Abstract
Background
Cerebral palsy (CP) is the most cause of motor dysfunction in children. Selective dorsal rhizotomy (SDR) plays a major role in long term spasticity control. However, limited data exists on the effect of SDR on postoperative spasticity treatment requirements and supraspinal effects, and the stimulation responses of dorsal nerve roots in those with CP.
Methods
The current study included the outcome for 35 individuals undergoing SDR for motor functional outcome, spasticity, baclofen dose changes, botulinum toxin injection frequency, and spasticity related orthopedic procedures. We also report on the stimulation responses in 112 individuals who underwent SDR at our institution.
Results
There was a significant difference in gross motor function measures (GMFM)-66 scores at last follow up that remained present when considering only ambulatory children but not with non-ambulatory children. Ashworth scores were significantly decreased for both upper and lower extremities after SDR at all follow up points. There was a significant decrease in Baclofen dose and botulinum toxin injections requirements after SDR, but no significant difference in the need for orthopedic intervention. A total of 5502 dorsal nerve roots were tested showing a decrease in stimulation intensity and increase in grade on the right side and for descending lumbosacral levels.
Conclusions
SDR improves gross motor scores during short term follow up but has additional benefits in decreasing baclofen dosing and botulinum toxin injections requirements after surgery. They stimulation responses of sectioned dorsal nerve roots adds to the limited available data and our understanding of the pathological changes that occur in CP.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00701-024-06187-8.
Keywords: Selective dorsal rhizotomy, Cerebral palsy, Spasticity, Baclofen, Botulinum toxin
Introduction
Cerebral palsy (CP) is the most common cause of motor dysfunction in children, with spasticity playing a prominent role in the lives of these individuals [11, 65]. The degree to which spasticity impacts an individual’s function can be assessed by the gross motor function measure (GMFM), originally developed to follow the effects of physical therapy in patients with CP [67], and commonly used to follow the natural history and effects of treatments. The 66-itemized version of the GMFM is a reliable tool to assess individuals with CP on five dimensions [68]. Based on the GMFM, the gross motor function classification system (GMFCS) was later developed based on the GMFM tasks into five levels, whereby those classified as levels I to III are predominately ambulatory and IV and V are not [57]. These measures serve to stratify individuals in quality of life surveys, treatment outcomes, and clinical research purposes.
Spasticity is a significant burden on individuals with CP and their caregivers [24]. In 1911, Foerster reported on early attempts to manage spasticity surgically with posterior spinal nerve root resection [17]. However, despite its benefits in tone reduction, there were significant postoperative complications, and its utilization remained limited to a small subset of surgeons until its revival by Gros in the 1960’s [23]. In the 1970’s, Fasano et al. reported on their experience with a functional approach to selective dorsal rhizotomy (SDR) using intraoperative stimulation and recording of electromyography (EMG) responses thus forming the basis for stimulation parameters used today [16]. The two modern approaches most commonly employed include the multi-level laminectomy SDR introduced by Peacock in 1987[62] and a refined limited laminectomy SDR at the level of the conus described by Park in 1993 [58]. Around the same time, other non-permanent options were also being sought for individuals with spasticity. Baclofen was introduced in the 1970’s as a potential treatment for spasticity in CP [8], but intolerance remained a major issue for some individuals. In the 1990’s, botulinum toxin injections into target muscles also showed benefit for treating spasticity [35]. These treatments, although reversable, are not without their cost and side effect profile.
The effect of SDR on the need for ongoing spasticity therapies remains limited. The current study aimed to assess multiple pre- and postoperative parameters of individuals with CP treated with SDR at our institution. We aimed to report not only functional changes after the procedure, but also its effect on requiring other spasticity treatments. In addition, we report on the intraoperative stimulation responses of sectioned dorsal nerve roots in individuals with CP undergoing SDR to add to the limited published electrophysiological data.
Methods and materials
Study design
This is a retrospective review of 117 patients who underwent SDR by the senior author (J.R.L) between January 2015 and August 2021 at Nationwide Children’s Hospital. Some patients were referred from out of city/state/country and long term follow up was performed elsewhere. The study had two objectives, analysis of participant outcome data with follow up at least 10 months postoperatively (available for 35 patients) and analysis of the intraoperative electrophysiological data (available for 112 patients). The outcome group baseline data included age at surgery, gender, spasticity type, presence of dystonia, preoperative Ashworth grade [2], GMFCS level, GMFM-66 score and percentile [24]. Outcome measures included postoperative Ashworth grade, GMFCS level, GMFM-66 scores, baclofen use and dose, botulinum toxin injections, and spasticity-related orthopedic procedures. The Ashworth scale is used more commonly by our physiatrists and was therefore used for analysis. Electrophysiological baseline data included age, gender, GMFCS level, and spasticity type. The Intuitional Review Board at Nationwide Children’s Hospital approved the current study.
Individuals with CP related spasticity referred to our center are candidates for SDR if: spasticity affects their quality of life, they are ambulatory with or without assistance, they are able to participate in rigorous postoperative physiotherapy, dystonia is not a major component of their presentation, they have spastic diplegia, and other causes of spasticity are ruled out by clinical assessment and cranial neuroimaging. Non-ambulatory individuals are considered for SDR as a palliative option on a case-by-case basis after discussion with the primary caregivers if decreasing spasticity is likely to have a positive impact on quality of life and individual care. Both types of candidates were included in this cohort.
All ambulatory individuals were enrolled in postoperative physiotherapy on day 4 – 5, non-ambulatory patients are discharged home when ready. Postoperative physiotherapy generally includes inpatient rehabilitation for approximately 2 weeks, followed by outpatient sessions approximately 4 – 5 times per week for six months, then 2 – 3 times per week for six to twelve months modified based on the individual needs and progress. All individuals were assessed by a trained physiotherapist and/or physiatrist on admission to rehabilitation. Based on follow up times among participants with GMFM-66 assessments, this was divided into short term (0 – 7 months), intermediate term (8 – 19 months), and long term follow up (≥ 19 months). Based on available follow up times among patients with Ashworth assessments, the timeframe was grouped into four time periods: 0 – 3 months, 4 – 9 months, 10 – 19 months, and > 19 months to reflect changes over time.
Surgical technique
Surgical approach is similar to that described by Park and Johnston [61]. The patients are positioned prone with EMG monitoring of the lower extremity and the anal sphincter. No botulinum toxin injections are given within 6 months of surgery for reliable monitoring. The incision is made along the midline over the L1 spinous process and the L1-L2 interspinous space is visualized with the intraoperative ultrasound to identify the conus and cauda equina transition level (Fig. 1). Once the transition level is confirmed, a single level laminectomy is performed. The dura is opened and the dorsal nerve roots on the more severely affected side (if one is present on exam or expressed by the parents) are targeted first in the event surgery cannot be completed due to any unforeseeable complications. The roots are isolated with a silicone strip without including the smaller midline sacral nerve roots (S3-S5). Each nerve root is then isolated and stimulated with rhizotomy probes to identify the level. Depending on size, the root is then divided into 3 – 5 rootlets and sectioned rootlets are stimulated for threshold and grade. We aim to cut approximately two-thirds of each dorsal nerve root and therefore, non-sectioned rootlets were not routinely stimulated or recorded. If a nerve root produces compound muscle action potentials with stimulation < 0.5 mA then mechanical stimulation of the nerve root is performed to help define whether this is a ventral nerve root which should be spared. Any nerve root with an isolated or predominant sphincter response is spared. After sectioning is completed on one side, the L1 nerve root is identified at its foraminal exit, and 50% is cut without stimulation. The same procedure is then carried out on the contralateral side. The dura is closed in watertight fashion, and an epidural catheter is then placed. The muscle, fascia and skin are then closed in layers. Post-operative pain control includes epidural analgesia for three days and as needed oral and intravenous analgesia; this postoperative pain protocol has been described previously [27].
