Abstract
Animal tuberculosis significantly challenges global health, agriculture, and wildlife conservation efforts. Mycobacterial cultures are resource-intensive, time-consuming, and challenged by heterogeneous populations. In this study, we employed a culture-independent approach, using targeted long-read-based next-generation sequencing (tNGS), to investigate the mycobacterial composition in 60 DNA samples extracted from Mycobacterium bovis infected culture-confirmed African buffalo tissue. We detected mycobacterial DNA in 93.3% of the samples and the sensitivity for detecting Mycobacterium tuberculosis complex (MTBC) was 91.7%, demonstrating a high concordance of our culture-independent tNGS approach with mycobacterial culture results. In five samples, we identified heterogenous mycobacterial populations with various non-tuberculous mycobacteria, including members of the Mycobacterium avium complex (MAC), M. smegmatis, and M. komaniense. The latter Mycobacterium species was described in South Africa from bovine nasal swabs and environmental samples from the Hluhluwe-iMfolozi Park, which was the origin of the buffalo samples in the present study. This finding suggests that exposure to environmental mycobacteria may confound detection of MTBC in wildlife. In conclusion, our approach represents a promising alternative to conventional methods for detecting mycobacterial DNA. This high-throughput technique enables rapid differentiation of heterogeneous mycobacterial populations, which will contribute valuable insights into the epidemiology, pathogenesis, and microbial synergy during mycobacterial infections.
Keywords: African buffaloes, Culture-independent, Mycobacteriome, Mycobacterium bovis, Oxford nanopore technology, Targeted next-generation sequencing
Subject terms: Infectious-disease diagnostics, Pathogens
Introduction
Animal tuberculosis (TB), caused by Mycobacterium bovis (M. bovis) and other members of the Mycobacterium tuberculosis complex (MTBC), is a threat to livestock and wildlife populations1,2. Among the different mycobacterial species, M. bovis is a significant pathogen capable of causing severe chronic infectious disease, particularly in animals such as African buffaloes (Syncerus caffer)3. African buffaloes are well-known wildlife maintenance hosts for M. bovis, contributing to the potential direct and indirect transmission of the pathogen to domestic livestock, other susceptible wildlife species like African lions (Panthera leo), rhinoceros (Ceratotherium simum, Diceros bicornis), African elephants (Loxodonta africana), and humans4–7.
In African buffaloes, ante-mortem detection of infection is based on the in vivo or in vitro measurement of M. bovis antigen-specific cell-mediated immunological (CMI) responses, using the tuberculin skin test (TST) or the interferon-γ (IFN-γ) release assay (IGRA), respectively8–10. Although conventional TST uses purified protein derivatives (PPDs) from M. bovis and M. avium, in vitro cytokine stimulation assays have employed PPDs or mycobacterial peptides, such as early secretory antigen target 6 kDa (ESAT-6) and culture filtrate protein 10 kDa (CFP-10), for identifying infected individuals. Unfortunately, cross-reactive host responses to non-tuberculous mycobacteria (NTMs), especially when using PPDs to stimulate antigen-specific cell-mediated immunity (CMI) responses, lead to diagnostic interference11. Except for well-documented pathogenic members, such as M. leprae, M. marinum, and M. avium subsp. paratuberculosis, NTM—previously regarded as benign environmental organisms prevalent in soil and water—are now recognized as potential pathogens with significant implications for human and animal health, particularly in immunocompromised individuals12. Infection with NTMs can cause diseases collectively referred to as mycobacteriosis, affecting not only humans but also various livestock and wildlife species11,13,14.
Currently, the gold standard for definitive confirmation and characterisation of TB relies on culture-based methods, where bacterial isolates obtained from infected animals undergo phenotypic and genotypic analyses15. However, the application of mycobacterial culture may not be feasible, especially in certain epidemiological contexts where the movement of animal samples is restricted. In South Africa, most of the Foot-and-Mouth Disease (FMD)-endemic areas coincide with well-known M. bovis-endemic wildlife parks. In these regions, the movement of cloven-hooved animals including samples is subjected to regulatory restrictions. As a result, there is a need for DNA-based culture-independent M. bovis diagnostic techniques as well as an unbiased approach to investigate complex host-mycobacterial interactions.
To date, a few commercially available real-time PCR assays have been reported to detect MTBC members and NTMs directly from animal specimens, including oronasal swabs, tissue homogenates, and respiratory secretions, including bronchoalveolar lavage samples. Assays include but are not limited to the Xpert® MTB/RIF Ultra (Cepheid, Sunnyvale, California, USA)16, artus® M. tuberculosis PCR Kit (Qiagen, Venlo, Limburg, Netherlands)17,18, COBAS® TaqMan® MTB Test kit (Roche Diagnostics, Indianapolis, Indiana, United States)19, BactoReal® kit (Ingenetix, Vienna, Austria)20, and VetMAX™ M. tuberculosis Complex PCR Kit (Thermo Fisher Scientific, Waltham, Massachusetts, United States)21,22 for MTBC DNA detection, and the Hain Geno‐Type CMdirect VER 1.0-line probe assay5 for MTBC/NTM DNA detection and identification of commonly found NTM species in human clinical samples. However, these assays cannot differentiate between the different members of the MTBC, in addition, the Hain Geno‐Type CMdirect VER 1.0-line probe assay has been reported to be less successful when applied to samples that had not undergone prior culture5. In contrast, PCR amplification of housekeeping genes such as the beta subunit of RNA polymerase (rpoB), partial heat-shock protein (hsp65), and the Ku genes, in conjunction with amplicon sequencing, have successfully detected Mycobacterium genus DNA and provided species identification in ante-mortem samples from wildlife species, circumventing the need for culture-based methods5. However, these targets are unable to differentiate specific MTBC members and require an additional region-of-difference PCR (RD-PCR) for speciation23. Unfortunately, RD-PCRs were initially tailored for the identification of MTBC in mycobacterial cultures, posing significant challenges in adapting them for use with DNA extracted directly from samples23. Sequencing of specific genetic targets has shown promise as an alternative tool for discerning phylogenetically related slow-growing mycobacteria by interrogating DNA sequence polymorphisms24. Genes encoding DNA gyrase subunits gyrA and gyrB have demonstrated satisfactory discriminatory power, facilitating precise taxonomic resolution and classification of distinct MTBC members25,26.
The 16S rRNA gene is widely used for bacterial identification, aiding in the discovery of new species and serving as an alternative to traditional identification methods. Direct sequencing of amplified 16S rRNA gene DNA in Mycobacterium species is well-documented27,28. Over the past decade, however, several authors suggested that the inter- and intraspecies discriminatory power of 16S rRNA gene sequences was insufficient for some bacterial genera, including Mycobacterium species29,30. Despite being a sub-optimal gene target for Mycobacterium genus speciation, studies have demonstrated the advantages of using full-length 16S rRNA amplicon sequencing for taxonomic classification, as opposed to short-read amplicon sequencing31–33. Nonetheless, previous methods for high-throughput full-length 16S rRNA gene amplicon sequencing using Oxford Nanopore Technologies (ONT) technology often relied solely on reference database alignment34,35 rather than employing de novo generation of sequence features, such as amplicon sequence variants (ASV) or operational taxonomic units (OTU).
Currently, the generation of whole-genome sequences for epidemiological investigations of animal TB relies on mycobacterial culture; however, analyses can be impeded by the presence of other microorganisms that outcompete MTBC growth in culture36–38. Over the past decade, an increasing number of publications have focused on direct whole genome sequencing (WGS) using hybridization-based target enrichment techniques to bypass mycobacterial culture and generate high-quality MTBC genomes directly from infected tissue samples39,40. However, their current considerable commercial cost limits their application in the veterinary field in low-income settings, especially when analyzing large numbers of samples. Furthermore, the presence of genetically closely related microorganism communities in samples poses a challenge for de novo whole genome assembly41. Therefore, identifying these communities before sequencing is crucial.
Co-infections can also modulate the immune response, particularly in infections with closely related microorganisms that share antigenic properties42. This study describes the use of three housekeeping genes (hsp65, rpoB, and 16S rRNA) in a culture-independent in-house multiplex PCR method to detect and identify Mycobacteria spp., including MTBC, followed by single-nucleotide polymorphism (SNP) for speciation of the MTBC (gyrA and gyrB), directly from culture-confirmed M. bovis infected tissue.
Using a subset of M. bovis infected tissue samples from African buffaloes, a novel culture-independent targeted next-generation sequencing (tNGS) approach has been recently developed43. In this study, we aimed to characterize the mycobacteriome, which refers to the community of mycobacteria present in a sample, of a larger group of samples obtained from the same animal species and geographical region. For this purpose, we applied a novel reference-free sorting tool44 on ONT-sequenced amplicons and based on their similarity in sequence and length, we were able to build consensus sequences revealing the mycobacteriome present in each sample45. This approach contributes to the refinement of diagnostic strategies, enhancing our ability to comprehensively study the mycobacterial landscape in wildlife and ensuring the effective surveillance of M. bovis in regions where it poses a significant threat to both animal health and conservation efforts.
