Abstract
We previously showed that an envelope A27L protein of intracellular mature virions (IMV) of vaccinia virus binds to cell surface heparan sulfate during virus infection. In the present study we identified another viral envelope protein, D8L, that binds to chondroitin sulfate on cells. Soluble D8L protein interferes with the adsorption of wild-type vaccinia virions to cells, indicating a role in virus entry. To explore the interaction of cell surface glycosaminoglycans and vaccinia virus, we generated mutant viruses from a control virus, WR32-7/Ind14K (A27L+ D8L+) to be defective in expression of either the A27L or the D8L gene (A27L+ D8L− or A27L− D8L+) or both (A27L− D8L−). The A27L+ D8L+ and A27L− D8L+ mutants grew well in BSC40 cells, consistent with previous observations. However, the IMV titers of A27L+ D8L− and A27L− D8L− viruses in BSC40 cells were reduced, reaching only 10% of the level for the control virus. The data suggested an important role for D8L protein in WR32-7/Ind14K virus growth in cell cultures. A27L protein, on the other hand, could not complement the functions of D8L protein. The low titers of the A27L+ D8L− and A27L− D8L− mutant viruses were not due to defects in the morphogenesis of IMV, and the mutant virions demonstrated a brick shape similar to that of the control virions. Furthermore, the infectivities of the A27L+ D8L− and A27L− D8L− mutant virions were 6 to 10% of that of the A27L+ D8L+ control virus. Virion binding assays revealed that A27L+ D8L− and A27L− D8L− mutant virions bound less well to BSC40 cells, indicating that binding of viral D8L protein to cell surface chondroitin sulfate could be important for vaccinia virus entry.
Vaccinia virus is a large enveloped DNA virus that belongs to the poxvirus family. It is the prototypic member of the orthopox viruses and is known to infect cells of many different origins. Although the viral genome has been sequenced, the nature of the broad host range remains to be explored. Vaccinia virus also contains three different forms of infectious virions: intracellular mature virus (IMV), intracellular enveloped virus (IEV), and extracellular enveloped virus (EEV) (1, 17, 48). These different forms of vaccinia virus virions contain different membrane structures and are responsible for various routes of infection (1, 30, 43). Of the three forms, IMV is the most abundant, and many of its surface envelope proteins have been identified (20, 47). Several proteins have been suggested to be involved in virus entry. For example, A27L and D8L proteins bind to the cell surface (22–24). A27L protein is required for fusion of virus-infected cells (12, 34, 38, 50). Another envelope protein, L1R, may play a role in virus penetration, since a monoclonal antibody (MAb) recognizing L1R protein blocked virus entry at a postbinding step (18, 19, 32, 51). Other proteins such as A14L and A17L also interact with A27L protein, but their roles in virus entry have yet to be determined (34, 39, 40).
Our previous data showed that viral A27L protein mediates IMV binding to cell surface glycosaminoglycans (GAGs), mainly heparan sulfate (HS) (6). The N-terminal region of A27L protein contains a cluster of positively charged amino acids that are important for binding to HS (16). In addition, binding to HS is essential for fusion of virus-infected cells, suggesting that conformational changes triggered after HS-A27L protein interaction initiate membrane fusion (16). To further clarify the mechanism of vaccinia virus entry, we wished to identify other viral proteins that may also participate in the entry process. In this report we identified D8L protein as the second envelope protein that binds to GAGs on cells. However, D8L protein binds to chondroitin sulfate (CS) on cells, revealing a different type of virus-GAG interaction.
Mutant viruses with defective A27L or D8L gene expression were also constructed in order to determine whether virus growth in cell cultures was affected by the impairment of GAG binding. These mutant viruses were purified, and their growth in cell cultures, virion morphology, and kinetics of binding to cells were analyzed. The data revealed a role of D8L protein in the binding of vaccinia virus virions to cells.
MATERIALS AND METHODS
Reagents and viruses.
Soluble heparin (HP), CS, and dermatan sulfate (DS) were purchased from Sigma Inc. Spurr resin was purchased from Electron Microscopy Sciences. An antiserum against TrpE-D8L protein, D8-1, was obtained from E. Niles (26). L, gro2C, and sog9 cells were obtained from F. Tufaro (2, 13). Wild-type vaccinia virus (WR strain) was grown in BSC40 cells. A recombinant vaccinia virus, WR32-7/Ind14K, was obtained from G. Smith (36, 37). IMV virions were purified by centrifugation of cell lysates through a 36% sucrose layer, and the pellets were saved as virus stocks (21). EEV was collected from fresh medium of the infected cell cultures and was used directly without freezing.
Protein expression and purification.
For expression of soluble D8L, two primers were made for PCR amplification. The 5′ primer (5′-AAAGAATTCATGCCGCAACAACTATCT) and the 3′ primer (5′-AAAAAGCTTTGAAAAACATGTCTCTCT) were used with a vaccinia virus DNA template in PCR amplification with a program of 94°C for 1 min, 42°C for 1.5 min, and 72°C for 1.5 min for 25 cycles. The amplified DNA fragment contained sequences corresponding to D8L amino acids 1 to 264. The DNA fragment was digested with EcoRI and HindIII and cloned into pET21a (Novagen). The resulting plasmid expressed an ectodomain with a T7 tag peptide at the N terminus for easy identification and hexahistidine sequences at the C terminus for purification with a nickel column.
For protein expression, the bacterial cultures were transformed with the above plasmid, and the cultures were induced with 0.8 mM isopropyl-β-d-thiogalactoside (IPTG) for 4 h at 37°C and then harvested. The bacterial pellets were sonicated, and the supernatant recovered after centrifugation was loaded onto a nickel column as recommended in the pET system manual (Novagen). The column was washed, and the bound protein was eluted with 0.3 M imidazole and dialyzed against phosphate-buffered saline (PBS) at 4°C overnight as described elsewhere (6).
Rabbit serum production and neutralization assays.
New Zealand White rabbits were immunized subcutaneously with 750 μg of soluble D8L protein and subsequently boosted three times, at 2-week intervals, with another 500 μg of protein before being sacrificed. One rabbit serum, C8, was used in neutralization assays. For neutralization assays, preimmune or postimmune sera at various dilutions (1:100, 1:500, and 1:1,000) were mixed with 150 PFU of wild-type vaccinia virus at 4°C for 30 min. The mixtures were added to BSC40 cells in 60-mm dishes in duplicate and incubated at 37°C for another hour. Cells were then washed with PBS and overlaid with 1% agar. Plaque numbers were determined after 3 days.
Biotinylation of D8L protein and cell binding assays.
D8L protein was biotinylated with an ECL biotinylation system purchased from Amersham Life Science, Inc. In brief, 1 mg of purified D8L protein was mixed with 40 μl of the biotinylation reagent N-hydroxysuccinamide ester in 40 mM bicarbonate buffer (pH 8.6) at room temperature for 1 h according to the manufacturer’s recommendation. The biotinylated mixture was then loaded on a 9-ml Sephadex G-25 column previously equilibrated with PBS. Biotinylated D8L protein was collected as 500-μl aliquots from fractions 7 to 9, and the extent of biotinylation was confirmed by Western blot analysis with horseradish peroxidase-conjugated streptavidin as described elsewhere (6).
For cell binding assays, BSC40 cells (106) were washed with cold PBS and incubated with biotinylated D8L protein (10 μg/125 μl) in staining medium (PBS–4% fetal bovine serum–10 mM HEPES [pH 7.2]). In some experiments, different GAGs (10, 100, or 1,000 μg/ml) were also added as competitors. The mixture was incubated at 4°C for 60 min with gentle rotation. Cells were subsequently washed with cold PBS, and phycoerythrin-conjugated streptavidin (1:100) was added for another 60 min at 4°C. Cells were again washed three times with cold PBS and analyzed with a fluorescence-activated cell sorter (FACS) (excitation wavelength, 488 nm; emission wavelength, 578 nm) as described elsewhere (16).
