Abstract
Micro-Abstract:
Based on JAVELINBladder100 trial, avelumab maintenance was approved for patients with advanced urothelial carcinoma (aUC) without progression on first-line platinum-based chemotherapy. We examined patient characteristics, prognostic factors and outcomes in patients who received avelumab switch maintenance in a “real-world” (outside trials) setting. Our results appear similar with those from the clinical trial and recent “real world” studies.
Background:
Platinum-based chemotherapy (PBC) followed by avelumab switch maintenance in non-progressors is standard first line (1L) treatment for advanced urothelial carcinoma (aUC). We describe clinical features and outcomes in a ‘real-world’ cohort treated with avelumab maintenance for aUC.
Methods:
This was a retrospective cohort study of patients (pts) who received 1L switch maintenance avelumab after no progression on PBC for aUC. We calculated progression-free survival (PFS) and overall survival (OS) from initiation of maintenance avelumab. We also described OS and PFS for specific subsets using Cox regression and observed response rate (ORR).
Results:
A total of 108 pts with aUC from 14 sites treated with maintenance avelumab were included. There was a median of 6 weeks (1–30) from end of PBC to avelumab initiation; median follow-up time from avelumab initiation was 8.8 months (1–42.7). Median [m]PFS was 9.6 months (95%CI 7.5–12.1) and estimated 1-year OS was 72.5%. CR/PR (vs SD) to 1L PBC (HR=0.33, 95%CI 0.13–0.87) and ECOG PS 0 (vs ≥1), (HR=0.15, 95%CI 0.05–0.47) were associated with longer OS. The presence of liver metastases was associated with shorter PFS (HR=2.32, 95%CI 1.17–4.59). ORR with avelumab maintenance was 28.7% (complete response 17.6%, partial response 11.1%), 29.6% stable disease, 26.9% progressive disease as best response (14.8% best response unknown).
Conclusions:
Results seem relatively consistent with findings from JAVELIN Bladder100 trial and recent “real world” studies. Prior response to platinum-based chemotherapy, ECOG PS 0, and absence of liver metastases were favorable prognostic factors. Limitations include the retrospective design, lack of randomization and central scan review, and possible selection/confounding biases.
Keywords: avelumab, anti-PD(L)1, bladder cancer, urinary tract cancer, urothelial carcinoma
Introduction
Bladder cancer is one of the most common malignancies with estimated 81,180 new cases and 17,100 deaths in United States in 2022 (1). Advanced urothelial carcinoma (aUC) which is defined as locally advanced / unresectable, or metastatic UC, is the most aggressive stage and is associated with poor quality of life and short survival (2). Standard of care first line (1L) induction treatment is platinum-based chemotherapy (PBC) for platinum-eligible patients with aUC (3). This is followed by switch maintenance avelumab for those without progression on/after PBC.
The benefit of switch maintenance avelumab was demonstrated in the landmark phase 3 JAVELIN Bladder (JB) 100 trial, which investigated switch maintenance avelumab (anti-PD-L1) plus best supportive care (BSC) compared to BSC alone in patients with aUC without progression after 4–6 cycles of PBC (4, 5). Maintenance avelumab resulted in significantly prolonged overall survival (OS) and progression-free survival (PFS) leading to level I evidence and approval by regulatory agencies in several countries, including FDA approval on June 30, 2020 (6).
We conducted a multicenter retrospective cohort study to examine the “real word” (outside clinical trials) patient characteristics and clinical outcomes with avelumab switch maintenance. We also examined outcomes of specific subgroups to investigate potential prognostic factors. We hypothesized that our data would align with what was reported in the JB 100 trial.
Methods
Study design and data collection
This is a retrospective cohort study which has been conducted according to the STROBE guidelines for cohort studies (7). After obtaining institutional review board (IRB) approval and with respect to the Declaration of Helsinki ethical principles (8), we conducted a retrospective study including patients from academic centers from the United States and Europe. Eligible patients for this study were identified through a larger cohort, previously described, in which data of patients who received immune checkpoint inhibitor (ICI) for aUC have been collected (9–17). Each institution independently identified consecutive patients through provider-driven and electronic health record search algorithms. Data collection and storage was performed using the web-based, secure, and standardized REDCap capture tools hosted at the Institute of Translational Sciences at the University of Washington (18, 19). Data was collected using a pre-specified web-based form and included demographic/baseline characteristics, cancer-related information, treatment details and clinical outcomes. Assessment of response was based on the report of the investigators.
Eligibility Criteria
We included adult patients with aUC who received 1L switch maintenance avelumab after induction PBC (cisplatin or carboplatin-based regimens). Patients were excluded if they had received avelumab for progressive disease (PD) or in cases that they received other regimens between PBC and avelumab. Patients who received (neo)adjuvant ICI (as standard of care or experimental) were considered eligible if they received switch maintenance avelumab as standard of care. Patients with mixed urothelial histology were included, but those with pure non-urothelial carcinoma were excluded.
Endpoints
Primary endpoints included OS and PFS from the start of avelumab maintenance. For both OS and PFS, we investigated specific subsets and potential prognostic factors. In particular, we investigated the following subsets: sex (male or female), smoking history (ever or never smoker), primary tumor site (upper tract [ureter or renal pelvis] or lower tract [bladder or urethra]), histology (pure or mixed UC), platinum (cisplatin or carboplatin), number of cycles of PBC received (≤4 or >4), presence of liver metastases at PBC start (yes or no), ECOG PS at PBC start (0 or ≥1), best response to PBC (Complete response [CR]/partial response [PR] or stable disease [SD]), weeks from PBC end to avelumab initiation (≤3 or 4–10; >10 or 4–10). We also examined the response rate, disease control rate (SD), primary progression rate with avelumab maintenance and calculated the observed response rate (ORR) (PR+CR) as well as the reasons for avelumab discontinuation. Response to avelumab was investigator-assessed without central scan review.
Statistical Analysis
Baseline characteristics were summarized using descriptive statistics. We calculated OS and PFS using the Kaplan Meier method. OS was calculated from the initiation of avelumab maintenance until the date of death, and PFS was calculated from the date of avelumab initiation until the date of investigator-assessed clinical and/or radiographic disease progression or death. In both OS and PFS, patients who did not have an event were censored at the date of last follow-up. We also calculated the estimated OS rate at 1 year. Univariate cox proportional-hazards models were used for our subset analyses. All statistical analyses were performed using R version 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
We identified 108 eligible patients from a total of 1,514 patients in our database. Included patients were from 14 centers and had received 1L avelumab maintenance after no progression on PBC for aUC. Baseline characteristics can be found in Table 1. Median age was 69 years, most patients were White (92.6%), male (80.6%) with lower tract primary (85.2%); 71 patients (65.7%) received cisplatin-based chemotherapy. One patient had received neoadjuvant nivolumab for localized muscle invasive bladder cancer (MIBC) and two patients received adjuvant nivolumab for MIBC.
