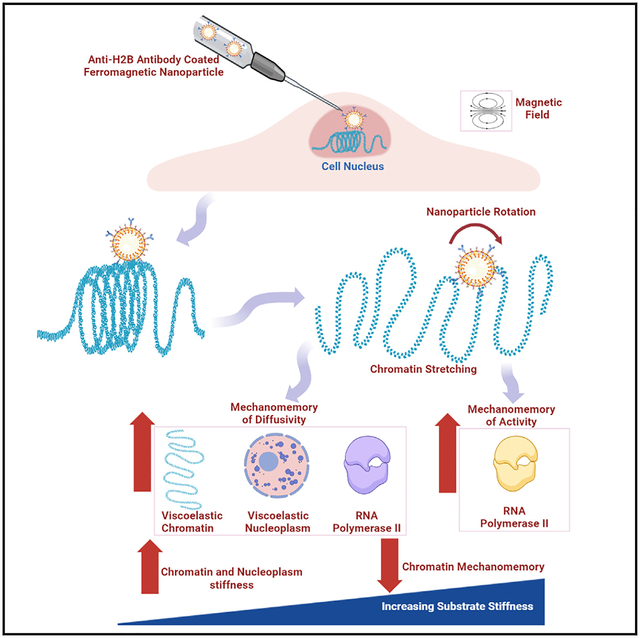
SUMMARY
Increasing evidence suggests that the mechanics of chromatin and nucleoplasm regulate gene transcription and nuclear function. However, how the chromatin and nucleoplasm sense and respond to forces remains elusive. Here, we employed a strategy of applying forces directly to the chromatin of a cell via a microinjected 200-nm anti-H2B-antibody-coated ferromagnetic nanoparticle (FMNP) and an anti-immunoglobulin G (IgG)-antibody-coated or an uncoated FMNP. The chromatin behaved as a viscoelastic gel-like structure and the nucleoplasm was a softer viscoelastic structure at loading frequencies of 0.1–5 Hz. Protein diffusivity of the chromatin, nucleoplasm, and RNA polymerase II (RNA Pol II) and RNA Pol II activity were upregulated in a chromatin-stretching-dependent manner and stayed upregulated for tens of minutes after force cessation. Chromatin stiffness increased, but the mechanomemory duration of chromatin diffusivity decreased, with substrate stiffness. These findings may provide a mechanomemory mechanism of transcription upregulation and have implications on cell and nuclear functions.
Graphical abstract
In brief
Rashid et al. show that chromatin and nucleoplasm in cells behave as viscoelastic materials. Chromatin stretching mediates the mechanomemory of chromatin and nucleoplasm diffusivity as well as of RNA polymerase II activity. The mechanomemory of RNA polymerase II activity provides a mechanism for sustained transcription upregulation tens of minutes after force cessation.
INTRODUCTION
It is increasingly clear that the mechanical forces and mechanics of the cellular microenvironment have a profound impact on the nuclear structure and function of living cells.1–4 However, the mechanotransduction pathways are less understood. We have shown that a cell surface force of physiologic magnitudes via the integrins can directly stretch the chromatin in the living cell to induce rapid gene upregulation independent of calcium signaling and YAP translocation.5–8 These findings suggest that the forces can be rapidly transmitted from the integrins on the cell surface to the cytoskeleton, from the cytoskeleton to the LINC (linker of nucleoskeleton and cytoskeleton), from the LINC to the chromatin via lamina-associated polypeptide 2 beta8 to induce rapid transcription upregulations of transgene BAC DHFR (bacterial artificial chromosome dihydrofolate reductase), and endogenous genes egr-1 (early growth response factor 1) and Cav1 (caveolin-1)6 within tens of seconds of force application. Recently, we demonstrated that protein diffusivity of the chromatin and nucleoplasm is elevated by force and stays elevated long (tens of minutes) after cessation of a force applied for only a short duration of 2–10 min,9 showing evidence of nuclear mechanosensation and mechanomemory in response to a very short (minutes) force application, extending previously published reports of mechanomemory or mechanoplasticity of the cells after they switch from stiff substrates to soft substrates, or vice versa, following being on the original substrates for days to weeks.10–16 Despite our recent progress in understanding the nuclear basis of mechanomemory, due to the limitation that, in our published study, the mechanical force is applied at the cell surface and needs to be transmitted all the way into the nucleus, it remains unclear if other factors in the cytoplasm and/or at the nuclear envelope play a critical role in initiating the memory effect. In addition, increasing evidence suggests that complex chromatin structures regulate the activity of the self-organizing genome17 and that the mechanics of chromatin and nucleoplasm may play critical roles in gene regulation. A recent study using microinjected 28-nm magnetic nanoparticles reveals that the chromatin behaves like a liquid,18 conflicting with a published report that the chromatin is a solid material.19 Therefore, whether chromatin behaves like a liquid or a solid is a fundamental and unresolved question. Furthermore, values of the stiffness (modulus) of the nucleus published from different labs using various methods vary by 3 orders of magnitude: ~0.5 Pa for an isolated nucleus using magnetic microbeads on the nuclear envelope20 and ~1,000 Pa for isolated nuclei using micropipette aspiration.21 Therefore, a direct quantification of nucleus mechanical properties in living cells is needed to resolve these discrepancies.
Motivated by these important unresolved issues surrounding the nucleus and chromatin, we employed a strategy of microinjecting 200-nm ferromagnetic nanoparticles (FMNPs) into the nucleus to attach them to the chromatin specifically via histone 2B or to the nucleoplasm network nonspecifically. We quantified moduli of the chromatin and nucleoplasm after the FMNPs were rotated remotely via the external sinusoidal magnetic fields to directly deform the chromatin or nucleoplasm and found that the chromatin behaved as a viscoelastic gel-like structure and the nucleoplasm was a 30-fold softer viscoelastic structure. We found that the mechanomemory in protein diffusivity of the chromatin and nucleoplasm, which depended on the chromatin stretching, lasted for tens of minutes after cessation of the force via the anti-H2B-antibody (ab)-coated FMNP. We also found that chromatin stiffness and chromatin mechanomemory duration are regulated by substrate stiffness. Remarkably, we found that mechanomemory existed in RNA polymerase II (RNA Pol II) diffusivity and activity, providing a mechanomemory explanation for the observation of sustained transcription upregulation after force cessation.
RESULTS
Microrheology of chromatin and nucleoplasm
To directly apply a stress to the chromatin, we microinjected anti-H2B-ab-coated FMNPs (~200 nm in diameter; 202 ± 6 nm [mean ± SD]; Figures S1A and S1B) into the nucleus of a CHO (Chinese hamster ovary) cell at 1–4 FMNPs per injection (Figures 1A and 1B). To avoid complications of force effect interferences from multiple nanoparticles, we chose those cells whose nuclei were injected with only 1 nanoparticle per nucleus for experiments. After microinjection, the medium was changed, and the dish with the cell that had the microinjected FMNP was placed in a 37°C 5% CO2 incubator for 30 min to allow sufficient time for the FMNP to bind its target. A single anti-H2B-ab-coated FMNP bound to the histone 2B in the chromatin was used to probe the nuclear micromechanics (Figure 1C). The uncoated FMNPs were pre-calibrated in a viscous fluid, and their magnetic moment constant was determined to be 0.1 Pa per Gauss (0.10 ± 0.005 Pa per Gauss magnetic field [mean ± SD]) (Figures S1C and S1D; STAR Methods). The CHO cells, with a double deletion of the endogenous DHFR locus, had a 10-copy insertion of a BAC (the cell clone DHFR D10) with a 170-kb mouse genomic insert containing the 34-kb DHFR gene in the same chromatin domain.22 The cells were plated on fibronectin-coated gridded rigid glass-bottomed dishes overnight for easy localization of the precise position of the FMNPs and DHFR green fluorescent protein (GFP) spots (Figures 1A and 1C). The cell clone DHFR D10 that we used in our experiments stably expressed enhanced GFP (EGFP)-dimer lactose (lac) repressor (GFP-LacI; i.e., diffusive GFPs in the nucleoplasm, Figure 1C) and enabled visualization of the DHFR BAC that was tagged with a 256-mer lac operator (LacO) repeat (10 kb) because of the tight binding of GFP-LacI to LacO.22 DHFR reduces dihydrofolate to tetrahydrofolate and is an essential enzyme for synthesizing thymidine. At each focal plane, 2 (or more) DHFR GFP spots could be visualized (Figure 1C), and the relative deformation of these spots was used to calculate chromatin strains. After being magnetized along the y direction with a strong magnetic pulse (1,000 Gauss for 10 ms), the FMNPs were rotated and displaced via the application of a weak sinusoidal magnetic field in the z direction (50 Gauss at 0.3 Hz, i.e., 5-Pa peak stress at 0.3 Hz) that resulted in a 5-Pa rotational stress on each FMNP (Figure 1D). The individual FMNPs were displaced at the same frequency and waveform as the input stress but with phase lags (Figure 1D), indicating that these displacements were induced by the external magnetic fields and not the spontaneous thermal motions. The stress from the anti-H2B FMNPs resulted in the deformation and strain of the chromatin. From the applied stress and chromatin strain, the chromatin complex modulus (applied stress divided by chromatin strain) was obtained. The chromatin in the living CHO cell had a complex shear modulus G of 122 Pa, a storage (elastic) modulus G′ of 97 Pa, and a loss modulus G′′ of 73 Pa (Figures 1E–1G). In contrast, after immunoglobulin G (IgG)-coated or uncoated FMNPs were injected into the nucleus, they did not bind the chromatin and induced little chromatin stretching with stress when compared to that without stress (Figure S2), suggesting that these FMNPs only probed the rheology of the nucleoplasm network. The G, G′, and G′′ probed from IgG-coated or uncoated FMNPs were similar in magnitude and were 3.8, 3.2, and 2.2 or 3.7, 3.0, and 2.0 Pa, respectively (Figures 1E–1G), ~30-fold lower than those of the chromatin. Because the storage modulus was higher than the loss modulus for both the chromatin and nucleoplasm, the results from direct measurements inside the nucleus via the 200-nm FMNP suggest that the chromatin behaves like a viscoelastic gel-like structure, while the nucleoplasm is a much softer viscoelastic gel-like structure.
Figure 1. Microrheology of chromatin and nucleoplasm.

(A) Schematic of anti-histone-H2B-ab-coated 200-nm FMNP microinjection to a CHO (Chinese hamster ovary) cell nucleus.
(B) Applying a sinusoidal cyclic stress after magnetizing 200-nm FMNPs in the y direction and twisting toward the z direction using the magnetic twisting cytometry (MTC).
(C) Representative images from 4 different cell nuclei in 4 different rows (the same cell in each row) of anti-H2B-ab-coated 200 nm FMNPs and DHFR (dihydrofolate reductase)-GFP spots or free GFP of CHO cell nucleus. Scale bar, 5 μm.
(D) Displacements of anti-H2B-ab-coated FMNPs in the same four CHO cells as in (C).
(E) Chromatin and nucleoplasm complex moduli (ratio of applied stress to measured chromatin strain) with FMNP applied stress (5 Pa at 0.3 Hz) in CHO cell nuclei.
(F) Chromatin and nucleoplasm storage modulus G′ (G′ = (σ0/ε0) cos δ) in CHO cell nuclei.
(G) Chromatin and nucleoplasm loss modulus G′′ (G′′ = (σ0/ε0) sin δ) in CHO cell nuclei.
Data (E, F, and G) are boxplots with minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. *p < 0.05, **p < 0.01, and ***p < 0.001, ns = not statistically different using one-way ANOVA with Tukey and Mann-Whitney tests. n = 3 biological replicates.
See also Figures S1 and S2.
To further quantify the rheological properties of the chromatin, we performed force-frequency response experiments of the chromatin using the anti-H2B-ab-coated FMNPs. At loading frequencies increased from 0.1 to 5 Hz (covering human resting breathing frequency to heart rates during exercise), chromatin stretching decreased, and thus chromatin moduli (G, G′, and G′′) increased (Figures 2A and 2B). Additional analyses from the data of Figures 2A and 2B showed that the power law exponent alpha of the frequency-complex modulus curve was 0.19 (Figure 2C), suggesting that the chromatin polymer followed the weak power law behavior, following the weak power law observed in the whole cell.6,23 As expected, both the storage modulus (power law constant alpha = 0.15) and loss modulus (alpha = 0.32) increased with the loading frequency, although the loss modulus increased at a faster rate (Figure 2C), suggesting that the chromatin dissipates more energy as the loading frequency increases. These results of chromatin microrheology suggest that the loading frequency has a substantial influence on the chromatin modulus.
Figure 2. Microrheology of chromatin probed with microinjected anti-H2B-ab-coated FMNPs.
