Abstract
Study Design
Retrospective Cohort Study.
Objective
To assess the predictive value of early C-reactive protein (CRP) trends following diagnosis of spinal epidural abscess (SEA). Non-operative management with intravenous antibiotics has not demonstrated equivalent outcomes with regard to mortality and morbidity. Knowledge of specific patient and disease factors associated with worse outcomes may predict treatment failure.
Methods
All patients treated for spontaneous SEA in a tertiary centre in New Zealand over a 10-year period were followed for at least 2 years. CRP at diagnosis and day 4-5 following treatment initiation was analyzed to determine predictors of CRP reduction of at least 50%. Proportional Cox hazards regression investigated mortality over 2 years.
Results
94 patients met inclusion criteria and with CRP values available for analysis. Median age was 62 years (+/− 17.7) and 59 (63%) were treated operatively. Kaplan-Meier analysis estimate of 2-year survival was .81 (95% CI .72-.88). CRP reduction by 50% was seen in 34 patients. Patients who did not experience a 50% reduction were more likely to have thoracic infection (27 vs 8, P = .02) or multifocal sepsis (41 vs 13, P = .002). Failure to achieve a 50% reduction by day 4-5 was associated with worse post-treatment Karnofsky scores (70 vs 90, P = .03) and longer hospital stay (25 days vs 17.5 days, P = .04). Cox regression model showed mortality predicted by Charlson Comorbidity Index, thoracic location of infection, pre-treatment Karnofsky score, and failure to achieve a 50% CRP reduction by day 4-5.
Conclusions
Patients who fail to reduce CRP values by 50% at day 4-5 following treatment initiation are more likely to experience prolonged hospital stay, have poorer functional outcome and have greater mortality risk at 2 years. This group has severe illness regardless of treatment type. Failure to achieve a biochemical response to treatment should prompt reassessment.
Keywords: pyogenic spinal column infection, epidural abscess, conservative management, treatment failure
Introduction
Spinal epidural abscess (SEA) is a rare but potentially debilitating diagnosis with the potential for severe neurologic sequelae. 1 Patient factors associated with the development of SEA include older age, diabetes, immunosuppression, and intravenous drug use. 2 Once diagnosed, regardless of treatment type, patients may experience a spectrum of illness ranging from isolated disease to multifocal sepsis, systemic upset, neurologic compromise up to paralysis and prolonged hospital stay. 3 Historically, treatment has been urgent surgical decompression. A recent trend toward non-operative management with intravenous antibiotics has not demonstrated equivalent outcomes with regard to mortality and morbidity and remains controversial. 4
Specific patient and disease factors are associated with worse outcomes in the literature, such as presenting with a neurologic deficit, raised inflammatory markers, and delay to surgery. 5 The role of laboratory factors in predicting outcome of disease has been previously investigated, particularly with regards to failure of non-operative management. Elevated inflammatory markers such as C-reactive protein (CRP) and white cell count (WCC) have been shown to predict failure of non-operative management in multiple studies.6-8
The trend of certain laboratory markers has also been studied in the setting of pyogenic spinal column infection with elevated CRP at 14 days post treatment onset for vertebral osteomyelitis considered a poor prognostic indicator. 9
Monitoring laboratory values provides an opportunity for clinicians to consider the disease response to treatment decisions. 10 Laboratory response to therapy predicted treatment failure in cases of VO in a study by Yoon et al. 11 The authors argued that a patient with persistently elevated CRP should be re-evaluated, for example, with repeat imaging to search for a drainable collection. These objective changes in inflammatory markers need to be detectable early in the course of treatment if a change in therapy is warranted. Furthermore, falling inflammatory markers could help plan treatment de-escalation such as a switch from intravenous to oral antibiotics. 12 Faster resolution of inflammatory markers may indicate success of treatment together with likelihood of a better final clinical outcome.
The prognostic value of CRP in cases of SEA has yet to be fully investigated in the literature. The aims of this study were firstly to determine the influence of failure to suppress CRP on outcome in patients with SEA and secondly to identify risk factors for failure to achieve a 50% reduction in CRP.