Results
A total of 60 tissue samples from 57 individual African buffaloes43 from the Hluhluwe-iMfolozi Park (HiP), South Africa (SA), were selected in the present study and included pooled head lymph nodes (n = 5), pooled thoracic lymph nodes (n = 7), lung tissue (n = 7), tonsil (n = 5), tracheobronchial lymp nodes (n = 12), mediastinal lymph nodes (n = 8), parotid (n = 4), retropharyngeal lymph nodes (n = 6), prescapular lymph nodes (n = 3), two mandibular lymph nodes, and one subiliac lymph node. All buffalo samples had previously been confirmed as infected using the aforementioned culture methods, and the presence of the M. bovis region of difference 4 (RD4) signature was confirmed from culture-positive crude DNA extracts23. Spacer oligonucleotide typing hybridization assay (spoligotyping) revealed that all samples fit one of two profiles, SB0130 (n = 39) and SB1474 (n = 21) (Suppl. Table 1).
ONT targeted amplicon sequencing
All samples showing amplification of > 1 target gene (n = 56; 93.3%) were included for amplicon sequencing, whereas four samples (2 tonsils, one lung, and one parotid) for which none of the targets could be visualized after electrophoresis were excluded from downstream analysis. The hsp65, rpoB, and 16S rRNA gene regions were successfully amplified from 55 samples (Fig. 1). One pooled head lymph node sample did not amplify the rpoB target. The number of reads per specimen with Q12 or greater ranged from 63,705 to 897,809 (Mean (M) = 320,985 reads, Standard Deviation (SD) = 154,129), and the total number of bases sequenced ranged from 38,921,972 to 554,606,081 (M = 199,128,119 bases, SD = 95,521,803). The N50 read length (median read length of the longest contigs) varied from 558 to 781 bases. The mean read length for the samples ranged from 576 to 658 bases. The mean read quality, as indicated by the Phred score, ranged between 14.2 and 14.5. A total of 200,000 reads with > Q12 were randomly selected from each sample for downstream analysis (Fig. 2).
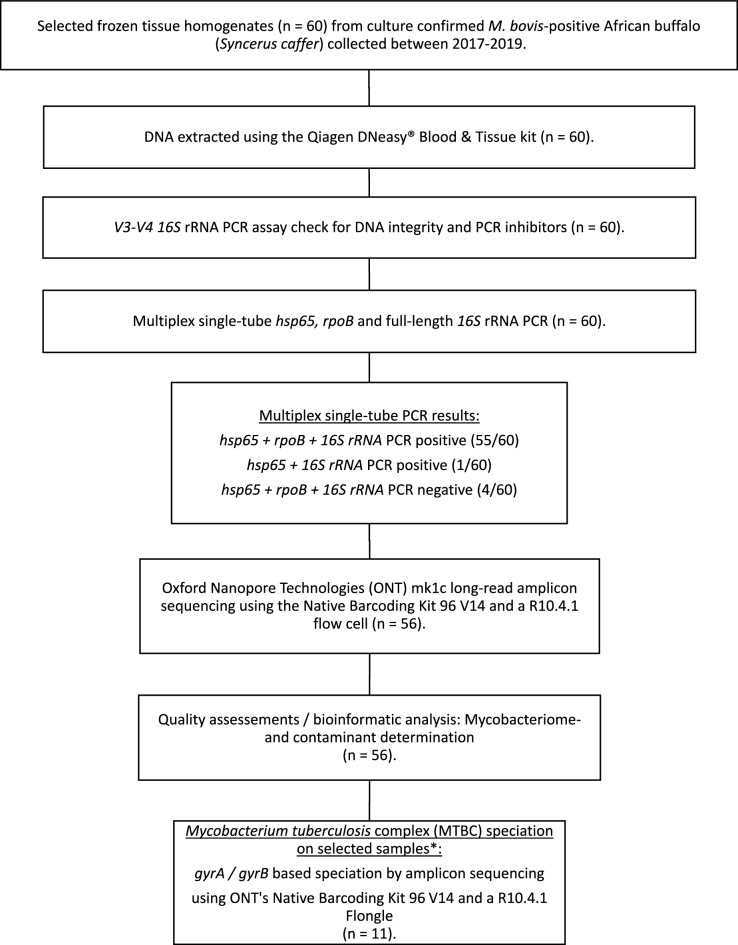
Figure 1.
Study overview illustrating the methods applied to DNA extracted from culture-confirmed M. bovis-positive tissue samples (n = 60) from 57 different African buffalo, selected and included in the study following mycobacterial species identification. After amplifying three target genes (hsp65, rpoB, 16S rRNA), long-read amplicon sequencing was conducted on 56 samples using Oxford Nanopore Technologies (ONT), the Native Barcoding Kit 96 V14, and a single R10.4.1 flow cell. Mycobacterium tuberculosis complex (MTBC) speciation was achieved by amplifying and sequencing the gyrA and gyrB gene targets on 11 randomly selected samples using a single Flongle R10.4.1 flow cell (ONT).
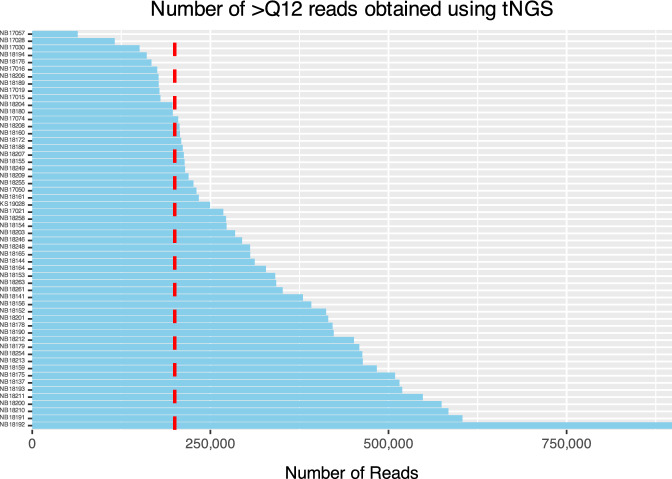
Figure 2.
Number of reads with quality score > Q12 obtained using Oxford Nanopore Technologies (ONT) targeted next-generation sequencing (tNGS) across 56 African buffalo tissue samples. Among 78.6% of the samples (n = 44), over 200,000 reads were generated, with a maximum of 897,809 reads. Conversely, the remaining 21.4% (n = 12) of samples yielded reads ranging between 63,705 and 197,343, all of which were included in the analysis. The vertical red dotted line highlights the threshold for the number of reads used for downstream analysis.
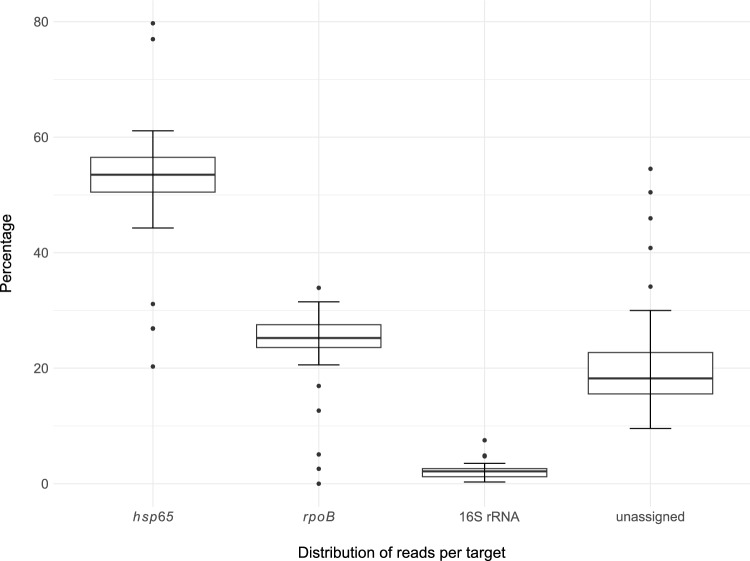
Since amplification of the three specific genetic targets occurred in a single tube, amplified PCR products were not normalized, and therefore shorter sequences were predominant. The proportion of reads for each target varied, with hsp65 ranging from 29 to 80% (M = 52.6%; mean number of reads 101,132), rpoB 2–34% (M = 24.4%; mean number of reads 47,325), and 16S rRNA 0.3–8% (M = 2.2%; mean number of reads 4,007) (Fig. 3). Singletons that could not be assigned to any group because of their length or sequence similarity were reported as unassigned 9–54% (M = 20.8%; mean number of reads 39,330) and excluded from the downstream analysis.
Figure 3.
Distribution plot of read counts for the 56 African buffalo tissue samples and each genetic target (hsp65, rpoB, and 16S rRNA). Reference-free sorting and assembly of consensus sequences were performed on a maximum of 200,000 randomly selected reads (> Q12) for each sample. For tissue samples with fewer reads, the entire available dataset was included. Reads were generated using Oxford Nanopore Technologies (ONT) on selected targeted genes shown on the x-axis. Singletons that could not be included in any group because of their length or sequence similarity are reported as unassigned and were excluded from the analysis.
