Abstract
Study design
Cross-sectional study
Objectives
To examine the relationship between morphological changes of the deep extensor neck muscles in patients with degenerative cervical myelopathy (DCM) and the level of maximum spinal cord compression (MSCC) and canal compromise (MCC). A secondary objective was to examine the relationship between muscle morphological changes with neck pain and functional scores related to neck pain and interference.
Methods
A total of 171 patients with DCM were included. Total cross-sectional area (CSA), functional CSA (fat free area, FCSA), ratio of FCSA/CSA (fatty infiltration) and asymmetry of the multifidus (MF) and semispinalis cervicis (SCer) together, and cervical muscle as a group (eg, MF, SCer, semispinalis capitis, splenius capitis) were obtained from T2-weighted axial MR images at mid-disc, at the level of maximum cord compression and the level below. The relationship between the muscle parameters of interest, MSCC, MCC and functional scores including the Neck Disability Index (NDI) was assessed using multivariate linear regression models, adjusting for age, body mass index and sex.
Results
Greater MF + Scer fatty infiltration was associated with greater MCC (P = .032) and MSCC (P = .049) at the same level. Greater asymmetry in MF + SCer CSA was also associated with greater MCC (P = .006). Similarly, greater asymmetry in FCSA and FCSA/CSA of the entire extensor muscle was associated with greater MCC (P = .011, P = .013). There was a negative association between asymmetry in FCSA MF + SCer, FCSA/CSA MF + SCer and FCSA/CSA group muscles with NDI score at the level below.
Conclusion
Greater MCC is associated with increased fatty infiltration and greater asymmetry of the deep cervical muscles in patients with DCM. A negative association between muscle asymmetry and NDI scores was also observed which has implications for clinical prediction around axial neck pain.
Keywords: extensor neck muscles, degenerative cervical myelopathy, magnetic resonance images, maximum spinal cord compression, maximum canal compromise, total cross-sectional area, muscle fat infiltration
Introduction
Degenerative cervical myelopathy (DCM) is a major cause of disability in the adult population. 1 This condition is characterized by a narrowing of the cervical spinal canal, leading to pain and neurological impairments.1-3 Symptoms often varies from neck stiffness, gait impairment and numbness of the hands to tetraplegia. 1 Spinal degenerative changes begin with the degeneration and loss of the structural integrity of the spine, and as the severity of the disease increases failure of the annulus fibrosus may result in disc bulging or herniation through the annulus into the spinal canal, causing compression of the neural elements.3,4 Therefore, decompressive surgery is considered a practical option for patients with progressive DCM. While patients with moderate and severe DCM are mostly managed using surgical decompression, nearly 40% of patients undergoing surgery report partial recovery (eg, <50% improvement).4-6
Recent studies have demonstrated the deep extensor neck muscles, especially the cervical multifidus (MF) and semispinalis cervicis (Scer), are often impaired in patients with cervical disorders7,8 and atrophied in patients with whiplash-type injury or chronic neck pain.8,9 However, few studies have evaluated the deep extensor neck muscles of patients with DCM.4,10 Therefore, the presence and extent of morphologic muscle changes in patients with DCM warrants further attention.
The deep extensor paraspinal muscles have shown to be associated with poor clinical symptoms and functional outcomes in DCM.10,11 Fortin et al. 10 reported an association between greater fatty infiltration and lower modified Japanese Orthopedic Association (mJOA) functional scores in patients with DCM. A significant correlation between the deep cervical extensor muscle morphology, clinical signs, and symptoms 10 as well as cervical muscle strength 11 was also observed. This may occur because the deep extensor neck muscles of the cervical spine, including the MF and Scer, play a critical role in maintaining normal cervical curvature, cervical spinal stability, and activity through their deep attachments to the cervical spine.3,10-12 Deep extensor neck muscles are innervated by the cervical plexus (C1-C4), cranial nerves, or dorsal rami of upper cervical nerves. 12 Furthermore, previous evidence demonstrated the impact of DCM on muscles innervated by the brachial and lumbar plexus, suggesting that muscle denervation may progress at the same level, or level below the spinal cord compression in patients with DCM. 11 However, while numerous mechanisms may lead to muscle morphological alterations of deep extensor (and flexor) neck muscles, 13 we hypothesized that greater morphological changes including size, fat infiltration and asymmetry of deep extensor neck muscles is correlated with greater spinal compression and canal compromise. Additionally, the relation between cervical muscle morphology, clinical outcome, and functional status requires additional examination, given that muscle morphology alterations have an impact on their function and behaviour.
