Abstract
The oil obtained from black cumin (Nigella sativa) seeds has many health-effective properties, which is used in food applications and in traditional medicine. One practical method to extract its oil is mixing with other seeds such as sunflower (Helianthus anuus) seeds before oil extraction by press. The effectiveness of the cold-press oil obtained from the mixture of black cumin seeds (BS) and sunflower seeds (SF) in different proportions 100:0, 95:5, 90:10, 85:15 and 0:100 (w/w) was studied to evaluate their qualitative properties including peroxide value (PV), acid value, p-anisidine value (AnV), pigments (carotenoid and chlorophyll) content, polyphenols, and profile of fatty acids during heating process (30–150 min at 180 °C). The results revealed that the acid and p-anisidine value of the all samples enhanced with the extension of the heating time, and the peroxide value increased at the beginning of the heating and then decreased with the prolongation of the heating time (p < .05). With the increase of temperature and heating time, the peroxide of sunflower oil increased with a higher slope and speed than that of black seed and blends oil. Changes in the PV and AnV were the fastest in sunflower oil. Blending and heating caused considerable changes in the fatty acid composition of oils, especially myristic, palmitic, and stearic acids. Moreover, the levels of certain unsaturated fatty acids, namely linoleic, oleic, and linolenic acids declined after heating. The carotenoids, chlorophyll and total phenol content decreased gradually during heating treatments. Among extracted oils, SF:BS (15%) had the good potential for stability, with total phenol content of 95.92 (Caffeic acid equivalents/100 g), PV of 2.16 (meq O2/kg), AV of 2.59 (mg KOH/g oil), and AnV of 8.08 after the heating. In conclusion, oil extracted from the mixture of SF and BS can be used as salad and cooking oils with a high content of bioactive components and positive nutritional properties.
Keywords: Black cumin sunflower oil, Cold press, Physicochemical properties, Thermal stability
Subject terms: Environmental sciences, Chemistry
Introduction
Sunflower oil is one of the most popular edible oils which is generally utilized in cooking and frying processes. This traditional oil contains relatively high content of polyunsaturated fatty acids (PUFA) (85–95%) including omega-6 FAs and especially linoleic acid, which makes it prone to thermooxidative degradation, discoloration, off-flavor, rancidity and limits their applications as frying oils1,2. The fatty acid profile of sunflower oil varies due to factors such as genetic modifications, environmental conditions, and geographical locations. Studies have shown that sunflower oil composition can be modified through conventional breeding techniques and genetic modifications, leading to changes in the levels of oleic, linoleic, palmitic, and stearic acids3,4.To overcome the low oxidative stability of frying oils and retardation of oil degradation, various methods have been applied, such as hydrogenation, blending and using natural or synthetic antioxidants5–10 .However, synthetic antioxidants may cause numerous health risks11. Animal experiments have shown that high-dose exposure to these synthetic antioxidants can be toxic, leading to DNA damage, mismatches, and the development of cancerous tumors12. The application of most common synthetic antioxidants, butylated hydroxyl anisole (BHA) and butylated hydroxyl toluene (BHT), has been limited due to their side effects and health risks, including carcinogenic effects13,14. For that reason, the importance of replacing synthetic antioxidants with natural components from oil seeds and other plant resources has noticeably increased.
Black cumin seed (Nigella sativa L.) (BS) belongs to the Ranunculaceae family, is an emerging source of vegetable oil used as a multi-purpose product for food industry, nutritional, cosmetic and therapeutic applications15,16. Among the numerous sources of oils, BS oil has high levels of bioactive components, including total phenol content, carotenoids, and essential fatty acids. Also, a high amount of natural antioxidants such as polyphenols (245–309 mg/kg), thymoquinone (26.8–54.8 mg/kg) and tocopherols (91–246 mg/kg) make this oil nutritious and health effective17. However, this oil has low application in food industry as it has strong flavour and relatively dark color, which needs to be improved to enhance its application as it has many positive health promoting effects.
Frying is one of the well-known procedures for cooking, which leads to making products with the desired flavor and distinctive sensorial properties18. Through frying at high temperatures between 150 and 190 °C, which occurs in the presence of oxygen and water, various reactions might occur including oxidation, pyrolysis, hydrolysis, cyclization and polymerization, which lead to changes in the nutritional quality of frying oils19. Moreover, toxic products such as aldehydes, ketones, cyclic and epoxy constituents, dimers, oligomers, trans-isomers, polar compounds, peroxides and epoxides can be formed. Also, oxidation can produce undesirable off-flavors and reduce the shelf life of products20.
Using thermally effective antioxidants in frying oils can be highly efficient in oil stability. Previous research confirmed enhanced stabilization of SF oil blended with individual and mixtures of some natural antioxidants such as Tiger nut (Cyperus esculentus L.), Moringa oleifera, and oleoresin rosemary5,21,22. In particular, Kiralan et al.studied the blending of SF oil with black cumin oil as an innovative practice with several benefits, such as oxidation stability improvement due to the high amount of thymoquinone and γ‐tocopherol of Nigella sativa oil.
BS is rich source of bioactive components which remains in the cake produced during oil extraction by press. Mixing BS with other oilseeds with high content of oil such as sunflower oil can help to extract bioactive components as much as possible from BS during oil extraction by press, and also can affect the extracted oil quality and oxidative stability as well. Therefore, the present investigation aimed to compare the effectiveness of oil extracted from a combination of different portions of seeds (BS: SF) by cold press on the deterioration rate of SF oil subjected to heating process at a temperature of 180 °C. Moreover, it aimed to evaluate the impact of BS as a rich source of natural bioactive components on the oxidative stability of extracted oils at the high temperature (180 °C).
Materials and methods
Ingredients
The BS (Nigella sativa L.) and SF (Helianthus annuus) seeds were bought from a local market (Tabriz city, Iran). The solvents, solutions, and reagents such as sodium hydroxide, sodium thiosulfate, chloroform, ethanol, phenolphthalein, n-hexane and acetic acid glacial were purchased from Merck & Co., Inc. (Darmstadt, Germany). Also, the standards, the caffeic acid and the Folin–Ciocalteu were obtained from Sigma–Aldrich Co. (St. Louis, MO, USA).
Sample processing
The BS and SF seeds were mixed at a ratio of 100:0, 95:5, 90:10, 85:15, and 0:100 (SF:BS) (w/w) and then oil from the seed mixtures were extracted by cold press (screw press 85 Mm, Kern Kraft, Germany) based on the method mentioned by Mazaheri et al.24. The obtained oils were filtered using Whatman No.2 filter paper. The final oil samples were put in sealed 100 ml dark glass bottles and kept at 4 °C.
