLOW‐UP)
Following treatment with three intravenous doses of methylprednisolone and maintenance therapy with mycophenolate mofetil, the patient's laboratory parameters, including hemoglobin levels, platelet counts, and renal and liver function test results, started to improve. The acute kidney injury gradually resolved. The patient requested discharge from the hospital following an improvement in symptoms. She was discharged following renal biopsy after a hospital stay of 18 days.
When she returned with her renal biopsy report, it showed class IV diffuse LN. There was evidence of increased mesangial and matrix cellularity with segmental/global endocapillary proliferation and variable intercapillary/mesangial neutrophil infiltration. Crescents, subendothelial deposits, and segmental tuft sclerosis were also noted (Figure 1). On immunofluorescence microscopy, full‐house nephropathy was observed with 3+ granular staining for IgG and kappa and lambda light chains and 2+ granular staining for C3, C1q, IgA, and IgM along both the mesangial and capillary walls. The modified NIH+ indices of disease activity and chronicity were 13/24 and 2/12, respectively. The patient was then advised to continue her medication, which included prednisolone, hydroxychloroquine, mycophenolate mofetil, furosemide, vitamin D, calcium, and folic acid supplementation. Previously, after she was kept on 500 mg mycophenolate mofetil BD, her dosage was increased to 500 mg TDS at this follow‐up. She was then asked to follow‐up at the nephrology outpatient department (OPD) after 2 weeks, and the dosage of mycophenolate mofetil was increased to 1 g twice daily. The patient is advised to follow‐up at the nephrology OPD every 3 months.
FIGURE 1.
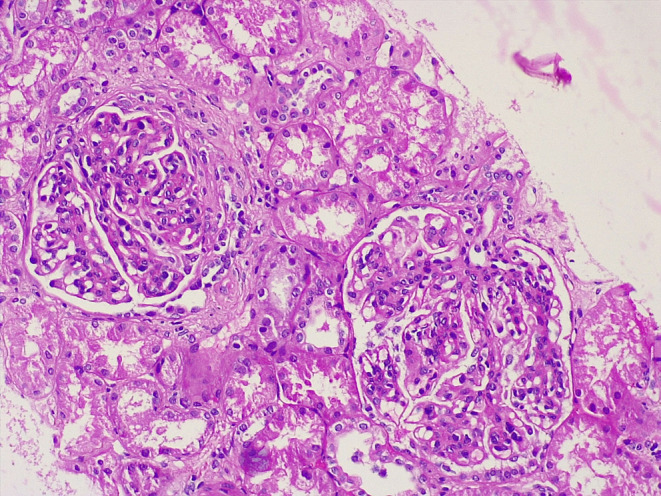
Renal section showing diffuse moderate mesangial cellularity and matrix and segmental/global endocapillary cell proliferation with variable intercapillary/mesangial neutrophil infiltration. Four glomeruli had overlying crescents, and five had subendothelial deposits.
5. DISCUSSION
This case highlights the complexities and challenges faced by clinicians when diagnosing a patient with SLE. Given its diverse manifestations, lupus can mimic various other medical conditions, 8 and serological investigations often play a crucial role in establishing a definitive diagnosis. The classification systems for lupus, though not primarily designed for clinicians, are often used in clinical scenarios. The most recent EULAR/ACR criterion mandates ANA as an entry criterion. 6 In that light, this patient's diagnosis could have been missed if only a single classification criterion was relied upon. An important drawback for the reliability of ANA as a diagnostic tool lies in the fact that people without SLE can test positive for ANA 9 ; therefore, clinicopathological characteristics must be present for an SLE diagnosis.
A study of ANA‐negative SLE in an international inception cohort that aimed to redefine negative ANA as the absence of any intracellular immunofluorescence staining revealed that only about 92% cases of SLE patients were ANA‐positive as per conventional nuclear staining patterns. 10 Around 1.5% of these patients were positive for cytoplasmic and mitotic cell patterns (CMPs) and remaining 6% were negative for nuclear staining or any CMPs, referred to as anti‐cellular‐negative. This study further emphasized that misclassifying CMP‐positive cases as ANA‐negative could lead to misdiagnosis of a significant cohort of patients. Also, complete anti‐cellular antibody‐negative patterns were seen in older patients and in patients who were already under high dose of glucocorticoids. Our patient, however, was a young female and was never previously under steroids. Newer insights into SLE reveal that as many as 30% of patients with SLE enrolled into clinical trials are ANA‐negative. This highlights the limitations of using ANA as a universal criterion and indicates that some patients might actually transition into an ANA‐negative stage in the disease course. 11
Several explanations have been proposed to explain the factors responsible for the negative serological profile in patients with SLE. A literature review of existing studies suggests that the development of seropositivity for SLE might be delayed by up to 10 years and is eventually inevitable once disease activity becomes more extensive. 7 Additionally, immune complex formation and deposition in end organs could mask the presence of autoantibodies. 12 In the case of our patient, the presence of hypocomplementemia, specifically a reduced C3 level, strongly suggested activation of the immune system's complement pathway through immune mediation. It is worth noting that reduced C3 alone qualifies as an immunological criterion as per the SLICC guidelines. 4 C4 was normal in our patient. Though both C4 and C levels can fall during a renal flare, C3 is a more sensitive indicator of LN than C4, and C3 activation rather than C4 is involved in active tissue damage in LN. One study suggested that the absence of autoantibodies could be attributed to urinary loss owing to severe nephrotic syndrome 13 ; however, our patient's urine protein excretion was well below the nephrotic range. False‐negative ANA could also be attributed to faulty sample handling and processing, owing to which repeat testing may alleviate doubts in cases of strong clinical suspicion.