Materials and Methods
Using hospital coding we identified all patients who were admitted with a diagnosis of SEA between January 2007 and January 2017. Our hospital is a Level 1 Trauma centre and tertiary referral spine centre for a population of just over 900, 000. The years were selected to allow confirmation of the diagnosis using the digital imaging record. Clinical Audit Support Unit approval was granted for an outcome analysis. The study was deemed exempt from requirement for informed consent. All patients were followed for a minimum of 2 years.
Inclusion criteria were age >16 and first presentation with SEA. Recurrent cases and surgical site infections were excluded. Patients without available CRP levels on days 4 or 5 following treatment initiation were excluded. Diagnosis was confirmed based on radiological evidence with magnetic resonance imaging (MRI) and positive intra-operative cultures where available. Patients with unrelated cause of death (e.g., road traffic accident or similar) were excluded.
Data Collection
Explanatory variables and outcomes were collected retrospectively. Demographic details, past medical history, concurrent infection and social factors were recorded. Information about presenting complaint (pain, motor function, sensory function), laboratory values (CRP (mg/L), white cell count (WCC/L), haemoglobin (Hb g/dl), albumin (Alb, mg/L), and microbiology (cultures) were reviewed.
CRP values between day 0-5 were collected. In this study, day 0 was considered to be the day of diagnosis and treatment initiation. We chose this as a starting point because we wanted to capture a response to treatment in serial CRP measurements.
For examples, a patient admitted through the Emergency Department with back pain and fever who proceeded for urgent diagnostic magnetic resonance imaging (MRI) and surgery would have ‘day 0’ on admission. Conversely, a patient admitted to hospital in a delayed fashion after several days of symptoms would have day ‘0’ when the diagnostic MRI was obtained and treatment was initiated, not on the day of admission. This enables us to pinpoint the day that treatment for their epidural abscess commenced. Therefore, the changes in laboratory values after day 0 reflect response to treatment.
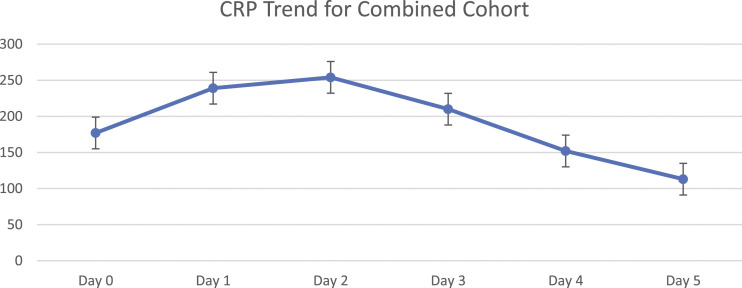
We have focused on laboratory values taken over the 5-day period following diagnosis and treatment initiation, to see whether there is an association between CRP resolution and treatment success. The 5-day period was chosen for several reasons. Firstly, the majority of patients had a CRP value available at either day 4 or 5, whereas later testing of inflammatory markers was more variable as directed by the responsible clinician(s). Secondly, the general CRP trend for patients in our cohort demonstrates elevation until day 2-3, followed by either plateau or decline (Figure 1). Finally, persistently elevated CRP at day 4-5 could guide clinicians to re-evaluate treatment early, providing an opportunity for timely further investigation or change in management if appropriate. In paediatric bone and joint infection a link between severity of illness and prolonged CRP elevation, using CRP at days 4-5, has been described. 13
Figure 1.
Early trends in median C-reactive protein.
Neurological function was scored with pre- and post-treatment Frankel Grade. For the purposes of this study, a patient with concurrent infection such as pneumonia, psoas infection, or urinary tract infection (UTI) was defined as having ‘multifocal sepsis’. Details from past medical history included established risk factors for SEA: intravenous drug use (IVDU), immunosuppression, diabetes mellitus (DM), renal disease, and liver disease. To capture the wider burden of disease the Charlson Comorbidity Index (CCI) was calculated for each patient as well as the American Society of Anaesthesiologist’s (ASA) Score. Where available, a Body Mass Index (BMI, kg/m2) has been included.