Heating technique
The 70 g of extracted oil samples was weighed, put into an aluminum cupcake foil, and placed into the oven (Astar DKL-40, Guangdong, China) at 180 ± 2 °C for 0 (control samples), 30, 60, 90, 120 and 150 min25.
Fatty acid profile
The oil was transformed to fatty acid methyl esters (FAMEs) according to the method of Savage et al.26. FAMEs were identified via a gas chromatograph (GC-1000, DANI, Italy).
Peroxide and acid values
The acid value (AV: mg KOH/g) and the peroxide value (PV: meqO2/kg oil) were detected by titration based on the methods mentioned by the American Oil Chemists’ Society (AOCS 2017)27.
Anisidine value
The p-anisidine value (AnV; mg KOH/g oil) of the oil samples was measured by AOCS Method27.
Calculation of totox value (TV)
Totox value was calculated based on PV and AnV, by using the following equation28:
For each determination, three replicates were analyzed per sample.
Carotenoid and chlorophyll contents
The chlorophyll and carotenoid pigments were analyzed29. For chlorophyll content, absorption was determined at 630, 670 and 710 nm; for carotenoids, absorption was measured at 670 nm; and the contents were measured by the following equations:
| 1 |
| 2 |
where A is the absorbance and L is the spectrophotometer cell thickness (10 mm).
Total phenol content
The total phenol content of the oils was evaluated as mg of caffeic acid/kg of the oil using the Folin-Ciocalteu reagent. Absorption was determined at 765 nm via an Aquaris 1100 spectrometer (Cecil Instruments Ltd., Cambridge, UK)30.
Statistical analysis
All the values were calculated with three replications. The records were analyzed by use of ANOVA in factorial experiments in a completely randomized design by 20.0 SPSS statistical software (Chicago, IL, USA). The final data was reported as the mean ± standard deviation (SD). To determine the significant differences at 0.05 level, Duncan’s multiple ranges post hoc experiment was employed.
Results and discussion
Oil yield
Different factors, including temperature, pressure, and the process of oil extraction affect oil yield17. Oil yield of SF, BS, and from their mixtures were determined. The oil yield from SF (51.7%) was higher than the oil yield reported previously31. The oil yield from BS was 22.0%, which was similar to the oil yield reported by Suri et al.32. The co-pressing of BS with SF seeds demonstrated a significant (P < 0.05) effect on the oil extraction yield. SF seeds in the mixture of seeds could help to increase the efficiency of BS oil extraction. The oil yield was increased to 47.23%, 48.04% and 49.7% by co-pressing BS with 85%, 90% and 95% SF, respectively. Gharby et al.34 showed that the oil extraction yield of Nigella sativa using solvent or cold press was 37% and 27%, respectively. Also it was observed that co-pressing Jatropha curcus kernels with rapeseed, maize, or soybean seeds increased the oil extraction yield33,34.
Changes in major fatty acids
The profile of fatty acid (FA) has a considerable effect on oxidative stability of the oils and nutritional properties of oils33. The most predominant FA which have been detected in BS oil were linoleic acid (58.06%) followed by oleic acid (23.88%) and palmitic acid (12.88%). SF oil showed the greatest percentages of oleic acid (46.42%) followed by linoleic acid (44.12%), and palmitic acid (5.53%). These findings are in agreement with the other reported data1,24,36–38. However, it should be mentioned that according to the fatty acid composition reported by the Codex Alimentarius, this sunflower oil belongs to the mid oleic acid class, which oleic acid and linoleic acid are 43.1–71.8 and 18.7–45.3%, respectively39. Increased BS quantity in the mixture of oilseeds before cold pressing could affect the amount of linoleic acid. However, a significant increase (P < 0.05) in the palmitic and myristic acid compared with pure SF oil was observed. It has been reported that blending can make a noticeable change in the fatty acid composition of obtained oils40.
During heating, considerable changes (P < 0.05) was observed in saturated fatty acids, especially myristic, palmitic, and stearic acids in all oils. Also, the high temperature (180 °C) lessens the level of some unsaturated fatty acids such as linoleic, oleic, and linolenic acids (P < 0.05) (Table 1). The decreased unsaturated fatty acid percentage over the process of heating at high temperatures may be due to destructive reactions such as hydrolysis, oxidation, and polymerization41. Therefore, because of a reduction in the percentage of the unsaturated fatty acids, there has been a noticeable increase in the level (percentage) of saturated fatty acids after the heating treatment (P < 0.05). This result was in agreement with Casal et al.42, who reported that there was significant decrease in unsaturated fatty acids in SF oil during the heating, with a consequent rise in saturated fatty acid levels.
Table 1.
Fatty acid composition (%) of sunflower, black seed and their mixtures oil in different time at heating condition (180 ± 2 °C).
| Fatty acid | Sunflower (SF) | Black seed (BS) | SF:BS (95:5, W/W)* | SF:BS (90:10, W/W) | SF:BS (85:15, W/W) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Time (min) | Time (min) | Time (min) | Time (min) | ||||||
| 0 | 150 | 0 | 150 | 0 | 150 | 0 | 150 | 0 | 150 | |
| C14:0 | 0.05 ± 0.005f | 0.06 ± 0.52e | 0.18 ± 0.00b | 0.19 ± 0.01a | 0.06 ± 0.002e | 0.06 ± 0.005e | 0.06 ± 0.01e | 0.06 ± 0.03e | 0.07 ± 0.00d | 0.08 ± 0.01c |
| C16:0 | 5.53 ± 0.07g | 6.78 ± 0.04c | 12.88 ± 0.26b | 13.22 ± 0.05a | 5.56 ± 0.02fg | 6.01 ± 0.05de | 5.70 ± 0.00f. | 5.88 ± 0.02e | 5.80 ± 0.02e | 6.10 ± 0.06d |
| C18:0 | 3.36 ± 0.11c | 3.75 ± 0.02a | 2.50 ± 0.05f | 2.76 ± 0.00e | 3.84 ± 0.05c | 3.92 ± 0.011cb | 3.30 ± 0.02c | 3.53 ± 0.02b | 3.24 ± 0.05cd | 3.31 ± 0.02c |
| C18:1 | 46.42 ± 0.02a | 46.96 ± 0.07a | 23.88 ± 0.11d | 24.18 ± 0.10d | 45.50 ± 0.02b | 45.70 ± 0.12b | 45.04 ± 0.05b | 45.30 ± 0.12b | 44.87 ± 0.13c | 44.89 ± 0.1c |
| C18:2 | 44.12 ± 0.05c | 42.87 ± 0.06d | 58.06 ± 0.24a | 57.24 ± 0.01b | 42.80 ± 0.1d | 41.50 ± 0.02e | 44.36 ± 0.01c | 43.21 ± 0.05d | 44.5 ± 0.02c | 43.34 ± 0.00d |
| C18:3 | 0.13 ± 0.01b | 0.03 ± 0.11d | 0.34 ± 0.00a | 0.32 ± 0.01a | 0.12 ± 0.05b | 0.07 ± 0.00c | 0.13 ± 0.00b | 0.09 ± 0.01c | 0.12 ± 0.01b | 0.08 ± 0.00c |
Different letters represent significant differences (P < 0.05).
