Abstract
Aleutian mink disease parvovirus (ADV) is the etiological agent of Aleutian disease of mink. Several ADV isolates have been identified which vary in the severity of the disease they elicit. The isolate ADV-Utah replicates to high levels in mink, causing severe Aleutian disease that results in death within 6 to 8 weeks, but does not replicate in Crandell feline kidney (CrFK) cells. In contrast, ADV-G replicates in CrFK cells but does not replicate in mink. The ability of the virus to replicate in vivo is determined by virally encoded determinants contained within a defined region of the VP2 gene (M. E. Bloom, J. M. Fox, B. D. Berry, K. L. Oie, and J. B. Wolfinbarger. Virology 251:288–296, 1998). Within this region, ADV-G and ADV-Utah differ at only five amino acid residues. To determine which of these five amino acid residues comprise the in vivo replication determinant, site-directed mutagenesis was performed to individually convert the amino acid residues of ADV-G to those of ADV-Utah. A virus in which the ADV-G VP2 residue at 534, histidine (H), was converted to an aspartic acid (D) of ADV-Utah replicated in CrFK cells as efficiently as ADV-G. H534D also replicated in mink, causing transient viremia at 30 days postinfection and a strong antibody response. Animals infected with this virus developed diffuse hepatocellular microvesicular steatosis, an abnormal accumulation of intracellular fat, but did not develop classical Aleutian disease. Thus, the substitution of an aspartic acid at residue 534 for a histidine allowed replication of ADV-G in mink, but the ability to replicate was not sufficient to cause classical Aleutian disease.
Aleutian mink disease parvovirus (ADV) causes both chronic and acute diseases in mink. The chronic disease, termed Aleutian disease (AD), is associated with a persistent infection of adult mink and is characterized by hypergammaglobulinemia, plasmacytosis, increased CD8+ lymphocytes and an immune complex disorder (10). Affected animals maintain viremia and high levels of antiviral antibodies throughout the course of disease. Macrophages have been identified as sites of restricted virus replication, and infection of these cells is thought to lead to the immune disturbances (2, 33, 34). The acute disease is a fulminant, fatal interstitial pneumonitis resulting from permissive ADV infection of type II alveolar cells in newborn mink. In addition, milder forms of both diseases have been reported and inapparent infections have been recognized (3, 5, 6, 10, 24).
Although host factors contribute to the outcome of ADV infections, the major determinants of disease variability and severity are virally encoded (8, 9, 14, 37). Highly virulent isolates of ADV such as ADV-Utah and ADV-TR cause severe disease in both newborn and adult mink of either the Aleutian or non-Aleutian genotypes, but have not been successfully propagated in cell culture (1, 4, 25, 37). In contrast, ADV-G does not replicate to detectable levels in adult mink of either genotype, but does replicate permissively in cultures of Crandell feline kidney (CrFK) cells (1, 4, 14, 37). Thus, the ability of ADV to replicate either in vitro or in vivo is regulated by sequences within the viral genome.
The development of full-length infectious molecular clones of ADV-G has greatly facilitated attempts to identify virally encoded host range and pathogenicity determinants (7–10). Subgenomic clones have been used to determine the ADV-Utah sequence and to construct chimeric viruses between ADV-G and ADV-Utah in an attempt to identify regions of the viral genome responsible for encoding host range and/or replication determinants (8, 9). Experiments with these chimeras map sequences governing in vitro and in vivo viral replication to the VP2 capsid gene (8, 9).
Recent work has identified two chimeric ADV viruses, G/U-8 and G/U-10, that are capable of replicating both in vitro and in vivo (9). Both of these viruses contain a short segment of the ADV-Utah VP2 gene (corresponding to amino acid residues 360 to 589) substituted into the ADV-G genome. Both induce viremia, anti-ADV antibodies, and typical but mild pathological changes. This segment of VP2 is the minimal ADV-Utah VP2 region necessary to impart in vivo replication competence to ADV-G. The G/U-8 virus replicated better in vivo, inducing higher antibody titers and persistent viremia, whereas the G/U-10 virus produced only transient viremia (9). The G/U-8 virus contains an additional VP2 mutation, I352V, and a small segment of the ADV-Utah NS1 protein not present in G/U-10. The G/U-8 and G/U-10 viruses are the first molecularly cloned ADVs that can replicate both in vitro and in vivo.
In this study, we prepared site-directed mutants of ADV-G to determine how substitutions at defined locations in the VP2 protein affected in vivo replication. We tested each virus for the ability to replicate in vitro, and those that replicated in cell culture were injected into mink. The ability of each mutant virus to induce viremia, an antibody response, and pathology was compared to those of ADV-Utah and G/U-10.
MATERIALS AND METHODS
Viruses, cells, and plasmids.
Replication-competent mutant viruses were propagated and assayed in CrFK cells as previously described (8). The in vivo propagation and assay of ADV-Utah in adult Aleutian genotype (sapphire) mink have also been described previously (37).
Cloning and mutagenesis.
Full-length molecular clones were transformed and amplified in Escherichia coli JC8111 (recBCsbcrecF) by standard techniques (7, 15, 31). Other plasmids used were maintained in either E. coli JM109 or Epicurian Coli XL1-Blue (Stratagene, La Jolla, Calif.). Maintenance of the unstable right-hand hairpin was determined by restriction enzyme analysis as previously described (8, 9).