MRI reports were obtained to quantify the level and position of the abscess relative to the thecal sac. A pre-treatment and post-treatment Karnofsky Performance Score (KPS) was calculated at admission and last follow-up to determine the functional status of each patient. The KPS score was calculated using available information from clinical letters and electronic case review. The days until death have been calculated for those that passed away during the study period, with a maximum number of 730 days.
Statistical Analysis
The cohort has been represented by descriptive statistics with relative frequencies, percentages, inter-quartile range, and mean/median calculated. Univariate analysis for descriptive statistics was either t-test (numerical variables) or Fisher’s Exact Test (categorical variables). The variation in CRP resolution over 5 days has been presented graphically for several subgroups. The cohort has been dichotomized into those who experienced 50% reduction in CRP at day 4-5 and those who did not. Both groups were evaluated with regards to parameters suggesting more severe illness, such as length of stay, post-treatment Karnofsky score, and mortality at 30 days and 2 years. The decision to use a 50% reduction to differentiate patients was made after testing several different cut-offs; a 50% reduction had a stronger association with disease outcomes than either lower (25%) or higher *75%) values and was a better discriminator in outcome. The hazard ratios for 2-year mortality were calculated and presented in Kaplan-Meier analysis. Finally, Cox proportional hazards models for 2-year survival were tested. The model with the lowest Akaike Information Criterion (AIC) was selected. 14 All statistical methodology completed using Excel with StatPlus.
Results
Over the 10-year period 120 patients were identified with SEA. A total of 94 met inclusion criteria, with CRP measured on day 4-5 following diagnosis and treatment initiation. Of the 94 patients, 32 were female and the median age was 62 (+/− 17.7, IQR17.8) (Table 1). On presenting to hospital 70 were febrile (75%) and 41 (44%) had neurological compromise with Frankel Grade worse than E.
Table 1.
Descriptive Statistics.
| Variable | Number | *IQR/Percentage |
|---|---|---|
| Demographics | ||
| Median age | 62 | 17.75 |
| Female | 32 | 34.0 |
| Male | 62 | 66.0 |
| Fever on arrival | 70 | 74.5 |
| Frankel grade not E | 41 | 43.6 |
| Radiculopathy | 38 | 40.4 |
| Paralysis | 8 | 8.5 |
| Laboratory values | ||
| Median white cell count | 12.45 | 6.6 |
| Median haemoglobin | 121 | 24.5 |
| Median CRP day 0 | 170 | 175 |
| Median CRP day 4/5 | 120 | 102 |
| Median albumin | 29.5 | 8.8 |
| Positive blood culture | 69 | 73.4 |
| Staphylococcus aureus isolated | 52 | 55.3 |
| Comorbidity | ||
| Multifocal sepsis | 54 | 57.4 |
| Endocarditis | 4 | 4.3 |
| Median charlson comorbidity index | 3 | 4 |
| Median ASA* | 3 | 3.2 |
| Diabetes | 15 | 16.0 |
| Immunosuppression | 11 | 11.7 |
| IVDU* | 9 | 9.6 |
| Liver disease | 14 | 14.9 |
| Renal disease | 15 | 16.0 |
| Median BMI | 29 | 9.5 |
| Median pre-treatment Karnofsky Performance score | 90 | 30 |
| Abscess location | ||
| Cervical | 20 | 21.3 |
| Thoracic | 35 | 37.2 |
| Lumbosacral | 42 | 44.7 |
| Multilevel disease | 24 | 25.5 |
| Bony involvement | 55 | 58.5 |
| Treatment type | ||
| Non-operative | 35 | 37.2 |
| Operative (including converted) | 59 | 62.8 |
| Converted to operative management | 21 | 22.3 |
| CT-Guided Drainage | 9 | 9.6 |
| Outcomes | ||
| Median length of stay | 17.5 | 21.3 |
| Median post-treatment Karnofsky score | 80 | 30 |
| Mortality within 30 days | 10 | 10.6 |
| Mortality within 2 years | 18 | 19.1 |
Legend: IQR = Interquartile range, ASA=American Society of Anaesthesiologists, IVDU= Intravenous Drug Use, CT= Computed Tomography.