All values are the mean of three replicates ± standard deviation of the mean.
*Sunflower: Black seed oil.
Peroxide value
The peroxide value (PV) is an indicator of the hydroperoxide compounds and shows the amount of primary products through the initial stages of lipid oxidation43. Peroxides are important intermediate substances of oxidative reactions due to their capability to undergo decomposition via transition metal irradiation and elevated temperatures, thus resulting in the production of free radicals44. The PVs of BS and SF oils were 5.6 and 0.24 (meq O2/ oil kg), respectively. The PV of extracted oil samples were increased as BS increased in the seed mixtures (Table 2).
Table 2.
Physicochemical properties of sunflower, black seed and their mixtures oil in different time at heating condition (180 ± 2 °C).
| Time (min) | Acid value (mg KOH/g oil) | Peroxide value (meq O2/kg oil) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Treatments | |||||||||
| Sunflower | Black seed | SF:BS (95:5)* | SF:BS (90:10) | SF:BS (85:15) | Sunflower | Black seed | SF:BS (95:5) | SF:BS (90:10) | SF:BS (85:15) | |
| 0 | 0.04 ± 0.00e | 0.68 ± 0.01d | 0.056 ± 0.00e | 0.073 ± 0.00e | 0.083 ± 0.01b | 0.24 ± 0.03d | 5.6 ± 0.52f. | 0.35 ± 0.05e | 0.48 ± 0.02e | 0.85 ± 0.09e |
| 30 | 0.8 ± 0.01d | 10.45 ± 0.11c | 0.85 ± 0.01d | 0.88 ± 0.02d | 2.41 ± 0.12a | 4.11 ± 0.12a | 92.4 ± 0.25a | 7.43 ± 0.4a | 8.76 ± 0.25a | 9.58 ± 0.07b |
| 60 | 0.87 ± 0.01d | 10.59 ± 0.28c | 0.89 ± 0.01c | 0.93 ± 0.01c | 2.51 ± 0.04a | 5.46 ± 0.15c | 84.6 ± 0.53b | 7.9 ± 0.28a | 8.62 ± 0.14a | 13.45 ± 0.31a |
| 90 | 0.88 ± 0.01bc | 11.28 ± 0.3b | 0.92 ± 0.01b | 0.94 ± 0.01bc | 2.56 ± 0.1a | 7.78 ± 0.25a | 24.06 ± 0.25c | 3.31 ± 0.2b | 5.37 ± 0.32b | 4.38 ± 0.25c |
| 120 | 0.9 ± 0.0b | 11.61 ± 0.3b | 0.93 ± 0.01b | 0.96 ± 0.00ab | 2.52 ± 0.07a | 5.72 ± 0.1c | 18.48 ± 0.44d | 2.74 ± 0.25c | 3.9 ± 0.1c | 2.69 ± 0.1d |
| 150 | 0.93 ± 0.01a | 12.26 ± 0.36a | 0.96 ± 0.01a | 0.97 ± 0.01a | 2.59 ± 017a | 6.12 ± 0.12b | 16.7 ± 0.25e | 1.99 ± 0.13d | 2.7 ± 0.05d | 2.16 ± 0.19d |
Values (mean ± SD, n = 3) denoted by the same letter do not constitute statistically significant differences at P ˂ 0.05 among treatments.
*Sunflower: Black seed oil (w/w).
PV of oil samples was increased significantly during the heating process and was strongly correlated with the prolonging of the heating period and BS level in the mixture of seeds (P < 0.05) (Table 2). Peroxide products are not stable and decompose to secondary compounds such as carbonyl and aldehydes under frying conditions45. Hence, it was reduced after 60 min of heating (Table 2). Karakaya and Simsek46 found that the PV changes for edible oils (corn, hazelnut, soybean, and olive oils) demonstrated different patterns in repeated deep fat frying. Akil et al.47 found the similar findings after the process and preparation of French fries. As well, a substantial rise in PV was found in high oleic SF oil after 50 min frying (1.63–38.80 meq O2/kg)48.
Among the samples, the BS oil had a high PV. The nature of the seed is the most effective reason for the high peroxide value that is because of the high lipoxygenase activity and unsaturated fatty acid content in BS17,49.
In a study conducted by Quiles et al.50, the PV of SF oil enhanced gradually up to 10 (meq O2/kg oil) through 60 min of frying process. Mudawi et al.51 reported that the PV of SF oil was assessed as 8.8 (meq O2/kg) in fried potatoes after four days, but PV quickly decreased to 4.7 (meq O2/kg) on the fifth day of frying. On the other hand, pure BS oil had the highest PV, but blending BS with SF seeds prior to extraction resulted in more stable oils at heating temperatures (180 °C). In fact, SF in the blend can cause effective delay in oils oxidation. There are many antioxidants in the used oils such as tocopherols and thymoquinone, which could have positive effects on the oxidation stability of the oils52. The obtained results showed that using pure BS is not suitable and by mixing the BS with other seeds such as SF could give oils with high enough bioactive components with good oxidative stability. This can be a practical approach to take advantage of the nutritional properties of BS.
Acid value
Acid value was utilized to evaluate the frying oil quality and indicate free fatty acids initiated from the triglycerides hydrolysis53. a considerable and gradual (P < 0.05) rise in the acid values was observed after heating (180 °C ± 2 °C, 150 min) (Table 2). The acid values were 0.04 and 0.68 (mg KOH/g oil) for SF and BS oil, respectively. By increasing the heating time, the AV of all oil samples was increased (P < 0.05). At the end of the frying process, the lowest and highest AVs (0.93 and 12.26 mg KOH/g oil) were determined for SF (100%) and BS oil (100%), respectively. The oils with higher quantities of BS displayed higher AV compared to the other blends.