Table 1:
Baseline characteristics of patients who received avelumab maintenance.
| Overall population (N=108) n (%) | |
|---|---|
| Age of cancer diagnosis | 69 [31.3, 96.2] * |
| Sex | |
| Male | 87 (80.6) |
| Female | 21 (19.4) |
| Race | |
| White | 100 (92.6) |
| Not white | 6 (5.6) |
| Unknown | 2 (1.9) |
| Smoking history | |
| Yes | 63 (58.3) |
| No | 43 (39.8) |
| Missing | 2 (1.9) |
| Tumor site | |
| Lower urinary tract | 92 (85.2) |
| Upper urinary tract | 16 (14.8) |
| Pure UC histology | |
| Yes | 85 (78.7) |
| No | 23 (21.3) |
| ECOG PS at PBC start | |
| 0 | 51 (47.2) |
| 1 | 38 (35.2) |
| 2 | 3 (2.8) |
| Missing | 16 (14.8) |
| Cycles of 1L PBC | |
| >4 cycles | 62 (57.4) |
| ≤4 cycles | 42 (38.9) |
| Missing | 4 (3.7) |
| Liver metastases | |
| No | 95 (88) |
| Yes | 13 (12) |
| Weeks from last PBC to avelumab initiation | |
| ≤3 weeks | 18 (16.7) |
| 4–10 weeks | 76 (70.3) |
| >10 weeks | 14 (13) |
| Platinum agent | |
| Carboplatin | 37 (34.3) |
| Cisplatin | 71 (65.7) |
| Best response to PBC | |
| Complete Response | 18 (16.7) |
| Partial Response | 69 (63.9) |
| Stable Disease | 21 (19.4) |
PBC: Platinum Based Chemotherapy
Median [Min, Max]
At the time of PBC start, 13 (12%) patients had liver metastases, 51 (47.2%) had ECOG PS 0 and 41 (38%) ECOG PS ≥1 (16 unknown). The median time from avelumab maintenance initiation to the last follow up was 8.8 months (min-max: 1–42.7). The median time interval between the last chemotherapy dose and avelumab maintenance initiation was 6 weeks (min-max: 1–30). At the time of the analysis, 48 patients (44.4%) were still on avelumab maintenance and among the 60 (55.6%) patients who had discontinued avelumab, 46 (76.7%) had radiographic and/or clinical progression, 6 (10%) had a treatment-related adverse event (TRAE) and 8 (13.3%) discontinued treatment due to other reasons, including treatment holiday or completion, death, loss to follow up, and other unrelated to avelumab treatment (Table 2).