Using a subset of eleven tissue DNA samples, the gyrA and gyrB genes were amplified and sequenced on a single Flongle flow cell (ONT). The number of reads with Q12 or greater ranged from 118 to 1918 (M = 1157 reads, SD = 489), and the total number of bases sequenced ranged from 20,411 to 328,224 (M = 200,948 bases, SD = 85,764). The mean read quality, as indicated by the Phred score, was consistently above 13 (11/11). All reads with > Q12 quality scores were selected from each sample for downstream analysis. Specifically, the gyrA amplicon sequences confirmed the presence of MTBC DNA in approximately three-quarters of the amplified samples (8 out of 11), while gyrB1 and gyrB2 confirmed the RD and spoligotyping speciation results obtained from culture-derived DNA in all samples.
Mycobacterial DNA detection and mycobacteriome composition
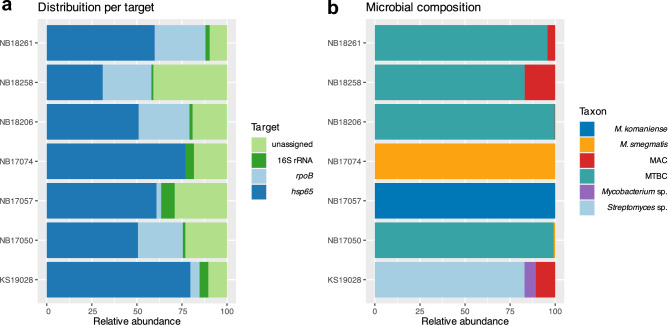
Evidence of Mycobacterium spp. DNA presence was detected in 93.3% (56/60) of the DNA samples extracted directly from tissue homogenates. Among the 56 samples subjected to amplicon generation and subsequent deep sequencing for the three specific genetic targets, the rpoB PCR exhibited the highest sensitivity 91.7% for MTBC detection (55/60), followed by hsp65 88.3% (53/60), and 16S rRNA 86.7% (52/60). Overall, the mycobacterial DNA detection and composition of the mycobacteriome varied depending on the three independent targets. The shorter target PCR (hsp65) was predominant (Fig. 4a). It demonstrated the highest efficacy in detecting NTM and heterogeneous mycobacterial populations compared to alternative targets (Table 1), as well as closely related microorganisms such as Streptomyces sp. (Fig. 4b). Overall, the presence of NTM and heterogeneous mycobacterial communities was identified in seven African buffaloes tissue samples, including one parotid (NB17057), two pooled head lymph nodes (NB17074 and KS19028), one retropharyngeal lymph node (NB17050), one subiliac lymph node (NB18206), one mediastinal lymph node (NB18261), and one lung (NB18258).
Figure 4.
(a) Visualization depicting the percentage of reads across different target genes (16S rRNA, rpoB, hsp65) for DNA from each African buffalo tissue sample (n = 7) where the presence of non-tuberculous mycobacteria or heterogeneous mycobacterial communities was detected. Reference-free sorting and assembly of consensus sequences were performed on randomly selected reads. The relative abundance of the target genes amplified using a multiplex PCR and sequenced using Oxford Nanopore Technologies is shown. (b) The relative abundance of reads appertaining to different mycobacteria and closely related organisms including Streptomyces sp. is illustrated. Identification of mycobacterial communities based on the amplification of the hsp65 gene. Mycobacterium tuberculosis complex (MTBC); Mycobacterium avium complex (MAC); NB18261 mediastinal lymph node; NB18258 lung; NB18206 subiliac lymph node; NB17074 and KS19028 pooled head lymph nodes; NB17057 parotid; NB17050 retropharyngeal lymph node.
Table 1.
Synopsis of the reference-free sorting and assembly outcomes of consensus sequences for the three Mycobacterium spp. target genes (hsp65, rpoB, 16S rRNA) included in the culture-independent next-generation sequencing approach.
| hsp65 | rpoB | 16S rRNA | |
|---|---|---|---|
| Mycobacterium tuberculosis complex (MTBC) | 49 | 54 | 52 |
| Non-tuberculous mycobacteria (NTM) | 3 | 0 | 2 |
| MTBC + NTM | 4 | 1 | 0 |
| Total | 56 | 55a | 54b |
The mycobacterial DNA detection and composition of the mycobacteriome varied depending on the three independent targets. Notably, the shorter target PCR (hsp65) demonstrated the ability to identify the highest proportion of mycobacteria, with amplification and Mycobacterium spp. sequences detected in 93.3% of the samples tested (56/60). The rpoB PCR displayed the highest sensitivity for MTBC detection, identifying MTBC in 91.7% of all samples included (55/60).
aOne sample could not be amplified using the rpoB target.
bFor two samples (NB18154 and KS19028), the 16S rRNA consensus sequences were classified as organisms not belonging to the Mycobacterium genus. Further analysis using NCBI Basic Local Alignment Search Tool (BLAST) identified the presence of Niallia sp. in NB18154 and Streptomyces sp. in KS19028.
Based on previous molecular analyses, which involved RD-PCRs and spoligotyping used to confirm the presence of M. bovis within the selected samples, our goal was to assess the ability of the novel culture-independent methodology to detect and speciate MTBC members. The three additional targets employed for confirming the presence of M. bovis DNA (gyrA, gyrB1, and gyrB2) were successfully amplified in all 11 selected samples. For two samples (NB17057 and KS19028), however, the total number of reads (656 and 118) and the number of consensus sequences identified as MTBC in one or more genetic targets (92 and 43) were lower compared to the corresponding mean values generated for the other samples (total number of reads 10,149).
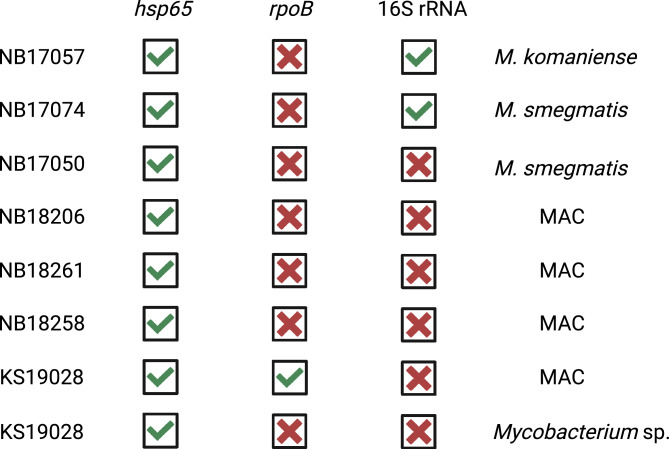
In addition to the detection of MTBC DNA, tNGS of the hsp65 gene successfully identified different mycobacterial species in various tissue specimens, including two pooled head lymph nodes, one lung, one mediastinal lymph node, one parotid, one retropharyngeal lymph node, and one subiliac lymph node. Mycobacterium avium complex (MAC) was detected in 6.7% samples (4/60), M. smegmatis in 3.3% (2/60), M. komaniense and an unclassified Mycobacterium sp. in one sample each (1.7%). Additionally, DNA of mycobacteria’s closely related organisms such as Streptomyces sp. were amplified and further identified using the hsp65 target gene (Fig. 4b). In the DNA sample NB17074, originating from lung tissue, M. smegmatis was identified using hsp65 and 16S rRNA targets, but no amplification was observed using rpoB PCR (Fig. 5). Conversely, in one retropharyngeal lymph node (sample NB17057), M. komaniense was detected using hsp65 and 16S rRNA PCRs, while MTBC DNA was detected using rpoB. For the three samples where NTM were detected by more than one target PCR—M. komaniense and M. smegmatis (hsp65 and 16S rRNA) and a member of the MAC (hsp65 and rpoB)—a 100% agreement between the different target results was observed (Fig. 5).
Figure 5.
Agreement between different genetic targets in detecting and identifying non-tuberculous mycobacteria (NTM) in culture-confirmed Mycobacterium bovis infected African buffalo tissue sample (n = 7). The shorter target PCR (hsp65) demonstrated the highest efficacy in detecting NTM and heterogeneous mycobacterial populations compared to alternative targets. The latter target identified eight NTM in seven samples, rpoB one member of the Mycobacterium avium complex (MAC), and 16S rRNA detected two NTM in distinct samples. In sample KS19028 (bottom two rows), obtained from pooled head lymph nodes, a member of the MAC and an unclassified Mycobacterium sp. were detected in addition to M. bovis. A complete consensus of results was observed across different target PCRs when NTM were detected by two separate genes.
At least two distinct strains of MAC were detected, exhibiting 15 genomic modifications, including single nucleotide variations and indels distributed over the 441 bp of the hsp65 amplicon. These strains displayed the highest hsp65 sequence homology to M. avium and M. colombiense and possibly represent different strains of yet-to-be-characterized species. The MAC sequences were exclusively detected in tissue samples exhibiting heterogeneous bacterial populations (4/4), including MTBC in three samples, and a combination of Streptomyces sp. and Mycobacterial sp. in one sample (Table 2). The samples originated from various locations including lung tissue, mediastinal lymph nodes, head lymph nodes, and subiliac lymph nodes. Finally, the relative abundance of reads classified as MAC varied between 0.3 and 17% for the hsp65 target in the above-mentioned samples (Fig. 4b).
