Abstract
The changes in tongue coating metabolites in patients with chronic gastritis (CG) under different gastroscopy indicators were analyzed, and these metabolites were screened for potential non-invasive biomarkers to assist in the diagnosis of chronic gastritis. The technology of gas chromatography and liquid chromatography combined with mass spectrometry has been used to more comprehensively detect tongue coating metabolites of 350 CG patients. Spearman correlation analysis and random forest algorithm were used to screen metabolites that can serve as potential biomarkers. Compared with healthy individuals, CG group showed significant changes in the content of 101 metabolites, with an increase in the content of 54 metabolites and a decrease in the content of 47 metabolites. These differential metabolites are mainly composed of 47 lipids and lipid like substances. 1 metabolite was associated with bile reflux, 1 metabolite was associated with gastric mucosal erosion, 10 metabolites were associated with atrophy, 10 metabolites were associated with intestinal metaplasia, and 3 metabolites were associated with Helicobacter pylori infection. The ROC model composed of 5 metabolites can distinguish between CG group and healthy individuals, with an accuracy of 95.4%. The ROC model composed of 5,6-Dihydroxyindole can distinguish between chronic superficial gastritis group and chronic atrophic gastritis group, with an accuracy of 75.3%. The lipids and lipid like metabolites were the main abnormal metabolites in patients with chronic gastritis. It was worth noting that the content of Sphinganine 1-phase, 4-Ipomenol, and Nervonic acid in tongue coating increased, and the content of 1-Methyladenosine and 3-Hydroxycapric acid decreased, which helped to identify CG patients. The decrease in the content of 5,6-dihydroxyindole reminded patients that the development trend of CG was shifting from superficial to atrophic or even intestinal metaplasia. The detection of these metabolic markers of tongue coating was expected to be developed as a non-invasive and convenient technology in the future to assist us in monitoring and diagnosing the occurrence and development of CG.
Keywords: Metabolomics, Gastroscopy indicators, Gastric mucosal pathology, Non-invasive diagnosis
Subject terms: Gastrointestinal diseases, Oesophagogastroscopy
Introduction
Nowadays, with the faster pace of people’s lives and the change of people’s dietary structure, more and more people are suffering from digestive diseases. Chronic gastritis (CG) as one of the most common digestive diseases, has affected more than half the world's population in recent years. The development of CG generally progresses from superficial gastritis to atrophic gastritis, and atrophic gastritis has a certain chance of transforming into gastric cancer1. Therefore, early detection and treatment of CG are of great significance in preventing further injury and improving the quality of life. Although gastroscopy is currently the most reliable method for diagnosing CG, it cannot be performed frequently in a short time2. Thus, finding a noninvasive and repeatable monitoring method to observe the progress of CG is one of the main directions of our research.
According to the theory of traditional Chinese medicine (TCM), the formation of tongue coating is influenced by the physiological function of the stomach. Therefore, TCM doctor often diagnoses gastric disease by observing changes in the tongue3, which is non-invasive and repeatable. Some researchers have used liquid chromatography-mass spectroscopy technology to discover differences in tongue coating metabolites between CG patients and healthy individuals4,5. However, these studies only used one metabolomics detection method and had a small sample size, which resulted in incomplete detection of metabolites. Meanwhile, studies on the correlation of tongue coating metabolites and gastroscopic indexes in CG patients have not been reported.
The gas chromatography-time-of-light-mass spectroscopy (GC-TOF-MS) and ultra high-performance liquid chromatography-Q-exactive-mass spectroscopy (UHPLC-QE-MS) are the two most commonly used techniques for metabolomics detection. The two detection methods target different levels of metabolite molecular weight, volatility, and solubility6. Therefore, for the complex composition of metabolites in tongue coating, a combination of two methods can be used to achieve a more comprehensive detection.
Therefore, this study used a combination of GC-TOF-MS and UHPC-QE-MS to detect tongue coating samples from 350 CG patients, and then used bioinformatics and statistical methods to identify potential metabolic markers in the development of CG.
Materials and methods
Samples
This study diagnosed 350 patients with CG through gastroscopy and gastric mucosal pathological examination at Longhua Hospital. Additionally, 50 healthy controls from Shanghai University of TCM were selected. The healthy controls had no history of stomach disease or current stomach discomfort. The health of the control group was confirmed by a comprehensive health assessment including haematological cytology, liver and renal function, blood glucose, blood lipids, tumour markers, blood pressure, colour Doppler ultrasound of the neck and abdomen, and 13C urea breath test. The participants included in this study were Han Chinese (self-reported), and the sample was collected from December 2018 to October 2019. Gastroscopy and gastric mucosal pathology were performed promptly after obtaining tongue coating specimens from the participants.
Ethics approval and statement
The ethics approval for the study was granted by the Ethics Committee of Shanghai University of TCM, with the approval obtained in December 2018. Prior to sample collection, all the participants provided their informed consent by signing consent agreements, indicating their voluntary participation in the study. The experimental procedures were carried out in strict accordance with the Declaration of Helsinki and local laws.
Criteria
With reference to the first and second editions of the Kyoto Classification of Gastritis, patients were subjected to endoscopic examination and biopsies were performed from suspected lesions of gastric antrum, gastric angle, gastric body, and cardia7. The gastroscopic report includes six gastroscopic and pathological indicators: bile reflux, gastric mucosal erosion, inflammatory activity, atrophy (mild, moderate, or severe), intestinal metaplasia (mild, moderate, or severe), and Helicobacter pylori (Hp) infection (mild, moderate, or severe).
The inclusion and exclusion criteria followed our previous study which were shown in Supplementary file and Fig. S18.
Tongue coating collection
The tongue coating was collected using our previous collection method8. The specific acquisition method was shown in Supplementary file.
Metabolomics testing
In the GC-TOF-MS testing, the reagents used were listed in Table S3a. The experimental steps were presented in Supplementary file. The original data were analyzed by using Chroma TOF (V 4.3x, LECO) software9. The mass spectrum and retention index identification of metabolites were performed by using the LECO-Fiehn Rtx5 database. The peaks detected in QC samples were less than half of the peaks were removed in QC samples with RSD > 30%10.
In the UHPLC-QE-MS testing, the reagents used were listed in Supplementary Table S3b. The experimental steps were presented in Supplementary file. The cutoff value for annotation was set to 0.311.
Statistical analysis
The Simca-p+13.0 software (Umea, Sweden) was used for OPLS-DA. To further validate the model, a drive substitution experiment was carried out. The significance of metabolite differences between two groups was determined using various statistical criteria, including the false discovery rate (FDR) of the rank-sum test, P-values (P < 0.05), variable importance in the projection (VIP) of the first principal component of the OPLS-DA model (VIP > 1)11, similarity value (SV) identified through GC-TOF-MS (SV > 700)12, and MS2 score obtained from UHPLC-QE-MS detection (MS2 score > 0.6)13. Metabolite peak areas' mean values were compared between the two groups, and the log fold change (FC) value was subsequently calculated to assess the magnitude of differences.
The significantly different metabolites of the two groups were randomly combined, and the receiver operating characteristic (ROC) curve was used to evaluate model accuracy. Finally, the combination with the highest AUC value was selected as the diagnostic model.
The Spearman algorithm was used to analyze correlation between gastroscopic indicators and differential metabolites. The Corr matrix, correlation P-values were calculated for subsequent analysis and graphically plotted.
Among the metabolites that have significant correlation with gastroscopic indicators, Analysis of variance (ANOVA) was used to compare the standardized peak areas of these metabolites in patients with chronic gastritis when there were different levels of pathological indicators.
We performed the total ion flow normalisation method on the measured peaks and tested for normality. The data did not conform to a normal distribution, so metabolites with significant differences were screened by the rank sum test (see “Materials and methods”). Using significantly different metabolites that can be retrieved in the Fiehn database, ROC curves were plotted for the CG group versus the healthy control group and AUC values were obtained. The AUC values were sorted in descending order, and the top 10 metabolites with the highest AUC values in the ROC curves were selected for random combination. The ROC curves of the CG group and the healthy control group were plotted again. The ROC curve with the highest AUC value and involving the lowest number of differential metabolites was derived. As a diagnostic model for the CG group versus healthy controls.