Table 2:
Response and outcomes to avelumab maintenance treatment
| (N=108) | |
|---|---|
| Best response to Avelumab maintenance | |
| Complete Response | 19 (17.6) |
| Partial Response | 12 (11.1) |
| Stable Disease | 32 (29.6) |
| Progressive Disease | 29 (26.9) |
| Unknown* | 16 (14.8) |
| Overall Response Rate (ORR) % | 28.7% |
| Reason of avelumab discontinuation | |
| Clinical Progression | 12 (11.1) |
| Radiographic Progression | 34 (31.5) |
| Toxicity | 6 (5.6) |
| Other | 8 (7.4) |
| Patient still on treatment | 48 (44.4) |
Most of those patients did not have response evaluation or were lost to follow-up
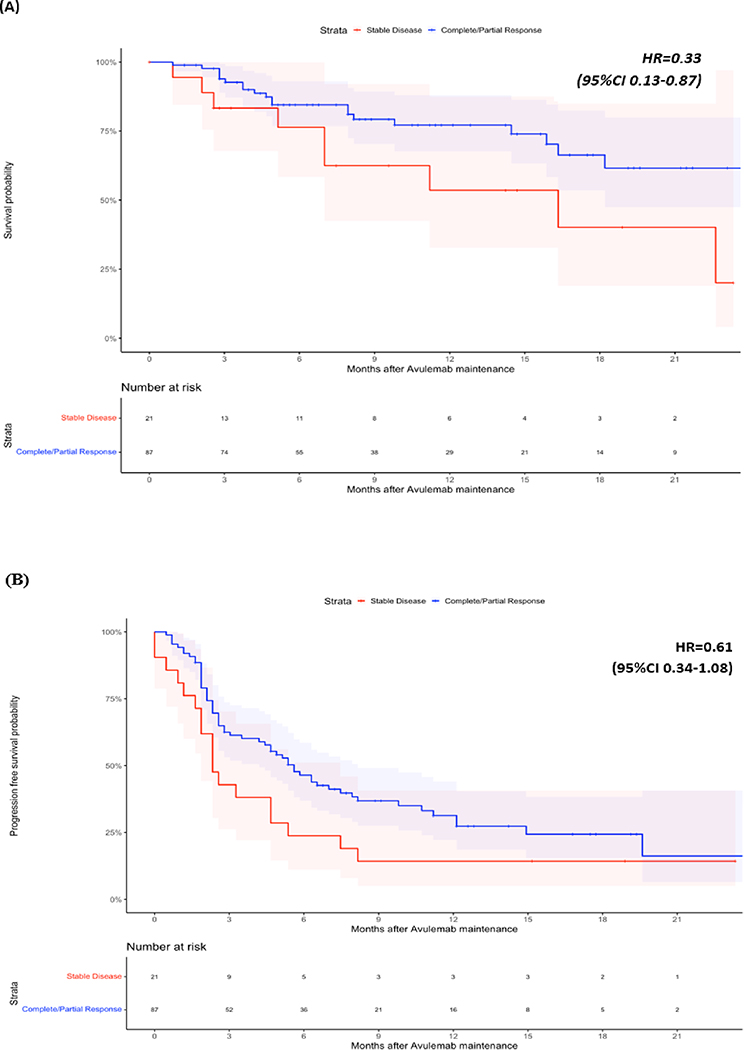
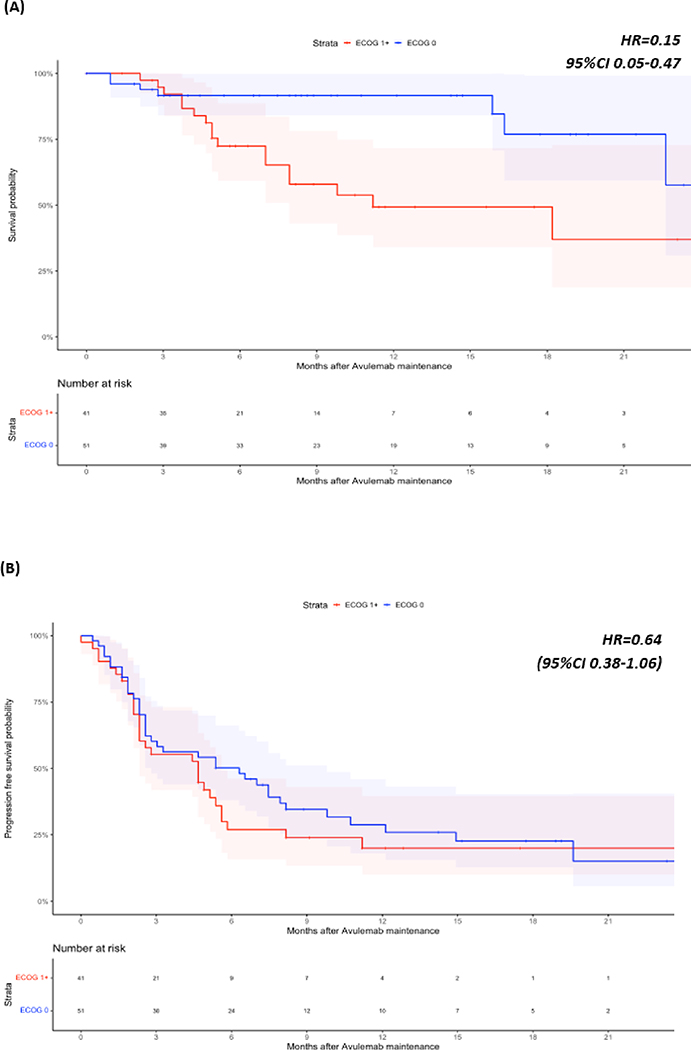
At last follow-up 76 (70.3%) patients were alive, 30 (27.8%) patients had died, and two (1.9%) patients had unknown vital status. Median OS was not reached, but the landmark OS rate at 1 year was 72.5% (CI: 63.2–83.1%), median PFS was 9.6 months (95%CI 7.5–12.1) (Figure 1). Results after examining specific subsets of interest regarding OS and PFS can be found in Table 3. We found that response to PBC (CR/PR) vs SD (HR=0.33, 95%CI 0.13–0.87) as best response to PBC as well as ECOG PS 0 vs ≥1 at PBC initiation (HR=0.15, 0.05–0.47) were associated with longer OS. We also found that patients with liver metastases at the time of PBC initiation had shorter PFS vs patients without liver metastases (HR=2.32, 95%CI 1.17–4.59) (Figures 2 and 3). Notably, response to PBC and ECOG PS did not have a statistically significant impact on PFS on avelumab. Sex, smoking history, site of primary tumor, pure vs mixed histology, type of platinum agent used, number of cycles of PBC and the time interval from PBC end to avelumab initiation were not significantly associated with OS or PFS.
