Abstract
Here we report a rare case of immunoglobulin G4 (IgG4)–related pleural disease diagnosed using a thoracoscopic pleural biopsy. A 66‐year‐old man was admitted to our hospital with right‐dominant bilateral pleural effusions and gradually worsening dyspnoea. Chest radiographs revealed right‐dominant pleural effusions, while chest computed tomography showed bilateral pleural effusions without parenchymal lesions. Although the bilateral pleural effusions were exudative with an increased number of lymphocytes, the definitive diagnosis was initially elusive. High IgG4 levels in the serum and pleural effusions were observed. A pathological evaluation of a right pleural biopsy specimen collected via video‐assisted thoracoscopic surgery showed fibrosis‐associated lymphoplasmacytic infiltration, 45–60 IgG4‐positive plasma cells per high‐power field, and an IgG4/immunoglobulin G ratio of 40%. Consequently, the patient was diagnosed with IgG4‐related pleural disease. The bilateral pleural effusions improved after corticosteroid therapy.
Keywords: corticosteroid, IgG4‐related pleural disease, pleural biopsy, video‐assisted thoracoscopic surgery (VATS)
Here we report a rare case of immunoglobulin G4 (IgG4)–related pleural disease diagnosed using a thoracoscopic pleural biopsy
INTRODUCTION
Immunoglobulin G4 (IgG4)‐related disease (IgG4‐RD) is a chronic disease that presents with inflammation and fibrosis of the involved tissue. 1 IgG4‐RD is characterized by IgG4‐positive plasma cells and lymphocyte infiltration into the tissues. 1 It is known to affect any organ system including the biliary tree, kidneys, retroperitoneum, prostate, aorta, pericardium, lungs, thyroid, lymph nodes, meninges, and skin. 2 In the lungs, it variably presents as interstitial pneumonia, an inflammatory pseudotumour, bronchial inflammation, and pleuritis. 2 Although pleuritis is a rare finding, several lines of evidence indicate that IgG4‐RD causes a proportion of cases of idiopathic pleural effusions. 2 Here we report a rare case of IgG4‐related pleural disease diagnosed via a pleural biopsy collected by video‐assisted thoracoscopic surgery (VATS).
CASE REPORT
A 66‐year‐old man was admitted to our hospital with right‐dominant bilateral pleural effusions and gradually worsening dyspnoea. One year before admission, he experienced subjective symptoms of submandibular gland swelling, leading to the diagnosis of IgG4‐RD. The submandibular gland swelling diminished spontaneously without treatment. On admission, his body temperature was 37.1°C. No swelling of the submandibular or parotid glands was noted. Breath sounds were diminished predominantly on the right, and no abnormal heart sounds were noted. Bilateral slightly indurated oedema from the dorsal surfaces of the feet was noted. The white blood cell count was 7480/μL, while the C‐reactive protein level was 0.19 mg/dL. The brain natriuretic protein level was not significantly elevated (24.9 pg/mL), T‐SPOT tuberculosis and collagen‐related antibody tests were negative, and tumour markers were within reference values. The albumin level was normal (4.0 g/dL), while renal function was slightly impaired (creatinine, 1.13 mg/dL). Right‐dominant pleural effusions were observed on chest radiography (Figure 1A). Chest computed tomography confirmed the presence of right‐dominant bilateral pleural effusions without parenchymal lesions (Figure 1B,C). A sample of the right pleural effusion was cloudy yellow with lymphocyte predominance (89.5%), and the total protein and lactic acid dehydrogenase levels were 5.2 g/dL and 84 U/L, respectively, indicating exudative type. A left pleural effusion sample showed comparable results. The adenosine deaminase (ADA) level was normal (30.4 IU/L). Bacterial culture, polymerase chain reaction analysis, and Mycobacterium tuberculosis culture results were all negative. No malignant cells were detected. High IgG4 levels were observed in the serum (240 mg/dL) and pleural effusion (394 mg/dL). Chest drainage of the right pleural effusion was performed to ease the dyspnoea. However, fluid retention was observed, and a right pleural biopsy was collected by VATS. A pathological evaluation revealed fibrosis‐associated lymphoplasmacytic infiltration (Figure 2A), 45–60 IgG4‐positive plasma cells per high‐power field, and an IgG4/IgG ratio of 40% (Figure 2B). Finally, IgG4‐RD was diagnosed. The bilateral pleural effusions improved following corticosteroid therapy (oral prednisolone [PSL] 35 mg/day) (Figure 1D). The dose was reduced to PSL 3 mg/day over the course of 1 year. However due to increasing pleural effusion volume and IgG4 levels, the dose was increased to PSL 15 mg/day, and the pleural effusion improved. The dose was reduced to PSL 7.5 mg/day as maintenance, and the pleural effusion did not worsen over the next 15 months.