Table 2.
Percentage of hsp65 PCR target reads (%) indicating the presence of non-tuberculous mycobacteria or heterogeneous mycobacterial communities in each African buffalo tissue sample where the DNA of these organisms was detected.
| NB17057 Parotid |
NB17074 Head Lnn |
NB17050 Retrop Ln |
NB18206 Subiliac Ln |
NB18261 Mediast Ln |
NB18258 Lung |
KS19028 Head Lnn |
|
|---|---|---|---|---|---|---|---|
| M. komaniense | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| M. smegmatis | 0 | 100 | 0.86 | 0 | 0 | 0 | 0 |
| MTBC | 0 | 0 | 99.14 | 99.68 | 95.7 | 83.01 | 0 |
| MAC | 0 | 0 | 0 | 0.32 | 4.3 | 16.99 | 10.9 |
| Mycobacterium sp. | 0 | 0 | 0 | 0 | 0 | 0 | 6.13 |
| Streptomyces sp. | 0 | 0 | 0 | 0 | 0 | 0 | 82.97 |
Lymph nodes (Lnn); retropharyngeal lymph node (Retrop Ln); mediastinal lymph node (Mediast Ln).
Discussion
While M. bovis culture is regarded as the gold standard technique for detecting animal TB, it necessitates a substantial bacterial load and is time-intensive, often taking eight weeks or more to confirm a diagnosis definitively15,46. To overcome these challenges, various direct molecular techniques, such as real-time PCR, have been developed for rapid and specific identification of MTBC DNA40. The advantages of this approach include high diagnostic sensitivity (Se), specificity (Sp), and shorter turnaround time for a definitive diagnosis. For instance, recent studies reported Se varying between 87.70 and 100%21,47–49 and Sp of 93.66–100%49–52 for targeted PCR detection of M. bovis in tissue samples from infected cattle herds compared to mycobacterial culture. However, while direct molecular techniques enable rapid and accurate diagnosis, they require careful design and validation to avoid false positive results due to imperfect specificity. Cross-reactivity of IS6110-based real-time PCRs, which is one of the most commonly used genetic targets for MTBC detection, has been reported for non-MTBC mycobacterial isolates, such as M. marinum, M. wolinskyi, and specific lineages of “M. avium subsp. hominissuis”. At the same time, MTBC strains lacking commonly used real-time PCR target genes such as IS6110 have been described from human clinical samples, leading to false negative outcomes53,54. Therefore, despite their advantages, the design and validation of real-time PCR-based detection of MTBC in animals strongly depend on factors such as the target population, genetic target, and epidemiological situation. These considerations are crucial when employing direct molecular techniques for M. bovis DNA detection and animal TB surveillance, given the vast and complex nature of MTBC molecular detection and diagnosis.
Our culture-independent detection method applied to 60 samples, independently confirmed to harbour M. bovis through culture and molecular characterisation (RD-PCRs and spoligotyping), showed a Se of 91.7%, which was comparable to previous observations using different molecular means21,47–49. The majority of samples exhibited visible lesions (VL), with one-third of the animals (n = 30) being heavily infected and showing prominent lesions with scores of 2–3. However, over 20% (n = 13) exhibited no visible lesions (NVL), which are known to be more susceptible to false-negative results in culture46. The lower culture-positive rates of NVL reactors may be attributed to the low bacterial load in tissues and the detrimental effects of decontamination, which can potentially reduce the recovery of bacilli46. Notably, in the present study, 10/13 of the animals (16.7% of the entire dataset), had NVL and performed well using the mycobacteriome assay, leading to the amplification of mycobacterial-specific amplicons. It is promising to observe that, among these 10 samples, M. bovis DNA was detected in at least one target in nine samples. In contrast, in the remaining sample, only M. smegmatis was detected using hsp65 and 16S rRNA. Finally, of the four samples (2 tonsils, one lung, and one parotid) for which none of the targets could be visualized after electrophoresis, three were NVL samples and one presented a grade 1 lesion.
The advantage of the approach reported in this study is the ability to distinguish between various mycobacterial species and, with careful consideration due to differences in primer affinities estimate the relative abundance of each population present in one sample, facilitating characterisation of the mycobacteriome. This approach targets a wide range of mycobacterial species, which may lead to interference in culture or in vivo diagnostic test results. Furthermore, the single-tube amplification of multiple genetic targets allows the user to obtain a more comprehensive understanding of the sample composition. However, caution is advised when interpreting the abundances of amplicons obtained for the three target genes, as the affinity of the primer pairs used may vary significantly. Finally, the inclusion of discriminatory genes, such as gyrA and gyrB, provides additional information, especially for complex clinical settings, where mixed infections may occur in various hosts and the environment55–61. Mycobacterial species differentiation can have significant implications for antimicrobial treatment of zoonotic TB, since most M. bovis strains are naturally resistant to pyrazinamide, an essential first-line antibiotic used to treat TB patients62.
This study explores the capabilities of long-read Nanopore-based sequencing to provide high-throughput and high-accuracy profiles of housekeeping genes for the genus Mycobacterium, alongside de novo generated consensus sequences utilizing full-length 16S rRNA gene amplicon sequencing in the context of animal TB. The presence of NTM in animals and people infected with MTBC carries significant medical consequences. Firstly, their presence may hinder the detection of MTBC through culture methods, potentially resulting in false negative outcomes. Secondly, NTM could impact immune responses to MTBC, influence the progression of infection, and modulate host response to existing live attenuated vaccines such as the Bacillus Calmette–Guérin (BCG) strain63–65.
The most common NTM found in this study through targeted deep sequencing were members of the MAC, followed by M. smegmatis, M. komaniense, and an uncharacterized Mycobacterium sp. This finding aligns with previous observations in wild and domestic ungulates such as cattle14,66, pigs67, wild boar68, and African buffalo13, suggesting a high presence of M. avium and M. colombiense in tissue samples due to transient colonisation or co-infection with MTBC. In this study, M. komaniense, a rapidly growing NTM closely related to M. moriokaense, was identified in a single parotid showing a lesion score of 2. Notably, this newly described mycobacterial species was initially documented in bovine nasal swabs, soil, and water samples collected from 2010 to 2012 in South Africa69. One of the four isolates previously reported by Gcebe et al. was obtained from a soil sample taken in the same geographic region (HiP, KwaZulu Natal Province) where the buffaloes in the current study were sampled69. These findings suggest potential geographic adaptation of certain mycobacterial species and temporal persistence. However, the clinical significance of M. komaniense is unknown. The concurrent detection of M. bovis and M. komaniense in a retropharyngeal lymph node exhibiting a small focal lesion with a diameter < 10 mm (lesion score 1) does not necessarily preclude the possibility of an incidental finding. With the rising incidence of NTM infections, particularly those caused by MAC, among both immunocompromised and immunocompetent human patients globally, there is a need for further investigation of the clinical implications posed by these emerging pathogens. A One Health approach, that includes mycobacterial strains from human, animal, and environmental origins, is required to investigate common etiological determinants of infection, comorbidities, and virulence factors influencing clinical outcomes.
In this study, a targeted PCR deep-sequencing culture-independent approach, using DNA from tissues confirmed to contain M. bovis, was used to explore the composition of the mycobacteriome. The shorter target PCR (hsp65) demonstrated superior capability in detecting NTM and heterogeneous populations of mycobacteria compared to alternative targets. However, since none of the three specific genetic targets amplified simultaneously in the multiplex PCR could distinguish between M. bovis and other members of the MTBC using amplicon sequencing, the incorporation of additional ecotype-specific markers (gyrA and gyrB genes) was imperative for the diagnostic pipeline70. Implementing the outlined method in future studies, particularly those involving samples containing multiple mycobacterial species and from microbially complex sites, such as the upper respiratory tract, will enhance understanding of the diverse interactions and within-host coexistence of mycobacterial species.
One of the main limitations of the study is the lack of confirmation for the presence of NTM in MGIT cultures, complicated by the concomitant growth of M. bovis. Further studies investigating heterogeneous mycobacterial populations using selective culture media will enable the determination of NTM viability in M. bovis-affected animals and assess the ability of different target PCRs in detecting NTM compared to culture. Another limitation of the mycobacteriome approach is that mycobacteria-specific primers may encounter more amplification difficulties with the targeted genes compared to the more conserved targets used in metagenomic investigations71. To address this issue, we used multiple targets. The observed differences in amplification between these targets likely reflect their varying affinities for the species detected.