The median day ‘0’ CRP was 170 mg/dL. In the majority of cases, CRP rose until day 2 and then tended to reduce (Figure 1). A total of 68 (72%) patients experienced a net CRP reduction by day 4-5. The remainder had unchanged CRP (within 10-20 mg/dL) or persistently elevated CRP values (increased by >/= 20 mg/dL).
Median day 4/5 CRP for the combined cohort was 120 mg/dL. Median percentage reduction in CRP was 36.2% (+/−77.7%). A reduction to 50% of the initial level by day 4-5 was seen in 35 patients (37%). Patients who experienced this reduction were assessed for relevant differences in disease outcomes (see below).
With regard to comorbidity, 15 patients were diabetic, 11 were immunocompromised and the median CCI was 3 (IQR = 4). The median pre-treatment KPS for the cohort was 90, suggesting the majority of patients were functionally independent and able to work before developing SEA.
Abscess location was typically lumbosacral (45%), with infection spanning multiple levels in 24 patients (25%). A significant number (n = 54) had multifocal sepsis, with infection in other locations. This included endocarditis (n = 4), pneumonia (n = 21), and urinary infection (n = 11).
The majority of patients received operative intervention (n = 59, 63%) however, of these, 21 (22%) converted to operative management after starting intravenous antibiotics with therapeutic intent. The treatment decision was taken by the attending Spine Surgical team with antibiotic duration and selection advised by Infectious Disease physicians. Operative intervention was most commonly decompression alone (n = 49, 83%) and the remainder of patients required decompression with instrumented fusion (n = 10, 17%).
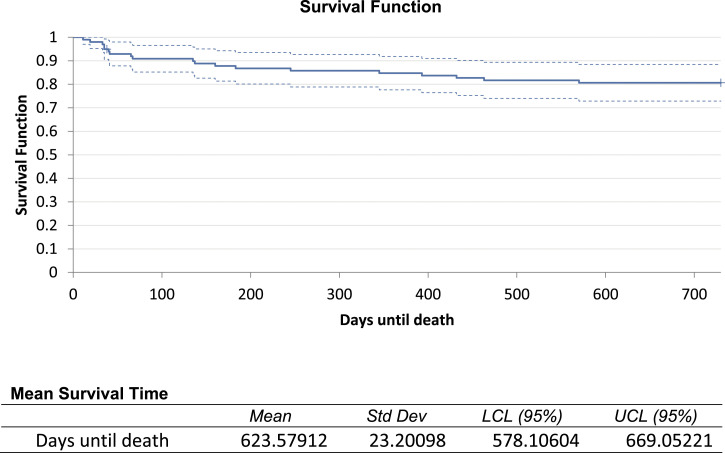
The median length of hospital stay was 17.5 days (+/−22.4). A total of 18 patients died within 2 years of developing SEA, 10 within 30 days of diagnosis. Kaplan-Meier analysis of the combined cohort showed a mean survival time of 623 days (+/−23.2) with survival estimate at 2 years of .81 (95% CI .72-.88). Cumulative hazard for the cohort at 2 years was .21 (Figure 2).
Figure 2.
Kaplan-Meier survival estimate over 2 -year follow-up.
Following treatment, those who survived had a median KPS of 80. Of 46 patients with neurological deficit, only 19 (41%) experienced recovery by at least one Frankel Grade; 9 patients (30%) were grade A or B at final follow-up with complete loss of motor function.
