Abstract
Background
Inhaled beta‐agonist therapy is central to the management of acute asthma. This review evaluates the benefit of an additional use of intravenous beta2‐agonist agents.
Objectives
To determine the benefit of adding intravenous (IV) beta2‐agonists to inhaled beta2‐agonist therapy for acute asthma treated in the emergency department.
Search methods
Randomised controlled trials (RCTs) were identified using the Cochrane Airways Group Register which is a compilation of systematic searches of MEDLINE, EMBASE, CINAHL, and CENTRAL as well as handsearching of 20 respiratory journals. Bibliographies from included studies and known reviews were also searched. Primary authors and content experts were contacted to identify eligible studies. The search was performed in September 2012.
Selection criteria
Only RCTs were considered for inclusion. Studies were included if patients presented to the emergency department with acute asthma and were treated with IV beta2‐agonists with inhaled beta2‐agonist therapy and existing standard treatments versus inhaled beta2‐agonists and existing standard treatments.
Data collection and analysis
Two review authors independently extracted data and confirmed their findings with corresponding authors of trials. We obtained missing data from authors or calculated from data present in the papers. We used fixed‐effect model for odds ratios (OR) and for mean differences (MD) we used both fixed‐effect and random‐effects models and reported 95% confidence intervals (CI).
Main results
From 109 potentially relevant studies only three (104 patients) met our inclusion criteria: Bogie 2007 (46 children), Browne 1997 (29 children) and Nowak 2010 (29 adults). Bogie 2007 investigated the addition of intravenous terbutaline to high dose nebulised albuterol in children with acute severe asthma, requiring intensive care unit (ICU) admission. Browne 1997 investigated the benefit of adding intravenous salbutamol to inhaled salbutamol in children with acute severe asthma in the emergency department. Nowak 2010 investigated addition of IV bedoradrine to standard care (nebulised albuterol, ipratropium and oral corticosteroids) among adults, and was reported as a conference abstract only.
There was no significant advantage (OR 0.29; 95%CI 0.06 to 1.38, one trial, 29 adults) for adding IV bedoradrine to standard care (nebulised albuterol, ipratropium and oral corticosteroids) with regard to hospitalisation rates.
Various outcome indicators for the length of stay were reported among the trials. Browne 1997 reported a significantly shorter recovery time (in terms of cessation of 30 minute salbutamol) for children in the IV salbutamol with inhaled salbutamol group (four hours) versus the 11.1 hours for the inhaled salbutamol group (P = 0.03). Time to cessation of hourly nebuliser was also significantly shorter (P = 0.02) for the IV plus inhaled salbutamol group (11.5 hours versus 21.2 hours), and they were ready for emergency patient discharge on average 9.7 hours earlier than the inhaled salbutamol group (P < 0.05). In a paediatric ICU study Bogie 2007 reported no significant advantage in length of paediatric ICU admission (hours) for adding IV terbutaline to nebulised albuterol (MD ‐12.95, 95% CI: ‐38.74, 12.84).
Browne 1997 reported there were only six out of 14 children with a pulmonary index score above six in the IV plus inhaled salbutamol group at two hours compared with 14 of the 15 in the inhaled salbutamol group (P = 0.02)
In Browne 1997 there was a higher proportion of tremor in the IV plus inhaled salbutamol group than in the inhaled salbutamol group (P < 0.02). Nowak 2010 did not report any statistically significant adverse effects associated with adding IV bedoradrine to standard care (nebulised albuterol, ipratropium and oral corticosteroids). Troponin levels were elevated in three children in the IV terbutaline + nebulised albuterol group at 12 and 24 hours in Bogie 2007
Authors' conclusions
There is very limited evidence from one study (Browne 1997) to support the use of IV beta2‐agonists in children with severe acute asthma with respect to shorter recovery time, and similarly there is limited evidence (again from one study Browne 1997) suggesting benefit with regard to pulmonary index scores; however this advantage needs to be considered carefully in relation to the increased side effects associated with IV beta2‐agonists. We identified no significant benefits for adults with severe acute asthma. Until more, adequately powered, high quality clinical trials in this area are conducted it is not possible to form a robust evaluation of the addition of IV beta2‐agonists in children or adults with severe acute asthma.
Keywords: Adult; Child; Humans; Acetamides; Acetamides/administration & dosage; Acute Disease; Administration, Inhalation; Administration, Intravenous; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Adrenergic beta‐2 Receptor Agonists; Adrenergic beta‐2 Receptor Agonists/administration & dosage; Albuterol; Albuterol/administration & dosage; Anti‐Asthmatic Agents; Anti‐Asthmatic Agents/administration & dosage; Asthma; Asthma/drug therapy; Drug Therapy, Combination; Drug Therapy, Combination/methods; Emergencies; Ipratropium; Ipratropium/administration & dosage; Naphthalenes; Naphthalenes/administration & dosage; Randomized Controlled Trials as Topic; Terbutaline; Terbutaline/administration & dosage
Plain language summary
Addition of intravenous beta2‐agonists to inhaled beta2‐agonists for acute asthma
Beta2‐agonist drugs are used for the treatment of asthma and work by opening the airways to help people breathe more easily. Beta2‐agonists can be given to people in two different ways – intravenously (directly thorough a vein) and via an inhaler. Inhalers are one of the most important treatments for people with acute severe asthma. The question this review considered was whether treatment would offer additional benefit if patients received these drugs both ways (by breathing them via an inhaler and receiving them directly through a vein) than by just inhaling them alone. This review examined all the randomised controlled trials on the use of intravenous beta2‐agonists in addition to inhaled beta2‐agonists with existing standard care (such as steroids either taken as tablets of by injection) in severe acute asthma.
We found three trials involving 104 people (75 children and 29 adults) with acute asthma. There was no significant difference in adults receiving intravenous beta‐agonists as well as standard care in the one small trial considering this comparison. We also looked at length of stay in the emergency department. Two reported shorter recovery time or quicker discharge from the emergency department in patients also receiving intravenous beta‐agonists. One trial reported that more children experienced tremor if they had received injected beta‐agonists whereas another trial, with adults, reported no significant difference in adverse effects. As there are so few trials and so few included patients we cannot be sure about the reliability of these findings.
This review found that until more, larger, high quality clinical trials in this area are conducted it is not possible to judge whether there is any enhanced benefit using additional intravenous beta2‐agonists in children or adults with severe acute asthma compared with inhaled beta2‐agonists alone.
Summary of findings
Summary of findings for the main comparison. IV + inhaled beta agonist compared with inhaled beta‐agonist for acute asthma.
| IV + inhaled beta agonist compared to inhaled beta‐agonist for acute asthma | ||||||
| Patient or population: patients with acute asthma Settings: ED and ICU Intervention: IV + inhaled beta agonist Comparison: inhaled beta‐agonist | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| inhaled beta‐agonist | IV + inhaled beta agonist | |||||
| Admissions to hospital | 54 per 100 | 25 per 100 (7 to 62) | OR 0.29 (0.06 to 1.38) | 29 (1 study) | ⊕⊕⊝⊝ low1 | |
| Length of stay in emergency department | mean control group stay was 57 (SD 56) minutes | The mean length of stay in the intervention groups was 12.95 minutes lower (38.74 lower to 12.84 higher) | MD ‐12.95 (‐38.74 to 12.84) | 46 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| Pulse rate at 2 hours | the mean heart rate in the control group was 142 (SD 10) | The mean heart rate at 2 hours in the intervention groups was 10 beats per minute higher (1.07 lower to 21.07 higher) | MD 10.00 (‐1.07 to 21.07) | 29 (1 study) | ⊕⊕⊕⊝ moderate2 | |
|
Clinical Failure (children with a severe to moderate overall clinical assessment score at two hours) |
93 per 100 | 56 per 100 (22 to 84) | OR 0.09 (0.02 to 0.38) | 29 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Admissions: one point deducted for risk of bias due to lack of clarity in randomisation and blinding procedures, and an additional point deducted as data contributed by only one study
2 An additional point deducted as data contributed by only one study
Background
Description of the condition
In the period 2005 to 2006 there were 65,732 hospital admissions for asthma in the UK (NHS 2011), and 10 million people experience asthma exacerbations in the US each year (Krishnan 2006). Approximately 10% to 20% of acute asthma cases in the US lead to hospital admission, and a similar proportion of those discharged from the emergency department relapse within the following 14 days (Emerman 1999; Emerman 2001). Over the last two decades several national (e.g. Boulet 1999; BTS 1997; BTS/SIGN 2011; NAEPP 1997; NIH 2007) and international (e.g. GINA 2011; NHLBI/WHO 1995) guidelines have been produced for the management of acute asthma.
Description of the intervention
Over the last 25 years there have been numerous examples of studies investigating the role of IV beta2‐agonists in the management of acute asthma. In North America and Europe practice guidelines have recommended inhaled beta2‐agonist therapy for all emergency department management of asthma (Beveridge 1996; BTS/SIGN 2011; Ernst 1996; GINA 2011; Lipworth 1997; NAEPP 1997; NIH 2007).
How the intervention might work
The conventional and recommended management of severe acute asthma is to use beta2‐agonist bronchodilators and corticosteroids. When aiming to ensure efficient bronchodilatation, especially in severe acute asthma, penetration of an inhaled drug to the affected small conducting airways may be limited, and positive reactions may be a result of the drug reaching the receptors via the systemic circulation. In these circumstances, if bronchodilatation occurs predominantly in response to the systemic distribution of the drug, intravenous (IV) in addition to inhaled administration of bronchodilators may provide an earlier clinical response (Browne 1997).
Why it is important to do this review
For patients unresponsive to inhaled bronchodilator and systemic corticosteroid therapy IV beta2‐agonists are considered as second line therapy. Alternatively they are considered if the inhaled route is not practical for the patient (Beveridge 1996; BTS/SIGN 2011; Ernst 1996; GINA 2011; Lipworth 1997; NAEPP 1997; NIH 2007). However, the benefit of this route of delivery remains a matter of intense debate. The previous systematic review by Travers (Travers 2001), concluded that 'There is no evidence to support the use of IV beta2‐agonists in patients with severe acute asthma. These drugs should be given by inhalation. No subgroups were identified in which the IV route should be considered.' This is a new review based on the previous protocol for Travers 2001. The review aims to evaluate that conclusion with regard to relevant randomised controlled trials published over the last 11 years investigating the addition of intravenous beta agonists to inhaled beta agonist therapy.