In this study, the high acid value is mainly due to the lipolitic enzyme activity of BS. The AV of BS oil can be declined considerably by thermal pretreatments including microwaving and roasting of BS before oil extraction by press17. Likewise, refining could be the most effective step in removing the free fatty acids by saponification42.
The increase in AV during heat treatment and frying is commonly due to the triacylglycerol hydrolysis and degradation of secondary oxidation compounds resulting from the process of heating and the existing of oxygen. The moisture can help and increase the formation of free fatty acids54. Rehab and El Anay21 reported the maximum change in the acid value of SF oil, which AV raised from 0.13 to 0.69 KOH/g oil after frying duration 3 h, while by enhancing the blending ratio of tiger nut oil, the lowest change was reported. This was due to antioxidative and anti-hydrolytic properties of the high amount of phenol content of tiger nut during the frying process.
Anisidine value
Although the peroxide value is a good indicator for determination of the main compounds of oxidation at room temperature, it is not a sufficient biomarker or accurate criterion for estimating the secondary by-products of oxidation at high degrees55. At high temperature, hydroperoxides decompose and aldehydes form, that are known as secondary oxidation compounds. The p-anisidine value (AnV) is well-known and also the most beneficial experiment for the evaluation of progressive oxidative rancidity in the edible oils.
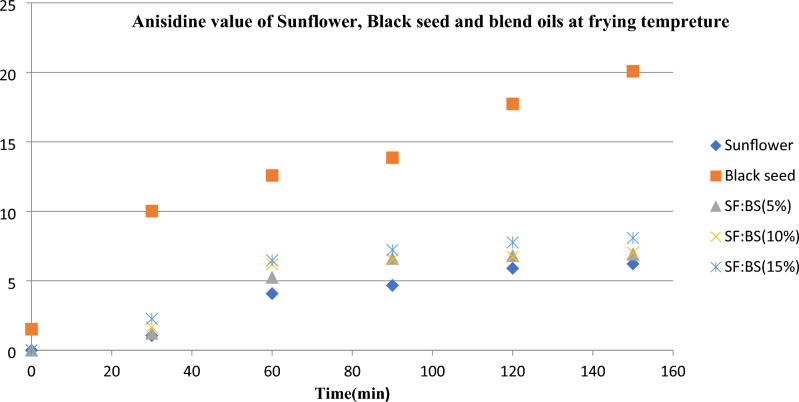
The AnV values before heating were measured for oil samples, while BS oil showed the highest initial AnV (1.52). At the end of the 2.5 h of heating, the AnV was observed 20.08 for BS and 6.22 SF oils, respectively. Also, blended oils demonstrate a steady increase in the AnV, representing the formation of secondary oxidation products (Fig. 1). Formation of aldehydes and their conversion into secondary oxidation compounds are highly related to the oils composition and conditions of processing5 .In fact, there were no significant differences between the AnV values of the obtained oils from the mixture of seeds at proportion of 5%, 10 and 15% (P < 0.05) during heating process. However, extracted oil with 15% BS presents the highest AnV compared to the oils obtained from the seed mixtures (2.26–8.08). However, this value was in the range of standards which is determined for high stable vegetable oils (less than 10)56.
Figure 1.
Anisidine value of sunflower, black seed and blend oils at frying temperature (180 ± 2 °C).
Totox value
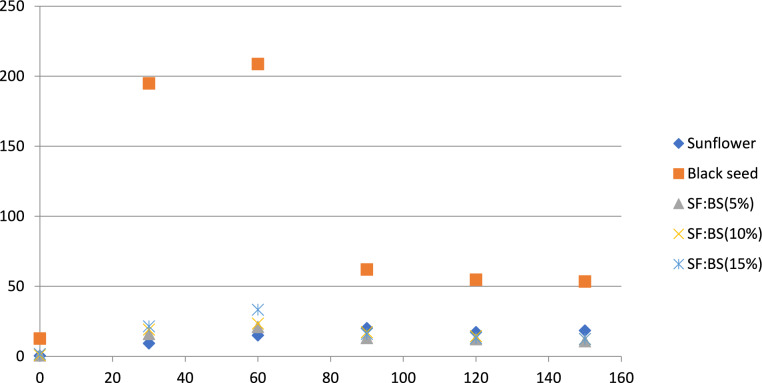
The totox index is frequently employed for the evaluation of lipid oxidative degradation due to its capability to integrate the levels of primary oxidation byproducts (hydroperoxides) and secondary byproducts (mainly alkenals and alkadienals) within fats or oil28. As shown in Fig. 1, BS showed the highest totox value (12.72–53.48) and SF oil displayed the lowest (0.48–18.46). Additionally, no significant differences was observed between the Totox indexs of the extracted oils from the mixture of seeds the 5%, 10 and 15% (P < 0.05) at the end of 2.5 h heating process (Fig. 2).
Figure 2.
Totox value of sunflower, black seed and blend oils at frying temperature (180 ± 2 °C).
Carotenoid and chlorophyll content
Carotenoids are valuable fraction of the BS oil composition with high antioxidant activity and nutritional effect57,58. The initial carotenoid contents of BS and SF oils extracted from seeds were 3.79 and 0.6 (mg/kg oil), respectively. With an increased heating time, a significant reduction in the carotenoid content was observed in all samples (P < 0.05) (Table 3). The heating time and the type of edible oils showed an important influence on the pigment composition. The reduction in the carotenoid content in SF oil was quite very significant and degraded at a faster rate compared to the other oils (0.19–0.6-mg/kg). However, this reduction in the carotenoid content was less in BS oil and the oils made up of a mixture of 15%, 10%, and 5%, respectively (Table 3). These results were in accordance with the previous studies stating that only low levels of β-carotene were detected in the SF oil (0.23 mg/kg) and that the amount of carotenoids was so low that it could not be measured at the end of 3 h of the frying process42. This degradation could be associated with the changes in the level of β-carotene. On the other hand, β-carotene, through frying temperature and exposure to the air, polymerization occurs as a main side-reaction and the heating process causes the loss of carotenoid pigments59.
Table 3.