FIGURE 1.
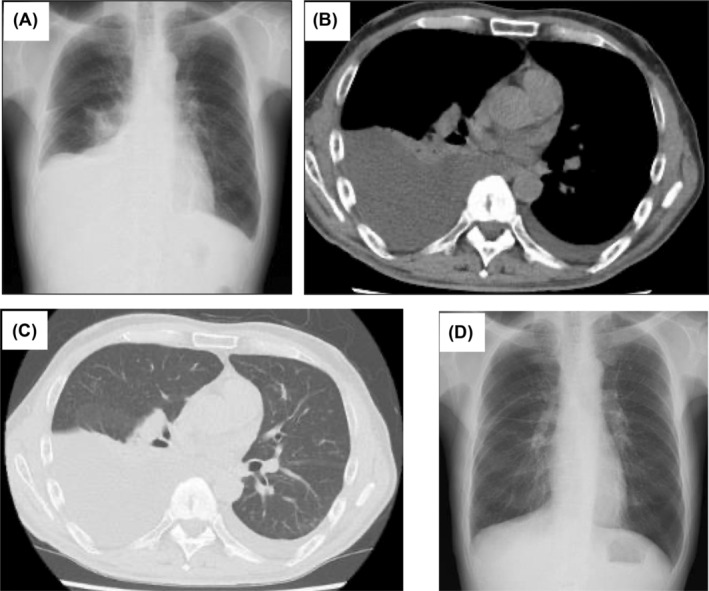
(A) Chest radiograph on admission showing right‐dominant bilateral pleural effusions. (B) and (C) Chest computed tomography scan showing right‐dominant bilateral pleural effusions without any parenchymal lesions. (D) The pleural effusion was improved on a chest radiograph after 1 month of corticosteroid therapy (35 mg/day oral prednisolone).
FIGURE 2.
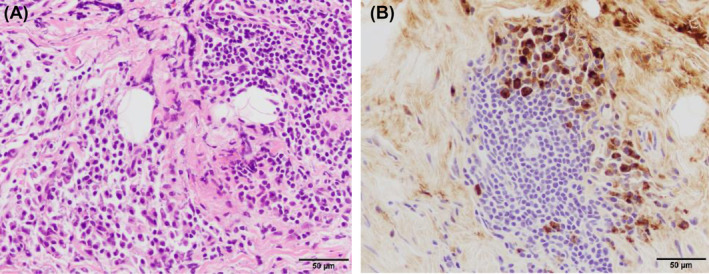
(A) Haematoxylin and eosin staining of pleural tissues collected via video‐assisted thoracoscopic surgery (VATS) showed fibrosis‐associated lymphoplasmacytic infiltration. (B) Immunostaining of pleural tissues via VATS showed 45–60 immunoglobulin G4 (IgG4)‐positive plasma cells per high‐power field and an IgG4/immunoglobulin G ratio of 40%.
DISCUSSION
Here we described a case of IgG4‐related pleural effusions diagnosed via histological evaluation. The case met the diagnostic criteria for IgG4‐RD. 3 Previous reports indicated that IgG4‐RD may exhibit periods of exacerbation and improvement or even remit spontaneously. 2 , 3 Similarly, in the case discussed herein, a lesion had initially appeared in the submandibular gland but resolved spontaneously. Lesions were subsequently observed in the pleura.
