Abstract
Brain electrical stimulation, particularly non-invasive brain stimulation (NIBS) techniques such as transcranial electrical stimulation (tES), have emerged as a promising treatment for various psychiatric disorders, including depression, anxiety, and post-traumatic stress disorder. tES techniques, such as transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and transcranial random noise stimulation (tRNS), are cost-effective and safe interventions that are designed to affect neuronal circuits in the brain using various modalities. Although tES has shown effectiveness in the treatment of psychiatric disorders, there is a lack of comprehensive papers that consider its clinical implications. Therefore, this review aims to evaluate the clinical implications of tES and provide practical guidance for the treatment of psychiatric illnesses. Moreover, this review provides an overview of tES techniques and their mechanisms of action and summarizes recent clinical studies that have examined the use of tES for psychiatric disorders.
Keywords: Transcranial electrical stimulation, Transcranial direct current stimulation, Transcranial alternating current stimulation, Transcranial random noise stimulation
INTRODUCTION
Brain electrical stimulation is a pre-eminent treatment for patients with various psychiatric disorders, such as depression [1], anxiety [2], and post-traumatic stress disorder [3]. Specifically, many studies have focused on non-invasive brain stimulation (NIBS) based on the concept of neuroplasticity, an ability of the brain to change that occurs in response to intrinsic or extrinsic experience. The most commonly used NIBS in mental health is transcranial electrical stimulation (tES) [4,5].
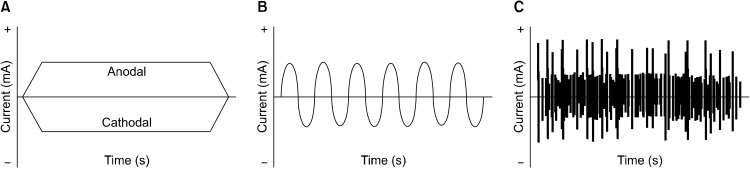
tES is designed to affect neuronal circuits in the brain using multiple modalities including constant currents and alternating currents (Fig. 1): transcranial direct current stimulation (tDCS), transcranial altering current stimulation (tACS), and transcranial random noise stimulation (tRNS). In particular, tDCS is the most widely used technique, which delivers low-intensity direct currents (∼1−2 mA) to the scalp through several electrodes. Additionally, tACS delivers sinusoidal current to the scalp in order to examine cortical oscillation patterns [6,7] and tRNS delivers fluctuating currents at frequencies ranging from 0.1 to 640 Hz [8]. These techniques are characterized by relatively greater cost-effectiveness and adaptability, especially in the level of safety than other interventions.
Fig. 1.
Various types for transcranial electrical stimulation (tES). (A) Transcranial direct current stimulation (tDCS) delivers direct and monopolar current which fades in/fades out at the beginning and end of the stimulation until the desired intensity is achieved. (B) Transcranial alternating current stimulation (tACS) delivers biphasic sinusoidal current alternates between positive and negative voltages and enables entrainment of brains’ endogenous oscillations. (C) Transcranial random noise stimulation (tRNS) delivers fluctuating currents within a specific range.
Furthermore, tES is useful in neuroscience research as well as the treatment of psychiatric disorders. Specifically, the effectiveness of tES has been found in depressed patients who do not respond to traditional medications or psychotherapies in recent studies [9]. Despite its importance, there have been fewer comprehensive papers considering the clinical implication of tES. Therefore, the aim of this review is to evaluate the clinical implication of tES as well as practice for the treatment of psychiatric illness.
tDCS
The Basis of tDCS: Mechanism
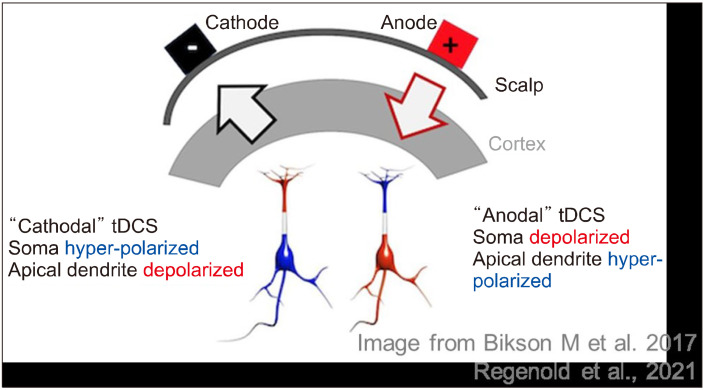
Direct current (DC) stimulation to the scalp is used to stimulate neuronal activity by depolarizing or hyperpolarizing the membrane potential [10-12]. The anode placed on the cortex depolarizes the basal dendrites and hyperpolarizes the apical dendrites, while the cathode depolarizes the apical dendrites and hyperpolarizes the basal dendrites (Fig. 2) [13]. In other words, neuronal excitability is increased by anodal tDCS while decreased by cathodal tDCS [14]. This implies that anodal current leads to an increase in alertness, mood, and motor activity, whereas cathodal current results in calmness and apathy [15]. However, unlike the primary effect, neuronal membrane polarization cannot sufficiently explain the after-effects of tDCS. Considering that dextromethorphan, an antagonist of the N-Methyl-D-aspartate (NMDA) receptor, blocks the after-effects of tDCS, it may depend on NMDA receptor sensitivity [16].
Fig. 2.
Mechanism of transcranial direct current stimulation (tDCS).
tDCS Electrode Placement: Montages
One or more anodal and cathodal electrodes have been used to deliver current, with one electrode positioned over the target area and the other placed on either another area within the cranial (intracephalic) or extracranial (extracephalic) area on the body [17]. The multiple arrangements of the anode and cathode electrode on the scalp are referred to as tDCS montages. Several studies have been conducted using various electrode placements, among which most of the tDCS research is conducted targeting the motor cortex and dorsolateral cortex [17].
Generally, it has been suggested that M1 stimulation is probably effective on Parkinson’s disease, other movement disorders, motor stroke, and especially physical pain [18]. Specifically, in studies related to chronic or acute pain, anodal stimulation was administered to the primary motor cortex (M1) of the hemisphere opposite the site of pain. The cathodal stimulation was applied to the contralateral supraorbital region [18]. This M1 stimulation can alleviate pain by activating different neural circuits within the precentral gyrus [18]. These circuits may involve connections with structures responsible for the sensory and emotional aspects of pain processing, such as the thalamus or the dorsolateral prefrontal cortex (DLPFC) [19]. Thus, tDCS can be used to treat several symptoms targeting the regions associated with motor-sensory functions.
Furthermore, the anode electrode is commonly placed over the left DLPFC, and the cathode electrode over the contralateral supraorbital area as a reference electrode [18]. This electrode placement montage has been shown to improve mood, alleviate anxiety, and reduce depressive symptoms [20]. Moreover, it has been illustrated that by targeting the DLPFC region, tDCS can improve cognitive and behavioral skills in healthy individuals [17]. For example, the anodal electrode positioned over the left DLPFC and the cathodal electrode over the right orbital PFC improves selective attention and working memory [21].