Ethics approval and consent to participate
The Ethics Committee of Shanghai University of TCM approved our study. The study obtained informed consent from all participants.
Results
Basic information analysis results
It can be seen from Table S1 that there was no significant difference in the sex ratio between the CG group and healthy controls, but there was significant difference in the age. Therefore, the orthogonal projections to late structures-discriminant analysis (OPLS-DA) were used to observe the interference of age factors between the two groups. From Fig. S2, it can be shown that the metabolites were not significantly distinguished, which suggested that age does not affect the reliability of our results.
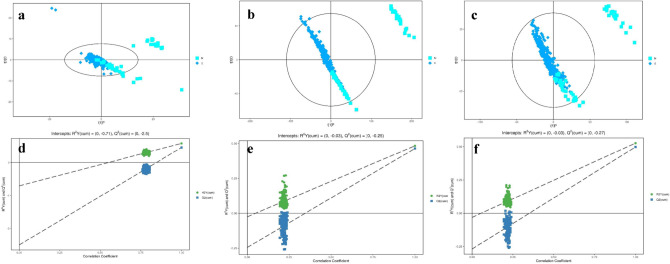
The horizontal axist[1]P represents the predicted component scores of the first principal component, while the vertical axis t[1]O represents the scores of the orthogonal component. The scatter plot uses different shapes and colors to represent distinct groups. According to the results from the OPLS-DA score plot, it is evident that the differentiation between sample groups is significant, and the samples generally fall within the 95% confidence interval (show in Fig. 1).
Figure 1.
OPLS-DA analysis of the chronic gastritis group and healthy controls. (a) GC-TOF-MS scatterplot of OPLS-DA model scores. (b) UHPLC-QE-MS positive ion modes scatterplot of OPLS-DA model scores. (c) UHPLC-QE-MS negative ion modes scatterplot of OPLS-DA model scores. (d) GC-TOF-MS plot of permutation test results for the OPLS-DA model. (e) UHPLC-QE-MS positive ion modes plot of permutation test results for the OPLS-DA model. (f) UHPLC-QE-MS negative ion modes plot of permutation test results for the OPLS-DA model.
The Q2 values of the random model were consistently lower than those of the original model. The intercept of the regression line between Q2 and the vertical axis was less than zero. Simultaneously, as the permutation retention rate gradually decreased, the proportion of permuted Y variables increased, leading to a gradual decline in the Q2 of the random model. This indicates that the original model exhibits good robustness and does not show signs of overfitting (show in Fig. 1).
Different metabolic MS peaks in tongue coating samples
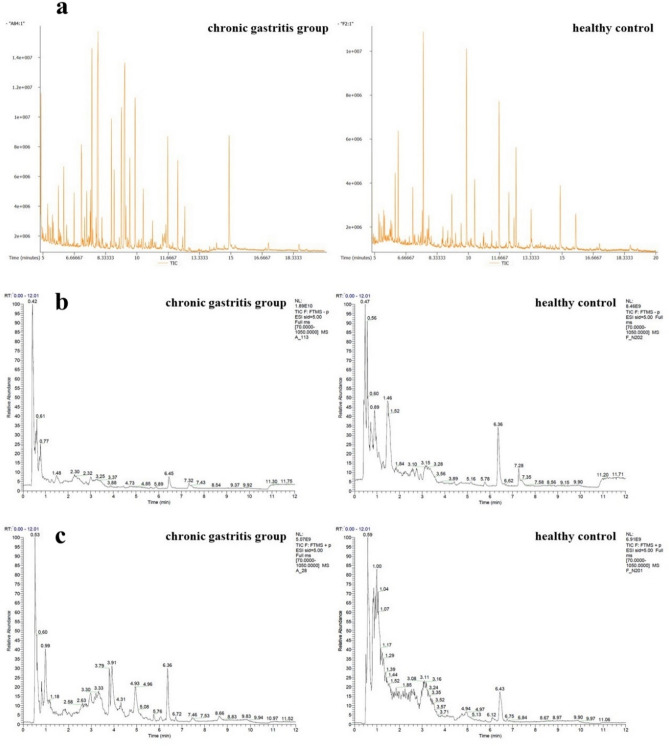
As depicted in Fig. 2a–c, discernible differences in MS peaks were observed between CG patients and healthy controls.
Figure 2.
Mass spectrum peaks of chronic gastritis group and healthy control group. (a) GC-TOF-MS, (b) UHPLC-QE-MS negative ion modes, (c) UHPLC-QE-MS positive ion modes. As shown in (a–c), there were some different mass spectrum peaks between the chronic gastritis patients and healthy control people.
Applying the criteria of P-value < 0.05 and VIP > 1, the analysis using GC-TOF-MS identified 32 peaks in the tongue coating of CG patients (20 increased and 12 decreased). Similarly, UHPLC-QE-MS positive analysis revealed 98 peaks (62 increased and 36 decreased), while UHPLC-QE-MS negative analysis detected 14 peaks (10 increased and 4 decreased).
When employing the criteria of similarity (SV > 700) or MS2 score (> 0.6), GC-TOF-MS analysis unveiled 19 peaks (11 increased and 8 decreased) in the tongue coating of CG patients. For UHPLC-QE-MS positive analysis, 72 peaks were identified (36 increased and 36 decreased), and UHPLC-QE-MS negative analysis revealed 10 peaks (7 increased and 3 decreased). These findings highlight the distinct metabolic profiles present in the tongue coating of CG patients compared to healthy controls.
Different metabolites analysis
In Table 1, 85 metabolites were divided into 11 categories, of which the largest category was benzenoids (2 metabolites), lipids and lipid-like molecules (47 metabolites), organic acids and derivatives (13 metabolites), organic nitrogen compounds (6 metabolites), organic oxygen compounds (4 metabolites), and organoheterocyclic compounds (8 metabolites). There was one metabolite in homogeneous non-metal compounds, hydrocarbons, nucleosides, nucleotides, and analogues, organosulfur compounds, and phenylpropanoids and polyketides.
Table 1.
Identification of significant different metabolites in tongue coating by comparison of chronic gastritis patients and healthy controls.