Therefore, the purpose of this study was to examine the relationship between morphological changes of the deep extensor neck muscles in patients with DCM and the level of maximum spinal compression and canal compromise. A secondary objective was to investigate the relationship between morphological changes of the deep extensor muscles the level of maximum spinal compression and canal compromise with neck pain and functional status scores.
Materials and Methods
Subjects
Of the 479 symptomatic DCM subjects from the Controlled Prospective AOSpine DCM-International cohort study database (16 global institutions), a total of 171 subjects with good quality pre-surgery T2-weighted axial magnetic resonance images (MRI) were identified and included in the current study. All DCM patients included in this cohort were scheduled for surgical treatment. As we were interested in testing the hypothesis that cervical muscle morphological alterations occur in relation to the DCM level of maximum compression and spinal compromised, subjects were eligible for the current study if they satisfied the following inclusion criteria: (1) aged 18 years or older; (2) presenting with symptomatic DCM with at least one clinical sign or symptom of myelopathy; (3) available pre-operative MRIs T2-axial images and (4) no previous cervical spine surgery. Related signs and symptoms of myelopathy and sources of stenosis of the included subjects are presented in Table 1. Subjects were excluded if they were asymptomatic or diagnosed with active infection, neoplastic disease, rheumatoid arthritis, ankylosing spondylitis, or concomitant lumbar stenosis.
Table 1.
Demographic and Baseline Characteristics of Patients (n = 171).
| Characteristics of patients | Mean (SD) or frequency (%) |
|---|---|
| Age (year) | 54.92 (11.85) |
| BMI (kg/m2) | 25.77 (5.43) |
| Sex | |
| Male | 112 (65.5%) |
| Female | 59 (34.5%) |
| mJOA | 12.05 (2.71) |
| NDI | 39.24 (19.32) |
| SF-36 | 36.84 (12.13) |
| DCM duration (month) | 30.23 (39.63) |
| MCC | 45.38% (14.96) |
| MSCC | 42.84% (17.7) |
| C3- C4 (max level of compression) | 39 (22.8%) |
| C4- C5 (max level of compression) | 48 (28.07%) |
| C5- C6 (max level of compression) | 68 (39.76%) |
| C6- C7 (max level of compression) | 16 (9.35%) |
| DCM symptoms | |
| Numb hands | 89.8% |
| Clumsy hands | 71.7% |
| Impairment of gait | 80.7% |
| Bilateral arm paresthesia | 57.2% |
| L’Hermitte’s phenomena | 19.9% |
| Weakness | 79.5% |
| DCM signs | |
| Corticospinal distribution motor deficits | 73.5% |
| Atrophy of hand intrinsic muscles | 36.1% |
| Hyperreflexia | 84.3% |
| Positive hoffman sign | 66.9% |
| Upgoing plantar responses | 51.2% |
| Lower limb spasticity | 64.5% |
| Broad based, unstable gait | 65.7% |
| DCM sources of stenosis | |
| Spondylosis | 84.3% |
| Disk | 73.49% |
| Ossified posterior longitudinal ligament | 33.7% |
| Hypertrophic ligamentum flavum | 33.7% |
| Subluxation | 5.4% |
| Other | 0% |
mJOA: modified Japanese orthopedic association, NDI: Neck disability index, SF-36: Short form 36 health survey questionnaire, DCM: Degenerative cervical myelopathy, BMI: Body mass index, MCC: Maximum canal compromise, MSCC: Maximum spinal cord compression, SD: Standard deviation.
Subjects provided written informed consent, acknowledging that their data would be used to improve the understanding of DCM. The Controlled Prospective AOSpine DCM-International study was approved by research ethics boards at each centre. The Research Ethics Board at University Health Network (Toronto) approved the study at the principal coordinating site (Toronto Western Hospital: PI Michael Fehlings). The Ethics Research Board of McGill University also approved this study (#14-085-GEN).