Multifocal tDCS and Multichannel tDCS: Multisite Stimulation
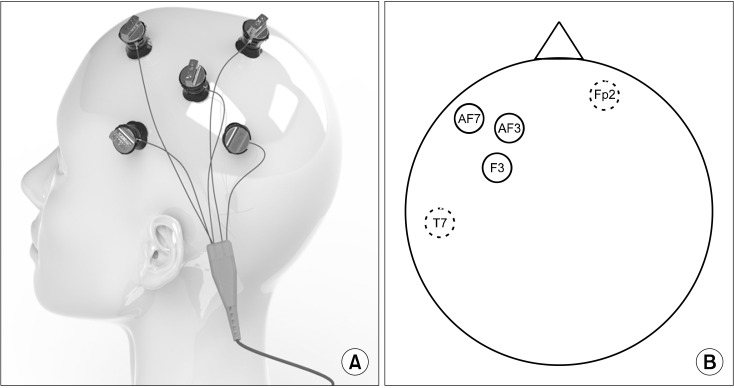
Multifocal or multichannel tDCS refers to an electrical stimulation technique to target multiple regions of the brain simultaneously (Fig. 3) [22]. The multifocal (multisite) montages can be employed to more comprehensively modulate several brain networks and regulate brain activity and excitability [23]. Multiple electrodes with varying intensities and polarities are utilized to specifically target a distributed brain network, resulting in potentially higher modulatory efficacy compared to a similar two-electrode tDCS approach, particularly in younger samples [24]. The montages are placed in a circular or rectangular configuration on the scalp. These newer specialized stimulation techniques may have enhanced neuromodulation [25].
Fig. 3.
Multichannel transcranial direct current stimulation (tDCS) mon-tages. (A) Multichannel tDCS device. Image courtesy of Soterix Medical, Inc. (www.soterixmedical.com). (B) Solid line circles represent anodal electrodes and dotted line circles represent reference electrodes.
For example, high-definition tDCS (HD-tDCS) is a technically advanced form of tDCS, which is more localized, precise, and longer-lasting [26]. HD-tDCS utilizes multiple smaller-sized electrodes and electrode montages to limit the spread of current flow outside of the targeted stimulated region [26,27]. HD-tDCS also comprises anode and cathode, where the most commonly used montage is the 4 × 1-ring configuration widely adopted in studies [28]. Specifically, one active electrode is placed on the area of interest, and four return electrodes on the surroundings [29]. An advantage of using a 4 × 1-ring configuration in HD-tDCS is its ability to selectively stimulate the specific brain area directly beneath the electrodes, where the current enters through the central electrode and is collected across the remaining four electrodes [27]. The primary advantage of HD-tDCS lies in its focused delivery of the current to the target site.
HD-tDCS has several advantages over conventional tDCS. First, it has higher spatial resolution and precision compared to conventional tDCS, characterized by its lower spatial resolution as the stimulation is typically based on a broader cortical region [27]. Second, in comparison to conventional tDCS, HD-tDCS demonstrated more focused cortical effects. For example, HD-tDCS resulted in a more pronounced reduction in excitability and a longer-lasting reduction in excitability, meaning that it induces polarity-dependent plasticity stimulation in the motor cortex of healthy individuals [26]. Lastly, HD-tDCS has better tolerability, which is an important factor when administering any type of intervention. Specifically, a pilot study supports the tolerability of HD-tDCS on pain perception [30].To ascertain the beneficial effects of multifocal tDCS, however, it is necessary to conduct more controlled studies on a larger sample.
Practice: Why is tDCS, not FDA Approved?
According to the United States Food and Drug Admin-istration (FDA), tDCS is currently not an approved treatment since there is limited evidence to support its safety and effectiveness for most uses. First, although several previous studies have examined that the adverse effects of tDCS are mild [31], most reviews did not systematically assess these side effects. There are still concerns regarding potential adverse effects and long-term safety [31]. Addi-tionally, it might be difficult to determine the appropriate level of regulation due to the uncertain potential benefits and risks to consumers. The effectiveness of tDCS for various clinical conditions is still debated, with some studies reporting positive results and others reporting inconclusive or negative results [32]. Finally, it might be easy to misuse tDCS resulting in lower efficacy or side effects [31]. Given that tDCS devices are available for home use, users need to have accurate information about the proper administration and potential risks of tDCS. Further research is needed to fully understand the potential benefits and limitations of tDCS.
tDCS in Depression
Major depressive disorder (MDD) is a severe mental disorder that drastically interferes with daily life. Although many effective treatments were used for patients with MDD, several studies found that there were inadequate responses to antidepressants or no responses to specific treatments including electroconvulsive therapy [33]. Specifically, about 50% of patients with MDD reported insufficient responses to an individual antidepressant trial [34]. Moreover, even about 10% of patients with MDD showed severe cognitive dysfunction without remission [35]. Accordingly, there is a need for other therapies or combinations for patients with MDD to increase the effect of antidepressants or psychotherapy.
Recently, tDCS is increasingly being investigated as a possible effective treatment. It is a useful method characterized to be non-invasive, reliable, and relatively simple with little side effects. Previous studies indicate that tDCS applied to patients with MDD, resulting in lower depression scores, lower dropout rates, higher response rates, and higher remission rates. The efficacy of tDCS on MDD can be influenced depending on depression subtypes, severity, and montages. The rationale for the use of tDCS in MDD is based on the studies using transcranial magnetic stimulation (TMS) in patients with depression and neuroimaging studies examining structural and functional changes in brain regions. Being contingent on the findings that the left DLPFC is underactive but the right DLPFC is hyperactive in depressive patients, tDCS has targeted excitatory stimulation of the left DLPFC and the inhibitory stimulation of the right DLPFC.
Moreover, tDCS is often combined with other psychological and pharmacological treatments in clinical practice. There was some evidence that combining a cognitive behavioral intervention with tDCS improves depressive symptoms. For example, a combination of tDCS and cognitive control training (CCT) sustained antidepressant effects at three weeks of follow-up [36]. This study was conducted using a three-arm randomized sham-controlled design to compare the therapeutic effects of five sessions. It investigated the effect of either 2 mA tDCS + CCT, sham tDCS + CCT, or sham CCT + 2 mA tDCS in participants with MDD. In contrast to other participants, those who received tDCS + CCT exhibited a further reduction in depression scores, measured by the Montgomery–Asberg Depression Rating Scale, which were significantly lower compared to their pretreatment levels after three weeks of follow-up [36].
According to the systematic review, the combination of tDCS with medications was more stable and useful in the treatment of depression, having a large effect size for depression score and a 2.7 times greater response rate than the sham group [37]. Brunoni and colleagues [38] examined that the combined treatment of sertraline with tDCS was better than monotherapy with sertraline or tDCS. In this study, the participants were given a six-week treatment that involved administering 2 mA of anodal stimulation to the left prefrontal cortex and cathodal stimulation to the right prefrontal cortex, along with the administration of sertraline hydrochloride. As a result, only the combined treatment group showed a significant improvement at 2 weeks and those reported higher response rates than groups with sertraline + sham tDCS.