| Metabolite name | RT (min) | M/Z | Chronic gastritis Mean | Healthy control Mean | P | VIP | Log fold change | Detection method |
|---|---|---|---|---|---|---|---|---|
| Lipids and lipid-like molecules | ||||||||
| Arachidonic acid | 37.582 | 303 | 17.019 | 4.430 | 1.509E−09 | 1.569 | 1.942 | LC− |
| Prostaglandin D2 | 46.555 | 351 | 1.937 | 1.098 | 8.949E−10 | 1.657 | 0.819 | LC − |
| 9,10-DHOME | 81.276 | 313 | 0.133 | 0.634 | 0.001 | 1.256 | − 2.256 | LC − |
| Azelaic acid | 359.713 | 187 | 0.191 | 0.796 | 1.889E−12 | 1.731 | − 2.062 | LC − |
| Ethyl oleate | 41.892 | 309 | 0.692 | 0.332 | 4.658E−12 | 1.241 | 1.060 | LC − |
| 8,11,14-Eicosatrienoic acid | 37.965 | 305 | 0.760 | 0.229 | 1.003E−12 | 1.364 | 1.727 | LC − |
| Nervonic acid | 37.630 | 365 | 0.309 | 0.128 | 6.539E−15 | 1.521 | 1.275 | LC − |
| Tetracosanoic acid | 37.748 | 367 | 0.561 | 0.357 | 4.645E−07 | 1.040 | 0.651 | LC − |
| Foeniculoside VII | 71.454 | 349 | 0.517 | 1.452 | 2.235E−04 | 1.168 | −1.490 | LC + |
| Glycerol tripropanoate | 61.345 | 261 | 1.163 | 2.718 | 0.002 | 1.078 | − 1.225 | LC + |
| Solavetivone | 115.309 | 219 | 0.950 | 0.134 | 7.717E−10 | 1.408 | 2.826 | LC + |
| Patchoulenone | 61.062 | 219 | 11.219 | 3.010 | 9.843E−06 | 1.438 | 1.898 | LC + |
| Gibberellin A79 | 71.856 | 365 | 0.064 | 0.152 | 2.553E−04 | 1.063 | − 1.247 | LC + |
| Oleic acid | 33.290 | 283 | 0.263 | 0.421 | 9.784E−06 | 1.132 | − 0.678 | LC + |
| Dexamethasone | 77.202 | 393 | 0.262 | 1.323 | 1.264E−04 | 1.249 | − 2.335 | LC + |
| 3-O-Methylniveusin A | 77.445 | 409 | 0.044 | 0.277 | 2.619E−07 | 1.373 | − 2.641 | LC + |
| Hoduloside VII | 248.651 | 932 | 0.926 | 0.579 | 2.553E−05 | 1.398 | 0.678 | LC + |
| Fucoxanthin | 259.139 | 659 | 0.077 | 0.392 | 2.217E−10 | 1.576 | − 2.344 | LC + |
| N-Cyclopropyl-trans-2-cis-6-nonadienamide | 46.068 | 194 | 0.174 | 0.114 | 2.907E−07 | 1.364 | 0.611 | LC + |
| 3-Hydroxyisovalerylcarnitine | 57.688 | 262 | 0.119 | 1.688 | 4.202E−09 | 1.457 | − 3.830 | LC + |
| Lyciumoside III | 142.803 | 649 | 0.025 | 2.219 | 3.956E−11 | 1.524 | − 6.491 | LC + |
| Eicosadienoic acid | 33.311 | 309 | 0.795 | 3.662 | 4.891E−10 | 1.584 | − 2.203 | LC + |
| 3beta-Acetoxy-11alpha-methoxy-12-ursen-28-oic acid | 193.697 | 543 | 0.092 | 0.044 | 5.348E−12 | 1.400 | 1.071 | LC + |
| Antibiotic X 14889C | 257.759 | 615 | 0.082 | 0.406 | 7.246E−11 | 1.561 | − 2.308 | LC + |
| 2-Ethyl-2-Hydroxybutyric acid | 58.656 | 133 | 0.727 | 2.302 | 6.578E−06 | 1.406 | − 1.663 | LC + |
| Palmitoyl glucuronide | 293.193 | 419 | 0.408 | 0.261 | 8.303E−06 | 1.422 | 0.648 | LC + |
| Physalin O | 314.512 | 529 | 0.129 | 0.082 | 3.070E−05 | 1.426 | 0.663 | LC + |
| Desglucocoroloside | 220.265 | 505 | 0.030 | 0.017 | 4.319E−13 | 1.078 | 0.792 | LC + |
| 3-Methylglutarylcarnitine | 84.851 | 290 | 0.257 | 0.182 | 0.008 | 1.289 | 0.502 | LC + |
| Smilanippin A | 113.780 | 725 | 0.594 | 0.375 | 2.235E−04 | 1.408 | 0.662 | LC + |
| PS(16:1(9Z)/18:4(6Z,9Z,12Z,15Z)) | 207.222 | 754 | 0.069 | 0.573 | 2.189E−13 | 1.592 | − 3.047 | LC + |
| CPA(18:0/0:0) | 278.918 | 421 | 0.210 | 0.131 | 1.435E−04 | 1.368 | 0.682 | LC + |
| Cucurbitacin I 2-glucoside | 205.358 | 677 | 1.712 | 1.092 | 1.325E−04 | 1.092 | 0.648 | LC + |
| Glycerol tributanoate | 125.367 | 303 | 0.170 | 0.563 | 1.158E−05 | 1.081 | − 1.728 | LC + |
| Fasciculic acid C | 116.186 | 710 | 0.470 | 1.367 | 3.248E−07 | 1.406 | − 1.539 | LC + |
| Hebevinoside I | 38.382 | 809 | 0.097 | 0.365 | 2.113E−06 | 1.375 | − 1.917 | LC + |
| 2,4,6-Octatriynoic acid | 440.442 | 133 | 0.296 | 0.478 | 6.027E−12 | 1.127 | − 0.692 | LC + |
| Lycoperoside D | 154.407 | 740 | 0.568 | 11.651 | 8.613E−06 | 1.352 | − 4.358 | LC + |
| Chondrillasterol 3-[glucosyl-(1->4)-glucoside] | 97.620 | 737 | 0.687 | 1.821 | 2.655E−11 | 1.488 | − 1.406 | LC + |
| Physapruin B | 227.103 | 603 | 0.099 | 0.058 | 5.799E−08 | 1.112 | 0.778 | LC + |
| Traumatic acid | 281.345 | 229 | 0.497 | 0.421 | 0.047 | 1.196 | 0.239 | LC + |
| Lactosylceramide (d18:1/26:0) | 31.388 | 1003 | 0.003 | 1.964 | 2.231E−16 | 1.364 | − 9.313 | LC + |
| Sphinganine 1-phosphate | 51.580 | 382 | 0.166 | 0.069 | 2.606E−14 | 1.554 | 1.256 | LC + |
| 3-Benzoyloxy-11-oxo-12-ursen-28-oic acid | 34.377 | 575 | 2.140 | 1.439 | 0.002 | 1.278 | 0.573 | LC + |
| Momordicoside K | 69.766 | 649 | 0.674 | 1.394 | 2.049E−11 | 1.508 | − 1.049 | LC + |
| PI(22:5(4Z,7Z,10Z,13Z,16Z)/16:0) | 244.507 | 886 | 0.154 | 0.528 | 7.818E−08 | 1.368 | − 1.779 | LC + |
| Vinaginsenoside R3 | 84.853 | 932 | 0.466 | 1.065 | 1.241E−09 | 1.445 | − 1.192 | LC + |
| Organic acids and derivatives | ||||||||
| Glycolic acid | 5.611 | 147 | 0.001 | 0.002 | 0.005 | 1.229 | − 0.518 | GC |
| Pantothenic acid | 11.358 | 103 | 4.356E-04 | 2.233E-04 | 1.183E−08 | 1.143 | 0.964 | GC |
| 3-Hydroxycapric acid | 83.306 | 187 | 0.214 | 0.441 | 3.691E−15 | 1.383 | − 1.043 | LC − |
| Bortezomib | 285.056 | 407 | 0.080 | 0.811 | 4.942E−05 | 1.026 | − 3.350 | LC + |
| Ustiloxin D | 287.091 | 495 | 0.044 | 0.450 | 5.519E−04 | 1.239 | − 3.339 | LC + |
| Symmetric dimethylarginine | 158.848 | 203 | 4.835 | 6.288 | 1.124E−12 | 1.032 | − 0.379 | LC + |
| Oseltamivir | 314.576 | 313 | 0.145 | 0.096 | 2.488E−04 | 1.397 | 0.593 | LC + |
| Arginyl-Arginine | 296.631 | 331 | 2.106 | 1.462 | 0.045 | 1.439 | 0.526 | LC + |
| Arginyl-Histidine | 166.723 | 312 | 0.423 | 0.284 | 0.010 | 1.342 | 0.574 | LC + |