Virion binding assays.
To test if soluble D8L protein blocked vaccinia virus infection at the binding step, BSC40 cells were incubated first with various amounts of soluble D8L protein (0, 1, 10, or 50 μg in 200 μl) at 4°C for 30 min. The cultures were subsequently infected with wild-type vaccinia virus at a multiplicity of infection (MOI) of 10 PFU per cell at 4°C for another 30 min. After a wash, these cells were immediately harvested, and cell lysates were freeze-thawed three times, sonicated, and used for plaque assays on BSC40 cells. The number of plaques obtained from cells infected in the absence of D8L protein was taken as 100%.
To measure viral early gene expression, BSC40 cells were infected with vMJ360, which expresses lacZ from an early promoter, at an MOI of 10 PFU per cell. The infected cells were harvested at 2 h postinfection (p.i.) for β-galactosidase (β-Gal) assays as described elsewhere (16).
To determine the IMV binding kinetics of WR32-7/Ind14K or D8L− WR32-7/Ind14K virus, BSC40 cells were infected with each virus at an MOI of 5 PFU per cell and incubated at 4°C for various times (0, 1, 3, 4, and 5 h). At each time point, cells were washed three times with cold PBS and lysates were harvested. The virions were released from cell lysates by freeze-thawing three times and were sonicated, and aliquots were removed for plaque assays on BSC40 cells in the presence of 5 mM IPTG.
Construction of mutant vaccinia viruses defective in A27L and D8L gene expression.
Plasmid pSC11-5 was digested with SalI and PstI to release a DNA fragment containing a lacZ gene regulated by the p11K promoter (5). The lacZ cassette was blunt ended and cloned into a filled-in BssHII site of plasmid p770 so that the lacZ cassette was flanked by D8L sequences (26). The lacZ gene is transcribed in the same direction as the D8L gene.
The resulting plasmid was transfected into CV-1 cells and infected with WR32-7/Ind14K at an MOI of 0.1 PFU per cell in the presence of 5 mM IPTG as described previously (37). The lysates were harvested after 3 days, and the viruses were titered on agar containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (300 μg/ml) and 5 mM IPTG. The recombinant virus that expresses β-Gal, D8L− WR32-7/Ind14K, was isolated after three rounds of plaque purification. To facilitate plaque picking, IPTG was always added to the agar so that large plaques were formed, and subsequently isolations were repeated until pure plaques were obtained. To confirm that we did not introduce extra mutations during recombinant virus isolation, we obtained two independent D8L− WR32-7/Ind14K virus isolates. Viral DNA was purified from these clones, and restriction digestions were performed to confirm that the lacZ gene was inserted into the D8L locus. Since these two virus isolates behaved indistinguishably in cell cultures, we present data obtained from experiments with one isolate.
A27L+ D8L+, A27L+ D8L−, A27L− D8L+, and A27L− D8L− virus stock preparations.
In order to make virions of four different phenotypes, A27L+ D8L+, A27L+ D8L−, A27L− D8L+, and A27L− D8L−, BSC40 cells were infected with WR32-7/Ind14K or D8L− WR32-7/Ind14K at an MOI of 0.05 PFU per cell in duplicate sets. After infection, one set of cells infected by WR32-7/Ind14K or D8L− WR32-7/Ind14K was maintained in medium with 5 mM IPTG for 3 days, and the cell lysates were harvested and prepared as A27L+ D8L+ and A27L+ D8L− virus stocks, respectively. Another set of infected cultures was maintained in medium without IPTG for 3 days and harvested as A27L− D8L+ and A27L− D8L− virus stocks. All these IMV stocks were partially purified with 36% sucrose cushions as previously described (21).
Determination of virion infectivity by confocal microscopy.
IMV virions of A27L+ D8L+, A27L+ D8L−, A27L− D8L+, and A27L− D8L− viruses were sonicated, and virus stocks were serially diluted. One part of each dilution was used for plaque determination on BSC40 cells. Another part of the dilution was spotted onto glass slides and fixed for confocal microscopy analysis with an antiserum (1:500) against IMV as described previously (49). Images of fluorescent virion particles were photographed from three randomly selected fields. The virion particle numbers were counted, and averages of three fields per sample are presented in Table 2.
TABLE 2.
Determination of relative infectivities of virions produced by A27L and D8L mutant viruses
| Virus | No. of virion particles/fielda | Virus titerb (PFU/ml) | Relative infectivityc |
|---|---|---|---|
| Expt 1 | |||
| A27L+ D8L+ | 136 | 6.7 × 108 | 1 |
| A27L− D8L+ | 31 | 4.8 × 107 | 0.31 |
| A27L+ D8L− | 258 | 1.3 × 108 | 0.1 |
| A27L− D8L− | 283 | 9.3 × 107 | 0.066 |
| Expt 2 | |||
| A27L+ D8L+ | 136 | 1.8 × 109 | 1 |
| A27L− D8L+ | 20 | 9.6 × 107 | 0.36 |
| A27L+ D8L− | 254 | 2.5 × 108 | 0.07 |
| A27L− D8L− | 110 | 1.5 × 108 | 0.10 |
Number of virions counted by confocal microscopy as described in Materials and Methods. Three fields per sample were randomly photographed, and particle numbers were determined.
The aliquots of virus stocks used in particle determination were titered at the same time by plaque assays.
Calculated as (a/b for A27L+ D8L+)/(a/b for mutant virus) where a is the number of virion particles per field, b is the virus titer (in PFU per milliliter), and mutant virus is either A27L+ D8L−, A27L− D8L+, or A27L− D8L−.
One-step growth curve analysis.
BSC40 cells were infected with A27L+ D8L+, A27L− D8L+, A27L+ D8L−, or A27L− D8L− virus, respectively, at an MOI of 5 PFU per cell. Cells were cultured in the presence (for A27L+ D8L+ and A27L+ D8L− viruses) or absence (for A27L− D8L+ and A27L− D8L− viruses) of 5 mM IPTG and were harvested at various times (0, 2, 4, 8, 19, and 24 h) p.i. Virus IMV titers of each lysate were determined on BSC40 cells in the presence of 5 mM IPTG.
To determine the titers of EEV, medium was collected at 24 h p.i. after brief centrifugation, and a MAb (2D5) against IMV L1R protein (1:1,000) was added to block IMV contamination in the medium (27). The mixtures were put on BSC40 cells and incubated at 37°C for 1 h. Cells were then washed, and an agar-IPTG overlay was added for plaque determination.
Electron microscopy of virus morphogenesis.
BSC40 cells were infected with A27L+ D8L+, A27L− D8L+, A27L+ D8L−, or A27L− D8L− virus, respectively, at an MOI of 20 PFU per cell. These cells were fixed at 24 h p.i. in 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) at room temperature for 1 h and rinsed in three changes of the same buffer for 15 min each time. Cells were dehydrated with an ethanol series, and Spurr’s resin was used for infiltration and embedding (45). Sectioning was done with an Ultracut E ultramicrotome. Thin sections of 90 nm were stained with uranyl acetate and lead citrate and were subsequently analyzed with a Zeiss 902 transmission electron microscope (33).
RESULTS
Soluble vaccinia virus D8L protein bound to cell surface CS and blocked IMV binding to cells.
