Abstract
Following a request from the European Commission, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of liquid l‐lysine base produced with a genetically modified strain of Corynebacterium glutamicum as a nutritional feed additive for all animal species. The l‐lysine base liquid produced with C. glutamicum NRRL B‐67535 and NRRL B‐67439 is currently authorised as a nutritional additive for all animal species. The present application is aimed at modifying the current authorisation to include C. glutamicum NRRL B‐68248 as a production strain. The new production strain qualifies for the qualified presumption of safety approach when used for production purposes. It was unambiguously identified as C. glutamicum and was shown not to harbour acquired antimicrobial resistance determinants for antibiotics of human and veterinary importance. All the introduced sequences or mutations were considered to be safe, and no viable cells or DNA of the NRRL B‐68248 strain was detected in the final product. Therefore, the final product does not pose any safety concern associated with the production strain. l‐Lysine base produced using C. glutamicum NRRL B‐68248 does not represent a risk for the target species, the consumer or the environment. The additive was considered to be neither irritant to skin or the eyes, nor a dermal sensitiser. l‐Lysine base produced with C. glutamicum NRRL B‐68248 is considered to be an efficacious source of the essential amino acid l‐lysine for non‐ruminant animal species. For the supplemental l‐lysine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
Keywords: amino acid, Corynebacterium glutamicum, efficacy, liquid lysine base, NRRL B‐68248, nutritional additive, safety
1. INTRODUCTION
1.1. Background and Terms of Reference
Regulation (EC) No 1831/2003 1 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 13(3) of that Regulation lays down that if the holder of an authorisation proposes changing the terms of the authorisation by submitting an application to the Commission, accompanied by the relevant data supporting the request for the change, the authority shall transmit its opinion on the proposal to the Commission and the Member States.
The European Commission received a request from ADM Specialty Ingredients (Europe) B.V. 2 for the modification of the terms of the authorisation of the additive consisting of liquid l‐lysine base produced with Corynebacterium glutamicum NRRL B‐68248, when used as a feed additive for all animal species (category: nutritional additives; functional group: amino acids, their salts and analogues).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 13(3) (modification of the authorisation of a feed additive). The dossier was received on 11 July 2023 and the general information and supporting documentation are available at https://open.efsa.europa.eu/questions/EFSA‐Q‐2023‐00482. The particulars and documents in support of the application were considered valid by EFSA as of 01 December 2023.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the feed additive consisting of liquid l‐lysine base produced with Corynebacterium glutamicum NRRL B‐68248, when used under the proposed conditions of use (see Section 3.1.4 ).
1.2. Additional information
The EFSA Panel on Additives and Products or Substances used in Animal feed (FEEDAP) issued two opinions on the safety and efficacy of liquid l‐lysine base produced with the C. glutamicum strains NRRL B‐67439 NRRL B‐67535 and NRRL B‐50775 when used as a nutritional feed additive for all animal species (EFSA FEEDAP Panel, 2019a, 2019b).
This liquid l‐lysine base is currently authorised for use as a nutritional additive in complete feed and water for drinking for all animal species (3c320). 3
2. DATA AND METHODOLOGIES
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier 4 in support of the authorisation request for the use of liquid l‐lysine base produced with C. glutamicum NRRL B‐68248 as a feed additive.
In accordance with Article 38 of the Regulation (EC) No 178/2002 5 and taking into account the protection of confidential information and of personal data in accordance with Articles 39 to 39e of the same Regulation, and of the Decision of EFSA's Executive Director laying down practical arrangements concerning transparency and confidentiality, 6 a non‐confidential version of the dossier has been published on Open.EFSA.
According to Article 32c(2) of Regulation (EC) No 178/2002 and to the Decision of EFSA's Executive Director laying down the practical arrangements on pre‐submission phase and public consultations, EFSA carried out a public consultation on the non‐confidential version of the technical dossier from 22 May to 14 June 2024 for which no comments were received.
The confidential version of the technical dossier was subject to a target consultation of the interested Member States from 01 December 2023 to 01 March 2024; the comments received were considered for the assessment.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA, other scientific reports and experts' knowledge, to deliver the present output.