| Dihydro-3-(2-octenyl)-2,5-furandione | 281.391 | 211 | 0.127 | 0.101 | 0.012 | 1.196 | 0.323 | LC + |
| N-Acetylleucine | 265.067 | 174 | 0.154 | 0.099 | 2.133E−04 | 1.231 | 0.632 | LC + |
| Valyl-Isoleucine | 221.874 | 231 | 0.092 | 0.032 | 3.496E−12 | 1.161 | 1.498 | LC + |
| l-cis-3-Amino-2-pyrrolidinecarboxylic acid | 75.782 | 131 | 0.032 | 0.683 | 1.979E−12 | 1.399 | − 4.437 | LC + |
| Organoheterocyclic compounds | ||||||||
| 1-(1-Pyrrolidinyl)-2-propanone | 53.003 | 128 | 1.823 | 2.480 | 7.682E−13 | 1.031 | − 0.444 | LC + |
| Pyrimidine | 112.505 | 81 | 0.097 | 0.320 | 1.230E−04 | 1.275 | − 1.728 | LC + |
| 5,6-Dihydroxyindole | 45.066 | 150 | 0.251 | 0.169 | 1.267E−05 | 1.128 | 0.574 | LC + |
| N1-Methyl-4-pyridone-3-carboxamide | 79.822 | 153 | 0.212 | 0.176 | 0.032 | 1.002 | 0.269 | LC + |
| 2-Methylpiperidine | 299.990 | 100 | 3.360 | 2.030 | 1.331E−06 | 1.371 | 0.727 | LC + |
| Alpha-Carboxy-delta-decalactone | 125.959 | 215 | 0.080 | 0.336 | 9.623E−07 | 1.087 | − 2.065 | LC + |
| I-Urobilin | 174.113 | 591 | 0.121 | 0.476 | 3.184E−06 | 1.214 | − 1.974 | LC + |
| Garcimangosone C | 282.076 | 413 | 0.857 | 0.656 | 0.048 | 1.287 | 0.387 | LC + |
| Organic nitrogen compounds | ||||||||
| 1-Butylamine | 268.847 | 74 | 12.807 | 7.239 | 2.310E−08 | 1.402 | 0.823 | LC + |
| Nervonyl carnitine | 237.324 | 102 | 636.919 | 429.936 | 1.702 | 1.351 | 0.567 | LC + |
| Isobutylpropylamine | 231.650 | 116 | 0.444 | 0.294 | 7.682E−04 | 1.294 | 0.596 | LC + |
| 2-Diethylaminoethanol | 300.009 | 118 | 196.915 | 117.700 | 2.547E−06 | 1.441 | 0.742 | LC + |
| Dipyridamole | 47.761 | 253 | 0.366 | 9.287 | 0.016 | 1.060 | − 4.666 | LC + |
| l-Histidinol | 62.697 | 142 | 0.424 | 0.160 | 2.656E−07 | 1.289 | 1.406 | LC + |
| Organic oxygen compounds | ||||||||
| Glycerol | 7.006 | 85 | 1.092E-04 | 2.011E-04 | 2.605E−08 | 1.884 | − 0.882 | GC |
| 4-Ipomeanol | 72.941 | 167 | 0.405 | 0.072 | 1.036E−17 | 1.626 | 2.499 | LC − |
| Leonuriside A | 372.037 | 333 | 0.012 | 0.235 | 6.978E−12 | 1.365 | − 4.293 | LC + |
| Trimethylaminoacetone | 206.235 | 116 | 0.123 | 0.095 | 2.258E−04 | 1.324 | 0.380 | LC + |
| Benzenoids | ||||||||
| Dictagymnin | 60.998 | 203 | 0.237 | 0.059 | 8.304E−05 | 1.007 | 2.008 | LC + |
| Salmeterol | 57.091 | 416 | 0.458 | 1.005 | 4.693E−09 | 1.466 | − 1.134 | LC + |
| Homogeneous non-metal compounds | ||||||||
| hydroxylamine | 5.968 | 85 | 6.208E-04 | 0.001 | 2.224E−08 | 1.240 | − 0.834 | GC |
| Nucleosides, nucleotides, and analogues | ||||||||
| 1-Methyladenosine | 185.868 | 282 | 0.375 | 0.692 | 3.673E−14 | 1.015 | − 0.884 | LC + |
| Phenylpropanoids and polyketides | ||||||||
| Sofalcone | 285.882 | 451 | 0.064 | 0.639 | 9.614E−05 | 1.259 | − 3.311 | LC + |
| Organosulfur compounds | ||||||||
| (±)-2-Pentanethiol | 32.769 | 105 | 0.419 | 0.279 | 1.489E−05 | 1.195 | 0.590 | LC + |
| Hydrocarbons | ||||||||
| 1-Methyl-1,3-cyclohexadiene | 32.571 | 95 | 0.476 | 0.315 | 3.084E−09 | 1.290 | 0.595 | LC + |
| Others | ||||||||
| 6-Deoxyglucitol | 10.173 | 117 | 1.979E−04 | 1.416E−04 | 1.789E−04 | 2.679 | 0.483 | GC |
| Myristic acid | 10.642 | 117 | 9.197E−04 | 0.002 | 1.892E−09 | 1.264 | − 1.213 | GC |
| Octadecanol | 12.158 | 327 | 2.588E−05 | 3.633E−05 | 6.106E−05 | 1.036 | − 0.489 | GC |
| 1,2,4-Benzenetriol | 9.344 | 124 | 0.006 | 0.003 | 0.002 | 2.238 | 0.835 | GC |
| Threitol | 8.562 | 191 | 4.846E−05 | 1.651E−04 | 4.677E−11 | 1.286 | − 1.769 | GC |
| 2,4-Dichloro-1-(2-chloroethenyl)-benzene | 7.925 | 170 | 0.012 | 0.008 | 0.011 | 2.553 | 0.645 | GC |
| Asparagine dehydrated | 8.628 | 110 | 2.811E−04 | 2.228E−04 | 0.002 | 1.493 | 0.335 | GC |
| Conduritol-beta-expoxide | 11.541 | 103 | 0.005 | 0.003 | 1.260E−05 | 1.674 | 0.696 | GC |
| Methylmaleic acid | 7.923 | 170 | 0.012 | 0.008 | 0.037 | 2.782 | 0.601 | GC |
| 4-Methylcatechol | 7.928 | 170 | 0.012 | 0.008 | 0.036 | 2.120 | 0.607 | GC |
| Glucose-6-phosphate major | 12.806 | 111 | 5.894E−05 | 4.413E−05 | 1.142E−04 | 2.808 | 0.417 | GC |
| Uridine minor | 13.354 | 145 | 0.002 | 0.001 | 0.015 | 2.825 | 0.387 | GC |
| Methylmalonic acid | 6.698 | 85 | 7.146E−05 | 1.287E−04 | 9.368E−08 | 1.764 | − 0.849 | GC |
| 5-Hydroxynorvaline | 8.88 | 85 | 9.138E−04 | 0.002 | 2.404E−06 | 1.157 | − 0.977 | GC |
| Sulfuric acid | 6.326 | 117 | 1.023E−04 | 7.394E−05 | 0.003 | 1.921 | 0.469 | GC |
| (10S,11R)-Pterosin C 4-glucoside | 282.232 | 397 | 15.388 | 11.382 | 0.024 | 1.345 | 0.435 | LC + |
GC, GC-TOF-MS; LC +, UHPLC-QE-MS positive ion; LC −, UHPLC-QE-MS negative ion.
Diagnostic model of CG
As shown in Fig. 3, the diagnostic model was constructed by identifying distinctive metabolites of 350 CG patients and 50 healthy individuals. The optimal diagnostic model comprised 5 tongue coating metabolites. Comparative analysis with healthy controls revealed significant upregulation of 4-Ipomeanol, Nervonic acid, and Sphinganine 1-phosphate in CG patients, whereas 1-Methyladenosine and 3-Hydroxycapric acid exhibited significant downregulation in CG group. These findings underscore the potential of these identified metabolites as valuable markers for distinguishing CG patients from healthy individuals in the diagnostic context.
Figure 3.
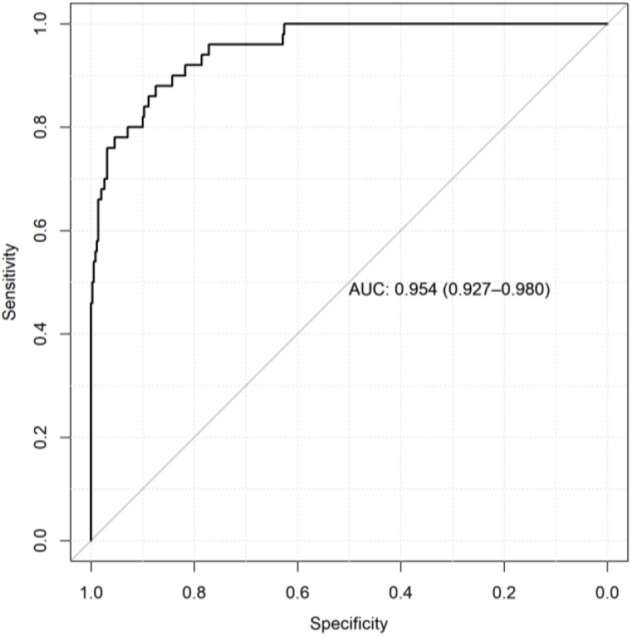
Diagnostic model of tongue coating metabolites in patients with chronic gastritis. The accuracy of the diagnostic model is 95.4%, the specificity is 87.4%, and the sensitivity is 88.0%.