All site-directed mutagenesis was performed with the QuickChange Site-Directed Mutagenesis kit (Stratagene). The oligonucleotide primers used and their corresponding mutations are shown in Fig. 1. The mutated primers were designed so that each construct also contained noncoding changes that either introduced or eliminated a restriction enzyme recognition site.
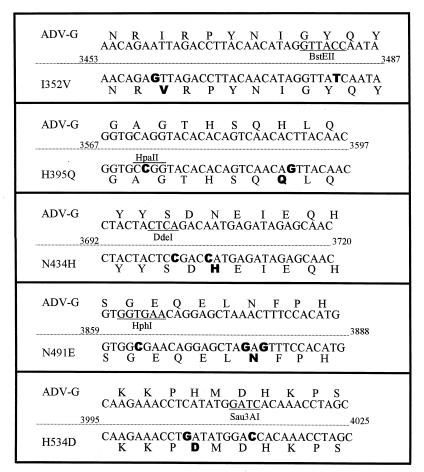
FIG. 1.
Site-directed mutagenesis of ADV-G. The top line in each box shows the ADV-G VP2 amino acid sequence with the nucleotide sequence below it. In the bottom of the box are the corresponding sequences of the oligonucleotides used for mutagenesis. The mutagenic nucleotides are shown in boldface type. Restriction enzyme recognition sequences altered during mutagenesis (for screening) are designated. In construction of the H395Q mutant, the HpaII site was created, and in all other mutants, the displayed restriction enzyme recognition site was destroyed during mutagenesis.
To facilitate mutagenesis, the EcoRI-HindIII fragment of ADV-G was cloned into pBluescript II SK(+) (Stratagene). This clone was denoted p33F-11. Mutagenesis was performed on p33F-11, and mutations were confirmed by restriction enzyme digestion and DNA sequence analysis. The mutated EcoRV-HindIII fragments (contained within the EcoRI-HindIII fragment) were excised and subcloned back into the clone pIC4-2. Clone pIC4-2 is a full-length ADV-G clone (7) in which the EcoRV-HindIII region has been replaced with the 32-bp EcoRV-HindIII fragment from pBR322. The final clones were transformed into E. coli JC8111, amplified, and purified with Wizard Maxiprep kits (Promega, Madison, Wis.). The integrity of the DNA was assessed by agarose gel electrophoresis, and the concentration was determined spectrophotometrically. The DNA was aliquoted and stored in ethanol at −70°C.
Individual 35-mm-diameter culture dishes of CrFK cells were transfected with 5 μg of purified plasmid DNA and incubated at 31.8°C for 5 days (7, 8). Cell pellets were collected in 1 ml of medium, freeze-thawed three times, sonicated, and passaged to T-25 culture flasks of CrFK cells. This process was repeated three more times with the final two passages being performed in T-150 culture flasks. Upon harvesting of the final passage, the cells were scraped into 5 ml of PBBS, freeze-thawed three times, sonicated, and centrifuged at 4,000 rpm for 20 min in a Beckman J-6 centrifuge. The supernatants were collected, aliquoted, and stored at −20°C. Viruses were titrated by infecting CrFK cells with supernatants and using immunofluorescent microscopy with a fluorescein-conjugated polyclonal anti-ADV mink serum as previously described (9).
In vivo infectivity and pathogenicity.
Adult sapphire (Aleutian genotype) mink (Mustela vison) obtained from an ADV-negative mink farm were housed in modified primate cages within sheds as previously described (37). All experimental procedures involving animals were performed under the guidelines of the Rocky Mountain Laboratories Animal Care and Use Committee, and animals were anesthetized with ketamine-acepromazine for all procedures.
Two weeks prior to injection, the animals were distributed into physically separated groups of four. The ADV-Utah group was housed in a separate shed. Immediately prior to injection, sera (day 0) were collected. The mink were injected with 104 focus-forming units (FFU) of in vitro-propagated viruses. The positive control group was injected with 105 50% mink infectious doses of ADV-Utah. Control mink were injected with CrFK cell lysates in 0.5 ml of PBBS diluent. Animals were bled at 10, 30, 60, 90, and 120 days postinjection (dpi). Sera were obtained by jugular venipuncture into Vacutainer Plus plastic centrifuge tubes containing SST gel and Clot Activator (Becton-Dickinson).
Serological responses to ADV infections were assessed by counterimmune electrophoresis (13, 37) and determination of the percent serum gamma globulin level by serum protein electrophoresis with a horizontal thin-layer agarose gel system (Ciba-Corning). The change in serum gamma globulin between 0 and 120 dpi was determined by laser densitometry (8).
The presence of viremia was determined with serum-based PCR (12, 37). This assay, which amplifies a 692-bp fragment in the VP2 region of the genome, can detect <1 fg of viral DNA (<10 genomes) in 2.5 μl of serum (37).
Tissue blocks of liver, spleen, kidney, and mesenteric lymph node collected at necropsy were formalin fixed, embedded in paraffin, sectioned, and stained with hematoxylin-eosin according to standard histological procedures. The slides were evaluated for the presence of microscopic lesions (22, 23, 26).