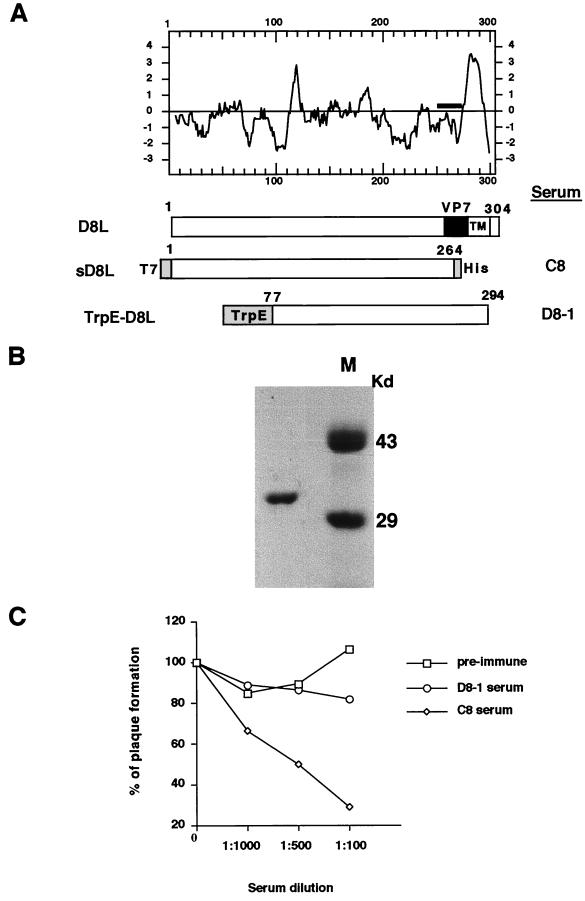
The extracellular domain region of D8L protein from amino acid (aa) 1 to aa 264 was cloned into a bacterial expression vector so that the fusion protein, soluble D8L, contains a T7 tag at the N terminus for identification and hexahistidine sequences at the C terminus (Fig. 1A). The construct was expressed in bacteria, and soluble D8L protein was purified through a nickel column (Fig. 1B). This soluble D8L protein was used for generation of a rabbit serum, C8. We obtained another antiserum, D8-1, previously raised from a denatured fusion protein, TrpE-D8L, that contains D8L sequences of aa 77 to 294 (26). Both rabbit sera, C8 and D8-1, were tested for neutralization activity in vaccinia virus infections (Fig. 1C). The C8 serum readily neutralized vaccinia virus infection of BSC40 cells, whereas preimmune and D8-1 sera had no effect. The lack of neutralization ability of D8-1 serum was probably due to the fact that it recognized only denatured epitopes. Thus, the results indicated that the C8 serum recognized a native structure of D8L protein and blocked virus infections.
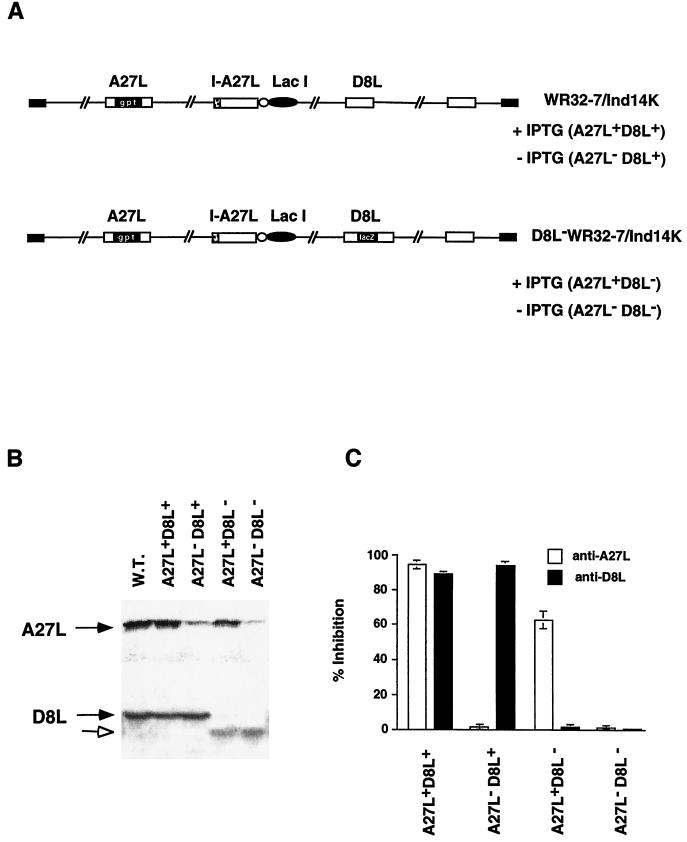
FIG. 1.
(A) Hydropathy plot of D8L amino acids translated from the DNA sequences (24) and diagrams of D8L, soluble D8L (sD8L), and TrpE-D8L. The D8L diagram shows the open reading frame from aa 1 to aa 304. TM, transmembrane region; VP7, aa sequences with homology to rotavirus glycoprotein VP7 (24). sD8L is the ectodomain of D8L protein fused with T7 and hexahistidine tag (His) sequences, shown as shaded areas at the N and C termini, respectively. TrpE-D8L contains D8L from aa 77 to aa 294 (26). Sera C8 and D8-1 were generated from sD8L and TrpE-D8L proteins, respectively (26). (B) Purified sD8L protein stained with Commassie blue on a sodium dodecyl sulfate–12% polyacrylamide gel. Protein markers (M) are shown. (C) Neutralization of vaccinia virus infections by antiserum C8. BSC40 cells were infected with wild-type vaccinia virus in the absence or in the presence of each serum at various dilutions as shown along the x axis. Cells were then washed, and 1% agar was added for plaque determination. These plaque assays were performed in duplicate, and the averages are presented. The number of plaques obtained from control cells without serum addition was around 150 PFU, which was taken as 100%. (D) sD8L protein blocked vaccinia virus early gene expression. BSC40 cells were mock infected (M) or infected with vMJ360 expressing lacZ at an MOI of 10 PFU per cell in the presence of various amounts of sD8L (0, 1, 10, or 50 μg) and were harvested at 2 h p.i. for β-Gal assays (8). (E) sD8L protein blocked vaccinia virus adsorption to cells. BSC40 cells were mock infected (M) or infected with vaccinia virus at an MOI of 10 PFU per cell at 4°C for 30 min. After a wash, these cells were immediately harvested, and the number of cell-associated virions was determined by plaque assays on BSC40 cells.
To determine if D8L protein plays any role in vaccinia virus entry, we used soluble D8L protein as a competitor during virus infection. Addition of soluble D8L protein interfered with vaccinia virus infection, and expression of a viral early marker gene was greatly reduced (Fig. 1D). Furthermore, virion binding assays revealed that soluble D8L protein blocked vaccinia virus infection at the adsorption stage (Fig. 1E). The inhibitory effect of D8L protein was not due to the T7 or the His tag sequences, since other proteins with identical tags had no effect on vaccinia virus binding (16). The above data thus indicated a role of D8L protein in vaccinia virus adsorption to cells, and the N-terminal region could be important for such a process.