Correlation between different metabolites and gastroscopy indexes
As depicted in Fig. 4a, conduritol-beta-expoxide and octadecanol exhibited negative correlations with atrophy, while conduritol-beta-expoxide demonstrated a negative correlation with intestinal metaplasia. Moreover, 6-deoxyglucitol displayed a negative correlation with bile reflux, and 2,4-dichloro-1-(2-chloroethenyl)-benzene, 4-methylcatechol, and methylmaleic acid were negatively correlated with Hp infection. (Data with correlation P values less than 0.05 were marked with “*” in the figures. Data with correlation P values less than 0.01 were marked with “+” in the figures.)
Figure 4.
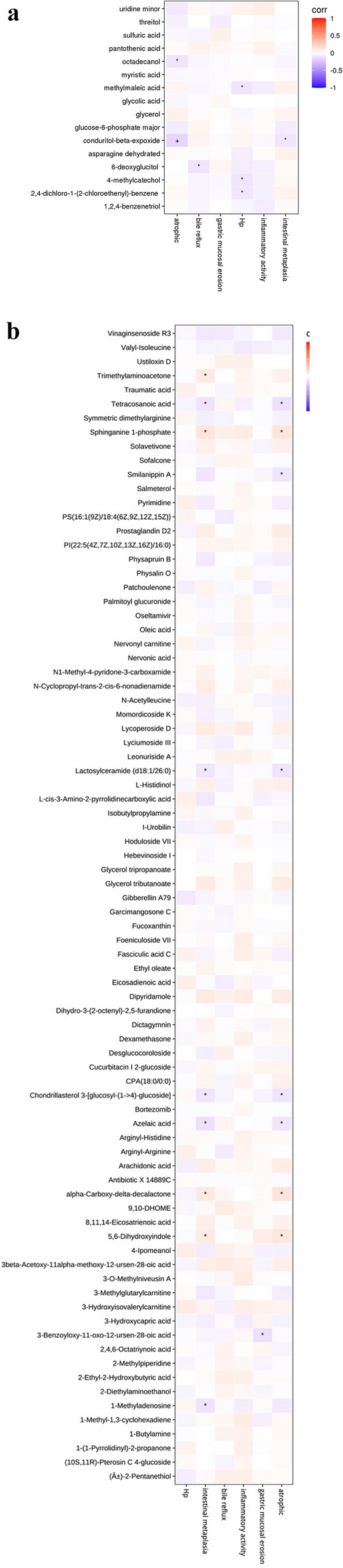
(a) Correlation figure between metabolic markers of tongue coating and gastroscopic indexes and pathological indexes in patients with chronic gastritis analyzed by GC-TOF-MS. Draw a thermodynamic diagram to show the correlation analysis results, which were represented by red (corr = 1), blue (corr = − 1) and white (corr = 0). Data with correlation P values less than 0.05 were marked with “*” in the figures. Data with correlation P values less than 0.01 were marked with “+” in the figures. The abscissa was the differential metabolic marker and the ordinate was the gastroscopic characteristic indicator. (b) Correlation figure between metabolic markers of tongue coating and gastroscopic indexes and pathological indexes in patients with chronic gastritis analyzed by UHPLC-QE-MS. Draw a thermodynamic diagram to show the correlation analysis results, which were represented by red (corr = 1), blue (corr = − 1) and white (corr = 0). Data with correlation P values less than 0.05 were marked with “*” in the figures. The abscissa was the differential metabolic marker, and the ordinate was the gastroscopic characteristic indicator.
Figure 4b reveals that, 5,6-Dihydroxyindole, alpha-Carboxy-delta-decalactone, Sphinganine1-phosphate, and Trimethylaminoacetone were positively correlated with intestinal metaplasia. Conversely, 1-Methyladenosine, Azelaic acid, Chondrillasterol 3-[glucosyl-(1->4)-glucoside], Lactosylceramide(d18:1/26:0), and Tetracosanoic acid were negatively correlated with intestinal metaplasia. Furthermore, 3-benzoyloxy-11-oxo-12-ursen-28-oic acid was negatively correlated with gastric mucosal erosion. 5,6-Dihydroxyindole, alpha-Carboxy-delta-decalactone, and Sphinganine1-phosphate were positively correlated with atrophy. Conversely, Azelaic acid, Chondrillasterol 3-[glucosyl-(1->4)-glucoside], Lactosylceramide(d18:1/26:0), Smilanippin A, and Tetracosanoic acid displayed negative correlations with atrophy. These correlations elucidate the potential associations between specific metabolites and various pathological features observed in CG patients. (Data with correlation P values less than 0.05 were marked with “*” in the figures.)
Change trends of differential metabolite in different pathological stages
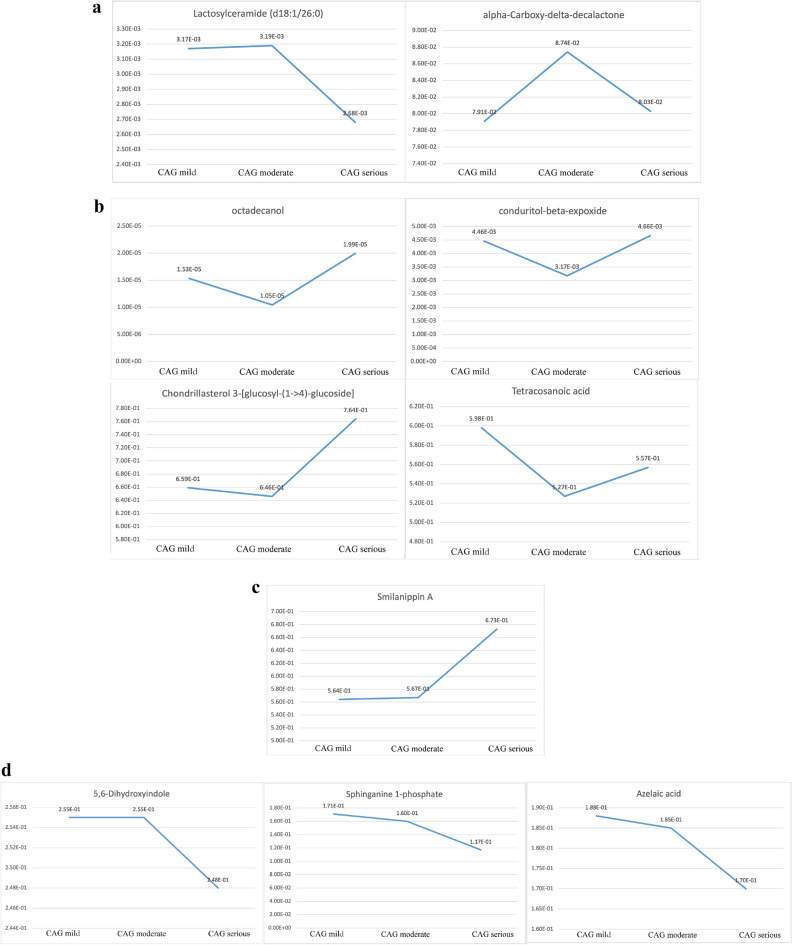
According to Table 2, the contents of alpha-Carboxy-delta-decalactone, Azelaic acid, Chondrillasterol 3-[glucosyl-(1->4)-glucoside], Lactosylceramide (d18:1/26:0), and Octadecanol in the three stages of atrophic were lower than those in the healthy controls. The contents of 5,6-Dihydroxyindole, Conduritol-beta-expoxide, Smilanippin A, Sphinganine 1-phosphate, and Tetracosanoic acid in the three stages of atrophic were higher than those in the healthy controls. The content of conduritol-beta-expoxide decreased significantly in mild atrophic compared with moderate atrophic (P < 0.05). The changing trend of Lactosylceramide and alpha-Carboxy-delta-decalactone (d18:1/26:0) content of atrophic patients was first increased and then decreased (Fig. 5a). The changing trend of Octadecanol, Conduritol-beta-expoxide, Chondrillasterol 3-[glucosyl-(1->4)-glucoside], and Tetracosanoic acid contents of atrophic patients was first decreased and then increased (Fig. 5b). The content of Smilanippin A in the tongue coating of atrophic patients showed an upward trend (Fig. 5c). The contents of 5,6-Dihydroxyindole, Sphinganine 1-phosphate, and Azelaic acid of atrophic patients showed a downward trend (Fig. 5d). We further modelled random forests based our randomization combinations on metabolites with significant correlations. It was used to distinguish patients with chronic superficial gastritis 195 cases (no intestinal metaplasia) and those with atrophic gastritis 106 cases (some are accompanied by intestinal metaplasia). As shown in Fig. 6, the accuracy of the model was 75.3%, sensitivity was 82.9%, and specificity was 60.0%.