Chlorophyll and carotenoid pigments of the oil samples at heating condition (180 ± 2 °C).
| Time (min) | Carotenoid content (mg/kg) | Chlorophyll content (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Treatments | |||||||||
| Sunflower | Black seed oil | SF:BS* | SF:BS** | SF:BS*** | Sunflower | Black seed | SF:BS* | SF:BS** | SF:B** | |
| 0 | 0.60 ± 0.01a | 3.79 ± 0.1a | 0.65 ± 0.03a | 1.10 ± 0.1a | 1.52 ± 0.1a | 2.9 ± 0.08a | 75.63 ± 0.55a | 6.9 ± 0.1a | 13.77 ± 0.25a | 16.2 ± 0.29a |
| 30 | 0.34 ± 0.0b | 2.83 ± 0.15b | 0.62 ± 0.0a | 0.78 ± 0.01b | 0.79 ± 0.01b | 1.5 ± 0.5b | 63.33 ± 5.6b | 5.7 ± 0.26b | 8.7 ± 0.25b | 12.4 ± 0.36b |
| 60 | 0.34 ± 0.01b | 2.86 ± 0.06b | 0.60 ± 0.03a | 0.67 ± 0.02c | 0.72 ± 0.03b | 0.63 ± 0.15c | 61.26 ± 1.1b | 5.1 ± 0.38c | 8.03 ± 0.15bc | 12.1 ± 0.15b |
| 90 | 0.27 ± 0.01c | 2.80 ± 0.1b | 0.51 ± 0.01b | 0.58 ± 0.01d | 0.59 ± 0.01c | 0.53 ± 0.05c | 57.83 ± 1.89b | 5.1 ± 0. 1c | 7.72 ± 0.25c | 11.23 ± 0.25c |
| 120 | 0.23 ± 0.0c | 2.09 ± 0.09c | 0.34 ± 0.04c | 0.33 ± 0.01e | 0.41 ± 0.01d | 0.47 ± 0.01c | 48.33 ± 9.5b | 4.9 ± 0.15c | 6.79 ± 0.97d | 10.77 ± 0.11c |
| 150 | 0.19 ± 0.01d | 2.06 ± 0.11c | 0.23 ± 0.01c | 0.31 ± 0.01e | 0.34 ± 0.02e | 0.32 ± 0.02d | 43.6 ± 1.5c | 4.43 ± 0.37d | 5.23 ± 0.25e | 9.56 ± 0.48d |
Values (mean ± SD, n = 3) denoted by the same letter do not constitute statistically significant differences at P˂ 0.05 among treatments.
*Sunflower: Black seed oil (95:5, W/W).
**Sunflower: Black seed oil (90:10, W/W).
***Sunflower: Black seed oil (90:15, W/W).
Also, Boukandoul et al.5 found a remarkable decrease in the amount of carotenoids in SF oil in the initial hours of frying from the reaction of these components with free radicals. However, by adding Moringa oleifera oil (MO) to sunflower oil, the maximum antioxidant effect was found in the SF:MO blend over frying because of high levels of carotenoids .Table 3 depicts the chlorophyll content changes of the oils that were subjected to the heating process. With the increase in the heating temperature, a similar way was observed. Chlorophyll content decreased gradually, as well as carotenoids. In Luaces et al.60 study, heat process can change the amount of edible oil pigment. The highest chlorophyll content was shown by the BS oil (43.6–75.63-mg/kg), followed by the BS: SF oil with 15% (9.56–16.2-mg/kg), the BS: SF oil with 10% (5.23–13.77-mg/kg), and the BS: SF oil with 5% (4.43–6.9-mg/kg), in priority orders. BS oil had a higher chlorophyll content compared with SF oil and blends, of course, the SF:BS (15%) oil. However, at the end of the heating, the total chlorophyll (the dark green color of the oils is because of the high content of chlorophyll) contents of BS oil, SF oil, and the blends significantly reduced (P < 0.05). The thermal process leads to the loss of chlorophyll content, which could be due to high degradation of the green pigments, which cause the color of the oil. It may be related to an oxidative reaction and polymerization of polyphenolic compounds61.
Total phenol content
Total Phenol content has been observed to have considerable antioxidant properties and an important effect in preventing different diseases such as cancer and cardiovascular diseases. By comparison to the other vegetable oils, BS oils are getting lots of attention for the presence of phenolic compounds, which provide desirable antioxidative properties and show a major role in preventing diseases62. Table 4 shows the changes in the total phenol content (TPC) of SF, BS and blended oils through thermal oxidation. The primary total phenol content in the SF oil (27.17 mg GAE kg−1 oil) was detected as the lowest, but TPC in the BS oil (592.66 mg GAE kg−1 oil) was determined as the maximum.
Table 4.
Total phenol content (mg Caffeic acid/Kg oil) of treatments during heating (180 ± 2 °C).
| Treatments | Time (min) | |||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | |
| Total phenol content (mg Caffeic acid/kg oil) | ||||||
| Sunflower | 27.17 ± 0.48a | 0.12 ± 0.02b | nd | nd | nd | nd |
| Black seed | 592.66 ± 11a | 474.18 ± 24b | 406.83 ± 11.9c | 264.68 ± 39.3d | 191.93 ± 10.1e | 197.28 ± 23.4e |
| SF:BS* | 141.92 ± 2.91a | 103.1 ± 3.2b | 90.68 ± 4.6c | 60.87 ± 5.1d | 56.48 ± 0.82d | 40.18 ± 6.01e |
| SF:BS* | 231.47 ± 1.3a | 172.8 ± 20b | 99.58 ± 1.22c | 79.56 ± 0.97d | 74.68 ± 1.85de | 62.13 ± 6.18e |
| SF:BS* | 322.62 ± 1.5a | 273.08 ± 16.54b | 189.36 ± 9.8c | 143.43 ± 7.2d | 118.41 ± 6.6e | 95.92 ± 2.3f. |
Values (mean ± SD, n = 3) denoted by the same letter do not constitute statistically significant differences at P ˂ 0.05 among treatments.
*Sunflower: Black seed oil (95:5, W/W).
**Sunflower: Black seed oil (90:10, W/W).
***Sunflower: Black seed oil (90:15, W/W).
Generally, the total phenol content decreased with increasing the heating time and the heating procedure significantly lessened the content of the phenols (P < 0.05). At high temperatures, the TPC of the SF oil disappeared after 60 min of heating. This could prove that thermal treatment leads to the thermal oxidation and also polymerization of the polyphenols63. A close connection between TPC and the stability of oils towards oxidation has been observed in other investigations. The higher content for BS can confirm more stability and higher resistance in frying conditions. TPC prevents the harmful effects of free radicals and protects the oils against oxidation reactions50. It is noteworthy that polyphenol content is related predominantly to their antioxidant properties. They also demonstrate high biological activity in vivo and may be useful in preventing diseases correlated with extreme oxygen radical improvement exceeding the antioxidant defense capacity of the human body63. Addition to BS with SF was strongly effective in the total phenol content of the blend oils, and the stability of SF oil and other mixture of edible oil considerably increased during heating. On the whole, the oxidative stability of oils obtained from seed blends was better than sunflower oil alone, most probably as high levels of components such as tocopherols and total phenol content with high antioxidative activity, and low content of bioactive lipids in BS oils17,62,64.