One possible explanation for this effect might involve the influence of tDCS on cortical structures, leading to network-related alterations in deeper brain regions like the thalamus, amygdala, and striatum; antidepressant affects serotonergic and noradrenergic structures in the brainstem and their connections to the amygdala and ventral striatum [39]. It has been suggested that compared to monotherapy, combined treatments might affect corticolimbic neuronal circuits related to several psychiatric disorders. Therefore, tDCS is one of the promising therapeutics as a potential method for treatment-resistant patients, especially. Most patients with psychiatric disorders cannot adequately respond to interventions such as antidepressants or psychotherapy. tDCS combined with cognitive-emotional training may be more effective in medication-resistant patients.
tDCS in Anxiety Disorder
Anxiety disorder is also a pervasive mental disorder that includes generalized anxiety disorder (GAD), panic disorder, social anxiety disorder (SAD), agoraphobia, and specific phobia according to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) [40]. Although the treatment of anxiety disorder is also based on pharmacological and cognitive behavior therapy, these interventions would have side effects or less treatment efficacy.
tDCS is one of the alternative methods that modulate anxiety levels, which has been commonly used to regulate trait anxiety, an essential aspect of all anxiety disorders. In one study [41], individuals with trait anxiety were required to carry out an attentional task disregarding threatening face distractors following tDCS. Additionally, the participants engaged in an functional magnetic resonance imaging emotional task involving facial expressions displaying fear or neutrality after tDCS ends. The result indicated that the influence of threatening stimulus on the accuracy of attentional tasks was reduced after tDCS. These effects were accompanied by reduced activity of the amygdala, which is relevant for fear induction, through alteration of DLPFC activity [41].
While it has been established that an imbalance of activities between the bilateral DLPFC is characteristic of patients with MDD [42], anxiety symptoms have also been related to an imbalance in activity between the bilateral DLPFC, specifically manifested as decreased activity in the left and increased activity in the right [43,44]. The reduced activity in the right DLPFC is associated with negative emotional judgment, whereas the increased activity is linked to attentional modulation [43]. Considering this mechanism, the main tDCS montage of patients with anxiety symptoms is usually bicephalic DLPFC.
More concretely, patients with GAD typically present persistent and intensive worries about several situations or things such as health, work, finance, or family. According to previous studies, when cathodal tDCS was performed over the right DLPFC, the anxiety level was significantly diminished in individuals with GAD symptoms [45,46]. Therefore, tDCS can be a potential treatment in GAD, targeting the right DLPFC through cathodal stimulation. Additionally, SAD is a common mental disorder characterized by excessive fear and anxiety about the scrutiny of others, resulting in avoiding social situations. One example demonstrating the effects of tDCS on SAD is the study by Heeren et al. [47]. In this study, anodal tDCS applied over the left DLPFC was found to reduce attentional bias for threat in female patients with SAD, thereby minimizing the tendency to misinterpret external non-threat cues as actual threats. This finding suggests that anodal tDCS over the left DLPFC is an effective treatment for SAD [47].
tDCS in Schizophrenia
Schizophrenia is a multifaceted neurological illness related to severe cognitive and behavioral disabilities. Although antipsychotic drugs have been a cornerstone in treating schizophrenia, their efficacy in addressing cognitive impairment is limited. As an alternative therapeutic approach, several studies have investigated the use of tDCS in the treatment of cognitive dysfunction in patients with schizophrenia [48]. The montage mainly used to investigate the effects of tDCS on schizophrenia is the left DLPFC as the anode and the left temporoparietal junction as the cathode [49].
Moreover, previous studies examined the effect of tDCS on positive symptoms of schizophrenia such as auditory hallucination [50-52]. Brunelin et al. [50] demonstrated that the application of sham-controlled tDCS decreased auditory verbal hallucinations. Not only in the positive symptoms of schizophrenia but also in the negative symptoms, a significant effect was observed. For example, according to the positive and negative symptom scale, Palm et al. [53] noted significant improvement in both positive and negative symptoms in a patient with treatment-resistant schizophrenia, and Gomes et al. [54] found a reduction in negative symptoms following active tDCS versus sham tDCS.
It is notable that according to the meta-analysis studies [55], tDCS was effective for the treatment of refractory patients having psychotic symptoms without remission despite at least one or two antipsychotic medications. Specifically, among the 7 studies included in the analysis [50,56-61], 6 studies reported on the effect of tDCS on auditory hallucinations in patients with treatment-refractory conditions [50,57-61].
These patients were defined as individuals who continued to experience persistent auditory hallucinations or negative symptoms, even after receiving treatment with one or two antipsychotic medications. Brunelin and colleagues [50] showed that a notable decrease in the intensity of auditory hallucinations was observed in individuals with refractory schizophrenia following tDCS treatment. These effects of tDCS were observed to persist for a duration of three months post-stimulation. Similar results indicated by other studies [57,60] and all three studies [50,57,60] employed a protocol in which tDCS was administered twice daily over a period of five days, resulting in a total of 10 stimulation sessions.
tDCS in Mild Cognitive Impairment and Dementia
As the aging population gradually grows, the prevalence of mild cognitive impairment (MCI) and dementia is on the rise worldwide. MCI is a neurocognitive disorder that mainly manifests memory loss, indicating a transition from normal aging to Alzheimer’s disease or other dementia [62]. Each year, around 10−15% of patients with MCI progress to develop dementia [62,63]. Dementia is characterized by severe impairment of cognitive function including memory, language, and decision-making abilities. Although pharmacological therapy is primarily used to treat MCI and dementia, tDCS has recently attracted considerable attention as a nonpharmacological intervention for addressing symptoms in individuals with MCI and dementia.
Several studies have found a link between preserved or enhanced cognitive performance and the use of tDCS in patients with MCI or AD. According to Meinzer et al. [64], after receiving anodal stimulation, patients with MCI showed significant improvement in their scores for semantic word retrieval, performing at a level comparable to that of the healthy controls. André and their colleagues [65] found that anodal tDCS resulted in improved visual recall and enhanced performance on tasks measuring working memory (n-back) and response inhibition (go/no-go) when compared to the sham condition. Furthermore, in another clinical trial involving 34 patients with Alzheimer’s disease (AD), both the cathodal and anodal stimulation groups showed improved scores on the Mini-Mental State Examination for global cognition compared to the sham group [66].
Furthermore, several studies have investigated the effects of combining cognitive training (CT) and tDCS on cognitive performance in patients with AD. In a study [67], it was found that bipolar tDCS resulted in a more significant improvement on a picture naming test compared to the sham tDCS group. Additionally, other studies demonstrated that the combination of bipolar tDCS and CT resulted in sustained stability in overall cognitive function and memory improvements [68,69].
tDCS in Healthy Individuals
tDCS is also applied to healthy individuals in several studies focusing on the effectiveness of tDCS in a non-psychiatric conditions. Most of those studies concentrate on working memory, language, and motor learning in healthy individuals. First, tDCS is shown to be efficacious in working memory in healthy individuals. For example, studies on healthy elderly individuals [70,71] have investigated working memory improvement with anodal tDCS (a-tDCS) over the prefrontal cortex (PFC). Furthermore, tDCS is used to improve language functions in healthy people. For instance, a-tDCS over both Wernicke’s area [72] and the left DLPFC [73] causes naming latencies to be shorter in healthy individuals. Moreover, tDCS has a specific effect on motor learning in healthy individuals [74]. Specifically, a-tDCS was applied over the primary motor cortex (M1) to boost the effects of training during a variety of task paradigms in several studies [75-78]. However, only a limited number of studies in healthy individuals have been conducted; thus, further research is needed to examine the effectiveness of tDCS in normal conditions.
tACS
The Basis of tACS: Mechanism
tACS applies a low-intensity sinusoidal electrical current to the brain through electrodes on the scalp, boosting the brain’s oscillation. Specifically, some of the alternating currents reach the brain when tACS is applied to the skull, causing the cortical neurons to change in membrane potential towards depolarization or hyperpolarization [79]. During the tACS, the external driving current influences the endogenous brain oscillations to align in frequency and phase [80,81]. Compared to the tDCS, the tACS current does not include the directional voltage component [82]. tACS utilizes the manipulation and entrainment of intrinsic oscillations, leading to physiological entrainment through frequency stimulation at current strengths that are nearly imperceptible [82].
tACS in Depression
Patients with depression show a comparably complex picture of altered brain oscillations. In terms of power and coherence, during the resting state in individuals with depression, there was an enhancement in the low-frequency bands (specifically delta and alpha); alpha oscillations continue to persist even after the transition from a closed-eyes state to an open-eyes state [83-85]. Increased alpha activity in depression was found to be correlated with a better response to antidepressant therapies [86,87]. Furthermore, gamma oscillations were reduced in the anterior cingulate cortex and frontal regions during the resting state in participants who have depressive symptoms [88].