RESULTS
Site-directed mutagenesis of ADV-G.
Previous studies demonstrated that control of ADV replication in vivo localizes to a region of the VP2 protein (9). Analysis of a sequence alignment within the defined replication region revealed five amino acid residues that were highly conserved among pathogenic isolates and that differed from ADV-G VP2: amino acid residues 352, 395, 434, 491, and 534 (Fig. 2). These five residues were selected for site-directed mutagenesis by a PCR-based method (Fig. 1). Mutant viruses, which contained single ADV-Utah amino acid residues in the ADV-G background, were constructed as detailed in Materials and Methods.
FIG. 2.
ADV VP2 protein. (A) Schematic of the VP2 protein illustrating the location of the previously defined in vivo replication determinant present in the G/U-10 and G/U-8 viruses (9). aa, amino acids. (B) Protein sequence alignment of VP2 amino acid residues 341 to 590 of ADV-G and other pathogenic ADV isolates Utah (Ut), Tucker (Tr), Pullman (Pu), Utah1 kit (U1k), and Zk8. Identical residues are shown as dots, while divergent residues are shown in the single letter code. Arrows indicate the amino acid residues targeted for site-directed mutagenesis in this study.
In vitro analysis of site-directed mutant ADV clones.
In order to assess in vitro replication competence, the mutant DNA clones were individually transfected into CrFK cells. The lysates harvested from the initial transfections were blindly passaged three successive times in CrFK cells to determine if virus could be rescued from the clones and to amplify the viruses for subsequent analysis. After a total of four passages, the lysates were titrated on CrFK cells by fluorescent-antibody microscopy. Three of the site-directed mutants, I352V, N491E, and H534D, replicated to levels equivalent to those of ADV-G, achieving titers in the 107-FFU/ml range (Fig. 3A). However, two of the clones, H395Q and N434H, appeared replication defective and did not yield any virus at the end of four passages (Fig. 3A).
FIG. 3.
Replication of mutant ADV viruses in CrFK cells. (A) Titration of mutant viruses following DNA transfection and four passages in CrFK cells. The fourth-passage cell lysates were used to infect CrFK cells, and 3 days postinfection, the cells were examined for virus replication by fluorescent-antibody microscopy. (B) The parental ADV-G molecular clone along with each of the mutant molecular clones was transfected into CrFK cells and assayed 3 days later for virus replication by fluorescent-antibody microscopy. Mocks containing buffer only were used as negative controls.
To confirm that the H395Q and N434H mutants were replication defective, the study was repeated and the titers were determined at each passage level. Three days after DNA transfection, the cells were assayed for viral replication by fluorescent-antibody microscopy (Fig. 3B). All clones replicated to similar levels as the parental ADV-G clone. Western blot analysis of the lysates showed that each of the mutants, as well as the ADV-G parental virus, expressed the viral proteins NS1, VP1, and VP2 to similar levels (data not shown). Thus, all coding sequences were intact and our manipulations had not unexpectedly interrupted the sequences. However, upon subsequent passage of the primary cell lysates, virus could not be detected with H395Q and N434H mutants (data not shown). Thus, while these clones are capable of replicating when DNA is transfected into cells, they are not able to produce virus that is capable of initiating additional rounds of infection in vitro.
Infection of mink with mutant ADV viruses.
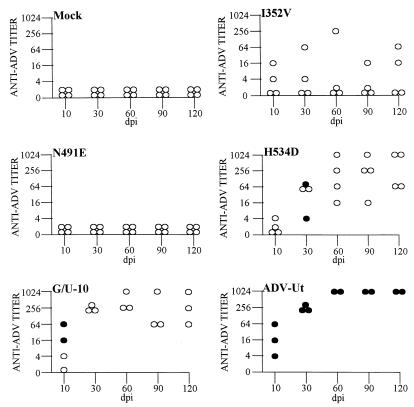
To determine if the replication-competent mutant viruses were infectious for mink, we injected adult Aleutian mink with the I352V, N491E, and H534D viruses. In vivo infectivity was assessed by testing for both viremia and antiviral antibody titers at 10, 30, 60, 90, and 120 dpi. Mink injected with either the mock control or N491E virus did not develop viremia or detectable viral antibody levels throughout the 120-day study (Fig. 4). Thus, the N491E virus acted like ADV-G (8, 9), indicating that the 491 residue alone did not confer in vivo replication competence. However, mink injected with I352V and H534D or G/U-10 and ADV-Utah positive controls showed signs of infection, as evidenced by antiviral antibodies and/or viremia (Fig. 4). Two of the four animals injected with the I352V virus developed an antibody response, but did not have a detectable viremia (Fig. 4). The H534D virus induced high titers of antiviral antibodies in all four animals within the group, and two of the animals had a transient viremia at 30 dpi (Fig. 4). The G/U-10 virus gave results similar to those previously published, in that all animals developed high antiviral antibody titers and two of the animals were transiently viremic at 10 dpi (Fig. 4). All animals injected with ADV-Utah became persistently viremic and demonstrated high antiviral antibody titers throughout the experimental study (Fig. 4).
FIG. 4.