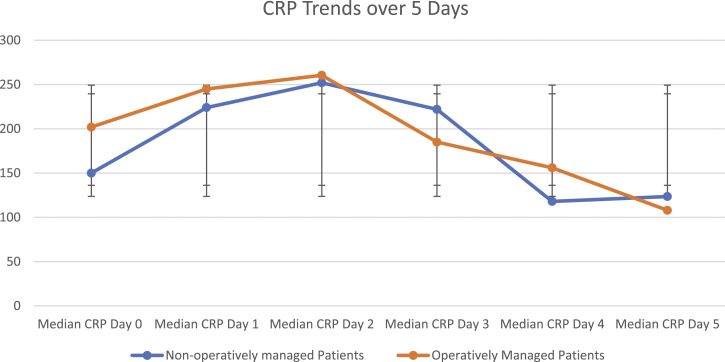
Comparing patients who experienced 50% reduction in CRP with those that did not, there were several statistically significant differences in univariate analysis (Table 2). Patients who did not experience a CRP reduction were more likely to have ASA >1 (53 vs 26, P = .04). There were more patients with thoracic infection (27 vs 8, P = .02) or multifocal sepsis (41 vs 13, P = .002). Those with CRP reduction of 50% were not more likely to have undergone operative management (37 vs 22, P = .98). Plotting the median daily CRP for operative and non-operatively managed patients shows overlapping confidence intervals at every time point (Figure 3).
Table 2.
Univariate Analysis Comparing Patients with and without CRP Normalisation.
| Patients without CRP normalisation | Patients with CRP normalisation | T-TEST/CHI2 | |
|---|---|---|---|
| Total | 59 | 35 | 94 |
| Male | 38 | 24 | 0.6 |
| Female | 21 | 11 | / |
| Median age | 63 | 61 | .45 |
| Median CCI | 4 | 3 | .31 |
| Bacteraemia | 44 | 25 | .73 |
| Frankel grade not E | 28 | 13 | .32 |
| Diabetes | 10 | 5 | .73 |
| Renal disease | 9 | 6 | .81 |
| Liver disease | 8 | 6 | .22 |
| Immunosuppression | 9 | 2 | .16 |
| IVDU | 3 | 6 | .054 |
| Median BMI (kg/m2) | 29 | 28 | 0.6 |
| ASA >1 | 53 | 26 | *0.04 |
| Thoracic disease | 27 | 8 | *0.02 |
| Multifocal sepsis | 41 | 13 | *0.002 |
| Conservative management only | 22 | 13 | .98 |
| Surgical management at any point | 37 | 22 | .98 |
| Converted to surgical management | 16 | 5 | .06 |
| >1 surgical debridement | 18 | 8 | .42 |
| Instrumentation | 5 | 5 | .36 |
| Bony involvement | 36 | 19 | .52 |
| S. aureus | 25 | 27 | *0.001 |
| Median length of stay (excluding patients who died in hospital) | 25 | 17.5 | *0.04 |
| Median pre-treatment Karnofsky Performance score | 90 | 90 | .12 |
| Median post-treatment Karnofsky Performance score | 70 | 90 | *0.03 |
| Pre-treatment Karnofsky score <80 | 19 | 6 | .11 |
| 30 Day mortality | 9 | 1 | *0.05 |
| 2 Year mortality | 16 | 2 | *0.01 |
Legend: * = Statistically significant, p =</= .05, CCI = Charlson Comorbidity Index, BMI = Body Mass Index, ASA = American Society of Anaesthesiologist Classification, IVDU= Intravenous Drug Use.
Figure 3.
CRP trends For Operatively and Non-Operatively Managed Patients.
The group who did not experience CRP reduction includes more patients with delayed operative management however, this difference does not reach statistical significance (16 vs 5, P = .06).
With regards to outcomes, the group that did not reduce CRP had worse post-treatment KPS (70 vs 90, P = .03) and a longer median hospital stay (25 days vs 17.5 days, P = .04). Mortality at 30 days and 2 years was higher in the group that did not experience 50% CRP reduction (16 (27%) vs 2 (6%) deaths, P = .01).