In a double-blind randomized controlled trial, tACS targeted abnormally elevated alpha waves on the left frontal region compared to the right frontal region, and this resulted in a significant reduction in alpha power within the left DLPFC [89]. In addition, one of the case reports included in the study focused on the effect of tACS on depression and provided an extension of a previous clinical trial [90]. In this case, an additional 12 weekly sessions of 10-Hz tACS were administered to a single participant. Following the original study, the patient showed a positive response to the treatment, but remission was not achieved.
However, after the 12 additional sessions, the participant achieved remission, which was sustained for at least a 2-month follow-up period [90]. Another case report investigated the impact of frontal tACS targeting the gamma frequency band, which is typically diminished in the frontal region of individuals with depression [91]. Following 9 stimulation sessions and a two-week follow-up period, the patient demonstrated symptom improvement and achieved remission after three months.
tACS in Schizophrenia
Several studies have proven that there are alterations in brain oscillations in patients with schizophrenia. A common pattern of oscillatory disturbances observed in patients with SZ is a reduction in amplitude and altered phase synchronization in all frequency bands [92,93]. According to case reports, one session of 20-minute tACS was able to improve performance in working memory tasks [94]. It was applied to the left DLPFC and the left posterior parietal region in theta frequency (6 Hz) and the same protocol was used in other case reports, resulting in improved working memory [95]. These studies were based on the previous findings that reduction of theta and gamma oscillation was related to working memory. Moreover, one study examined the effectiveness and safety of theta tACS, resulting in reduced negative symptoms, general symptoms, and improved insight into illness [96].
tACS in Mild Cognitive Impairment and Dementia
tACS has the potential to modify abnormal oscillatory patterns observed in individuals with MCI or AD and restore cortical oscillations associated with improved cognitive and memory function [97,98]. There is compelling evidence suggesting that cognitive enhancements induced by tACS in individuals with MCI or AD are particularly linked to gamma stimulation [99,100]. Gamma frequency range plays a crucial role in hippocampal-mediated memory processes, which are typically compromised in the early stages of the disease [99-101].
MCI and AD, both of which have significant effects on cognition and memory, have been consistently associated with impaired gamma oscillatory activity [102,103]. This abnormal gamma oscillatory activity is closely related to impaired performance in various memory tasks [104,105], including working memory and long-term episodic memory processing, which are key features of AD and MCI [106]. In addition, the abnormal gamma oscillations are linked to desynchronization of theta-gamma coupling [104], leading to reduced connectivity between brain regions involved in memory networks [107].
Furthermore, according to a review article [108], there is a study investigating the potential role of tACS as a predictor for the progression from MCI to AD [109]. This study was based on previous research indicating decreased gamma-band connectivity in AD patients [110] and increased local gamma-band power compared to those MCI during resting and task conditions [111]. The objective of the study was to explore whether the response to gamma tACS could differentiate MCI from AD and serve as a predictor for MCI progression to AD. Each participant received both sham stimulation over the left primary motor area (M1) and five active stimulation sessions, with Gamma tACS applied for 10 minutes to different brain regions, including M1, premotor area, supplementary motor area, DLPFC, and dorsomedial prefrontal cortex, in a random order every week.
tACS in Healthy Individuals
Several studies have also applied tACS to healthy individuals to assess its effectiveness in treating non-psychiatric conditions. Previous studies have investigated the regulatory impact of tACS on a wide range of perception and cognition, with emphasis on behavioral consequences. As tACS can influence neural firing patterns in memory-related frequencies [112], it has gained popularity as a technique for modulating memory [113]. Specifically, tACS can interact with endogenous synchronization mechanisms during working memory, in particular when the stimulation protocol resembles the intrinsic oscillatory properties of neural networks [114]. Several experiments showed reliable effects of frontoparietal theta tACS on working memory performance [115-118].
tRNS
The Basis of tRNS: Mechanism
tRNS is a relatively novel technique that alternates at a random frequency and amplitude within a specific range. tRNS is not limited to a particular direction of current flow and it provides stimulation at both electrode locations [119]. Compared to other transcranial electric stimulation methods such as tDCS and tACS, tRNS is the most effective for enhancing cortical excitability in the motor cortex [120]. The stimulation frequency of tRNS typically follows a normal distribution ranging from 0.1 to 640 Hz [119]. However, according to Fertonani and colleagues [121], it is common to categorize tRNS into low-frequency stimulation (0.1−100 Hz) or high-frequency stimulation (101−640 Hz).
There are some studies regarding the mechanisms of tRNS [122,123]. A study conducted on rats suggests that repetitive high-frequency stimulation can cause inward sodium currents in the neurons and weak depolarization [123]. Chaieb and colleagues [122] showed that in the case of humans, the excitability-increasing effects of tRNS were significantly reduced by blocking voltage-gated sodium channels. When central nervous system active drugs are combined with single-pulse TMS, the effects of tRNS are likely to operate independently of NMDA receptors [122]. This indicates a distinct mechanism of action compared to tDCS [16,124].
The precise mechanism underlying this cortical activity is not fully understood. Nonetheless, it has been postulated to be associated with neuroplasticity [119]. However, one commonly proposed hypothesis is the stochastic resonance phenomenon, which suggests that tRNS introduces random activity (noise) in the targeted neurons, thereby enhancing their sensitivity to external inputs [125,126]. Specifically, stochastic resonance is a general mechanism that amplifies the response of nonlinear systems to weak subthreshold signals by introducing an optimal level of random noise [127,128]. Fertonani and colleagues suggest that the action of tRNS is believed to involve repeated subthreshold stimulations, which can disrupt the homeostasis of the neural system and enhance task-related neural activity [121].
tRNS in Depression
tRNS might be an effective intervention for improving mood in individuals with severe low mood who meet the diagnostic criteria for MDD. According to a previous study [129], there was a reduction in the severity of depressive symptoms when the patients with MDD underwent an open-label tRNS. This reduction was greater than that observed in the two prior tDCS trials. Specifically, in the two previous trials of tDCS, the reduction in the severity of depressive symptoms at the end of the acute treatment phase was 31% and 25%, while in the tRNS trials, by the 15th session, there was a more substantial reduction of 63% compared to baseline. Across all three trials, patients had similar depression scores at baseline [129]. However, the patient reported faster improvement and fewer skin sensations with tRNS compared to tDCS. Thus, tRNS may produce fewer skin-related effects compared to tDCS, resulting in a stable option for studies that involve administering both active and sham tES [130]. Additional research is needed to further evaluate the potential effects of tRNS on depression.
tRNS in Schizophrenia
tRNS applied at its high-frequency range (hf-tRNS) between 101 and 640 Hz has been reported to increase cortical excitability of the primary motor cortex with sustained after-effects [119,120] and to improve performance on the tasks assessing sensory or cognitive functions [131]. The treatment of schizophrenia with hf-tRNS has been reported in two cases. Palm et al. [132] reported on a case of schizophrenia whose negative symptoms and cognitive deficits were improved by the add-on treatment of unidirectional (i.e., delivering current flow analogously to tDCS via switching the offset from zero to 1 mA to prohibit the oscillations from being negatively polarized) anodal hf-tRNS targeting the left dlPFC. Haesebaert et al. [133] reported on the other drug-free case of schizophrenia whose positive and negative symptoms, and insight impairment were alleviated by the monotherapy of unidirectional hf-tRNS with fronto-temporal montage. Thus, the therapeutic effects of hf-tRNS in schizophrenia merit systematic investigation.