Table 2.
The standardized peak areas of metabolites in the tongue coating of chronic gastritis patients at different pathological stages.
| Metabolites | Mild mean | Moderate mean | Severe mean |
|---|---|---|---|
| Atrophic | |||
| Octadecanol | 1.534E−05 | 1.045E−05 | 1.994E−05 |
| Conduritol-beta-expoxide | 4.46E−03 | 3.17E−03* | 4.66E−03 |
| Tetracosanoic acid | 5.98E−01 | 5.27E−01 | 5.57E−01 |
| Sphinganine 1-phosphate | 1.71E−01 | 1.60E−01 | 1.17E−01 |
| Smilanippin A | 5.64E−01 | 5.67E−01 | 6.73E−01 |
| Lactosylceramide (d18:1/26:0) | 3.17E−03 | 3.19E−03 | 2.68E−03 |
| Chondrillasterol 3-[glucosyl-(1->4)-glucoside] | 6.59E−01 | 6.46E−01 | 7.64E−01 |
| Azelaic acid | 1.88E−01 | 1.85E−01 | 1.70E−01 |
| alpha-Carboxy-delta-decalactone | 7.91E−02 | 8.74E−02 | 8.03E−02 |
| 5,6-Dihydroxyindole | 2.55E−01 | 2.55E−01 | 2.48E−01 |
| Intestinal metaplasia | |||
| Conduritol-beta-expoxide | 4.62E−03 | 3.20E−03* | 4.66E−03 |
| Trimethylaminoacetone | 1.24E−01 | 1.16E−01 | 1.12E−01 |
| Tetracosanoic acid | 6.08E−01 | 5.24E−01 | 5.57E−01 |
| Sphinganine 1-phosphate | 1.64E−01 | 1.58E−01 | 1.17E−01 |
| Lactosylceramide (d18:1/26:0) | 3.51E−03 | 3.34E−03 | 2.68E−03 |
| Chondrillasterol 3-[glucosyl-(1->4)-glucoside] | 6.75E−01 | 6.56E−01 | 7.64E−01 |
| Azelaic acid | 1.84E−01 | 1.85E−01 | 1.70E−01 |
| alpha-Carboxy-delta-decalactone | 8.14E−02 | 8.77E−02 | 8.03E−02 |
| 5,6-Dihydroxyindole | 2.54E−01 | 2.56E−01 | 2.48E−01 |
| 1-Methyladenosine | 3.60E−01 | 3.92E−01 | 4.52E−01 |
| Hp infection | |||
| Methylmaleic acid | 1.17E−02 | 1.19E−02 | 1.22E−02 |
| 4-methylcatechol | 1.03E−02 | 1.13E−02 | 1.22E−02 |
| 2,4-dichloro-1-(2-chloroethenyl)-benzene | 1.17E−02 | 1.13E−02 | 1.22E−02 |
*There were significant differences between mild and moderate, P < 0.05.
Figure 5.
(a) Compounds with increasing and decreasing then content in atrophic gastritis of different degrees. The changing trend of Lactosylceramide and alpha-Carboxy-delta-decalactone (d18:1/26:0) content of atrophic patients was first increased and then decreased. (b) Compounds with decreasing and then increasing content in atrophic gastritis of different degrees. The changing trend of Octadecanol, Conduritol-beta-expoxide, Chondrillasterol 3-[glucosyl-(1->4)-glucoside], and Tetracosanoic acid contents of atrophic patients was first decreased and then increased. (c) Compounds with an increasing trend in content in atrophic gastritis of different degrees. The content of Smilanippin A in the tongue coating of atrophic patients showed an upward trend. (d) Compounds with a decreasing trend in content in atrophic gastritis of different degrees. The contents of 5,6-Dihydroxyindole, Sphinganine 1-phosphate, and Azelaic acid of atrophic patients showed a downward trend.
Figure 6.

Diagnostic model of tongue coating metabolites in patients with with chronic superficial gastritis and chronic atrophic gastritis. The accuracy of the diagnostic model is 73.50%, the specificity is 60.00%, and the sensitivity is 82.93%.
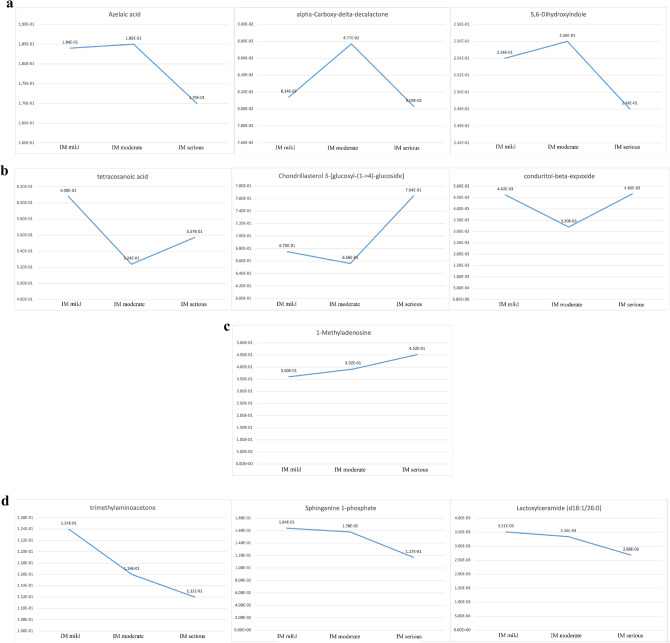
In intestinal metaplasia patients, the contents of 1-Methyladenosine, alpha-Carboxy-delta-decalactone, Azelaic acid, Chondrillasterol 3-[glucosyl-(1->4)-glucoside], and Lactosylceramide (d18:1/26:0) in the three stages of intestinal metaplasia were lower than those in healthy controls. The contents of 5,6-Dihydroxyindole, Conduritol-beta-expoxid, Sphinganine 1-phosphate, Tetracosanoic acid, and Trimethylaminoacetone in the three stages of intestinal metaplasia were higher than those in healthy controls. The content of Conduritol-beta-expoxide decreased significantly in mild intestinal metaplasia compared with moderate intestinal metaplasia (P < 0.05). The changing trend of Azelaic acid, alpha-Carboxy-delta-decalactone, and 5,6-Dihydroxyindole contents of intestinal metaplasia patients was first increased and then decreased (Fig. 7a). The changing trend of Tetracosanoic acid, Chondrillasterol 3-[glucosyl-(1->4)-glucoside], and Conduritol-beta-expoxide contents of intestinal metaplasia patients was first decreased and then increased (Fig. 7b). The content of 1-Methyladenosine of intestinal metaplasia patients showed an upward trend (Fig. 7c). The contents of Trimethylaminoacetone, Sphinganine 1-phosphate, and Lactosylceramide (d18:1/26:0) of intestinal metaplasia patients showed a downward trend (Fig. 7d).
Figure 7.
(a) Compounds with increasing and decreasing then content in intestinal metaplasia (IM) of different degrees. The changing trend of Azelaic acid, alpha-Carboxy-delta-decalactone, and 5,6-Dihydroxyindole contents of intestinal metaplasia patients was first increased and then decreased. (b) Compounds with decreasing and increasing then content in intestinal metaplasia (IM) of different degrees. The changing trend of Tetracosanoic acid, Chondrillasterol 3-[glucosyl-(1->4)-glucoside], and Conduritol-beta-expoxide contents of intestinal metaplasia patients was first decreased and then increased. (c) Compounds with an increasing trend in content in intestinal metaplasia (IM) of different degrees. The content of 1-Methyladenosine of intestinal metaplasia patients showed an upward trend. (d) Compounds with a decreasing trend in content in intestinal metaplasia (IM) of different degrees. The contents of Trimethylaminoacetone, Sphinganine 1-phosphate, and Lactosylceramide (d18:1/26:0) of intestinal metaplasia patients showed a downward trend.