Similar to prior research reports72–74, our findings indicate that Nanopore-based targeted amplicon sequencing can generate comparable results to existing high-throughput assays for the detection and characterisation of MTBC organisms. However, it is important to note that Nanopore costs may fluctuate significantly based on batch sizes and the utilization of flow cells. Moreover, because of the frequent updates in chemistry and base calling algorithms, constant validation of new kits and pipelines might pose a hurdle for clinical applications. One of the main drawbacks is the short longevity of flow cells, which may impede progress in longer-term projects. Finally, transitioning entirely from mycobacterial cultures, which usually enhance DNA yield and indicate viability, poses a challenge in demonstrating the transmission and actual infection potential of the mycobacteria detected via a culture-independent approach. Both culturing and PCR may introduce bias, leading to findings that might not faithfully reflect the original mycobacteriome75,76. Therefore, innovative research efforts should focus on refining RNA-based detection methods to differentiate viable bacilli and remnant DNA draining through the lymph system in clinical samples77.
Conclusion
By applying a culture-independent targeted next-generation sequencing approach to DNA extracted from previously culture-confirmed M. bovis infected African buffalo tissues, we were able to detect mycobacterial DNA in 93.3% of the samples. Of these, 98.2% were positive for MTBC DNA. Mycobacterial-specific amplicons were sequenced and identified positive tissue samples using a one-tube multiplex amplicon sequencing approach. This technique provided rapid and comparable results to previously published molecular methods. This study presents a promising alternative to culture-based detection methods for the detection of animal TB and for characterisation of mycobacterial communities in wildlife tissue samples.
Materials and methods
Study population
Between 2017 and 2019, a routine veterinary annual test-and-slaughter program was conducted in Hluhluwe-iMfolozi Park (HiP), South Africa (SA), on different herds of African buffaloes3,10. All animals were captured, immobilized, and whole blood collected, as previously described9. Positive animals were euthanized by gunshot based on the detection of cell-mediated immunity (CMI) responses toward M. bovis-specific antigens (n = 67). All buffaloes were handled by the Enzemvelo Wildlife Services and KZN state veterinarians. Various tissue samples (lymph nodes from the head, thorax, and lung) were collected during necropsies, and lesion scores were assigned as previously described (Suppl. Table 1)8. For each sample, tissue homogenate aliquots were split for mycobacterial culture and culture-independent analyses, with processing occurring in a BSL-3 laboratory up to the step of DNA extraction. Briefly, approximately 10 g of tissue was homogenized in 50-mL skirted tubes (Becton Dickinson, Franklin Lakes, New Jersey, USA) containing eight 4.8-mm metal beads and 4 mL of sterile phosphate-buffered saline (PBS) using a blender (Bullet Blender 50; Next Advance, Averill Park, NY, USA) for 15 min at maximum speed, as previously described78. After decontamination, performed with BD MycoPrep™ (N-acetyl L-cysteine sodium hydroxide) and PBS following the manufacturer’s instructions, all samples were centrifuged for 15 min at 1500×g and the supernatant was decanted. Each pellet was resuspended in 1 ml PBS and 500 μl of this suspension was transferred to a Mycobacteria Growth Indicator Tube (MGIT™) supplemented with PANTA and incubated in a BACTEC™ MGIT™ 960 Mycobacterial Detection System (both Becton Dickinson). An additional aliquot of tissue homogenate was subsequently preserved (− 80 °C) for DNA extractions, repeat culturing, and/or future sequencing. All Mycobacteria Growth Indicator Tubes (MGIT™) were incubated for 56 days and culture-positive crude extracts were subjected to speciation using a PCR targeting genetic regions of difference to confirm M. bovis infections, as previously described23. Further genetic speciation using spoligotyping was performed79.
Sample selection and processing
A total of 60 frozen native tissue homogenates from 57 individual African buffaloes, including seven animals previously involved in the development of the mycobacteriome assay43, were retrospectively selected from the cohort mentioned above and included for downstream analysis (Fig. 1) based on M. bovis culture and PCR confirmation. A total of 78.3% (47/60) of the samples exhibited macroscopic lesions, with one-third (30/60) classified with lesion scores of 2–3. Previously frozen tissue homogenates (1 mL aliquot) were subjected to genomic DNA extraction using the DNeasy Blood and Tissue kit (Qiagen) with modifications, as previously described43. Briefly, tissue homogenates were heat-inactivated (98 °C for 45 min), then centrifuged (1500×g for 10 min), after which 300 μL of Buffer ATL (Qiagen) was added to the cell pellet. Subsequently, 25 μL of Proteinase K (Qiagen) was added and allowed to digest overnight at 56 °C with agitation at 600 rpm. The sample was centrifuged at 5500×g for 5 min, after which 500 μL of supernatant was collected and transferred to a 1.5 mL tube. An additional 400 μL of buffer AL and 400 μL of ethanol was mixed with the supernatant and then transferred to a Mini Spin Column (Qiagen). Finally, DNA purification was conducted using wash buffers AW1 and AW2 (Qiagen), followed by elution in 60 μL of buffer AE (Qiagen) pre-warmed to 54 °C. Concentrations of DNA were quantified using the Qubit 1 × dsDNA High Sensitivity Assay kit (Thermo Fisher Scientific), following the manufacturer's instructions. Subsequently, DNA integrity and presence of PCR inhibitors were assessed by amplification of the variable regions V3-V4 within the 16S gene, using previously published primers (prbac1/prbac2) and PCR conditions80. Finally, the PCR products were visualized by 1% agarose gel electrophoresis.
Targeted amplicon sequencing using Oxford Nanopore Technologies (ONT)
A multiplex PCR-based amplification targeting three housekeeping genes, namely hsp65 (441 bp), rpoB (680 bp) and the full-length 16S rRNA gene (~ 1500 bp), was performed culture-independently using DNA extracted from buffalo tissue homogenates, as previously described43. Presence of the amplified products was confirmed by 1% agarose gel electrophoresis. All samples showing amplification of > 1 target were included for amplicon sequencing. Using the Native Barcoding Kit 96 V14 kit (ONT), amplicons were end-repaired, individually barcoded, pooled into a single library, native adapter-ligated, loaded onto a single R10.4.1 flow cell (> 1250 pores), and sequenced on a MinION mk1C device (ONT). Building upon prior molecular analyses, which encompassed RD-PCRs and spoligotyping, confirming the presence of M. bovis within the selected tissues, we aimed to verify the efficacy of the novel culture-independent methodology on a subset of samples. Therefore, eleven DNA samples were randomly selected to confirm the presence of M. bovis using gyrA and gyrB targeted amplicon sequencing. Three independent 25 μL reactions containing 14 μL Q5 High-Fidelity 2X Master Mix (New England Biolabs Inc., Ipswich, Massachusetts, United States), 0.5 μL of each 50 μM primer stock solution25, 6 μL sterile, nuclease free water, and 2 μL undiluted extracted DNA. The PCR cycling conditions were as follows: 1 cycle initial denaturation at 98 °C for 15 min, followed by 40 cycles of denaturation (98 °C for 30 s), annealing (62.5 °C for 30 s) and elongation (72 °C for 2 min). Final elongation took place at 72 °C for 5 min. The presence of the amplified products was confirmed by 1% agarose gel electrophoresis. Amplicons were end-repaired, individually barcoded, and native adapter-ligated using the Native Barcoding Kit 96 V14 kit (ONT). Finally, the pooled library was loaded onto a single Flongle R10.4.1 flow cell (> 60 pores) and sequenced using the MinION mk1C device (both ONT).
Data analyses
For the targeted amplicon sequencing datasets, base-calling, demultiplexing, and trimming of the barcodes were performed in real-time using Guppy [v6.4.6] (260 bps—High-Accuracy)81. Data acquisition and base-calling were stopped after 72 h for the three specific genetic targets run using an R10.4.1 flow cell and after 22 h for the gyrA and gyrB run using a Flongle flow cell (Supplementary Materials 1 and 2). Quality control, filtering, and summary reports for Nanopore reads were generated using nanoq v0.10.082 and reads with a Q score of < 12 were discarded. Thereafter, reference-free reads sorting, based on similarity and length, was performed using the amplicon sorter tool [v2023-06-19]44. Since over 80% of the samples yielded more than 200,000 reads and computational analysis remained feasible, we opted to increase the number of reads compared to our previous study43. A total of 200,000 randomly chosen reads with minimum and maximum lengths of 300 bp and 2000 bp, respectively, were selected for each barcode generated, using the three target amplicon approach (hsp65, rpoB, and 16S rRNA). Finally, ABRicate (https://github.com/tseemann/abricate) and custom databases, generated as previously described43, were used for the screening of consensus sequences and summarizing the report files. For the analysis, the following interpretation criteria were established:
Coverage ≥ 90% and identity ≤ 90%, the sequence was reported as unclassified.
Coverage ≥ 90% and identity fell within the range of 90–98%, the sequence was classified as Mycobacterium sp.
Coverage ≥ 90% and identity ≥ 98%, the sequence was reported according to the results table.
Since the database only contained mycobacterial sequences and to characterise bacterial contaminants, all consensus sequences with a corresponding length to 16S rRNA and showing an identity lower than 98% were manually searched using the NCBI Basic Local Alignment Search Tool (BLAST).