A separate review is available on The Cochrane Library for 'Continuous versus intermittent beta2‐agonists for acute asthma' (Camargo 2011) and reviews of epinephrine for acute asthma and intravenous beta2‐agonists versus intravenous aminophylline are currently in preparation.
Objectives
To determine if the evidence from randomised trials supports the use of IV beta2‐agonists in addition to inhaled beta2‐agonists in the treatment of patients with severe acute asthma who present to the emergency department.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
We included studies of adult or paediatric patients with severe acute asthma presenting to an emergency room (or its equivalent).
Types of interventions
The target intervention was the administration of IV selective or non‐selective beta1 and beta2‐agonists.
We compared IV beta2‐agonists used in addition to inhaled beta2‐agonists and existing standards of care with inhaled beta2‐agonists and standard care alone.
Types of outcome measures
Primary outcomes
Hospital admission
Length of stay
Secondary outcomes
Pulmonary function
Vital signs
Adverse effects
Clinical scores
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched records in the CAGR coded as 'asthma' using the terms given in Appendix 2. We also conducted a search of ClinicalTrials.gov and the search strategy is given in Appendix 2. All databases were searched from their inception to the present and there was no restriction on language of publication. The searches were conducted in November 2011 and updated in September 2012.
Searching other resources
Enquiries regarding other published or unpublished studies known and/or supported by the authors of the primary studies were made so that these results could be included in this review. Several pathways were used to locate authors including letters to an address presented in the article, Internet 'People and Hospital Searches', electronic author searches in library databases for the address on the most recent article published by the author, and contact with other review authors in the Cochrane Airways Group. Scientific advisors of the various pharmaceutical companies (Glaxo) that manufacture beta2‐agonists were contacted for any unpublished, published, or interim results on beta2‐agonist research. Personal contact with colleagues, collaborators and other trialists working in the field of asthma was made to identify potentially relevant studies. We searched reference lists of included papers and other systematic reviews for additional relevant studies.
Data collection and analysis
Selection of studies
The reference lists from the search strategy was independently reviewed by two review authors (AHT, SJM), and clearly irrelevant articles were discarded. If the title, abstract, or descriptors suggested any potential relevance, the full text article was retrieved. Two review authors (SJM, AHT) then assessed each relevant paper for inclusion in this review. The review authors were not blinded to the authors, journal of publication, or results of the studies as investigator bias was deemed unlikely. Disagreement would have been resolved by consensus or third party adjudication (CC).
Data extraction and management
Two review authors (AHT, SJM) independently extracted data and one review author (SJM) entered the data into The Cochrane Collaboration software program (Review Manager Version 5.1 Revman 2011).
Assessment of risk of bias in included studies
The risk of bias of included studies was assessed using the Collaboration's risk of bias methodology see Chapter 8 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). Two review authors (AHT and SJM) assessed the risk of bias for all included studies with regard to random sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting. Each item was assessed as high, low or unclear risk of bias along with relevant information reported in the randomised controlled trial.
Measures of treatment effect
For dichotomous variables, data are expressed as odds ratios (OR) with 95% confidence intervals (CI). Data for continuous variables were reported as mean differences (MD) with 95% CIs.
Unit of analysis issues
The unit of analysis was the patient.
Dealing with missing data
We planned to contact authors If outcome data or information on trial design was missing; however, this need did not arise.
Assessment of heterogeneity
Heterogeneity was assessed with regard to the forest plots. The Chi2 test was similarly considered (P value < 0.10) but interpreted with caution owing to the low power associated with this test. I2 (Higgins 2011) was also considered and interpreted in relation to the following guidance:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
Examination of publication bias was planned, using funnel plots, if there was an adequate number of trials aggregated in the analyses. It is however recognised that an asymmetrical funnel plot can reflect heterogeneity, outcome reporting bias and small study effects and is therefore, not necessarily a reflection of publication bias, especially in reviews of less than 10 trials..
Data synthesis
We planned to combine data using the Review Manager 5.1 software (Revman 2011), however we were unable to pool any data. For continuous variables, a random‐effects MD and 95% CI were calculated for each study. For dichotomous variables, a random‐effects OR with 95% CI was calculated for individual studies. All similar studies were pooled using random‐effects OR or MD and 95% CIs.
Subgroup analysis and investigation of heterogeneity
The following subgroup analysis was planned; however, the small number of available trials did not make this possible.
Population: adult versus paediatric
Sensitivity analysis
Sensitivity analyses were planned but the paucity of data meeting the inclusion criteria for the review precluded us from conducting this assessment.
Results
Description of studies
Results of the search
The Cochrane Airways Group database searches identified a total of 425 references, and an additional 49 references were identified from Travers 2001. Independent review of the abstracts and titles of these publications identified three studies assessed as eligible for inclusion in the review (Figure 1).
1.
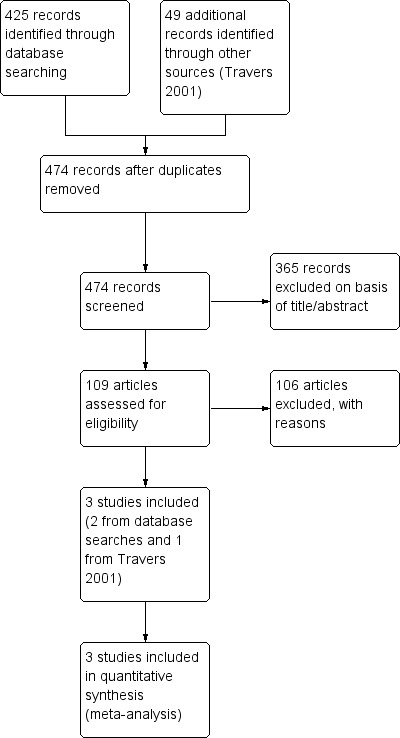
Study flow diagram.
Included studies
Three studies on 104 people met our inclusion criteria Bogie 2007 (46 children), Browne 1997 (29 children) and Nowak 2010 (29 adults). Bogie 2007 and Browne 1997 were paediatric studies of severe acute asthma, but differed in the clinical setting. Bogie 2007 investigated the benefit of adding intravenous terbutaline to high‐dose nebulised albuterol in children with acute severe asthma who required intensive care unit (ICU) admission. Browne 1997 investigated the benefit of adding intravenous salbutamol to inhaled salbutamol in children with acute severe asthma in the emergency department (ED).
Nowak 2010 is reported as a conference abstract and the information available is relatively limited. The objective was to assess the benefit of adding IV bedoradrine to standard care (nebulised albuterol, ipratropium and oral corticosteroids) in the treatment of severe acute exacerbations of asthma.
Excluded studies
One hundred and six studies failed to meet the eligibility criteria of this review. Forty (38%) were not randomised and in 22 (21%) the focus was on epinephrine (rather than IV beta2‐agonists). Thirteen (12%) trials assessed inhaled β2 agonists versus IV β2 agonists and 11 (10%) compared IV β2 agonists versus IV methylxanthines. In a further five studies (5%) the focus was on subcutaneous (rather than IV) β2 agonists and in three (3%) trials the patients has chronic asthma rather than acute asthma. A further two (2%) were reviews and two (2%) trials compared IV β2 agonists versus nebulised ipratropium. Another two (2%) were excluded as they were conducted in a laboratory setting rather than in the emergency department. The remaining six were excluded because one (1%) trial compared IV terbutaline versus IV prenalterol, one (1%) trial compared β2 agonists against steroids, another (1%) trial compared two IV β2 agonists, and another (1%) trial compared ipratropium plus standard care versus standard care alone. One (1%) trial compared IV β2 agonists versus IV atrial natriuretic factor (ANF) and another (1%) trial compared IV salbutamol versus IV epinephrine. The reasons for the exclusion of each reference is given in Characteristics of excluded studies.
Risk of bias in included studies
Allocation
Two studies were judged to be at low risk of selection bias (Bogie 2007; Browne 1997) and in the third (reported as a conference abstract) it was unclear (Nowak 2010). Full details of our 'Risk of bias' judgements can be found in Characteristics of included studies and the judgements are presented graphically in Figure 2 and Figure 3.
2.
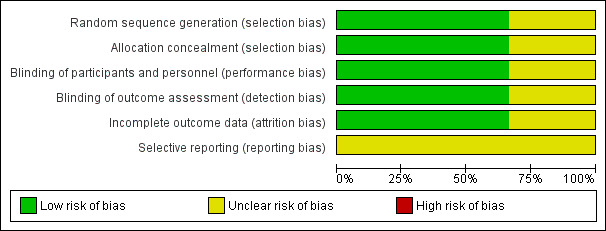
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
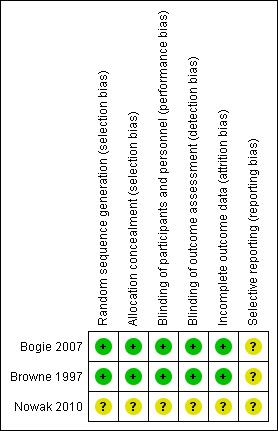
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Blinding
Two of the included studies were assessed as low risk of bias (Bogie 2007; Browne 1997) and in (Nowak 2010) the risk was assessed as unclear.
Incomplete outcome data
Two of the included studies were judged to be at low risk of attrition bias Bogie 2007; Browne 1997 and in the third the risk was judged to be unclear Nowak 2010.
Selective reporting
In all three studies the risk of reporting bias was assessed as unclear.
Effects of interventions
See: Table 1
Very little data from the three included studies were reported in a form that could be incorporated in meta‐analyses.
Admissions
One study Nowak 2010 with 29 adult patients indicates there was no significant advantage for adding IV bedoradrine to standard care (nebulised albuterol, ipratropium plus oral corticosteroids) with regard to hospitalisation rates (OR 0.29; 95% CI 0.06 to 1.38; Analysis 1.1).
1.1. Analysis.
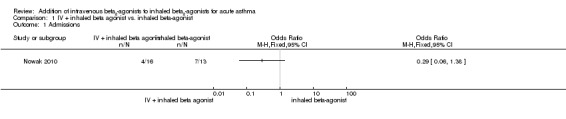
Comparison 1 IV + inhaled beta agonist vs. inhaled beta‐agonist, Outcome 1 Admissions.