Cervical Muscle Measurements
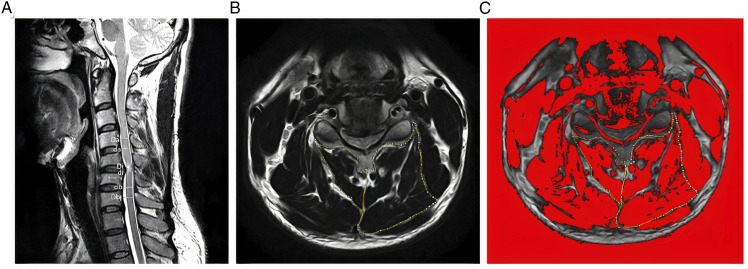
Quantitative measurements of the deep extensor neck cervical muscles were acquired from T2-weighted axial MR images at the level of max compression and level below using ImageJ imaging software (version 1.43; National Institutes of Health, Bethesda, Maryland, downloadable at http://rsbweb.nih.gov/ij/download.html) after multiplanar reconstruction (3D MPR) using the 32-bitOsiriX software program (version 3.8.1; Pixmeo, Geneva, Switzerland) to position the image slices perpendicular to the muscle mass, when required. The level and degree maximum spinal cord compression (MSCC) and maximum canal compromise (MCC) was determined using the following formulas MSCC = [1 −di (da + db)/2] × 100, and MCC = [1 −Di (Da + Db)/2] × 100 as defined by Fehlings et al. 14 (Figure 1A). Cervical measurement of the MF+SCer together, and deep extensor muscles as a group (eg, MF, SCer, semispinalis capitis, splenius capitis) were obtained bilaterally at mid-disc, at the level of maximum cord compression and the level below (Figure 1B and C). The measurements of interest included: total cross-sectional area (CSA), functional CSA (fat free area, FCSA), ratio of FCSA/CSA (fatty infiltration) and asymmetry. The mean value of the sum of the muscle CSAs or FCSAs on right and left side at all levels, and the means for the FCSA/CSA ratio were calculated and used in the analysis.
Figure 1.
(A) Measurements required for MCC and MSCC calculation. Di, Da, and Db measure the diameter of the spinal canal at the site of compression and at the normal site above and below, respectively; di, da, and db indicate the diameter of the spinal cord at the site of compression and at the normal site above and below respectively. (B) Measurements of the Total CSA of the MF + SCer muscles and extensor muscles group on axial T2-weighted image at the C5-C6 level. (C) The image shows the application of a signal threshold filter (ImageJ) to highlight the fat-free muscle are and obtain the FCSA muscle measurements.
The FCSA was measured using a highly reliable thresholding technique described in a previous study 15 (Figure 1C). In brief, 6 small sample regions of interest within the bilateral paraspinal muscle group of each axial slice were taken from areas of lean muscle tissue to detect the maximum value. The maximum value was used as the highest cut-off value to distinguish muscle tissue from fat, and 0 was regarded as the lower limit. The relative percent asymmetry of the paraspinal muscles on axial view was calculated as follows: the relative asymmetry rate = [(L−S)/L)] x 100, where L is the larger side and S is the smaller side. 10
Self-Reported Questionnaires
Subjects included in this cohort study completed the following functional tests at the time of recruitment; mJOA, Neck Disability Index (NDI) and Short Form 36 health survey questionnaire (SF-36). The mJOA is an 18-point scale scoring way of measuring and quantifying upper and lower extremity motor and sensory function which has been previously validated.16,17 The NDI is a self-reported questionnaire used to measure related pain and disability; higher scores (out of 100) are indicative of greater disability. This questionnaire has previously demonstrated good levels of reliability and validity for neck pain.18,19 The SF-36 health survey is a reliable and valid questionnaire, consisting of 8 classified scores to measure health-related quality of life.20,21
Statistical Analysis
Means and standard deviations were calculated for the cervical muscle measurements. The relationship between the muscle parameters of interest, MSCC and MCC was assessed using multivariate linear regression models, adjusting for age, body mass index (BMI) and sex. Separate models were used for each muscle group and spinal level. Similarly, multivariable linear regression models were used to investigate the association between muscle parameters of interest and functional status scores, adjusting for age, BMI and sex. P < .05 were considered statistically significant. All data analysis were performed with STATA (version 12.0; StataCorp, LP, College Station, TX).