Reads generated from the gyrB and gyrA amplicons were selected based on lengths with a minimum of 50 bp to a maximum of 200 bp. Consensus sequences were then generated for each species amplified and target gene. Relative abundances were determined by analyzing the representative pool of reads. Sequence comparison with a custom database, generated with gyrB and gyrA sequences for all members of the MTBC, was performed as above.
For all bioinformatics tools, default settings were used unless stated otherwise. The proportion of consensus sequences generated per target gene and relative abundance of Mycobacterium sp. identified across different samples was visualized using the R package ggplot2.
Ethics
South African Veterinary Council (SAVC)-registered wildlife veterinarians were responsible for all procedures, including immobilization of animals, blood collection, euthanasia, and tissue sampling. No animal was specifically immobilized, sampled, or sacrificed for this study. Ethical approval for this study was granted by Stellenbosch University Animal Care and Use Research Ethics Committee (SU-ACUD16-00072; SU-ACU-2019-9081) and the Stellenbosch University Biological and Environmental Safety Research Ethics Committee (SU-BEE-2021-22561). Section 20 approval was granted by the South African Department of Agriculture, Land Reform and Rural Development (DALRRD 12/11/1/7/6 and 12/11/1/7/2). The study was conducted following the local legislation and institutional requirements and the authors complied with the ARRIVE guidelines.
Supplementary Information
Acknowledgements
We thank Dr David Cooper, Alicia and Warren McCall, Debbie Cooke, Dr Rowan Leeming, Dumisani Zwane, JP van Heerden, and the Game Capture staff from KwaZulu-Natal Ezemvelo Wildlife for the capture and sampling of buffaloes, and their support of this study. This work was supported by funding from the South African government through the South African Medical Research Council and the National Research Foundation South African Research Chair Initiative (Grant #86949), Wellcome Trust Foundation (222941/Z/21/Z), European Union supported by the Global Health EDCTP3 Joint Undertaking and its members [Project 101103171], and American Association of Zoo Veterinarians Wild Animal Health Fund (S005651). The content is the sole responsibility of the authors and does not necessarily represent the official views of the funders.
Author contributions
GG: conceptualization, formal analysis, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing. TK: data curation, writing—review and editing. SKM: methodology, writing—review and editing. ES, NB: investigation, methodology, writing—review and editing. AGL: funding acquisition, resources, writing—review and editing. RMW: funding acquisition, resources, writing—review and editing. MAM: conceptualization, methodology, resources, supervision, writing—review and editing. WG: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing—original draft, writing—review and editing.
Data availability
The datasets presented in this study were submitted to the European Nucleotide Archive (ENA) under project reference number PRJEB75236, https://www.ebi.ac.uk/ena/browser/view/PRJEB75236.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Michele A. Miller and Wynand J. Goosen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68189-x.
References
- 1.Meiring, C., van Helden, P. D. & Goosen, W. J. TB control in humans and animals in South Africa: A perspective on problems and successes. Front. Vet. Sci.5, 298. 10.3389/fvets.2018.00298 (2018). 10.3389/fvets.2018.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller, B. et al. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg. Infect. Dis.19, 899–908. 10.3201/eid1906.120543 (2013). 10.3201/eid1906.120543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernitz, N. et al. Test characteristics of assays to detect Mycobacterium bovis infection in high-prevalence African buffalo (Syncerus caffer) herds. J. Wildl. Dis.56, 462–465 (2020). 10.7589/2019-06-173 [DOI] [PubMed] [Google Scholar]
- 4.Dwyer, R. A., Witte, C., Buss, P., Goosen, W. J. & Miller, M. Epidemiology of tuberculosis in multi-host wildlife systems: Implications for black (Diceros bicornis) and white (Ceratotherium simum) rhinoceros. Front. Vet. Sci.7, 580476. 10.3389/fvets.2020.580476 (2020). 10.3389/fvets.2020.580476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goosen, W. J. et al. Culture-independent PCR detection and differentiation of Mycobacteria spp. in antemortem respiratory samples from African elephants (Loxodonta africana) and rhinoceros (Ceratotherium simum, Diceros bicornis) in South Africa. Pathogens10.3390/pathogens11060709 (2022). 10.3390/pathogens11060709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goosen, W. J. et al. Identification and molecular characterization of Mycobacterium bovis DNA in GeneXpert(R) MTB/RIF ultra-positive, culture-negative sputum from a rural community in South Africa. One Health18, 100702. 10.1016/j.onehlt.2024.100702 (2024). 10.1016/j.onehlt.2024.100702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller, M. et al. Detection of antibodies to tuberculosis antigens in free-ranging lions (Panthera leo) infected with Mycobacterium bovis in Kruger National Park, South Africa. J. Zoo Wildl. Med.43, 317–323. 10.1638/2011-0171.1 (2012). 10.1638/2011-0171.1 [DOI] [PubMed] [Google Scholar]
- 8.Bernitz, N. et al. Impact of Mycobacterium bovis-induced pathology on interpretation of QuantiFERON(R)-TB Gold assay results in African buffaloes (Syncerus caffer). Vet. Immunol. Immunopathol.217, 109923. 10.1016/j.vetimm.2019.109923 (2019). 10.1016/j.vetimm.2019.109923 [DOI] [PubMed] [Google Scholar]
- 9.Parsons, S. D. et al. Modification of the QuantiFERON-TB Gold (In-Tube) assay for the diagnosis of Mycobacterium bovis infection in African buffaloes (Syncerus caffer). Vet. Immunol. Immunopathol.142, 113–118. 10.1016/j.vetimm.2011.04.006 (2011). 10.1016/j.vetimm.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Smith, K. et al. Optimisation of the tuberculin skin test for detection of Mycobacterium bovis in African buffaloes (Syncerus caffer). Prev. Vet. Med.188, 105254. 10.1016/j.prevetmed.2020.105254 (2021). 10.1016/j.prevetmed.2020.105254 [DOI] [PubMed] [Google Scholar]
- 11.Biet, F. & Boschiroli, M. L. Non-tuberculous mycobacterial infections of veterinary relevance. Res. Vet. Sci.97(Suppl), S69-77. 10.1016/j.rvsc.2014.08.007 (2014). 10.1016/j.rvsc.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 12.Augustynowicz-Kopec, E. et al. Interferon gamma release assays in patients with respiratory isolates of non-tuberculous mycobacteria—A preliminary study. Pol. J. Microbiol.68, 15–19. 10.21307/pjm-2019-002 (2019). 10.21307/pjm-2019-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gcebe, N., Rutten, V., Gey van Pittius, N. C. & Michel, A. Prevalence and distribution of Non-Tuberculous Mycobacteria (NTM) in cattle, African buffaloes (Syncerus caffer) and their environments in South Africa. Transbound. Emerg. Dis.60, 74–84. 10.1111/tbed.12133 (2013). 10.1111/tbed.12133 [DOI] [PubMed] [Google Scholar]
- 14.Ghielmetti, G. et al. Non-tuberculous mycobacteria isolated from lymph nodes and faecal samples of healthy slaughtered cattle and the abattoir environment. Transbound. Emerg. Dis.65, 711–718. 10.1111/tbed.12793 (2018). 10.1111/tbed.12793 [DOI] [PubMed] [Google Scholar]
- 15.WHOA. Bovine Tuberculosis. Terrestrial Manual, https://www.woah.org/app/uploads/2021/03/3-04-06-bovine-tb.pdf (2018).