Length of stay
Browne 1997 reported a significantly shorter recovery time (in terms of cessation of 30 minute salbutamol) for children in the IV plus inhaled salbutamol group (four hours) versus 11.1 hours for the inhaled salbutamol group (P = 0.03). Time to cessation of hourly nebuliser was also significantly shorter (P = 0.02) for the IV plus inhaled salbutamol group (11.5 hours versus 21.2 hours), and they were ready for emergency patient discharge on average 9.7 hours earlier than the inhaled salbutamol group (P < 0.05). In a paediatric ICU study, Bogie 2007 reported no significant advantage in length of PICU admission (hours) for adding IV terbutaline to nebulised albuterol (mean difference (MD) ‐12.95; 95% CI ‐38.74 to 12.84; Analysis 1.2).
1.2. Analysis.
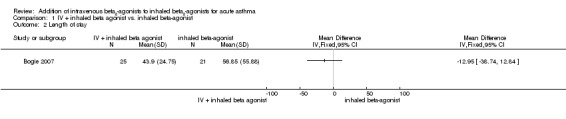
Comparison 1 IV + inhaled beta agonist vs. inhaled beta‐agonist, Outcome 2 Length of stay.
Heart rate
There was no significant difference in heart rates for paediatric patients between the IV plus inhaled salbutamol and inhaled salbutamol groups at two hours (one study, 29 participants; Analysis 1.3).
1.3. Analysis.
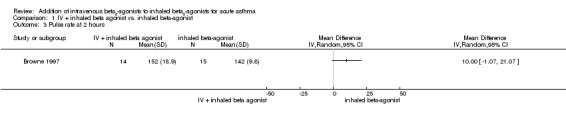
Comparison 1 IV + inhaled beta agonist vs. inhaled beta‐agonist, Outcome 3 Pulse rate at 2 hours.
Need for supplemental oxygen
In Browne 1997 only two of the 14 children in the IV plus inhaled salbutamol group were on medical oxygen at two hours, contrasting with eight of the 15 in the inhaled salbutamol group (P = 0.05).
Asthma severity score
A study in 46 children in ICU (Bogie 2007) reported a significant benefit in adding IV terbutaline to nebulised albuterol in relation to the improvement of Clinical Asthma Severity Scores over the first 24 hours of 6.5 points in the IV plus inhaled group compared with 4.8 points in inhaled group (P = 0.073). In another paediatric study, Browne 1997 reported a significant benefit of adding IV salbutamol to nebulised salbutamol in relation to the improvement in both the National Australian Asthma Campaign Severity clinical assessment scale and the pulmonary index score. The National Australian Asthma Campaign Severity clinical assessment scale was measured at two hours where (36% (5/14) had persistent moderate to severe asthma in the IV plus inhaled salbutamol group compared with 93% (14/15) in the inhaled salbutamol only group (P < 0.002); these data are included in Analysis 1.4 as a measure of clinical failure, and in view of the small number of participants in this single study should be interpreted with caution. The pulmonary index score was ≥ seven at two hours in six out of 14 (43%) participants in the IV plus inhaled salbutamol group versus 13 out of 15 (93%) in the inhaled salbutamol only group (P < 0.02)).
1.4. Analysis.

Comparison 1 IV + inhaled beta agonist vs. inhaled beta‐agonist, Outcome 4 Clinical Failure.
Adverse effects
Although we were unable to pool data, all three studies reported information on adverse effects. In Browne 1997 there was a higher proportion of tremor in the IV plus inhaled salbutamol group than in the inhaled salbutamol group (P < 0.02). Nowak 2010 did not report any statistically significant adverse effects associated with adding IV bedoradrine to standard care (nebulised albuterol, ipratropium and oral corticosteroids). Troponin levels were elevated in three children in the IV terbutaline + nebulised albuterol group at 12 and 24 hours in Bogie 2007.
Discussion
Summary of main results
The randomised controlled trial literature is very limited regarding the addition of intravenous beta2‐agonists to inhaled beta2‐agonists in the treatment of acute asthma, and we were able to include only three small trials in this review. The two paediatric trials (Browne 1997; Bogie 2007) were generally judged to be at low risk of bias, whereas there were more uncertainties in methodological rigour and other risks of bias due to the study being published in abstract form only and with insufficient information to draw firm conclusions in the adult study (Nowak 2010).
In terms of recovery time, there is only very limited evidence from one study (Browne 1997) to support the use of IV beta2‐agonists in children with severe acute asthma, and there is also very limited evidence (again from a single study Browne 1997) indicating benefit with regard to pulmonary index scores; however this advantage needs to be considered carefully in relation to the increased side effects associated with IV beta2‐agonists (Browne 1997) Moreover, these positive results were reported for secondary outcomes, which are less likely to be clinically important outcomes to either patients or physicians. We identified no apparent benefits for adults with severe acute asthma from the one available study broadly meeting our inclusion criteria (Nowak 2010). A conservative interpretation of these findings is appropriate and final conclusions should be reserved until more, adequately powered, high quality clinical trials are available. On the basis of the data currently available meeting our inclusion criteria, it is not possible to form a robust evaluation of the addition of IV beta2‐agonists to inhaled beta2‐agonists in children or adults with severe acute asthma
Overall completeness and applicability of evidence
In view of the limited number of trials and patients included in this review, and the lack of opportunity for statistical aggregation, no firm conclusions can be made. The completeness and applicability of the evidence available from randomised controlled trials indicates that we should be cautious in generalising from the data. There is slightly more information available from the included studies with regard to children with severe acute asthma from the Bogie 2007 and Browne 1997 trials; however, only the latter was conducted in the emergency department. The paucity of data relating to the addition of IV beta2‐agonists to inhaled beta2‐agonists for adults with severe acute asthma is striking and the only available randomised study (Nowak 2010), with 29 patients, is reported in conference proceedings.
Quality of the evidence
With regard to random sequence generation only two trials were judged to be low in risk of selection bias (Bogie 2007; Browne 1997); the risk of bias for Nowak 2010 was judged as unclear as details of the random sequence generation were not described in the trial report. In terms of the blinding of participants and personnel, Bogie 2007 and Browne 1997 were judged to be at low risk of performance and detection bias, and Nowak 2010 was assessed as unclear. Two of the included studies were judged to be at low risk of attrition bias (Bogie 2007; Browne 1997) and in the third trial, the risk was judged to be unclear Nowak 2010.
Potential biases in the review process
The support provided by the Cochrane Airways Group in the identification of potentially relevant trials is of a very high order; however, there is inevitably a concern regarding study selection bias or publication bias in this review. There is a concern that failure to identify unpublished trials may lead to an incomplete estimation of the effects IV beta2‐agonists may have when given in addition to inhaled beta2‐agonists in the treatment of acute severe asthma. Having said that, an exhaustive search of the published literature, without language restrictions, for potentially relevant clinical trials was undertaken using a systematic search strategy to minimise the likelihood of bias; however, we recognize that additional unidentified trials may exist. The standardisation of reporting would improve the opportunities to draw comparisons among trials. It is also of concern that the assessment of adverse effects was hampered by a lack of standardised reporting.
Agreements and disagreements with other studies or reviews
The earlier review (Travers 2001) included an additional comparison of inhaled beta2‐agonists versus IV beta2‐agonists, together with an evaluation of IV aminophylline versus IV beta2‐agonists. With regard to this particular comparison there is consistency with Travers 2001 in as much as the opportunity to draw robust conclusions of the effects IV beta2‐agonists may have when given in addition to inhaled beta2‐agonists is limited by paucity of data and a lack of standardisation in reporting outcomes.
Authors' conclusions
Implications for practice.
The current evidence is insufficient to provide recommendations regarding the addition of IV beta2‐agonists to inhaled beta2‐agonists as a standard treatment for severe acute asthma. The clinical benefits and adverse effects that IV beta2‐agonists may have when given in addition to inhaled beta2‐agonists in the paediatric and adult population remains unclear since too few clinical trials were available.
Implications for research.
Population
The clinical benefits and adverse effects that IV beta2‐agonists may have when given in addition to inhaled beta2‐agonists in patients with severe acute asthma needs to be clarified in adequately powered, high quality randomised trials.
Interventions
Further research is required to clarify whether IV beta2‐agonists improve outcomes when given in addition to nebulised bronchodilator (beta2‐agonists and anticholinergics) and systemic corticosteroid therapy.
The evidence for subcutaneous routes of beta2‐agonists (both selective and non‐selective) must be formally evaluated via a systematic review.
Outcomes
Future research on acute asthma must concentrate on well defined outcomes which may lead to more informative overviews in the future. More specifically the following areas must be refined:
Statistical planning and sample size calculations must be more carefully considered. Trials should be large enough to protect against type II error, and when multiple statistical tests are performed the increased risk of type I error should be addressed.
Standardisation and complete reporting of clinically relevant outcomes such as: admission to hospital, admission to intensive care department, length of hospital stay, relapse rates.
Complete reporting of pulmonary function tests (PFT) data in a systematic and standardised fashion would assist in further work (i.e. reporting of % predicted peak expiratory flow rate (PEFR) and changes in % predicted PEFR).
The inherent variability of these PFTs, particularly in acute asthma, emphasizes the need for further research into alternative measures, particularly assessment of factors that are important to the patient.
Standardisation and complete reporting of Asthma Severity Scores.
Standardisation and complete reporting of adverse reactions and side effects.