Injection of mink with ADV mutant viruses. Mink were injected with mock, I352V, N491E, H534D, G/U-10, and ADV-Utah viruses and monitored for 120 dpi. At each time point, sera were collected and analyzed for the presence of viral antibody and viremia. Open circles represent antibody titers, and solid circles indicate viremia. Each circle represents one animal. One animal in each of the G/U-10 and ADV-Utah groups died of irrelevant causes early in the study.
To compare the viral antibody titers of the different virus groups, the geometric mean titers (GMTs) were calculated and plotted (Fig. 5). Clear trends were observed, but the small group size and individual variability between animals did not allow for statistical differences between all groups. The I352V virus, although variable, gave a final GMT of 1:6 at 120 dpi. The H534D and G/U-10 viruses gave relatively high GMTs, with both final titers being 1:257 at 120 dpi. ADV-Utah resulted in a characteristically high GMT with a final value of 1:1,024 at 120 dpi. Thus, the H534D virus is capable of replicating to equivalent levels as G/U-10 in vivo.
FIG. 5.
Geometric mean viral antibody titers. The GMT is plotted for each animal group at the time points of serum collection during the study. The standard mean error is displayed with error bars. The symbols and the corresponding viruses used to plot the graph are shown in the upper left-hand corner. The final GMT is printed on the right-hand side of the graph.
A hallmark of progressive AD is pronounced hypergammaglobulinemia. To determine if any of the viruses induced elevated gamma globulin levels, sera from each animal at 0 and 120 dpi were compared. Only ADV-Utah induced a hypergammaglobulinemia (Table 1). Thus, in spite of clear evidence of in vivo infectivity, the H534D and G/U-10 viruses did not induce the hypergammaglobulinemia characteristic of progressive AD.
TABLE 1.
Induction of hypergammaglobulinemiaa
| Virus | % Gamma globulin (SE)b
|
||
|---|---|---|---|
| Prebleed | Terminal bleed | Change | |
| Mock | 16.25 (4.15) | 15.78 (2.88) | −0.48 (2.72) |
| I352V | 13.63 (4.23) | 14.48 (4.48) | 0.85 (2.55) |
| N491E | 11.78 (4.58) | 13.40 (1.90) | 1.63 (3.67) |
| H534D | 14.43 (1.73) | 16.65 (2.65) | 2.23 (2.37) |
| G/U-10 | 5.50 (0.70) | 6.33 (3.77) | 0.80 (3.10) |
| ADV-Utah | 12.45 (0.95) | 38.65 (4.55) | 26.20 (3.60) |
Gamma globulin levels of prebleed and final bleed serums were determined as described in Materials and Methods.
Values represent gamma globulin levels as a percentage of the total serum proteins. Values in the parentheses indicate errors from the mean within each group.
Throughout the course of the study noticeable signs of infection were observed in the animals infected with ADV-Utah, G/U-10, and H534D. Weight loss, changes in pelt condition, and lethargy were evident in animals within these groups. The animals injected with the other mutant viruses did not show these clinical signs of infection. These results suggested that the animals infected with these three viruses were being impacted by the viral infections.
To more precisely determine if the animals had lesions typical of AD, the liver, spleen, kidney, and mesenteric lymph node, harvested at 120 dpi, were examined. The livers of animals infected with ADV-Utah showed typical AD pathology, including widespread lymphocyte and plasma cell infiltration (Fig. 6). In addition, plasma cell infiltration and mesangial thickening of the glomeruli within the kidney, erythropoiesis within the spleen, and infiltration of plasma cells within the paracortex of the mesenteric lymph nodes were all evident (data not shown).
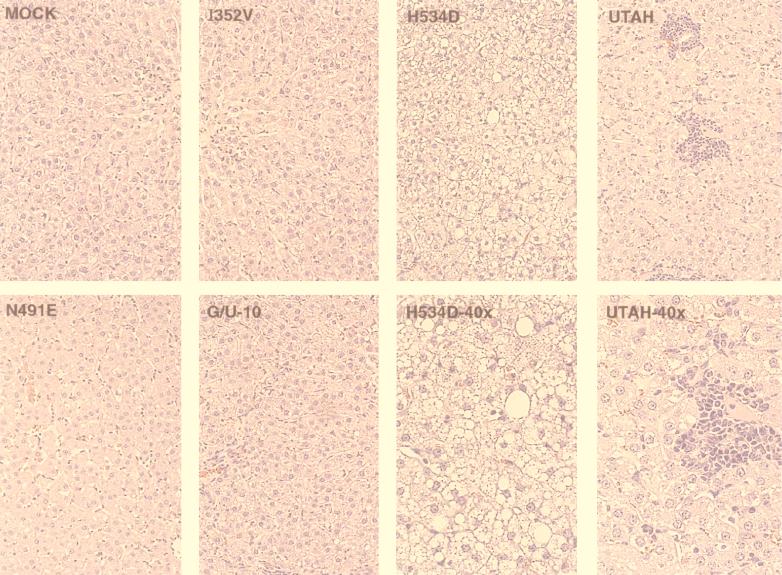
FIG. 6.