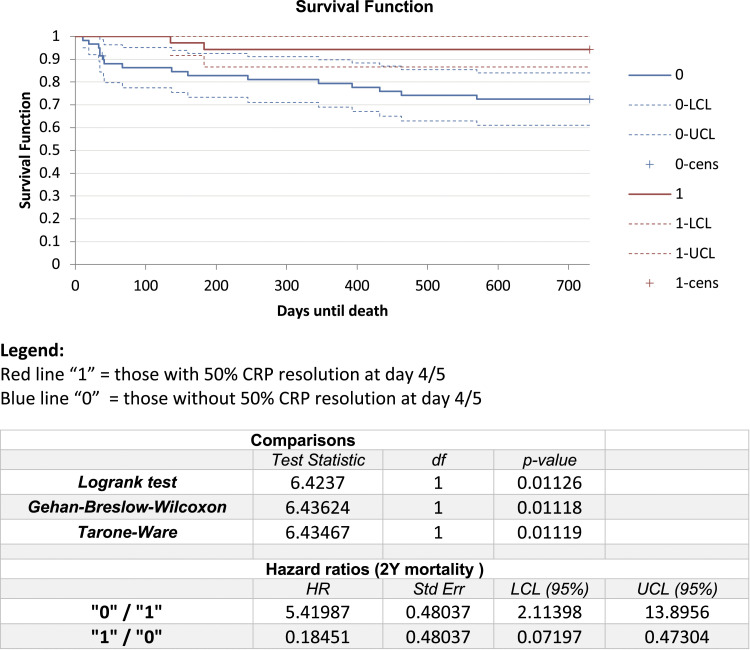
Examining the mortality differences more closely, the Kaplan-Meier survival function was plotted for each group and hazard ratios compared (Figure 4). The median survival time was greater in those that had 50% CRP reduction. These patients had a statistically lower hazard ratio (HR .18 vs 5.41, P = .01).
Figure 4.
Kaplan- Meier survival analysis with and without CRP resolution.
Proportional Cox hazard models were tested using known variables associated with greater mortality risk, including median values for white cell count, haemoglobin, and albumin. The model with best AIC included 4 variables: Charlson Comorbidity Index, thoracic location of infection, pre-treatment KPS, and 50% CRP reduction at day 4/5 (Table 3).
Table 3.
Cox Hazard Regression Model for 2-Year Mortality.
| Covariate | Beta | Standard error | Wald | P-value | LCL [beta] | UCL [beta] | Risk Ratio |
|---|---|---|---|---|---|---|---|
| Karnofsky Performance score pre-treatment | −.02685 | .01492 | 3.23789 | .07195 | −.0561 | .0024 | .97351 |
| Charlson comorbidity index | .3934 | .11432 | 11.8427 | .00058 | .16934 | .61746 | 1.48201 |
| CRP 50% reduction | −1.4466 | .76688 | 3.5583 | .05925 | −2.94968 | .05648 | .23537 |
| Thoracic infection | .91316 | .5354 | 2.90891 | .08809 | −.13623 | 1.96255 | 2.49218 |
Discussion
The major finding of this study is that regardless of treatment type, early CRP resolution predicts greater recovery from spinal epidural abscess. The failure to reduce CRP by 50% by day 4-5 is associated with longer hospital stay, worse functional outcome, and greater mortality risk within 2 years. This finding is significant because it allows clinicians timely insight into the course of disease and suggests that failure to respond biochemically within 4-5 days should prompt a search for other foci of infection and/or consider modification of treatment regimen.
Previous studies looking at CRP have examined timeframes for complete normalization. In studies focused on lumbar epidural abscess, complete CRP normalization took a median of 4.8-7.6 weeks depending on treatment type. 3 In cases of vertebral osteomyelitis, CRP normalization by 14 days is associated with lower mortality. 9 However, by this stage, clinicians are unlikely to have the capacity to make decisions significantly altering the course of illness. Instead, we consider that persistently elevated CRP at day 4-5 could guide clinicians to re-evaluate treatment early and thereby influence the outcome in the short term let alone the long term.