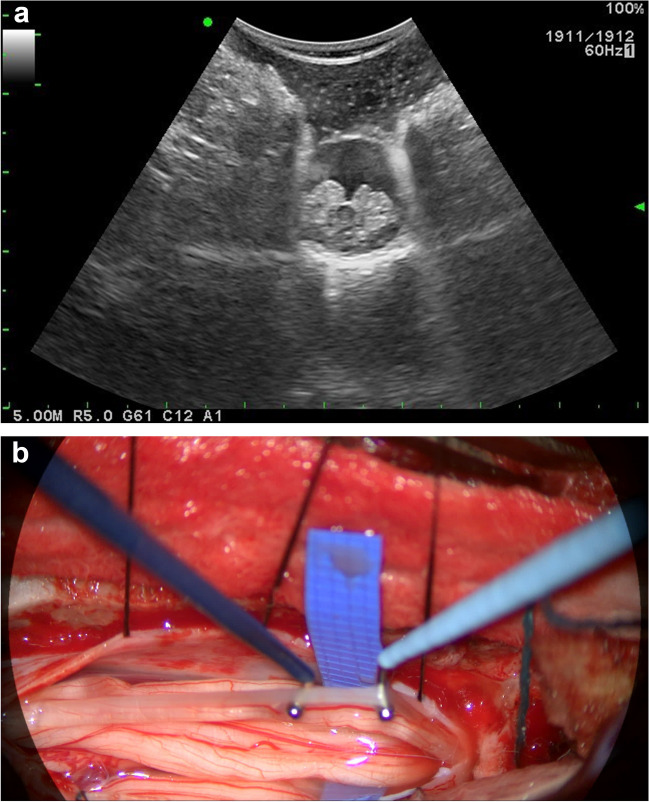
Fig. 1.
(a) Intraoperative ultrasound prior to laminectomy to determine level of transition from conus (central dark grey) to nerve roots (surrounding light grey structures). (b) Intraoperative microscopic image of isolated right sided dorsal nerve roots before starting rhizotomy, terminal end of conus appreciated (superior [left] midline structure)
All SDR procedures at our institution are done with neurophysiologic intraoperative monitoring (NIOM). NIOM utilizes free run and stimulated EMG recordings of muscles representing innervation from the L2-S4 spinal levels. These consist of the bilateral adductor longus (L2-L4), vastus lateralis (L2-L4), tibialis anterior (L4-L5), short head of the biceps femoris (L5-S1), medial gastrocnemius (S1-S2), abductor hallucis brevis (S2-S3), and external anal sphincter (S3-S4). The spinal level for each root is determined based on what muscles demonstrate compound muscle potentials with 1 Hz stimulation. Intensity threshold for each rootlet is determined by the lowest level of stimulation needed to produce a consistent compound muscle potential. Each rootlet then undergoes tetanic 50 Hz stimulation to allow for grading of the EMG response. The Philips and Park grading scheme defines a 0–4 scale to grade responses with Grade 4 being the most severe [63]. Neuromonitoring data recorded for each nerve root included laterality, spinal level based on nerve root stimulation at 1 Hz, rootlet threshold intensity (mA) at 1 Hz, rootlet grade at 50 Hz, and number of rootlets cut. The spinal level for each root was designated as lumbar (L2-L5 spinal levels), lumbosacral (spanning lumbar and sacral spinal levels), or sacral (S1-S4 spinal levels).
Statistical analysis
Data analysis was performed with IBM SPSS Statistics® v29 (IBM Corp, Armonk, NY, USA). Descriptive data was presented as mean, standard error (SE) and SE of the mean, and percentage. For continuous data, a paired-sample t-test was used to compare preoperative and postoperative results. For categorical data, Chi-squared test or Fisher’s exact test was used when appropriate, and odds ratio (OR). For continuous independent variables, independent-sample t-test was used to assess differences in means. Pearson correlation coefficient was used to assess the association between continuous variables, while Spearman’s Rank-Order Correlation was used to assess the association between continuous and ordinal data. Changes over time were plotted using one-way repeated measures analysis of variance (ANOVA) for continuous variables and Wilcoxon Signed-Rank Test for categorical variables, and McNemar test for nominal data comparing pre- and postoperative baclofen use, spasticity related orthopedic surgery and botulinum toxin injections. The Mann–Whitney U test was used to compare differences between groups in pre- and postoperative dichotomous outcome measures (baclofen use, orthopedic surgery, and lower extremity botulinum toxin injection) for ordinal variables (grade at 50 Hz). One-way ANOVA was used to assess differences in electrophysiological data based on patient variables, with Tukey honestly significant difference (HSD) post hoc analysis used when a significance difference was encountered. Linear regression was used to assess relationship between intraoperative electrophysiological parameters and age and percentage of rootlets cut. Standard error (SE) or standard deviation (SD) and 95% confidence intervals (CI) were reported were possible and confirmed with bias-corrected and accelerated (BCa) bootstrap confidence intervals (1000 samples).
To ensure equal weighting of NIOM data with the variation in the number of rootlets per participant, comparisons with other variables were performed against the average stimulation and grade per participant and per participant side and level. For subgroup analysis, GMFCS levels were divided into ambulatory (GMFCS levels I – III) and non-ambulatory (GMFCS levels IV and V). The Ashworth scale was divided into upper and lower extremity, and the lower extremity was further divided into lumbar (L1-L5): hip flexion and adduction and knee extension; lumbosacral (L5-S1): hip extension and abduction, knee flexion, and ankle dorsiflexion; sacral (S1-S3): ankle plantarflexion; in addition to laterality.
Results
Outcome group characteristics for 35 patients
A total of 35 patients were available. Mean age was 7.11 ± 3.73 years (range 3 – 18), and 15 (42.9%) patients were female. The most common spasticity subtype was diplegic spastic CP in 18 participants (51.4%). Twenty-eight (96.65%) participants had periventricular white matter changes on 29 available MRI brain images, and 8/32 (25%) had ventriculoperitoneal shunts. Patient characteristics are shown in Table 1. Follow up beyond 19 months was available for 31/35 patients (88.57%), and mean last available follow up was 28.2 ± 12.6 months (range 10 – 62 months).
Table 1.
Participant characteristics undergoing selective dorsal rhizotomy with outcome data (n = 35)
| Patient Characteristics | N | % |
|---|---|---|
| Age (mean ± SD) | 7.11 | ± 3.73 |
| < 10 years | 28 | 80 |
| ≥ 10 years | 7 | 20 |
| Gender | ||
| Male | 20 | 57.1 |
| Female | 15 | 42.9 |
| GMFCS Level | ||
| I | 11 | 31.4 |
| II | 7 | 20 |
| III | 9 | 25.7 |
| IV | 5 | 14.3 |
| V | 3 | 8.6 |
| Spasticity Type | ||
| Diplegia | 18 | 51.4 |
| Hemiplegia | 4 | 11.4 |
| Tetraplegia | 5 | 14.3 |
| Quadriplegia | 8 | 22.9 |
| Dystonia | ||
| Present | 2 | 5.7 |
| Not present | 33 | 94.3 |
| Ventriculoperitoneal Shunt | ||
| Yes | 8 | 22.86 |
| No | 24 | 68.57 |
| Not available | 3 | 8.57 |
| MRI findings | ||
| No abnormality | 1 | 2.86 |
| Periventricular leukomalacia | 27 | 77.14 |
| Ventriculomegaly/Periventricular gliosis | 1 | 2.86 |
| Not available | 6 | 17.14 |
Abbreviations: SD standard deviation, GMFCS gross motor function classification system, MRI magnetic resonance imaging
GMFCS, GMFM-66, and Ashworth scores
There was no statistically significant change in GMFCS level at any follow up point (for change in level at ≥ 19 m, Z = -0.816, p = 0.414). GMFM-66 score before SDR and ≥ 19 months follow up were strongly and positively correlated (r = 0.84, p < 0.001). When considering only ambulatory patients, a paired-samples t-test showed a significant difference between preoperative and ≥ 19 m scores;(t16) = -2.75, p = 0.014). On average, ≥ 19 month follow up scores were 5.6 points higher than preoperative scores (95% CI 9.96, -1.28). There was no significant change between the preoperative score and any earlier time points, and no significant change for percentile before and after surgery (supplementary Table 1).