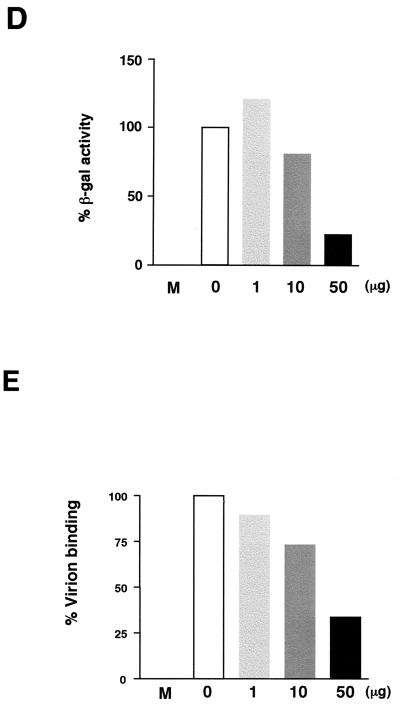
D8L protein was previously reported to bind to cells (22, 24). However, it was not clear what cell surface molecules it interacts with. To investigate the surface binding property, soluble D8L protein was biotinylated, incubated with BSC40 cells, and analyzed for cell surface staining with a FACS (Fig. 2). D8L protein bound to cells, and a significant shift in cell surface staining was detected (Fig. 2A through C). Soluble GAGs at various concentrations (10, 100, or 1,000 μg/ml) were added as competitors. Soluble CS revealed a dosage-dependent competition with D8L protein for binding to cells (Fig. 2A), whereas HP had no inhibitory effect at any concentration (Fig. 2C). DS showed no inhibition at the lower concentrations of 10 and 100 μg/ml and showed slight inhibition at 1,000 μg/ml (Fig. 2B). Since DS differs from CS due to epimerization of glucuronic acid at the C-5 position, the inhibition we observed may be due to incomplete epimerization of CS contaminating the commercial DS preparation (31, 42). These findings indicated that D8L protein binds to cell surface CS. To demonstrate that this binding specificity was unique to D8L protein, another biotinylated envelope protein, A27L, was tested in parallel as a control. Consistent with our previous data, binding of A27L protein was competed by HP (Fig. 2F) and not by CS (Fig. 2D) or DS (Fig. 2E) (6). We have also tested D8L protein binding to CS on other cell lines, such as HeLa and L cells, and the results were consistent with those for BSC40 cells (data not shown).
FIG. 2.
Soluble D8L protein bound to BSC40 cell surface CS. Live BSC40 cells were incubated with PBS (black lines) and biotinylated D8L (red lines in panels A through C) or A27L (red lines in panels D through F) protein alone and analyzed with a FACS as described elsewhere (16). Alternatively, biotinylated proteins were mixed with 10, 100, or 1,000 μg of soluble GAGs/ml and analyzed with a FACS, and the cell staining under each condition was shown as a green, blue, or orange line, respectively. The soluble GAGs used in competitions were CS (A and D), DS (B and E), and heparin (C and F).
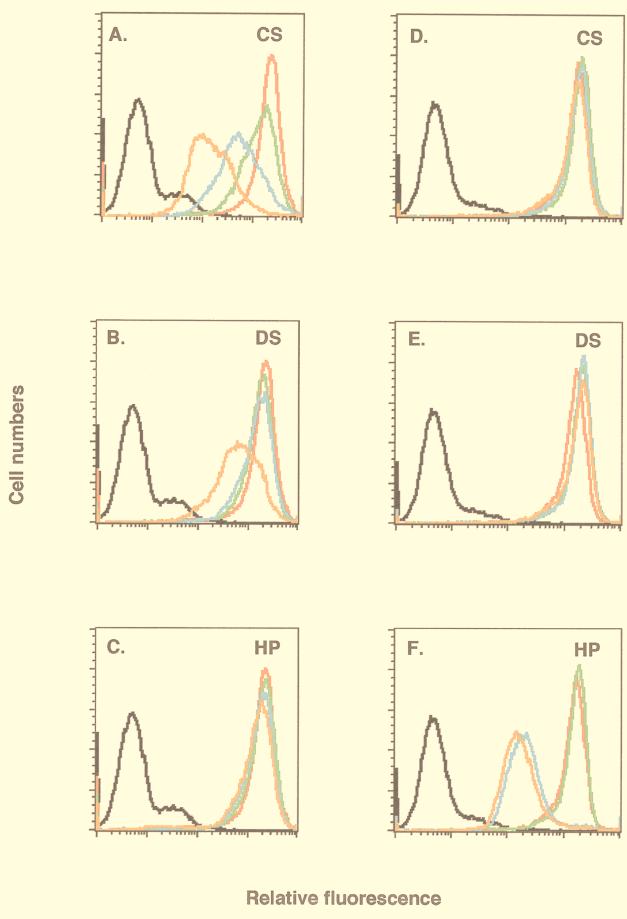
Although competition assays with soluble GAGs have been widely used in various cell binding assays, they are nevertheless indirect measurements of cell surface interactions. To provide direct evidence of D8L protein interaction with cell surface CS, we tested binding of D8L protein on cell lines expressing different GAGs (2, 13). Parental mouse L cells express both HS and CS on cell surfaces, whereas a mutant cell line selected from L cells, gro2C, expresses only CS (13). Another mutant cell line, sog9, was further selected from gro2C cells and expresses neither HS nor CS (2). These mutant cells have been useful for studies of binding of herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) and gC proteins to cell surface GAGs (3, 9).
D8L and A27L proteins were tested with these cells for their binding specificity (Fig. 3). Biotinylated D8L protein bound to L and gro2C cells to comparable extents (Fig. 3A and B). This indicated that expression of CS on gro2C cells is sufficient for D8L protein binding, and the lack of HS on gro2C cells did not reduce D8L protein binding. On the other hand, D8L protein bound poorly to sog9 cells, at a level similar to the background level (Fig. 3C). Comparison between D8L binding to gro2C cells and to sog9 cells indicated an essential role of CS in D8L protein binding. In summary, these results were consistent with the GAG competition data and directly demonstrated that D8L protein interacts with CS on cells.
FIG. 3.
Binding of soluble D8L protein to mutant cells defective in GAG expression. Biotinylated D8L protein (A through C) or A27L protein (D through F) was incubated with L (A and D), gro2C (B and E), or sog9 (C and F) cells as described for Fig. 2, and the surface staining (solid lines) was analyzed with a FACS. Dotted lines, background staining of cells in the absence of biotinylated proteins.
When biotinylated A27L protein was incubated with L, gro2C, and sog9 cells, it showed a binding pattern different from that of D8L protein (Fig. 3D through F). Strong staining of A27L protein was detected on L cells, but only weak staining was observed on gro2C and sog9 cells. This pattern was consistent with the ability of A27L protein to bind cell surface HS (6, 16). We thus concluded that both A27L and D8L proteins are vaccinia virus IMV envelope proteins that bind to cell surface GAGs. However, D8L protein demonstrated a preference to bind CS, whereas A27L protein binds to HS, on cells (6, 16).
Although GAGs are ubiquitously expressed on multiple cell types in vitro and in vivo, their distributions and saccharide modifications remain heterogeneous among different cell types (10). It is attractive to postulate that the fact that vaccinia virus contains envelope proteins capable of binding to different GAGs may broaden its host range, whereas mutant viruses defective in expression of these genes may be more restricted or attenuated in cell entry.
Construction of a double-mutant vaccinia virus defective in expression of both the A27L and the D8L protein.
Mutant viruses defective in A27L or D8L gene expression have been described previously (26, 36, 37, 41). A recombinant A27L− virus, WR32-7/Ind14K, that expresses A27L protein under IPTG regulation exhibited a small-plaque phenotype in the absence of IPTG (36, 37). Despite the difference in the plaque size, the titers of IMV produced by WR32-7/Ind14K were not affected by IPTG (37). Recombinant D8L− viruses have also been reported, and these mutant viruses formed normal plaques and exhibited no loss of IMV infectivity in cell cultures (26, 41). These data indicated that A27L and D8L proteins are dispensable for IMV growth in cell cultures. However, since vaccinia virus has two (and perhaps more) envelope proteins for binding to cell surface GAGs, the contribution of an individual protein in virus entry is difficult to assess by single-gene mutation due to functional redundancy. The absence of any one protein on virions does not eliminate functional virus attachment; instead, both proteins must be impaired in order to render the virus defective in binding to cell surface GAGs. Based on the above rationale, we constructed a mutant virus that is defective in both A27L and D8L gene expression in order to study the IMV entry process.