Previous work by Uchida et al. found that operatively managed patients with lumbar SEA normalized CRP earlier than non-operatively managed patients, in 4.8 weeks vs 7.6 weeks (P<.05). 15 This suggested that surgically managed patients recover earlier. In contrast, work by Zadran et al. looking at vertebral osteomyelitis found CRP levels were more likely to drop to <30 mg/dL by day 14 in conservatively managed patients. 9 Their postulated theory is that surgical insult prolongs elevated CRP. It appears that the net effect of operative intervention on CRP resolution is yet to be well-defined in the setting of pyogenic spinal infection.
Overshadowing both concepts is the selection bias inherent in surgical decision making. A patient with overwhelming sepsis who is an unfit or poor operative candidate is likely to have sustained CRP elevation and may pass away as a result of their illness. In contrast, a young patient with isolated SEA and a drainable collection could undergo early surgery and experience quicker normalization of inflammatory markers. Clinicians need objective measures to predict mortality and morbidity across a spectrum of patients with SEA. The advantage of considering CRP reduction at day 4-5 following diagnosis is that the early recovery of inflammatory markers predicts a better outcome regardless of treatment type.
CRP normalization also allows clinicians to stratify patients by severity of illness. It is expected that those with features of more severe disease, such as disseminated infection and thoracic involvement, would be less likely to experience normalization of inflammatory markers. This is in keeping with our findings and with the broader literature. Considering risk factors for severe neurological deficit in VO, Lemaignen et al. showed that an initial CRP >150 mg/L predicted poorer functional outcome at 3 months following treatment. 16 Higher CRP is also associated with failed non-operative treatment. For example, in 2013 Patel et al. found 4 variables including CRP greater than 115 mg/dL predicted failure of medical management. 6 Confirming this in a larger cohort, a 2020 paper by Lyons et al. found admission CRP was statistically higher in those who failed medical management.
Currently, there is no universally accepted score or scale for severity of illness in cases of epidural abscess, although it is evident that a spectrum of illness exists. Whilst other variables such as neurologic deterioration should be evaluated in the development of severity scales, we believe that future research to refine this concept will be of value to treatment planning.
With regards to mortality at 2 years following treatment, the variables identified in Cox regression are supported by other findings in the literature. Thoracic infections have a known association with mortality and are considered to have increased speed and severity of progression. 17 The Charlson Comorbidity Index represents baseline risk of death and burden of disease and should influence mortality in statistical analysis. CRP reduction has previously been shown to have an association with mortality when measured later time point (failure to reduce to <30 mg/dL at 14 days) but this is the first investigation of CRP trends early in the course of illness. Pre-treatment KPS has also been previously shown to predict 2-year mortality in cases of VO, but has not been added to analysis in cases of SEA. 9
Limitations of this study include small sample size and heterogeneity. Patients were included in our evaluation whether they underwent operative, non-operative, or delayed operative management. However, the impact of treatment type on CRP is not elucidated. Also, our analysis was dependent on the availability of laboratory results and the timing of these is dependent on the responsible clinicians ordering the markers. Other cut-off percentages were tested and not found to be useful, but the predictive value of declining CRP at other time points was not evaluated.