LN is a serious complication of SLE. Our patient had class IV diffuse nephritis, the most common and most severe variant of LN, along with full‐house nephropathy, as observed via immunofluorescence microscopy. 14 Clinically evident kidney disease occurs in approximately half of patients with SLE, and 44% of those with class IV LN are likely to progress to end‐stage kidney disease in a span of 15 years. 15 In patients with class IV LN, especially in patients with active disease, elevated anti‐dsDNA and hypocomplementemia are said to occur. 16 Anti‐dsDNA antibodies, along with other essential serological parameters, such as ANA, anti‐Smith, anti‐cardiolipin, and anti‐GBM antibodies, were markedly negative; however, low complement could suggest immune‐mediated activation of the complement pathway, leading to its consumption. Notably, this patient was positive for anti‐Ro52 antibodies, which is observed in patients with SLE and Sjogren's syndrome. Autoantibodies against Ro‐52 is found in a subset of SLE patients and is seen to associate with clinical and laboratory features of the disease. 17 While positive in patients with SLE, Ro52 antibody positivity does not directly qualify as a marker for SLE. Notably, our patient did not have other clinical features suggestive of Sjogren's syndrome, and biopsy of the salivary glands was negative for Sjogren's syndrome.
Prior studies have highlighted the necessity of establishing distinct guidelines for individuals with ANA‐negative lupus, as such cases have been documented multiple times. 7 Successful diagnosis of these cases often hinges on the clinician's strong clinical suspicion. ANA positivity has both diagnostic and therapeutic implications in cases of SLE as ANA titers are often used to monitor therapeutic response and ANA‐positive patients show increased treatment responses to certain biologics compared to ANA‐negative patients. 11 Hence, incorporating ANA‐negative cases into clinical trials may help in understanding the heterogeneity of SLE and designing tailored approach for its management and monitoring of treatment response.
In this case, given the patient's thrombocytopenia and anemia, renal biopsy was delayed. As a result, a swift decision was made to administer methylprednisolone pulses without waiting for the biopsy. On the other hand, the clinical features of this patient were strongly positive for SLE and included malar rashes, photosensitivity, hair loss, and deterioration of renal function. The constellation of these symptoms, coupled with the patient's profile as a 25‐year‐old female, was another pointer toward the diagnosis of SLE, as SLE is mostly reported in young women of reproductive age. 18 Hence, a diagnosis of SLE was made purely based on clinical suspicion, and prompt treatment was administered, which in turn proved to be life‐saving to the patient.
This case report, however, has its limitations. Due to financial constraints to the patient, repeat testing of the antibodies was not performed, except for ANA, which was confirmed to be negative by both ELISA and later by immunofluorescence. Additionally, several studies have indicated that a cohort of patients with LN who are initially negative for ANA can exhibit seroconversion later in the disease course. 7 This potential for seroconversion remains unexplored in our patient. Further research regarding disease progression and dynamic changes in serological markers in a cohort of ANA‐negative SLE patients could help us draw definite conclusions regarding the intricacies of disease behavior and inform effective patient management strategies.
6. CONCLUSIONS
This case report underscores the challenges in diagnosing SLE, as its varied manifestations and the limitations of serological tests can lead to a diagnostic dilemma. While the recent EULAR/ACR classification criteria necessitate positive ANA as an entry criterion, this case highlights that relying solely on ANA positivity can lead to missed diagnoses, especially in patients with ANA‐negative SLE. Instead, a comprehensive approach considering clinical features, immunological markers, and patient demographics is crucial for accurate diagnosis. Early diagnosis and prompt management can be life‐saving in patients with LN.