Results
The average MSCC and MCC was 42.84 ± 17.7% and 45.38 ± 14.96%, respectively. Patients’ clinical characteristics and functional scores are presented in Table 1 and cervical muscle MRI measurements of interest Table 2.
Table 2.
Mean (Standard Deviation) of Cervical Paraspinal Muscle Measurements at the Level of Maximum Compression and Level Below.
| Paraspinal muscle measurements | Max level | Level below |
|---|---|---|
| FCSA/CSA MF + SCer | .6 (.16) | .6 (.11) |
| FCSA/CSA group | .68 (.09) | .69 (.09) |
| FCSA/CSA asy MF + Scer | 11.07 (9.95) | 11.09 (9.03) |
| FCSA/CSA asv group | 5.8 (5.06) | 6.52 (5.44) |
| CSA asy MF + SCer | 10.48 (8.33) | 9 (6.97) |
| CSA asy group | 7.16 (6.36) | 6.65 (5.17) |
| FCSA asy MF + SCer | 13.31 (11.37) | 13.13 (10.25) |
| FCSA asy group | 7.6 (7.5) | 7.21 (6.34) |
CSA: Cross-sectional area, FCSA: Functional cross-sectional area, MF: Multifidus muscle, SCer: Semispinalis Cervicis, Asy: Asymmetry.
Association Between Muscle Parameters and MCC and MSCC
The associations between the muscle parameters of interest with MCC and MSCC are presented in Table 3 and Table 4. Greater MF + Scer fatty infiltration (eg, lower FCSA/CSA) was associated with greater MCC (P = .032) and MSCC (P = .049) at the same level. Greater asymmetry in MF + SCer CSA was also associated with greater MCC (P = .006). Similarly, greater asymmetry in FCSA and FCSA/CSA of the entire extensor muscle group was associated with greater MCC (P = .011, P = .013). There was no significant association between muscle measurements obtained at the level below of the maximum level of compression and MCC and MSCC.
Table 3.
Results of Multivariable Regression Analyses and MCC.
| Paraspinal muscle measurements | Regression coefficient | P-value (95% CI) |
|---|---|---|
| Max level | ||
| FCSA/CSA MF + SCer | −.0018 | .032 [−.0034, −.0001]* |
| FCSA/CSA group | −.0006 | .212 [−.0016, .0003] |
| CSA asy MF + SCer | .1161 | .006 [.0334, .1988]* |
| FCSA asy Group | .0975 | .011 [.0228, .1722]* |
| FCSA/CSA asy MF + SCer | .0159 | .755 [−.0849, .1169] |
| FCSA/CSA asy group | .0643 | .013 [.0139, .1147]* |
| Level below | ||
| FCSA/CSA MF + SCer | −.0006 | .280 [−.0017, .0005] |
| FCSA/CSA group | −.00007 | .880 [−.0009, .0008] |
| CSA asy MF + SCer | .0296 | .408 [−.0409, .1002] |
| CSA asy Group | .0129 | .626 [−.0394, .0653] |
| FCSA/CSA asy MF + SCer | .0478 | .302 [−.0434, .1391] |
| FCSA/CSA asy group | −.0266 | .340 [−.0817, .0283] |
CI = Confidence interval, * = P < .05.
Table 4.
Results of Multivariable Regression Analyses and MSCC.
| Paraspinal muscle measurements | Regression coefficient | P-value (95% CI) |
|---|---|---|
| Max level | ||
| FCSA/CSA MF + SCer | −.0013 | .049 [−.0027, −3.86]* |
| FCSA/CSA group | −.0004 | .283 [−.0013, .0003] |
| CSA asy MF + SCer | .054 | .135[−0. 017, .1251] |
| FCSA asy Group | .0446 | .170 [−.0193, .1086] |
| FCSA/CSA asy MF + SCer | −.0427 | .323 [−.1278, .0423] |
| FCSA/CSA asy group | .0275 | .210 [−.0156, .0707] |
| Level below | ||
| FCSA/CSA MF + SCer | −.0001 | .830 [−.001, .0008] |
| FCSA/CSA group | .0002 | .534 [−.0005, .001] |
| CSA asy MF + SCer | .0188 | .533 [−.0408, .0786] |
| CSA asy Group | .0098 | .662 [−.0345, .0541] |
| FCSA/CSA asy MF + SCer | .0089 | .819 [−.0684, .0864] |
| FCSA/CSA asy group | −.0463 | .049 [−.0925, −.0002] |
CSA: Cross-sectional area, FCSA: Functional cross-sectional area, CI: Confidence interval, * = P < .05.