Figure 1:
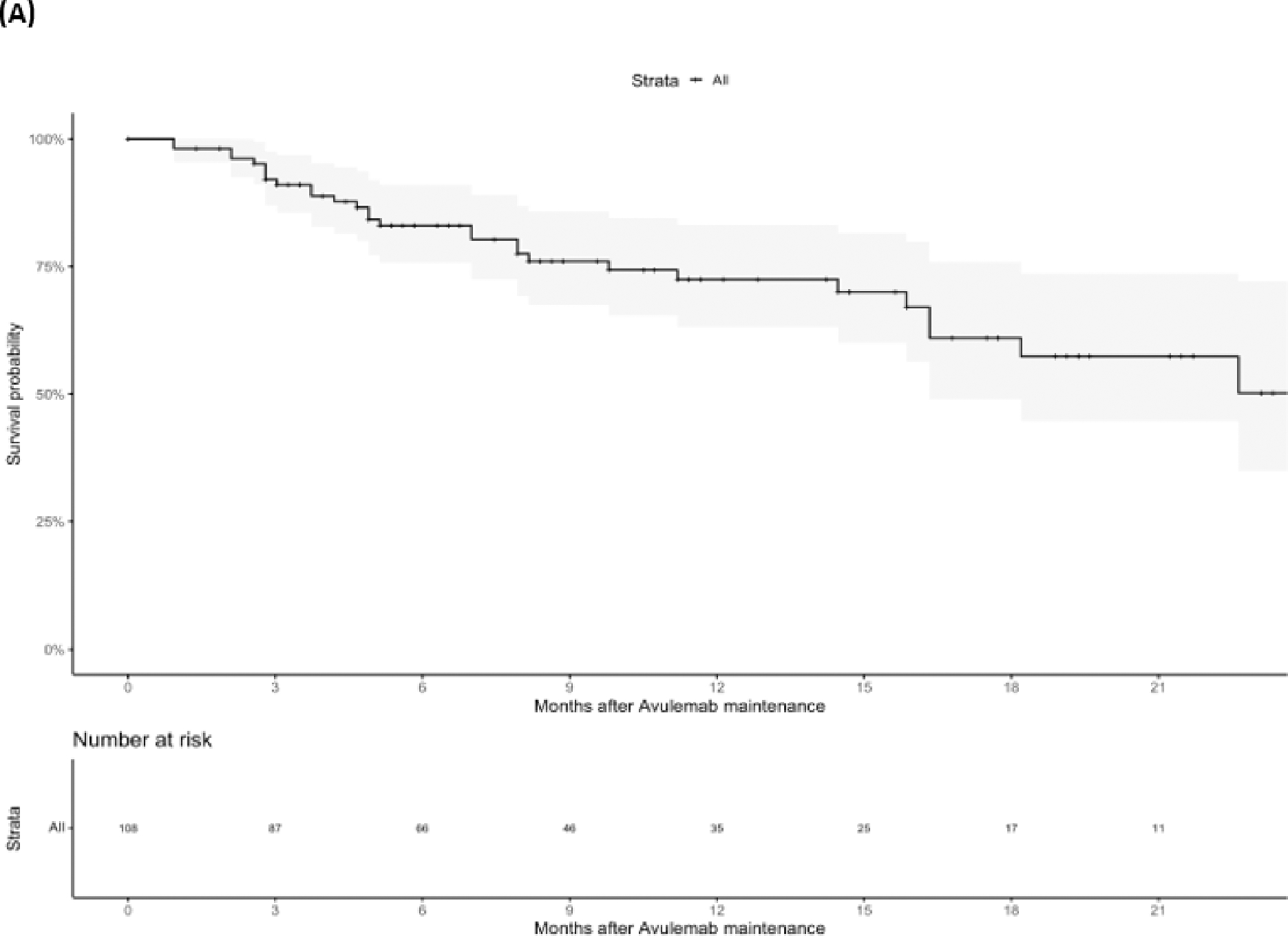
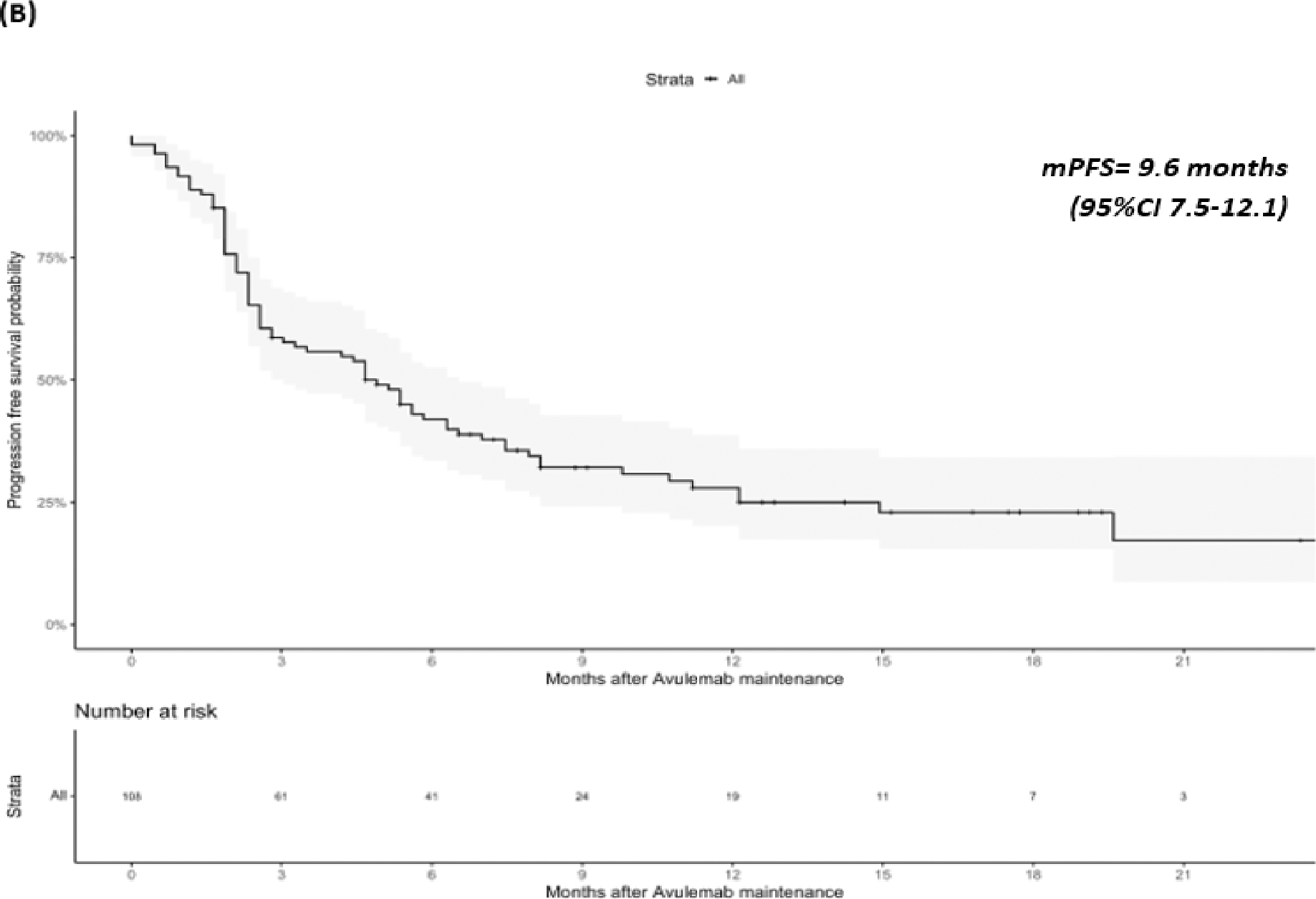
Overall Survival (OS) (A) and Progression Free Survival (PFS) (B) for the entire population.
Table 3:
Hazard ratios (HR) for Overall Survival (OS) and Progression-free survival (PFS) for specific subsets
| Variable | OS | PFS | ||
|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | |
| Sex (Female vs Male) | 1.76 | 0.68–4.50 | 1.29 | 0.70–2.36 |
| Smoking History (No vs Yes) | 0.70 | 0.27–1.78 | 0.94 | 0.57–1.55 |
| Upper vs Lower tract | 1.19 | 0.33–4.29 | 1.32 | 0.64–2.74 |
| Histology (pure vs mixed UC) | 1.09 | 0.37–3.21 | 0.91 | 0.52–1.57 |
| PBC Regimen (Cis vs Carbo) | 2.25 | 0.88–5.77 | 1.58 | 0.90–2.76 |
| Cycles of PBC (≤4 vs >4) | 0.80 | 0.30–2.15 | 1.13 | 0.67–1.91 |
| Liver mets at start of PBC (Yes vs No)* | 1.06 | 0.35–3.18 | 2.32 | 1.17–4.59 |
| ECOG PS (0 vs ≥1) at start of PBC* | 0.15 | 0.05–0.47 | 0.64 | 0.38–1.06 |
| Best response to PBC (CR/PR vs SD)* | 0.33 | 0.13–0.87 | 0.61 | 0.34–1.08 |
| Weeks from PBC end to avelumab initiation (≤3 vs 4–10) | 1.46 | 0.48–4.41 | 1.59 | 0.84–3.00 |
| Weeks from PBC end to avelumab initiation (>10 vs 4–10) | 0.59 | 0.13–2.75 | 0.44 | 0.19–1.05 |
CI: Confidence Interval, CR: Complete Response, ECOG PS: Eastern Cooperative Oncology Group Performance Status, Mets: Metastases, OS: Overall Survival, HR: Hazard Ratio, PBC: Platinum-based Chemotherapy, PFS: Progression-Free Survival, PR: Partial Response, SD: Stable disease
Significant variables (a = 0.05)
Figure 2:
Overall Survival (OS) (A) and Progression Free Survival (PFS) (B) of subjects stratified by best response to Platinum Based Chemotherapy.