We obtained a recombinant virus, WR32-7/Ind14K, that expresses A27L protein under IPTG regulation (Fig. 4A) (36, 37). With IPTG, the virus behaved similarly to the wild-type virus with regard to expression of A27L and D8L proteins (A27L+ D8L+). Production of EEV and cell fusion, known to be dependent on A27L protein expression, were detected only upon the addition of IPTG in cells infected by WR32-7/Ind14K (37). In the absence of IPTG, this virus expressed no A27L protein and formed small plaques (A27L− D8L+). Despite the small-plaque phenotype, the A27L− D8L+ virus showed normal growth characteristics, similar to those of the A27L+ D8L+ virus, with no loss of IMV infectivity in cell cultures (37).
FIG. 4.
Characterization of A27L and D8L mutant viruses. (A) Schematic representations of four phenotypic viruses produced from the parental virus WR32-7/Ind14K and D8L− WR32-7/Ind14K virus. (B) Expression of A27L and D8L proteins in virus-infected cells. BSC40 cells were infected with wild-type (WT) vaccinia virus or with either WR32-7/Ind14K or D8L− WR32-7/Ind14K virus at an MOI of 10 in the presence (A27L+ D8L+ and A27L+ D8L−) or absence (A27L− D8L+ and A27L− D8L−) of 5 mM IPTG. Expression of A27L and D8L proteins was determined by Western blot analyses with sera (1:500) against A27L and D8L proteins, respectively. Solid arrows indicate the positions of A27L and D8L proteins. The open arrow indicates the truncated D8L protein. (C) Neutralization of A27L and D8L mutant viruses. BSC40 cells were infected with each of the mutant viruses shown in panel A in the absence or presence of antiserum (1:100) against A27L or D8L protein. Cells were washed and overlaid with agar, and plaque numbers were determined after 3 days. The plaque count obtained from virus infection without serum, around 150 plaques per 60 mm, was used as a control and taken as 100%. The values on the y axis represent percentages of inhibition and were calculated as [1 − (number of plaques obtained with serum/number of plaques obtained without serum)] × 100. Results shown here are averages from four experiments.
We therefore inactivated the D8L gene locus within the genome of WR32-7/Ind14K by inserting a lacZ gene cassette into the D8L locus by double recombination (Fig. 4A). The resulting mutant virus, D8L− WR32-7/Ind14K, expresses only A27L protein in the presence of IPTG (A27L+ D8L−) and expresses neither protein (A27L− D8L−) without IPTG.
Expression of A27L protein was compared in BSC40 cells infected with WR32-7/Ind14K and D8L− WR32-7/Ind14K viruses (Fig. 4B). Cells infected by either virus expressed A27L protein when IPTG was added to the medium (Fig. 4B) (37). Cells infected by the same viruses and cultured in the absence of IPTG expressed very little A27L protein (Fig. 4B).
Expression of D8L protein was also investigated in these cells. Full-length D8L protein of 32 kDa was detected in cells infected by the parental WR32-7/Ind14K virus, which contains an intact D8L gene (Fig. 4B). At the same time, a smaller, truncated D8L protein was detected in cells infected by D8L− WR32-7/Ind14K virus (Fig. 4B). Because the lacZ cassette was inserted into the BssHII site of the D8L gene, which is at position 736, the open reading frame upstream of the BssHII site presumably could encode a protein of 28 kDa. However, this truncated D8L protein does not contain the transmembrane region located downstream of the BssHII site and thus would not be expressed on the virion surface. This observation is similar to that described in a previous report when a D8L− mutant virus (v-ΔBssHII), in which the BssHII site was also used to generate a frameshift of the D8L gene, expressed a smaller, 28-kDa D8L protein (26). However, this altered D8L protein was not functional and did not affect the mutant phenotype as well (26).
To be certain that these viruses indeed exhibit the respective mutant phenotypes as we expected, we performed neutralization assays with antisera specific to the A27L and D8L proteins (Fig. 4C). Preimmune sera had no effect on titers of any of the four viruses (data not shown). A postimmune serum specific to A27L protein neutralized virions of the A27L+ D8L+ and A27L+ D8L− phenotypes (16). Mutant viruses harvested in the absence of IPTG did not express A27L protein and thus were resistant to this serum (A27L− D8L+ and A27L− D8L−). At the same time, a postimmune serum specific to D8L protein, C8, readily neutralized A27L+ D8L+ and A27L− D8L+ viruses. Viruses expressing the truncated D8L protein (A27L+ D8L− and A27L− D8L−) were resistant to the C8 serum, as expected. In contrast to A27L expression, which was regulated by IPTG, the neutralization effect on D8L protein was independent of IPTG. In summary, the sensitivities of these viruses to serum neutralization were consistent with the expression profile of the A27L and D8L envelope proteins.
Inactivation of D8L protein from vaccinia virus WR32-7/Ind14K reduced virus titers in cell cultures, and further removal of A27L protein revealed no additional effects.
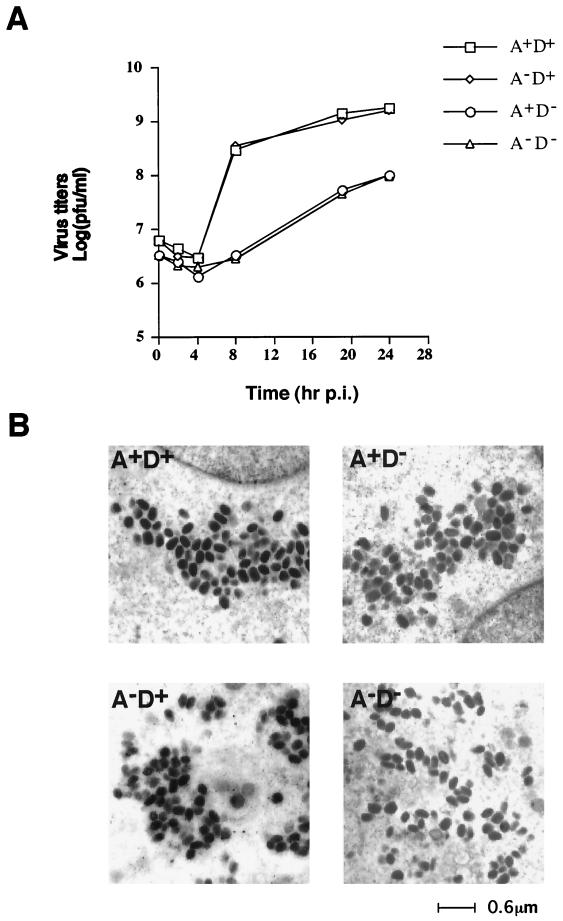
To monitor the growth characteristics of A27L and D8L mutant viruses in cell cultures, BSC40 cells were infected with each of the four viruses at an MOI of 5 PFU per cell. These infected cells were cultured in appropriate media as described in Materials and Methods to ensure that virion progenies maintained their respective phenotypes. The lysates were harvested after a single round of virus infection and were titrated for IMV production on BSC40 cells (Fig. 5A). The titers of A27L+ D8L+ and A27L− D8L+ viruses at 24 h p.i. were comparable, suggesting that inactivation of A27L protein expression had no effect. This was consistent with the previous report (37). However, the titers of A27L+ D8L− virus were 1 log unit lower than those of the A27L+ D8L+ control virus. This was surprising to us because in previous reports D8L− mutant viruses yielded progeny titers similar to those of wild-type viruses in cell cultures (26, 41). We have independently isolated another D8L− WR32-7/Ind14K mutant virus clone, and the results were identical (data not shown). Thus, it is unlikely that the difference in titers was due to extra mutations generated during recombinant virus isolation. Finally, the titers of A27L− D8L− virus appeared to overlap those of the A27L+ D8L− virus, with no further attenuation.