Association Between Muscle Parameters and functional scores
Our results demonstrated an association between less asymmetry in FCSA MF + SCer, FCSA/CSA MF + SCer and FCSA/CSA group muscles (negative regression coefficient) with higher NDI scores at the level below the maximum level of compression Table 5. There were no significant association between the muscle parameters of interest and mJOA or SF-36 at the level of maximum compression or level below, except for a positive relationship between asymmetry in FCSA/CSA group and mJOA at the level below (P = .041).
Table 5.
Results of Multivariable Regression Analyses and NDI.
| Paraspinal muscle measurements | Regression coefficient | P-value (95% CI) |
|---|---|---|
| Max level | ||
| FCSA/CSA MF + SCer | .000 | .792 [.533, .663] |
| FCSA/CSA group | .000 | .483 [.644, .718] |
| CSA asy MF + SCer | −.006 | .884 [−.082, .071] |
| FCSA asy Group | −.017 | .635 [−.086, .052] |
| FCSA/CSA asy MF + SCer | .047 | .334 [−.049, .142] |
| FCSA/CSA asy group | .007 | .761 [−.040, .054] |
| Level below | ||
| FCSA/CSA MF + SCer | .000 | 0. 570 [.544, .628] |
| FCSA/CSA group | .000 | .772 [−.001, .001] |
| CSA asy MF + SCer | −.014 | .686 [−.079, .052] |
| FCSA asy MF + SCer | −.129 | .005 [−.219, −.039]* |
| FCSA/CSA asy MF + SCer | −.105 | .014 [−.188, −.022]* |
| FCSA/CSA asy group | −.062 | .016 [−.112, −.012]* |
CSA: Cross-sectional area, FCSA: Functional cross-sectional area, CI: Confidence interval, * = P < .05.
Discussion
Previous studies have found that patients with neck pain demonstrated degenerative changes in morphology (size, asymmetry, fatty infiltration) and functional deficit (delay contraction, decrease muscle strength) in deep extensor neck muscles compared with asymptomatic individuals.22-25 This innovative study demonstrates that the degree of cervical muscle fatty infiltration and asymmetry in patients with DCM is associated with the degree of spinal cord compression and canal compromise at the maximum level of compression. We found a significant association between MF + Scer FCSA/CSA and MCC and MSCC at the level of maximum compression, suggesting greater spinal compression is associated with more fatty infiltration. The level of maximum compression was also associated with greater MF + SCer CSA asymmetry and MCC. Our findings also revealed a significant association between greater asymmetry in FCSA and FCSA/CSA of the entire extensor muscle group and greater MCC at the maximum level of compression in patients with DCM. Importantly, a negative association between muscle asymmetry and NDI scores was also observed which may have implications for understanding the mechanisms of axial neck pain and for managing this challenging issue.
The structure and innervation of the architecturally complex deep extensor neck muscles (MF and Scer), which attach between the transverse process and spinal process of cervical vertebral column are innervated by the cervical plexus (C1-C4), cranial nerves, or dorsal branches of upper cervical nerves 26 may partly explain the results of this study. Indeed, the structure of the MF+ Scer may be affected by spinal cord compression and specific nerve root, 27 which could be a plausible explanation for the observed increase in fatty infiltration and atrophy of the paraspinal muscles at the level of spinal cord compression. Cornwall et al. 28 reported that muscular bundles of paraspinal muscle extending across 2 or 3 segments do not have consecutive/in-series architecture, and that multisegmental nerves are unlikely to supply a single muscle bundle of the paraspinal muscle. Polysegmental innervation for lumbar MF muscle has been argued in several studies, however there is no such evidence of cervical MF muscle innervation. In addition, muscle denervation has been previously reported in patients with DCM, 11 and skeletal muscle denervation is known to cause increase intramuscular fat and atrophy.10,11 Hou et al 12 examined the correlation between deep cervical paraspinal morphology alteration and the level of spinal cord compression in patients with C4-5 single level DCM. Their findings showed the degree of cervical paraspinal fat infiltration and atrophy were greatest at the level of spinal cord compression.