Figure 3:
Overall Survival (OS) (A) and Progression Free Survival (PFS) (B) of subjects stratified by ECOG Performance Status (PS)
Among all 108 patients treated with avelumab maintenance, CR and PR as best observed response were reported in 19 (17.6%) patients and 12 (11.1%) patients, respectively (ORR 28.7% of all patients) (Table 2); 32 additional patients (29.6%) had SD and 29 patients (26.9%) had progression as best response. Response to avelumab maintenance was unknown for 16 patients due to mortality, loss to follow-up or no documented evaluation.
Discussion
In this multi-institutional retrospective study, we aimed to examine the “real-world” data of patients who received 1L avelumab switch maintenance after no progression on PBC for aUC. Our results support the key findings of the JB 100 trial findings, showing an estimated OS at 1 year (72.5% alive) but higher ORR and median PFS in patients with aUC who received avelumab maintenance relative to JB 100 trial data. Moreover, we found that response to PBC and ECOG PS 0 were associated with longer OS and the absence of liver metastases was associated with longer PFS in patients receiving avelumab maintenance. Our findings also align well with other “real world” studies discussed below, while a few others are ongoing, e.g. PATRIOT-II (20). Impaired ECOG PS (≥2) and liver metastases have been associated with worse outcomes according to a new survival prognostic model in patients with aUC, which align and lend credibility to our results (10).
In line with our findings (mPFS 9.6 months and 12-months OS 72,5%), results from other studies aiming to describe “real-world” data, such as READY and AVENANCE studies, corroborate the favorable outcomes and support the avelumab maintenance strategy (20–23). In the READY study in the Italian population, the 12-months PFS was 38.9% and the median PFS 6.6 months. The 12-month OS rate was 69.1% (95%CI 64.4%–73.6%) (22). Moreover, in the AVENANCE study in the French population, median PFS was 5.7 months and the 1-year OS rate 64.8% (23)
An important question has been whether the number of induction PBC cycles and platinum agent (cisplatin vs carboplatin) may affect outcomes with switch maintenance avelumab. According to our results, no significant difference was found between the number of cycles (≤4 vs >4 cycles) of PBC and survival with avelumab neither the platinum agent. In the same pattern, data from published studies did not show a significant association between the number of 1L PBC cycles and outcomes with maintenance avelumab (24, 25). The DISCUS trial (EudraCT number 2021–001975-17) in Europe will help elucidate if receiving 3 cycles of induction chemotherapy followed my switch maintenance avelumab results in better quality of life (based on patient-reported outcomes) whilst maintaining similar levels of efficacy compared to 6 cycles of induction PBC (26).
Interestingly, our study showed 28.7% ORR to avelumab, which is higher than the reported 9.7% ORR with avelumab in JB 100 (16 patients with no response evaluation were included in the denominator). This discrepancy may likely be attributed to the limitation of using retrospective chart review rather than central scan review to guide response assessment A recent Japanese study also examined “real-world” data with 1L avelumab maintenance after no progression on PBC in 27 patients (27). According to that study, 16 patients (59%) had disease control including CR, PR, or SD as best response (27). It is hard to ascertain in this setting whether response should be attributed to avelumab maintenance and/or to prior PBC. The JB 100 trial and ‘real-world’ studies (e.g. READY, AVENANCE), did not show significant PFS benefit based on best response to PBC (4, 22, 23).
In our study, only 6% of patients discontinued avelumab due to a TRAE, which aligns well with data from JB100 trial. However, we did not rigorously collect the incidence, type, and grade of TRAEs. Data regarding avelumab toxicity from the READY study reported that grade 3–4 TRAEs were observed in 7.1% (22). A similarly low rate of high grade TRAEs (10.3%) was reported in the AVENANCE study (23). The incidence of grade 3–4 TRAEs with avelumab monotherapy has been low, while rare events might occur (28). In the Japanese study, 12 patients (44%) experienced immune related adverse events of any grade; one patient (3.7%) had a grade ≥3 TRAE leading to avelumab discontinuation (27). Data from the JB 100 trial showed that the survival benefit with avelumab maintenance was not associated with significant detriment in patient reported outcomes and the quality of life of patients (25).
Data from the long-term follow up analysis of the JB 100 trial confirms the survival benefit in patients who received avelumab maintenance (29). Biomarker analysis investigated the potential use of several candidate biomarkers (e.g. PD-L1, TMB, APOBEC, other gene signatures, mutations in DNA damage response [DDR] etc.) as prognostic or predictive tools in this setting; however, such molecular biomarkers are not used in clinical practice for selection of patients for avelumab maintenance (30). Interestingly, despite the JB100 trial outcomes reported and the level I evidence, results from a recent abstract showed a relatively modest uptake of avelumab maintenance in practice (31); this may be possibly due to the awareness regarding the data and/or patient preference to avoid long-term therapy burden.
As switch maintenance has been identified as a successful treatment approach for patients with aUC, other agents have been investigated or are currently undergoing investigation in this setting. A randomized phase II trial investigating switch maintenance pembrolizumab after achieving at least SD on 1L induction PBC showed prolonged PFS with pembrolizumab vs BSC (median 5.4 vs 3 months, respectively HR=0.65) without significant OS benefit (32). ATLANTIS is a randomized multi-arm phase II biomarker-directed umbrella trial of 1L switch maintenance therapy after response or SD to induction PBC in aUC (33, 34). There was no benefit with switch maintenance cabozantinib vs placebo (33), whereas switch maintenance rucaparib showed longer median PFS vs placebo (35.3 vs 15.1 weeks) in patients with DDR gene alterations, which was a hypothesis-generating result (34). Other ongoing maintenance therapy trials include JAVELIN Bladder Medley, a phase Ib/II, multi-arm trial that aims to evaluate switch maintenance avelumab alone vs avelumab plus sacituzumab govitecan (anti-body drug conjugate against TROP-2), avelumab plus M6223 (an anti T-cell-immune-receptor) and avelumab plus NKTR-255 (a novel recombinant human IL-15) (35). The MAIN-CAV study is a phase III trial evaluating maintenance cabozantinib plus avelumab vs avelumab alone (36), and the TALASUR is a phase II trial investigating maintenance talazoparib (PARP-inhibitor) plus avelumab (37). These and other ongoing studies, e.g. TROPHY U-01 (NCT03547973) and PRESERVE 3 (NCT04887831) can help further refine the optimal switch maintenance strategy for aUC.