Liver pathology. Liver sections that have been hematoxylin and eosin stained are shown for a representative animal from each virus group. All pictures were taken at an original magnification of ×20, except for the H534D and Utah panels, which were taken at an original magnification of ×40. Notice the intracellular fat accumulation in the H534D virus frames.
Consistent with the previous report, the G/U-10 virus induced lesions typical of mild AD (9). These included mild lymphocytic infiltration of the liver portal tracts (Fig. 6) and paracortex of the mesenteric lymph nodes (data not shown). Animals injected with the mock control, I352V, and N491E viruses did not show any significant pathological findings in the livers (Fig. 6) or other tissues.
The tissues of H534D virus-infected animals did not show typical signs of AD, but the livers of these animals displayed microvesicular steatosis, a hepatocellular condition not previously reported with ADV and not seen in the livers of other animals in this study (Fig. 6). It was noted during necropsy of these animals that the livers were mildly swollen and yellow, with brown mottling over the entire external surface of the tissue. Thus, while the H534D mutant virus did not cause typical symptoms of AD, it induced a pathological change that had not been previously reported for ADV.
DISCUSSION
In this study, we identified a single amino acid residue, aspartic acid 534 (D534), that when present in ADV-G, results in a virus capable of inducing a persistent antibody response and transient viremia. The replication of this virus resembled that seen with the previously reported G/U-10 virus, an ADV-G-like virus containing four amino acid changes from ADV-Utah in the VP2 protein (9). The similar antibody response and transient viremia induced by both the H534D and G/U-10 viruses suggested that D534 is the major determinant within G/U-10 that allows it to replicate in vivo. If the additional three amino acid changes present in the G/U-10 VP2 protein play a role in in vivo replication, it is minimal. Furthermore, the data suggest that D534 is a major determinant for ADV-Utah replication in vivo.
The I352V virus induced a persistently low antibody response in two of the four animals injected. While this response was low relative to the H534D, G/U-10, or ADV-Utah viruses, it appeared relevant when compared to either the N491E or ADV-G (8, 9) viruses, neither of which induced an antibody response. The I352V mutation was the only difference within the VP2 protein between the previously reported G/U-8 and G/U-10 viruses (9). It is not known if the I352V VP2 mutation or three additional mutations in the NS1 protein are responsible for the enhanced in vivo replication and pathogenicity of G/U-8. However, our data suggested that the I352V mutation may be a reason the G/U-8 virus replicates better than the G/U-10 virus (9). Experiments are under way to determine if the I352V mutation is the reason for enhanced in vivo replication and to determine what role, if any, the mutations in the NS1 protein have on virus replication and pathogenicity.
In vivo replication of the H534D virus was transient as demonstrated by the lack of persistent viremia. Furthermore, H534D induced a lower overall antibody response than ADV-Utah. These differences from ADV-Utah suggest there are other determinants within ADV-Utah, not present in H534D, contributing to persistent virus replication. We are interested in mapping these differences and defining their role in ADV replication and pathogenicity. The differences may lie within the nonstructural proteins NS1 and/or NS2 or within other regions of the VP2 protein. There is precedent for determinants of parvovirus host range residing in both the nonstructural and structural proteins. The NS2 protein of minute virus of mice is responsible for determining its virus host range (16, 35). In addition, the NS1 protein and capsid proteins together determine the host range of porcine parvovirus (47). Finally, amino acid residues within the VP2 protein of both canine parvovirus and feline panleukopenia virus are responsible for determining the ability of the viruses to differentially replicate in various host cells (17, 30, 40, 41, 45, 46). There are few reports on the analysis of the nonstructural proteins of ADV with regard to host range or replication determinants. One report by Bloom et al. (8) demonstrated that replacement of the majority of the ADV-G NS1 and NS2 protein coding sequences with ADV-Utah sequences resulted in viruses that were ADV-G like (i.e., replicated in vitro but not in vivo). However, none of these viruses had H534D, a required VP2 residue needed for in vivo replication.
It is interesting to speculate about the possible mechanistic differences between ADV-Utah and H534D virus replication and disease induction. A hallmark of ADV infection is the pronounced immune disturbances that occur as a result of persistent virus infection (22, 23, 26). It is believed that many of the immune disturbances are a direct consequence of persistent ADV replication occurring within macrophages (2, 27, 33, 34). It is believed ADV gains entry into macrophages via Fc-Fc receptor-mediated interactions, a process termed antibody-dependent enhancement (21, 28, 38). Thus, antibodies raised against the virus assist the virus in entering the target cell where replication occurs. One difference between ADV-Utah and H534D may be a different immune response induced by the two viruses. Since ADV-Utah contains several additional amino acid differences in the VP2 protein, there may be significant antigenic or structural variation on the virion surface that results in a differing immune response relative to H534D. If the H534D virus induces a different response, the types of antibodies needed to infect macrophages and persistently replicate may not be generated. Recent work on the structure of ADV suggests many of these various amino acid residues are in regions of VP2 that are immunodominant (11, 32). Thus, it seems possible that changes in these regions could alter the antigenicity in a subtle fashion.