Acknowledgements
We would particularly like to acknowledge the excellent support and assistance from Emma Welsh, Liz Stovold, and Emma Jackson of the Cochrane Airways Review group, together with the greatly appreciated guidance from Chris Cates (Cochrane Airways Review Group Co‐ordinating Editor). We are most grateful to Karen Kelly and Samantha Barker for their assistance with data extraction, entry and review at an early stage of this review's development, and we are particularly grateful to Toby Lasserson, Luis Nannini and Taixiang Wu for their help with the translation of potentially relevant non‐English trials. The support provided by librarians Judith Scammel, Jane Appleton and Hilary Garrett at St George's University of London is also greatly appreciated.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Hand‐searches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Database search strategies
Cochrane Airways Group Register of trials (CAGR)
(status* or emergenc* or ED or ER or trauma* or emergicent* or casualty or observation* or holding* or admit* or admission* or discharg* or hospitali* or outpatient* or acute* or exacerbat* or sever*) AND (bronchodilat* or "adrenergic beta‐agonists" or beta‐agonist or "beta agonist" or beta2* or beta‐2* or albuterol or salbutamol or levalbuterol or levosalbutamol or ventolin* or proventil or ventosol or proair or isoproterenol or metaproterenol or aluprent or terbutaline or brethine or bricanyl or fenoterol or bedoradrine or reproterol or clenbuterol ) AND ( intraven* or IV or I.V. or bolus or infus* or inject*)
[This search was limited to records coded as 'asthma']
Clinicaltrials.gov
search terms = intravenous study type = interventional studies conditions = asthma
Data and analyses
Comparison 1. IV + inhaled beta agonist vs. inhaled beta‐agonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Admissions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Length of stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pulse rate at 2 hours | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Clinical Failure | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bogie 2007.
| Methods | Prospective, randomised double blind, placebo‐controlled trial. | |
| Participants | 46 paediatric patients with severe acute asthma who present to the emergency department | |
| Interventions | IV terbutaline with nebulised albuterol, nebulised ipratropium and systemic corticosteroids versus IV placebo with nebulised albuterol, nebulised ipratropium and systemic corticosteroids. Details of dosage used is included below. The independent variable (whether IV beta2‐agonist or placebo) was initially given as loading dose of 10 mg/kg per minute over 10 to 20 minutes, and then as a continuous infusion of 1 mg/kg per minute. It was increased to 2 mg/kg per minute at the discretion of the attending physician or as a result of deteriorating CASS. If there was an additional decline in the participant's condition the dose was increased to 4 mg/kg per minute and aminophylline was also introduced. The children in both arms of the study received continuous nebulized albuterol as follows 'at a dose determined by weight; 10 mg/h for children less than 20 kg, 15 mg/h for children between 20 and 40 kg, and 20 mg/h for children larger than 40 kg. For children less than 40 kg, the dose of albuterol could be increased every hour to a maximum of 20 mg/h. Ipratropium bromide nebulisation at 250 mg every 6 hours for children less than or equal to 10 kg and 500 mg every 6 hours for children equal to or greater than 11 kg was given to all study patients. Methylprednisolone was provided at a dose of 2 mg/kg loading dose followed by 1 mg/kg every 6 hours. All patients received a normal saline bolus of 20 mL/kg followed by maintenance intravenous fluids containing D5 1/2 NS with 20 mEq/L of KCl.' |
|
| Outcomes | 46 patients enrolled into study: 25 in IV terbutaline arm, and 21 in placebo arm. Clinical Asthma Severity Score: mean improvement at 24 hours of 6.5 with IV terbutaline arm versus 4.8 with placebo arm (P = 0.073). Continuous nebulised albuterol duration of therapy: 38.19 hours in IV terbutaline arm versus 51.93 hours in placebo arm (P = 0.25). Pediatric Intensive Care Unit Length of Stay: mean 43.9 hours in IV terbutaline vs mean 56.85 hours in placebo arm (P = 0.345). |
|
| Notes | Contamination of IV terbutaline arm where discretion of the attending physician allowed titrating the study drug to 4ug/kg/min from baseline 2ug/kg/min. For this higher dose, aminophylline was added to the component therapy. 5/21 (24%) of placebo group received aminophylline, and 9/25 (36%) of terbutaline group received aminophylline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 49 patients enrolled, 5 did not complete the study, but unclear which treatment arm. |
| Selective reporting (reporting bias) | Unclear risk | No apparent indication of reporting bias |
Browne 1997.
| Methods | Randomisation: yes (table of random numbers) Blinding: double‐blind Number excluded: 13 Withdrawals: none Baseline characteristics: Heart rate 127.8 (15.4 ) intravenous + nebulised group (iv), 146.2 (13.6) nebulised group; Respiratory rate 38.9 (11.9) iv, 45.8 (9.9) nebulised; glucose 7.5 (2.7) iv, 8.5 (3.1) nebulised; potassium 3.9 (0.5) iv, 4.2 (0.6) nebulised; pulmonary index 12 iv, 15 nebulised; accessory muscle use 12 iv, 15 nebulised; Dyspnea12 iv, 13 nebulised; wheeze 13 iv, 14 nebulised; fatigue 7 iv, 9 nebulised | |
| Participants | Location: Westmead, Australia Participants: initially 50, 37 eligible, 29 final (8 gave no consent), 1‐12 yrs (mean 8.4 iv, 6.3 nebulised); males 7 iv, 12 nebulised; females 7 iv, 3 nebulised; height 1.3m (0.2) iv, 1.2m (0.2) nebulised; weight 29.2 kg (10.1) iv, 22.5 kg (8.1) nebulised Asthma definition and severity: severe acute asthma as per National Australian Asthma Campaign guidelines Exclusion criteria: mild, moderate or life‐threatening asthma, congenital heart disease,, family history or past episode of supraventricular tachycardia (SVT), respiratory illness, diabetes mellitus weighed < 10kg or > 50kg, aged < 12months or > 12yrs, or had already received the maximum iv dose for the day Inhaled corticosteroid use: no details | |
| Interventions | Standard care: Coincident with iv drugs, O2 NPV 30%, continuous salbutamol 2.5 mg (< 2 yrs ) or 5 mg ( > 2 yrs ), hydrocortisone 5 mg/kg iv, then from 2 hrs onwards continuous salbutamol, then at 30 minutes, 60 minutes, 2 hours, 3 hours, 4 hours depending on clinical state Treatment group: salbutamol iv 15 ug/kg over 10 min at 0 min Placebo: saline | |
| Outcomes | PFTs: not done Timing: not done Admissions: all patients admitted to high‐dependency ward Side effects: higher proportion with tremor at 2 hours (specifics unknown) Complications: | |
| Notes | Run in period of 30 min where patients given salbutamol nebulised of 2.5 or 5 mg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by hospital pharmacy using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Investigators unaware as to order of randomisation sequence. Allocation sequence was retained by the pharmacy and released only when all clinical and laboratory assessments had been completed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Staff, investigators and patients were blinded and physicians who had administered the intravenous solution were surveyed to assess whether they had been aware of the solution being salbutamol or saline at the time of the bolus infusion and effective blinding was demonstrated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Details of allocation released by pharmacy only when all clinical and laboratory assessments had been completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients withdrawn from trial |
| Selective reporting (reporting bias) | Unclear risk | No indication of reporting bias |
Nowak 2010.
| Methods | Randomised, placebo‐controlled, dose‐escalation, multicentre trial | |
| Participants | 29 patients with severe acute asthma in emergency department setting | |
| Interventions | All patients received nebulised albuterol and ipratropium with oral corticosteroids. Patients with FEV1< 55% were randomised to MN‐221 (bedoradrine) or placebo | |
| Outcomes | 13 patients received placebo and 16 patients received MN‐221. MN‐221 was administered at the following doses: 5 at 240 ug over 15 min, 6 at 450 ug over 15 min, and 5 at 1080 ug over two hours (2 received 1995 ug). Reduced hospitalisation rate: MN‐221 4/16 (25%) vs placebo 7/13 (54%). Improved FEV1: change in baseline AUC1‐5hr was 43% higher in the MN‐221 arm compared to placebo. No significant difference in adverse events between arms: ECG, heart rate, etc |
|
| Notes | Abstract format only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. Conference abstract – limited information |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Conference abstract – limited information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear. Conference abstract – limited information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. Conference abstract – limited information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. Conference abstract – limited information |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Conference abstract – limited information |
CASS: Clinical Asthma Severity Score FEV1: forced expiratory volume in 1 sec iv: intravenous NPV: negative predictive value PFTs: pulmonary function tests
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd 1989 | Epinephrine (rather than IV β2 agonists). |
| Aggarwal 1986 | Excluded on basis of non randomised clinical trial, unclear asthma severity of patients, and IV aminophylline versus IV epinephrine versus SC salbutamol. |
| Anonymous 1978 | Non‐experimental study (not randomised controlled clinical trial). |
| Appel 1989 | Epinephrine (rather than IV β2 agonists). |
| Arnaud 1977 | Not a randomised controlled clinical trial. |
| Arnaud 1982 | D: RCT, (P): severe acute asthma, (I): IV terbutaline & steroids vs (C) IV steroids, (O): PEF. Excluded on basis of comparing IV terbutaline & steroids vs IV steroids. |
| Badatcheff 1989 | D: Single blind RCT, (P): severe acute asthma, (I): IV salbutamol vs (C) IV EPI, (O): PEF. Excluded on basis of comparing IV salbutamol vs IV EPI. |
| Baur 1988 | D: non RCT, (P): severe acute asthma, (I): IV fenoterol vs (C) no comparator, (O): various outcomes including serum levels, PF, HR. Excluded on basis of comparing IV fenoterol vs no comparator. |
| Becker 1983 | T: Epinephrine (rather than IV β2 agonists). |
| Ben‐Zvi 1980 | D: RCT, (P): severe acute asthma, (I): SC EPI, SC sus‐phrine salbutamol vs (C) nebulised fenoterol, (O): vitals, PEF. Excluded on basis of comparing SC EPI vs SC sus‐phrine salbutamol vs nebulised fenoterol. |
| Ben‐Zvi 1982 | Epinephrine (rather than IV β2 agonists). |
| Ben‐Zvi 1983 | Epinephrine (rather than IV β2 agonists). |
| Beswick 1975 | Not a randomised controlled clinical trial. |
| Bloomfield 1979 | This trial does not compare the addition of iv β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between salbutamol 5 mg IPPB at 0 min or at 60 min vs. salbutamol 500 ug iv over 3 minutes at 0 min or at 60 min. |
| Blumenthal 1979 | Letter, not a clinical trial. |
| Boe 1985 | Not randomised controlled clinical trial. Intravenous beta‐agonists use was not the primary research question (no control; compared 2 doses of terbutaline ‐ dose response curve). |
| Bohn 1984 | Not a randomised controlled clinical trial. |
| Brandstetter 1980 | Epinephrine (rather than IV β2 agonists). |
| Browne 2002 | Excluded on basis of IV salbutamol with standard of care vs nebulised ipratropium with standard of care vs IV salbutamol and nebulised ipratropium vs standard of care. |