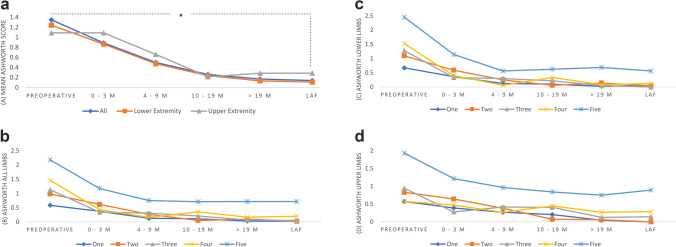
A total of 1050 limbs were assessed for spasticity, lower extremity joints included 560 (53.3%) limbs and upper extremity were 490 (46.7%) limbs, equally divided on the right and left. A paired-samples t-test was conducted to compare Ashworth scores before and after SDR. The results indicate a significant difference between Ashworth scores before SDR (M = 1.06; SD = 1.085) and after SDR at follow up > 19 months (M = 0.12; SD = 0.47); [t(625) = -23.213, p < 0.001]. A significant decrease was seen for all follow up time points and when considering upper and lower extremities separately (Fig. 2, supplementary Table 2).
Fig. 2.
Mean Ashworth scores preoperatively and postoperatively based on (a) limbs and (B-D) GMFCS level, (b) all limbs, (c) lower extremity, (d) upper extremity. Asterisk indicates statistically significant difference obtained at p < 0.001
Outcome measures
Twenty-seven (77.1%) patients were receiving preoperative botulinum toxin injections to the lower extremities. There was no significant difference between ambulatory and non-ambulatory patients to receive lower extremity botulinum toxin injections (p = 0.648), but postoperatively non-ambulatory patients were 21 times more likely to continue to receive injections compared to ambulatory patients (OR 21.15, 95% CI 0.9 – 495.9, p = 0.047). There was a significant difference in the proportion of botulinum toxin injections pre- and postoperatively overall (p < 0.001) and for ambulatory patients (p < 0.001), but was not significant when considering only non-ambulatory patients (p = 0.063).
Mean recorded follow up for postoperative baclofen use was 23.75 ± 18.67 months (range 2 – 56 months). Non-ambulatory patients were 14 times more likely to be taking baclofen preoperatively (OR 14.09, 95% CI 1.486 – 125, p = 0.013 using Fisher’s exact test), but were not significantly different when considering dose (mg/24H) despite higher doses in non-ambulatory patients (M = 9.096, SD = 24.357) than ambulatory patients (M = 17.563, SD = 11.121); (t(32) = -0.945, p = 0.351). There was a significant difference in the proportion of those receiving baclofen pre- and postoperatively overall (p = 0.003) and for ambulatory patients (p = 0.021), but was not significant when considering only non-ambulatory patients (p = 0.25). Mean baclofen dose differed before and after SDR (F(1262.485, 242.644) = 5.203, p = 0.029). Baclofen dose was decreased from preoperatively to 12 months postoperatively (mean difference = 8.618 mg/24H, 95% CI 0.931 – 16.304, p = 0.029). This significance was lost in the subgroup analysis based on ambulatory status Table 2.
Table 2.
Preoperative and postoperative comparisons for spasticity interventions (n = 35): baclofen, botulinum toxin, and Orthopedic surgery
| Preop GMFCS Level | Preop Baclofen | Postop Baclofen at 12 months | Preop Botox (N, %) | Postop Botox (N, %) | Preop Ortho Srx | Postop Ortho Srx | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | µDose (mg/24H) | SD | N | % | µDose (mg/24H) | SD | Any | % | LE | % | UE | % | Any | % | LE | % | UE | % | N | % | N | % | |
| Overall | 16 | 45.7 | 11.1 | 22.1 | 5 | 14.3 | 2.5 | 7 | 28 | 80 | 27 | 77.1 | 4 | 11.4 | 5 | 14.3 | 2 | 5.7 | 5 | 14.7 | 6 | 17.1 | 10 | 28.6 |
| p-value* | 0.003 | 0.029 | < 0.001 | < 0.001 | 1 | 0.344 | ||||||||||||||||||
| A (n = 27) | 9 | 33.3 | 9.1 | 24.4 | 1 | 3.7 | 0.2 | 1 | 21 | 77.8 | 20 | 74.1 | 3 | 11.1 | 3 | 11.1 | 0 | 0 | 3 | 11.5 | 3 | 11.1 | 6 | 22.2 |
| 1 (n = 11) | 3 | 27.3 | 6.36 | 11.4 | 1 | 9.1 | 0.45 | 1.5 | 9 | 81.8 | 9 | 81.8 | 1 | 9.1 | 1 | 9.1 | 0 | 0 | 1 | 9.1 | 1 | 9.1 | 1 | 9.1 |
| 2 (n = 7) | 2 | 28.6 | 17.9 | 45.1 | 0 | 0 | 0 | - | 6 | 85.7 | 5 | 71.4 | 1 | 14.3 | 1 | 14.3 | 0 | 0 | 1 | 14.3 | 1 | 14.3 | 3 | 42.9 |
| 3 (n = 9) | 4 | 44.4 | 5.2 | 9.2 | 0 | 0 | 0 | - | 6 | 66.7 | 6 | 66.7 | 1 | 11.1 | 1 | 11.1 | 0 | 0 | 1 | 12.5 | 1 | 11.1 | 2 | 22.2 |
| p-value* | 0.021 | 0.075 | < 0.001 | < 0.001 | 1 | 0.375 | ||||||||||||||||||
| NA (n = 8) | 7 | 87.5 | 17.6 | 11.1 | 4 | 50 | 9.9 | 12.1 | 7 | 87.5 | 7 | 87.5 | 1 | 12.5 | 2 | 25 | 2 | 25 | 2 | 25 | 3 | 37.5 | 4 | 50 |
| 4 (n = 5) | 5 | 100 | 17.3 | 9.5 | 3 | 60 | 9.8 | 10.3 | 5 | 100 | 5 | 100 | 1 | 20 | 2 | 40 | 2 | 40 | 2 | 40 | 1 | 20 | 3 | 60 |
| 5 (n = 3) | 2 | 66.7 | 18 | 15.9 | 1 | 33.3 | 10 | 17.3 | 2 | 66.7 | 2 | 66.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 66.7 | 1 | 33.3 |
| p-value* | 0.25 | 0.12 | 0.063 | 0.063 | 1 | 1 | ||||||||||||||||||
*McNemar test and ANOVA repeated measures (baclofen dose) used as appropriate, all tests are two-sided
Percentages are based on total numbers for each subgroup
Abbreviations: GMFCS gross motor function classification system, A ambulatory, NA non-ambulatory, Preop preoperative, Postop postoperative, Ortho orthopedic, Srx surgery, N number, µ mean, mg milligrams, SD standard deviation, LE lower extremities, UE upper extremities
There was no significant difference between the rates of preoperative and postoperative orthopedic surgeries for spasticity (p = 0.344). Non-ambulatory participants had a higher rate of postoperative orthopedic surgeries compared to ambulatory patients but the difference was not significant (22.2 vs 50%, p = 0.186). Results are shown in Table 4.
Table 4.