CONCLUSION
This study sheds light on the potential of tES as a promising tool for modulating brain activity in a variety of contexts. Our findings suggest that tES can enhance cognitive function, improve motor skills, and even alleviate symptoms of depression and chronic pain. However, it is important to note that the effects of tES can be highly variable across individuals and depend on a variety of factors such as the specific brain region targeted, the stimulation parameters used, and the individual’s baseline brain activity. Further research is needed to fully understand the underlying mechanisms of tES and to develop optimal protocols for safe and effective use. With continued advancements in tES technology and growing interest in its potential applications, we are optimistic that tES will become an increasingly valuable tool in the field of neuroscience and beyond.
Funding Statement
Funding This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (1711138348, KD000169) and by the Korea Brain Research Institute Basic Research Program (Grant no. 22-BR-02-02).
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Chaeyeon Yang, Seung-Hwan Lee. Original draft: Chaeyeon Yang. Critical revision: Bori Jung, Seung-Hwan Lee. Supervision: Seung-Hwan Lee.
References
- 1.Razza LB, Palumbo P, Moffa AH, Carvalho AF, Solmi M, Loo CK, et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. 2020;37:594–608. doi: 10.1002/da.23004. [DOI] [PubMed] [Google Scholar]
- 2.Stein DJ, Fernandes Medeiros L, Caumo W, Torres IL. Transcranial direct current stimulation in patients with anxiety: Current perspectives. Neuropsychiatr Dis Treat. 2020;16:161–169. doi: 10.2147/NDT.S195840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kan RLD, Zhang BBB, Zhang JJQ, Kranz GS. Non-invasive brain stimulation for posttraumatic stress disorder: A systematic review and meta-analysis. Transl Psychiatry. 2020;10:168. doi: 10.1038/s41398-020-0851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathak H, Sreeraj VS, Venkatasubramanian G. Transcranial alternating current stimulation (tACS) and its role in schizophrenia: a scoping review. Clin Psychopharmacol Neurosci. 2023;21:634–649. doi: 10.9758/cpn.22.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh J, Jeon S, Ha TH, Myung W, Lee SH, Ko YH, et al. Effect of home-based self-administered transcranial direct stimula-tion in patients with mild to moderate major depressive disorder: a single-arm, multicentral trial. Clin Psychopharmacol Neurosci. 2023;21:271–278. doi: 10.9758/cpn.2023.21.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 2014;24:333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 7.Polanía R, Nitsche MA, Korman C, Batsikadze G, Paulus W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol. 2012;22:1314–1318. doi: 10.1016/j.cub.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Moret B, Donato R, Nucci M, Cona G, Campana G. Transcranial random noise stimulation (tRNS): A wide range of frequencies is needed for increasing cortical excitability. Sci Rep. 2019;9:15150. doi: 10.1038/s41598-019-51553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palm U, Schiller C, Fintescu Z, Obermeier M, Keeser D, Reisinger E, et al. Transcranial direct current stimulation in treatment resistant depression: A randomized double-blind, placebo-controlled study. Brain Stimul. 2012;5:242–251. doi: 10.1016/j.brs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- 11.Gartside IB. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: Role of protein synthesis. Nature. 1968;220:383–384. doi: 10.1038/220383a0. [DOI] [PubMed] [Google Scholar]
- 12.Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. Direct current stimulation modulates LTP and LTD: Activity dependence and dendritic effects. Brain Stimul. 2017;10:51–58. doi: 10.1016/j.brs.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippold OC, Redfearn JW. Mental changes resulting from the passage of small direct currents through the human brain. Br J Psychiatry. 1964;110:768–772. doi: 10.1192/bjp.110.469.768. [DOI] [PubMed] [Google Scholar]
- 16.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 17.Nasseri P, Nitsche MA, Ekhtiari H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front Hum Neurosci. 2015;9:54. doi: 10.3389/fnhum.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol. 2017;128:56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 19.Lefaucheur JP, Antal A, Ahdab R, Ciampi de Andrade D, Fregni F, Khedr EM, et al. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul. 2008;1:337–344. doi: 10.1016/j.brs.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Murphy DN, Boggio P, Fregni F. Transcranial direct current stimulation as a therapeutic tool for the treatment of major depression: Insights from past and recent clinical studies. Curr Opin Psychiatry. 2009;22:306–311. doi: 10.1097/YCO.0b013e32832a133f. [DOI] [PubMed] [Google Scholar]
- 21.Gladwin TE, den Uyl TE, Fregni FF, Wiers RW. Enhancement of selective attention by tDCS: Interaction with interference in a Sternberg task. Neurosci Lett. 2012;512:33–37. doi: 10.1016/j.neulet.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 22.Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage. 2014;89:216–225. doi: 10.1016/j.neuroimage.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefaucheur JP, Wendling F. Mechanisms of action of tDCS: A brief and practical overview. Neurophysiol Clin. 2019;49:269–275. doi: 10.1016/j.neucli.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Fischer DB, Fried PJ, Ruffini G, Ripolles O, Salvador R, Banus J, et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage. 2017;157:34–44. doi: 10.1016/j.neuroimage.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan T, Nikolin S, Loo CK, Martin DM. Effects of high-definition transcranial direct current stimulation and theta burst stimulation for modulating the posterior parietal cortex. J Int Neuropsychol Soc. 2019;25:972–984. doi: 10.1017/S1355617719000766. [DOI] [PubMed] [Google Scholar]
- 26.Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: A neurophysiological study. Brain Stimul. 2013;6:644–648. doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–207.:207.e1. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parlikar R, Vanteemar SS, Shivakumar V, Narayanaswamy CJ, Rao PN, Ganesan V. High definition transcranial direct current stimulation (HD-tDCS): A systematic review on the treatment of neuropsychiatric disorders. Asian J Psychiatr. 2021;56:102542. doi: 10.1016/j.ajp.2020.102542. [DOI] [PubMed] [Google Scholar]