FIG. 5.
(A) One-step growth curve analysis of A27L and D8L mutant viruses. BSC40 cells were infected with each mutant virus at an MOI of 5 PFU per cell, and cells were cultured under appropriate conditions as described in Materials and Methods. Cell lysates were harvested at 0, 2, 4, 8, 19, and 24 h p.i., and virus titers on BSC40 cells in the presence of IPTG were determined. (B) Electron microscopy of ultrathin sections of BSC40 cells infected by mutant viruses at 24 h p.i. A, A27L; D, D8L.
Reduction of A27L+ D8L− and A27L− D8L− virus IMV titers was not due to a blockage of the virion assembly process.
There are two possible explanations for the significant reductions in IMV titers produced by A27L+ D8L− and A27L− D8L− viruses. It is possible that A27L+ D8L− and A27L− D8L− mutant viruses were defective at some stages in cell cultures, such as morphogenesis. Alternatively, A27L+ D8L− mutant viruses may have grown well but produced virion particles with lower infectivities than A27L+ D8L+ and A27L− D8L+ viruses. To differentiate between these two possibilities, we first tested if the titer reduction resulted from abnormal virion morphogenesis in these cells. Cells infected by the A27L+ D8L− and A27L− D8L− mutant viruses at 24 h p.i. often contained mature IMV virions that appeared compact, with dark staining (Fig. 5B). Immature virion structures were also observed in some of these infected cells, but the distribution of these intermediate virion structures was comparable to those seen in cells infected by A27L+ D8L+ and A27L− D8L+ viruses (data not shown). Furthermore, IMV virions were purified from these infected cells and directly analyzed by electron microscopy. The A27L+ D8L+ virions had a brick-like shape and a width of roughly 200 nm. The other three mutant virions, A27L+ D8L−, A27L− D8L+, and A27L− D8L−, also had comparable shapes and sizes (data not shown). We therefore concluded that the reductions in the titers of A27L+ D8L− and A27L− D8L− viruses were not due to IMV assembly defects.
Reductions in A27L+ D8L− and A27L− D8L− IMV titers were not due to EEV.
Although it is unlikely, EEV released from the infected cells could have reinfected the same cultures and influenced the IMV titers we observed in Fig. 5. We therefore collected the medium at 24 h p.i. and determined the titers of EEV produced from these infected cells (Table 1). The EEV titers produced from cells infected by A27L+ D8L+ and A27L+ D8L− viruses were highest, roughly two- to threefold higher than those for A27L− D8L+ virus and sixfold higher than those for A27L− D8L− virus. The reduction in EEV titers from cultures infected by A27L− D8L+ and A27L− D8L− viruses was consistent with a role for A27L protein in EEV wrapping (36). Most importantly, the titers of IMV from these four cultures did not correlate with the respective titers of EEV. For example, A27L+ D8L− virus produced high titers of EEV, similar to those with A27L+ D8L+ virus, but the IMV yields were 10-fold lower. We therefore concluded that the EEV produced from these cultures did not contribute to the IMV yield difference.
TABLE 1.
Production of IMV and EEV in BSC40 cells infected by A27L and D8L mutant virusesa
A27L+ D8L− and A27L− D8L− viruses produced IMV of low infectivity.
The above results raised the possibility that the low IMV titers of A27L+ D8L− and A27L− D8L− viruses produced in one-step growth analysis could reflect progeny particles with low infectivity. We directly compared the infectivities of these mutant IMV virions. All four IMV virions were purified, and the numbers of virion particles were determined by particle counting under a confocal microscope (49). The biological titers of infectious units of these viruses were simultaneously determined by plaque assays on BSC40 cells. Comparison of the number of virion particles required to form a plaque provides an index of infectivity of these IMV virions. We have assigned the control A27L+ D8L+ virus infectivity a value of 1, and the relative infectivities of the other three viruses are shown in Table 2. Analysis by confocal microscopy showed that A27L+ D8L+ virus, which had higher biological titers, contained fewer particles. A27L− D8L+ mutant viruses had intermediate infectivity, with a slight, threefold reduction. A27L+ D8L− and A27L− D8L− mutant viruses, on the other hand, contained more virion particles and had the lowest titers. The relative infectivities of A27L+ D8L− and A27L− D8L− mutant virions were only 7 to 10% of that of A27L+ D8L+ virions, i.e., an average 10- to 14-fold reduction in virion infectivity. These results suggested that D8L protein acts as an important determinant for virion infectivity.
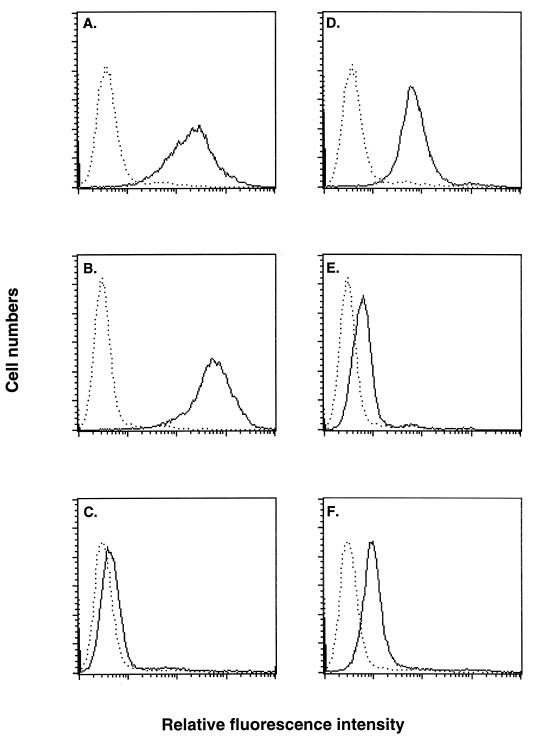
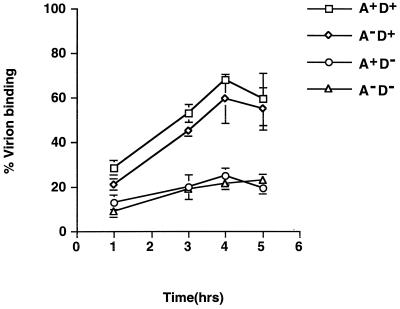
D8L protein affects the infectivity of WR32-7/Ind14K IMV at the cell binding step.
To investigate the mechanism that renders these IMV particles less infectious, we performed virion binding assays with these mutant viruses (Fig. 6). The abilities of these mutant viruses to bind to cells differed. Both A27L+ D8L+ and A27L− D8L+ virions bound to cells to a comparable extent. However, they both bound to cells better than A27L+ D8L− and A27L− D8L− virions. The binding data were all consistent with the infectivity assay results and indicated an important role of D8L protein in virion adsorption. There was a slight increase in virion binding to cells when A27L protein was expressed on virions, but the difference was not significant, consistent with all previous data (34, 35). In summary, the data suggested that the loss of D8L protein reduced virion adsorption to cells and, consequently, resulted in virions of low infectivity.
FIG. 6.