In conclusion, our findings confirm the known mortality risk of certain variables in pyogenic spinal infection and highlight the value of measuring inflammatory markers within the 5 days following diagnosis and treatment initiation for spinal epidural abscess. Failure to reduce CRP by 50% on day 4-5 is associated with longer hospital stay, worse post-treatment Karnofsky Performance Score, and higher mortality risk within 2 years. Close monitoring of inflammatory markers will aid clinicians in identifying potential treatment failure and provide and earlier opportunity to reconsider management strategy.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Sarah Hunter https://orcid.org/0000-0002-6436-3244
Joseph F. Baker https://orcid.org/0000-0002-8518-8780
References
- 1.Artenstein AW, Friderici J, Holers A, et al. Spinal epidural abscess in adults: A 10-year clinical experience at a tertiary care academic medical center. Open Forum Infect Dis. 2016;3. doi: 10.1093/ofid/ofw191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arko L, Quach E, Nguyen V, et al. Medical and surgical management of spinal epidural abscess: A systematic review. Neurosurg Focus. 2014;37:1-9. [DOI] [PubMed] [Google Scholar]
- 3.de Leeuw CN, Fann PR, Tanenbaum JE, et al. Lumbar epidural abscesses: A systematic review. Global Spine J. 2018. doi: 10.1177/2192568218763323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein NE. What are we waiting for? An argument for early surgery for spinal epidural abscesses. Surg Neurol Int. 2015. doi: 10.4103/2152-7806.166894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karhade AV, Shah AA, Bono CM, et al. Development of machine learning algorithms for prediction of mortality in spinal epidural abscess. Spine J. 2019. doi: 10.1016/j.spinee.2019.06.024 [DOI] [PubMed] [Google Scholar]
- 6.Patel AR, Alton TB, Bransford RJ, et al. Spinal epidural abscesses: Risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14:326-330. [DOI] [PubMed] [Google Scholar]
- 7.Kim SD, Melikian R, Ju KL, et al. Independent predictors of failure of nonoperative management of spinal epidural abscesses. Spine J. 2014;14:1673-1679. [DOI] [PubMed] [Google Scholar]
- 8.Hunter S, Chb MB, Cussen R, et al. Predictors of Failure for Nonoperative Management of Spinal Epidural Abscess; 2019. 10.1177/2192568219887915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zadran S, Pedersen PH, Eiskjær S. Vertebral osteomyelitis: A mortality analysis comparing surgical and conservative management. Global Spine J. 2020;10. doi: 10.1177/2192568219862213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong GX, Crawford AM, Striano B, et al. The NIMS framework: an approach to the evaluation and management of epidural abscesses. Spine J. 2021. doi: 10.1016/j.spinee.2021.05.012 [DOI] [PubMed] [Google Scholar]
- 11.Yoon SH, Chung SK, Kim K-J, et al. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19. doi: 10.1007/s00586-009-1216-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babouee Flury B, Elzi L, Kolbe M, et al. Is switching to an oral antibiotic regimen safe after 2 weeks of intravenous treatment for primary bacterial vertebral osteomyelitis? BMC Infect Dis. 2014;14. doi: 10.1186/1471-2334-14-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copley LAB, Kinsler MA, Gheen T, et al. The impact of evidence-based clinical practice guidelines applied by a multidisciplinary team for the care of children with osteomyelitis. Journal of Bone and Joint Surgery - Series A. 2013;95. doi: 10.2106/JBJS.L.00037 [DOI] [PubMed] [Google Scholar]
- 14.Bozdogan H. Model selection and Akaike’s Information Criterion (AIC): The general theory and its analytical extensions. Psychometrika. 1987;52. doi: 10.1007/BF02294361 [DOI] [Google Scholar]
- 15.Uchida K, Nakajima H, Yayama T, et al. Epidural abscess associated with pyogenic spondylodiscitis of the lumbar spine; evaluation of a new MRI staging classification and imaging findings as indicators of surgical management: a retrospective study of 37 patients. Arch Orthop Trauma Surg. 2010;130. doi: 10.1007/s00402-009-0928-3 [DOI] [PubMed] [Google Scholar]
- 16.Lemaignen A, Ghout I, Dinh A, et al. Characteristics of and risk factors for severe neurological deficit in patients with pyogenic vertebral osteomyelitis. Medicine. 2017;96. doi: 10.1097/MD.0000000000006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howie BA, Davidson IU, Tanenbaum JE, et al. Thoracic epidural abscesses: A systematic review. Global Spine J. 2018;8. doi: 10.1177/2192568218763324 [DOI] [PMC free article] [PubMed] [Google Scholar]