- 16.Goosen, W. J. et al. The Xpert MTB/RIF ultra assay detects Mycobacterium tuberculosis complex DNA in white rhinoceros (Ceratotherium simum) and African elephants (Loxodonta africana). Sci. Rep.10, 14482. 10.1038/s41598-020-71568-9 (2020). 10.1038/s41598-020-71568-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghielmetti, G. et al. Tuberculosis in Swiss captive Asian elephants: Microevolution of Mycobacterium tuberculosis characterized by multilocus variable-number tandem-repeat analysis and whole-genome sequencing. Sci. Rep.7, 14647. 10.1038/s41598-017-15278-9 (2017). 10.1038/s41598-017-15278-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghielmetti, G. et al. Epidemiological tracing of bovine tuberculosis in Switzerland, multilocus variable number of tandem repeat analysis of Mycobacterium bovis and Mycobacterium caprae. PLoS ONE12, e0172474. 10.1371/journal.pone.0172474 (2017). 10.1371/journal.pone.0172474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoning, J. M. et al. Surveillance of bovine tuberculosis and risk estimation of a future reservoir formation in wildlife in Switzerland and Liechtenstein. PLoS ONE8, e54253. 10.1371/journal.pone.0054253 (2013). 10.1371/journal.pone.0054253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorn-In, S. et al. Shedding of Mycobacterium caprae by wild red deer (Cervus elaphus) in the Bavarian alpine regions, Germany. Transbound. Emerg. Dis.67, 308–317. 10.1111/tbed.13353 (2020). 10.1111/tbed.13353 [DOI] [PubMed] [Google Scholar]
- 21.Courcoul, A. et al. Estimation of sensitivity and specificity of bacteriology, histopathology and PCR for the confirmatory diagnosis of bovine tuberculosis using latent class analysis. PLoS ONE9, e90334. 10.1371/journal.pone.0090334 (2014). 10.1371/journal.pone.0090334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goosen, W. J. et al. The VetMAX M. tuberculosis complex PCR kit detects MTBC DNA in antemortem and postmortem samples from white rhinoceros (Ceratotherium simum), African elephants (Loxodonta africana) and African buffaloes (Syncerus caffer). BMC Vet. Res.16, 220. 10.1186/s12917-020-02438-9 (2020). 10.1186/s12917-020-02438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warren, R. M. et al. Differentiation of Mycobacterium tuberculosis complex by PCR amplification of genomic regions of difference. Int. J. Tuberc. Lung Dis.10, 818–822 (2006). [PubMed] [Google Scholar]
- 24.Kasai, H., Ezaki, T. & Harayama, S. Differentiation of phylogenetically related slowly growing mycobacteria by their gyrB sequences. J. Clin. Microbiol.38, 301–308 (2000). 10.1128/JCM.38.1.301-308.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landolt, P., Stephan, R., Stevens, M. J. A. & Scherrer, S. Three-reaction high-resolution melting assay for rapid differentiation of Mycobacterium tuberculosis complex members. Microbiologyopen10.1002/mbo3.919 (2019). 10.1002/mbo3.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niemann, S., Harmsen, D., Rüsch-Gerdes, S. & Richter, E. Differentiation of clinical Mycobacterium tuberculosis complex isolates by gyrB DNA sequence polymorphism analysis. J. Clin. Microbiol.38, 3231–3234. 10.1128/Jcm.38.9.3231-3234.2000 (2000). 10.1128/Jcm.38.9.3231-3234.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogall, T., Flohr, T. & Bottger, E. C. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. J. Gen. Microbiol.136, 1915–1920. 10.1099/00221287-136-9-1915 (1990). 10.1099/00221287-136-9-1915 [DOI] [PubMed] [Google Scholar]
- 28.Turenne, C. Y., Tschetter, L., Wolfe, J. & Kabani, A. Necessity of quality-controlled 16S rRNA gene sequence databases: Identifying nontuberculous Mycobacterium species. J. Clin. Microbiol.39, 3637–3648. 10.1128/JCM.39.10.3638-3648.2001 (2001). 10.1128/JCM.39.10.3638-3648.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beye, M., Fahsi, N., Raoult, D. & Fournier, P. E. Careful use of 16S rRNA gene sequence similarity values for the identification of Mycobacterium species. New Microbes New Infect.22, 24–29. 10.1016/j.nmni.2017.12.009 (2018). 10.1016/j.nmni.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo, P. C., Lau, S. K., Teng, J. L., Tse, H. & Yuen, K. Y. Then and now: Use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin. Microbiol. Infect.14, 908–934. 10.1111/j.1469-0691.2008.02070.x (2008). 10.1111/j.1469-0691.2008.02070.x [DOI] [PubMed] [Google Scholar]
- 31.Callahan, B. J. et al. High-throughput amplicon sequencing of the full-length 16S rRNA gene with single-nucleotide resolution. Nucleic Acids Res.47, e103. 10.1093/nar/gkz569 (2019). 10.1093/nar/gkz569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earl, J. P. et al. Species-level bacterial community profiling of the healthy sinonasal microbiome using Pacific Biosciences sequencing of full-length 16S rRNA genes. Microbiome6, 190. 10.1186/s40168-018-0569-2 (2018). 10.1186/s40168-018-0569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, J. S. et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun.10, 5029. 10.1038/s41467-019-13036-1 (2019). 10.1038/s41467-019-13036-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curry, K. D. et al. Emu: Species-level microbial community profiling of full-length 16S rRNA Oxford Nanopore sequencing data. Nat. Methods19, 845–853. 10.1038/s41592-022-01520-4 (2022). 10.1038/s41592-022-01520-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zorz, J. et al. SituSeq: An offline protocol for rapid and remote Nanopore 16S rRNA amplicon sequence analysis. ISME Commun.3, 33. 10.1038/s43705-023-00239-3 (2023). 10.1038/s43705-023-00239-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopalaswamy, R., Shanmugam, S., Mondal, R. & Subbian, S. Of tuberculosis and non-tuberculous mycobacterial infections—A comparative analysis of epidemiology, diagnosis and treatment. J. Biomed. Sci.27, 74. 10.1186/s12929-020-00667-6 (2020). 10.1186/s12929-020-00667-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maiga, M. et al. Failure to recognize nontuberculous mycobacteria leads to misdiagnosis of chronic pulmonary tuberculosis. PLoS ONE7, e36902. 10.1371/journal.pone.0036902 (2012). 10.1371/journal.pone.0036902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riello, F. N. et al. Diagnosis of mycobacterial infections based on acid-fast bacilli test and bacterial growth time and implications on treatment and disease outcome. BMC Infect. Dis.16, 142. 10.1186/s12879-016-1474-6 (2016). 10.1186/s12879-016-1474-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lozano, N. et al. Detection of minority variants and mixed infections in Mycobacterium tuberculosis by direct whole-genome sequencing on noncultured specimens using a specific-DNA capture strategy. mSphere6, e0074421. 10.1128/mSphere.00744-21 (2021). 10.1128/mSphere.00744-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeineldin, M. M., Lehman, K., Camp, P., Farrell, D. & Thacker, T. C. Diagnostic evaluation of the IS1081-targeted real-time PCR for detection of Mycobacterium bovis DNA in bovine milk samples. Pathogens10.3390/pathogens12080972 (2023). 10.3390/pathogens12080972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson, N. D. et al. Metagenomic assembly through the lens of validation: Recent advances in assessing and improving the quality of genomes assembled from metagenomes. Brief. Bioinform.20, 1140–1150. 10.1093/bib/bbx098 (2019). 10.1093/bib/bbx098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorsich, E. E. et al. Opposite outcomes of coinfection at individual and population scales. Proc. Natl. Acad. Sci. USA115, 7545–7550. 10.1073/pnas.1801095115 (2018). 10.1073/pnas.1801095115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghielmetti, G. et al. Advancing animal tuberculosis surveillance using culture-independent long-read whole-genome sequencing. Front. Microbiol.10.3389/fmicb.2023.1307440 (2023). 10.3389/fmicb.2023.1307440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vierstraete, A. R. & Braeckman, B. P. Amplicon_sorter: A tool for reference-free amplicon sorting based on sequence similarity and for building consensus sequences. Ecol. Evol.12, e8603. 10.1002/ece3.8603 (2022). 10.1002/ece3.8603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macovei, L. et al. The hidden “mycobacteriome” of the human healthy oral cavity and upper respiratory tract. J. Oral Microbiol.7, 26094. 10.3402/jom.v7.26094 (2015). 10.3402/jom.v7.26094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart, L. D., McNair, J., McCallan, L., Gordon, A. & Grant, I. R. Improved detection of Mycobacterium bovis infection in bovine lymph node tissue using immunomagnetic separation (IMS)-based methods. PLoS ONE8, e58374. 10.1371/journal.pone.0058374 (2013). 10.1371/journal.pone.0058374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costa, P. et al. Enhanced detection of tuberculous mycobacteria in animal tissues using a semi-nested probe-based real-time PCR. PLoS ONE8, e81337. 10.1371/journal.pone.0081337 (2013). 10.1371/journal.pone.0081337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorente-Leal, V. et al. Validation of a real-time PCR for the detection of Mycobacterium tuberculosis complex members in bovine tissue samples. Front. Vet. Sci.6, 61. 10.3389/fvets.2019.00061 (2019). 10.3389/fvets.2019.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorente-Leal, V. et al. Direct PCR on tissue samples to detect Mycobacterium tuberculosis complex: An alternative to the bacteriological culture. J. Clin. Microbiol.10.1128/JCM.01404-20 (2021). 