AUTHOR CONTRIBUTIONS
Reechashree Dhungana: Conceptualization; data curation; formal analysis; methodology; project administration; visualization; writing – original draft; writing – review and editing. Bibhav Bashyal: Conceptualization; data curation; investigation; resources; supervision; validation. Sagar Paudel: Investigation; resources; supervision; validation. Bibek Shrestha: Data curation; formal analysis; writing – original draft. Saket Jha: Investigation; supervision; validation. Nishan Bhurtyal: Investigation; supervision; validation.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
DISCLOSURE
This case report has been presented as a poster in the IRACON 2023, that is, the 38th Annual Conference of the Indian Rheumatology Association.
ETHICS STATEMENT
The Institutional Review Board of the Institute of Medicine, Nepal, does not mandate ethical approval for the writing or publication of case reports, and patient consent was obtained. Informed written consent was obtained from the patient before writing this case report.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Dhungana R, Bashyal B, Paudel S, Shrestha B, Jha S, Bhurtyal N. Full‐house nephropathy in antinuclear antibody‐negative systemic lupus erythematosus: A case report. Clin Case Rep. 2024;12:e9231. doi: 10.1002/ccr3.9231
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Parikh SV, Almaani S, Brodsky S, Rovin BH. Update on lupus nephritis: Core curriculum 2020. Am J Kidney Dis. 2020;76(2):265‐281. http://www.ajkd.org/article/S0272638619311709/fulltext. [DOI] [PubMed] [Google Scholar]
- 2. Appel GB, Jayne D, Rovin BH. Lupus nephritis. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Elsevier/Saunders; 2015:303‐315. [Google Scholar]
- 3. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. https://pubmed.ncbi.nlm.nih.gov/9324032/. [DOI] [PubMed] [Google Scholar]
- 4. Petri M, Orbai A, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677‐2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oku K, Atsumi T, Akiyama Y, et al. Evaluation of the alternative classification criteria of systemic lupus erythematosus established by systemic lupus international collaborating clinics (SLICC). Mod Rheumatol. 2018;28(4):642‐648. 10.1080/14397595.2017.1385154. [DOI] [PubMed] [Google Scholar]
- 6. Aringer M, Costenbader K, Daikh D, et al. European league against rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151‐1159. http://www.ncbi.nlm.nih.gov/pubmed/31383717. [DOI] [PubMed] [Google Scholar]
- 7. Simmons SC, Smith ML, Chang‐Miller A, Keddis MT. Antinuclear antibody‐negative lupus nephritis with full house nephropathy: a case report and review of the literature. Am J Nephrol. 2016;42(6):451‐459. 10.1159/000443747 [DOI] [PubMed] [Google Scholar]
- 8. Calixto OJ, Franco JS, Anaya JM. Lupus mimickers. Autoimmun Rev. 2014;13(8):865‐872. [DOI] [PubMed] [Google Scholar]
- 9. Tan EM, Feltkamp TEW, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40(9):1601‐1611. 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- 10. Choi MY, Clarke AE, St. Pierre Y, et al. Antinuclear antibody–negative systemic lupus erythematosus in an international inception cohort. Arthritis Care Res. 2019;71(7):893‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16(10):565‐579. https://www.nature.com/articles/s41584‐020‐0480‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blomjous FJEM, Feltkamp‐Vroom TM. Hidden anti‐nuclear antibodies in sero‐negative systemic lupus erythematosus patients and in NZB and (NZB × NZW) F1 mice. Eur J Immunol. 1971;1(5):396‐398. 10.1002/eji.1830010519. [DOI] [PubMed] [Google Scholar]
- 13. Persellin R, Takeuchi A. Antinuclear antibody‐negative systemic lupus erythematosus: loss in body fluids. J Rheumatol. 1980;7:547‐550. [PubMed] [Google Scholar]
- 14. Lewis EJ, Schwartz MM, Korbet SM. Severe lupus nephritis: importance of re‐evaluating the histologic classification and the approach to patient care. J Nephrol. 2001;14(4):223‐227. https://europepmc.org/article/med/11506244. [PubMed] [Google Scholar]
- 15. Tektonidou MG, Dasgupta A, Ward MM. Risk of end‐stage renal disease in patients with lupus nephritis, 1971–2015: a systematic review and Bayesian meta‐analysis. Arthritis Rheumatol. 2016;68(6):1432‐1441. 10.1002/art.39594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lloyd W, Schur PH. Immune complexes, complement, and anti‐DNA in exacerbations of systemic lupus erythematosus (SLE). Medicine. 1981;60(3):208‐217. https://pubmed.ncbi.nlm.nih.gov/7231154/. [DOI] [PubMed] [Google Scholar]
- 17. Kvarnström M, Dzikaite V, Ottosson L, Gunnarsson I, Svenungsson E, Wahren‐Herlenius M. Autoantibodies to the functionally active RING‐domain of Ro52/SSA associate with clinical activity in a subset of patients with lupus. Ann Rheum Dis. 2012;71(Suppl 1):A40. [Google Scholar]
- 18. Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2002;16(5):847‐858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.