The European Union Reference Laboratory (EURL) considered that the conclusions and recommendations reached in the previous assessment regarding the methods used for the control of the liquid l‐lysine base produced with C. glutamicum strain NRRL B‐67535 in animal feed are valid and applicable for the current application. 7
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of liquid l‐lysine base produced with C. glutamicum strain NRRL B‐68248 is in line with the principles laid down in Regulation (EC) No 429/2008 8 and the relevant guidance documents: Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017a), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018b), Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019c), EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain (EFSA, 2021) and Guidance on the assessment of the safety of feed additives for the users (EFSA FEEDAP Panel, 2023).
3. ASSESSMENT
The l‐lysine base liquid produced with Corynebacterium glutamicum NRRL B‐67535 is currently authorised as a nutritional additive (functional group: amino acids, their salts and analogues) for all animal species. The present application is aimed at modifying the current terms of the authorisation to include C. glutamicum NRRL B‐68248 as a production strain.
3.1. Characterisation
3.1.1. Characterisation of the production organism
The l‐lysine base present in the additive is obtained by fermentation with a genetically modified strain of C. glutamicum which is deposited in the US Agricultural Research Culture Collection under the accession number NRRL B‐68248. 9 This production strain shares the parental strain (C. glutamicum ■■■■■) with the one previously evaluated and currently authorised (C. glutamicum NRRL B‐67535, EFSA FEEDAP Panel, 2019a).
The production strain was identified as C. glutamicum by whole genome sequence (WGS) analysis with an average nucleotide identity value of 98.3% with the type strain C. glutamicum ATCC 13032T. 10 In a phylogenetic analysis conducted based on the alignment of 146 genes between the production strain and other strains from the same genus, strain NRRL B‐68248 clustered with the C. glutamicum strains ATCC 13869 and BF100, confirming that the production strain (NRRL B‐68248) belongs to the C. glutamicum species.
The susceptibility of the production strain to the battery of antibiotics recommended by the FEEDAP Panel (EFSA FEEDAP Panel, 2018b) was tested and all the minimum inhibitory concentration values fell below the cut‐off values for Gram positives. 11 Therefore, the strain is susceptible to all the tested antibiotics.
The WGS data of the production strain were analysed for the presence of antimicrobial resistance genes using the ResFinder and the NCBI Bacterial Antimicrobial Resistance Reference Gene databases. No hits were identified above the thresholds established by EFSA (EFSA, 2021). 12
Information related to the genetically modified microorganism
Characterisation of the recipient or parental microorganism
The recipient strain is C. glutamicum ■■■■■ which was obtained by classical mutagenesis. 13
Description of the genetic modification
The production strain C. glutamicum NRRL B‐68248 ■■■■■ The absence of the antimicrobial resistance genes transiently introduced during the genetic modification process was confirmed by the WGS interrogation described above.
■■■■■:
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■.
■■■■■:
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■.
3.1.2. Manufacturing process
According to the applicant, the manufacturing process has not been modified as compared to the one previously assessed for the additive, except for the change of the production strain.
l‐Lysine is produced ■■■■■ The applicant states that no antimicrobials, including antibiotics, are employed in the manufacturing processes.
3.1.3. Characterisation of the active substance/additive
l‐Lysine (IUPAC name (2S)‐2,6 diaminohexanoic acid; synonym α,ε diaminocaproic acid) is identified with the CAS No 56‐87‐1 and the EINECS No 200‐294‐2, has a molecular weight of 146.2 g/mol. The molecular formula is NH2‐(CH2)4‐CH(NH2)‐COOH. The molecular structure is given in Figure 1.
FIGURE 1.
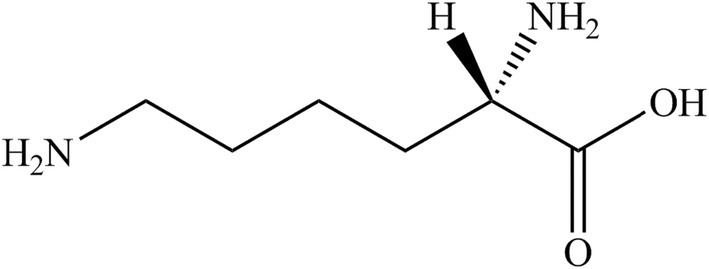
Molecular structure of l‐lysine.
l‐Lysine base, liquid, is currently authorised as an aqueous solution of l‐lysine with a minimum of 50% l‐lysine.
Analytical data of eight batches showed an average content of l‐lysine ‘as is’ of ■■■■■ (range ■■■■■) and water of ■■■■■ (range ■■■■■). 14 Other analysed constituents (average values) were ■■■■■ 15
On a ‘as is’ basis, the sum of the quantified components was on average 98.5% (range 98.2%–99.4%).