Limitations of our study include the moderate sample size, the relatively limited number of events and the retrospective design which is characterized by lack of randomization and of central scan review, with potential selection and confounding biases. Furthermore, the involvement of multiple institutions can lead to slightly different clinical practices (e.g. imaging scheduled intervals, which can affect PFS) as well as interpretation of scan review regarding therapy response or SD vs progression. Further, while our study aimed to investigate “real-world” outcomes, contributing sites are academic centers, so application of results to broader community practice settings may be relatively limited. We also did not evaluate quality of life, patient reported outcomes and molecular biomarker data.
Despite inherent limitations, to our knowledge this is one of the first analyses of “real world” data after the approval of 1L switch maintenance avelumab in aUC, aiming to complement clinical trial data. Our results support the data from JB100 and align with other recent “real world” studies.
Clinical Practice Points.
Avelumab has been approved in several countries with level I evidence as first line maintenance therapy in patients with advanced urothelial carcinoma (aUC) without progression on platinum-based chemotherapy (PBC) based on the JAVELINBladder100 (JB100) trial, which showed longer overall and progression-free survival (OS, PFS) with switch maintenance avelumab plus best supportive care (BSC) vs BSC alone.
We conducted a retrospective cohort study investigating patient characteristics, prognostic factors and outcomes in patients treated with avelumab switch maintenance after no progression on first line PBC for aUC in the “real-world” setting (outside clinical trials).
Our results support JB100 trial data; we noted higher response rate and longer median PFS (9.6 months) with avelumab maintenance and an estimated 73% OS rate at 1st year, which align with recent “real world” studies in this setting.
We also found that response (vs stable disease) to PBC, better performance status and absence of liver metastasis were favorable prognostic factors.
Limitations include retrospective nature, lack of randomization and of central scan review, as well as possible selection and confounding biases.
Acknowledgements
DR Bakaloudi acknowledges support from KureIt Cancer Research.
EY Yu and P Grivas acknowledges the Seattle Translational Tumor Research program.
David J. Pinato acknowledges grant funding from the Wellcome Trust Strategic Fund (PS3416) the AIRC MFAG Programme Grant and funding from the Cancer Treatment and Research Trust (CTRT) and acknowledges the infrastructure support provided by Imperial Experimental Cancer Medicine Centre and the Imperial College Healthcare NHS Trust Tissue Bank
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. RT was supported by training grant 5T32CA009515.
Abbreviations
- 1L
First Line
- APOBEC
Apolipoprotein B mRNA-editing enzyme catalytic polypeptide
- aUC
Advanced Urothelial carcinoma
- BSC
Best supportive care
- CI
Confidence Interval
- CR
Complete response
- DDR
DNA Damage Response
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- FDA
Food and Drug Administration
- HR
Hazard Ratio
- JB
JAVELIN Bladder
- IRB
Institutional review board
- ITT
Intent-to-treat
- MIBC
Muscle invasive bladder cancer
- Mo
Months
- ORR
Observed response rate
- OS
Overall survival
- PBC
Platinum-based chemotherapy
- PFS
Progression-free survival
- PR
Partial response
- SD
Stable disease
- TMB
Tumor mutational burden
- TRAE
Treatment-related adverse event
Footnotes
Declaration of Competing Interest
None.
Disclosure
Dimitra Rafailia Bakaloudi, Rafee Talukder, Genevieve Ihsiu Lin, Dimitrios Makrakis, Leonidas N. Diamantopoulos, Nishita Tripathi, Roubini Zakopoulou, James Korolewicz, Tanya Jindal, Vadim S. Koshkin, Jure Murgić, Marija Miletić, Jeffrey Johnson, David Marmorejo Castañeda, Lucia Alonso Buznego, Clara Castro Carballeira, Tyler Stewart, Andrew Thomas Ruplin: No conflicts to disclosure. Neeraj Agarwal (lifetime disclosures): No personal COIs since April 15, 2021. Consultancy to Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics. Research funding to Neeraj Agarwal’s institution: Arnivas, Astellas, Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Crispr, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Lava, Medivation, Merck, Nektar, Neoleukin, New Link Genetics, Novartis, Oric, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. Aristotelis Bamias: Honoraria, Advisory, Research support: AZ, MSD, BMS, Ipsen, Pfizer, Roche, Astellas Jason R. Brown: Received funding from EMD-Serono for a Speaker’s Bureau. David J Pinato received lecture fees from ViiV Healthcare, Bayer Healthcare, Astra Zeneca, Roche, IPSEN and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, Astra Zeneca, DaVolterra, Exact Sciences, MURSLA, Avamune, BMS; received research funding (to institution) from MSD, BMS, GSK. Ana Frōbe are following: Ana Fröbe has done consulting for Astellas, AstraZeneca, Janssen, Merck. Yousef Zakharia: Advisory Board: Bristol Myers Squibb, Amgen, Roche Diagnostics, Novartis, Janssen, Eisai, Exelixis, Castle Bioscience, Genzyme Corporation, Astrazeneca, Array, Bayer, Pfizer, Clovis, EMD serono, Myovant. Grant/research support from: Institution clinical trial support from NewLink Genetics, Pfizer, Exelixis, Eisai. DSMC: Janssen Research and Development.Consultant honorarium: Pfizer, Novartis. Alexandra Drakaki has served as consultant for Bristol-Myers Squibb, AstraZeneca, RADMETRIX, Seattle Genetics, Janssen, PACT Pharma, Merck, Roche/Genentech, Exelixis, Dyania Health, has received research funding from Kite/Gilead, AstraZeneca, Genentech/Roche, BMS, Merck Sharp & Dohme, Jounce Therapeutics, Infinity Pharmaceuticals, Seattle Genetics/Astellas, and has received travel expenses from Lilly, AstraZeneca and Seattle Genetics. Alejo Rodriguez-Vida has served as advisor for MSD, Pfizer, BMS, Astellas, Janssen, Bayer, Clovis, Ipsen and Roche has received honoraria or travel expenses from Pfizer, MSD, Astellas, BMS, Janssen, Astra Zeneca, Roche, Bayer, Ipsen and Sanofi Aventis, and has received research funding from Takeda, Pfizer, and