- 29.Masina F, Arcara G, Galletti E, Cinque I, Gamberini L, Mapelli D. Neurophysiological and behavioural effects of conventional and high definition tDCS. Sci Rep. 2021;11:7659. doi: 10.1038/s41598-021-87371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 2012;13:112–120. doi: 10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Fregni F, Nitsche MA, Loo CK, Brunoni AR, Marangolo P, Leite J, et al. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): Review and recommendations from an expert panel. Clin Res Regul Aff. 2015;32:22–35. doi: 10.3109/10601333.2015.980944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto H, Ugawa Y. Adverse events of tDCS and tACS: A review. Clin Neurophysiol Pract. 2016;2:19–25. doi: 10.1016/j.cnp.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang R, Lam CLM, Peng X, Zhang D, Zhang C, Huang R, et al. Efficacy and acceptability of transcranial direct current stimulation for treating depression: A meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021;126:481. doi: 10.1016/j.neubiorev.2021.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 35.Duval F, Lebowitz BD, Macher JP. Treatments in depression. Dialogues Clin Neurosci. 2006;8:191–206. doi: 10.31887/DCNS.2006.8.2/fduval. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segrave RA, Arnold S, Hoy K, Fitzgerald PB. Concurrent cognitive control training augments the antidepressant efficacy of tDCS: A pilot study. Brain Stimul. 2014;7:325–331. doi: 10.1016/j.brs.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Luo H, Schülke R, Geng X, Sahakian BJ, Wang S. Is transcranial direct current stimulation, alone or in combination with antidepressant medications or psychotherapies, effective in treating major depressive disorder? A systematic review and meta-analysis. BMC Med. 2021;19:319. doi: 10.1186/s12916-021-02181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–391. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 39.Palm U, Hasan A, Strube W, Padberg F. tDCS for the treatment of depression: A comprehensive review. Eur Arch Psychiatry Clin Neurosci. 2016;266:681–694. doi: 10.1007/s00406-016-0674-9. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association; 2013. [DOI] [Google Scholar]
- 41.Ironside M, Browning M, Ansari TL, Harvey CJ, Sekyi-Djan MN, Bishop SJ, et al. Effect of prefrontal cortex stimulation on regulation of amygdala response to threat in individuals with trait anxiety: A randomized clinical trial. JAMA Psychiatry. 2019;76:71–78. doi: 10.1001/jamapsychiatry.2018.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W, Mao Y, Wei D, Yang J, Du X, Xie P, et al. Structural asymmetry of dorsolateral prefrontal cortex correlates with depressive symptoms: Evidence from healthy individuals and patients with major depressive disorder. Neurosci Bull. 2016;32:217–226. doi: 10.1007/s12264-016-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Nitschke JB, Heller W. Distinguishing neural substrates of heterogeneity among anxiety disorders. Int Rev Neurobiol. 2005;67:1–42. doi: 10.1016/S0074-7742(05)67001-8. [DOI] [PubMed] [Google Scholar]
- 45.Shiozawa P, Leiva AP, Castro CD, da Silva ME, Cordeiro Q, Fregni F, et al. Transcranial direct current stimulation for generalized anxiety disorder: A case study. Biol Psychiatry. 2014;75:e17–e18. doi: 10.1016/j.biopsych.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Lin Y, Zhang C, Wang Y. A randomized controlled study of transcranial direct current stimulation in treatment of generalized anxiety disorder. Brain Stimul. 2019;12:403. doi: 10.1016/j.brs.2018.12.302. [DOI] [Google Scholar]
- 47.Heeren A, Billieux J, Philippot P, De Raedt R, Baeken C, de Timary P, et al. Impact of transcranial direct current stimulation on attentional bias for threat: A proof-of-concept study among individuals with social anxiety disorder. Soc Cogn Affect Neurosci. 2017;12:251–260. doi: 10.1093/scan/nsw119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mervis JE, Capizzi RJ, Boroda E, MacDonald AW., 3rd Transcranial direct current stimulation over the dorsolateral prefrontal cortex in schizophrenia: A quantitative review of cognitive outcomes. Front Hum Neurosci. 2017;11:44. doi: 10.3389/fnhum.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kekic M, Boysen E, Campbell IC, Schmidt U. A systematic review of the clinical efficacy of transcranial direct current stimulation (tDCS) in psychiatric disorders. J Psychiatr Res. 2016;74:70–86. doi: 10.1016/j.jpsychires.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719–724. doi: 10.1176/appi.ajp.2012.11071091. [DOI] [PubMed] [Google Scholar]
- 51.Bose A, Shivakumar V, Narayanaswamy JC, Nawani H, Subramaniam A, Agarwal SM, et al. Insight facilitation with add-on tDCS in schizophrenia. Schizophr Res. 2014;156:63–65. doi: 10.1016/j.schres.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 52.Shivakumar V, Chhabra H, Subbanna M, Agarwal SM, Bose A, Kalmady SV, et al. Effect of tDCS on auditory hallucinations in schizophrenia: Influence of catechol-O-methyltransferase (COMT) Val158Met polymorphism. Asian J Psychiatr. 2015;16:75–77. doi: 10.1016/j.ajp.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 53.Palm U, Keeser D, Blautzik J, Pogarell O, Ertl-Wagner B, Kupka MJ, et al. Prefrontal transcranial direct current stimulation (tDCS) changes negative symptoms and functional connectivity MRI (fcMRI) in a single case of treatment-resistant schizophrenia. Schizophr Res. 2013;150:583–585. doi: 10.1016/j.schres.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 54.Gomes JS, Shiozawa P, Dias ÁM, Valverde Ducos D, Akiba H, Trevizol AP, et al. Left dorsolateral prefrontal cortex anodal tDCS effects on negative symptoms in schizophrenia. Brain Stimul. 2015;8:989–991. doi: 10.1016/j.brs.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 55.Kim J, Iwata Y, Plitman E, Caravaggio F, Chung JK, Shah P, et al. A meta-analysis of transcranial direct current stimulation for schizophrenia: "Is more better?". J Psychiatr Res. 2019;110:117–126. doi: 10.1016/j.jpsychires.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Smith RC, Boules S, Mattiuz S, Youssef M, Tobe RH, Sershen H, et al. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: A randomized controlled study. Schizophr Res. 2015;168:260–266. doi: 10.1016/j.schres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull. 2016;42:318–326. doi: 10.1093/schbul/sbv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fröhlich F, Burrello TN, Mellin JM, Cordle AL, Lustenberger CM, Gilmore JH, et al. Exploratory study of once-daily transcranial direct current stimulation (tDCS) as a treatment for auditory hallucinations in schizophrenia. Eur Psychiatry. 2016;33:54–60. doi: 10.1016/j.eurpsy.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Fitzgerald PB, McQueen S, Daskalakis ZJ, Hoy KE. A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul. 2014;7:813–816. doi: 10.1016/j.brs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Bose A, Shivakumar V, Agarwal SM, Kalmady SV, Shenoy S, Sreeraj VS, et al. Efficacy of fronto-temporal transcranial direct current stimulation for refractory auditory verbal hallucinations in schizophrenia: A randomized, double-blind, sham-controlled study. Schizophr Res. 2018;195:475–480. doi: 10.1016/j.schres.2017.08.047. [DOI] [PubMed] [Google Scholar]
- 61.Chang CC, Tzeng NS, Chao CY, Yeh CB, Chang HA. The effects of add-on fronto-temporal transcranial direct current stimulation (tDCS) on auditory verbal hallucinations, other psychopathological symptoms, and insight in schizophrenia: A randomized, double-blind, sham-controlled trial. Int J Neuropsychopharmacol. 2018;21:979–987. doi: 10.1093/ijnp/pyy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 63.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 64.Meinzer M, Lindenberg R, Phan MT, Ulm L, Volk C, Flöel A. Transcranial direct current stimulation in mild cognitive impairment: Behavioral effects and neural mechanisms. Alzheimers Dement. 2015;11:1032–1040. doi: 10.1016/j.jalz.2014.07.159. [DOI] [PubMed] [Google Scholar]
- 65.André S, Heinrich S, Kayser F, Menzler K, Kesselring J, Khader PH, et al. At-home tDCS of the left dorsolateral prefrontal cortex improves visual short-term memory in mild vascular dementia. J Neurol Sci. 2016;369:185–190. doi: 10.1016/j.jns.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 66.Khedr EM, Gamal NF, El-Fetoh NA, Khalifa H, Ahmed EM, Ali AM, et al. A double-blind randomized clinical trial on the efficacy of cortical direct current stimulation for the treatment of Alzheimer's disease. Front Aging Neurosci. 2014;6:275. doi: 10.3389/fnagi.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roncero C, Kniefel H, Service E, Thiel A, Probst S, Chertkow H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer's disease and frontotemporal dementia. Alzheimers Dement (N Y) 2017;3:247–253. doi: 10.1016/j.trci.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Penolazzi B, Bergamaschi S, Pastore M, Villani D, Sartori G, Mondini S. Transcranial direct current stimulation and cognitive training in the rehabilitation of Alzheimer disease: A case study. Neuropsychol Rehabil. 2015;25:799–817. doi: 10.1080/09602011.2014.977301. [DOI] [PubMed] [Google Scholar]