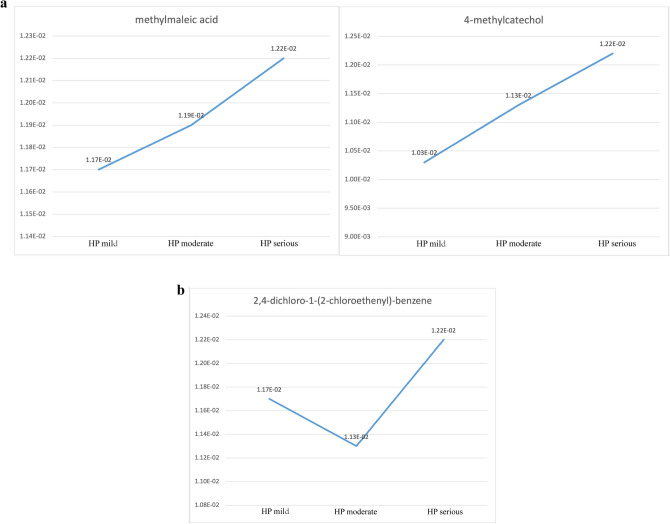
In Hp infection patients, the contents of 2,4-dichloro-1-(2-chloroethenyl)-benzene, 4-methylcatechol, and Methylmaleic acid in the three stages of Hp infection were higher than those in the healthy controls. The contents of Methylmaleic acid and 4-methylcatechol of Hp infection patients showed an upward trend (Fig. 8a). The changing trend of 2,4-dichloro-1-(2-chloroethenyl)-benzene content of Hp infection patients was first decreased and then increased (Fig. 8b).
Figure 8.
(a) Compounds with an increasing trend in content in HP of different degrees. The contents of Methylmaleic acid and4-methylcatechol of Hp infection patients showed an upward trend. (b) Compounds with decreasing and increasing then content in HP of different degrees. The changing trend of 2,4-dichloro-1-(2-chloroethenyl)-benzene content of Hp infection patients was first decreased and then increased.
Discussion
We detected the tongue coating metabolites of CG patients by metabolomics, compared with the controls, the number of lipids and lipid-like molecules of CG patients was the largest (there were 47 substances in total, of which 22 up regulated and 25 down regulated), accounting for 55.29% of the total metabolites. Researchers have found that Lipid oxidative stress can lead to a variety of chronic inflammatory diseases14.
Some researchers also have found that lipids and lipid-like molecules as the main different serum metabolites in the CG patients may promote the development of CG to gastric cancer. This study15 focuses on the diagnostic significance of lipid metabolites in serum. Linoleamide, which is mentioned in the paper as a metabolite upregulated in both chronic gastritis (CG) and gastric cancer (GC) patients, was also observed to change in the tongue coating of CG patients. Sphinganine 1-phosphate, identified as a candidate biomarker in the paper, was also found in our study to have increased levels in CG patients and is correlated with pathological indicators. However, our study involves a richer variety of lipid compounds with greater abundance variations. We speculate that the microbial metabolism in the oral cavity and digestive tract may affect the types and abundance of lipid compounds, leading to significant differences from those found in serum. Further research is needed to confirm this hypothesis.
Among the different metabolites of lipids and lipid-like molecules there was a substance called Sphinganine 1-phosphate, which was significantly elevated compared to healthy individuals. Sphinganine 1-phosphate was one of the markers of the diagnostic model of chronic gastritis. It has also been found to have significant correlation with atrophy and intestinal metaplasia which was positive. Studies have also shown that Sphinganine 1-phosphate may inhibition of apoptosis16, It can also significantly inhibit liver necrosis and cell apoptosis caused by liver ischemia–reperfusion17. In addition, we found that the level of Sphinganine 1-phosphate decreased gradually with the severity increases of gastric mucosal atrophic and intestinal metaplasia in CG patients. We speculated that the decrease of this substance may be related to the aggravation of the disease. Among the different metabolites screened by the diagnostic model of CG, except Sphinganine 1-phosphate, there was another substance belonging to the different metabolites of lipids and lipid-like molecules, which was named Nervonic acid. Some researchers found Nervonic acid in the mucosa of gastric cancer patients18. Nervonic acid exists not only in the upper digestive tract but also in the lower digestive tract. Moreover, as one kind of lipid, Nervonic acid can increase fatty acid oxidation in the liver and reduce the level of circulating triglycerides leading to obesity19.
As shown in Fig. 5c the content of Smilanippin A of severe atrophic patients was higher than that of mild and moderate atrophic patients. The content of Lactosylceramide (d18:1/26:0) of severe atrophic patients was lower than that of mild and moderate atrophic patients. In addition, according to Fig. 7d, the content of Lactosylceramide (d18:1/26:0) gradually decreased with the aggravation of intestinal metaplasia. Lactosylceramide can promote the growth of colon cancer cells20, and such substance also exists in the serum of children with inflammatory bowel disease21. This substance will aggravate digestive system diseases. Moreover, oxidative stress environment can promote inflammation22. Glycosphingolipids readily lead to the occurrence of free radical induced oxidation, this oxidation may be related to the increase of lactosylceramide content23.
In Figs. 5b and 7b, the content of Chondrillasterol 3-[glucosyl-(1->4)-glucoside] of severe atrophic and intestinal metaplasia patients was higher than that of patients with mild and moderate atrophic and intestinal metaplasia. Moreover, the content of this substance in the three stages of atrophic and intestinal metaplasia was lower than that in the tongue coating of healthy controls. The content of Azelaic acid gradually decreases with the aggravation of atrophic, and its content in the tongue coating of subjects with different degrees of atrophic was lower than healthy people. Moreover, the content of Azelaic acid in severe intestinal metaplasia subjects was lower than that in mild and moderate patients, and the content in the three stages of intestinal metaplasia was lower than that in the tongue coating of healthy controls. Azelaic acid is a marker of lipid peroxidation24. Researchers found that the content of Azelaic acid in the plasma of gastric cancer patients was significantly increased25. However, we found that the content of Azelaic acid in the tongue coating of gastric precancerous lesion patients was lower than healthy people. This phenomenon deserves our in-depth study in the future.
Organic acids and derivatives were the second largest metabolites. There were 13 metabolites (7 up regulated and 6 down regulated), among which pantothenic acid was up regulated. Some researchers have found that this substance in gastric cancer was a potential biomarker26. The different metabolite 3-Hydroxycapric acid screened by the diagnostic model of CG also belongs to Organic acids and derivatives.
In other metabolic species, l-histidinol expression was up regulated. Some studies have shown that histidinol can inhibit gastric acid secretion and alleviate stomach pain. In addition, it can also inhibit the growth and metastasis of lung cancer cells27,28. Dictagymnin expression was up regulated, and this substance has anti-inflammatory and tumor growth inhibitory effects29. Patients with chronic gastritis were probably undergoing self-repair by the body during their illness. From the correlation between chronic gastritis and pathological indexes, we found that the differential metabolites belonging to Organoheterocyclic compounds were 5,6-Dihydroxyindole and alpha-Carboxy-delta-decalactone. In Figs. 5d and 7a, the content of 5,6-Dihydroxyindole of severe atrophic and intestinal metaplasia patients was lower than that of patients with mild and moderate atrophic. The content of alpha-Carboxy-delta-decalactone of moderate atrophic and intestinal metaplasia patients was higher than that of patients with mild and severe atrophic.
Among the different metabolites screened by the diagnostic model of chronic gastritis, 4-Ipomeanol belongs to Organic oxygen compounds, and 1-Methyladenosine belongs to Nucleosides, nucleotides, and analogues. 1-Methylladenosine was also found in the results of correlation of pathological indexes. In the trend figures of chronic gastritis with intestinal transformation, the content of 1-Methylladenosine increased with the increase of intestinal degree.
In addition, as shown in Fig. 8a. The contents of methylmaleic acid and 4-methylcatechol gradually increased with the aggravation of Hp infection. The content of 2,4-dichloro-1-(2-chloroethenyl)-benzene of moderate intestinal metaplasia patients was lower than mild and severe patients. Experiments on rats showed that 4-methylcatechol and Hp infection may cause gastric cancer30,31. We suggest that 4-methylcatechol might be relate to the transformation from CG with Hp infection to gastric cancer. In Table 2, we also found that the content of Conduritol-beta-expoxide decreases significantly with the severity increases of atrophic and intestinal metaplasia. However, we have not found the content change of this substance in digestive system diseases.