IgG4‐RD in the pleura reportedly comprises only 1.6% of all IgG4‐RD cases. 2 The pleural fluid is typically lymphocytic in nature. 2 , 4 Diagnosing IgG4‐RD can be difficult for several reasons: first, while an elevated serum IgG4 level is a diagnostic criterion, such levels are elevated in only 50% of active IgG4‐RD cases. 2 Conversely, serum IgG4 levels are also elevated in approximately 5% of healthy individuals. 2 Therefore, relying solely on serum IgG4 levels for the diagnosis is challenging. Second, IgG4‐RD often affects multiple organs. In such cases, the presence of extrapulmonary lesions may provide a diagnostic clue. However, IgG4‐related pleural effusions frequently lack extra‐ or intrapulmonary lesions, further complicating the diagnostic process. 2 Third, although an elevated ADA level is useful for detecting an IgG4‐related pleural effusion, it is important to note that the pleural‐fluid ADA level may also be elevated in other diseases. 2 , 4 Both tuberculous pleurisy and IgG4‐related pleural effusions present as lymphocytic pleural fluid and must be differentiated, particularly considering the high ADA level in tuberculous pleurisy. In contrast, in the present case, the ADA level was not elevated; rather, the diagnosis was based on a history of extrapulmonary IgG4‐RD and elevated serum and pleural‐fluid IgG4 levels. Therefore, although pleural‐fluid ADA levels can serve as a diagnostic adjunct, a comprehensive assessment incorporating other information is crucial to diagnostic accuracy.
Idiopathic IgG4‐related pleural effusions were recently reported. Murata et al. reported that 34% of idiopathic pleural effusions were associated with IgG4‐related pleurisy. 5 Therefore, IgG4 related pleurisy should be considered even in the absence of elevated serum IgG4 levels once other sources such as malignancy have been excluded. 2 , 5 Accordingly, an unexplained uni‐ or bilateral pleural effusion should be considered a possible sign of IgG4‐RD. High serum IgG4, pleural‐fluid IgG4, and pleural‐fluid ADA levels or extrapulmonary involvement may aid the diagnosis, but often none of these factors is present. Although recent reports suggested that IgG4 staining of cell blocks in pleural effusions is useful, 2 pathological analyses remain essential to archieving a difinitive diagnosis. Specifically, a pleural biopsy via VATS is considered valuable. In conclusion, this case underscores the importance of a proactive pathological evaluation using VATS when pleural IgG4‐RD is suspected.
AUTHOR CONTRIBUTIONS
Azusa Miyoshi and Hideki Katsura contributed substantially to writing the manuscript. Tomohiro Akaba, Mitsuko Kondo, and Etsuko Tagaya contributed substantially to its critical review. All authors have read and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and the accompanying images.
ACKNOWLEDGMENTS
Presented as an abstract at the ATS international meeting, May, 2020.
Miyoshi A, Katsura H, Akaba T, Kondo M, Tagaya E. IgG4‐related pleural disease diagnosed by thoracoscopic pleural biopsy: A case report. Respirology Case Reports. 2024;12(8):e01442. 10.1002/rcr2.1442
Associate Editor: Yet Hong Khor
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Stone JH, Zen Y, Deshpande V. IgG4‐related disease. N Engl J Med. 2012;366:539–551. [DOI] [PubMed] [Google Scholar]
- 2. Murata Y, Aoe K, Mimura Y. Pleural effusion related to IgG4. Curr Opin Pulm Med. 2019;25:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Handa T, Matsui S, Yamamoto H, Waseda Y, Iwasawa T, Johkoh T, et al. The 2022 revised diagnostic criteria for IgG4‐related respiratory diseases. Respir Investig. 2023;61:755–759. [DOI] [PubMed] [Google Scholar]
- 4. Saito Z, Yoshida M, Kojima A, Tamura K, Kuwano K. Characteristics of pleural effusion in IgG4‐related pleuritis. Respir Med Case Rep. 2020;29:101019. 10.1016/j.rmcr.2020.101019 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murata Y, Aoe K, Mimura‐Kimura Y, Murakami T, Oishi K, Matsumoto T, et al. Association of immunoglobulin G4 and free light chain with idiopathic pleural effusion. Clin Exp Immunol. 2017;190:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