Virion binding assays for A27L (A) and D8L (D) mutant viruses. BSC40 cells were infected with each mutant virus at an MOI of 5 PFU per cell at 4°C for various times and then washed with cold PBS, and cell-associated virions were determined by plaque assays on BSC40 cells in the presence of IPTG.
DISCUSSION
The amino acid sequences of D8L protein show homology with cellular carbonic anhydrase, although D8L protein does not contain any detectable enzymatic activity (24). In addition, a region with short homology to the attachment glycoprotein VP7 of rotaviruses exists on D8L protein near the C-terminal transmembrane region (26). However, the significance of these homologies is not clear. In addition, D8L protein is dispensable for cell culture, since D8L− mutant virus forms plaques of normal size (26, 41). These data indicated that D8L protein plays no important role in cell culture systems.
In the present study, we found that vaccinia virus D8L protein binds to cell surface CS and is involved in virus entry. The evidence came from several experiments. First of all, binding of soluble D8L protein to cells was competed by CS. In addition, D8L protein bound poorly to mutant cells defective in CS expression. Furthermore, soluble D8L protein blocked the binding of vaccinia virus to cells. Finally, inactivation of D8L expression by vaccinia virus WR32-7/Ind14K resulted in a 10-fold reduction in IMV titers in cell culture growth. The reduction in IMV titers was a specific effect of D8L protein, since removal of A27L protein expression by withdrawal of IPTG from the culture medium did not change IMV titers.
In one-step growth experiments as described above, BSC40 cells were infected with each of four mutant viruses at identical MOIs of 5 PFU per cell so that the cell populations in all four situations were equally infected, regardless of how many particles were initially put on cells at the beginning of infection. After entering cells, all four mutant viruses finished one round of the life cycle and produced comparable amounts of IMV progeny particles. When these particles were harvested and titered on BSC40 cells, the resulting plaques of D8L− WR32-7/Ind14K viruses, including A27L+ D8L− and A27L− D8L− viruses, were 10 times fewer than those of A27L+ D8L+ and A27L− D8L+ WR32-7/Ind14K viruses, suggesting that D8L− WR32-7/Ind14K virus required more particles to initiate a plaque formation. Indeed, this interpretation was confirmed by the infectivity analysis for which results are shown in Table 2, which demonstrated that D8L− WR32-7/Ind14K mutant viruses were 10 times less infectious than their D8L+ counterparts. In summary, these results revealed that D8L protein is important for IMV virion infectivity in BSC40 cells. Since GAGs are ubiquitously expressed in vitro and in vivo, we may expect that the results obtained with BSC40 cells will be applicable to other cell lines.
We demonstrated that the low infectivity of D8L− WR32-7/Ind14K IMV virions was partly due to inefficient binding of virions to BSC40 cells. Since we detected only a threefold difference in virion binding, these mutant virions may have additional defects in postbinding fusion that account for the remainder of the infectivity loss. Resolution of this issue awaits further investigation.
The effect of D8L protein on IMV virion infectivity that we obtained is different from the findings of previous studies, which indicated that inactivation of D8L protein had no effect on IMV infectivity (26, 41). Previous studies introduced a frameshift mutation into the open reading frame of the D8L gene, whereas we inserted a lacZ cassette into the D8L locus. The direction of lacZ transcription was the same as that of the endogenous D8L gene in order to allow minimal perturbation of gene alignment on the viral genome. It is possible that insertion of the lacZ cassette somehow exerted a polar effect on the expression of other genes adjacent to the D8L locus, such as D7R and D9R (11). However, D7R encodes a subunit of viral RNA polymerase, and a mutation of D7R was reported to be lethal, which does not explain the phenotype of our D8L− mutants (7). Thus, we think it unlikely that the phenotype difference was due to technical problems. Alternatively, the phenotype difference could be due to genetic deletions of virus genomes. Previous D8L− mutants were generated from wild-type vaccinia virus, whereas the D8L− mutant we worked with was from a different parental virus, WR32-7/Ind14K (36, 37). WR32-7/Ind14K was derived from vaccinia virus 48-7, which was reported to have some deletions in the left end of the viral genome (29, 36). Initially, we were not concerned about the difference because the published results indicated that the deleted region was nonessential for vaccinia virus growth in cell cultures (28, 29). In addition, the growth characteristics and A27L expression of WR32-7/Ind14K virus were indistinguishable from those of the wild-type virus (36, 37). Only after we inactivated expression of the D8L gene of WR32-7/Ind14K virus did we find that the resulting D8L− mutant behaved differently from the same D8L− mutant built on a wild-type virus background. This surprising result raised the interesting possibility that the deleted viral genomic sequences in WR32-7/Ind14K encode a protein that also binds to CS. This hypothesis of gene redundancy would posit that the D8L− mutant generated from the wild-type virus backbone remained fully infectious because of functional complementation. However, when these sequences were deleted from the WR32-7/Ind14K virus, inactivation of D8L expression seriously impaired virus infectivity, since no other gene could compensate for D8L functions. Similar observations were reported for the HS-binding proteins, gB and gC, on HSV (4, 14). gC is the principal HS-binding protein on HSV-1, whereas binding of the virus to cells became gB dependent only when gC was inactivated (4). Another possible mechanism of gene redundancy is that the deleted region encodes an HS-binding protein that normally binds more strongly to cells and “masks” CS interactions. This is based on previous studies of HSV-1 that bound to cell surface GAGs; binding of HSV through CS became evident only when the HS was removed (3). Regardless which mechanism is operative, D8L− WR32-7/Ind14K mutant virus revealed a previously unknown situation in which D8L protein becomes important in vaccinia virus entry. Currently, boundaries of these deletions in WR32-7/Ind14K are not molecularly defined, and at least the HindIII C fragment appeared to be missing (data not shown). Precise mapping of the deleted regions and identification of viral genes within those regions will help us clarify the difference in the future.
Even with a 10-fold drop in IMV infectivity, the D8L− WR32-7/Ind14K mutant viruses (both A27L+ D8L− and A27L− D8L− phenotypes) were nevertheless viable. This finding indicated that, besides A27L and D8L proteins, other viral proteins exist to mediate virus-cell interactions. Other viruses, such as HSV, are known to contain two proteins, gB and gC, for GAG binding (15, 25, 44, 46). Considering the broad host range of vaccinia virus, it would not be surprising to discover that vaccinia virus has evolved multiple envelope proteins for interaction with different GAGs on cells, including HS and CS.
In summary, for the understanding of IMV entry into mammalian cells, identification of viral and cellular components important for vaccinia virus binding and fusion is only the beginning. Further exploration of the biochemical interactions of these molecules will allow us to discover the mechanisms and obtain new insights into vaccinia virus entry.
ACKNOWLEDGMENTS
We thank E. Nile for plasmid p770, rabbit serum D8-1, and helpful suggestions on our manuscript; G. Smith for the virus WR32-7/Ind14K; and F. Tufaro for L, gro2C, and sog9 cells. We also thank Sue-Ping Lee for excellent techniques in electron microscopy.
This work is supported by grants from Academia Sinica and the National Science Council (NSC88-2311-B-001-103) of the Republic of China.