| Bruguerolle 1991 | Not a randomised controlled clinical trial. |
| Chanez 1990 | Excluded on basis of IV terbutaline vs IV ANF. |
| Cheong 1988 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between salbutamol 5 mg NEB at 30 min and at 120 min vs. salbutamol iv infusion 12.5 ug/min for four hrs at 30 min. |
| Chiang 2000 | Excluded on basis of no comparative cohort. |
| Claybo 1985 | Excluded on basis of design: Letter to editor only, no data available. |
| Crompton 1990 | Review. |
| Davis 1977 | Subcutaneous (rather than IV) β2 agonists. |
| Downes 1973 | Not a randomised controlled clinical trial. |
| Edmunds 1981 | Not a randomised controlled clinical trial. |
| Elenbaas 1985 | Epinephrine (rather than IV β2 agonists). |
| Evans 1979 | Exclude on basis of IV salbutamol vs. IV methylxanthines. |
| Evans 1980 | Not a randomised controlled clinical trial ‐ cohort study. |
| Fanta 1986 | Epinephrine (rather than IV β2 agonists). |
| Femi‐Pearse 1977 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between i.v. salbutamol vs. i.v. aminophylline. |
| Fitchett 1975 | Not a randomised controlled clinical trial ‐ cohort study. |
| Gotz 1981 | Epinephrine (rather than IV β2 agonists). |
| Grant 1976 | Letter to editor. |
| Greefhorst 1983 | D: non RCT, (P): chronic stable asthma, (I): IV terbutaline vs (C) IV prenaterol, (O): PEF, vitals. Excluded on basis of comparing IV terbutaline vs IV prenaterol. |
| Greif 1985 | Not a randomised controlled clinical trial ‐ cohort study. |
| Hambleton 1979 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between intravenous salbutamol vs. intravenous aminophylline. |
| Hayday 2002 | D: Double blind RCT, (P): severe acute peds asthma, (I): nebulised ipratropium with standard care vs (C) standard care (nebulised albuterol and IV hydrocortisone), (O): PEF. Excluded on basis of comparing nebulised ipratropium with standard care vs. standard care (NEB albuterol and IV hydrocortisone). |
| Herman 1983 | Not a randomised controlled clinical trial ‐ cohort study. |
| Hetzel 1976 | Not a randomised controlled clinical trial ‐ cohort study. |
| Hirsch 1979 | Case report. |
| Hussein 1986 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between IV reproterol vs. inhaled reproterol. |
| Hussein 1986a | D: RCT, (P): severe acute asthma, (I): IV repoterol IV theoph, IV steroids vs (C) nebulised salbutamol, IV theoph, IV steroids EPI, (O): PEF. Excluded on basis of comparing IV repoterol IV theophylline, IV steroids vs nebulised salbutamol, IV theophylline IV steroids EPI. |
| Hutton 2002 | D: RCT, (P): severe acute asthma, (I): IV salbutamol with standard care vs (C) nebulised ipratropium; vs (C2) IV salbutamol and nebulised ipratropium and standard care, (O): PEF. Excluded on basis of comparing IV salbutamol with standard care vs (C) nebulised ipratropium; vs (C2) IV salbutamol and nebulised ipratropium and standard care. |
| Iodice 1980 | Not a randomised controlled clinical trial ‐ cohort study. |
| Janson 1988 | (D) Non‐RCT, (P) stable asthmatics in outpatient setting, (I) terbutaline SC with amino IV, (C) terbutaline SC with delayed ipratropium nebulised, (C) terbutaline SC with concurrent ipratropium nebulised (C), (O). Exclude on basis of terbutaline SC with amino IV vs terbutaline SC with delayed ipratropium nebulised vs terbutaline SC with concurrent ipratropium nebulised. |
| Janson 1992 | Not a randomised controlled clinical trial. |
| Johnson 1978 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between aminophylline infusion 1 mg/min at 75 min and 'control group' of inhaled salbutamol vs. salbutamol iv infusion at 10 ug/min at 75 min. |
| Karetzky 1980 | Epinephrine (rather than IV β2 agonists). |
| Kornberg 1991 | Epinephrine (rather than IV β2 agonists). |
| Lawford 1978 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between salbutamol 10 mg nebulised at 0 min lasting for 45 min vs. salbutamol iv infusion 20 ug/min at 0 min lasting for 45 min. |
| Lebovitz 2004 | Excluded on basis of design: (D) dose finding/pharmacokinetic study. |
| Li 2002 | D: RCT, (P): severe acute asthma, (I): IV terbutaline (domestic source); (C) IV terbutaline (local source), (O): PEF. Excluded on basis of comparing IV terbutaline (domestic source) vs IV terbutaline (local source). |
| Lin 1996 | Epinephrine (rather than IV β2 agonists). |
| Lowell 1987 | Epinephrine (rather than IV β2 agonists). |
| Marlin 1975 | Chronic asthma. |
| May 1975 | Not a randomised controlled clinical trial ‐ cohort study. |
| Monie 1979 | Letter referring to a trial comparing aminophylline versus β2‐agonists. |
| Naspitz 1987 | Epinephrine (rather than IV β2 agonists). |
| Ngamphaiboon 1989 | Epinephrine (rather than IV β2 agonists). |
| Nogrady 1977 | Case series. |
| Noseda 1989 | Review. |
| O'Connell 1990 | Not a randomised controlled clinical trial ‐ cohort study. |
| Pang 1977 | Excluded on basis of design and no comparison group: (D) non RCT, (P) peds, (I) terbutaline SC, (C) nil. |
| Parry 1976 | Not a randomised controlled clinical trial ‐ cohort study. |
| Phanichyakarn 1989 | D: RCT, (P): severe acute asthma, (I): IV terbutaline vs (C) nebulised terbutaline, (O): PEF. Excluded on basis of comparing IV terbutaline vs nebulised terbutaline. |
| Pierce 1981 | Patients were not seen in an emergency setting (study done in a lab setting). |
| Prego 2001 | (D) non RCT, (P) peds severe asthma, (I) IV salbutamol, (C) NEB salbutamol, (O) various. Excluded on basis of comparing IV salbutamol vs nebulised salbutamol. |
| Quadrel 1995 | Epinephrine (rather than IV β2 agonists) study to be considered in separate Cochrane review. |
| Quijada 1992 | D: RCT, (P): severe acute asthma, (I): SC salbutamol vs (C) nebulised salbutamol, (O): PEF. Excluded on basis of comparing SC salbutamol vs nebulised salbutamol. |
| Rahman 1990 | D: non RCT, (P): severe acute asthma, (I): SC salbutamol vs (C) ‘nebuhaler’, (O): PEF. Excluded on basis of SC salbutamol vs ‘nebuhaler’. |
| Roberts 2003 | Intravenous salbutamol bolus compared with an aminophylline infusion. |
| Rodrigo 1994 | Addition of IV aminophylline to inhaled β2 agonists. Study to be considered in separate Cochrane review. |
| Rossing 1980 | Epinephrine (rather than IV β2 agonists). |
| Ruddy 1986 | Epinephrine (rather than IV β2 agonists). |
| Salmeron 1988 | D: Double blind RCT, (P): severe acute asthma, (I): IV salbutamol vs (C) nebulised Salbutamol, (O): PEF. Excluded on basis of comparing IV salbutamol vs nebulised Salbutamol. |
| Salmeron 1989 | D: Double blind RCT, (P): severe acute asthma, (I): IV salbutamol vs (C) nebulised Salbutamol, (O): PEF. Excluded on basis of comparing IV salbutamol vs nebulised Salbutamol. |
| Salmeron 1994 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between albuterol 10 mg NEB (two 5 mg nebs over 15 min for one hour), then if successful continue Rx 5 mg nebulised q2h for 7 h vs. albuterol iv infusion of 8.3 ug/min for 60 min (total 500 ug) at 0 min lasting for 1 hr, then if successful continue Rx 500 ug/hr for 7h. |
| Salmeron 1995 | Letter to editor. |
| Schiavi 1987 | Not a randomised controlled clinical trial. |
| Schwartz 1980 | D: unknown RCT, (P): severe acute asthma, (I): SC EPI or terbutaline vs (C) nebulised isoetharine HCL, (O): PEF, various other. Excluded on basis of comparing SC EPI or terbutaline vs nebulised isoetharine HCL. |
| Sharma 1984 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between intravenous salbutamol vs. iv aminophylline. |
| Smith 1986 | Non‐experimental study (not randomised controlled clinical trial). |
| Subias 1989 | Not a randomised controlled clinical trial. |
| Swedish Society 1990 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between salbutamol 0.15 mg/kg nebulised at 0 min lasting 7 min, repeat x1 at 30 min (total nebulised = 0.30 mg/kg in 1 hour) vs. salbutamol 5 ug/kg iv over 10 min at 0 min. |
| Tarala 1981 | Excluded on basis of type of patients: stable adults in outpatient setting. |
| Teoh 1979 | Non emergency patients. Not randomised controlled clinical trial ‐ cohort study. |
| Thiringer 1976 | Non‐experimental study (not randomised controlled clinical trial). Patients were not seen in an emergency setting (study done in a lab setting). |
| Thompson 1977 | Study on non‐severe asthmatics in ambulatory setting. |
| Ting 1991 | Not a randomised controlled clinical trial. |
| Tirot 1992 | Not a randomised controlled clinical trial. |
| Tribe 1976 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between theophylline 250 mg iv at 0 min over ?5 min vs. salbutamol 100 ug iv at 0 min. |
| Tripathi 1989 | Not a randomised controlled clinical trial. |
| Uden 1985 | Epinephrine (rather than IV β2 agonists). |
| Van Renterghem 1987 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between terbutaline 0.1 mg/kg nebulised over 5 min at 0 min and 60 min vs. terbutaline 6 ug/kg iv over 5 min (q60min x1) at 0 min and 60 min. |
| Victoria 1989 | Trial compared subcutaneous epinephrine and terbutaline injections. |
| Wheeler 2005 | Comparing theophylline versus terbutaline. |
| Williams 1975 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between aminophylline 500 ug iv at 0 min infused over 60 min vs. salbutamol 500 ug iv at 0 min infused over 60 min (8.33 ug/min). |
| Williams 1977 | Non‐experimental study (not randomised controlled clinical trial). |
| Williams 1981 | This trial does not compare the addition of intravenous β2‐agonists to inhaled β2‐agonists versus inhaled β2‐agonists for acute asthma. The comparison was between terbutaline 2.5 mg nebulised over 10 min (repeat X 2 for each time FEV1 maxed) vs. terbutaline 250 ug iv over 10 min at 0 min (repeat X 2 for each time FEV1 maxed). |
| Wood 1972 | Not a randomised controlled clinical trial. |
| Wood 1973 | Not a controlled clinical trial. |
| Zehner 1995 | D: Double blind RCT, (P): severe acute asthma, (I): SC terbutaline vs (C) nebulised albuterol, (O): PEF Excluded on basis of comparing subcutaneous terbutaline vs nebulised albuterol. |
| Zhang 2004 | Not a randomised controlled clinical trial. |
ANF: atrial natriuretic factor. EPI: epinephrine FEV1: forced expiratory volume in 1 sec HR: heart rate IPPB: intermittent positive‐pressure breathing iv: intravenous NPV: negative predictive value peds: paedtrics PF: peak flow PEF: peak expiratory flow PFTs: pulmonary function tests RCT: randomised controlled trial sc: subcutaneous vs: versus Study characteristics D: design P: participants I: interventions O: outcomes
Contributions of authors
Travers A: Initiated the review, wrote the protocol, performed searches, performed quality assessments, entered data and performed analysis, and primary author of review. Jones AP: study selection, quality assessment, review of protocol; Camargo CA Jr: Protocol development, methodological input, statistical support, manuscript review at an early stage of this review's development; Rowe BH: Co‐authored protocol, performed selection for inclusion and quality assessment, data extraction and data entry, manuscript review, conversion to RevMan at an early stage of this review's development, and as assigned editor for the Cochrane Airways Group. Milan SJ and Welsh E independently selected trials for inclusion from initial searches, and Travers A and Milan SJ independently selected trials for inclusion from full trial reports. Milan SJ and Travers A updated the 'Risk of bias' tables for trials already included in the review and similarly for any new trials identified in the update. Milan SJ entered data and this was verified by Cates C. Milan SJ drafted the review and further development was provided by Travers A and Cates C.