We did not find a relationship between cervical muscle measurements and MCC and MSCC at the level below maximum compression. To our knowledge, there are no other studies investigating the structure of deep neck extensor muscle in relation to the degree of maximum compression, and therefore comparison to our results is not possible. However, previous studies such as Fortin et al’s 11 and Cloney et al’s 4 studies investigating paraspinal muscle morphology in patients with DCM have reported increase cervical muscle fat infiltration at the level below the myelopathy. In both studies, the amount of MF fat infiltration was compared above, same and level below the myelopathy. In the current study, only the relationship between morphological changes of the deep extensor neck muscles with MCC and MSCC at the level of maximum compression and the level below was assessed and no direct comparison was made between 2 levels. On the other hand, other factors, such as the selected measurement levels could have influenced the results and related paraspinal muscle measurements. Fortin et al. 11 only included symptomatic patients with DCM where their most cranial level of spinal cord compression was observed at C4-C5 and C5-C6 levels. Moreover, in Cloney et al’s 4 study, MF fat infiltration was calculated from C3 to C7 on MRI images, and a modified Torg-Pavlov ratio (eg Ratio = Canal Diameter/VB Anterior-Posterior Diameter was used to identify the level of myelopathy. While in the current study, quantitative measurements of the deep extensor neck cervical muscles were acquired at the maximum level of compression, which was selected between C2 and C7 using the formula as defined by Fehlings et al. 14 It is vital to build a consistent operating method to improve the comparability from the findings of other available studies. Additional studies are needed to clarify the role of fat infiltration and atrophy of deep extensor (and flexor) neck muscles in development of DCM. Although contributing causes of non-traumatic neck pain remain unclear, considering changes in cervical muscle morphology and their relationship with the MCC and MSCC as muscle degeneration markers may be helpful for clinical decision making pre- and post-surgery, in addition to informed rehabilitation.
Our results showed a negative association between FCSA MF + SCer asymmetry, FCSA/CSA MF + SCer asymmetry and FCSA/CSA group muscles asymmetry and NDI score at the level below the maximum level of compression. These findings suggest an association between lower asymmetry in cervical muscle morphology and increased NDI scores. In a prospective study involving 36 participants following a whiplash injury, higher NDI scores were associated with greater cervical muscle fatty infiltration, both at 2 weeks and 3 months post injury. 29 One is likely to observe a higher pain intensity in population with acute injury vs chronic disorders. Fortin et al. 10 revealed that greater asymmetry in semispinalis capitis muscle fatty infiltration was associated with higher NDI scores in patients with diagnosed DCM. The inconsistent findings may be explained by the variations in muscle group examined, as Fortin et al.’ study examined deep extensor neck muscle separately, rather than muscle group combined. Conversely, Elliott et al 8 reported no association between cervical muscle fatty infiltration and NDI in subjects with persistent whiplash disorders, possibly due to the population sharing a clinical course of chronic pain and significant impairment. Similarly, no association between NDI score and the amount of relative fat infiltration in cervical extensor muscles was reported in Cloney et al.’ study in 2018, 4 but muscle morphology, particularly muscle asymmetry, was not examined. Another possible reason that may explain the disparities between our findings and previous studies include the heterogeneity of the clinical populations, especially with regards to symptom duration. In this study, the average symptom duration of patients with DCM was 30.23 months, while the mean symptom duration from Elliott et al.’ study 8 (eg whiplash related disorders) and Cloney et al’s study 4 (eg DCM patients) was 20.3 months and 10.1 months, respectively. Furthermore, we measured cervical neck extensor neck muscles in reference to the level of maximum spinal cord compression.