Differences and predictors for stimulation intensity and grading
| Variable | Stimulation Intensity (mA) | Grading at 50 Hz | ||
|---|---|---|---|---|
| One-way ANOVA for differences between groups | ||||
| Gender | F(1, 112) = 2.485 | p = 0.118 | F(1, 112) = 0.272 | p = 0.603 |
| Spinal Level | F(2, 326) = 8.08 | p < 0.001 | F(2, 326) = 49.906 | p < 0.001 |
| Laterality | F(2, 220) = 11.294 | p < 0.001 | F(1,220) = 56.263 | p < 0.001 |
| Gender | F(1, 110) = 0.364 | p = 0.547 | F(1, 110) = 0.379 | p = 0.54 |
| GMFCS | F(4,105) = 1.345 | p = 0.258 | F(4, 105) = 1.24 | p = 0.298 |
| Spasticity | F(3, 103) = 0.066 | p = 0.978 | F(3, 103) = 1.322 | p = 0.271 |
| Linear regression for possible predictors | ||||
| Age | R2 = 0.001, F(1, 110) = 0.153 | p = 0.696 | R2 = 0.003, F(1, 110) = 0.313 | p = 0.577 |
| GMFM-66 | R2 = 0.027, F(1, 57) = 1.593 | p = 0.212 | R2 = 0.004, F(1, 57) = 0.2 | p = 0.656 |
| % Rootlets cut | R2 = 0.002, F(1, 5384) = 8.794 | p = 0.003 | R2 = 0.004, F(1, 5379) = 23.058 | p < 0.001 |
One-way ANOVA reported as: F(df between groups, df within groups) = F-value, p-value
Linear regression reported as: R squared value, F(df regression, df residual) = F-value, p-value
Abbreviations: mA milliamperes, Hz hertz, ANOVA analysis of variance, GMFCS gross motor function classification system, GMFM gross motor function measure
Clinical characteristics and sectioned nerve root stimulation responses for 112 patients
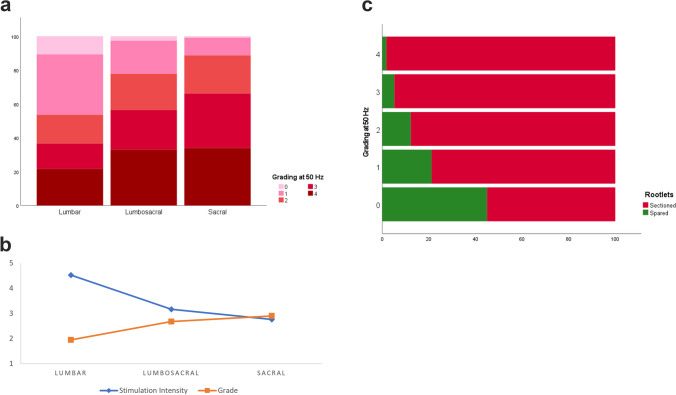
A total of 5502 sectioned nerve rootlets for 112 patients were included in the final analysis. Mean age was 8.34 ± 5.43 years (range 3 – 28), and 62 were males (55.36%). CP type was available for 105 patients of which the majority were spasticity without dystonia (n = 100) and 5 were mixed. The average number or nerve roots per patient was 48 ± 10.84 (mode 47, min 20 and max 84). Nerve roots were divided on average to 3.38 ± 0.848 rootlets (range 1 to 7, mode 3), and 65.6% ± 8.04% on average was cut. Stimulation responses and patient characteristics are shown in Table 3. Differences and predictors between variables and electrophysiological data are shown in Table 4. There was a significant difference between sides (left and right) for stimulation intensity (left side mean increase of 1.59 mA) and grading (left side mean decrease of 0.716). The percentage of rootlets cut was significantly predicted by stimulation intensity (p < 0.001) and grading (p < 0.001). The fitted regression model was: percentage of rootlets cut = 65.85%—0.067 (stimulation intensity in mA), and 64.61% + 0.407 (grading at 50 Hz). A one-way ANOVA showed a significant difference between spinal levels in stimulation intensity (p < 0.001) and grading (p < 0.001); a Tukey’s HSD Test for multiple comparisons was performed and results are shown in Table 5. Figure 3 graphically shows this relationship.
Table 3.
Nerve root characteristics among 112 participants who underwent selective dorsal rhizotomy
| Nerve Roots | N | % | Stimulation intensity (mA) | Grading at 50 Hz | Rootlets Cut (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Overall | 5502 | 100 | 3.711 | 4.988 | 2.41 | 1.287 | 65.6 | 8.04 | |
| Age | < 10 years | 77 | 68.75 | 3.81 | 5.154 | 2.44 | 1.28 | 65.83 | 8.39 |
| ≥ 10 years | 35 | 31.25 | 3.506 | 4.618 | 2.36 | 1.3 | 65.1 | 7.2 | |
| Gender | Male | 62 | 55.36 | 3.235 | 4.77 | 2.38 | 1.287 | 65.88 | 8.67 |
| Female | 50 | 44.64 | 4.31 | 5.189 | 2.45 | 1.286 | 65.23 | 7.11 | |
| Level | Lumbar | 2616 | 47.5 | 4.655 | 5.824 | 2.01 | 1.34 | 65.4 | 8.5 |
| Lumbosacral | 1344 | 24.4 | 3.07 | 3.935 | 2.65 | 1.204 | 65.38 | 7.8 | |
| Sacral | 1542 | 28 | 2.67 | 3.848 | 2.88 | 1.025 | 66.11 | 7.39 | |
| Laterality | Left | 2763 | 50.7 | 3.549 | 4.708 | 2.52 | 1.287 | 65.91 | 8.17 |
| Right | 2683 | 49.3 | 3.887 | 5.289 | 2.31 | 1.285 | 65.27 | 7.89 | |
| GMFCS | I | 26 | 23.6 | 4.327 | 5.115 | 2.54 | 1.261 | 64 | 8.46 |
| II | 26 | 23.6 | 4.913 | 6.977 | 2.28 | 1.24 | 65.39 | 5.49 | |
| III | 33 | 30 | 2.78 | 3.128 | 2.4 | 1.317 | 65.74 | 8.51 | |
| IV | 17 | 15.5 | 2.683 | 2.926 | 2.39 | 1.3 | 66.17 | 6.6 | |
| V | 8 | 7.3 | 4.577 | 4.855 | 2.62 | 1.29 | 67.78 | 5.58 | |
| Spasticity | Diplegia | 63 | 60 | 3.559 | 5.011 | 2.4 | 1.264 | 65.31 | 8.03 |
| Hemiplegia | 12 | 11.4 | 3.839 | 4.373 | 2.61 | 1.333 | 67.07 | 8.98 | |
| Triplegia | 9 | 8.6 | 5.056 | 6.955 | 2.19 | 1.372 | 65.56 | 10.13 | |
| Quadriplegia | 21 | 20 | 3.497 | 4.536 | 2.39 | 1.301 | 65.69 | 7.55 | |
Missing data: Laterality = 56, GMFCS = 2, Spasticity = 7
Abbreviations: mA milliamperes, Hz hertz; SD standard deviation, GMFCS gross motor function classification system
Table 5.
Tukey post hoc analysis for stimulation intensity and grading based on spinal level
| Spinal Level | Stimulation Intensity (mA) | Grading at 50 Hz | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MD | SE | Sig | 95% CI | MD | SE | Sig | 95% CI | ||||
| Lumbar | LS | 1.360 | 0.462 | 0.01 | 0.271 | 2.448 | -0.728 | 0.100 | < 0.001 | -0.962 | -0.493 |
| Lumbar | Sacral | 1.759 | 0.459 | < 0.001 | 0.678 | 2.840 | -0.947 | 0.099 | < 0.001 | -1.181 | -0.713 |
| LS | Sacral | 0.399 | 0.464 | 0.666 | -0.694 | 1.492 | -0.220 | 0.100 | 0.074 | -0.456 | 0.016 |
Abbreviations: mA milliamperes, Hz hertz, MD mean difference, SE standard error, Sig significance, CI confidence interval, LS lumbosacral
Fig. 3.