Conclusions
The obtained results display that the heating of SF, BS and blend oils for 150 min at 180 °C makes considerable changes in the qualitative properties of the extracted oils. Outcomes indicated that the heating process of BS oil noticeably improved by blending it with SF. By enhancing the blending ratio of BS before pressing, the lowest change in AV, AnV and PV was observed. Thus, this study proposed that blending BS with SF seeds prior to cold press resulted in producing oil blends with high stability in high temperature heating. Among oils extracted from mixture of seeds, SS:BS (15%) was identified as the best treatment, which revealed higher oxidative stability due to high level of carotenoids (0.34 mg/kg oil) and total phenol content (95.92–322.62 mg caffeic acid/kg oil) at the end of heating processing (150 min). BS oil has many nutritional compounds; however, it is not suitable to be used in the pure form due to its strong flavor and relatively dark color. This study showed that extraction of oil from a blend of BS with other oil seeds such as sunflower seed could be a promising way to bring the BS oil in the multipurpose a use to benefit from its high number of bioactive components.
Acknowledgements
The research protocol was approved and supported by Student Research Committee, Tabriz University of Medical Sciences (registration code: 64801).
Author contributions
Mehran Naderi: conceptualization, methodology, investigation, formal analysis, writing—original draft. Yeganeh Mazaheri, Sodeif Azadmard-Damirchi, Alieh Rezagholizade-shirvan, Samira Shokri: investigation, methodology, writing—review and editing. Mohammadali Torbati: investigation, methodology, writing—review and editing, supervision.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramadan, M. F. & Wahdan, K. M. M. Blending of corn oil with black cumin (Nigella sativa) and coriander (Coriandrum sativum) seed oils: Impact on functionality, stability and radical scavenging activity. Food Chem.132, 873–879 (2012). 10.1016/j.foodchem.2011.11.054 [DOI] [Google Scholar]
- 2.Ezazi, H. et al. The influence of dietary sunflower oil, rich in n-6 polyunsaturated fatty acids, in combination with vitamin C on ram semen parameters, sperm lipids and fertility. J. Sci. Food Agric.99, 3803–3810 (2019). 10.1002/jsfa.9602 [DOI] [PubMed] [Google Scholar]
- 3.Aristizabal-Henao, J. J., Stark, K. D. Macrolipidomic profiling of vegetable oils: the analysis of sunflower oils with different oleic acid content. Plant Metabolic Eng. Methods Protocols. 161–73 (2022). [DOI] [PubMed]
- 4.Carvalho, C. d., Caldeira, A., de Carvalho, L. M., de Carvalho, H. W., Ribeiro, J. L., Mandarino, J. M. et al. Fatty acid profile of sunflower achene oil from the brazilian semi-arid region. J. Agric. Sci.10, 144–150 (2018).
- 5.Boukandoul, S., Santos, C. S., Casal, S. & Zaidi, F. Oxidation delay of sunflower oil under frying by moringa oil addition: More than just a blend. J. Sci. Food Agric.99, 5483–5490 (2019). 10.1002/jsfa.9809 [DOI] [PubMed] [Google Scholar]
- 6.Rezagholizade-Shirvan, A., Shokri, S., Dadpour ,S. M. & Amiryousefi, M. R. Evaluation of physicochemical, antioxidant, antibacterial activity, and sensory properties of watermelon rind candy. Heliyon. 9, e17300 (2023). [DOI] [PMC free article] [PubMed]
- 7.Falahi, E., Delshadian, Z., Ahmadvand, H. & Shokri Jokar, S. Head space volatile constituents and antioxidant properties of five traditional Iranian wild edible plants grown in west of Iran. AIMS Agric. Food. (2019).
- 8.Bahmani, M., Shokri, S., Akhtar, Z. N., Abbaszadeh, S. & Manouchehri, A. The effect of pomegranate seed oil on human health, especially epidemiology of polycystic ovary syndrome; a systematic review. JBRA Assist. Reprod.26, 631 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saki, K. et al. Identification of Plant Flora Affecting Anti-Anxiety and Anti-Depression Disorders Based on Ethnobotanical Knowledge of the Arasbaran Region, Azerbaijan. Iran. Adv. Life. Sci.9, 589–594 (2023). [Google Scholar]
- 10.Shokri, S. et al. Synthesis and characterization of a novel magnetic chitosan–nickel ferrite nanocomposite for antibacterial and antioxidant properties. Sci. Rep.13, 15777 (2023). 10.1038/s41598-023-42974-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botterweck, A. A., Verhagen, H., Goldbohm, R. A., Kleinjans, J. & Van den Brandt, P. A. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands cohort study. Food Chem. Toxicol.38, 599–605 (2000). 10.1016/S0278-6915(00)00042-9 [DOI] [PubMed] [Google Scholar]
- 12.Wang, W. et al. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res.201, 111531 (2021). 10.1016/j.envres.2021.111531 [DOI] [PubMed] [Google Scholar]
- 13.Hajera, S., Rani, A. S. & Sulakshana, G. Determination of antioxidant potential in Spilanthes acmella using DPPH assay. Int. J. Curr. Microbiol. Appl. Sci.3, 219–223 (2014). [Google Scholar]
- 14.Sun, W., Chu, H., Zha, X., Lu, S. & Wang, Y. Enhanced electrochemical sensing of butylated hydroxy anisole through hollow metal-organic frameworks with gold nanoparticles and enzymes. ACS Appl. Nano Mater.6, 15183–15192 (2023). 10.1021/acsanm.3c02832 [DOI] [Google Scholar]
- 15.Zadeh, A. R, et al. Nigella sativa extract in the treatment of depression and serum Brain-Derived Neurotrophic Factor (BDNF) levels. J. Res. Med. Sci. 27 (2022). [DOI] [PMC free article] [PubMed]