10.1128/JCM.01404-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araujo, C. P. et al. Detection of Mycobacterium bovis in bovine and bubaline tissues using nested-PCR for TbD1. PLoS ONE9, e91023. 10.1371/journal.pone.0091023 (2014). 10.1371/journal.pone.0091023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parra, A. et al. Development of a molecular diagnostic test applied to experimental abattoir surveillance on bovine tuberculosis. Vet. Microbiol.127, 315–324. 10.1016/j.vetmic.2007.09.001 (2008). 10.1016/j.vetmic.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 52.Thacker, T. C., Harris, B., Palmer, M. V. & Waters, W. R. Improved specificity for detection of Mycobacterium bovis in fresh tissues using IS6110 real-time PCR. BMC Vet. Res.7, 50. 10.1186/1746-6148-7-50 (2011). 10.1186/1746-6148-7-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lok, K. H. et al. Molecular differentiation of Mycobacterium tuberculosis strains without IS6110 insertions. Emerg. Infect. Dis.8, 1310–1313. 10.3201/eid0811.020291 (2002). 10.3201/eid0811.020291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steensels, D., Fauville-Dufaux, M., Boie, J. & De Beenhouwer, H. Failure of PCR-based IS6110 analysis to detect vertebral spondylodiscitis caused by Mycobacterium bovis. J. Clin. Microbiol.51, 366–368. 10.1128/JCM.02524-12 (2013). 10.1128/JCM.02524-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker, M. G., Lopez, L. D., Cannon, M. C., De Lisle, G. W. & Collins, D. M. Continuing Mycobacterium bovis transmission from animals to humans in New Zealand. Epidemiol. Infect.134, 1068–1073. 10.1017/S0950268806005930 (2006). 10.1017/S0950268806005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibson, A. L. et al. Molecular epidemiology of disease due to Mycobacterium bovis in humans in the United Kingdom. J. Clin. Microbiol.42, 431–434. 10.1128/JCM.42.1.431-434.2004 (2004). 10.1128/JCM.42.1.431-434.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guven Gokmen, T. et al. Molecular characterization of Mycobacterium bovis strains isolated from cattle and humans by spoligotyping and 24-locus MIRU-VNTR, and prevalence of positive IGRA in slaughterhouse workers in Southern Turkey. Iran J. Vet. Res.23, 210–218. 10.22099/IJVR.2022.42580.6186 (2022). 10.22099/IJVR.2022.42580.6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lari, N., Rindi, L., Bonanni, D., Tortoli, E. & Garzelli, C. Molecular analysis of clinical isolates of Mycobacterium bovis recovered from humans in Italy. J. Clin. Microbiol.44, 4218–4221. 10.1128/JCM.01216-06 (2006). 10.1128/JCM.01216-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortiz, A. P. et al. Whole genome sequencing links Mycobacterium bovis from cattle, cheese and humans in Baja California, Mexico. Front. Vet. Sci.8, 674307. 10.3389/fvets.2021.674307 (2021). 10.3389/fvets.2021.674307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palaniyandi, K. et al. Isolation and comparative genomics of Mycobacterium tuberculosis isolates from cattle and their attendants in South India. Sci. Rep.9, 17892. 10.1038/s41598-019-54268-x (2019). 10.1038/s41598-019-54268-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahdan, A., Riad, E. M. & Enany, S. Genetic differentiation of Mycobacterium bovis and Mycobacterium tuberculosis isolated from cattle and human sources in, Egypt (Suez Canal area). Comp. Immunol. Microbiol. Infect. Dis.73, 101553. 10.1016/j.cimid.2020.101553 (2020). 10.1016/j.cimid.2020.101553 [DOI] [PubMed] [Google Scholar]
- 62.Allix-Beguec, C. et al. Importance of identifying Mycobacterium bovis as a causative agent of human tuberculosis. Eur. Respir. J.35, 692–694. 10.1183/09031936.00137309 (2010). 10.1183/09031936.00137309 [DOI] [PubMed] [Google Scholar]
- 63.Poyntz, H. C. et al. Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis. Tuberculosis94, 226–237. 10.1016/j.tube.2013.12.006 (2014). 10.1016/j.tube.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanner, R., Villarreal-Ramos, B., Vordermeier, H. M. & McShane, H. The humoral immune response to BCG vaccination. Front. Immunol.10, 1317. 10.3389/fimmu.2019.01317 (2019). 10.3389/fimmu.2019.01317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verma, D., Chan, E. D. & Ordway, D. J. Non-tuberculous mycobacteria interference with BCG-current controversies and future directions. Vaccines10.3390/vaccines8040688 (2020). 10.3390/vaccines8040688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mareledwane, V., Adesiyun, A. A. & Hlokwe, T. M. Absence of tuberculosis-causing cycobacteria from slaughtered livestock tissues and environmental samples, Gauteng Province, South Africa. Int. J. Microbiol.2024, 4636652. 10.1155/2024/4636652 (2024). 10.1155/2024/4636652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komijn, R. E. et al. Prevalence of Mycobacterium avium in slaughter pigs in The Netherlands and comparison of IS1245 restriction fragment length polymorphism patterns of porcine and human isolates. J. Clin. Microbiol.37, 1254–1259. 10.1128/JCM.37.5.1254-1259.1999 (1999). 10.1128/JCM.37.5.1254-1259.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghielmetti, G. et al. Mycobacterial infections in wild boars (Sus scrofa) from Southern Switzerland: Diagnostic improvements, epidemiological situation and zoonotic potential. Transbound. Emerg. Dis.68, 573–586. 10.1111/tbed.13717 (2021). 10.1111/tbed.13717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gcebe, N., Rutten, V. P. M. G., van Pittius, N. G., Naicker, B. & Michel, A. L. Mycobacterium komaniense sp. nov., a rapidly growing non-tuberculous Mycobacterium species detected in South Africa. Int. J. Syst. Evol. Microbiol.68, 1526–1532. 10.1099/ijsem.0.002707 (2018). 10.1099/ijsem.0.002707 [DOI] [PubMed] [Google Scholar]
- 70.Smith, N. H. et al. Ecotypes of the Mycobacterium tuberculosis complex. J. Theor. Biol.239, 220–225. 10.1016/j.jtbi.2005.08.036 (2006). 10.1016/j.jtbi.2005.08.036 [DOI] [PubMed] [Google Scholar]
- 71.Varela-Castro, L. et al. Beyond tuberculosis: Diversity and implications of non-tuberculous mycobacteria at the wildlife-livestock interface. Transbound. Emerg. Dis.69, e2978–e2993. 10.1111/tbed.14649 (2022). 10.1111/tbed.14649 [DOI] [PubMed] [Google Scholar]
- 72.Murphy, S. G. et al. Direct detection of drug-resistant Mycobacterium tuberculosis using targeted next generation sequencing. Front. Public Health11, 1206056. 10.3389/fpubh.2023.1206056 (2023). 10.3389/fpubh.2023.1206056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szoboszlay, M. et al. Nanopore is preferable over Illumina for 16S amplicon sequencing of the gut microbiota when species-level taxonomic classification, accurate estimation of richness, or focus on rare taxa is required. Microorganisms10.3390/microorganisms11030804 (2023). 10.3390/microorganisms11030804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tafess, K. et al. Targeted-sequencing workflows for comprehensive drug resistance profiling of Mycobacterium tuberculosis cultures using two commercial sequencing platforms: Comparison of analytical and diagnostic performance, turnaround time, and cost. Clin. Chem.66, 809–820. 10.1093/clinchem/hvaa092 (2020). 10.1093/clinchem/hvaa092 [DOI] [PubMed] [Google Scholar]
- 75.Damene, H. et al. Broad diversity of Mycobacterium tuberculosis complex strains isolated from humans and cattle in Northern Algeria suggests a zoonotic transmission cycle. PLoS Negl. Trop. Dis.14, e0008894. 10.1371/journal.pntd.0008894 (2020). 10.1371/journal.pntd.0008894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanoussi, C. N., Affolabi, D., Rigouts, L., Anagonou, S. & de Jong, B. Genotypic characterization directly applied to sputum improves the detection of Mycobacterium africanum West African 1, under-represented in positive cultures. PLoS Negl. Trop. Dis.11, e0005900. 10.1371/journal.pntd.0005900 (2017). 10.1371/journal.pntd.0005900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beissner, M., Phillips, R. O. & Bretzel, G. Determining viability of M. ulcerans by 16S rRNA RT reverse transcriptase real-time PCR. Methods Mol. Biol.2387, 81–86. 10.1007/978-1-0716-1779-3_9 (2022). 10.1007/978-1-0716-1779-3_9 [DOI] [PubMed] [Google Scholar]
- 78.Goosen, W. J. et al. Improved detection of Mycobacterium tuberculosis and M. bovis in African wildlife samples using cationic peptide decontamination and mycobacterial culture supplementation. J. Vet. Diagn. Investig.34, 61–67. 10.1177/10406387211044192 (2022). 10.1177/10406387211044192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belakehal, F. et al. Evaluation of the discriminatory power of spoligotyping and 19-locus mycobacterial interspersed repetitive unit-variable number of tandem repeat analysis (MIRU-VNTR) of Mycobacterium bovis strains isolated from cattle in Algeria. PLoS ONE17, e0262390. 10.1371/journal.pone.0262390 (2022). 10.1371/journal.pone.0262390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rupf, S., Merte, K. & Eschrich, K. Quantification of bacteria in oral samples by competitive polymerase chain reaction. J. Dent. Res.78, 850–856. 10.1177/00220345990780040501 (1999). 10.1177/00220345990780040501 [DOI] [PubMed] [Google Scholar]
- 81.Wick, R. R., Judd, L. M. & Holt, K. E. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol.20, 129. 10.1186/s13059-019-1727-y (2019). 10.1186/s13059-019-1727-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinig, E. & Coin, L. Nanoq: Ultra-fast quality control for nanopore reads. JOSS7, 2991 (2022). 10.21105/joss.02991 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study were submitted to the European Nucleotide Archive (ENA) under project reference number PRJEB75236, https://www.ebi.ac.uk/ena/browser/view/PRJEB75236.