(a) Grading distribution (percent) based on spinal level. (b) Mean stimulation intensity and grading at 50 Hz based on spinal level. (c) Stacked bar chart for rootlets sectioned (percent) based on grade
Discussion
Functional outcomes after SDR
This study considered both GMFCS level and GMFM-66 changes after SDR. GMFCS level did not significantly change, but a difference was found in GMFM for ambulatory individuals. The natural history of motor function over time suggests that major gains in ambulatory children with CP occur up to the age of 6 to 7 years and then plateaus [4, 26, 66]. This would not explain earlier or later gains seen at various age groups in our study. One factor that may play a role is the wide variation in reported outcomes between and among GMFCS levels, and that motor gains can occur earlier and to a much lesser degree in non-ambulatory individuals [66, 71]. Hanna et al. found that some functional decline may occur in GMFCS levels IV and V after reaching their early childhood peak as they enter into adolescence and early adulthood, and the decline was most prominent among those with GMFCS level III [25]. This decline has not been demonstrated by other reports [71]. McLaughlin et al. found the greatest gain in GMFM was among non-ambulatory children although this did not reach significance [42]. Clearly, variation exist in the outcome for older children and this may be secondary to other factors, as shown by Bartlett et al. where range of motion, scoliosis, and pain correlated with a decline in adolescents with GMFCS III, IV, and V [3].
GMFCS level III formed nearly a third of our cohort. A systematic review of walking performance from childhood to adulthood found that GMFCS level III carries the highest variability to improve, remain stable, or decline with time, while those above or below this level largely remained unchanged [10]. Two of three randomized control trials on SDR involving ambulatory individuals found a significant change in GMFM scores at 9 to 12 months [42, 73, 83], and a meta-analysis of these trials similarly found significant improvement when data was pooled [43]. Long term significant improvement in GMFM scores for ambulatory children has been reported 5 to 10 years after SDR [6, 15, 31, 76], but not for non-ambulatory children [15]. Tedroff et al. reported a gradual decline at ten years compared to earlier gains [76, 77], but remained a significant improvement from baseline at ten years [76], and was no longer significant by 17 years [77]. Their study only included 18 participants of which 7 were non-ambulatory. A larger cohort of 100 individuals reported by Park et al. included childhood SDR from all GMFCS levels with sustained or even improved function at 20 to 28 years [59]. Unfortunately, neither Tedroff et al. or Park et al. stratified their analysis based on ambulatory status to provide further insight into long term outcomes in each of these groups. These studies show the wide variations both in the natural history of CP and in those undergoing SDR. However, similar to our findings, early and sustained short term gains occur for ambulatory children undergoing SDR, but further subgroup analysis including long-term functional outcome is needed to better predict who will continue to benefit and who may not.
Limb spasticity and SDR
We found a significant decrease in spasticity in all limbs at last follow up after SDR, this included the upper and lower extremities. This is in keeping with reported SDR benefits in decreasing spasticity in the lower extremities in three randomized controlled trials at 9 to 12 months [43], and these effects were maintained long term [1, 39, 77]. Its effect on upper extremity tone and function however is less discussed. Early studies on changes in tone in the upper extremities after SDR in ambulatory children were not consistent [7, 62]. Ambulatory children are less likely to have spasticity in the upper extremity and therefore detection of a meaningful change may be difficult especially in smaller cohorts. In studies including individuals with spastic quadriplegia both tone and function of the upper extremity appear to improve with SDR [21, 46]. Other studies have also shown improvement in upper extremity function that continues several years after SDR [55, 72]. These changes may be due to suprasegmental effects, with a small case series suggesting changes at the cortical level [55], including cognitive gains compared to controls in a slightly larger cohort [12]. The evidence and quality of current available studies is not enough to make conclusive remarks, but strongly suggests that SDR likely has effects well above the level of operation and is an important aspect to be included in future research on SDR outcomes as this effects the indications for utilizing the procedure.
Baclofen use and botulinum toxin injections after SDR
Our study found a significant post-operative decrease in the use of baclofen and botulinum toxin injections in ambulatory individuals, and this was sustained over the follow-up period. Additionally, there was a decrease in utilization in the non-ambulatory group, but this did not reach significance. Although the majority of SDR studies focus on motor function outcomes after SDR, an important and much less reported outcome is the change in baclofen use and botulinum toxin injections. A significant decrease in postoperative muscle relaxant use among SDR participants at one year follow up has been reported [79]. Of those that report on post-SDR anti-spasmodic medication or botulinum toxin injections, this varies from 0 to 38% and 12.5 to 53%, respectively, but were not compared to preoperative rates [15, 30, 60, 77].
The rate of adverse events reported from botulinum toxin injections ranges from 3.6 to 23.2% [36, 47, 52, 56]. While in one study of family reported adverse events, 95 events were reported in 45 individuals [5]. These rates may also be underestimated [75]. Ambulatory children may not have significant clinical benefit between physiotherapy alone compared to physiotherapy and botulinum toxin injections, with higher costs for the latter [69]. In a population-based study that included children enrolled in Medicaid, those with CP had average annual costs 15 times higher than average, of which pharmacy costs accounted for 11% and overall oral baclofen use was reported in 13.5% [64]. Side effects have been reported in 20—40%[22, 41, 44] and fatigue, lethargy, or drowsiness in more than a third of individuals [13]. These are important factors to consider for individuals and their families living with CP where both cost and risk of side effects over time may play a large role in their quality of life, however, dedicated studies are lacking and our findings further support the benefit of SDR in the ongoing need for these treatments.
Orthopedic interventions after SDR
We did not find a significant difference in the rate of orthopedic procedures before and after SDR (17 vs 29%, respectively), although rates were higher in non-ambulatory participants (22 vs 50%). Prior orthopedic surgery was not found to be associated with “poor” outcome after SDR [34]. Comparative studies show the rate of orthopedic surgery after the first year of SDR may be lower than those receiving intrathecal baclofen therapy (19 vs 41%, respectively) [32], however this is limited by the short follow up as other studies found no orthopedic surgery done within the first year after SDR but reached 18% by five years[45] and 84% by 10 years [51]. Post-SDR orthopedic procedure rates at approximately five years varies at 18% [45], 24% [9], 42% [38, 48], and 66% [50]. Longer follow up studies beyond 15 years reported even higher rates of 28% [15], 57% [59], and 74% [30]. Age at SDR may play a role, Chicoine et al. reported a significantly higher rate of any orthopedic procedure (before or after SDR) in those 5 years and older compared to those younger than 5 years (45 vs 22%, respectively), but similar post-SDR rates (both 19%) [9]. This age related difference was also found by O’Brien et al. but their analysis included only post-SDR rates and did not reach significance [50]. This may be less related to the SDR procedure itself, but to the age and functional status of participants included in the SDR study. Individuals with CP undergoing orthopedic surgeries at a younger age may be more likely to require further surgeries in the future [49]. In a cohort of independent versus assisted walkers, the rate of post-SDR orthopedic surgery was significantly higher in the latter (24 vs 51%, respectively) [51]. Therefore, the effect of SDR on the rate of orthopedic surgery is likely more complex than can be answered with the current state of evidence, with age and functional status playing a significant role, and larger long term data is needed.
Sectioned dorsal nerve roots stimulation responses in SDR
In our cohort, the left side had lower stimulation intensity thresholds and higher grades compared to the right side. Stimulation intensity thresholds were lower at lumbar levels compared to sacral levels, and grade also increased from lumbar to sacral levels. There was no association found with age, gender, or GMFCS level. This pattern was also reported by De Vloo et al. in a cohort of 145 participants [78], and similar findings regarding increasing grade in lower levels have been reported by others [19, 81, 82]. We divide spinal levels into lumbar, lumbosacral and sacral rather than individual spinal levels based on our experiences and that of others that muscle response will often occur with stimulation of more than one spinal level [20, 63, 70]. We found a significant asymmetry in grading, higher on the left, which was also reported by Wolter et al. [82] but not found by others [78]. It is difficult to ascertain whether these findings are due to a higher proportion of participants with left more than right spasticity being captured in any one study resulting in a type 1 error, if addressing one side effects the response on the contralateral side which was not controlled for in our study, or whether a true asymmetry in CP individuals exists and further investigation into this distribution is needed.