With further investigation, these metabolites present in the tongue coatings of CG patients hold promise as potential non-invasive diagnostic markers, augmenting gastroscope-based diagnosis and monitoring of patients with chronic gastritis. Continued research in this area may offer valuable insights into improving diagnostic methods and enhancing patient care for CG.
Supplementary Information
Acknowledgements
The study was assisted by Shanghai Biotree Biotech Co. Ltd.
Author contributions
In the execution of this study, Y. H took on the responsibility of overseeing the research. Z. Y and Y. X contributed to the writing of the manuscript. J. L responsible for the management of the data. R. Z played a key role in the collection of clinical samples, while Z. Z provided assistance with the experimental procedures. Y. W contributed valuable ideas to the conceptualization of the study. All authors involved in this research unanimously agreed to be accountable for all aspects of the work, demonstrating a collaborative and collective commitment to the integrity and completeness of the study. The authors all agreed to publish.
Funding
The study was funded by the NNSF of China (82174279 and 81703982) and Shanghai Science and Technology Planning Program (21DZ2271000).
Data availability
In the study, experimental data other than personal information of the subjects can be obtained by contacting the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-68261-6.
References
- 1.Sipponen, P. & Maaroos, H. I. Chronic gastritis. Scand. J. Gastroenterol.50(6), 657–667 (2015). 10.3109/00365521.2015.1019918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui, J. et al. Tongue coating microbiome as a potential biomarker for gastritis including precancerous cascade. Protein Cell10(7), 496–509 (2019). 10.1007/s13238-018-0596-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan, L. et al. Development of a tongue image-based machine learning tool for the diagnosis of gastric cancer: A prospective multicentre clinical cohort study. EClinicalMedicine57, 101834 (2023). 10.1016/j.eclinm.2023.101834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li, F. F. et al. Metabolite changes in the greasy tongue coating of patients with chronic gastritis. Zhong Xi Yi Jie He Xue Bao10(7), 757–765 (2012). 10.3736/jcim20120706 [DOI] [PubMed] [Google Scholar]
- 5.Sun, Z. M. et al. Metabolic markers and microecological characteristics of tongue coating in patients with chronic gastritis. BMC Complement Altern. Med.13, 227 (2013). 10.1186/1472-6882-13-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeki, Ö. C. et al. Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal.25(190), 113509 (2020). 10.1016/j.jpba.2020.113509 [DOI] [PubMed] [Google Scholar]
- 7.Sugano, K. et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut64(9), 1353–1367 (2015). 10.1136/gutjnl-2015-309252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu, Y. et al. Metabolite characteristics in tongue coating from damp phlegm pattern in patients with gastric precancerous lesion. Evid. Based Complement Alternat. Med.2(2021), 5515325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kind, T. et al. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem.81(24), 10038–10048 (2009). 10.1021/ac9019522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn, W. B. et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc.6(7), 1060–1083 (2011). 10.1038/nprot.2011.335 [DOI] [PubMed] [Google Scholar]
- 11.Smith, C. A. et al. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem.78(3), 779–787 (2006). 10.1021/ac051437y [DOI] [PubMed] [Google Scholar]
- 12.Yang, C. et al. GC–TOF/MS-based metabolomics studies on the effect of protein sources in formulated diet for pearl oyster Pinctada fucata martensii. Aquaculture486, 139–147 (2018). 10.1016/j.aquaculture.2017.12.020 [DOI] [Google Scholar]
- 13.Wang, W. et al. Metabolomics-based evidence of the hypoglycemic effect of Ge-Gen-JiaoTai-Wan in type 2 diabetic rats via UHPLC-QTOF/MS analysis. J. Ethnopharmacol.219, 299–318 (2018). 10.1016/j.jep.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 14.Leuti, A., Maccarrone, M. & Chiurchiù, V. Proresolving lipid mediators: Endogenous modulators of oxidative stress. Oxid. Med. Cell Longev.18(2019), 8107265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu, L. et al. Serum metabolic profiling analysis of chronic gastritis and gastric cancer by untargeted metabolomics. Front. Oncol.11(11), 636917 (2021). 10.3389/fonc.2021.636917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, D. H. et al. Elevation of sphinganine 1-phosphate as a predictive biomarker for fumonisin exposure and toxicity in mice. J. Toxicol. Environ. Health A69(23), 2071–2082 (2006). 10.1080/15287390600746215 [DOI] [PubMed] [Google Scholar]
- 17.Park, S. W. et al. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock33(1), 31–42 (2010). 10.1097/SHK.0b013e3181c02c1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacuszka, T. et al. Neutral glycolipid composition in human cancer. Cancer Biochem. Biophys.5(1), 1–6 (1980). [PubMed] [Google Scholar]
- 19.Keppley, L. J. W. et al. Nervonic acid limits weight gain in a mouse model of diet-induced obesity. FASEB J.34(11), 15314–15326 (2020). 10.1096/fj.202000525R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machala, M. et al. Colon cancer and perturbations of the sphingolipid metabolism. Int. J. Mol. Sci.20(23), 6051 (2019). 10.3390/ijms20236051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filimoniuk, A. et al. Sphingolipid analysis indicate lactosylceramide as a potential biomarker of inflammatory bowel disease in children. Biomolecules10(7), 1083 (2020). 10.3390/biom10071083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee, S., Balram, A. & Li, W. Convergence: Lactosylceramide-centric signaling pathways induce inflammation, oxidative stress, and other phenotypic outcomes. Int. J. Mol. Sci.22(4), 1816 (2021). 10.3390/ijms22041816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couto, D. et al. Glycosphingolipids and oxidative stress: Evaluation of hydroxyl radical oxidation of galactosyl and lactosylceramides using mass spectrometry. Chem. Phys. Lipids191, 106–114 (2015). 10.1016/j.chemphyslip.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 24.Schallreuter, K. U. & Wood, J. M. Azelaic acid as a competitive inhibitor of thioredoxin reductase in human melanoma cells. Cancer Lett.36(3), 297–305 (1987). 10.1016/0304-3835(87)90023-1 [DOI] [PubMed] [Google Scholar]
- 25.Yu, L. et al. Metabolomic phenotype of gastric cancer and precancerous stages based on gas chromatography time-of-flight mass spectrometry. J. Gastroenterol. Hepatol.26(8), 1290–1297 (2011). 10.1111/j.1440-1746.2011.06724.x [DOI] [PubMed] [Google Scholar]
- 26.Jiang, W. et al. Metabolic profiles of gastric cancer cell lines with different degrees of differentiation. Int. J. Clin. Exp. Pathol.11(2), 869–887 (2018). [PMC free article] [PubMed] [Google Scholar]
- 27.Zu, G. X. et al. Urine metabolomics of rats with chronic atrophic gastritis. PLoS One15(11), e0236203 (2020). 10.1371/journal.pone.0236203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escobar-Reséndiz, R. et al. Evaluation of antitumoral effect of the combination of L-histidine methyl esther hydrochloride of anfotericin B with antineoplastics on A549 cells. FASEB J.34, 1 (2020). 10.1096/fasebj.2020.34.s1.00724 [DOI] [Google Scholar]
- 29.Wang, J. Y. et al. Dictamnine promotes apoptosis and inhibits epithelial-mesenchymal transition, migration, invasion and proliferation by downregulating the HIF-1α and Slug signaling pathways. Chem. Biol. Interact.25(296), 134–144 (2018). 10.1016/j.cbi.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 30.Asakawa, E. et al. Carcinogenicity of 4-methoxyphenol and 4-methylcatechol in F344 rats. Int. J. Cancer56(1), 146–152 (1994). 10.1002/ijc.2910560126 [DOI] [PubMed] [Google Scholar]
- 31.Malfertheiner, P. et al. Management of Helicobacter pylori infection–the Maastricht IV/ florence consensus report. Gut61(5), 646–664 (2012). 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In the study, experimental data other than personal information of the subjects can be obtained by contacting the corresponding author.