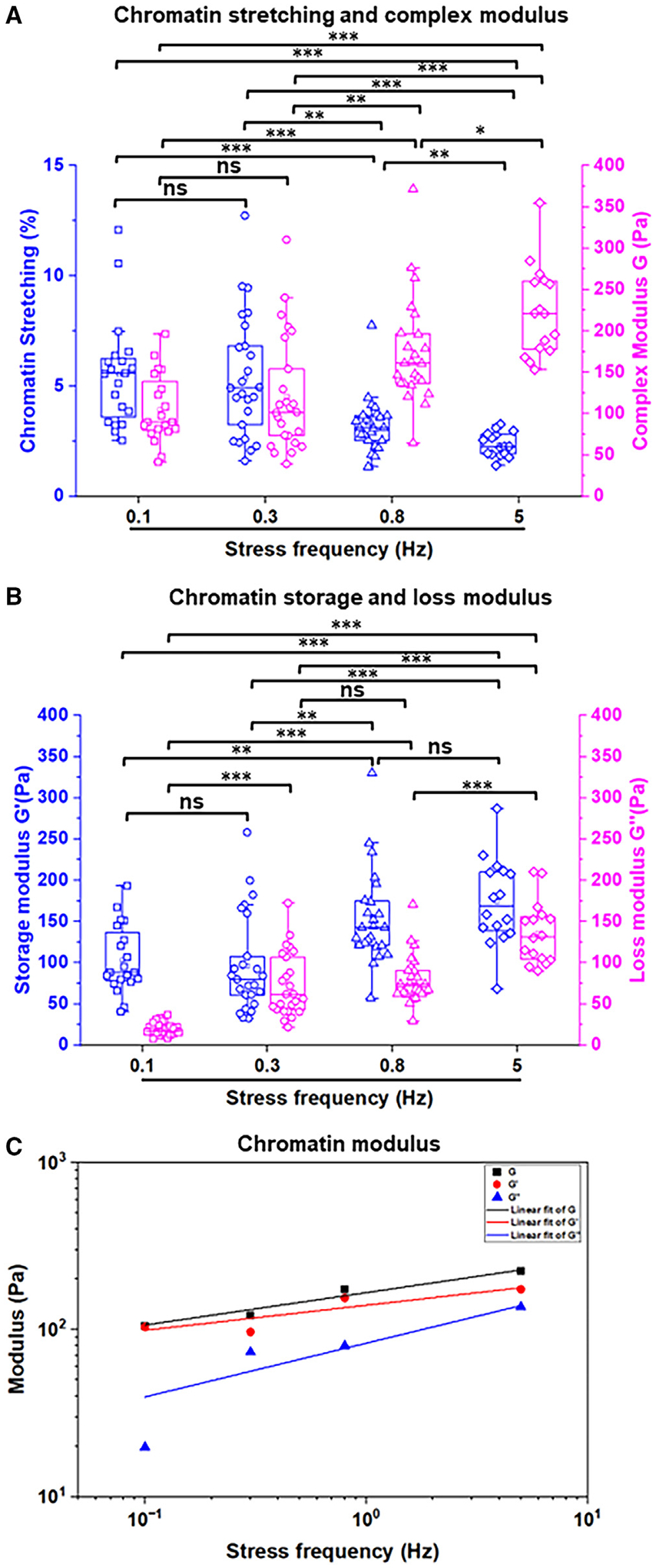
(A) Chromatin stretching and complex modulus G of CHO cells in the same chromatin domain for anti-H2B-ab-coated FMNP with applied stress 5 Pa at 0.1, 0.3, 0.8, or 5 Hz.
(B) Chromatin storage and loss modulus in CHO cell for anti-H2B-ab-coated FMNP with applied stress 5 Pa at 0.1, 0.3, 0.8, or 5 Hz.
(C) Chromatin average complex, storage, and loss moduli versus stress frequency on a log-log scale for CHO cells.
Data (A and B) are boxplots with minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. *p < 0.05, **p < 0.01, and ***p < 0.001, ns = not statistically different using one-way ANOVA with Tukey and Mann-Whitney tests. n = 3 biological replicates.
Direct gene upregulation by FMNP forces on chromatin
To probe the potential gene expression regulation effect from these FMNPs, we first measured relative displacements (deformation) of DHFR GFP chromatin spots as a function of the distance of an FMNP to the two DHFR GFP spots (Figure 3A). The results show that the chromatin displacements in response to a stress of 5 Pa were greater than 75 nm when the FMNP was within 5 μm of the DHFR GFP spots (Figure 3A), higher than the maximum spontaneous displacements (i.e., spontaneous movements, which were less than 50 nm) of the DHFR GFP spots induced by the anti-H2B-ab-coated nanoparticles in the absence of stress (Figure S3A). Furthermore, there was distance-dependent chromatin deformation from the anti-H2B-ab-coated FMNPs (Figure 3B). In contrast, the IgG-ab-coated or uncoated FMNPs did not exhibit any stress-induced chromatin deformation, nor there was any deformation that was dependent on the distance between the nanoparticle and the DHFR GFP spots with or without stress (Figures S3B–S3E). These results suggest that the IgG-ab-coated or uncoated FMNPs did not probe the chromatin mechanics, only the nucleoplasm mechanics. Based on these results, we focused on and analyzed the displacement data from the anti-H2B-ab-coated FMNPs that were less than 5 μm away from the chromatin GFP spots. From the relative displacements of any two chromatin GFP spots induced by a stress of 5 Pa from a single anti-H2B-ab FMNP that was within 5 μm of the DHFR GFP spots (Figures 3A and S3F), we computed chromatin stretching and found that the average stretching was 5.4% (Figure 3B). In contrast, the chromatin Δ (relative) displacements and chromatin stretching induced by the 5-Pa stress from IgG-ab-coated or uncoated FMNPs were almost zero (Figures 3B, S3G, and S3H). Immediately after 2- or 10-min stress application, the cells were fixed, and fluorescence in situ hybridization assays were performed to quantify DHFR transcription (Figure 3C). The stress via anti-H2B-ab-coated FMNPs, but not IgG-ab-coated or uncoated FMNPs, induced duration-dependent DHFR transcription upregulation (Figures 3C and 3D). As a positive control, the stress applied for 10 min with an Arg-Gly-Asp (RGD)-coated 4-μm magnetic bead on the cell surface via integrins also induced DHFR upregulation (Figures 3C and 3D), consistent with the published reports.5–8 However, for the same magnitude and duration, the stress applied on the cell surface induced less DHFR upregulation than the stress directly applied to the chromatin (Figure 3D), and the chromatin stretching by the surface force was only 3.4% (Figure 3B), which might be, in part, due to the fact that the stress from the cell surface decreased as it was being transmitted from the plasma membrane into the nucleus. Together, these results suggest that the stress from the anti-H2B-ab-coated FMNPs directly stretches the chromatin to upregulate DHFR transcription.
Figure 3. Direct gene upregulation by FMNP force on chromatin.

(A) Chromatin displacements depend on DHFR-GFP spots distance from the anti-H2B-ab-coated FMNP.
(B) Chromatin stretching (percentage) for microinjected anti-histone-H2B-ab-coated, anti-IgG-ab-coated, or uncoated magnetic FMNPs or a 4-μm RGD (Arg-Gly-Asp)-coated ferromagnetic bead on CHO cell surface.
(C) Representative images of CHO cell bright-field (BF) and Cy3 (red) DHFR fluorescence in Situ hybridization (FISH) (same cell in BF and Cy3 [red] fluorescence image for each condition). Scale bar, 5 μm.
(D) Normalized DHFR gene transcription from original data in (C).
Data (B) are boxplots with minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. Data (D) are mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001, ns = not statistically different using one-way ANOVA with Tukey and Mann-Whitney tests. n = 3–4 biological replicates.
See also Figure S3.
Chromatin and nucleoplasm mechanomemory after FMNP force release
To determine if there was mechanomemory of the protein diffusivity of the chromatin GFP spots or the nucleoplasm free GFPs, we directly applied stress via the microinjected anti-H2B-ab-coated FMNPs for 2 or 10 min, and then the stress was terminated. There was a 5- or 60-min waiting time post-force cessation before we quantified the diffusivity of the DHFR GFP spots or the free GFP in the nucleoplasm. We employed our recently published method where the diffusion coefficients were calculated from the MSDs (mean-squared displacements) for the DHFR GFP spots or from the autocorrelation function of the fluorescence correlation spectroscopy for the nucleoplasm free GFPs.9 The diffusion coefficient of the chromatin or the nucleoplasm free GFPs was elevated by the stress from the 200-nm FMNP in a stress-duration-dependent manner and stayed elevated 5 min after stress cessation, showing evidence of mechanomemory (Figures 4A and 4B). The diffusivity stayed elevated for 30 min (Figures S4A–S4C) and returned to the prior stress level 60 min after stress cessation (Figures 4A and 4B). When the FMNPs were more than 5 μm away (5–10 μm) from the nucleoplasm free GFPs, the increase in the diffusivity coefficient was smaller than that within the 5-μm distance (Figure 4B), suggesting that the effect of the nanoparticle decayed with distance. In contrast, anti-IgG-ab-coated or uncoated FMNPs did not induce any mechanomemory of the protein diffusivity of the chromatin or the nucleoplasm free GFPs (Figures S4D–S4G), suggesting that chromatin deformation is necessary for the mechanomemory effect. As a positive control, an RGD-coated 4-μm magnetic bead on the cell surface induced mechanomemory effects similar to the anti-H2B-ab-coated nanoparticles (Figures 4A and 4B), consistent with the published results.9 These results show that a very small rotational force of ~1pN (shear stress × surface area of the FMNP = 5 Pa times 4π(100 nm)2 = 1 pN) on the 200-nm FMNP and the equivalent 200-pNnm torque (the torque = 1 pN × 200 nm = 200 pNnm) directly applied to the chromatin can produce mechanomemory effects on the protein diffusivity of the chromatin and of the nucleoplasm that is near the FMNP.
Figure 4. Mechanomemory of chromatin and nucleoplasm diffusivity after FMNP force release.
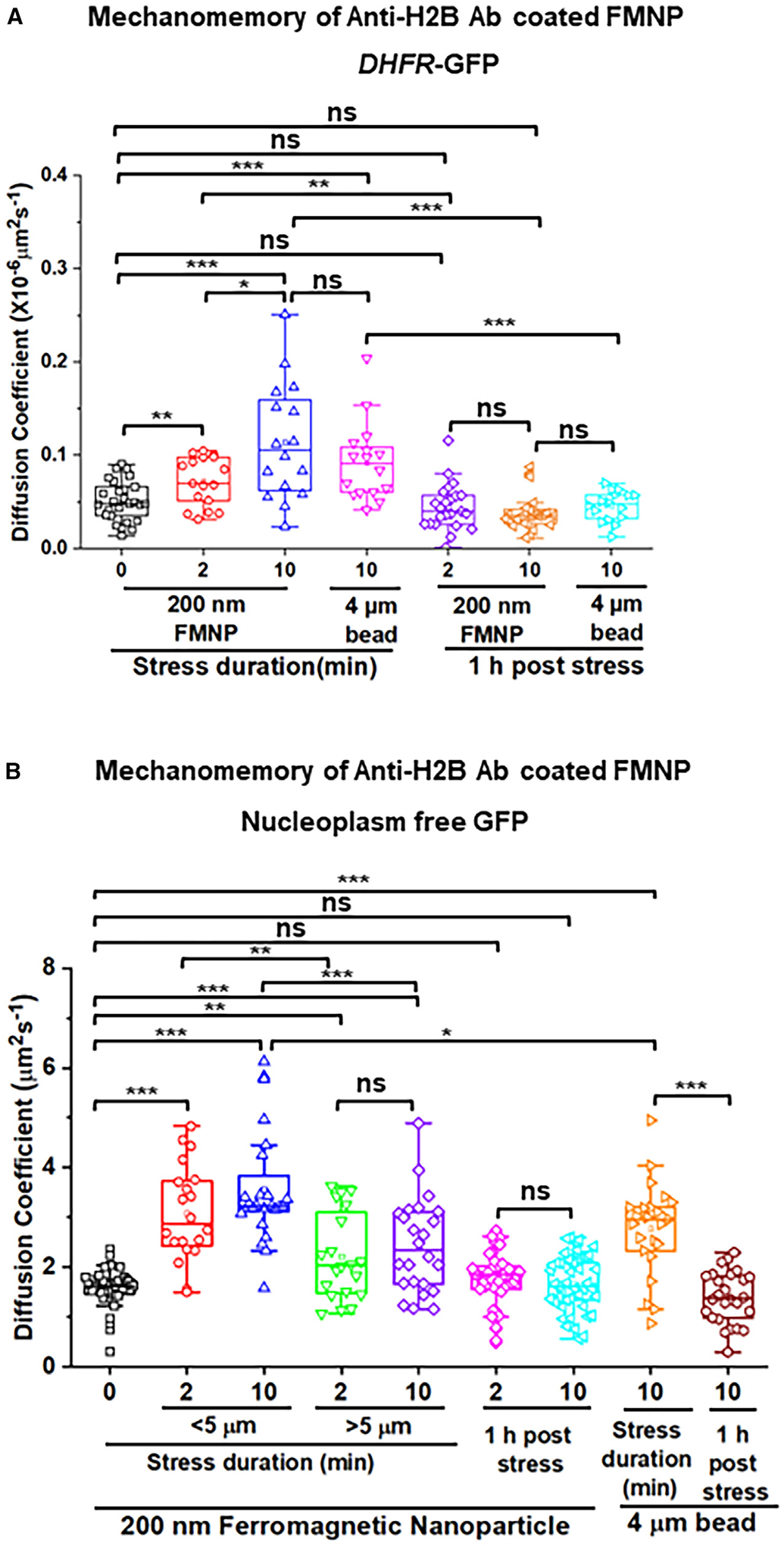
(A) Diffusion coefficient of DHFR-GFP on the chromatin 5 min after 0-, 2-, or 10-min stress (5 Pa at 0.3 Hz), 1 h post 2- and 10-min FMNP stress and 5 min after 10-min stress, or 1 h post-10-min stress from a 4-μm RGD-coated bead attached to CHO cell surface with the same stress (5 Pa at 0.3 Hz).
(B) Diffusion coefficient of free GFP inside the nucleoplasm (free GFPs <5-μm and >5-μm distance from location of DHFR-GFP spots) 5 min after 0-, 2-, or 10-min stress and 1 h post 2- or 10-min stress from anti-H2B-ab-coated FMNP and 10 min or 1 h post-10-min stress from the 4-μm RGD-coated bead attached to a CHO cell surface.
Data are boxplots with minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. *p < 0.05, **p < 0.01, and ***p < 0.001, ns = not statistically different using one-way ANOVA with Tukey and Mann-Whitney tests. n = 3–6 biological replicates.
See also Figure S4.
Mechanomemory of RNA Pol II diffusivity
Because force-induced DHFR transcription exhibits mechanomemory and stays upregulated >30 min after cessation of the force,6 we wondered what the underlying mechanism might be. We hypothesized that RNA Pol II should exhibit mechanomemory to show transcription upregulation memory. To evaluate this hypothesis, we first examined the diffusivity of human induced pluripotent stem cells (hiPSCs) with a mono-allelic monomeric enhanced green fluorescent protein (mEGFP)-tagged RNA Pol II spot (Figure 5A). These hiPSCs were used instead of the CHO cells because they expressed fluorescent RNA Pol II markers, so we were able to quantify the diffusivity of RNA Pol II spots. After the force was applied via either the anti-H2B-ab-coated FMNP or the 4-μm RGD-coated magnetic bead for 2 or 10 min, the diffusion coefficient measured via the MSD method on the RNA Pol II spot stayed elevated for 5 and 30 min and returned to the baseline value 60 min after force cessation (Figures 5B and S5A). The fluorescence correlation spectroscopy measurements of single RNA Pol IIs in the nucleoplasm in the vicinity (<0.5 μm) of the RNA Pol II spot revealed that there was force-duration-dependent elevation of the diffusion coefficients induced by both the anti-H2B-ab-coated 200-nm FMNP bound to the chromatin and the 4-μm RGD-coated magnetic bead bound to the integrins (Figure 5C); the diffusion coefficients stayed elevated for 5 and 30 min but returned to the baseline values 60 min after force release (Figures 5C and S5B). In sharp contrast, the force applied via an uncoated FMNP that did not cause any stretching of the chromatin (Figure 3B) did not induce an elevation in the diffusivity of the RNA Pol II spot nor the mechanomemory of the RNA Pol II diffusivity (Figure S5C), suggesting that chromatin stretching is necessary for mechanomemory of the RNA Pol II diffusivity. These results suggest that direct stretching of the chromatin via the FMNP inside the nucleus or via the 4-μm magnetic bead at the cell surface can induce mechanomemory of the RNA Pol II diffusivity.