The use of electrophysiology during SDR has been debated. Arguments to forgo neuromonitoring include shorter operative time [74], similar postoperative outcomes [37, 74], and potentially non-reproducible results with repeat stimulation in a small cohort [80]. One study provided a mathematical probability, stating that even if 50 – 75% of rootlets were sectioned at random this would include enough abnormal rootlets to achieve clinical benefit. It is the senior author’s practice to always cut approximately two-thirds of the dorsal nerve roots. A meta-analysis of three randomized controlled trials found a significant inverse relationship between changes in GMFM scores and the percent of dorsal nerve roots sectioned [43]. This was also reflected in other studies comparing the rate of nerve root sectioning and spasticity outcome [28, 72]. We include L1 and S2 in our rhizotomy procedure to avoid residual spasticity at the hip adductors and ankles respectively as has been shown by some authors [33]. However, the benefit of neuromonitoring is beyond simply spasticity outcomes. Bladder and bowel innervation can receive contributions from S1 and S2 [14, 29, 53]. Overlap in stimulation above this level triggering anal sphincter response has also been put forth by some authors as an unreliable use of NIOM [54]. Nerve roots with more abnormal responses have more degenerative changes on histology further supporting the use of electrophysiological sectioning in SDR [18]. Additionally, monitoring has been used for more tailored approaches by some authors [19, 84]. We have also found that monitoring helps guide the surgeon performing single level SDR when anatomy is distorted to ensure adequate representation of all levels and avoiding ventral nerve roots with lower stimulation thresholds as has been reported by others [40]. Therefore, it is our practice to continue to use neuromonitoring for these cases in addition to the neurophysiological data this adds to understanding the change in neuronal circuity in CP individuals with spasticity.
Limitations
The current study has several limitations. Including its retrospective nature, the smaller number of patients included in the outcome measures and length of follow up. The effect of SDR on upper limb spasticity is reported but we did not assess change in function or quality of life as it pertains to this improvement and believe this an important aspect to include in future studies. Absolute baclofen dose was used for analysis and not weight based dosing, however, as these were used to compare each participant’s pre- and postoperative change, we believe the results accurately reflect any changes. We did not include the type of orthopedic procedures done for spasticity which may provide frequencies and trends. Spared nerve rootlets were not included in the analysis which would add further information about the neurophysiological characteristics of these nerves; we sectioned the first 2/3rd if they were a grade 3 or 4 and only stimulated further if a grade 0 – 2 was encountered first. We believe this achieves satisfactory results and decreases the time under general anesthesia with shorter surgery times.
Conclusions
SDR improves GMFM-66 scores at short term follow up in GMFCS I to III levels. SDR also significantly decreases the need for postoperative botulinum injections and baclofen use. Lower stimulation threshold and higher grades were seen on the left side and with descending lumbosacral levels. The stimulation responses of sectioned nerve roots adds to limited available information regarding dorsal nerve root characteristics in patients undergoing SDR. These parameters should be included in future reports on SDR beyond the gross motor function as they also impact quality of life and our knowledge of the neurophysiology of the disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CP
Cerebral palsy
- GMFM
Gross motor function measure
- GMFCS
Gross motor function classification system
- SDR
Selective dorsal rhizotomy
- EMG
Electromyography
- NIOM
Neurophysiologic intraoperative monitoring
- ANOVA
One-way repeated measures analysis of variance
- SE
Standard error
- SD
Standard deviation
- CI
Confidence intervals
- BCa
Bias-corrected and accelerated
- OR
Odds ratio
- mA
Milliamperes
- Hz
Hertz
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ziyad Makoshi, Monica Islam and Jennifer McKinney. The first draft of the manuscript was written by Ziyad Makoshi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for this research.
Data availability
Data is available at request and per our institutional guidelines for data sharing.
Code availability
Not applicable.
Declarations
Ethics approval
Informed consent was obtained from all individual participants included in the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ailon T et al (2015) Long-term outcome after selective dorsal rhizotomy in children with spastic cerebral palsy. Childs Nerv Syst 31(3):415–423 10.1007/s00381-015-2614-9 [DOI] [PubMed] [Google Scholar]
- 2.Ashworth B (1964) Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 192:540–2 [PubMed] [Google Scholar]
- 3.Bartlett DJ et al (2010) Correlates of decline in gross motor capacity in adolescents with cerebral palsy in Gross Motor Function Classification System levels III to V: an exploratory study. Dev Med Child Neurol 52(7):e155–e160 10.1111/j.1469-8749.2010.03632.x [DOI] [PubMed] [Google Scholar]
- 4.Beckung E et al (2007) The natural history of gross motor development in children with cerebral palsy aged 1 to 15 years. Dev Med Child Neurol 49(10):751–756 10.1111/j.1469-8749.2007.00751.x [DOI] [PubMed] [Google Scholar]
- 5.Blaszczyk I et al (2015) Questionnaire about the adverse events and side effects following botulinum toxin A treatment in patients with cerebral palsy. Toxins (Basel) 7(11):4645–4654 10.3390/toxins7114645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolster EA et al (2013) Long-term effect of selective dorsal rhizotomy on gross motor function in ambulant children with spastic bilateral cerebral palsy, compared with reference centiles. Dev Med Child Neurol 55(7):610–616 10.1111/dmcn.12148 [DOI] [PubMed] [Google Scholar]
- 7.Buckon CE et al (1996) Assessment of upper-extremity function in children with spastic diplegia before and after selective dorsal rhizotomy. Dev Med Child Neurol 38(11):967–975 10.1111/j.1469-8749.1996.tb15057.x [DOI] [PubMed] [Google Scholar]
- 8.Calia R, Santomauro E, Traldi S (1976) The use of baclofen in children with cerebral palsy. Fohla Medica 3:199–202 [Google Scholar]
- 9.Chicoine MR, Park TS, Kaufman BA (1997) Selective dorsal rhizotomy and rates of orthopedic surgery in children with spastic cerebral palsy. J Neurosurg 86(1):34–39 10.3171/jns.1997.86.1.0034 [DOI] [PubMed] [Google Scholar]
- 10.Chiu HC, Ada L, Chen C (2020) Changes in walking performance between childhood and adulthood in cerebral palsy: a systematic review. Dev Neurorehabil 23(6):343–348 10.1080/17518423.2019.1648579 [DOI] [PubMed] [Google Scholar]
- 11.Christensen D et al (2014) Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning - Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol 56(1):59–65 10.1111/dmcn.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craft S et al (1995) Changes in cognitive performance in children with spastic diplegic cerebral palsy following selective dorsal rhizotomy. Pediatr Neurosurg 23(2): 68–74; discussion 75 [DOI] [PubMed]
- 13.Dai AI, Aksoy SN, Demiryürek AT (2016) Comparison of efficacy and side effects of oral baclofen versus tizanidine therapy with adjuvant botulinum toxin type A in children with cerebral palsy and spastic equinus foot deformity. J Child Neurol 31(2):184–189 10.1177/0883073815587030 [DOI] [PubMed] [Google Scholar]