The H534D virus did not cause pathological lesions typical of AD. However, infected animals showed weight loss and lethargy and developed rough coats, indicating virus infection was having a negative impact on the animals. The livers of mink injected with this virus consistently displayed microvesicular steatosis (Fig. 6). This lesion, an accumulation of small fatty deposits within hepatocytes, has not been previously noted for ADV-infected animals and is distinct from ADV-Utah-induced lesions (Fig. 6). Microvesicular steatosis has been reported to be associated with influenza virus B infections of mice, murine adenovirus infections of SCID mice, and arctic squirrel hepatitis virus infections of arctic ground squirrels (19, 20, 42–44). These reports have compared the liver lesions resulting from the virus infections with Reye’s syndrome in humans. While microvesicular steatosis has not been reported to be associated with any parvovirus infections, associations between parvovirus infections and liver dysfunction have been reported (18, 29, 36, 39, 48). The human parvovirus B19 infection is associated with fulminant liver failure and associated aplastic anemia. In addition, the cellular receptor for B19 can be detected in liver tissue. It is interesting that the single H534D mutation in ADV-G allowed it to replicate in vivo in a similar manner to the G/U-10 virus, but the disease that each induces is dramatically different. The additional three amino acid mutations present in the G/U-10 virus, while not controlling in vivo replication, may be involved in classical AD induction. Another explanation is the possibility that the H534D mutation altered the host range of the virus to be hepatotropic.
Two of the mutants we constructed, H395Q and N434H, were unable to replicate in CrFK cells. Currently it is not understood why these viruses are replication defective in vitro. The transfected full-length clones of both viruses are capable of expressing the viral proteins NS1, VP1, and VP2 at similar levels to ADV-G in CrFK cells. However, it is not possible to infect subsequent cells from lysates of these primary transfected cells. While these mutations abolish CrFK infectivity when present alone, they do not abolish infectivity when present together with the other divergent amino acid residues in either the G/U-8 or G/U-10 viruses (9). Both the G/U-8 and G/U-10 viruses replicate, albeit poorly, in CrFK cells. Perhaps the structural alterations being formed by the presence of single mutations H395Q and N434H can be partly compensated for by the presence of other mutations. It is possible that these residues contribute to ADV in vitro replication competence and when present with the H534D mutation would result in a virus that behaves more like ADV-Utah. Currently studies are under way to determine where the infection cycle of these mutants is disrupted.
In summary, we have identified a single VP2 amino acid residue, D534, that plays a role in determining in vivo replication competence. The D534 residue confers on ADV-G the ability to replicate in mink and induce a persistent antibody response. This virus clone will provide a starting point to determine which other features of ADV-Utah contribute to its viral persistence and pathogenicity. In addition, the H534D virus causes a disease that results in lesions, microvesicular steatosis of the liver, not previously seen with ADV. A more detailed study of this virus’ replication and pathogenic potential will further our understanding of ADV replication and its relationship to viral pathogenicity.
ACKNOWLEDGMENTS
We thank William Hadlow for assistance in evaluating the pathological results in this study. We thank Cynthia Favara for the preparation, sectioning, and staining of tissue blocks. We thank James Wolfinbarger and Julie Buss for technical assistance and Dirk Whitsitt, Don Dale, and Ralph Larson for assistance with the care and handling of the animals.
Mink for this study were provided by the Mink Farmers Research Foundation.
REFERENCES
- 1.Alexandersen S, Bloom M E. Studies on the sequential development of acute interstitial pneumonia caused by Aleutian disease virus in mink kits. J Virol. 1987;61:81–86. doi: 10.1128/jvi.61.1.81-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandersen S, Bloom M E, Wolfinbarger J. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J Virol. 1988;62:1495–1507. doi: 10.1128/jvi.62.5.1495-1507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandersen S, Larsen S, Aasted B, Uttenthal A, Bloom M E, Hansen M. Acute interstitial pneumonia in mink kits inoculated with defined isolates of Aleutian mink disease parvovirus. Vet Pathol. 1994;31:216–228. doi: 10.1177/030098589403100209. [DOI] [PubMed] [Google Scholar]
- 4.Alexandersen S, Storgaard T, Kamstrup N, Aasted B, Porter D D. Pathogenesis of Aleutian mink disease parvovirus infection: effects of suppression of antibody response on viral mRNA levels and on development of acute disease. J Virol. 1994;68:738–749. doi: 10.1128/jvi.68.2.738-749.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An S H, DePauli F J, Wright P, Ingram D G. Characteristics of inapparent Aleutian disease virus infection in mink. Res Vet Sci. 1978;24:200–204. [PubMed] [Google Scholar]