Merck. Macarena Rey Cárdenas: Pfizer, Bayer. Pfizer; Pierre Fabre; Ipsen; BMS; AstraZeneca; MSD; Roche; Kyowa Kirin; Accord. Daniel Castellano: Consulting or Advisory Role: Janseen Oncology, Roche/Genentech, Astellas Pharma, AstraZeneca, Pfizer, Novartis, Ipsen, Bristol-Myers Squibb, MSD Oncology, Bayer, Lilly, Sanofi, Pierre Fabre, Bpehringer Ingelheim. Research funding: Janseen Oncology. Travel, Accomondations, Expenses: Pfizer, Roche, Bristol-Myers Squibb, AstraZeneca Spain. Ignacio Duran: Research Grant to institution: Roche, AstraZeneca, Honoraria: Bristol Myers Squibb, MSD, Ipsen, Roche-Genentech, Janssen, Astellas Pharma, EUSA Pharma, Bayer, Novartis, Gilead, Bayer. Support for attending meetings and/or travel: Merck-Pfizer, Ipsen, Jansen, Bayer, AstraZeneca. Advisory board: Bristol Myers Squibb, MSD, Ipsen, Roche-Genentech, Astellas Pharma, EUSA Pharma, Bayer, Novartis, Eisai, Debio Pharma, Pharmacyclycs, Gilead. Rafael Morales-Barrera has served as consultant/advisor for
Sanofi, AstraZeneca, Astellas Pharma and MSD, has been in the speaker’s Bureau for Astellas Pharma, Merck/Pfizer and MSD Oncology, and has received travel accommodations from Sanofi, Pfizer, MSD, Astellas Pharma, Astra Zeneca, Bayer, Roche/Genentech. Rana R. McKay: Consulting/Advisory Board – Aveo, AstraZeneca, Bayer, Bristol Myers Squib, Calithera, Caris, Denderon, Exelixis, Esiai, Janssen, Lilly, Merck, Myovant, Novartis, Pfizer, Sanofi, SeaGen, Sorrento Therapeutics, Telix, Tempus. Institutional Research Funding – Bayer, Tempus, AstraZeneca, Oncternal, Exelixis, BMS. Shilpa Gupta has received personal fees from Bristol Myers Squibb, Merck, Janssen, Seattle Genetics, EMD Sorono and Pfizer, and has received grants from Astellas, BMS and Bristol Myers Squibb. Evan Y. Yu has received research funding to his institution Bayer, Blue Earth, Daiichi Sankyo, Dendreon, Lantheus, Merck, Seagen, Taiho and received consulting fees from Abbvie, Advanced Accelerator Applications, Bayer, Clovis, Exelixis, Janssen, Merck, Sanof Petros Grivas: has done consulting for 4D Pharma, Aadi Bioscience, Astellas, Asieris Pharmaceuticals, AstraZeneca, BostonGene, Bristol Myers Squibb, CG Oncology, Dyania Health, Exelixis, Fresenius Kabi, Genentech, Gilead Sciences, Guardant Health, ImmunityBio, Infinity Pharmaceuticals, Janssen, Lucence, Merck KGaA, Mirati Therapeutics, MSD, Pfizer, PureTech, QED Therapeutics, Regeneron, Roche, Seattle Genetics, Silverback Therapeutics, Strata Oncology, UroGen Pharma; and has received institutional research funding from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm Group, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Merck KGaA, Mirati Therapeutics, MSD, Pfizer, QED Therapeutics. Ali Raza Khaki has received honoraria from OncLive/MJH Life Sciences, has owned stocks of Merck and Sanofi, and has had uncompensated relationships with Seattle Genetics/Astellas.
This research did not receive other external funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Flannery K, Cao X, He J, Zhong Y, Shah AY, Kamat AM. Survival Rates and Health Care Costs for Patients With Advanced Bladder Cancer Treated and Untreated With Chemotherapy . Clinical genitourinary cancer. 2018;16(4):e909–e17. [DOI] [PubMed] [Google Scholar]
- 3.Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, et al. Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2017;15(10):1240–67. [DOI] [PubMed] [Google Scholar]
- 4.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma . New England Journal of Medicine. 2020;383(13):1218–30. [DOI] [PubMed] [Google Scholar]
- 5.Powles T, Park SH, Caserta C, Valderrama BP, Gurney H, Ullén A, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. Journal of Clinical Oncology. 2023:JCO.22.01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA approves avelumab for urothelial carcinoma maintenance treatment 2020. [Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-avelumab-urothelial-carcinoma-maintenance-treatment#:~:text=On%20June%2030%2C%202020%2C%20the,%2Dline%20platinum%2Dcontaining%20chemotherapy.
- 7.Cuschieri S The STROBE guidelines. Saudi journal of anaesthesia. 2019;13(Suppl 1):S31–s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–4. [DOI] [PubMed] [Google Scholar]
- 9.Khaki AR, Li A, Diamantopoulos LN, Bilen MA, Santos V, Esther J, et al. Impact of performance status on treatment outcomes: A real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer. 2020;126(6):1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khaki AR, Li A, Diamantopoulos LN, Miller NJ, Carril-Ajuria L, Castellano D, et al. A New Prognostic Model in Patients with Advanced Urothelial Carcinoma Treated with First-line Immune Checkpoint Inhibitors. European Urology Oncology. 2021;4(3):464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makrakis D, Talukder R, Diamantopoulos LN, Carril-Ajuria L, Castellano D, De Kouchkovsky I, et al. Association of prior local therapy and outcomes with programmed-death ligand-1 inhibitors in advanced urothelial cancer. BJU international. 2022;130(5):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makrakis D, Talukder R, Lin GI, Diamantopoulos LN, Dawsey S, Gupta S, et al. Association Between Sites of Metastasis and Outcomes With Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma. Clinical genitourinary cancer. 2022;20(5):e440–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talukder R, Makrakis D, Lin GI, Diamantopoulos LN, Dawsey S, Gupta S, et al. Association of the Time to Immune Checkpoint Inhibitor (ICI) Initiation and Outcomes With Second Line ICI in Patients With Advanced Urothelial Carcinoma. Clinical genitourinary cancer. 2022;20(6):558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talukder R, Makrakis D, Diamantopoulos LN, Carril-Ajuria L, Castellano D, De Kouchkovsky I, et al. Response and Outcomes to Immune Checkpoint Inhibitors in Advanced Urothelial Cancer Based on Prior Intravesical Bacillus Calmette-Guerin. Clinical genitourinary cancer. 2022;20(2):165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esagian SM, Khaki AR, Diamantopoulos LN, Carril-Ajuria L, Castellano D, De Kouchkovsky I, et al. Immune checkpoint inhibitors in advanced upper and lower tract urothelial carcinoma: a comparison of outcomes. BJU international. 2021;128(2):196–205. [DOI] [PubMed] [Google Scholar]