The same eight batches of the additive were analysed for cadmium, lead, mercury and arsenic levels and all batches showed concentrations below the limit of quantification of the method (LOQ) applied for all analytes. 16
Three out of the eight batches were further analysed for dioxins and dioxin‐like PCBs, mycotoxins and microbiological contamination. 17 Dioxins and the sum of dioxins plus dioxin like PCBs concentrations ranged 0.05–0.10 ng WHO‐PCDD/F‐TEQ/kg and 0.05–0.10 ng WHO‐PCDD/F‐PCB‐TEQ/kg, respectively. The analysis of mycotoxins included aflatoxins (B1, G1, B2, G2), fumonisins (B1, B2, B3), 3‐acetyl‐deoxynivalenol, deoxynivalenol, HT‐2 toxin, T2‐toxin, zearalenone and ochratoxin A. In all cases, the analysed concentration was below the LOQ of the analytical method, except for fumonisin B1, which ranged from 37 to 60 μg/kg. 18
Microbiological contamination was tested by determining levels of Pseudomonas spp., total coliforms, Escherichia coli, Salmonella spp., aerobic plate count, Enterobacteriaceae, yeasts and filamentous fungi. No Salmonella was detected in 25 g samples. The other microbial contaminants were < 10 CFU/g.
The FEEDAP Panel considers that the results of the microbiological analyses and the amounts of the detected impurities do not raise safety concerns.
The presence of viable cells of the production strain was analysed in three production batches of the additive, ■■■■■. 19 ■■■■■. No colonies were detected.
The presence of DNA of the production strain was analysed in three production batches of the additive, ■■■■■. 20 ■■■■■ The limit of detection of 1 ng/mL was determined. No DNA was detected in any of the samples.
The additive appears as a fluid liquid, soluble in water, and it has a pH (analysed in three batches) of 10.1. 21 The viscosity and density were measured in three batches of the additive. The viscosity (at 25°C) ranged from 56.0 to 57.9 cP, 22 and the density (at 20°C) was 1150 kg/m3 in all three batches. 23
The particle size of the additive was investigated in three batches using scanning electron microscopy. No small particles including nanoparticles (< 500 nm) were detected. 24
As the manufacturing process and the composition of the additive have not been modified, the outcome of the studies related to shelf‐life, stability and homogeneous distribution in feed of the previous assessment (EFSA FEEDAP Panel, 2019a) is considered valid.
3.1.4. Conditions of use
The additive is currently authorised for use in feed for all animal species, without a minimum or maximum content. 25
Under other provisions:
-
–
The lysine content shall be indicated on the labelling of the additive.
-
–
l‐lysine base, liquid, may be placed on the market and used as an additive consisting of a preparation.
-
–
For users of the additive and premixtures, feed business operators shall establish operational procedures and organisational measures to address potential risks by inhalation and for the skin and eyes. Where those risks cannot be eliminated or reduced to a minimum by such procedures and measures, the additive and premixtures shall be used with personal protective equipment, including breathing, skin and eye protection.
-
–
The additive may be also used via water for drinking.
-
–
Declarations to be made on the labelling of the additive and premixtures: ‘The supplementation with l‐lysine, in particular via water for drinking, should take into account all essential and conditional essential amino acids in order to avoid imbalances.’
The applicant is seeking the modification of the terms of the authorisation to allow the inclusion of C. glutamicum NRRL B‐68248 as production strain and the use of lysine base liquid produced with this strain in feed for all animal species, without a minimum or maximum content.
3.2. Safety
The safety of l‐lysine base produced with C. glutamicum NRRLB‐67535 was assessed by the FEEDAP Panel (EFSA FEEDAP Panel, 2019a). Considering that the manufacturing process and the composition of the additive have not been modified, except for the production strain, the Panel considers that the only safety aspect that needs to be considered is the change of the strain.
3.2.1. Safety for the target species, consumers, and environment
The production strain belongs to a species, C. glutamicum, that qualifies for the qualified presumption of safety (QPS) approach for safety assessment when used for production purposes (EFSA BIOHAZ Panel, 2023). The strain was unambiguously identified as C. glutamicum and was shown not to harbour acquired antimicrobial resistance determinants for antibiotics of human and veterinary importance. All the introduced sequences or mutations are considered to be safe. No viable cells or DNA of the production strain were detected in three batches of the additive. Consequently, no safety concerns for the target animals, consumers and the environment are expected from the additive concerning the production strain and the fermentation residues that may be present in the final additive.