Figure 5. Stress-induced mechanomemory of RNA Pol II diffusivity.

(A) Representative images from two different hiPSC nuclei. One cell shown on the left (BF image; white arrow points to the nanoparticle) and the middle left (GFP image) was microinjected with an anti-H2B-ab-coated FMNP; another cell shown on the middle right (BF image; white arrow points to the bead) and the right (GFP image) was bound to a 4-μm RGD-coated magnetic bead on its surface. Scale bars, 5 μm.
(B) Diffusion coefficient of RNA polymerase II (RNA Pol II) spots 5 min after 0-, 2-, or 10-min stress and 1 h post-2- or 10-min stress from the anti-H2B-ab-coated FMNP and the 4-μm RGD-coated bead on the hiPSC surface.
(C) Diffusion coefficient of single RNA Pol II molecule (<0.5 μm distance from RNA Pol II arrow shown in A) 5 min after 0-, 2-, or 10-min stress and 1 h post-2- or 10-min stress from the microinjected anti-H2B-ab-coated FMNP and the 4-μm RGD-coated bead on the hiPSC surface.
Data (B and C) are boxplots with minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. *p < 0.05, **p < 0.01, and ***p < 0.001, ns = not statistically different using one-way ANOVA with Tukey and Mann-Whitney tests. n = 3–4 biological replicates.
See also Figure S5.
Mechanomemory of RNA Pol II activation
Next, we wondered if the FMNP force for 2 and 10 min was able to induce changes and mechanomemory of the RNA Pol II activity. We assayed RNA Pol II serine 2 phosphorylation as an index of RNA Pol II activity 5, 30, or 60 min after force cessation. There was force-duration-dependent elevation of RNA Pol II serine 2 phosphorylation in human iPSCs and CHO cells; the elevation stayed high for 5 and 30 min for both the FMNP and the 4-μm magnetic bead (Figures 6A–6D, S6A, and S6B). On the other hand, the force applied via an uncoated FMNP did not induce elevation of RNA Pol II serine 2 phosphorylation nor the mechanomemory of RNA Pol II serine 2 phosphorylation in CHO cells (Figures S6C and S6D), suggesting that chromatin stretching is necessary for mechanomemory of the RNA Pol II activity. Because RNA Pol II activity is critical in gene transcription, these data of mechanomemory of RNA Pol II activity 30 min after force cessation in response to the 2-min stress may provide a partial explanation for the previously published results that DHFR transcription continues for 30 min after termination of the 2-min magnetic bead stress.6 Together, these results suggest that the mechanomemory of the RNA Pol II activation is the result of the direct force effect via the anti-H2B-ab-coated FMNPs.
Figure 6. Stress-induced mechanomemory of RNA Pol II activity.

(A) Representative images of BFs and immunofluorescence (red) in hiPSC nuclei. Scale bar, 5 μm.
(B) Normalized RNA Pol II activity in hiPSCs 5 min after 0-, 2-, or 10-min stress and 1 h post-2- or 10-min stress from the anti-H2B-ab-coated FMNP and the 4-μm RGD-coated magnetic bead at the cell surface.
(C) Representative images of BFs and immunofluorescence of RNA Pol II Ser 2 phosphorylation (red) in CHO cell nuclei. Scale bar, 5 μm.
(D) Normalized RNA Pol II activity in CHO cells 5 min after 0-, 2-, or 10-min stress and 1 h post-2- or 10-min stress for the anti-H2B-ab-coated FMNP and the 4-μm RGD-coated bead at the cell surface.
Data (B and D) are boxplots with minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. *p < 0.05, **p < 0.01, and ***p < 0.001, ns = not statistically different using one-way ANOVA with Tukey and Mann-Whitney tests. n = 3–4 biological replicates.
See also Figure S6.
Chromatin mechanomemory duration decreases with substrate stiffness
It is well known that substrate stiffness regulates cellular functions and behaviors.1 To explore if substrate stiffness plays any role in chromatin diffusivity, mechanomemory, and mechanics, we plated CHO cells on varying substrate stiffness of 1, 10, or 20 kPa made of polyacrylamide gels. In the absence of an externally applied stress, there was no change in spontaneous chromatin DHFR-GFP spot diffusivity with increasing substrate stiffness from 1 to 10 to 20 kPa (Figures 7A and 7B); these diffusion coefficients were similar in magnitude to the diffusivity value of the CHO cells plated on rigid glass (compare Figure 7B with Figure 4A). However, on the 1- or 10-kPa substrate, after cessation of the 2- or 10-min stress, chromatin diffusivity remained elevated even 60 min after cessation of 5-Pa stress from the anti-H2B-ab-coated FMNP (Figure 7B); in contrast, on the 20-kPa substrate, the chromatin diffusivity returned to the baseline (0 min stress) value 60 min after force cessation (Figure 7B), similar to those cells plated on rigid glass 60 min after force release (compare with those in Figure 4A). In addition, in response to the anti-H2B-ab-coated FMNP stress, chromatin D displacements and stretching decreased as substrate stiffness increased from 1 to 20 kPa (Figures S7A and 7C). As a result, the chromatin complex modulus, storage modulus, and loss modulus increased with substrate stiffness (Figures 7D–7F). Furthermore, the nucleoplasm complex modulus, storage modulus, and loss modulus (which were quantified when the 5-Pa stress from the microinjected uncoated FMNP was applied) increased with substrate stiffness (Figures 7G–7I). Together, these results show that the mechanomemory duration of chromatin diffusivity decreases, and moduli of chromatin/nucleoplasm increase with substrate stiffness.
Figure 7. Substrate stiffness increases chromatin stiffness but decreases its diffusivity mechanomemory.

(A) Representative images of CHO cell nuclei after microinjection of an anti-H2B-ab-coated FMNP per cell on polyacrylamide (PA) gel substrates of 1-, 10-, or 20-kPa stiffness. In each inset, an FMNP is within a dashed circle. Scale bar, 5 μm.
(B) Diffusion coefficient of DHFR-GFP on the chromatin of CHO cells on a 1-, 10-, or 20-kPa substrate stiffness at 0 or 5 min after 2- or 10-min stress or 1 h post-2-or 10-min stress from an anti-H2B-ab-coated FMNP.
(C) Chromatin stretching in CHO cells in response to microinjected anti-H2B-ab-coated FMNP stress on a 1-, 10-, or 20-kPa substrate stiffness.
(D) Chromatin complex modulus of CHO cells on 1-, 10-, or 20-kPa substrate stiffness in response to a microinjected anti-H2B-ab-coated FMNP stress.
(E) Chromatin storage modulus (G′ = (σ0/ε0) cos δ) of CHO cells on a 1-, 10-, or 20-kPa substrate stiffness in response to the anti-H2B-ab-coated FMNP stress.
(F) Chromatin loss modulus (G′′ = (σ0/ε0) sin δ) of CHO cells on a 1-, 10-, or 20-kPa substrate stiffness in response to the anti-H2B-ab-coated FMNP stress.
(G) Nucleoplasm complex modulus of CHO cells on a 1-, 10-, or 20-kPa substrate stiffness in response to a microinjected uncoated FMNP stress.
(H) Nucleoplasm storage modulus (G′ = (σ0/ε0) cos δ) of CHO cells on a 1-, 10-, or 20-kPa substrate stiffness in response to the uncoated FMNP stress.
(I) Nucleoplasm loss modulus (G′′ = (σ0/ε0) sin δ) of CHO cells on a 1-, 10-, or 20-kPa substrate stiffness in response to the uncoated FMNP stress.
The applied stress was 5 Pa at 0.3 Hz for (B)–(I). Data (B–I) are boxplots with minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. *p < 0.05, **p < 0.01, and ***p < 0.001, ns = not statistically different using one-way ANOVA with Tukey and Mann-Whitney tests. n = 3 biological replicates.
DISCUSSION
In this study, we show that a stress of physiologic magnitudes applied via the microinjected 200-nm anti-H2B-ab-coated FMNP for 2 or 10 min inside the nucleus directly stretches the chromatin and produces mechanomemory of the protein diffusivity of the chromatin and nucleoplasm tens of minutes after force release. The approach of microinjected magnetic nanoparticles into the nucleus is significant because it bypasses the force transmission pathways from the cell surface adhesion protein integrins, cytoskeleton, LINC, and nuclear lamins and avoids the potential contribution from signaling molecules across the cell surface or relaying molecules in the cytoplasm such as calcium, Piezo1/2, and/or YAP/TAZ. Importantly, we reveal that the RNA Pol II diffusivity and activity stay elevated for 30 min after force cessation, providing a mechanistic explanation for the observation that gene transcription lasts for over 30 min after cessation of a 2-min magnetic bead stress on the cell surface,6 extending our previous findings on the mechanomemory of protein diffusivity in the chromatin and nucleoplasm and of dissociation of heterochromatin protein 1 alpha clusters, suggesting a possible nuclear basis of mechanointelligence in a living cell.24 The mechanomemory of the nucleoplasm, whose duration is much longer than the time constant (<10 s) of the viscoelastic properties of the nucleus, is shown to be regulated by transport across the nuclear pore complexes (NPCs) and is predicted in a dissipative particle dynamics model, which shows that the local density reduction of the free nucleoplasmic proteins, as a result of the chromatin tensile strains, contributes to the mechanomemory.9 Furthermore, we have quantified the moduli of the chromatin and nucleoplasm using the FMNPs and found that the chromatin and nucleoplasm behave like a viscoelastic gel structure under physiological loading frequencies of 0.1–5 Hz. We find that substrate stiffness regulates the mechanomemory duration of chromatin diffusivity and moduli of chromatin and nucleoplasm. These findings of the viscoelastic behavior of the chromatin in live cells in response to a direct mechanical loading of physiologic magnitudes and frequencies provide concrete evidence that the chromatin does not behave like a pure liquid, as concluded in one study,18 nor like a solid, as concluded in another study.19
To evaluate the potential impact of the limitations of our approach, we performed chromatin diffusivity quantification and atomic force microscopy (AFM) experiments. The cell nuclei microinjected with an anti-H2B-ab-coated FMNP or medium alone (no FMNP) exhibited similar chromatin diffusion coefficients and local nuclear moduli above the site of the microinjected FMNP as the intact control cell nuclei (Figures S7B and S7C), suggesting that the mechanical impact of the microinjected anti-H2B-ab-coated FMNP on chromatin diffusivity and nuclear mechanics is small. However, additional experiments using transmission electron microscopy (TEM) showed some elevated intensity of gray contrast surrounding the microinjected FMNP, suggesting the presence of chromatin aggregation, when compared with the cell nuclei that are injected with medium only or the intact cell nuclei (Figure S7D). These results suggest that a microinjected anti-H2B-ab-coated FMNP induces some local aggregation of chromatin fibers but may not cause significant changes in the diffusivity of the nearby chromatin DHFR-GFP spots or the nuclear stiffness. However, these results do not rule out the possibility of FMNP inducing other local structural and mechanical changes in the nucleus that are not probed in this study.
In our study, our data show broad ranges in chromatin deformation and moduli. Currently, we do not know the exact reason for these broad data ranges because we do not have super-resolution images of the chromatin structure in these live cells to associate local chromatin structures with the location of the FMNP and the mechanical measurements. However, we suspect that this broad data range may in part be due to the local microenvironment of the microinjected nanoparticle. It is known from TEM images that chromatin structures are highly heterogeneous within the nucleus; some parts are more condensed than other parts. The condensed parts of the chromatin might have higher modulus than less condensed parts. However, we were not able to control the exact location of the microinjected FMNP in each nucleus, which is another limitation of our approach using the microinjected FMNP. It is possible that those FMNPs that are near the nuclear periphery (where the majority of heterochromatin is located) may probe the chromatin domains of higher moduli than those that are closer to the nuclear center. This interpretation is consistent with a previous study that shows that chromatin domains that are near the nuclear envelope are less deformable than the chromatin domains that are closer to the center of the nucleus.6 This interpretation is also consistent with the observation of variations in nuclear stiffness (3- to 5-fold) measured with AFM in the current study (Figure S7C), likely due to the variations in local chromatin structures, because the magnitude of the quantified local nuclear stiffness depends on the specific location of the AFM cantilever tip above the nucleus. Future studies might shed light on this matter.
There has been considerable uncertainty about and substantial variability in the mechanical properties of the nucleus, chromatin, and nucleoplasm. Earlier published work shows that that the modulus of a mitotic chromosome that is pulled out of a living cell is 1,000–5,000 Pa,25 while the modulus of isolated chromosomes is 300 Pa.26 However, a report shows that the modulus of an isolated nucleus, probed via a 2.8-μm magnetic bead pulling directly on the nuclear surface protein nesprins, is only 0.5 Pa.20 Although it is not clear why isolated nuclei exhibit such a low stiffness, it is possible that intranuclear structural proteins that link the lamins and the chromatin with the nuclear membrane are washed out in the isolated nuclei such that the stiffness of the nuclear envelope, rather than that of the whole nucleus, including the chromatin, is quantified in the previous study using the anti-nesprin-ab-coated magnetic bead.20 The nuclear elastic modulus from our current study is ~2 kPa, consistent with the published value of ~1 kPa for isolated nuclei.21 In one of our previous studies,6 using the 4-μm magnetic bead on the cell surface, we did not know the exact stress on the chromatin because of the stress decay along the stress transmission pathway into the nucleus; hence, we were not able to quantify the chromatin modulus. In contrast, our current study, using the microinjected 200-nm anti-H2B-ab-coated FMNP that directly deforms the chromatin, shows that the storage (elastic) modulus of the chromatin is 100 Pa, in the same range of modulus magnitude as the isolated chromosome of 300 Pa26 and the 250-Pa elastic modulus of the chromatin in the living cell measured with a 1-μm bead27 and in the same order of magnitude as the nuclear elastic modulus of 18 Pa measured with microinjected 100-nm nanoparticles in a published report.28 In our study, the loss modulus of the chromatin is ~70 Pa at a loading frequency of 0.3 Hz, lower than the elastic modulus of 100 Pa. Together, these results suggest that intranuclear probe size and binding specificity are important in assessing the mechanical properties of the nuclear structures.