- 16.Makrakis D, Bakaloudi DR, Talukder R, Lin GI, Diamantopoulos LN, Jindal T, et al. Treatment Rechallenge With Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma. Clinical genitourinary cancer. 2023;21(2):286–94. [DOI] [PubMed] [Google Scholar]
- 17.Miller NJ, Khaki AR, Diamantopoulos LN, Bilen MA, Santos V, Agarwal N, et al. Histological Subtypes and Response to PD-1/PD-L1 Blockade in Advanced Urothelial Cancer: A Retrospective Study. The Journal of urology. 2020;204(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivas P, Barata PC, Moon H, Hutson TE, Gupta S, Sternberg CN, et al. Baseline characteristics from a retrospective, observational, US-based, multicenter, real-world (RW) study of avelumab first-line maintenance (1LM) in locally advanced/metastatic urothelial carcinoma (la/mUC) (PATRIOT-II). Journal of Clinical Oncology. 2023;41(6_suppl):465-. [Google Scholar]
- 21.Bakaloudi DR, Talukder R, Makrakis D, Agarwal N, Tripathi N, Bamias A, et al. Response and outcomes of maintenance avelumab after platinum-based chemotherapy (PBC) in patients (pts) with advanced urothelial carcinoma (aUC): “Real world” experience. Journal of Clinical Oncology. 2023;41(6_suppl):472-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonuzzo L, Maruzzo M, De Giorgi U, Santini D, Tambaro R, Buti S, et al. READY: Real-world data from an Italian compassionate use program of avelumab first-line maintenance (1LM) treatment for locally advanced or metastatic urothelial carcinoma (la/mUC). Journal of Clinical Oncology. 2023;41(6_suppl):469-. [Google Scholar]
- 23.Barthelemy P, Loriot Y, Voog E, Eymard JC, Ravaud A, Flechon A, et al. Full analysis from AVENANCE: A real-world study of avelumab first-line (1L) maintenance treatment in patients (pts) with advanced urothelial carcinoma (aUC). Journal of Clinical Oncology. 2023;41(6_suppl):471-. [Google Scholar]
- 24.Grivas P, Park SH, Voog E, Caserta C, Gurney H, Bellmunt J, et al. Avelumab First-line Maintenance Therapy for Advanced Urothelial Carcinoma: Comprehensive Clinical Subgroup Analyses from the JAVELIN Bladder 100 Phase 3 Trial. European Urology. [DOI] [PubMed] [Google Scholar]
- 25.Grivas P, Kopyltsov E, Su P-J, Parnis FX, Park SH, Yamamoto Y, et al. Patient-reported Outcomes from JAVELIN Bladder 100: Avelumab First-line Maintenance Plus Best Supportive Care Versus Best Supportive Care Alone for Advanced Urothelial Carcinoma. European Urology. 2022. [DOI] [PubMed] [Google Scholar]
- 26.DISCUS - Cancer Research UK Barts Centre (bartscancer.london). A randomised phase II study comparing 3 vs 6 cycles of platinum-based chemotherapy prior to maintenance avelumab in advanced urothelial cance. [Google Scholar]
- 27.Miyake M, Shimizu T, Oda Y, Tachibana A, Ohmori C, Itami Y, et al. Switch-maintenance avelumab immunotherapy following first-line chemotherapy for patients with advanced, unresectable or metastatic urothelial carcinoma: the first Japanese real-world evidence from a multicenter study. Japanese Journal of Clinical Oncology. 2023;53(3):253–62. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Sharma N, Kazmierski D, Amjad MA, Dong Y, Wang Y, et al. Diffuse Alveolar Hemorrhage With Avelumab Maintenance Therapy. Cureus. 2021;13(6):e15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sridhar SS, Powles T, Gupta S, Climent MÁ, Aragon-Ching JB, Sternberg CN, et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): Long-term follow-up from the JAVELIN Bladder 100 trial in subgroups defined by 1L chemotherapy regimen and analysis of overall survival (OS) from start of 1L chemotherapy. Journal of Clinical Oncology. 2023;41(6_suppl):508-.36206505 [Google Scholar]
- 30.Powles T, Sridhar SS, Loriot Y, Bellmunt J, Mu XJ, Ching KA, et al. Avelumab maintenance in advanced urothelial carcinoma: biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nature Medicine. 2021;27(12):2200–11. [DOI] [PubMed] [Google Scholar]
- 31.Mamtani R, Zhang H, Parikh RB, Cohen AB, Patel K, Homet Moreno B, et al. Uptake of maintenance immunotherapy and changes in upstream treatment selection in patients with advanced urothelial cancer (aUC). Journal of Clinical Oncology. 2023;41(6_suppl):466-. [Google Scholar]
- 32.Galsky MD, Mortazavi A, Milowsky MI, George S, Gupta S, Fleming MT, et al. Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer. J Clin Oncol. 2020;38(16):1797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones RJ, Hussain SA, Birtle AJ, Song YP, Enting D, Faust G, et al. A randomised, double blind, phase II clinical trial of maintenance cabozantinib following chemotherapy for metastatic urothelial carcinoma (mUC): Final analysis of the ATLANTIS cabozantinib comparison. Journal of Clinical Oncology. 2022;40(17_suppl):LBA4505-LBA. [Google Scholar]
- 34.Crabb SJ, Hussain SA, Soulis E, Hinsley S, Dempsey L, Trevethan A, et al. A randomized, double blind, biomarker selected, phase II clinical trial of maintenance PARP inhibition following chemotherapy for metastatic urothelial carcinoma (mUC): Final analysis of the ATLANTIS rucaparib arm. Journal of Clinical Oncology. 2022;40(6_suppl):436-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman-Censits J, Grivas P, Powles T, Martincic D, Hawley J, Tyroller K, et al. 665 JAVELIN Bladder Medley: a phase 2 trial of avelumab in combination with other antitumor drugs as first-line maintenance therapy for advanced urothelial carcinoma. Journal for ImmunoTherapy of Cancer. 2022;10(Suppl 2):A695. [Google Scholar]
- 36.Gupta S, Ballman KV, Galsky MD, Morris MJ, Chen RC, Chan TA, et al. MAIN-CAV: Phase III randomized trial of maintenance cabozantinib and avelumab versus avelumab after first-line platinum-based chemotherapy in patients with metastatic urothelial cancer (mUC) (Alliance A032001). Journal of Clinical Oncology. 2022;40(16_suppl):TPS4607-TPS. [Google Scholar]
- 37.Coquan E, Clarisse B, Lequesne J, Brachet PE, Nevière Z, Meriaux E, et al. TALASUR trial: a single arm phase II trial assessing efficacy and safety of TALazoparib and Avelumab as maintenance therapy in platinum-Sensitive metastatic or locally advanced URothelial carcinoma. BMC Cancer. 2022;22(1):1213. [DOI] [PMC free article] [PubMed] [Google Scholar]