The study has a several limitations. Firstly, T2-weighted images were acquired in various institution, so imaging scanner parameters were not standardized. Secondly, the impact of pre-surgery conservative treatment on the deep extensor neck muscles morphology were not considered. Thirdly, we evaluated the paraspinal muscle morphology in different levels from C2 to C7; as the exact border between each muscle was not recognizable in all levels, the MF and Scer was considered as one group of muscle and paraspinal muscle as another group. The association between cervical muscle changes and the related sources of stenosis was not considered, as most subjects had a combination of pathologies, which is typical with DCM. Lastly, in this study, MRI was the sole method applied to evaluate the paravertebral muscles. Using other assessment methods, such as ultrasound or computed tomography scan simultaneously may increase the muscle measurements accuracy. Furthermore, deep learning automatic segmentation methods are rapidly evolving as in evidence of a convolutional neural network for the cervical muscles30,31 and this has been used in a clinical population of patients with DCM. 13
Conclusion
Greater MCC is associated with increased fatty infiltration and greater asymmetry of the deep extensor cervical muscles in patients with DCM. Our findings also suggest that MCC is a better indicator of cervical muscle morphological changes than MSCC. A negative relationship between deep extensor cervical muscle asymmetry in morphology and NDI scores was also observed. Whether such markers of muscle degeneration can be modified with pre- or post-operation rehabilitation exercise to impact patient health in related to the quality-of-life scores and neck function warrant further investigations. Additionally, it would be advantageous to investigate whether change in cervical lordosis and sagittal parameters contribute to variations in paraspinal muscle morphology and composition or functional outcomes. Given the importance that patients with DCM place on neck pain, our findings have important translational significance. We are now expanding this work to investigate whether cervical muscle morphology and composition are predictors of post-operative outcomes, in addition to assessing the impact of surgical treatment on cervical muscle characteristics in patients with DCM.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Neda Naghdi https://orcid.org/0000-0003-0072-1441
Michael G. Fehlings https://orcid.org/0000-0002-5722-6364
References
- 1.Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: The clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neurosci. 2013;19(4):409-421. [DOI] [PubMed] [Google Scholar]
- 2.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: Epidemiology, genetics, and pathogenesis. Spine. 2015;40(12):E675-E693. [DOI] [PubMed] [Google Scholar]
- 3.Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: Results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi: 10.1007/s00586-013-2658-z. [DOI] [PubMed] [Google Scholar]
- 4.Cloney M, Smith AC, Coffey T, et al. Fatty infiltration of the cervical multifidus musculature and their clinical correlates in spondylotic myelopathy. J Clin Neurosci. 2018;57:208-213. [DOI] [PubMed] [Google Scholar]
- 5.Matz P, Anderson P, Holly L, et al. Joint section on disorders of the spine and peripheral nerves of the american association of neurological surgeons and congress of neurological surgeons. J Neurosurg Spine. 2009;11(2):157-169.19769495 [Google Scholar]
- 6.Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: Recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(3_suppl):70S-83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae S-H, Lee S-J, Kim M-S, Kim T-U, Hyun J-K. Cervical multifidus muscle atrophy in patients with unilateral cervical radiculopathy. J Korean Acad Rehabil Med. 2010:743-751. [Google Scholar]
- 8.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: A magnetic resonance imaging analysis. Spine. 2006;31(22):E847-E855. [DOI] [PubMed] [Google Scholar]
- 9.Rezasoltani A, Ahmadipoor A, Khademi-Kalantari K, Javanshir K. The sign of unilateral neck semispinalis capitis muscle atrophy in patients with chronic non-specific neck pain. J Back Musculoskelet Rehabil. 2012;25(1):67-72. [DOI] [PubMed] [Google Scholar]
- 10.Fortin M, Dobrescu O, Courtemanche M, et al. Association between paraspinal muscle morphology, clinical symptoms, and functional status in patients with degenerative cervical myelopathy. Spine. 2017;42(4):232-239. [DOI] [PubMed] [Google Scholar]