A recent study using 28-nm paramagnetic nanoparticles that bind specifically to the chromatin showed that the chromatin behaves like a liquid,18 a conclusion that challenges an earlier finding that the chromatin behaves like a solid on the mesoscale in a living cell.19 In our current study, using the 200-nm anti-H2B-ab-coated FMNPs, we find that the chromatin behaves like a viscoelastic gel with a storage modulus that is higher than the loss modulus in the physiologic loading frequency range of 0.1–5 Hz. Therefore, our data support the notion that the chromatin in live cells is a dynamic material that behaves viscoelastically between a solid and a liquid. In response to the loading frequency elevation, the power law exponent of the loss modulus (0.32) is twice as that of the storage modulus (0.15). Extrapolating the experimental data predicts that the loss modulus will be greater than the storage modulus and the chromatin will behave more liquid-like at frequencies higher than 21.6 Hz (G′ and G′′ are equal at 21.6 Hz). However, at loading frequencies higher than 20 Hz, the anti-H2B-ab-coated FMNP-induced chromatin deformation is too small to be different from the spontaneous movements, and hence we are not able to verify this prediction experimentally in the current study. Nevertheless, our results suggest that in the physiological loading frequency range of 0.1–5 Hz, the differences among different studies with various probes are likely due to the differences in probe size and probe surface coating, which are known to be critical in probing the mechanics of the intranuclear structures. For the 28-nm nanoparticles, they are much smaller than the 50- to 200-nm mesh size of the chromatin29 and nucleoplasm. It is likely that an unfolded single chromatin is probed with the 28-nm probes, and thus, the liquid-like behavior of the chromatin is detected. Our results that the elastic (storage) modulus is much greater than the dissipative (loss) modulus within the 10-s loading period (i.e., a loading frequency of 0.1 Hz) (Figure 2B) do not support the notion that the chromatin behaves as a weakly crosslinked and short-lived (say, a few seconds) gel, as suggested by an early report.18 Instead, as the size of the probe that binds specifically to the chromatin increases and becomes greater than the mesh size of the nucleoplasm, the mechanics of the chromatin on the mesoscale that is relevant to the control of gene transcription is probed. To support this interpretation, IgG-ab-coated or uncoated 200-nm FMNPs do not bind specifically to the chromatin, do not deform the chromatin, and detect only the mechanics of the nucleoplasm; these probes reveal that the storage modulus and loss modulus of the nucleoplasm network are similar in magnitude (~3 Pa). In contrast, there are no storage modulus and loss modulus measurements and only estimated apparent viscosity values for the nucleoplasm from the spontaneous movements of the nucleoli (the nucleolar sizes range from 1 to 10 μm) in the geminal vesicles of Xenopus oocytes.30
One question is whether the 5-Pa stress in the nucleus of the CHO cell from the FMNP is the physiologically relevant magnitude of stress for the cell. The earlier published data show that the average traction the CHO cell generates on an 8-kPa substrate8 is 30 Pa, which is six times the 5-Pa applied stress. It is known that the traction at cell surface is generated via the endogenous actomyosin contractile forces that also pull on the nucleus of the cell via the LINC complex. Therefore the 5-Pa rotational stress via the FMNP, which is ~200-pNnm torque for the 200-nm nanoparticle, is equivalent to 50 kT energy (1 kT = 4 pNnm) and 4 times higher than the energy released from hydrolysis of ATP (adenosine triphosphate) to ADP. It is known that ATP depletion decreases chromatin spontaneous motions.31 To examine if the ATP depletion had similar effects in these CHO cells, ATP was depleted by changing the culture medium to a medium that contained ATP inhibitors. The quantified MSD and time-fitted data were used for calculating the diffusion coefficients of DHFR-GFP spots or uncoated 200-nm FMNP in CHO cells in the control (the regular medium or 20-mM D-glucose medium) or ATP-depleted conditions. As expected, the diffusion coefficient of the DHFR-GFP spots was reduced by 49% in 20-mM 2-deoxy-D-glucose medium and 48% in the 10-μM antimycin A (an inhibitor of oxidative phosphorylation) medium and that of the 200-nm FMNPs was reduced by 31% in the 20-mM 2-deoxy-D-glucose medium and 38% in the 10-μM antimycin A medium (Figures S7E and S7F), consistent with the published report.31 These data show that the spontaneous motions of the chromatin and nucleoplasm structures (using FMNP motions as an index) in CHO cells are regulated by the ATP-dependent processes in the nucleus and/or the cytoplasm, such as actomyosin forces.
Currently, the exact mechanism is unclear regarding the difference in chromatin deformation induced by the same magnitude of the applied stress between the surface stress by the 4-μm magnetic bead and the chromatin stress by the FMNP, but it may be partly explained by the fact that for the stress applied at the cell surface, because of the balance of the torque (or of the force) (force at the cell surface = stress at the cell surface × bead-cell contact area; force at the nucleus = stress at the nucleus × the impact area at the nucleus), the torque (or the force) at the cell surface must equal to the torque (or the force) at the nuclear plane. In addition, because the impact area of the force at the nucleus increases as the square of the distance from the bead to the nucleus and is larger than the bead-cell contact area, the effective stress transmitted to the nucleus and the chromatin is expected to be lower than the applied stress at the cell surface. However, cytoskeletal prestress and stress-fiber-dependent stress focus as well as long-distance force propagation substantially reduce the stress decay at the nucleus and the chromatin.7 This interpretation that is based on both the stress decay with distance and the stress concentration by the stress fiber cytoskeleton may explain the data that the chromatin stretching by the cell surface bead stress is only moderately lower than that by the FMNP stress. Other factors such as the efficiency/density of the coating and/or the force-dependent properties of the RGD-integrin bonds might also contribute to this difference in chromatin deformation. Alternatively, depending on the binding and effective surface interactions between the anti-H2B-ab-coated FMNP and the H2Bs on the chromatin fibers, the effective stress from the FMNP to the chromatin could be higher than 5 Pa, leading to higher chromatin deformation. Additional studies in the future may shed light on this issue.
Our data show that after cessation of the microinjected anti-H2B-ab-coated FMNP force, chromatin mechanomemory duration is longer on soft substrates than stiff substrates. Currently, we do not know the exact underlying mechanism. However, one possible interpretation is that because the diameters of the NPC are smaller on softer substrates,32 there is less transport across the NPCs, leading to longer nuclear mechanomemory. This interpretation is consistent with the recent finding that transport via NPCs regulates nuclear mechanomemory duration and the addition of a hypertonic medium that reduces the NPC size leads to longer (over 60 min) mechanomemory of nucleoplasm protein diffusivity.9 Furthermore, our data show that chromatin and nucleoplasm moduli increase with substrate stiffness. These results are in accord with and extend our published report5 that substrate stiffness regulates the extent of chromatin condensation and DHFR gene transcription via controlling actomyosin contractility. Nevertheless, future studies are needed to elucidate how substrate stiffness regulates chromatin and nucleoplasm structures and functions.
RNA Pol II is a key in the transcription process. In earlier studies, we have shown that in response to force applied for several minutes at the cell surface integrins, RNA Pol II is quickly recruited to the promoter site of the DHFR promoter sites, and RNA Pol II is rapidly activated.5,6,8 In the present study, we reveal that the diffusivity of the RNA Pol II is elevated by direct force applied to the chromatin via the 200-nm FMNP, and this elevation stays for 30 min after cessation of the stress, suggesting the mechanomemory of the RNA Pol II diffusivity. Notably, we find that the RNA Pol II activity (assayed by RNA Pol II serine 2 phosphorylation) exhibits mechanomemory for 30 min after cessation of the 2- or 10-min stress via either the 200-nm FMNP in the nucleus or the 4-μm magnetic bead on the cell surface. This finding extends our earlier findings5–9 and provides a mechanistic explanation of the earlier finding that DHFR gene transcription stays elevated for more than 30 min after cessation of the 2-min stress.6 The mechanomemory of the RNA Pol II activity is seen in both hiPSCs and CHO cells, suggesting that it is a general phenomenon and not a cell-type-specific response. Furthermore, earlier published results show that substrate stiffness, actomyosin contractility (pre-stress), and the integrity of the cytoskeleton regulate gene transcription via cell surface force transmission into the nucleus and/or chromatin condensation/decondensation.5,7 One question that still remains is whether the observed mechanomemory in the nucleus would cause any long-term biological effects in the nucleus and/or the cytoplasm. We hypothesize that the consecutive mechanomemory from the intermittent multiple applications of forces (e.g., via the 4-μm magnetic bead on the cell surface integrin receptors) for 2–10 min at a time may induce long-term effects (hours) on the physiological behaviors and responses of the cell. This possibility could be explored in the future. Although we have shown that forces applied to the chromatin increase the diffusivity of the chromatin, nucleoplasm, and RNA Pol II, the causal link among these is currently not clear and beyond the scope of this study. Further investigation is needed to shed light on this issue.
In summary, we have quantified moduli of the chromatin and nucleoplasm and found that the chromatin behaves as a viscoelastic gel-like structure and that the nucleoplasm network is a softer viscoelastic structure. We show that mechanomemory lasts for tens of minutes in protein diffusivity of the chromatin and nucleoplasm and in RNA Pol II diffusivity and activity, which may provide a direct mechanomemory mechanism for sustained transcription upregulation after force cessation.
Limitations of the study
Any method for mechanical manipulation and probing has its limitations. A published report critically evaluates mechanical properties of the same MCF-7 breast cancer cells and compares various mechanical probes such as magnetic twisting cytometry, AFM, particle-tracking microrheology, parallel-plate rheometry, cell monolayer rheology, and optical stretching.33 That study reveals that the moduli of the same breast cancer cells can vary 2–3 orders of magnitude depending on the probe being used and identifies that deformation rate, probe geometry, probe location in the cell, and the extracellular microenvironment all contribute to the differences in moduli. In our current approach, microinjection of a nanoparticle to penetrate the nuclear envelope may cause nuclear membrane damage that could potentially affect cytoplasm-nucleus transport. The injection of an anti-H2B-ab-coated nanoparticle may also induce chromatin realignment and aggregation around the particle to change local chromatin structure and nuclear function.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Any further information and requests for any resources should be sent to the lead contact, Dr. Ning Wang (ni.wang@northeastern.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data that are needed to evaluate the conclusions in the current study are shown in the paper and the supplemental information.
The MATLAB codes used for this research are available on GitHub (https://github.com/fazlurr2/Mechanomemory_FMNP_GFP_Pol-II.git).
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Cell lines
Chinese hamster ovary (CHO) DG44 cells and human induced pluripotent stem cells (hiPSCs) were used in this study. CHO cells were cultured with Ham’s F12 medium without thymidine and hypoxanthine but with 10% dialyzed fetal bovine serum (FBS) and hiPSCs were grown with complete mTeSR1 (cGMP, feeder-free medium from STEMCELL Technologies).
METHOD DETAILS
Cell culture
In this study, Chinese hamster ovary (CHO) DG44 cells and human induced pluripotent stem cells (hiPSCs) (Mono-Allelic mEGFP-Tagged RNA polymerase II subunit A (POLR2A) from Coriell Institute) were used. Dihydrofolate reductase (DHFR) BAC (bacterial artificial chromosome) inserted CHO (D10 subclone) cells were cultured in Ham’s F12 medium without thymidine and hypoxanthine but with 10% dialyzed fetal bovine serum (FBS) as mentioned previously.5,6,9 These cells express stable GFP-LacI (EGFP-Lac Repressor) that binds with DHFR-tagged 256-mer LacO (Lac Operator) to visualize and locate the transgene in chromatin.5 hiPSCs were cultured on a Matrigel matrix (Corning) coated cell culture dish by using complete mTeSR1 (cGMP, feeder-free medium from STEMCELL Technologies) supplemented with streptomycin (100 μg/mL), and penicillin (100 IU/mL) by following the manufacturer’s protocol. Both CHO cells and hiPSCs were cultured every 3–4 days using TrypLE Express (Thermo Fisher Scientific) and StemPro Accutase (Cell Dissociation Reagent from STEMCELL Technologies) respectively and grown in a 37°C humidified incubator with 5% CO2. Mycoplasma contamination was monitored visually and stained with DAPI (4′,6-diamidino-2-phenylindole) for both CHO and hiPS cells. No mycoplasma contamination was seen during cell culture and experiment for any of these two cell lines.