- 16.Rezagholizade-shirvan, A., Najafi, M. F., Behmadi, H. & Masrournia, M. Preparation of nano-composites based on curcumin/chitosan-PVA-alginate to improve stability, antioxidant, antibacterial and anticancer activity of curcumin. Inorg. Chem. Commun.145, 110022 (2022). 10.1016/j.inoche.2022.110022 [DOI] [Google Scholar]
- 17.Mazaheri, Y., Torbati, M., Azadmard-Damirchi, S. & Savage, G. P. A. comprehensive review of the physicochemical, quality and nutritional properties of Nigella sativa oil. Food. Rev. Int.35, 342–362 (2019). 10.1080/87559129.2018.1563793 [DOI] [Google Scholar]
- 18.Rababah, T. M., Feng, H., Yang, W. & Yücel, S. Fortification of potato chips with natural plant extracts to enhance their sensory properties and storage stability. J. Am. Oil. Chem. Soc.89, 1419–1425 (2012). 10.1007/s11746-012-2037-7 [DOI] [Google Scholar]
- 19.Melo, A., Viegas, O., Petisca, C., Pinho, O. & Ferreira, I. M. Effect of beer/red wine marinades on the formation of heterocyclic aromatic amines in pan-fried beef. J. Agric. Food Chem.56, 10625–10632 (2008). 10.1021/jf801837s [DOI] [PubMed] [Google Scholar]
- 20.Zhang, Q., Saleh, A. S., Chen, J. & Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids.165, 662–681 (2012). 10.1016/j.chemphyslip.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 21.Rehab, F. A. & El Anany, A. Physicochemical studies on sunflower oil blended with cold pressed tiger nut oil during deep frying process. Grasas Aceites.63, 455–465 (2012). 10.3989/gya.057612 [DOI] [Google Scholar]
- 22.Upadhyay, R. & Mishra, H. N. Classification of sunflower oil blends stabilized by oleoresin rosemary (Rosmarinus officinalis L.) using multivariate kinetic approach. J. Food. Sci.80, E1746-E54 (2015). [DOI] [PubMed]
- 23.Rękas, A., Wroniak, M. & Ścibisz, I. Microwave radiation and conventional roasting in conjunction with hulling on the oxidative state and physicochemical properties of rapeseed oil. Eur. J. Lipid. Sci. Technol.119, 1600501 (2017). 10.1002/ejlt.201600501 [DOI] [Google Scholar]
- 24.Mazaheri , Y., Torbati, M., Azadmard-Damirchi S. & Savage, G. P. Effect of roasting and microwave pre-treatments of Nigella sativa L. seeds on lipase activity and the quality of the oil. Food Chem.274, 480–6 (2019). [DOI] [PubMed]
- 25.Loganathan, R., Tarmizi, A. H. A., Vethakkan, S. R. & Teng, K-T. Retention of carotenes and vitamin E, and physico-chemical changes occurring upon heating red palm olein using deep-fat fryer, microwave oven and conventional oven. J. Oleo. Sci. 69, 167–83 (2020). [DOI] [PubMed]
- 26.Savage, G., McNeil, D. & Dutta, P. Lipid composition and oxidative stability of oils in hazelnuts (Corylus avellana L.) grown in New Zealand. J. Am. Oil. Chem. Soc. 74, 755–9 (1997).
- 27.AOCS. Official methods and recommended practices of the AOCS. 7th edn. (Champaign: AOCS Press, 2017).
- 28.Shahidi, F. & Wanasundara, U. N. Methods for measuring oxidative rancidity in fats and oils. Food Lipids. 484–507 (CRC Press, 2002).
- 29.Mínguez-Mosquera, M. I., Gandul-Rojas, B., Montaño-Asquerino, A. & Garrido-Fernández, J. Dertermination of chlorophylls and carotenoids by high-performance liquid chromatography during olive lactic fermentation. J. Chromatogr A.585, 259–266 (1991). 10.1016/0021-9673(91)85086-U [DOI] [Google Scholar]
- 30.Caponio, F. et al. First and second centrifugation of olive paste: Influence of talc addition on yield, chemical composition and volatile compounds of the oils. LWT-Food Sci. Technol.64, 439–445 (2015). 10.1016/j.lwt.2015.05.007 [DOI] [Google Scholar]
- 31.Nadeem, R., Iqbal ,A., Zia, M. A., Anwar, F., Shahid, S. A., Mahmood, Z. et al. Effect of cold-pressing and soxhlet extraction on the physico-chemical attributes of sunflower (Helianthus annuus L.) seed oil. Int. J. Chem. Biochem. Sci.7, 41–6 (2015).
- 32.Suri, K., Singh, B., Kaur, A. & Yadav, M. P. Physicochemical characteristics, oxidative stability, pigments, fatty acid profile and antioxidant properties of co-pressed oil from blends of peanuts, flaxseed and black cumin seeds. Food. Chem. Adv.2, 100231 (2023). 10.1016/j.focha.2023.100231 [DOI] [Google Scholar]
- 33.Romuli, S., Karaj, S., Latif, S. & Müller, J. Performance of mechanical co-extraction of Jatropha curcas L. kernels with rapeseed, maize or soybean with regard to oil recovery, press capacity and product quality. Ind. Crops. Prod . 104, 81–90 (2017).
- 34.Gharby, S., Harhar, H., Guillaume, D., Roudani, A., Boulbaroud, S., Ibrahimi, M. et al. Chemical investigation of Nigella sativa L. seed oil produced in Morocco. J. Saudi. Soci. Agric. Sci. 14, 172–7 (2015).
- 35.Naderi, M., Torbati, M., Azadmard-Damirchi, S., Asnaashari, S. & Savage, G. P. Common ash (Fraxinus excelsior L.) seeds as a new vegetable oil source. LWT. 131, 109811 (2020).
- 36.Mazaheri, Y., Torbati, M., Azadmard-Damirchi, S. & Savage, G. P. Oil extraction from blends of sunflower and black cumin seeds by cold press and evaluation of its physicochemical properties. J Food. Process. Preserv.43, e14154 (2019). 10.1111/jfpp.14154 [DOI] [Google Scholar]
- 37.Asdadi , A., Harhar, H., Gharby, S., Bouzoubaâ, Z., Yadini, A., Moutaj, R. et al. Chemical composition and antifungal activity of Nigella Sativa L. oil seed cultivated in Morocco. Int. J. Pharm. Sci. Inven.3, 09–15 (2014).
- 38.Abdi-Moghadam, Z., Mazaheri, Y., Rezagholizade-shirvan, A., Mahmoudzadeh, M., Sarafraz, M., Mohtashami, M. et al. The significance of essential oils and their antifungal properties in the food industry: A systematic review. Heliyon9(11), e21386 (2023). [DOI] [PMC free article] [PubMed] [Retracted]
- 39.Alimentarius, Codex. Standar for named vegetable oils-CXS 210–1999, Codex Alimentarius (2019).