- 14.Deletis V et al (1992) Intraoperative monitoring of the dorsal sacral roots: minimizing the risk of iatrogenic micturition disorders. Neurosurgery 30(1):72–75 10.1227/00006123-199201000-00013 [DOI] [PubMed] [Google Scholar]
- 15.Dudley RW et al (2013) Long-term functional benefits of selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg Pediatr 12(2):142–150 10.3171/2013.4.PEDS12539 [DOI] [PubMed] [Google Scholar]
- 16.Fasano, VA et al (1977) New aspects in the surgical treatment of cerebral palsy. Acta Neurochir (Wien) (Suppl 24) p 53–7. Springer [DOI] [PubMed]
- 17.Foerster (1911) Resection of the posterior spinal nerve-roots in the treatment of gastric crises and spastic paralysis. Proc R Soc Med 4(Surg Sect): 254 [PMC free article] [PubMed]
- 18.Fukuhara T et al (2011) Histological evidence of intraoperative monitoring efficacy in selective dorsal rhizotomy. Childs Nerv Syst 27(9):1453–1458 10.1007/s00381-011-1462-5 [DOI] [PubMed] [Google Scholar]
- 19.Georgoulis G, Brînzeu A, Sindou M (2018) Dorsal rhizotomy for children with spastic diplegia of cerebral palsy origin: usefulness of intraoperative monitoring. J Neurosurg Pediatr 22(1):89–101 10.3171/2018.1.PEDS17577 [DOI] [PubMed] [Google Scholar]
- 20.Georgoulis G, Sindou M (2020) Muscle responses to radicular stimulation during lumbo-sacral dorsal rhizotomy for spastic diplegia: Insights to myotome innervation. Clin Neurophysiol 131(5):1075–1086 10.1016/j.clinph.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 21.Gigante P et al (2013) Reduction in upper-extremity tone after lumbar selective dorsal rhizotomy in children with spastic cerebral palsy. J Neurosurg Pediatr 12(6):588–594 10.3171/2013.9.PEDS12591 [DOI] [PubMed] [Google Scholar]
- 22.Goyal V et al (2016) Prospective Randomized Study of Oral Diazepam and Baclofen on Spasticity in Cerebral Palsy. J Clin Diagn Res 10(6):RC01-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gros C et al (1967) Selective posterior radicotomy in the neurosurgical treatment of pyramidal hypertension. Neurochirurgie 13(4):505–518 [PubMed] [Google Scholar]
- 24.Hanna SE et al (2008) Reference curves for the Gross Motor Function Measure: percentiles for clinical description and tracking over time among children with cerebral palsy. Phys Ther 88(5):596–607 10.2522/ptj.20070314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna SE et al (2009) Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol 51(4):295–302 10.1111/j.1469-8749.2008.03196.x [DOI] [PubMed] [Google Scholar]
- 26.Harries N et al (2004) Changes over years in gross motor function of 3–8 year old children with cerebral palsy: using the Gross Motor Function Measure (GMFM-88). Isr Med Assoc J 6(7):408–411 [PubMed] [Google Scholar]
- 27.Hatef J et al (2020) Postoperative pain protocol in children after selective dorsal rhizotomy. Pediatr Neurosurg 55(4):181–187 10.1159/000509333 [DOI] [PubMed] [Google Scholar]
- 28.Hays RM et al (1998) Electrophysiological monitoring during selective dorsal rhizotomy, and spasticity and GMFM performance. Dev Med Child Neurol 40(4):233–238 10.1111/j.1469-8749.1998.tb15455.x [DOI] [PubMed] [Google Scholar]
- 29.Huang JC et al (1997) Preservation of pudendal afferents in sacral rhizotomies. Neurosurgery 41(2):411–415 10.1097/00006123-199708000-00015 [DOI] [PubMed] [Google Scholar]
- 30.Hurvitz EA et al (2013) Functional outcomes of childhood dorsal rhizotomy in adults and adolescents with cerebral palsy. J Neurosurg Pediatr 11(4):380–388 10.3171/2013.1.PEDS12311 [DOI] [PubMed] [Google Scholar]
- 31.Josenby AL et al (2012) Motor function after selective dorsal rhizotomy: a 10-year practice-based follow-up study. Dev Med Child Neurol 54(5):429–435 10.1111/j.1469-8749.2012.04258.x [DOI] [PubMed] [Google Scholar]
- 32.Kan P et al (2008) Surgical treatment of spasticity in children: comparison of selective dorsal rhizotomy and intrathecal baclofen pump implantation. Childs Nerv Syst 24(2):239–243 10.1007/s00381-007-0457-8 [DOI] [PubMed] [Google Scholar]
- 33.Kim DS et al (2002) Selective posterior rhizotomy for lower extremity spasticity: how much and which of the posterior rootlets should be cut? Surg Neurol 57(2):87–93 10.1016/S0090-3019(01)00680-2 [DOI] [PubMed] [Google Scholar]
- 34.Kim HS, Steinbok P, Wickenheiser D (2006) Predictors of poor outcome after selective dorsal rhizotomy in treatment of spastic cerebral palsy. Childs Nerv Syst 22(1):60–66 10.1007/s00381-005-1160-2 [DOI] [PubMed] [Google Scholar]
- 35.Koman LA et al (1993) Management of cerebral palsy with botulinum-A toxin: preliminary investigation. J Pediatr Orthop 13(4):489–495 10.1097/01241398-199307000-00013 [DOI] [PubMed] [Google Scholar]
- 36.Langdon K et al (2010) Adverse events following botulinum toxin type A treatment in children with cerebral palsy. Dev Med Child Neurol 52(10): 972–3; author reply 974 [DOI] [PubMed]
- 37.Logigian EL et al (1994) H reflex studies in cerebral palsy patients undergoing partial dorsal rhizotomy. Muscle Nerve 17(5):539–549 10.1002/mus.880170512 [DOI] [PubMed] [Google Scholar]
- 38.Lundkvist A, Hägglund G (2006) Orthopaedic surgery after selective dorsal rhizotomy. J Pediatr Orthop B 15(4):244–246 10.1097/01202412-200607000-00002 [DOI] [PubMed] [Google Scholar]
- 39.MacWilliams BA et al (2011) Functional decline in children undergoing selective dorsal rhizotomy after age 10. Dev Med Child Neurol 53(8):717–723 10.1111/j.1469-8749.2011.04010.x [DOI] [PubMed] [Google Scholar]
- 40.Martinez V et al (2020) Electrophysiology of sensory and motor nerve root fibers in selective dorsal rhizotomies. Pediatr Neurosurg 55(1):17–25 10.1159/000502326 [DOI] [PubMed] [Google Scholar]
- 41.McKinlay I, Hyde E, Gordon N (1980) Baclofen: a team approach to drug evaluation of spasticity in childhood. Scott Med J 25(4):S26–S28 10.1177/003693308002500440 [DOI] [Google Scholar]
- 42.McLaughlin JF et al (1998) Selective dorsal rhizotomy: efficacy and safety in an investigator-masked randomized clinical trial. Dev Med Child Neurol 40(4):220–232 10.1111/j.1469-8749.1998.tb15454.x [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin J et al (2002) Selective dorsal rhizotomy: meta-analysis of three randomized controlled trials. Dev Med Child Neurol 44(1):17–25 [DOI] [PubMed] [Google Scholar]
- 44.Milla PJ, Jackson AD (1977) A controlled trial of baclofen in children with cerebral palsy. J Int Med Res 5(6):398–404 [DOI] [PubMed] [Google Scholar]
- 45.Mittal S et al (2002) Long-term functional outcome after selective posterior rhizotomy. J Neurosurg 97(2):315–325 10.3171/jns.2002.97.2.0315 [DOI] [PubMed] [Google Scholar]
- 46.Mortenson P et al (2021) Long-term upper extremity performance in children with cerebral palsy following selective dorsal rhizotomy. Childs Nerv Syst 37(6):1983–1989 10.1007/s00381-020-05018-2 [DOI] [PubMed] [Google Scholar]
- 47.Naidu K et al (2010) Systemic adverse events following botulinum toxin A therapy in children with cerebral palsy. Dev Med Child Neurol 52(2):139–144 10.1111/j.1469-8749.2009.03583.x [DOI] [PubMed] [Google Scholar]
- 48.Nordmark E et al (2008) Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatr 8:54 10.1186/1471-2431-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norlin R, Tkaczuk H (1985) One-session surgery for correction of lower extremity deformities in children with cerebral palsy. J Pediatr Orthop 5(2):208–211 10.1097/01241398-198505020-00016 [DOI] [PubMed] [Google Scholar]
- 50.O’Brien DF et al (2004) Effect of selective dorsal rhizotomy on need for orthopedic surgery for spastic quadriplegic cerebral palsy: long-term outcome analysis in relation to age. J Neurosurg 101(1 Suppl):59–63 [DOI] [PubMed] [Google Scholar]