Therefore, the FEEDAP Panel reiterates its previous conclusions that the additive is safe for the target species, consumer and the environment.
3.2.2. Safety for the user
No specific studies were submitted to support the safety for the user of the product under assessment.
The applicant submitted an acute dermal irritation study, 26 an acute eye irritation study 27 and a skin sensitisation study 28 conducted with a l‐lysine liquid 50% produced with a different production strain (C. glutamicum KCTC 12307BP) and having a pH of 9.96. These studies have already been evaluated in the context of a previous opinion (EFSA FEEDAP Panel, 2019d) and the FEEDAP Panel concluded that the test item was neither irritant to skin or eyes nor a dermal sensitiser.
The FEEDAP Panel considered the following: (i) the physico‐chemical characteristics of both lysine products (≥ 50% lysine, ≤ 48% water, density 1.12–1.17 g/mL) are the same; (ii) the manufacturing process of both lysine products is similar; and (iii) historical data on pH measurements of several batches of the lysine under assessment show values not exceeding 10.4 and similar to the pH of the test item used in the user safety studies, and concluded that the outcome of the studies mentioned above are applicable to the product under assessment.
Therefore, the additive under assessment is not considered to be irritant to skin or eyes, nor a dermal sensitiser.
3.3. Efficacy
The Panel considers that the proposed change in the production strain will not have an impact on the efficacy of the additive. Therefore, l‐Lysine base produced using C. glutamicum strain NRRL B‐68248 is considered to be an efficacious source of the essential amino acid l‐lysine for non‐ruminant animal species. For the supplemental l‐lysine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation 29 and good manufacturing practice.
4. CONCLUSIONS
The production strain C. glutamicum NRRL B‐68248 is genetically modified and the FEEDAP Panel concludes that the genetic modification raises no safety concerns. No viable cells or DNA of the production strain were detected in the final product. Therefore, the final product does not pose any safety concern in regard to the production strain.
l‐Lysine base produced with C. glutamicum NRRL B‐68248 does not represent a risk for the target species, consumers or the environment.
On the basis of the information submitted, the additive was considered to be neither irritant to skin or the eyes, nor a dermal sensitiser.
l‐Lysine base produced with C. glutamicum NRRL B‐68248 is considered to be an efficacious source of the essential amino acid l‐lysine for non‐ruminant animal species. For the supplemental l‐lysine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
ABBERVIATIONS
- CAS
Chemical Abstracts Service
- CFU
colony forming unit
- DM
dry matter
- EINECS
European Inventory of Existing Chemical Substances
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed
- GC‐MS
gas chromatography–mass spectrometry
- IUPAC
International Union of Pure and Applied Chemistry
- LOD
limit of detection
- LOQ
limit of quantification
- MIC
minimum inhibitory concentration
- MW
molecular weight
- OECD
Organisation for Economic Co‐operation and Development
ACKNOWLEDGEMENTS
The Panel wishes to thank the following for the support provided to this scientific output (in alphabetical order of the last name): Ruud Woutersen and Birgit Dusemund.
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2023‐00482
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
LEGAL NOTICE
Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by EFSA. The full output has been shared with the European Commission, EU Member States (if applicable) and the applicant. The blackening may be subject to review once the decision on the confidentiality requests is adopted by EFSA and in case it rejects some of the confidentiality requests.
EFSA FEEDAP Panel (Panel on Additives and Products or Substances used in Animal Feed) , Villa, R. E. , Azimonti, G. , Bonos, E. , Christensen, H. , Durjava, M. , Dusemund, B. , Gehring, R. , Glandorf, B. , Kouba, M. , López‐Alonso, M. , Marcon, F. , Nebbia, C. , Pechová, A. , Prieto‐Maradona, M. , Röhe, I. , Theodoridou, K. , Herman, L. , Anguita, M. , … Brozzi, R. (2024). Modification of the terms of authorisation regarding the additive consisting of liquid l‐lysine base produced with Corynebacterium glutamicum NRRL B‐67439 and NRRL B‐67535 for all animal species (ADM specialty ingredients (Europe) B.V.). EFSA Journal, 22(7), e8950. 10.2903/j.efsa.2024.8950
Adopted: 4 July 2024
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the council of 22 September 2003 on the additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
ADM Specialty Ingredients (Europe) B.V., Kingsfordweg 43 GP Amsterdam The Netherlands – Netherlands.