- 69.Cotelli M, Manenti R, Brambilla M, Petesi M, Rosini S, Ferrari C, et al. Anodal tDCS during face-name associations memory training in Alzheimer's patients. Front Aging Neurosci. 2014;6:38. doi: 10.3389/fnagi.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoy KE, Emonson MR, Arnold SL, Thomson RH, Daskalakis ZJ, Fitzgerald PB. Testing the limits: Investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia. 2013;51:1777–1784. doi: 10.1016/j.neuropsychologia.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 71.Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 72.Sparing R, Dafotakis M, Meister IG, Thirugnanasambandam N, Fink GR. Enhancing language performance with non-invasive brain stimulation--a transcranial direct current stimulation study in healthy humans. Neuropsychologia. 2008;46:261–268. doi: 10.1016/j.neuropsychologia.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C. Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res. 2010;208:311–318. doi: 10.1016/j.bbr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 74.Hashemirad F, Zoghi M, Fitzgerald PB, Jaberzadeh S. The effect of anodal transcranial direct current stimulation on motor sequence learning in healthy individuals: A systematic review and meta-analysis. Brain Cogn. 2016;102:1–12. doi: 10.1016/j.bandc.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Prichard G, Weiller C, Fritsch B, Reis J. Effects of different electrical brain stimulation protocols on subcomponents of motor skill learning. Brain Stimul. 2014;7:532–540. doi: 10.1016/j.brs.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Hashemirad F, Zoghi M, Fitzgerald PB, Jaberzadeh S. The effect of anodal transcranial direct current stimulation on motor sequence learning in healthy individuals: A systematic review and meta-analysis. Brain Cogn. 2016;102:1–12. doi: 10.1016/j.bandc.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Saucedo Marquez CM, Zhang X, Swinnen SP, Meesen R, Wenderoth N. Task-specific effect of transcranial direct current stimulation on motor learning. Front Hum Neurosci. 2013;7:333. doi: 10.3389/fnhum.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang EK, Paik NJ. Effect of a tDCS electrode montage on implicit motor sequence learning in healthy subjects. Exp Transl Stroke Med. 2011;3:4. doi: 10.1186/2040-7378-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vöröslakos M, Takeuchi Y, Brinyiczki K, Zombori T, Oliva A, Fernández-Ruiz A, et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun. 2018;9:483. doi: 10.1038/s41467-018-02928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vosskuhl J, Strüber D, Herrmann CS. Non-invasive brain sti-mulation: A paradigm shift in understanding brain oscillations. Front Hum Neurosci. 2018;12:211. doi: 10.3389/fnhum.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deans JK, Powell AD, Jefferys JG. Sensitivity of coherent oscillations in rat hippocampus to AC electric fields. J Physiol. 2007;583:555–565. doi: 10.1113/jphysiol.2007.137711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tavakoli AV, Yun K. Transcranial alternating current stimulation (tACS) mechanisms and protocols. Front Cell Neurosci. 2017;11:214. doi: 10.3389/fncel.2017.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newson JJ, Thiagarajan TC. EEG frequency bands in psychiatric disorders: A review of resting state studies. Front Hum Neurosci. 2019;12:521. doi: 10.3389/fnhum.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Northoff G. How do resting state changes in depression translate into psychopathological symptoms? From 'Spatiotemporal correspondence' to 'Spatiotemporal Psychopathology'. Curr Opin Psychiatry. 2016;29:18–24. doi: 10.1097/YCO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 85.Leuchter AF, Cook IA, Hunter AM, Cai C, Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS One. 2012:e32508. doi: 10.1371/journal.pone.0032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pathak Y, Salami O, Baillet S, Li Z, Butson CR. Longitudinal changes in depressive circuitry in response to neuromodulation therapy. Front Neural Circuits. 2016;10:50. doi: 10.3389/fncir.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breitenstein B, Scheuer S, Holsboer F. Are there meaningful biomarkers of treatment response for depression? Drug Discov. Today. 2014;19:539–561. doi: 10.1016/j.drudis.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 88.Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: A 128-channel EEG study. Hum Brain Mapp. 2006;27:185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alexander ML, Alagapan S, Lugo CE, Mellin JM, Lustenberger C, Rubinow DR, et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD) Transl Psychiatry. 2019;9:106. doi: 10.1038/s41398-019-0439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Riddle J, Rubinow DR, Frohlich F. A case study of weekly tACS for the treatment of major depressive disorder. Brain Stimul. 2020;13:576–577. doi: 10.1016/j.brs.2019.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilkening A, Kurzeck A, Dechantsreiter E, Padberg F, Palm U. Transcranial alternating current stimulation for the treatment of major depression during pregnancy. Psychiatry Res. 2019;279:399–400. doi: 10.1016/j.psychres.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 94.Sreeraj VS, Shanbhag V, Nawani H, Shivakumar V, Damodharan D, Bose A, et al. Feasibility of online neuromodulation using transcranial alternating current stimulation in schizophrenia. Indian J Psychol Med. 2017;39:92–95. doi: 10.4103/0253-7176.198937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sreeraj VS, Shivakumar V, Sowmya S, Bose A, Nawani H, Narayanaswamy JC, et al. Online theta frequency transcranial alternating current stimulation for cognitive remediation in schizophrenia: A case report and review of literature. J ECT. 2019;35:139–143. doi: 10.1097/YCT.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 96.Kallel L, Mondino M, Brunelin J. Effects of theta-rhythm transcranial alternating current stimulation (4.5 Hz-tACS) in patients with clozapine-resistant negative symptoms of schizophrenia: A case series. J Neural Transm (Vienna) 2016;123:1213–1217. doi: 10.1007/s00702-016-1574-x. [DOI] [PubMed] [Google Scholar]
- 97.Kehler L, Francisco CO, Uehara MA, Moussavi Z. The effect of transcranial alternating current stimulation (tACS) on cognitive function in older adults with dementia. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:3649–3653. doi: 10.1109/EMBC44109.2020.9175903. [DOI] [PubMed] [Google Scholar]