Ferromagnetic bead and nanoparticle coating
Anti-Histone H2B (Abcam, ab52484) and Rabbit Anti-Mouse IgG H&L (Horseradish Peroxidase (HRP)) (Abcam, ab6728) antibody were used to coat ~200-nm (202 ± 6 nm (mean ± SD)) (Figure S1B) ferromagnetic nanoparticle (FMNP) (Fe3O4, cat. no. 17526, Polysciences, Warrington, PA) for microinjecting into a CHO cell nucleus or an hiPSC nucleus following the published protocol.34 We chose the 200-nm magnetic nanoparticles as the intranuclear mechanoprobes because they were larger than the 50–200 nm mesh size of the chromatin29 and thus of the nucleoplasm and large enough for being visualized in brightfield images for quantification of stress-induced FMNP displacements and calculation of the moduli but were small enough to cause minimal damage to the cell membrane and the nuclear envelope using the same microinjection pressure from the protocols that were published earlier.18,28 Before coating, the nanoparticles were briefly sonicated for 30–40 s and filtered using a 0.22 μm syringe filter to filter out nanoparticles that were greater than ~220 nm to avoid any aggregation in the Femtotip needle of 0.5 μm inner diameter (Catalog/Part#930000035 from Eppendorf North America; the inner diameter is 0.5 μm for the defined opening for the microinjection and 1 μm (±0.2 μm (SD)) outer diameter). The diameter of the ferromagnetic nanoparticle (Fe3O4) was measured using scanning electron microscopy (Hitachi S-4800 FE-SEM; accelerating voltage 500 V to 30 kV). First, ferromagnetic (Fe3O4) nanoparticles (~200 nm) were added with ethanol (5 mg/mL, ~4.2×1011 nanoparticles per mL). These nanoparticles were centrifuged to remove the supernatant and dried using nitrogen (N2). The nanoparticles were then functionalized for 30 min at room temperature in a borate buffer of pH 5.5 by using sulfo-NHS (N-hydroxysuccinimide, 1000:1 M ratio, Sigma-Aldrich) and EDC (1-ethyl-3, 3-dimethylaminopropyl carbodiimide hydrochloride, 2000:1 M ratio, Sigma-Aldrich). The NHS-EDC chemistry generates NHS ester outside the core of ~200 nm ferromagnetic nanoparticles. The pH was then adjusted to 8 to stabilize the conjugation of NHS ester with amine (-NH2) group from antibodies (Anti-Histone H2B or IgG H&L antibody).34 This functionalized nanoparticle was diluted two times to make it 840 nanoparticles per μL (~84,000 nanoparticles per 100 μL). One hundred-μl nanoparticle solution was taken in a 1.5 mL microcentrifuge tube and a 10 μL (1 μg/μL) of Anti-Histone H2B or IgG H&L antibody was added immediately. These nanoparticles were mixed well with antibody and incubated at room temperature in a rotator for 2 h. After that, a microloader tip (Eppendorf) was used to take 3 μL solution (~2520 nanoparticles) of antibody-conjugated ferromagnetic nanoparticle (FMNP-ab) to be loaded into a Femtotip needle for microinjection into a CHO cell nucleus and an hiPSC nucleus. The stability of FMNP was assessed in DPBS (Invitrogen), DPBS plus bovine serum albumin (BSA), and cell culture medium by incubating at 5% CO2 with a 37°C humidified incubator. The zeta potential before (−46.8 mV) and after (−34.3 mV) antibody conjugation with FMNP was measured using ZETASIZER (Nano series, Nano-ZS90).
Arginine–Glycine–Aspartic acid (RGD) peptides were used to coat the 4-μm ferromagnetic beads that were obtained from Dr. J. Fredberg’s laboratory (Boston, MA) by following the protocol as mentioned before.35 Briefly, two hundred μl of 4-μm-sized ferromagnetic beads were added to 1 mL carbonate buffer of pH 9.4. RGD was then added to a final concentration of 50 μg per mL and mixed overnight at 4°C on a rotator. These beads were used to apply force on integrin cell surface receptors.
Ferromagnetic nanoparticle (FMNP) microinjection
Anti-Histone H2B and IgG H&L (HRP) antibodies and uncoated ~200 nm FMNP were microinjected into the cell nucleus using the FemtoJet 4i and InjectMan 4 (Eppendorf) systems. Before that, around 90,000–100,000 cells were grown (CHO cells were plated on fibronectin (5 μg per mL) coated and hiPSCs were plated on Matrigel (1:30 dilution) coated 35-mm gridded glass-bottomed Petri dish (imprinted 50 μm cell location, 81148, Ibidi USA Incorporated, Madison, WI). After overnight incubation at 5% CO2 with a 37°C humidified incubator, cell media was changed with 10 mM HEPES buffer media containing 0.2% serum for FMNP microinjection at room temperature. A single spread nucleus in each CHO DG44 cell was observed after overnight incubation for interphase chromatin mechanics experiments. In the microinjection process, first, the Femtotip needle was set on the injection arm after loading three μL FMNP-ab or uncoated FMNP. By following published protocol and manufacturer’s guidelines (Eppendorf), injection parameters (injection mode: axial, injection speed: 300 μm per sec, injection angle: 45°, injection time: 0.2 s, injection pressure: 100 hPa (1 hPa = 100 Pa), compensation pressure: 30 hPa) were set to get high microinjection efficiency and maintain cell viability18,28. Microinjection was performed for a period of up to 30 min and immediately after that HEPES buffer medium was changed with fresh 2 mL medium for CHO cells or hiPSCs and kept at 5% CO2 with a 37°C humidified incubator for 30–40 min before starting the imaging experiment. To determine cell viability after FMNP microinjection, cells were washed twice with balanced salt solution followed by addition of 0.4% Trypan Blue Solution (Catalog#T8154; Sigma Aldrich). Microinjected cells were tracked on the gridded dish. Microinjected cells were kept in Trypan Blue Solution for up to 12 h. No Trypan Blue was taken up by the cells that were microinjected with FMNPs, suggesting that the potential damage to the cells was minimal and the plasma membrane recovered such that cell viability was not affected. Additionally, no abnormal nuclear shape changes or cell shape changes were observed after the microinjection of the FMNPs, and the cells appeared normal several days after the experiments.
Magnetic twisting cytometry (MTC)
Magnetic twisting cytometry (MTC) magnetizes ferromagnetic material in one axis direction (X, Y, or Z) and applies a twisted magnetic field orthogonally for in or out-of-plane rotation of these two axes.7 In this study, one-dimensional (1D)-MTC was used to magnetize in Y-direction and apply a weak homogeneous magnetic field in Z-direction directly to the cell nucleus via a microinjected 200 nm ferromagnetic nanoparticle and cell surface integrin receptors via Arg-Gly-Asp (RGD) coated 4-μm ferromagnetic beads.
For both CHO DG44 cells and hiPSCs, 1–4 FMNP per cell nucleus (3–5 nL per microinjection for both antibodies coated and uncoated 200-nm FMNP) and 1 bead per cell (about 2 × 105 RGD-coated 4 μm ferromagnetic beads on single dish (30 μL RGD at 50 μg per mL coated bead, 1 mg bead per mL cell culture medium) were added to 35-mm gridded glass-bottomed Petri dish. Cells were incubated for 15 min for RGD-coated 4-μm bead and 30 min for 200-nm microinjected FMNP at 37°C humidified incubator with 5% CO2. After that cells were washed with fresh medium to remove unattached 4-μm beads and to keep cell viability for microinjected 200-nm FMNPs. Finally, 2 mL fresh cell culture medium with 10 mM HEPES buffer was added to start live cell imaging.
For CHO DG44, cell dishes were placed in the chamber of the MTC coils and magnetized along the Y axis for 200-nm FMNP by using a magnetizing pulse of about 1000 Gauss for 10 ms and twisted along Z-direction by using a sinusoidal twisting field of 50 Gauss at 0.3 Hz. The corresponding shear stress was quantified by applying an external step-function twisting magnetic field of 50 Gauss for 10 s along the z axis on FMNP embedded in a pure viscous fluid of 1000 poise (100 Pa s) (Figures S2A and S2B). The quantified magnetic moment constant for 200-nm FMNPs was 0.10 ± 0.0054 Pa per Gauss (mean ± SD) which corresponds to 5 Pa at 50 Gauss magnetic field. A 50-Gauss magnetic twisting field at 0.1, 0.3, 0.8, or 5 Hz was then applied along Z-direction and at the same time DHFR-GFP movement and location for CHO cells and FMNPs were taken sequentially to quantify interphase chromatin and nucleoplasm complex, storage, loss modulus, chromatin stretching, and displacements.
For mechanomemory measurements, after performing experiments in spontaneous DHFR-GFP movement (no applied stress) for CHO cells or RNA Pol II for hiPSCs, sinusoidal stress of 5 Pa at 0.3 Hz was applied using MTC for 2 min or 10 min and waited 5 min, 30 min, or 60 min to quantify mean square displacement for diffusion coefficient. Similarly, DHFR-GFP movement for CHO and RNA Pol II for hiPSCs were quantified 5 min, 30 min, and 60 min after 0-min, 2-min, or 10-min stress by using MTC via an RGD-coated 4-μm bead (16.67 Gauss corresponding to 5 Pa, bead magnetic moment constant = 0.3 Pa per Gauss) but with a similar magnitude of FMNP stress (50 Gauss corresponding to 5 Pa) by following the work published previously.9
To quantify protein diffusivity, spontaneous movement of DHFR-GFP in CHO cells was quantified for control (no microinjection), medium microinjected (microinjection of same volume of medium without any nanoparticles) and microinjected anti-H2B-ab coated nanoparticle in the absence of external stresses.
Data analysis and RNA FISH
To quantify DHFR gene transcription, a customized RNA fish probe was designed and used (Bioresearch Technologies Inc. mouse 5′-end DHFR RNA fish probe (Quasar 570 dye) and full DHFR RNA fish probe (Quasar 670 fluorescent dye) (https://www.ncbi.nlm.nih.gov/nuccore/NM_010049.3). In short, after applying a sinusoidal stress (~5 Pa at 0.3 Hz) for 2 or 10 min via Anti-H2B, IgG H&L antibody, or uncoated ~200 nm FMNP microinjected to cell nucleus and an RGD coated 4-μm beads to integrin cell surface receptors, DHFR CHO cells were fixed using 4% PFA (paraformaldehyde, Santa Cruz) in phosphate-buffered saline (PBS) at room temperature for 10 min. CHO cells were then washed twice with PBS and permeabilized using 70% ethanol for ~8 h at 4°C by following the protocol published previously.5,6,9 Permeabilized cells were washed for 5 min at room temperature with wash buffer (10% deionized formamide (Thermo Fisher Scientific) added in 2X SSC (Sigma-Aldrich)). After washing, DHFR CHO cells were hybridized using the custom-designed RNA Fish probe (250 nM probe in 150 μL commercial hybridization buffer to each gridded glass-bottomed dish) of Quasar 570 for 2 min stress or mouse full probe (Quasar 670) for 10 min stress for 12 h in a humidified incubator (37°C at 5% CO2). After that, cells were washed with wash buffer for 30 min in a humidified incubator (37°C at 5% CO2). For nuclear staining, cells were then washed again with a wash buffer with DAPI (Thermo Fisher Scientific) for 30 min in a humidified incubator (37°C at 5% CO2). Before imaging, cells were rinsed two times (each of 5 min) with 2X SSC buffer at room temperature and continued for imaging.
Mouse full probe (Quasar 670) used for 10 min stress applied by microinjected anti-H2B or IgG H&L antibody or uncoated 200-nm FMNP and an RGD peptides-coated 4-μm bead at the cell surface:
gggaggcaaacggttctaag, cgcgcattctatttgtgtag, gtcaagtttggcgcgaaatc, gataaaatcctaccagcctt, gacgatgcagttcaatggtc, tccggttgtt caataagtct, accatgtctactttacttgc, taaacagaactgcctccgac, ctttcttctcgtagacttca, ttaggaggggagcagagaac, attatataggggctagggtt, accttgttagattactggga, tagctcctacttttatgagc, gctctgctgtttaaaacctg, gcagtatccattctcaattc, ttctctgacctgatgatctg, actaatttacaaggccaggc, atggacaccacactcacaag, agctctgaacagaccatttc, agcttacagacacaaggctg, ctgtgtttataccctgtatt, ctaggttttcagagtgcttt, ctactactgctgctttgtta, agtcatgggttctaaggaca, caatgcttcctagttggatt, catcaccagggagaaaagct, tgtcgtagccagatgacaag, gctcccaaaacaacacatct, gggcacccttaaaagtaact, acactggtggacaatgctta, ttcatctctttgttcatggt, tctagtgtacctggtcaatt, caaatgtctgttaaccccag, cattgtataacacacctcct, ctgccagggttacaaacata, ttgcaaatacactgccagtc, aatgctctataccctcattt, gcaagaaagcgctattgctt, tagctctgggagatgtcaac, ttttggcatgtatgaggtgt, acgacaatttccttgtgtct, ccttgaactaggtttcttgt, gggttgtcaatgggaatctg, cagtgtggaacatcgtgcaa, tttatgttagcagcttggga, cacaatgctttggtggaagc, atcatgtctccttttcagta, cctcgccttctaacacaaaa.
Mouse 5 ‘-end probe (Quasar 570) used for 2 min stress applied by microinjected anti-H2B antibody coated 200-nm FMNP:
tctgcgggaagcctaagatc, gggaggcaaacggttctaag, ctcttctgcacactgcaatg, cgcgcattctatttgtgtag, catcctatttgtgcagctaa, catcctatttgtgcagctaa, gtcaagtttggcgcgaaatc, cagccttcacgctaggattg, gatggcagcggggataaaat, tgcagttcaatggtcgaacc, atattttgggacacggcgac, agtacttgaactcgttcctg, agaggttgtggtcattcttt, agattctgtttaccttccac, aggttttcctacccataatc, ggtcgattcttctcaggaat, agtcttaaggcatcatccaa, tgccaattccggttgttcaa, tccaaaccatgtctacttta, taaacagaactgcctccgac, ctggttgattcatggcttcc, ccttgtcacaaagagtctga, gtcactttcaaattcctgca, ttccccaaatcaatttctgg, gggtattctgggagaagttt, ctttcttctcgtagacttca, ttaggaggggagcagagaac, agtcccatggtcttataaaa, ctcatagatctaaagccagc, accttgttagattactggga, gacatttcttaaggcacttt, gcactgagacctttatagca, cacttgaggtctcatgggag, cacagtaccctgtgcatatg, tacatcactggggtctcttg, tacttttatgagcccacaca, ctttggacttacctgcctag, ctctgctgtttaaaacctgt, ttctttatagtctgagttcc, agctttgctgcaagtgtgat, gattttctgtctgagtgagc, ttacataatcttccacctgc, gcagacaatttcagtgtttc.
For the quantification of DHFR gene transcription, the integrated intensity of the nucleus was quantified after subtracting background intensity by following the previously published protocol.5,6,9 The calculated background corrected integrated intensity of fluorescent nuclear areas for 0-min, 2-min, and 10-min stress was taken as the level of DHFR gene transcription for microinjected 200-nm and 4-μm bead at the cell surface.
Microscopy and live cell imaging
For dynamic tracking of FMNP, DHFR GFP movement, immunofluorescence, and RNA FISH in CHO cells and RNA pol II experiments in hiPSCs were performed using a DMIRE2 Leica inverted microscope. A 10X and 40X air objective was used for ~200 nm FMNP microinjection to cell nucleus, while a 63X objective (oil immersion, Numerical Aperture = 1.32), was used for DHFR GFP, RNA Pol II, and fluorescence imaging experiments. GFP (Chroma Technology Corp., Vermont), Quasar 570, DAPI, and 670 filters were used for fluorescence imaging experiments. DHFR GFP movements in CHO DG44 cells and RNA Pol II in hiPSCs were quantified in the X-Y plane. Occasionally, DHFR GFPs moved out of the focal plane and no data could be collected on those GFP spots.