- 11.Fortin M, Wilk N, Dobrescu O, Martel P, Santaguida C, Weber MH. Relationship between cervical muscle morphology evaluated by MRI, cervical muscle strength and functional outcomes in patients with degenerative cervical myelopathy. Musculoskelet Sci Pract. 2018;38:1-7. [DOI] [PubMed] [Google Scholar]
- 12.Hou X, Lu S, Wang B, Kong C, Hu H. Morphologic characteristics of the deep cervical paraspinal muscles in patients with single-level cervical spondylotic myelopathy. World Neurosurg. 2020;134:e166-e171. [DOI] [PubMed] [Google Scholar]
- 13.Paliwal M, Weber KA, Smith AC, et al. Fatty infiltration in cervical flexors and extensors in patients with degenerative cervical myelopathy using a multi-muscle segmentation model. PloS One. 2021;16(6):e0253863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehlings MG, Rao SC, Tator CH, et al. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury: Part II: Results of a multicenter study. Spine. 1999;24(6):605-613. [DOI] [PubMed] [Google Scholar]
- 15.Fortin M, Battié MC. Quantitative paraspinal muscle measurements: Inter-software reliability and agreement using OsiriX and ImageJ. Phys Ther. 2012;92(6):853-864. [DOI] [PubMed] [Google Scholar]
- 16.Kopjar B, Tetreault L, Kalsi-Ryan S, Fehlings M. Psychometric properties of the modified Japanese Orthopaedic Association scale in patients with cervical spondylotic myelopathy. Spine. 2015;40(1):E23-E28. [DOI] [PubMed] [Google Scholar]
- 17.Tetreault L, Kopjar B, Nouri A, et al. The modified Japanese Orthopaedic Association scale: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2017;26(1):78-84. [DOI] [PubMed] [Google Scholar]
- 18.Vernon H, Mior S. The neck disability index: A study of reliability and validity. J Manipulative Physiol Ther. 31, 491, 502, 1991. [PubMed] [Google Scholar]
- 19.Pietrobon R, Coeytaux RR, Carey TS, Richardson WJ, DeVellis RF. Standard scales for measurement of functional outcome for cervical pain or dysfunction: A systematic review. Spine. 2002;27(5):515-522. [DOI] [PubMed] [Google Scholar]
- 20.Findler M, Cantor J, Haddad L, Gordon W, Ashman T. The reliability and validity of the SF-36 health survey questionnaire for use with individuals with traumatic brain injury. Brain Injury. 2001;15(8):715-723. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr. SF-36 health survey update. Spine. 2000;25(24):3130-3139. [DOI] [PubMed] [Google Scholar]
- 22.Falla D, Jull G, Hodges P. Feedforward activity of the cervical flexor muscles during voluntary arm movements is delayed in chronic neck pain. Exp Brain Res. 2004;157(1):43-48. [DOI] [PubMed] [Google Scholar]
- 23.Wong AY, Harada G, Lee R, et al. Preoperative paraspinal neck muscle characteristics predict early onset adjacent segment degeneration in anterior cervical fusion patients: A machine-learning modeling analysis. J Orthop Res®. 2021;39(8):1732-1744. [DOI] [PubMed] [Google Scholar]
- 24.Pinter ZW, Wagner SC, Fredericks DR, Jr, et al. Higher paraspinal muscle density effect on outcomes after anterior cervical discectomy and fusion. Global Spine Journal. 2021;11(6):931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes RB. Clinical Epidemiology: How to do Clinical Practice Research. Philadelphia, PA: Lippincott williams & wilkins; 2012. [Google Scholar]
- 26.Macintosh JE, Valencia F, Bogduk N, Munro RR. The morphology of the human lumbar multifidus. Clin Biomech. 1986;1(4):196-204. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi N, Masumoto T, Abe O, Aoki S, Ohtomo K, Tajiri Y. Accuracy of abnormal paraspinal muscle findings on contrast-enhanced MR images as indirect signs of unilateral cervical root-avulsion injury. Radiology. 2002;223(2):397-402. [DOI] [PubMed] [Google Scholar]
- 28.Cornwall J, Deries M, Duxson M. Morphology of the lumbar transversospinal muscles examined in a mouse bearing a muscle fiber-specific nuclear marker. Anat Rec Adv Integr Anat Evol Biol. 2010;293(12):2107-2113. [DOI] [PubMed] [Google Scholar]
- 29.Elliott JM, Courtney DM, Rademaker A, Pinto D, Sterling MM, Parrish TB. The rapid and progressive degeneration of the cervical multifidus in whiplash: A MRI study of fatty infiltration. Spine. 2015;40(12):E694-E700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber KA, Abbott R, Bojilov V, et al. Multi-muscle deep learning segmentation to automate the quantification of muscle fat infiltration in cervical spine conditions. Sci Rep. 2021;11(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber KA, Smith AC, Wasielewski M, et al. Deep learning convolutional neural networks for the automatic quantification of muscle fat infiltration following whiplash injury. Sci Rep. 2019;9(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]