- 98.Sprugnoli G, Munsch F, Cappon D, Paciorek R, Macone J, Connor A, et al. Impact of multisession 40Hz tACS on hippocampal perfusion in patients with Alzheimer's disease. Alzheimers Res Ther. 2021;13:203. doi: 10.1186/s13195-021-00922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hampel H, Bürger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 100.Manippa V, Palmisano A, Nitsche MA, Filardi M, Vilella D, Logroscino G, et al. Cognitive and neuropathophysiological outcomes of gamma-tACS in dementia: A systematic review. Neuropsychol Rev. 2023 Mar 6; doi: 10.1007/s11065-023-09589-0. doi: 10.1007/s11065-023-09589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nissim NR, Pham DVH, Poddar T, Blutt E, Hamilton RH. The impact of gamma transcranial alternating current stimulation (tACS) on cognitive and memory processes in patients with mild cognitive impairment or Alzheimer's disease: A literature review. Brain Stimul. 2023;16:748–755. doi: 10.1016/j.brs.2023.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stam CJ, van Cappellen van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, Scheltens P, et al. Generalized synchronization of MEG recordings in Alzheimer's disease: Evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562–574. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reinhart RMG, Nguyen JA. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci. 2019;22:820–827. doi: 10.1038/s41593-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev. 2010;34:1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Irish M, Lawlor BA, Coen RF, O'Mara SM. Everyday episodic memory in amnestic mild cognitive impairment: A preliminary investigation. BMC Neurosci. 2011;12:80. doi: 10.1186/1471-2202-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lisman JE, Jensen O. The q-g neural code. Neuron. 2013;77:1002–1016. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elyamany O, Leicht G, Herrmann CS, Mulert C. Transcranial alternating current stimulation (tACS): From basic mechanisms towards first applications in psychiatry. Eur Arch Psychiatry Clin Neurosci. 2021;271:135–156. doi: 10.1007/s00406-020-01209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naro A, Corallo F, De Salvo S, Marra A, Di Lorenzo G, Muscarà N, et al. Promising role of neuromodulation in predicting the progression of mild cognitive impairment to dementia. J Alzheimers Dis. 2016;53:1375–1388. doi: 10.3233/JAD-160305. [DOI] [PubMed] [Google Scholar]
- 110.Moretti DV, Frisoni GB, Binetti G, Zanetti O. Anatomical substrate and scalp EEG markers are correlated in subjects with cognitive impairment and Alzheimer's disease. Front Psychiatry. 2011;1:152. doi: 10.3389/fpsyt.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Deursen JA, Vuurman EF, Verhey FR, van Kranen-Mastenbroek VH, Riedel WJ. Increased EEG gamma band activity in Alzheimer's disease and mild cognitive impair-ment. J Neural Transm (Vienna) 2008;115:1301–1311. doi: 10.1007/s00702-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vossen A, Gross J, Thut G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 2015;8:499–508. doi: 10.1016/j.brs.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klink K, Peter J, Wyss P, Klöppel S. Transcranial electric current stimulation during associative memory encoding: Comparing tACS and tDCS effects in healthy aging. Front Aging Neurosci. 2020;12:66. doi: 10.3389/fnagi.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alekseichuk I, Falchier AY, Linn G, Xu T, Milham MP, Schroeder CE, et al. Electric field dynamics in the brain during multi-electrode transcranial electric stimulation. Nat Commun. 2019;10:2573. doi: 10.1038/s41467-019-10581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jones KT, Arciniega H, Berryhill ME. Replacing tDCS with theta tACS provides selective, but not general WM benefits. Brain Res. 2019;1720:146324. doi: 10.1016/j.brainres.2019.146324. [DOI] [PubMed] [Google Scholar]
- 116.Guo X, Li Z, Zhang L, Liu Q. Modulation of visual working memory performance via different theta frequency stimulations. Brain Sci. 2021;11:1358. doi: 10.3390/brainsci11101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sahu PP, Tseng P. Frontoparietal theta tACS nonselectively enhances encoding, maintenance, and retrieval stages in visuospatial working memory. Neurosci Res. 2021;172:41–50. doi: 10.1016/j.neures.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 118.Polanía R, Nitsche MA, Korman C, Batsikadze G, Paulus W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol. 2012;22:1314–1318. doi: 10.1016/j.cub.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 119.Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28:14147–14155. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inukai Y, Saito K, Sasaki R, Tsuiki S, Miyaguchi S, Kojima S, et al. Comparison of three non-invasive transcranial electrical stimulation methods for increasing cortical excitability. Front Hum Neurosci. 2016;10:668. doi: 10.3389/fnhum.2016.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fertonani A, Pirulli C, Miniussi C. Random noise stimulation improves neuroplasticity in perceptual learning. J Neurosci. 2011;31:15416–15423. doi: 10.1523/JNEUROSCI.2002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaieb L, Antal A, Paulus W. Transcranial random noise stimulation-induced plasticity is NMDA-receptor independent but sodium-channel blocker and benzodiazepines sensitive. Front Neurosci. 2015;9:125. doi: 10.3389/fnins.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schoen I, Fromherz P. Extracellular stimulation of mammalian neurons through repetitive activation of Na+ channels by weak capacitive currents on a silicon chip. J Neurophysiol. 2008;100:346–357. doi: 10.1152/jn.90287.2008. [DOI] [PubMed] [Google Scholar]
- 124.Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation--technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–276. doi: 10.1016/S1567-424X(09)70230-2. [DOI] [PubMed] [Google Scholar]
- 125.Miniussi C, Harris JA, Ruzzoli M. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci Biobehav Rev. 2013;37:1702–1712. doi: 10.1016/j.neubiorev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 126.van der Groen O, Wenderoth N. Transcranial random noise stimulation of visual cortex: Stochastic resonance enhances central mechanisms of perception. J Neurosci. 2016;36:5289–5298. doi: 10.1523/JNEUROSCI.4519-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McDonnell MD, Abbott D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput Biol. 2009;5:e1000348. doi: 10.1371/journal.pcbi.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gingl Z, Kiss LB, Moss F. Non-dynamical stochastic resonance: Theory and experiments with white and arbitrarily coloured noise. Europhys Lett. 1995;29:191. doi: 10.1209/0295-5075/29/3/001. [DOI] [Google Scholar]
- 129.Chan HN, Alonzo A, Martin DM, Player M, Mitchell PB, Sachdev P, et al. Treatment of major depressive disorder by transcranial random noise stimulation: Case report of a novel treatment. Biol Psychiatry. 2012;72:e9–e10. doi: 10.1016/j.biopsych.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 130.Ambrus GG, Paulus W, Antal A. Cutaneous perception thresholds of electrical stimulation methods: Comparison of tDCS and tRNS. Clin Neurophysiol. 2010;121:1908–1914. doi: 10.1016/j.clinph.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 131.Antal A, Herrmann CS. Transcranial alternating current and random noise stimulation: Possible mechanisms. Neural Plast. 2016;2016:3616807. doi: 10.1155/2016/3616807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palm U, Hasan A, Keeser D, Falkai P, Padberg F. Transcranial random noise stimulation for the treatment of negative symptoms in schizophrenia. Schizophr Res. 2013;146:372–373. doi: 10.1016/j.schres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 133.Haesebaert F, Mondino M, Saoud M, Poulet E, Brunelin J. Efficacy and safety of fronto-temporal transcranial random noise stimulation (tRNS) in drug-free patients with schizophrenia: A case study. Schizophr Res. 2014;159:251–252. doi: 10.1016/j.schres.2014.07.043. [DOI] [PubMed] [Google Scholar]