- 40.Rudzińska, M., Hassanein, M. M., Abdel-Razek, A. G., Ratusz, K. & Siger, A. Blends of rapeseed oil with black cumin and rice bran oils for increasing the oxidative stability. J. Food. Sci. Technol.53, 1055–1062 (2016). 10.1007/s13197-015-2140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zambiazi, R. C., Przybylski, R., Zambiazi, M. W. & Mendonca, C. B. Fatty acid composition of vegetable oils and fats. Bol. Cent. Pesqui. Process. Aliment.. 25 (2007).
- 42.Casal, S., Malheiro, R., Sendas, A., Oliveira, B. P. & Pereira, J. A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol.48, 2972–2979 (2010). 10.1016/j.fct.2010.07.036 [DOI] [PubMed] [Google Scholar]
- 43.Sumnu, S. G. & Sahin, S. Advances in deep-fat frying of foods. (CRC press, 2008).
- 44.Uquiche, E., Jeréz, M. & Ortíz, J. Effect of pretreatment with microwaves on mechanical extraction yield and quality of vegetable oil from Chilean hazelnuts (Gevuina avellana Mol). Innov. Food Sci. Emerg. Technol.9, 495–500 (2008). 10.1016/j.ifset.2008.05.004 [DOI] [Google Scholar]
- 45.Abdulkarim, S., Long, K., Lai, O. M., Muhammad, S. & Ghazali, H. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem.105, 1382–1389 (2007). 10.1016/j.foodchem.2007.05.013 [DOI] [Google Scholar]
- 46.Karakaya, S. & Şimşek, Ş. Changes in total polar compounds, peroxide value, total phenols and antioxidant activity of various oils used in deep fat frying. J. Am. Oil Chem. Soc.88, 1361–1366 (2011). 10.1007/s11746-011-1788-x [DOI] [Google Scholar]
- 47.Akil, E., Castelo-Branco, V. N., Costa, A. M. M., do Amaral Vendramini, A. L., Calado, V. & Torres, A. G. Oxidative stability and changes in chemical composition of extra virgin olive oils after short-term deep-frying of French fries. J. Am. Oil Chem. Soc.92, 409–21 (2015).
- 48.Abenoza, M., De Las Heras, P., Benito, M., Oria, R. & Sánchez‐Gimeno, A. C. Changes in the physicochemical and nutritional parameters of Picual and Arbequina olive oils during frying. J. Food. Process. Preserv. 40, 353–61 (2016).
- 49.Chammem, N. et al. Improvement of vegetable oils quality in frying conditions by adding rosemary extract. Ind. Crop. Prod.74, 592–599 (2015). 10.1016/j.indcrop.2015.05.054 [DOI] [Google Scholar]
- 50.Quiles, J. L., Ramírez-Tortosa, M. C., Gomez, J. A., Huertas, J. R. & Mataix, J. Role of vitamin E and phenolic compounds in the antioxidant capacity, measured by ESR, of virgin olive, olive and sunflower oils after frying. Food Chem. 76, 461–8 (2002).
- 51.Mudawi, H. A., Elhassan, M. S. & Sulieman, A. M. E. Effect of frying process on physicochemical characteristics of corn and sunflower oils. Food. Publ. Health.4, 181–184 (2014). [Google Scholar]
- 52.Kiralan, M. et al. Blends of cold pressed black cumin oil and sunflower oil with improved stability: A study based on changes in the levels of volatiles, tocopherols and thymoquinone during accelerated oxidation conditions. J. Food. Biochem.41, e12272 (2017). 10.1111/jfbc.12272 [DOI] [Google Scholar]
- 53.Ramadan, M. F. Healthy blends of high linoleic sunflower oil with selected cold pressed oils: Functionality, stability and antioxidative characteristics. Ind. Crop. Prod.43, 65–72 (2013). 10.1016/j.indcrop.2012.07.013 [DOI] [Google Scholar]
- 54.Ndjouenkeu, R. & Ngassoum, M. Etude comparative de la valeur en friture de quelques huiles vegetales:(Comparative study of frying behaviour of some vegetable oils). J. Food. Eng.52, 121–125 (2002). 10.1016/S0260-8774(01)00093-0 [DOI] [Google Scholar]
- 55.Shahidi, F. & Wanasundara, U. N. Methods for measuring oxidative rancidity in fats and oils. Food Lipids: Chem Nutr. Biotechnol.3, 387–403 (2002). [Google Scholar]
- 56.Shahidi, F. Bailey's Industrial Oil and Fat Products, Industrial and Nonedible Products from Oils and Fats. (John Wiley & Sons, 2005).
- 57.Asdadi, A. et al. Chemical composition and antifungal activity of Nigella Sativa L. oil seed cultivated in Morocco. Int. J. Pharm. Sci. Inv.3, 9–15 (2014).
- 58.Kanter, M., Akpolat, M. & Aktas, C. Protective effects of the volatile oil of Nigella sativa seeds on β-cell damage in streptozotocin-induced diabetic rats: A light and electron microscopic study. J. Mol. Histol.40, 379–385 (2009). 10.1007/s10735-009-9251-0 [DOI] [PubMed] [Google Scholar]
- 59.Achir, N., Randrianatoandro, V. A., Bohuon, P., Laffargue, A. & Avallone, S. Kinetic study of β-carotene and lutein degradation in oils during heat treatment. Eur. J. Lipid. Sci. Technol.112, 349–361 (2010). 10.1002/ejlt.200900165 [DOI] [Google Scholar]
- 60.Luaces, P., Pérez, A. G., García, J. M. & Sanz, C. Effects of heat-treatments of olive fruit on pigment composition of virgin olive oil. Food Chem.90, 169–74 (2005).
- 61.Arroyo-López, F. N. et al. Instability profile of fresh packed “seasoned” Manzanilla-Aloreña table olives. LWT-Food. Sci. Technol.42, 1629–1639 (2009). 10.1016/j.lwt.2009.06.004 [DOI] [Google Scholar]
- 62.Mazaheri, Y., Torbati, M., Azadmard-Damirchi, S. & Savage, G. P. Oil extraction from blends of sunflower and black cumin seeds by cold press and evaluation of its physicochemical properties. J. Food. Process. Preserv.43, e14154 (2019). 10.1111/jfpp.14154 [DOI] [Google Scholar]
- 63.Siger, A., Nogala-kalucka, M. & Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food. Lipid.15, 137–149 (2008). 10.1111/j.1745-4522.2007.00107.x [DOI] [Google Scholar]
- 64.Mohamed, K. M., Elsanhoty, R. M. & Hassanien, M. F. Improving thermal stability of high linoleic corn oil by blending with black cumin and coriander oils. Int. J. Food. Prop.17, 500–510 (2014). 10.1080/10942912.2012.654560 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study available from the corresponding author on reasonable request.