Live cell chromatin and RNA pol II tracking for mean square displacement
To quantify the complex shear modulus (ratio of applied stress to chromatin strain) of interphase chromatin, BAC-inserted DHFR-GFP spots movement in CHO DG44 cells was tracked with the stress of anti-H2B antibody-coated microinjected FMNP or a 4-μm bead at the cell surface. After FMNP microinjection or adding RGD-coated 4-μm beads at the cell surface, a sinusoidal twisting stress of 5 Pa (corresponding to 50 Gauss for the FMNP or 16.67 Gauss for the 4-μm bead) was applied using MTC and at the same time, DHFR-GFP spot movement was tracked and captured sequentially for 10 to 30 s (exposure time 0.3 s) with minimal signs of photobleaching. A minimum of two DHFR-GFP spots on the same interphase chromatin domain in a single cell nucleus was taken to quantify chromatin strain with applied stress for complex shear modulus (G = Applied stress(σ0)/Chromatin strain (ε0)). Cells with one microinjected 200-nm FMNP were taken to quantify the complex shear modulus for an applied stress of 5 Pa. Storage (G’ = (σ0/ε0) cos δ) or loss (G” = (σ0/ε0) sin δ) modulus was calculated after quantifying phase lag (δ) of DHFR-GFP spots movements with microinjected FMNP applied stress of 5 Pa at 0.3 Hz
On the other hand, to quantify nucleoplasm modulus, Rabbit Anti-Mouse IgG H&L (HRP) or uncoated microinjected 200-nm FMNP were tracked with the stress of 5 Pa at 0.3 Hz. After IgG-ab or uncoated FMNP microinjection, their movements were tracked for a time interval of 0.3 s for a total of 20–40 s with applied stress of 5 Pa at 0.3 Hz. The phase lag (δ) of FMNP movement with applied stress was used to quantify the storage and loss complex shear modulus.
For the mechanomemory of the diffusion coefficient of DHFR-GFP spot in CHO DG44 cells and RNA Pol II in hiPSCs the diffusion coefficient was calculated from mean square displacement (MSD) by following the method described previously.9 Both DHFR-GFP spot movement in CHO DG44 and RNA Pol II in hiPSCs were tracked and measured for a time interval of 0.3 s and overlapped to quantify the MSD for a total time interval of 10–30 s without any photobleaching. The diffusion coefficient of DHFR-GFP and RNA Pol II was calculated using the best fit of MSD vs. total time interval. For the measurement of MSD, after microinjection of 200-nm FMNP or adding RGD-coated 4-μm bead at the cell surface, the spontaneous DHFR GFP movement in CHO DG44 cells or RNA Pol II in hiPSCs was quantified in living cells 5 min after for 0 min and 5, 30, or 60 min after 2- and 10-min stress. A sinusoidal cyclic stress of 5 Pa at 0.3 Hz was used for 2- and 10-min stress. After the quantification of MSD and diffusion coefficient of DHFR GFP spot or RNA Pol II spontaneous movement for 5 min after 2- and 10-min stress, both CHO DG44 cells and hiPSCs dishes were kept in a humidified incubator (37°C and 5% CO2) for 30 min and 1 h. After that, DHFR GFP spot or RNA Pol II spontaneous movement was quantified for 30-min and 1 h post 2- or 10-min stress.
ATP depletion experiments
CHO DG44 cells were seeded using the regular medium (Ham’s F12 medium without thymidine and hypoxanthine with 10% dialyzed FBS) on a 35 mm, gridded glass-bottomed dish (50 μm cell location, 81148, Ibidi USA Incorporated, Madison, WI) and incubated overnight at 37° C and 5% CO2. After that, uncoated 200-nm FMNPs were microinjected into the nucleus of CHO DG44 cells. Thirty minutes after microinjection, imaging experiments were performed for quantifying the spontaneous movement of DHFR-GFP or uncoated 200 nm FMNP for 0 min or no stress condition. Then the regular medium was changed to 20 mM D-glucose (Catalog# A2494001,Thermo Fisher Scientific) medium, 20 mM 2-deoxy-D-glucose (Catalog# D8375,Sigma-Aldrich)36 or 10 μM Antimycin A (Catalog# A8674, Sigma-Aldrich; an inhibitor of oxidative phosphorylation)37 containing medium and incubated in an incubator with 37° C and 5% CO2 for 15 min. Spontaneous movements of DHFR-GFP spots or uncoated 200-nm FMNP were quantified. Imaging was done for each DHFR-GFP or uncoated 200-nm FMNP for about 10–30 s to avoid photobleaching. The 20-mM D-glucose medium was used as a positive control for the 20-mM 2-deoxy-D-glucose containing medium.
Fluorescence correlation spectroscopy (FCS) for live cell imaging
Single molecule imaging (SMI) for nucleoplasm free-GFP in CHO DG44 cell or single Pol IIs in the nucleoplasm in the vicinity (<0.5 μm) from mEGFP-tagged RNA Pol II spots in hiPSCs were quantified using fluorescence correlation spectroscopy (FCS). For FCS, a confocal scanning time-resolved imaging system from ISS Alba (Champaign, USA) was used as mentioned previously.9,38 Briefly, a picosecond (ps) pulsed laser of 488 nm was used with a repetition rate of 50 MHz to excite EGFP (free-GFP in nucleoplasm and RNA Pol II) via apochromatic water (60X, NA = 1.2) or oil (100X, NA = 1.35) immersion objective from Olympus. These objectives (60X water or 100X oil) were then used to collect chroma dichoric filter reflected photons. Reflected photons passed through a 50-μm pinhole as well as a chroma band-pass filter and finally reaching to an avalanche photodiode (SPCM-AQRH-15) from Excelitas. This system stored and captured the reflected and detected photons in the format of Time-Tagged-Time-Resolved (TTTR) that was further used to analyze the diffusion coefficient of single protein molecules of nucleoplasm free-GFP and region <0.5 μm to RNA Pol II.
For FCS, CHO DG44 and hiPSCs were seeded on a 35-mm imprinted gridded glass-bottomed dish (50 μm cell location, 81148, Ibidi USA Incorporated, Madison, WI) for overnight incubation at 37° C and 5% CO2. After that, anti-H2B, IgG H&L (HRP) antibodies, and uncoated 200-nm FMNPs were microinjected or RGD-coated 4-μm beads were added. Thirty min after microinjection or 15 min after adding RGD-coated 4-μm bead, cells were washed with medium and added 2 mL fresh medium. After that, gridded cell dishes were transferred to a stage incubator (5% CO2; ONICS, Tokai, Japan) for FCS experiment to quantify the diffusion coefficient of nucleoplasm free-GFP in CHO DG44 and single Pol IIs in the nucleoplasm in the vicinity (<0.5 μm) of the RNA Pol II spots in an hiPSC for 0 min stress condition. Then, sinusoidal cyclic stress of 5 Pa at 0.3 Hz was applied using MTC for 2 or 10 min via the microinjected 200-nm FMNP and the 4-μm magnetic bead. FCS measurement was again done for free-GFP in CHO DG44 and single Pol IIs in the nucleoplasm in the vicinity (<0.5 μm) of the RNA Pol II spots in an hiPSC, 5 min, 30 min, or 60 min after 2- or 10-min stress.
For both cell lines, FCS measurement was done for 2 to 4 nuclear positions (each with 100 s; five segments of 20 s) at the nucleus (nucleoplasm free-GFP in a CHO DG44 cell and <0.5 μm RNA Pol II in an hiPSC) to avoid photo-bleaching. A 60X water immersion objective with ~0.2 μW back aperture was used for FCS measurement. VistaVision software was used for the quantification of the diffusion coefficient from the collected data of the FCS experiment by following the method as described previously.9,39
Briefly, rhodamine 110 was used for FCS calibration to quantify Kappa (k) before starting on each day of experiment by fitting with autocorrelation function G(τ).
| (Equation 1) |
| (Equation 2) |
Where δF(t) = F(t)- < F(t)>; <F> denotes average fluorescence intensity; , , where w0, z0, and D are lateral radii, axial radii of fluorescence detection volume, and diffusion coefficient respectively.
After calibration, for the measurement of the diffusion coefficient of nucleoplasm free-GFP in CHO DG44 cells and single Pol IIs in the nucleoplasm in the vicinity (<0.5 μm) of the RNA Pol II spots in hiPSCs, a 3D anomalous diffusion model was used to fit the acquired autocorrelation function G(τ):
| (Equation 3) |
Where, α denotes the degree of anomalous behavior.
Immunofluorescence imaging
For both CHO DG44 cells and hiPSCs, cells were fixed using paraformaldehyde (Santa Cruz; 4% in PBS at room temperature for 10 min) for 0 min (no stress) and 5 min, 30 min, or 60 min after 2 min, or 10 min stress via anti-H2B-ab or uncoated microinjected 200 nm FMNP and RGD-coated 4-μm bead. Cells were then washed three times with 1X PBS and added 0.3% Triton X-100 (Sigma-Aldrich) for permeabilization for 20 min. After washing again with 1X PBS, 5% normal donkey serum (Jackson ImmunoResearch Laboratories Inc.) was added to cells for about 4 h at room temperature. Overnight incubation was then done at 4°C with primary antibody (1:100 v/v in 1% BSA; Abcam, ab5095 (rabbit polyclonal anti-RNA Pol II CTD repeat YSPTSPS (phospho-S2)). After that, cells were washed three times with 1X PBS (each wash with 5 min) and followed by secondary antibody (Abcam, ab150062 (donkey anti-rabbit IgG H&L (Heavy & Light chains), Alexa Fluor 555); 1:200 v/v in PBS) incubation for about 2 h at 4°C. Finally, the samples were proceeded for imaging after three times washing using 1X PBS. For only primary antibody (rabbit polyclonal anti-RNA Pol II CTD repeat YSPTSPS (phospho-S2)) or secondary antibody (donkey anti-rabbit IgG H&L (Heavy & Light chains), Alexa Fluor 555) immunofluorescence, the samples were proceeded for imaging after adding and incubating them with these antibodies and wash three times with 1X PBS.
Polyacrylamide (PA) gel substrates
To quantify the effect of substrate stiffness on mechanomemory of DHFR-GFP, chromatin and nucleoplasm complex, storage, and loss modulus, CHO cells were grown on 1 kPa, 10 kPa, and 20 kPa Polyacrylamide (PA) gel substrates coated with fibronectin (30 μg per mL). Different PA-gel substrate stiffness was made on a 35-mm gridded glass-bottomed Petri dish (imprinted 50 μm cell location, 81148, Ibidi USA Incorporated, Madison, WI) by following the previously published protocol.40 Briefly, by mixing different concentrations of 40% acrylamide (Catalog #1610140; Biorad) and 2% Bis-acrylamide (Catalog #161–0142; Biorad) 1 kPa, 10 kPa, and 20 kPa substrate stiffness was created. For 1 kPa substrates, 1.25 mL of acrylamide from 40% stock solution and 0.15 mL of Bis-acrylamide from 2% stock concentration were added in a total of 10 mL solution with water. Similarly for 10 kPa substrates, 2.5 mL of 40% acrylamide mixed with 0.462 mL of 2% Bis-acrylamide, and 2.5 mL of 40% acrylamide added with 0.934 mL of 2% Bis-acrylamide for 20 kPa substrates PA gel in a total volume of 10 mL solution with water.40
For mechanomemory of DHFR-GFP protein diffusivity, anti-H2B-ab coated FMNP was microinjected to CHO cell nucleus after overnight incubation in 37° C and 5% CO2. 30 min after microinjection, cells were washed with fresh medium and added 2 mL medium to measure the spontaneous movement of DHFR-GFP for 10 to 30 s. The diffusion coefficient was calculated from the best fit of mean square displacement (MSD) by following the published method.9 After that, 2-min or 10-min sinusoidal cyclic stress (5 Pa at 0.3 Hz) was applied via MTC and measured the DHFR-GFP movement in living cells 5 min after 2- and 10-min stress. After the quantification of MSD and diffusion coefficient of DHFR GFP spot for 5 min after 2- or 10-min stress, CHO cells were kept in a humidified incubator (37° C and 5% CO2) for 1 h. Finally, DHFR GFP spot spontaneous movement was quantified for 1 h post 2- or 10-min stress for cells growing on 1 kPa, 10 kPa, and 20 kPa substrate stiffness.
For the measurement of chromatin complex moduli (ratio of applied stress to chromatin strain), DHFR-GFP spots movements were tracked with the stress of anti-H2B-ab coated FMNP microinjected CHO cells. After FMNP microinjection, a sinusoidal cyclic stress of 5 Pa (corresponding to a 50-Gauss magnetic field for FMNP) was applied via MTC, and at the same time, DHFR-GFP spot movements were tracked sequentially for 10 to 30 s. A minimum of two DHFR-GFP spots on the same interphase chromatin domain in a single cell nucleus was taken to quantify chromatin strain with applied stress for complex shear modulus (G = Applied stress(σ0)/Chromatin strain (ε0)). Chromatin storage (G’ = (σ0/ε0) cos δ) and loss (G” = (σ0/ε0) sin δ) modulus were quantified after calculating phase lag (δ) of DHFR-GFP spots movements with microinjected FMNP stress.
For nucleoplasm complex modulus, an uncoated microinjected FMNP was tracked with stress (5 Pa at 0.3 Hz) for a total time of 20–40 s. After quantifying the displacements to obtain the resultant strains, the nucleoplasm complex modulus (ratio of applied stress to strain; strain= (Peak FMNP displacement)/(FMNP radius)), the storage and loss modulus were calculated using the phase lag (δ) of FMNP movements in response to the applied stress.
Atomic force microscopy (AFM)
AFM was used to quantify live cell nuclear stiffness for control (intact cell, no microinjection), medium (same volume of medium microinjection without any nanoparticle), and anti-H2B-ab coated FMNP microinjected cells. CHO DG44 cells were grown on a 35-mm gridded glass-bottomed Petri dish (imprinted 50 μm cell location, 81148, Ibidi USA Incorporated, Madison, WI). After overnight incubation at 37° C with 5% CO2, microinjection was performed with anti-H2B-ab coated FMNP or medium only without any FMNP. There was a 30-min wait-time between microinjection and measurements to allow enough time for the FMNP to bind to the chromatin. Cells were then washed with medium and 2 mL fresh medium was added before starting the AFM measurement.