Commission Implementing Regulation (EU) 2020/997 of 9 July 2020 concerning the authorisation of l‐lysine base, liquid, l‐lysine sulfate and l‐lysine monohydrochloride, technically pure, as feed additives for all animal species. OJ L 221, 10.07.2020, p. 90.
Dossier reference: FEED‐2023‐14757.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–48.
Decision https://www.efsa.europa.eu/en/corporate‐pubs/transparency‐regulation‐practical‐arrangements.
Evaluation report available on the EU Science Hub https://joint‐research‐centre.ec.europa.eu/eurl‐fa‐eurl‐feed‐additives/eurl‐fa‐authorisation/eurl‐fa‐evaluation‐reports_en.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Annex 10.
Annex 13 and Annex 14.
Annex 15.
Annex 13 and Annex 14.
Annex 11.
Annex 1 COAs lysine ADM. Lysine analysed by AOAC Official method 999.13.
Annex 1 COAs lysine ADM. ■■■■■.
Annex 3 Heavy metals lysine ADM. LOQ in μg/kg was 4 for cadmium, lead, mercury and arsenic.
Annex 2 Dioxins + Microbio + Mycotoxins combined Lysine ADM.
LOQ in μg/kg was 1 for T2‐toxin. It was 5 for zearalenoe and ochratoxin A. It was 10 for Fumonisin B2 and B3, Aflatoxin B1, B2, G1 and G2, deoxynivalenol and HT‐2 toxin. It was 20 for 15‐acetyl‐deoxinivalenol.
Annex 4 and Answers from the applicant – characterisation.
Annex 5 and Answers from the applicant – characterisation.
Annex 12 pH lysine ADM.
Annex 6 Viscosity lysine ADM.
Annex 7 Density lysine ADM.
Annex 9 nano particles lysine ADM.
Commission implementing regulation (EU) 2020/997 of July 2020 concerning the authorisation of L‐lysine base, liquid, L‐lysine sulfate and l‐lysine monohydrochloride, technically pure, as feed additives for all animal species. OJ L 221, 10.7.2020, p. 90–94.
Annex 28 Safety acute dermal OECD 404 lysine ADM.
Annex 29 Safety acute eye OECD 405 lysine ADM.
Annex 30 Safety skin OECD 406 lysine ADM.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
REFERENCES
- EFSA (European Food Safety Authority) . (2021). EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA Journal, 19(7), 6506. 10.2903/j.efsa.2021.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis, K. , Allende, A. , Álvarez‐Ordóñez, A. , Bolton, D. , Bover‐Cid, S. , Chemaly, M. , De Cesare, A. , Hilbert, F. , Lindqvist, R. , Nauta, M. , Peixe, L. , Ru, G. , Simmons, M. , Skandamis, P. , Suffredini, E. , Cocconcelli, P. S. , Fernández Escámez, P. S. , Prieto Maradona, M. , … Herman, L. (2023). Scientific Opinion on the update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA Journal, 21(1), 7747. 10.2903/j.efsa.2023.7747 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen, G. , Aquilina, G. , Azimonti, G. , Bampidis, V. , Bastos, M. L. , Bories, G. , Chesson, A. , Cocconcelli, P. S. , Flachowsky, G. , Gropp, J. , Kolar, B. , Kouba, M. , López‐Alonso, M. , López Puente, S. , Mantovani, A. , Mayo, B. , Ramos, F. , Saarela, M. , … Innocenti, M. L. (2017a). Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal, 15(10), 5022. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen, G. , Aquilina, G. , Azimonti, G. , Bampidis, V. , Bastos, M. L. , Bories, G. , Chesson, A. , Cocconcelli, P. S. , Flachowsky, G. , Gropp, J. , Kolar, B. , Kouba, M. , López‐Alonso, M. , López Puente, S. , Mantovani, A. , Mayo, B. , Ramos, F. , Saarela, M. , … Innocenti, M. L. (2017b). Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal, 15(10), 5023. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen, G. , Aquilina, G. , Azimonti, G. , Bampidis, V. , Bastos, M. L. , Bories, G. , Chesson, A. , Cocconcelli, P. S. , Flachowsky, G. , Gropp, J. , Kolar, B. , Kouba, M. , López‐Alonso, M. , López Puente, S. , Mantovani, A. , Mayo, B. , Ramos, F. , Saarela, M. , … Martino, L. (2017c). Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal, 15(10), 5021. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen, G. , Aquilina, G. , Azimonti, G. , Bampidis, V. , Bastos, M. L. , Bories, G. , Chesson, A. , Cocconcelli, P. S. , Flachowsky, G. , Gropp, J. , Kolar, B. , Kouba, M. , López‐Alonso, M. , López Puente, S. , Mantovani, A. , Mayo, B. , Ramos, F. , Saarela, M. , … Martino, L. (2018a). Guidance on the assessment of the efficacy of feed additives. EFSA Journal, 16(5), 5274. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen, G. , Aquilina, G. , Azimonti, G. , Bampidis, V. , Bastos, M. L. , Bories, G. , Chesson, A. , Cocconcelli, P. S. , Flachowsky, G. , Gropp, J. , Kolar, B. , Kouba, M. , López‐Alonso, M. , López Puente, S. , Mantovani, A. , Mayo, B. , Ramos, F. , Saarela, M. , … Galobart, J. (2018b). Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal, 16(3), 5206. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V. , Azimonti, G. , Bastos, M. L. , Christensen, H. , Dusemund, B. , Kouba, M. , Kos Durjava, M. , López‐Alonso, M. , López Puente, S. , Marcon, F. , Mayo, B. , Pechová, A. , Petkova, M. , Ramos, F. , Sanz, Y. , Villa, R. E. , Woutersen, R. , Costa, L. , … Ramos, F. (2019a). Scientific Opinion on the safety and efficacy of l‐lysine monohydrochloride and concentrated liquid l‐lysine (base) produced by fermentation using Corynebacterium glutamicum strains NRRL‐B‐67439 or NRRL B‐67535 for all animal species. EFSA Journal, 17(11), 5886. 10.2903/j.efsa.2019.5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V. , Azimonti, G. , Bampidis, V. , Bastos, M. L. , Christensen, H. , Dusemund, B. , Kouba, M. , Kos Durjava, M. , López‐Alonso, M. , López Puente, S. , Marcon, F. , Mayo, B. , Pechová, A. , Petkova, M. , Ramos, F. , Sanz, Y. , Villa, R. E. , Woutersen, R. , … Wallace, R. J. (2019b). Scientific opinion on the safety and efficacy of l‐lysine monohydrochloride and concentrated liquid l‐lysine (base) produced by fermentation using Corynebacterium glutamicum strain NRRL B‐50775 for all animal species based on a dossier submitted by ADM. EFSA Journal, 17(1), 5537. 10.2903/j.efsa.2019.5537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V. , Bastos, M. , Christensen, H. , Dusemund, B. , Kouba, M. , Kos Durjava, M. , López‐Alonso, M. , López Puente, S. , Marcon, F. , Mayo, B. , Pechová, A. , Petkova, M. , Ramos, F. , Sanz, Y. , Villa, R. E. , Woutersen, R. , Brock, T. , de Knecht, J. , … Azimonti, G. (2019c). Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal, 17(4), 5648. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V. , Azimonti, G. , Bastos, M. L. , Christensen, H. , Dusemund, B. , Kouba, M. , Kos Durjava, M. , López‐Alonso, M. , López Puente, S. , Marcon, F. , Mayo, B. , Pechová, A. , Petkova, M. , Sanz, Y. , Villa, R. E. , Woutersen, R. , Costa, L. , Dierick, N. , … Ramos, F. (2019d). Scientific Opinion on the safety of concentrated l‐lysine (base), l‐lysine monohydrochloride and l‐lysine sulfate produced using different strains of Corynebacterium glutamicum for all animal species based on a dossier submitted by FEFANA asbl. EFSA Journal, 17(1), 5532. 10.2903/j.efsa.2019.5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V. , Azimonti, G. , Bastos, M. L. , Christensen, H. , Durjava, M. , Dusemund, B. , Kouba, M. , López‐Alonso, M. , López Puente, S. , Marcon, F. , Mayo, B. , Pechová, A. , Petkova, M. , Ramos, F. , Villa, R. E. , Woutersen, R. , Brantom, P. , Chesson, A. , … Galobart, J. (2023). Guidance on the assessment of the safety of feed additives for the users. EFSA Journal, 21(11), e8469. 10.2903/j.efsa.2023.8469 [DOI] [PMC free article] [PubMed] [Google Scholar]