Sources of support
Internal sources
University of Alberta, Faculty of Medicine & Dentistry, Canada.
Alberta Heritage Foundation for Medical Research (AHFMR), Canada.
NHS Research and Development, UK.
National Institute for Health Research (SJM), UK.
External sources
Canadian Association of Emergency Physicians (CAEP), Canada.
National Heart, Lung and Blood Institute (HL‐03533 NIH; CA Camargo, Jr), USA.
Canadian Institutes of Health Research (CIHR), Ottawa, ON (BH Rowe), Canada.
Declarations of interest
None. The authors are not involved in the primary research reported in this systematic review and have not represented the producers of these agents in the past.
New
References
References to studies included in this review
Bogie 2007 {published data only}
Browne 1997 {published data only}
- Browne GJ, Penna AS, Phung X, Soo M. Randomised trial of intravenous salbutamol in early management of acute asthma in children. Lancet 1997;349:301‐5. [DOI] [PubMed] [Google Scholar]
Nowak 2010 {published data only}
- Nowak R, Iwaki Y, Matsuda K, Johnson K, Dunton AW. Reduced hospital admission and improved pulmonary function following intravenous mn‐221 (bedoradrine), a novel, highly selective β2‐adrenergic receptor agonist, adjunctive to standard of care in severe acute exacerbation of asthma [Abstract]. Chest 2010; Vol. 138, issue 4:166A.
References to studies excluded from this review
Abd 1989 {published data only}
- Abd El‐Moneim MA, Hanafy HM, Gomaa K, Hussein MG. Comparison of inhaled salbutamol, inhaled reproterol and injected epinephrine in the treatment of acute asthma in children. Alexandria Journal of Pediatrics 1989 ;3(2):153‐60. [Google Scholar]
Aggarwal 1986 {published data only}
- Aggarwal P, Pande JN, Guleria JS. Bronchodilators in acute bronchial asthma : a comparative study. Indian Journal of Chest Diseases and Allied Sciences 1986; Vol. 28, issue 1:21‐7. [PubMed]
Anonymous 1978 {published data only}
- Anonymous. Intravenous versus inhaled salbutamol. Lancet 1978;1:80. [PubMed] [Google Scholar]
Appel 1989 {published data only}
- Appel D, Karpel JP, Sherman M. Epinephrine improves expiratory flow rates in patients with asthma who do not respond to inhaled metaproterenol sulfate. Journal of Allergy & Clinical Immunology 1989;84(1):90‐8. [DOI] [PubMed] [Google Scholar]
Arnaud 1977 {published data only}
- Arnaud A, Dugue P, Orehek J, Pommier de Santi P, Vervloet D, Charpin J. Treatment of acute asthma. Comparison of the effectiveness of corticosteroids and a combination of corticosteroids and an adrenergic beta‐stimulant. Nouvelle Presse Medicale 1977;6(45):4183‐6. [PubMed] [Google Scholar]
Arnaud 1982 {published data only}
- Arnaud A, Charpin J. Interaction between corticosteroids and beta 2‐agonists in acute asthma. European Journal of Respiratory Diseases 1982; Vol. 122 Suppl:126‐31. [PubMed]
Badatcheff 1989 {published data only}
- Badatcheff A, Person C, Raoult J, Meslier N, Racineux JL. Effects of salbutamol and intravenous adrenaline in severe asthmatic crisis. Revue des Maladies Respiratoires 1989; Vol. 6, issue Suppl 3:R168.
Baur 1988 {published data only}
Becker 1983 {published data only}
- Becker AB, Nelson NA, Simons FE. Inhaled salbutamol albuterol vs injected epinephrine in the treatment of acute asthma in children. Journal of Pediatrics 1983;102(3):465‐9. [DOI] [PubMed] [Google Scholar]
Ben‐Zvi 1980 {published data only}
- Ben‐Zvi Z, Lam C, Kattan M, et al. Emergency room assessment and treatment of asthma: Aerosol vs. injection. Pediatric Research 1980; Vol. 14, issue 4 II:No. 1280.
Ben‐Zvi 1982 {published data only}
- Ben‐Zvi Z, Lam C, Hoffman J, Teets‐Grimm KC, Kattan M. An evaluation of the initial treatment of acute asthma. Pediatrics 1982;70(3):348‐53. [PubMed] [Google Scholar]
Ben‐Zvi 1983 {published data only}
- Ben‐Zvi Z, Lam C, Spohn WA, Gribetz I, Mulvihill MN, Kattan M. An evaluation of repeated injections of epinephrine for the initial treatment of acute asthma. American Review of Respiratory Disease 1983;127(1):101‐5. [DOI] [PubMed] [Google Scholar]
Beswick 1975 {published data only}
- Beswick K, Davies J, Davey AJ. A comparison of intravenous aminophylline and salbutamol in the treatment of severe bronchospasm. The Practitioner 1975;214:561‐6. [PubMed] [Google Scholar]
Bloomfield 1979 {published data only}
- Bloomfield P, Carmichael J, Petrie GR, Jewell NP, Crompton GK. Comparison of salbutamol given intravenously and by intermittent positive‐pressure breathing in life‐threatening asthma. British Medical Journal 1979;1:848‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumenthal 1979 {published data only}
- Blumenthal I, Tormey W. Comparison of IV salbutamol with IV aminophylline in severe acute asthma. Archives of Disease in Childhood 1979;54:983‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boe 1985 {published data only}
- Boe J, Carlsson LG, Hetta L, Karlson B, Ljungholm K. Acute asthma ‐ plasma levels and effect of terbutaline i.v. injection. European Journal of Respiratory Diseases 1985;67:261‐8. [PubMed] [Google Scholar]
Bohn 1984 {published data only}
- Bohn D, Kalloghlian A, Jenkins J, Edmonds J, Barker G. Intravenous salbutamol in the treatment of status asthmaticus in children. Critical Care Medicine 1984;12:892‐6. [DOI] [PubMed] [Google Scholar]
Brandstetter 1980 {published data only}
- Brandstetter RD, Gotz VP, Mar DD. Optimal dosing of epinephrine in acute asthma . American Journal of Hospital Pharmacy 1980;37(10):1326‐9. [PubMed] [Google Scholar]
Browne 2002 {published data only}
- Browne GJ, Trieu L, Asperen P. Randomized, double‐blind, placebo‐controlled trial of intravenous salbutamol and nebulized ipratropium bromide in early management of severe acute asthma in children presenting to an emergency department. Critical Care Medicine 2002;30(2):448‐53. [DOI] [PubMed] [Google Scholar]
Bruguerolle 1991 {published data only}
- Bruguerolle B, Philip‐Joet F, Lagier F, Pierson F, Reynaud M, Leonardelli M, et al. Unequal day‐night terbutaline IV dosing in acute severe asthma: effect on nocturnal patency, heart rate, and arterial pressure. Chronobiology International 1991;8(3):194‐202. [DOI] [PubMed] [Google Scholar]
Chanez 1990 {published data only}
Cheong 1988 {published data only}
- Cheong B, Reynolds SR, Rajan G, Ward MJ. Intravenous B‐agonist in severe acute asthma. BMJ 1988;297:448‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiang 2000 {published data only}
Claybo 1985 {published data only}
Crompton 1990 {published data only}
- Crompton G. Nebulized or intravenous beta‐adrenoceptor agonist therapy in acute asthma. European Respiratory Journal 1990;3:125‐6. [PubMed] [Google Scholar]
Davis 1977 {published data only}
Downes 1973 {published data only}
- Downes J, Wood D, Harwood I. Intravenous isoproterenol infusion in children with severe hypercapnia due to status asthmaticus. Critical Care Medicine 1973;1(2):63‐8. [DOI] [PubMed] [Google Scholar]
Edmunds 1981 {published data only}
- Edmunds AT, Godfrey S. Cardiovascular response during severe acute asthma and its treatment in children. Thorax 1981;36:534‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elenbaas 1985 {published data only}
- Elenbaas RM, Frost GL, Robinson WA, Collier RE, McNabney WK, Ryan JL, et al. Subcutaneous epinephrine vs. nebulized metaproterenol in acute asthma. Drug Intelligence & Clinical Pharmacy 1985;19(17‐8):567‐71. [DOI] [PubMed] [Google Scholar]
Evans 1979 {published data only}
- Evans WV, Monie RDH. Aminophylline, salbutamol and combined intravenous infusions in acute severe asthma [abstract]. British Journal of Diseases of the Chest 1979; Vol. 73:423‐4. [PubMed]
Evans 1980 {published data only}
- Evans WV, Monie J, Crimmins J, Seaton A. Aminophylline, salbutamol and combined intravenous infusions in acute severe asthma. British Journal of Diseases of the Chest 1980;74:385‐9. [PubMed] [Google Scholar]
Fanta 1986 {published data only}
Femi‐Pearse 1977 {published data only}
- Femi‐Pearse D, George WO, Ilechukwu ST, Elegbeleye OO, Afonja AO. Comparison of intravenous aminophylline and salbutamol in severe asthma. British Medical Journal 1977;1:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitchett 1975 {published data only}
- Fitchett DH, McNicol MW, Riordan JF. Intravenous salbutamol in management of status asthmaticus. British Medical Journal 1975;1:53‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gotz 1981 {published data only}
Grant 1976 {published data only}
- Grant I. Effect of intravenous injection of salbutamol in asthma. British Journal of Clinical Pharmacology 1976;3:509‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greefhorst 1983 {published data only}
Greif 1985 {published data only}
- Greif J, Markovitz L, Topilsky M. Comparison of intravenous salbutamol (albuterol) and aminophylline in the treatment of acute asthmatic attacks. Annals of Allergy 1985;55:504‐6. [PubMed] [Google Scholar]
Hambleton 1979 {published data only}