- 6.An S H, Ingram D G. Detection of inapparent Aleutian disease virus infection in mink. Am J Vet Res. 1977;38:1619–1624. [PubMed] [Google Scholar]
- 7.Bloom M E, Alexandersen S, Garon C F, Mori S, Wei W, Perryman S, Wolfinbarger J B. Nucleotide sequence of the 5′-terminal palindrome of Aleutian mink disease parvovirus and construction of an infectious molecular clone. J Virol. 1990;64:3551–3556. doi: 10.1128/jvi.64.7.3551-3556.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloom M E, Berry B D, Wei W, Perryman S, Wolfinbarger J B. Characterization of chimeric full-length molecular clones of Aleutian mink disease parvovirus (ADV): identification of a determinant governing replication of ADV in cell culture. J Virol. 1993;67:5976–5988. doi: 10.1128/jvi.67.10.5976-5988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom M E, Fox J M, Berry B D, Oie K L, Wolfinbarger J B. Construction of pathogenic molecular clones of Aleutian mink disease parvovirus that replicate both in vivo and in vitro. Virology. 1998;251:288–296. doi: 10.1006/viro.1998.9426. [DOI] [PubMed] [Google Scholar]
- 10.Bloom M E, Kanno H, Mori S, Wolfinbarger J B. Aleutian mink disease: puzzles and paradigms. Infect Agents Dis. 1994;3:279–301. [PubMed] [Google Scholar]
- 11.Bloom M E, Martin D A, Oie K L, Huhtanen M E, Costello F, Wolfinbarger J B, Hayes S F, Agbandje-McKenna M. Expression of Aleutian mink disease parvovirus capsid proteins in defined segments: localization of immunoreactive sites and neutralizing epitopes to specific regions. J Virol. 1997;71:705–714. doi: 10.1128/jvi.71.1.705-714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloom M E, Oie K L, Christensen P, Durrant G. Evaluation of the polymerase chain reaction (pcr) as a tool for diagnosing infections with the Aleutian mink disease parvovirus (ADV) Scientifur. 1997;21:141–146. [Google Scholar]
- 13.Bloom M E, Race R E, Hadlow W J, Chesebro B. Aleutian disease of mink: the antibody response of sapphire and pastel mink to Aleutian disease virus. J Immunol. 1975;115:1034–1037. [PubMed] [Google Scholar]
- 14.Bloom M E, Race R E, Wolfinbarger J B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980;35:836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boissy R, Astell C. An Escherichia coli recBCsbc BrecF host permits the deletion-resistant propagation of plasmid clones containing the 5′-terminal palindrome of minute virus of mice. Gene. 1985;35:179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- 16.Brownstein D G, Smith A L, Johnson E A, Pintel D J, Naeger L K, Tattersall P. The pathogenesis of infection with minute virus of mice depends on expression of the small nonstructural protein NS2 and on the genotype of the allotropic determinants VP1 and VP2. J Virol. 1992;66:3118–3124. doi: 10.1128/jvi.66.5.3118-3124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang S-F, Sgro J-Y, Parrish C R. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J Virol. 1992;66:6858–6867. doi: 10.1128/jvi.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooling L L W, Koerner T A W, Naides S J. Multiple glycosphingolipids determine the tissue tropism of parvovirus B19. J Infect Dis. 1995;172:1198–1205. doi: 10.1093/infdis/172.5.1198. [DOI] [PubMed] [Google Scholar]
- 19.Davis L E. Influenza B virus model of Reye’s syndrome. Evidence for a nonpermissive infection of liver and brain. Lab Investig. 1987;56:32–36. [PubMed] [Google Scholar]
- 20.Davis L E, Cole L L, Lockwood S J, Kornfeld M. Experimental influenza B virus toxicity in mice. A possible model for Reye’s syndrome. Lab Investig. 1983;48:140–147. [PubMed] [Google Scholar]
- 21.Dworak L J, Wolfinbarger J B, Bloom M E. Aleutian mink disease parvovirus infection of K562 cells is antibody-dependent and is mediated via an Fc(gamma)RII receptor. Arch Virol. 1997;142:363–373. doi: 10.1007/s007050050082. [DOI] [PubMed] [Google Scholar]
- 22.Eklund C M, Hadlow W J, Kennedy R C, Boyle C C, Jackson T A. Aleutian disease of mink: properties of the etiologic agent and the host responses. J Infect Dis. 1968;118:510–516. doi: 10.1093/infdis/118.5.510. [DOI] [PubMed] [Google Scholar]
- 23.Hadlow W J. Pathologic lesions: they’re the worst kind. Vet Pathol. 1994;31:290–291. doi: 10.1177/030098589403100229. [DOI] [PubMed] [Google Scholar]
- 24.Hadlow W J, Race R E, Kennedy R C. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect Immun. 1983;41:1016–1023. doi: 10.1128/iai.41.3.1016-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn E C, Ramos L, Kenyon A J. Expression of Aleutian mink disease antigen in cell culture. Infect Immun. 1977;15:204–211. doi: 10.1128/iai.15.1.204-211.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmboldt C F, Jungherr E L. The pathology of Aleutian disease in mink. J Am Vet Med Assoc. 1958;29:212–222. [PubMed] [Google Scholar]