- 51.O’Brien DF et al (2005) Orthopedic surgery after selective dorsal rhizotomy for spastic diplegia in relation to ambulatory status and age. J Neurosurg 103(1 Suppl):5–9 [DOI] [PubMed] [Google Scholar]
- 52.O’Flaherty SJ et al (2011) Adverse events and health status following botulinum toxin type A injections in children with cerebral palsy. Dev Med Child Neurol 53(2):125–130 10.1111/j.1469-8749.2010.03814.x [DOI] [PubMed] [Google Scholar]
- 53.Ogiwara H, Morota N (2014) Pudendal afferents mapping in posterior sacral rhizotomies. Neurosurgery 74(2):171–175 10.1227/NEU.0000000000000235 [DOI] [PubMed] [Google Scholar]
- 54.Ojemann JG et al (1997) Lack of specificity in electrophysiological identification of lower sacral roots during selective dorsal rhizotomy. J Neurosurg 86(1):28–33 10.3171/jns.1997.86.1.0028 [DOI] [PubMed] [Google Scholar]
- 55.Ojemann JG et al (2005) Hand somatosensory cortex activity following selective dorsal rhizotomy: report of three cases with fMRI. Childs Nerv Syst 21(2):115–121 10.1007/s00381-004-1051-y [DOI] [PubMed] [Google Scholar]
- 56.Paget SP et al (2018) Systemic adverse events after botulinum neurotoxin A injections in children with cerebral palsy. Dev Med Child Neurol 60(11):1172–1177 10.1111/dmcn.13995 [DOI] [PubMed] [Google Scholar]
- 57.Palisano R et al (1997) Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39(4):214–223 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 58.Park TS et al (1993) Selective lumbosacral dorsal rhizotomy immediately caudal to the conus medullaris for cerebral palsy spasticity. Neurosurgery 33(5): 929–33; discussion 933–4 [DOI] [PubMed]
- 59.Park TS et al (2017) Functional outcomes of childhood selective dorsal rhizotomy 20 to 28 years later. Cureus 9(5):e1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park TS et al (2017) Beneficial Effects of Childhood Selective Dorsal Rhizotomy in Adulthood. Cureus 9(3):e1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park TS, Johnston JM (2006) Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Technical note. Neurosurg Focus 21(2):e7 10.3171/foc.2006.21.2.8 [DOI] [PubMed] [Google Scholar]
- 62.Peacock WJ, Arens LJ, Berman B (1987) Cerebral palsy spasticity Selective posterior rhizotomy. Pediatr Neurosci 13(2):61–66 10.1159/000120302 [DOI] [PubMed] [Google Scholar]
- 63.Phillips LH, Park TS (1991) Electrophysiologic mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve 14(12):1213–1218 10.1002/mus.880141213 [DOI] [PubMed] [Google Scholar]
- 64.Pulgar S et al (2019) Prevalence, patterns, and cost of care for children with cerebral palsy enrolled in medicaid managed care. J Manag Care Spec Pharm 25(7):817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddihough DS, Collins KJ (2003) The epidemiology and causes of cerebral palsy. Aust J Physiother 49(1):7–12 10.1016/S0004-9514(14)60183-5 [DOI] [PubMed] [Google Scholar]
- 66.Rosenbaum PL et al (2002) Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA 288(11):1357–1363 10.1001/jama.288.11.1357 [DOI] [PubMed] [Google Scholar]
- 67.Russell DJ et al (1989) The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol 31(3):341–352 10.1111/j.1469-8749.1989.tb04003.x [DOI] [PubMed] [Google Scholar]
- 68.Russell DJ et al (2000) Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther 80(9):873–885 10.1093/ptj/80.9.873 [DOI] [PubMed] [Google Scholar]
- 69.Schasfoort F et al (2018) Value of botulinum toxin injections preceding a comprehensive rehabilitation period for children with spastic cerebral palsy: A cost-effectiveness study. J Rehabil Med 50(1):22–29 10.2340/16501977-2267 [DOI] [PubMed] [Google Scholar]
- 70.Sharrard WJ (1964) The Segmental Innervation of the lower limb muscles in man. Ann R Coll Surg Engl 35(2):106–22 [PMC free article] [PubMed] [Google Scholar]
- 71.Smits DW et al (2013) Longitudinal development of gross motor function among Dutch children and young adults with cerebral palsy: an investigation of motor growth curves. Dev Med Child Neurol 55(4):378–384 10.1111/dmcn.12083 [DOI] [PubMed] [Google Scholar]
- 72.Steinbok P et al (1995) Relationship of intraoperative electrophysiological criteria to outcome after selective functional posterior rhizotomy. J Neurosurg 83(1):18–26 10.3171/jns.1995.83.1.0018 [DOI] [PubMed] [Google Scholar]
- 73.Steinbok P et al (1997) A randomized clinical trial to compare selective posterior rhizotomy plus physiotherapy with physiotherapy alone in children with spastic diplegic cerebral palsy. Dev Med Child Neurol 39(3):178–184 10.1111/j.1469-8749.1997.tb07407.x [DOI] [PubMed] [Google Scholar]
- 74.Steinbok P et al (2009) Electrophysiologically guided versus non-electrophysiologically guided selective dorsal rhizotomy for spastic cerebral palsy: a comparison of outcomes. Childs Nerv Syst 25(9):1091–1096 10.1007/s00381-009-0908-5 [DOI] [PubMed] [Google Scholar]
- 75.Sung KH et al (2013) Conflict of interest in the assessment of botulinum toxin A injections in patients with cerebral palsy: a systematic review. J Pediatr Orthop 33(5):494–500 10.1097/BPO.0b013e318288b42a [DOI] [PubMed] [Google Scholar]
- 76.Tedroff K et al (2011) Does loss of spasticity matter? A 10-year follow-up after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol 53(8):724–729 10.1111/j.1469-8749.2011.03969.x [DOI] [PubMed] [Google Scholar]
- 77.Tedroff K, Löwing K, Åström E (2015) A prospective cohort study investigating gross motor function, pain, and health-related quality of life 17 years after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol 57(5):484–490 10.1111/dmcn.12665 [DOI] [PubMed] [Google Scholar]
- 78.De Vloo P et al (2020) Intraoperative electrophysiology during single-level selective dorsal rhizotomy: technique, stimulation threshold, and response data in a series of 145 patients. J Neurosurg Pediatr 25(6):1–10 10.3171/2019.12.PEDS19372 [DOI] [PubMed] [Google Scholar]
- 79.Volpon Santos M et al (2021) Surgical Results of Selective Dorsal Rhizotomy for the Treatment of Spastic Cerebral Palsy. J Pediatr Neurosci 16(1):24–29 10.4103/jpn.JPN_26_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warf BC, Nelson KR (1996) The electromyographic responses to dorsal rootlet stimulation during partial dorsal rhizotomy are inconsistent. Pediatr Neurosurg 25(1):13–19 10.1159/000121090 [DOI] [PubMed] [Google Scholar]
- 81.Wolter S et al (2020) Frequency distribution in intraoperative stimulation-evoked EMG responses during selective dorsal rhizotomy in children with cerebral palsy-part 1: clinical setting and neurophysiological procedure. Childs Nerv Syst 36(9):1945–1954 10.1007/s00381-020-04734-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolter S et al (2020) Frequency distribution in intraoperative stimulation-evoked EMG responses during selective dorsal rhizotomy in children with cerebral palsy-part 2: gender differences and left-biased asymmetry. Childs Nerv Syst 36(9):1955–1965 10.1007/s00381-020-04735-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wright FV et al (1998) Evaluation of selective dorsal rhizotomy for the reduction of spasticity in cerebral palsy: a randomized controlled tria. Dev Med Child Neurol 40(4):239–247 10.1111/j.1469-8749.1998.tb15456.x [DOI] [PubMed] [Google Scholar]
- 84.Xiao B et al (2020) The role of intra-operative neuroelectrophysiological monitoring in single-level approach selective dorsal rhizotomy. Childs Nerv Syst 36(9):1925–1933 10.1007/s00381-019-04408-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available at request and per our institutional guidelines for data sharing.
Not applicable.