- Hambleton G, Stone MJ. Comparison of IV salbutamol with IV aminophylline in the treatment of severe, acute asthma in childhood. Archives of Disease in Childhood 1979;54:391‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayday 2002 {published data only}
- Hayday K, Stevermer JJ. In children hospitalized for asthma exacerbations, does adding ipratropium bromide to albuterol and corticosteroids improve outcome?. Journal of Family Practice 2002; Vol. 51, issue 3:280. [0094‐3509] [PubMed]
Herman 1983 {published data only}
- Herman JJ, Noah ZL, Moody RR. Use of intravenous isoproterenol for status asthmaticus in children. Critical Care Medicine 1983;11:716‐20. [DOI] [PubMed] [Google Scholar]
Hetzel 1976 {published data only}
- Hetzel MR, Clark TJH. Comparison of intravenous and aerosol salbutamol. British Medical Journal 1976;2(6014):919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsch 1979 {published data only}
- Hirsch SR. Intravenous therapy with terbutaline. Chest 1979;75:648. [DOI] [PubMed] [Google Scholar]
Hussein 1986 {published data only}
- Hussein A, Hardt H, Muller W, Schell SM. Intravenous infusion of reproterol in the treatment of acute severe asthma in children. Monatsschrift fur Kinderheilkunde 1986;134:192‐6. [PubMed] [Google Scholar]
Hussein 1986a {published data only}
- Hussein A, Hardt H, Muller W, Schell SM. Intravenous infusion of reproterol a beta‐2‐mimetic agent in the therapy of severe asthma attacks in childhood. Monatsschrift Kinderheilkunde 1986; Vol. 134, issue 4:192‐6. [PubMed]
Hutton 2002 {published data only}
- Hutton SF, Koval PG. Intravenous albuterol effective for acute severe asthma. Journal of Family Practice 2002; Vol. 51, issue 7:596. [0094‐3509] [PubMed]
Iodice 1980 {published data only}
- Iodice F, Rufolo L, Piscione F, Michele G. Hemodynamic and ventilatory effects of intravenous salbutamol in patients affected by cold. Respiration 1980;40:272‐7. [DOI] [PubMed] [Google Scholar]
Janson 1988 {published data only}
Janson 1992 {published data only}
- Janson C, Boman D. Intravenous theophylline after beta 2‐agonist treatment in severe acute asthma. Effect on patients who are not pre‐treated with theophylline. Upsala Journal of Medical Sciences 1992;97:149‐55. [DOI] [PubMed] [Google Scholar]
Johnson 1978 {published data only}
- Johnson AJ, Spiro SG, Pidgeon J, Bateman S, Clarke SW. Intravenous infusion of salbutamol in severe acute asthma. British Medical Journal 1978;1:1013‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karetzky 1980 {published data only}
- Karetzky MS. Acute asthma: the use of subcutaneous epinephrine in therapy. Annals of Allergy 1980;44(1):12‐4. [PubMed] [Google Scholar]
Kornberg 1991 {published data only}
- Kornberg AE, Zuckerman S, Welliver JR, Mezzadri F, Aquino N. Effect of injected long‐acting epinephrine in addition to aerosolized albuterol in the treatment of acute asthma in children. Pediatric Emergency Care 1991;7 (1):1‐3. [DOI] [PubMed] [Google Scholar]
Lawford 1978 {published data only}
- Lawford P, Jones BJM, Milledge JS. Comparison of intravenous and nebulised salbutamol in initial treatment of severe asthma. British Medical Journal 1978;1:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lebovitz 2004 {published data only}
- Lebovitz DJ, Smith PG, O'Riordan M, Reed MD. Pharmacokinetic properties and tolerability of single‐dose terbutaline in patients with severe asthma treated in the pediatric intensive care unit. Current Therapeutic Research, Clinical & Experimental 2004; Vol. 65, issue 1:98‐109. [0011‐393X] [DOI] [PMC free article] [PubMed]
Li 2002 {published data only}
- Li YL, Luo YA, Wu YM. Comparison of relieving asthma effects of domestic and imported terbutaline injection. Zhongguo Xinyao yu Linchuang Zazhi 2002; Vol. 21, issue 2:97‐9. [1007‐7669]
Lin 1996 {published data only}
- Lin YZ, Hsieh KH, Chang LF, Chu CY. Terbutaline nebulization and epinephrine injection in treating acute asthmatic children. Pediatric Allergy & Immunology 1996;7(2):95‐9. [DOI] [PubMed] [Google Scholar]
Lowell 1987 {published data only}
- Lowell DI, Lister G, Koss H, McCarthy P. Wheezing in infants: the response to epinephrine. Pediatrics 1987;79(6):939‐45. [PubMed] [Google Scholar]
Marlin 1975 {published data only}
- Marlin G, Turner P. Intravenous treatment with rimiterol and salbutamol. British Medical Journal 1975;2:715‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
May 1975 {published data only}
- May CS, Paterson JW, Spiro SG, Johnson AJ. Intravenous infusion of salbutamol in the treatment of asthma. British Journal of Clinical Pharmacology 1975;2:503‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monie 1979 {published data only}
- Monie R, Evans WV. Saving asthmatics. British Medical Journal 1979; Vol. 2:334. [0007‐1447] [DOI] [PMC free article] [PubMed]
Naspitz 1987 {published data only}
- Naspitz CK, Sole D, Wandalsen N. Treatment of acute attacks of bronchial asthma. A comparative study of epinephrine subcutaneous and fenoterol inhalation. Annals of Allergy 1987;59(1):21‐4. [PubMed] [Google Scholar]
Ngamphaiboon 1989 {published data only}
- Ngamphaiboon J, Chumdermpadetsuk S. Nebulized salbutamol vs injected epinephrine in the treatment of acute asthma in children. Chulalongkorn Medical Journal 1989;33(9):669‐73.. [Google Scholar]
Nogrady 1977 {published data only}
- Nogrady SG, Hartley JPR, Seaton A. Metabolic effects of intravenous salbutamol in the course of acute asthma. Thorax 1977;32:559‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noseda 1989 {published data only}
- Noseda A, Yernault JC. Sympathomimetics in acute severe asthma: inhaled or parenteral, nebulizer or spacer?. European Respiratory Journal 1989;2:377‐82. [PubMed] [Google Scholar]
O'Connell 1990 {published data only}
- O'Connell MB, Iber C. Continuous intravenous terbutaline infusions for adult patients with status asthmaticus. Annals of Allergy 1990;64:213‐8. [PubMed] [Google Scholar]
Pang 1977 {published data only}
Parry 1976 {published data only}
- Parry L, Martorano, Cotton E. Management of life‐threatening asthma with intravenous isoproterenol infusions. American Journal of Diseases of Children 1976;130:39‐42. [DOI] [PubMed] [Google Scholar]
Phanichyakarn 1989 {published data only}
- Phanichyakarn P, Kraisarin C, Sasisakulporn C. Comparison of inhaled terbutaline and terbutalifle injection in treatment of acute asthmatic attacks in children. Asian Pacific Journal of Allergy & Immunology 1989; Vol. 7, issue 1:29‐32. [PubMed]
Pierce 1981 {published data only}
- Pierce RJ, Payne CR, Williams SJ, Denison DM, Clark TJH. Comparison of intravenous and inhaled terbutaline in the treatment of asthma. Chest 1981;79:506‐11. [DOI] [PubMed] [Google Scholar]
Prego 2001 {published data only}
- Prego Petit J, Sehabiague Rigau GR, Leonardis Capelo DJ, Pujadas Ferrer MA. Treatment of asthmatic crisis with intravenous salbutamol and in continuous nebulization in an emergency. Archivos de Pediatra del Uruguay 2001; Vol. 72:S5‐S13.
Quadrel 1995 {published data only}
- Quadrel M, Lavery RF, Jaker M, Atkin S, Tortella BJ, Cody RP. Prospective, randomized trial of epinephrine, metaproterenol, and both in the prehospital treatment of asthma in the adult patient. Annals of Emergency Medicine 1995;26(4):469‐73. [DOI] [PubMed] [Google Scholar]
Quijada 1992 {published data only}
- Quijada C, Galleguillos F. Alternative treatment for acute asthmatic crisis. Revista Médica de Chile 1992; Vol. 120, issue 2:142‐6. [PubMed]
Rahman 1990 {published data only}
- Rahman M. Comparison of efficacy and safety of driphaler with nebular and injectable salbutamol in acute severe asthma. Pakistan Journal of Medical Research 1990;29(1):29‐35. [Google Scholar]
Roberts 2003 {published data only}
- Roberts G, Newsom D, Gomez K, Raffles A, Saglani S, Begent J, et al. Intravenous salbutamol bolus compared with an aminophylline infusion in children with severe asthma: a randomised controlled trial. Thorax 2003; Vol. 58, issue 4:306‐10. [0040‐6376] [DOI] [PMC free article] [PubMed]
Rodrigo 1994 {published data only}
- Rodrigo C, Rodrigo G. Treatment of acute asthma. Lack of therapeutic benefit and increase of the toxicity from aminophylline given in addition to high doses of salbutamol delivered by metered‐dose inhaler with a spacer. Chest 1994;106(4):1071‐6. [DOI] [PubMed] [Google Scholar]
Rossing 1980 {published data only}
- Rossing TH, Fanta CH, Goldstein DH, Snapper JR, McFadden ER, Jr. Emergency therapy of asthma: comparison of the acute effects of parenteral and inhaled sympathomimetics and infused aminophylline. American Review of Respiratory Disease 1980;122(3):365‐71. [DOI] [PubMed] [Google Scholar]
Ruddy 1986 {published data only}
- Ruddy RM, Kolski G, Scarpa N, Wilmott R. Aerosolized metaproterenol compared to subcutaneous epinephrine in the emergency treatment of acute childhood asthma. Pediatric Pulmonology 1986;2(4):230‐6. [DOI] [PubMed] [Google Scholar]
Salmeron 1988 {published data only}
- Salmeron S, Brochard L, Mal H, Tenaillon A, Henry‐Amar M, Renon D, et al. Inhaled and intravenous salbutamol comparison in status asthmaticus. Revue des Maladies Respiratoires 1988; Vol. 5, issue Suppl 1:R124.