- 27.Kanno H, Wolfinbarger J B, Bloom M E. Identification of Aleutian mink disease parvovirus transcripts in macrophages of infected adult mink. J Virol. 1992;66:5305–5312. doi: 10.1128/jvi.66.9.5305-5312.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanno H, Wolfinbarger J B, Bloom M E. Aleutian mink disease parvovirus infection of mink peritoneal macrophages and human macrophage cell lines. J Virol. 1993;67:2075–2082. doi: 10.1128/jvi.67.4.2075-2082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langnas A N, Markin R S, Cattral M S, Naides S J. Parvovirus B19 as a possible causative agent of fulminant liver failure and associated aplastic anemia. Hepatology. 1995;22:1661–1665. [PubMed] [Google Scholar]
- 30.Llamas-Saiz A L, Agbandje-McKenna M, Parker J S L, Wahid A T M, Parrish C R, Rossmann M G. Structural analysis of a mutation in canine parvovirus which controls antigenicity and host range. Virology. 1996;225:65–71. doi: 10.1006/viro.1996.0575. [DOI] [PubMed] [Google Scholar]
- 31.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 32.McKenna R, Olson N H, Chipman P R, Baker T S, Booth T F, Christensen J, Aasted B, Fox J M, Bloom M E, Wolfinbarger J B, Agbandje-McKenna M. Three-dimensional structure of Aleutian mink disease parvovirus: implications for disease pathogenicity. J Virol. 1999;73:6882–6891. doi: 10.1128/jvi.73.8.6882-6891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori S, Wolfinbarger J B, Dowling N, Wei W, Bloom M E. Simultaneous identification of viral proteins and nucleic acids in cells infected with Aleutian mink disease parvovirus. Microb Pathog. 1991;9:243–253. doi: 10.1016/0882-4010(90)90013-g. [DOI] [PubMed] [Google Scholar]
- 34.Mori S, Wolfinbarger J B, Miyazawa M, Bloom M E. Replication of Aleutian mink disease parvovirus in lymphoid tissues of adult mink: involvement of follicular dendritic cells and macrophages. J Virol. 1991;65:952–956. doi: 10.1128/jvi.65.2.952-956.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naeger L K, Cater J, Pintel D J. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990;64:6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naides S J, Karetnyi Y V, Cooling L L W, Mark R S, Langnas A N. Human parvovirus B19 infection and hepatitis. Lancet. 1996;347:1563–1564. doi: 10.1016/s0140-6736(96)90720-5. [DOI] [PubMed] [Google Scholar]
- 37.Oie K L, Durrant G, Wolfinbarger J B, Martin D, Costello F, Perryman S, Hogan D, Hadlow W J, Bloom M E. The relationship between capsid protein (VP2) sequence and pathogenicity of Aleutian mink disease parvovirus (ADV): a possible role for raccoons in the transmission of ADV infections. J Virol. 1996;70:852–861. doi: 10.1128/jvi.70.2.852-861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oleksiewicz M B, Wolfinbarger J B, Bloom M E. A comparison between permissive and restricted infections with Aleutian mink disease parvovirus (ADV): characterization of the viral protein composition at nuclear sites of virus replication. Virus Res. 1998;56:41–51. doi: 10.1016/s0168-1702(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 39.Pardi D S, Romero Y, Mertz L E, Douglas D D. Hepatitis-associated aplastic anemia and acute parvovirus B19 infection: a report of two cases and a review of the literature. Am J Gastroenterol. 1998;93:468–470. doi: 10.1111/j.1572-0241.1998.468_1.x. [DOI] [PubMed] [Google Scholar]
- 40.Parker J S L, Parrish C R. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J Virol. 1998;71:9214–9222. doi: 10.1128/jvi.71.12.9214-9222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parrish C R, Carmichael L E. Characterization and recombination mapping of an antigenic and host range mutation of canine parvovirus. Virology. 1986;148:121–132. doi: 10.1016/0042-6822(86)90408-3. [DOI] [PubMed] [Google Scholar]
- 42.Pirofski L, Horwitz M S, Scharff M D, Factor S M. Murine adenovirus infection of SCID mice induces hepatic lesions that resemble human Reye syndrome. Proc Natl Acad Sci USA. 1991;88:4358–4362. doi: 10.1073/pnas.88.10.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Lanier M, Davis L E, Blisard K S, Woodfin B M, Wallace J M, Caskey L S. Influenza A virus in the mouse: hepatic and cerebral lesions in a Reye’s syndrome-like illness. Int J Exp Pathol. 1991;72:489–500. [PMC free article] [PubMed] [Google Scholar]
- 44.Testut P, Renard C A, Terradillos O, Vitvitski-Trepo L, Tekaia F, Degott C, Blake J, Boyer B, Buendia M A. A new hepadnavirus endemic in arctic ground squirrels in Alaska. J Virol. 1996;70:4210–4219. doi: 10.1128/jvi.70.7.4210-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Truyen U, Evermann J F, Vieler E, Parrish C R. Evolution of canine parvovirus involved loss and gain of feline host range. Virology. 1996;215:186–189. doi: 10.1006/viro.1996.0021. [DOI] [PubMed] [Google Scholar]
- 46.Truyen U, Parrish C R. Canine and feline host ranges of canine parvovirus and feline panleukopenia virus: distinct host cell tropisms of each virus in vitro and in vivo. J Virol. 1992;66:5399–5408. doi: 10.1128/jvi.66.9.5399-5408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasudevacharya J, Compans R W. The NS and capsid genes determine the host range of porcine parvovirus. Virology. 1992;187:515–524. doi: 10.1016/0042-6822(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 48.Yoto Y, Kudoh T, Haseyama K, Suzuki N, Chiba S. Human parvovirus B19 infection associated with acute hepatitis. Lancet. 1996;347:868–869. doi: 10.1016/s0140-6736(96)91348-3. [DOI] [PubMed] [Google Scholar]