REFERENCES
- 1.Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- 2.Banfield B W, Leduc Y, Esford L, Schubert K, Tufaro F. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J Virol. 1995;69:3290–3298. doi: 10.1128/jvi.69.6.3290-3298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banfield B W, Leduc Y, Esford L, Visalli R J, Brandt C R, Tufaro F. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology. 1995;208:531–539. doi: 10.1006/viro.1995.1184. [DOI] [PubMed] [Google Scholar]
- 4.Byrne K M, Horohov D W, Kousoulas K G. Glycoprotein B of bovine herpesvirus-1 binds heparin. Virology. 1995;209:230–235. doi: 10.1006/viro.1995.1248. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung C S, Hsiao J C, Chang Y S, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condit R C, Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981;113:224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- 8.Davison A J, Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989;210:749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- 9.Dyer A P, Banfield B W, Martindale D, Spanner D-M, Tufaro F. Dextran sulfate can act as an artificial receptor to mediate a type-specific herpes simplex virus infection via glycoprotein B. J Virol. 1997;71:191–198. doi: 10.1128/jvi.71.1.191-198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evered D, Whelan J, editors. Ciba Foundation symposium. 124. Functions of the proteoglycans. Chichester, United Kingdom: John Wiley & Sons; 1986. [Google Scholar]
- 11.Goebel S J, Johnson G P, Perkus M E, Davis S W, Winslow J P, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- 12.Gong S C, Lai C F, Esteban M. Vaccinia virus induces cell fusion at acid pH, and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990;178:81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- 13.Gruenheid S, Gatzke L, Meadows H, Tufaro F. Herpes simplex virus infection and propagation in a mouse L cell mutant lacking heparan sulfate proteoglycans. J Virol. 1993;67:93–100. doi: 10.1128/jvi.67.1.93-100.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold B C, Visalli R J, Susmarski N, Brabdt C R, Spear P G. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 15.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao J-C, Chung C-S, Chang W. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. J Virol. 1998;72:8374–8379. doi: 10.1128/jvi.72.10.8374-8379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichihashi Y, Dales S. Biogenesis of poxviruses: interrelationship between hemagglutinin production and polykaryocytosis. Virology. 1971;46:533–543. doi: 10.1016/0042-6822(71)90057-2. [DOI] [PubMed] [Google Scholar]
- 18.Ichihashi Y, Oie M. Neutralizing epitope on penetration protein of vaccinia virus. Virology. 1996;220:491–494. doi: 10.1006/viro.1996.0337. [DOI] [PubMed] [Google Scholar]
- 19.Ichihashi Y, Takahashi T, Oie M. Identification of a vaccinia virus penetration protein. Virology. 1994;202:834–843. doi: 10.1006/viro.1994.1405. [DOI] [PubMed] [Google Scholar]
- 20.Jensen O N, Houthaeve T, Shevchenko A, Cudmore S, Ashford T, Mann M, Griffiths G, Krijnse Locker J. Identification of the major membrane and core proteins of vaccinia virus by two-dimensional electrophoresis. J Virol. 1996;70:7485–7497. doi: 10.1128/jvi.70.11.7485-7497.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joklik W K. The purification of four strains of poxvirus. Virology. 1962;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- 22.Lai C F, Gong S C, Esteban M. The 32-kilodalton envelope protein of vaccinia virus synthesized in Escherichia coli binds with specificity to cell surfaces. J Virol. 1991;65:499–504. doi: 10.1128/jvi.65.1.499-504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai C F, Gong S C, Esteban M. Structural and functional properties of the 14-kDa envelope protein of vaccinia virus synthesized in Escherichia coli. J Biol Chem. 1990;265:22174–22180. [PubMed] [Google Scholar]
- 24.Maa J S, Rodriguez J F, Esteban M. Structural and functional characterization of a cell surface binding protein of vaccinia virus. J Biol Chem. 1990;265:1569–1577. [PubMed] [Google Scholar]
- 25.Navarro D, Paz P, Pereira L. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992;186:99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- 26.Niles E G, Seto J. Vaccinia virus gene D8 encodes a virion transmembrane protein. J Virol. 1988;62:3772–3778. doi: 10.1128/jvi.62.10.3772-3778.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oie M, Ichihashi Y. Neutralizing epitope on penetration protein of vaccinia virus. Virology. 1996;220:491–494. doi: 10.1006/viro.1996.0337. [DOI] [PubMed] [Google Scholar]
- 28.Paez E, Dallo S, Esteban M. Virus attenuation and identification of structural proteins of vaccinia virus that are selectively modified during virus persistence. J Virol. 1987;61:2642–2647. doi: 10.1128/jvi.61.8.2642-2647.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paez E, Esteban M. Stability of vaccinia virus DNA during persistent infections: accumulation of left-end deletions and of tandem repeats at both ends of the viral genome and prevention by interferon. Virology. 1988;163:145–154. doi: 10.1016/0042-6822(88)90241-3. [DOI] [PubMed] [Google Scholar]
- 30.Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978;27:28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poole A R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986;236:1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravanello M P, Hruby D E. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virion assembly. J Virol. 1994;68:6401–6410. doi: 10.1128/jvi.68.10.6401-6410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds E. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;55:541–552. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez D, Rodriguez J R, Esteban M. The vaccinia virus 14-kilodalton fusion protein forms a stable complex with the processed protein encoded by the vaccinia virus A17L gene. J Virol. 1993;67:3435–3440. doi: 10.1128/jvi.67.6.3435-3440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez J F, Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J Virol. 1987;61:3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez J F, Smith G L. Inducible gene expression from vaccinia virus vectors. Virology. 1990;177:239–250. doi: 10.1016/0042-6822(90)90477-9. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez J F, Smith G L. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 1990;18:5347–5351. doi: 10.1093/nar/18.18.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez J F, Paez E, Esteban M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987;61:395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez J R, Risco C, Carrascosa J L, Esteban M, Rodriguez D. Characterization of early stages in vaccinia virus membrane biogenesis: implications of the 21-kilodalton protein and a newly identified 15-kilodalton envelope protein. J Virol. 1997;71:1821–1833. doi: 10.1128/jvi.71.3.1821-1833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez J R, Risco C, Carrascosa J L, Esteban M, Rodriguez D. Vaccinia virus 15-kilodalton (A14L) protein is essential for assembly and attachment of viral crescents to virosomes. J Virol. 1998;72:1287–1296. doi: 10.1128/jvi.72.2.1287-1296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez J-R, Rodriguez D, Esteban M. Insertional inactivation of the vaccinia virus 32-kilodalton gene is associated with attenuation in mice and reduction of viral gene expression in polarized epithelial cells. J Virol. 1992;66:183–189. doi: 10.1128/jvi.66.1.183-189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg L C, Choi H U, Poole A R, Lewandowska K, Culp L A. Biological roles of dermatan sulphate proteoglycans. Ciba Found Symp. 1986;124:47–68. doi: 10.1002/9780470513385.ch4. [DOI] [PubMed] [Google Scholar]
- 43.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 45.Spurr A. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 46.Svennerholm B, Jeansson S, Vahlne A, Lycke E. Involvement of glycoprotein C (gC) in adsorption of herpes simplex virus type 1 (HSV-1) to the cell. Arch Virol. 1991;120:273–279. doi: 10.1007/BF01310482. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi T, Oie M, Ichihashi Y. N-terminal amino acid sequences of vaccinia virus structural proteins. Virology. 1994;202:844–852. doi: 10.1006/viro.1994.1406. [DOI] [PubMed] [Google Scholar]
- 48.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia viruses and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- 49.Vanderplasschen A, Smith G L. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J Virol. 1997;71:4032–4041. doi: 10.1128/jvi.71.5.4032-4041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vazquez M-I, Rivas G, Cregut D, Serrano L, Esteban M. The vaccinia virus 14-kilodalton (A27L) fusion protein forms a triple coiled-coil structure and interacts with the 21-kilodalton (A17L) virus membrane protein through a C-terminal α-helix. J Virol. 1998;72:10126–10137. doi: 10.1128/jvi.72.12.10126-10137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolffe E J, Vijaya S, Moss B. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology. 1995;211:53–63. doi: 10.1006/viro.1995.1378. [DOI] [PubMed] [Google Scholar]