Salmeron 1989 {published data only}
- Salmeron S, Brochard L, Mal H, Tenaillon A, Henry‐Amar M, Renon D, et al. Inhaled and intravenous salbutamol in status asthmaticus?. Revue des Maladies Respiratoires 1989; Vol. 6, issue Suppl 2:R111.
Salmeron 1994 {published data only}
- Salmeron S, Brochard L, Mal H, Tenaillon A, Henry‐Amar M, Renon D, et al. Nebulized versus intravenous albuterol in hypercapnic acute asthma: A multicenter, double‐blind, randomized study. American Journal of Respiratory & Critical Care Medicine 1994;149:1466‐70. [DOI] [PubMed] [Google Scholar]
Salmeron 1995 {published data only}
- Salmeron S, Ellrodt A, Taravella O. Sympathomimetics in severe acute asthma. Lancet 1995;346:257. [PubMed] [Google Scholar]
Schiavi 1987 {published data only}
- Schiavi E. Acute effect of intravenous salbutamol in status asthmaticus. Article in Spanish [Efecto agudo salbutamol intravenosos en et astado de mal asmatico]. Medicina 1987;47:39‐44. [PubMed] [Google Scholar]
Schwartz 1980 {published data only}
Sharma 1984 {published data only}
- Sharma TN, Gupta RB, Gupta PR, Purohit SD. Comparison of intravenous aminophylline, salbutamol, and terbutaline in acute asthma. Indian Journal of Chest Diseases & Allied Sciences 1984;26:155‐8. [PubMed] [Google Scholar]
Smith 1986 {published data only}
- Smith SR, Kendall MJ. Potentiation of the adverse effects of intravenous terbutaline by oral theophylline. British Journal of Clinical Pharmacology 1986;21:451‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Subias 1989 {published data only}
- Subias J, Manrique N, Hidalgo V. Status asthmaticus treatment: beta‐agonist therapy experience in 71 cases. Anales Espanoles de Pediatria 1989;31:435‐9. [PubMed] [Google Scholar]
Swedish Society 1990 {published data only}
- Swedish Society of Chest Medicine, Janson C, Boe J, Boman G, Larsson S, Mossberg B, et al. High‐dose inhaled versus intravenous salbutamol combined with theophylline in severe acute asthma. European Respiratory Journal 1990;3:163‐70. [PubMed] [Google Scholar]
Tarala 1981 {published data only}
- Tarala RA, Martyn V, Paterson JW. Effect of intravenous injection of rimiterol in asthma. British Journal of Clinical Pharmacology 1981; Vol. 12, issue 3:333‐40. [DOI] [PMC free article] [PubMed]
Teoh 1979 {published data only}
- Teoh P. Clinical evaluation of intravenous hexoprenaline in bronchial asthma. Annals Academy of Medicine 1979;8:144‐7. [PubMed] [Google Scholar]
Thiringer 1976 {published data only}
- Thiringer G, Svedmyr N. Comparison of infused and inhaled terbutaline in patients with asthma. Scandinavian Journal of Respiratory Diseases 1976;57:17‐24. [PubMed] [Google Scholar]
Thompson 1977 {published data only}
- Thompson P, Friedman M. Intramuscular salbutamol in treatment of acute exacerbations of childhood asthma. Archives of Disease in Childhood 1977; Vol. 52, issue 7:551‐4. [DOI] [PMC free article] [PubMed]
Ting 1991 {published data only}
- Ting C. A comparative study of epinephrine injection and beta‐agonist inhalation in the treatment of childhood asthma. Chung‐Hua Min Kuo Hsiao Erh Ko i Hsueh Hui Tsa Chih 1991;32:372‐81. [PubMed] [Google Scholar]
Tirot 1992 {published data only}
- Tirot P, Bouachour G, Varache N, Harry P, Bourrier P, Chennebault JM, et al. The use of intravenous adrenaline in acute severe asthma. Revue des Maladies Respiratoires 1992;9(3):319‐23. [PubMed] [Google Scholar]
Tribe 1976 {published data only}
- Tribe AE, Wong RM, Robinson JS. A controlled trial of intravenous salbutamol and aminophylline in acute asthma. Medical Journal of Australia 1976;2:749‐52. [DOI] [PubMed] [Google Scholar]
Tripathi 1989 {published data only}
- Tripathi S. Management of acute bronchial asthma‐‐intravenous terbutaline or aminophylline?. Journal of the Indian Medical Association 1989;87:75‐6. [PubMed] [Google Scholar]
Uden 1985 {published data only}
- Uden DL, Goetz DR, Kohen DP, Fifield GC. Comparison of nebulized terbutaline and subcutaneous epinephrine in the treatment of acute asthma. Annals of Emergency Medicine 1985;14(3):229‐32. [DOI] [PubMed] [Google Scholar]
Van Renterghem 1987 {published data only}
- Renterghem D, Lamont H, Elinck W, Pauwels R, Straeten M. Intravenous versus nebulized terbutaline in patients with acute asthma: a double‐blind study. Annals of Allergy 1987;59:313‐6. [PubMed] [Google Scholar]
Victoria 1989 {published data only}
Wheeler 2005 {published data only}
Williams 1975 {published data only}
- Williams SJ, Parrish RW, Seaton A. Comparison of intravenous aminophylline and salbutamol in severe asthma. British Medical Journal 1975;4:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1977 {published data only}
- Williams S, Seaton A. Intravenous or inhaled salbutamol in severe acute asthma?. Thorax 1977;32:555‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1981 {published data only}
- Williams SJ, Winner SJ, Clark TJH. Comparison of inhaled and intravenous terbutaline in acute severe asthma. British Medical Journal 1975;4:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 1972 {published data only}
- Wood DW, Downes JJ, Scheinkopf H, Lecks HI. Intravenous isoproterenol in the management or respiratory arrest in childhood status asthmaticus. Journal of Allergy & Clinical Immunology 1972;50(2):75‐81. [DOI] [PubMed] [Google Scholar]
Wood 1973 {published data only}
- Wood D, Downes J. Intravenous isoproterenol in the treatment of respiratory failure in childhood status asthmaticus. Annals of Allergy 1973;31:607‐10. [PubMed] [Google Scholar]
Zehner 1995 {published data only}
Zhang 2004 {published data only}
- Zhang JX, Lin HQ, Chen JS. Clinical study on doxofylline injection in treatment of children with acute asthma attacks. Zhonghua Erke Zazhi 2004; Vol. 42, issue 2:143‐4. [PubMed]
Additional references
Beveridge 1996
- Beveridge RC, Grunfeld AF, Hodder RV, Verbeek PR. Guidelines for the emergency management of asthma in adults. Canadian Medical Association Journal (CMAJ) 1996;155:25‐37. [PMC free article] [PubMed] [Google Scholar]
Boulet 1999
- Boulet L‐P, Becker A, Berube D, Beveridge RC, Ernst P, on behalf of the Canadian Asthma Consensus Group. Canadian asthma consensus report. CMAJ 1999.;161:(11 suppl). [PMC free article] [PubMed] [Google Scholar]
BTS 1997
- The British Guidelines on Asthma Management: 1995 Review and Position Statement. Thorax 1997; Vol. 52:153‐6.
BTS/SIGN 2011
- British Guideline on the Management of Asthma. A national clinical guideline. British Thoracic Society www.brit‐thoracic.org.ukand Scottish Intercollegiate Guidelines Network www.sign.ac.uk May 2008. Revised May 2011.
Camargo 2011
- Camargo Jr CA, Spooner C, Rowe BH. Continuous versus intermittent beta‐agonists for acute asthma. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD001115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emerman 1999
- Emerman CL, Woodruff PG, Cydulka RK, Gibbs MA, Pollack CV Jr, Camargo CA Jr. Prospective multicenter study of relapse following treatment for acute asthma among adults presenting to the emergency department.. Chest 1999 115:919‐927. [DOI] [PubMed] [Google Scholar]
Emerman 2001
- Emerman CL, Cydulka RK, Crain EF, Rowe BH, Radeos MS, Camargo CA Jr. Prospective multicenter study of relapse after treatment for acute asthma among children presenting to the emergency department.. J Pediatrics 2001 2001;138:318‐324. [DOI] [PubMed] [Google Scholar]
Ernst 1996
- Ernst P, Fitzgerald J, Spier S. Canadian Asthma Consensus Conference: summary of recommendations. Canadian Respiratory Journal 1996;3:89‐100. [Google Scholar]
GINA 2011
- GINA Report, Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA). http://www.ginasthma.org December 2011.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Krishnan 2006
- Krishnan V, Diette GB, Rand CS, Bilderback AL, Merriman B, Hansel NN, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. American Journal of Respiratory and Critical Care Medicine 2006;174(6):633‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipworth 1997
- Lipworth BJ. Treatment of acute asthma. Lancet 1997;350:sii18‐sii23. [DOI] [PubMed] [Google Scholar]
NAEPP 1997
- National Asthma Education Program Expert Panel Report 2. Guidelines for the Diagnosis and Management of Asthma. Bethesda: NIH 1997:1. [PubMed]
NHLBI/WHO 1995
- NHLBI/WHO Workshop Report. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. Bethesda, MD: National Institutes of Health 1985.
NHS 2011
- NHS 2011 HES online hospital episode statistics. www.hesonline.nhs.uk.
NIH 2007
- NIH asthma guidelines, also known as Expert Panel Report 3. http://www.nhlbi.nih.gov/guidelines/asthma/.
Revman 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
References to other published versions of this review
Travers 2001
- Travers AA, Jones AP, Kelly KD, Camargo CA Jr, Barker SJ, Rowe BH. Intravenous beta2‐agonists for acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002988] [DOI] [PMC free article] [PubMed] [Google Scholar]