A DriveAFM (Nanosurf Inc.) coupled with an inverted microscope was used to quantify nuclear stiffness. A sphere AFM tip (CP-qp-CONT-SiO) of diameter 3.36 μm, spring constant 0.01 N/m, cantilever length 125 μm, the indentation up to 3 μm, the tip speed of 0.5 μm per s, and the force range up to 2 nN was used for the measurement. Before starting the AFM measurement, the spring constant was quantified using the thermal fluctuation of nanoscope. For control, medium, and medium+FMNP microinjected cells, approaching force curves were used to quantify the nuclear stiffness by placing the AFM tip directly above the nucleus of the microinjected FMNP site or the site of medium injection. To quantify the CHO cell nuclear stiffness, the Hertz-Sneddon model41 was used, as shown in Equation 4.
| (Equation 4) |
Where, E is the Young’s modulus, R is the radius of the sphere AFM tip, F is the applied force, δ is the depth of indentation, and Poisson’s ratio (n = 0.37) for CHO cells as measured previously.8
Transmission electron microscopy (TEM)
TEM imaging was performed for control (intact cell no microinjection), medium (same volume of medium without FMNP), and medium+FMNP (anti-H2B-ab coated FMNP) microinjected CHO cells using a 100 keV transmission electron microscope (JEOL JEM 1010 TEM) of accelerating voltage of 40 kV–100 kV. Images were taken using a 2k X 2k AMT CCD camera integrated with the TEM system. Before imaging, control, medium, and medium+FMNP microinjected CHO cells were prepared following standard TEM protocol. In brief, cells were first fixed using primary fixation (2.5% Glutaraldehyde and 2.5% Formaldehyde with 0.1 M Na Cacodylate Buffer pH 7.2) followed by two washes with 0.1 M Na Cacodylate Buffer of pH 7.2 and a final post fixation with 1% osmium Tetroxide with 0.1 M Na Cacodylate Buffer of pH 7.2 for 2 h at room temperature. After washing again with 0.1 M Na Cacodylate Buffer of pH 7.2, dehydration (30% ethanol treatment for 10–15 min, followed by 50%, 70%, 85%, and 95% ethanol, and finally 100% ethanol for 1 h), infiltration (1:1 and 2:1 Squetol resin to 100% ethanol and finally to 100% Squetol resin), embedding (embedded in fresh Squetol resin in BEEM capsules), and polymerization (BEEM capsules in an oven at 55–60°C for 20–24 h) was performed. Finally, the TEM section of 95 nm thickness was cut and set on a specified grid to proceed with imaging by using the transmission electron microscope.
Software and data analysis
Dynamic trackings of anti-H2B, IgG H&L, and uncoated 200-nm FMNPs, DHFR-GFP spots in CHO DG44 cells, and RNA Pol II in hiPSCs were performed using the MATLAB software (5–8).
QUANTIFICATION AND STATISTICAL ANALYSIS
In this work, One-way ANOVA with Tukey test and Mann-Whitney test were used for all statistical analysis (*p < 0.05, **p < 0.01, ***p < 0.001, ns = not statistically different). For image processing and data analysis, ImageJ, MATLAB, and VistaVision software’s were used.
Figure 1. (B) Applying a stress after magnetizing 200 nm FMNPs in the Y direction (1000 Gauss for 10 ms) and twisting toward the Z direction with a sinusoidal cyclic magnetic field (50 Gauss, i.e., 5 Pa at 0.3 Hz) using the magnetic twisting cytometry (MTC). (C) Representative images from 4 different cell nuclei in 4 different rows (the same cell in each row) of anti-H2B-antibody coated 200 nm FMNPs in Hoechst (nuclear staining) (2 columns on the left) and DHFR-GFP (green fluorescent protein) spots (mid right images) or free GFP (arrows) (right images) of CHO cell nucleus. Insets represent zoomed-in views of FMNPs and DHFR-GFPs. Note that the two GFP spots in nucleus #1 are close to each other and one of the GFP spots in nucleus #3 is dim. Scale bar, 5 μm. (D) Displacements of anti-H2B-antibody coated FMNPs in the same four cells as in Figure 1C. Each NP displacement value was an average of three sinusoidal cycles of 5 Pa at 0.3 Hz. In all ferromagnetic nanoparticles (FMNPs), the peak sinusoidal stress was constant at 5 Pa. (E) Chromatin complex modulus (ratio of applied stress to measured chromatin strain) for anti-H2B-ab coated FMNP and nucleoplasm complex modulus (ratio of applied stress to bead strain; NP strain = (Peak NP displacement)/(NP radius)) for Rabbit Anti-Mouse IgG H&L (HRP), and uncoated microinjected 200 nm magnetic NP with applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 16 cells for 36 DHFR-GFP spots for anti-H2B coated NP, n = 12 cells for 30 DHFR-GFP spots for IgG H&L antibody coated NP, and n = 10 cells for 23 DHFR-GFP spots for uncoated NP from three independent experiments; p= 1.23×10−6 between chromatin and nucleoplasm complex modulus for anti-H2B and Rabbit Anti-Mouse IgG H&L (HRP) antibody coated microinjected NP; p= 5.46×10−6 between chromatin and nucleoplasm complex modulus for anti-H2B and uncoated microinjected NPs. (F) Chromatin and Nucleoplasm storage modulus G’ (G’ = (σ0/ε0) cos δ); σ0 and ε0 are the stress and the strain; δ is the phase lag between the input stress and the bead displacement; see Figure 1D for the original phase lag images) for Anti-H2B, Rabbit Anti-Mouse IgG H&L (HRP), and uncoated microinjected NPs with applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 16 cells for 36 DHFR-GFP spots for anti-H2B coated NP, n = 12 cells for 30 DHFR-GFP spots for IgG H&L antibody coated FMNP, and n = 10 cells for 23 DHFR-GFP spots for uncoated NP from three independent experiments for (F) and (G). p= 4.39×10−6 between chromatin and nucleoplasm storage modulus for anti-H2B and Rabbit Anti-Mouse IgG H&L (HRP) antibody microinjected NP; p= 2.11×10−5 between chromatin and nucleoplasm storage modulus for anti-H2B and uncoated microinjected NP. (G) Chromatin and Nucleoplasm loss modulus G” (G” = (σ0/ε0) sin δ) for Anti-H2B, Rabbit Anti-Mouse IgG H&L (HRP) and uncoated microinjected 200 nm FMNP with applied stress (5 Pa at 0.3 Hz). p=3.84×10−7 between chromatin and nucleoplasm loss modulus for anti-H2B and Rabbit Anti-Mouse IgG H&L (HRP) antibody microinjected NP; p= 2.42×10−6 between chromatin and nucleoplasm loss modulus for anti-H2B and uncoated microinjected NP. One-way ANOVA with Tukey and Mann-Whitney test was used; n = 3 biological replicates.
Figure 2. (A) Chromatin stretching and chromatin complex modulus G in the same chromatin domain for anti-H2B-ab coated microinjected 200-nm FMNP with applied stress 5 Pa at 0.1, 0.3, 0.8, and 5 Hz. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 12 cells for 28 DHFR-GFP spots for 0.1 Hz, n = 16 cells for 36 DHFR-GFP spots for 0.3 Hz, n = 18 cells for 39 DHFR-GFP spots for 0.8 Hz, and n = 16 cells for 32 DHFR-GFP spots for 5 Hz from three independent experiments. p = 2.25×10−4 for chromatin stretching between 0.1 and 0.8 Hz; p = 2.66×10−6 for chromatin stretching between 0.1 and 5 Hz, p = 0.002 for chromatin stretching between 0.3 and 0.8 Hz, p = 9.03×10−5 for chromatin stretching between 0.3 and 5 Hz, p = 0.005 for chromatin stretching between 0.8 and 5 Hz; p = 0.0002 for chromatin complex modulus between 0.1 and 0.8 Hz; p = 1.54×10−8 for chromatin complex modulus between 0.1 and 5 Hz, p = 0.008 for chromatin complex modulus between 0.3 and 0.8 Hz, p = 1.34×10−5 for chromatin complex modulus between 0.3 and 5 Hz, p = 0.014 for chromatin complex modulus between 0.8 and 5 Hz. (B) chromatin storage and loss modulus for anti-H2B-ab coated microinjected 200-nm FMNP with applied stress 5 Pa at 0.1, 0.3, 0.8, and 5 Hz. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 12 cells for 28 DHFR-GFP spots for 0.1 Hz, n = 16 cells for 36 DHFR-GFP spots for 0.3 Hz, n = 18 cells for 39 DHFR-GFP spots for 0.8 Hz, and n = 16 cells for 32 DHFR-GFP spots for 5 Hz from three independent experiments. p = 0.002 for chromatin storage modulus between 0.1 and 0.8 Hz; p = 6.88×10−5 for chromatin storage modulus between 0.1 and 5 Hz, p = 0.0011 for chromatin storage modulus between 0.3 and 0.8 Hz, p = 0.0001 for chromatin storage modulus between 0.3 and 5 Hz, p = 2.59×10−7 for chromatin loss modulus between 0.1 and 0.3 Hz; p = 2.17×10−8 for chromatin loss modulus between 0.1 and 0.8 Hz, p = 3.82×10−7 for chromatin loss modulus between 0.1 and 5 Hz, p = 1.01×10−4 for chromatin loss modulus between 0.3 and 5 Hz, p = 1.76×10−5 for chromatin loss modulus between 0.8 and 5 Hz. Note that for chromatin stretching and complex modulus in (A) and storage modulus and loss modulus in (B), all data are collected at the exact loading frequencies of 0.1, 0.3, 0.8, or 5.0 Hz; the data points that are shown next to the loading frequency values are only for visual clarity. (C) Chromatin average complex, storage, and loss modulus versus stress frequency on a log-log scale. Data shown as scatter point of the average of chromatin complex, storage, and loss modulus. Chromatin average complex (y = 166.41×0.19; R2 = 0.96), storage (y = 139.81×0.15; R2 = 0.81), or loss (y = 82.93×0.32; R2 = 0.90) modulus was fitted with a power law. n = 12 cells for 28 DHFR-GFP spots for 0.1 Hz, n = 16 cells for 36 DHFR-GFP spots for 0.3 Hz, n = 18 cells for 39 DHFR-GFP spots for 0.8 Hz, and n = 16 cells for 32 DHFR-GFP spots for 5 Hz, from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure 3. (B) Chromatin stretching (percentage) depends on the binding specificity between the nanoparticles and the chromatin. The chromatin stretching is the distance changes between two DHFR GFP spots in the same chromatin domain divided by the original distance between the two GFP spots (~chromatin tensile strain) for anti-histone H2B, anti-IgG antibody, uncoated magnetic FMNPs, and a 4-μm RGD (Arg-Gly-Asp) coated ferromagnetic bead. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 16 cells for 36 DHFR-GFP spots for anti-H2B coated NP, n = 12 cells for 30 DHFR-GFP spots for Rabbit Anti-Mouse IgG H&L (HRP) coated NP, n = 11 cells for 25 DHFR-GFP spots for uncoated NP, and n = 15 cells for 30 DHFR-GFP spots for a 4-μm RGD-coated bead from three independent experiments. p= 2.68×10−9 between anti-H2B and IgG H&L coated FMNP; p= 2.95×10−8 between anti-H2B and uncoated FMNP; p= 0.02 between anti-H2B and a 4-μm RGD-coated bead; p= 2.20×10−7 between IgG H&L coated and a 4-μm RGD-coated bead; p= 8.43×10−7 between uncoated and a 4-μm RGD-coated bead. (C) Representative images of CHO cell brightfield (BF) and Cy3 (red) DHFR Fluorescence in Situ Hybridization (FISH) at 0 min (no stress), and 2 min, or 10-min for anti-H2B-ab coated 200-nm FMNP, 10 min for anti-IgG, and uncoated 200-nm FMNP, and a 4-μm RGD coated bead attached to the cell apical surface via integrins, induced by the same magnitude of the sinusoidal stress (5 Pa at 0.3 Hz). A dotted line shows the nucleus of the cell. Scale bar, 5 μm. (D) Normalized DHFR gene transcription from the original data in C. Data shown as bar plot with mean ± SD; n = 36 cells for 0 min, n = 39 cells for 2 min for anti-histone H2B coated FMNP from three independent biological replicates, n = 47 cells for 10 min for anti-histone H2B coated FMNP from four independent experiments, n = 29 for 10 min for anti-IgG antibody coated NPs, n = 39 for 10 min for uncoated NPs, n = 59 cells for 10 min for 4-μm RGD coated bead from three independent experiments. p= 4.05×10−8 between 0 and 2 min of anti-H2B coated NP, p= 6.48×10−8 between 0 and 10 min of anti-H2B coated NP, p= 0.00014 between 2 and 10 min of anti-H2B coated NP, p= 1.22×10−7 between 10 min anti-H2B coated microinjected NP and 10 min of 4-μm RGD coated bead at cell apical surface; p=3.86×10−8 between 0 min and 10 min of 4-μm RGD coated bead at cell apical surface. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure 4. (A) Diffusion coefficient (quantified by the best fit of time vs. mean square displacement (MSD) of DHFR-GFP spot movements) of DHFR-GFP on the chromatin 5 min after 0-, 2-, or 10-min stress (5 Pa at 0.3 Hz) or 1 h post 2- and 10-min stress for anti-H2B coated microinjected 200-nm FMNP and 5 min after 10 min or 1 h post 10-min stress for a 4-μm RGD-coated bead attached to cell surface with same stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 23 cells with 27 DHFR-GFP spots for 0 min, n = 14 cells with 17 DHFR-GFP spots for 2 min, n = 14 cells with 16 DHFR-GFP spots for 10 min, n = 17 cells with 23 DHFR-GFP spots for 1 h post 2 min, n = 21 cells with 24 DHFR-GFP spots from three independent experiments for anti-H2B coated microinjected FMNP and n = 13 cells and 16 DHFR-GFP spots from three independent experiments for 4-μm bead. p = 0.009 between 0 and 2 min stress for anti-H2B coated FMNP; p = 3.43×10−4 between 0- and 10-min stress for anti-H2B coated FMNP; p = 0.016 between 2- and 10-min stress for anti-H2B coated FMNP; p = 0.003 between 2 min and 1 h post 2-min stress for anti-H2B coated FMNP; p = 2.88×10−5 between 10-min stress and 1 h post 10-min stress for anti-H2B coated FMNP; p = 3.78×10−4 between 0-min and 10-min stress; p = 1.52×10−4 between 10-min and 1 h post 10-min stress for 4-μm RGD-coated bead. (B) Fluorescence correlation spectroscopy (FCS) based diffusion coefficient of free GFP inside the nucleoplasm (free GFP’s < 5 μm and >5 μm distance from location of DHFR-GFP spots) 5 min after 0-, 2-, or 10-min stress and 1 h post 2 or 10-min stress for anti-H2B-ab coated FMNP and 10 min or 1 h post 10-min stress for a 4-μm RGD-coated bead. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 40 cells for 0 min, n = 20 (free GFP’s < 5 μm), n = 20 (free GFP’s > 5 μm) for 2 min from five independent experiments, n = 24 (free GFP’s < 5 μm) and n = 24 (free GFP’s > 5 μm) for 10-min stress from six independent experiments, n = 37 for 1 h post 2-min from five independent experiments, n = 45 for 1 h post 10-min stress from six independent experiments for anti-H2B coated NP, and n = 23 cells for 10 min and n = 26 cells for 1 h post 10-min for a 4-μm RGD-coated bead. p = 7.84×10−8 between 0- and 2-min stress for free GFP’s < 5 μm distance from DHFR-GFP spots; p = 2.67×10−10 between 0- and 10-min stress for free GFP’s < 5 μm distance from DHFR-GFP spots; p = 0.009 between 0 and 2 min stress for free GFP’s > 5 μm distance from DHFR-GFP spots; p = 3.03×10−4 between 0- and 10-min stress for free GFP’s > 5 μm distance from DHFR-GFP spots; p = 0.004 between free GFP’s within (<) and outside (>) 5 μm distance from DHFR-GFP spots for 2-min stress; p = 2.52×10−4 between free GFP’s within and outside 5 μm distance from DHFR-GFP spots for 10-min stress for Anti-H2B coated FMNP; p = 1.67×10−6 between 0-min and 10-min stress for 4-μm RGD-coated bead; p = 2.90×10−6 between 10-min stress and 1 h post 10-min stress for 4-μm RGD-coated bead; p = 0.010 between 10-min stress for anti-H2B coated microinjected FMNP (free GFPs< 5 μm) and 10-min stress for 4-μm RGD-coated bead. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure 5. (B) Diffusion coefficient (quantified by the best fit of time vs. mean square displacement (MSD) of RNA Pol II movements) of RNA Polymerase II spots (Green bright spots shown in (A)) 5 min after 0-, 2-, or 10-min stress and 1 h post 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at cell surface receptors. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 42 cells with 55 RNA Polymerase II spots for 0 min, n = 20 cells with 20 RNA Polymerase II for 5 min after 2-min stress, n = 16 cells with 16 RNA Polymerase II for 5 min after 10-min stress, n = 14 cells with 14 RNA Polymerase II for 1 h post 2-min stress, and n = 17 cells with 17 RNA Polymerase II for 1 h post 10-min stress from three independent experiments for anti-H2B coated microinjected 200 nm FMNP, n = 22 cells with 32 RNA Polymerase II spots for 5 min after 2-min stress, n = 25 cells with 35 RNA Polymerase II for 5 min after 10-min stress, n = 34 cells with 40 RNA Polymerase II for 1 h post 2-min stress, and n = 43 cells with 48 RNA Polymerase II for 1 h post 10-min stress from four independent experiments for a 4-μm RGD-coated bead. p = 3.03×10−7 between 0 and 5 min after 2-min stress; p = 2.60×10−8 between 0 and 5 min after 10-min stress; p = 9.55×10−5 between 5 min and 1 h post 2-min stress; p = 2.71×10−5 between 5 min and 1 h post 10-min stress for anti-H2B coated microinjected 200-nm FMNP; p = 2.22×10−8 between 0 and 5 min after 2-min stress; p = 0.033 between 5 min after 2- and 10-min stress; p = 7.29×10−8 between 0 and 5 min after 10-min stress; p = 2.93×10−7 between 5 min and 1 h post 2-min stress; p = 6.48×10−8 between 5 min and 1 h post 10-min stress for the 4-μm RGD-coated bead. (C) Fluorescence correlation spectroscopy (FCS) based diffusion coefficient of RNA Polymerase II (<0.5 μm distance from RNA Pol II arrow shown in (A)) 5 min after 0-, 2-, or 10-min stress and 1 h post 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at cell surface receptors. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 32 cells for 0 min, n = 7 cells for 5 min after 2-min stress, n = 8 cells for 5 min after 10-min stress, n = 8 cells for 1 h post 2 min stress, n = 8 cells for 1 h post 10-min stress for anti-H2B coated microinjected 200 nm FMNP, n = 16 cells for 5 min after 2 min stress, n = 15 cells for 5 min after 10-min stress, n = 31 cells for 1 h post 2-min stress, and n = 30 cells for 1 h post 10-min stress for a 4-μm RGD-coated bead from three independent experiments. p = 4.49×10−5 between 0 and 5 min after 2-min stress; p = 0.032 between 5 min after 2-and 10-min stress; p = 1.62×10−5 between 0 and 5 min after 10-min stress; p = 0.0015 between 5 min and 1 h post 2-min stress; p = 9.39×10−4 between 5 min and 1 h post 10-min stress for anti-H2B coated microinjected 200-nm FMNP; p = 1.01×10−7 between 0 and 5 min after 2-min stress; p = 0.010 between 5 min after 2- and 10-min stress; p = 4.61×10−8 between 0 and 5 min after 10-min stress; p = 1.40×10−7 between 5 min and 1 h post 2-min stress; p = 1.10×10−7 between 5 min and 1 h post 10-min stress for a 4-μm RGD-coated bead. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure 6. (A) Representative images of brightfields (BF) and immunofluorescence (used both Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2) and Anti-Rabbit IgG H&L (Alexa Fluor 555) antibody) of RNA Polymerase II Ser 2 phosphorylation (red) in human induced pluripotent stem cells (hiPSCs) nucleus at 0-min (no stress) or 5 min after, 2-, or 10-min stress (5 Pa at 0.3 Hz) for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at the cell surface and 0-min stress for only primary antibody (Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2)) or only secondary antibody(Anti-Rabbit IgG H&L (Alexa Fluor 555) antibody) images. Scale bar, 5 μm. Note that the 4-μm bead appeared out of focus in brightfield images because it was on the cell surface. (B) Normalized RNA Polymerase II activity (RNA Pol II Ser 2 phosphorylation fluorescence intensity) 5 min after 0-, 2-, or 10-min stress and 1 h post 2- or 10-min stress for an anti-H2B coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at cell surface receptors for Anti-RNA polymerase II (phospho S2) or Anti-Rabbit IgG H&L in hiPSC nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 78 cells for 0 min, n = 16 cells for 5 min after 2-min stress, n = 18 cells for 5 min after 10 min stress, n = 16 cells for 1 h post 2-min, and n = 20 cells for 1 h post 10-min stress for anti-H2B coated microinjected 200-nm FMNP, n = 33 cells for 5 min after 2-min stress, n = 76 cells for 5 min after 10-min stress, n = 27 cells for 1 h post 2-min stress, and n = 39 cells for 1 h post 10-min stress for a 4-μm RGD-coated bead, n = 59 cells for 0-min stress for only Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2) and n = 39 cells for only Anti-Rabbit IgG H&L (Alexa Fluor 555) antibody from three independent experiments. p = 4.90×10−4 between 0 and 5 min after 2-min stress; p = 4.84×10−6 between 5 min after 2- and 10-min stress; p = 1.24×10−10 between 0 and 5 min after 10-min stress; p = 6.67×10−6 between 2-min stress and 1 h post 2-min stress; p = 6.21×10−7 between 10-min stress and 1 h post 10-min stress for anti-H2B coated FMNP; p = 3.12×10−8 between 0 and 5 min after 2-min stress; p = 1.51×10−4 between 5 min after 2- and 10-min stress; p = 1.77×10−8 between 0 and 5 min after 10-min stress; p = 1.64×10−8 between 2-min and 1 h post 2-min stress; p = 1.26×10−7 between 10-min stress and 1 h post 10-min stress; p = 2.44×10−8 for 60 min after 2- and 10-min stress for a 4-μm RGD-coated bead at cell surface receptors for a 4-μm RGD-coated bead at cell surface receptors; p = 2.16×10−4 for 2-min stress between Anti-H2B coated FMNP and 4-μm RGD-coated bead; p = 6.82×10−6 for 10-min stress between Anti-H2B coated FMNP and 4-μm RGD-coated bead; p = 3.66×10−8 between 0 min immunofluorescence and only Anti-RNA polymerase II CTD fluorescence intensity; p = 0.010 between 0 min immunofluorescence and only Anti-Rabbit IgG H&L fluorescence intensity; p = 4.00×10−8 between 0 min for only RNA Polymerase II Ser 2 phosphorylation or Anti-Rabbit IgG H&L fluorescence intensity. (C) Representative images of brightfields (BF) and immunofluorescence (used both Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2) and Anti-Rabbit IgG H&L (Alexa Fluor 555) antibody) of RNA Polymerase II Ser 2 phosphorylation (red) in CHO cell nucleus at 0-, 2-, or 10 min stress (5 Pa at 0.3125 Hz) for anti-H2B coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at cell surface receptors, and 0 min stress for only primary antibody (Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2)) or only secondary antibody(Anti-Rabbit IgG H&L (Alexa Fluor 555) antibody) images. Scale bar, 5 μm. Note that Pol II Ser 2 phosphorylation is distributed at considerable distances away from the FMNP, possibly because of the rapid induced movement of Pol IIs 5 s after chromatin stretching.5 (D) Normalized RNA Polymerase II activity (RNA Pol II Ser 2 phosphorylation fluorescence intensity) 5 min after 0-, 2-, or 10-min stress and 1 h post 2- or 10-min stress for anti-H2B coated microinjected 200 nm FMNP and a 4-μm RGD-coated bead at cell surface receptors for Anti-RNA polymerase II (phospho S2) or Anti-Rabbit IgG H&L in CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 80 cells for 0 min, n = 20 cells for 5 min after 2-min stress, n = 17 cells for 5 min after 10-min stress, n = 13 cells for 1 h post 2-min, and n = 13 cells for 1 h post 10-min stress for anti-H2B coated microinjected 200-nm FMNP from three independent experiments, n = 78 cells for 5 min after 2-min stress from four independent experiment, n = 79 cells for 5 min after 10-min stress, n = 49 cells for 1 h post 2-min, and n = 70 cells for 1 h post 10-min stress from three independent experiments for a 4-μm RGD-coated bead, n = 80 cells for 0-min stress for only Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2) and n = 68 cells for only Anti-Rabbit IgG H&L (Alexa Fluor 555) antibody from three independent experiments. p = 1.38×10−11 between 0 and 5 min after 2-min stress; p = 1.31×10−4 between 5 min after 2- and 10-min stress; p = 1.14×10−10 between 0 and 5 min after 10-min stress; p = 1.83×10−6 between 2-min stress and 1 h post 2-min stress; p = 4.15×10−6 between 10-min and 1 h post 10-min stress for anti-H2B coated FMNP; p = 1.75×10−8 between 0 and 5 min after 2-min stress; p = 1.70×10−8 between 5 min after 2- and 10-min stress; p = 1.74×10−8 between 0 and 5 min after 10-min stress; p =2.03 ×10−8 between 2-min stress and 1 h post 2-min stress; p = 4.43×10−8 between 10-min stress and 1 h post 10-min stress, p = 8.12×10−7 for 60 min after 2- and 10-min stress for a 4-μm RGD-coated bead at cell surface receptors; p = 0.008 for 2-min stress between Anti-H2B coated FMNP and 4-μm RGD-coated bead; p = 2.82×10−8 between 0-min stress immunofluorescence and only Anti-RNA polymerase II CTD fluorescence intensity; p = 4.36×10−8 between 0-min stress immunofluorescence and only Anti-Rabbit IgG H&L fluorescence intensity; p = 4.40×10−8 between 0-min stress for only RNA Polymerase II Ser 2 phosphorylation and Anti-Rabbit IgG H&L fluorescence intensity. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure 7. (A) Representative anti-H2B-ab coated ferromagnetic NP in Hoechst (nuclear staining) and GFP (DHFR-GFP images of CHO cell nucleus grown on a 1 kPa, 10 kPa, and 20 kPa stiffness Polyacrylamide (PA) gel substrate. Scale bar, 5 μm. (B) Diffusion coefficient (quantified by the best fit of time vs. mean square displacement (MSD) of DHFR-GFP spot movements) of DHFR-GFP on the chromatin of CHO cell nucleus grown on a 1 kPa, 10 kPa, and 20 kPa substrate stiffness at 0 min, 5 min after 2- or 10-min stress (5 Pa at 0.3 Hz) or 1 h post 2- and 10-min stress for anti-H2B coated microinjected 200-nm NP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 20 cells with 20 DHFR-GFP spots for 0 min, n = 20 cells with 20 DHFR-GFP spots for 2-min stress, n = 16 cells with 16 DHFR-GFP spots for 10-min stress, n = 18 cells with 18 DHFR-GFP spots for 1 h post 2-min stress, n = 16 cells with 16 DHFR-GFP spots for 1 kPa, n = 20 cells with 20 DHFR-GFP spots for 0 min, n = 17 cells with 17 DHFR-GFP spots for 2-min stress, n = 15 cells with 15 DHFR-GFP spots for 10-min stress, n = 17 cells with 17 DHFR-GFP spots for 1 h post 2-min stress, n = 15 cells with 15 DHFR-GFP spots for 10 kPa, n = 15 cells with 16 DHFR-GFP spots for 0 min, n = 15 cells with 15 DHFR-GFP spots for 2-min stress, n = 17 cells with 18 DHFR-GFP spots for 10-min stress, n = 15 cells with 15 DHFR-GFP spots for 1 h post 2-min stress, n = 18 cells with 18 DHFR-GFP spots for 20 kPa stiffness PA gel substrates from three independent experiments for anti-H2B-ab coated microinjected NP. p = 1.18×10−4 between 1 kPa and 20 kPa substrates for 2-min stress; p = 0.04 between 10 kPa and 20 kPa substrates for 2-min stress; p = 0.005 between 1 kPa and 20 kPa substrates for 1 h post 2-min stress; p = 0.001 between 10 kPa and 20 kPa substrates for 1 h post 2-min stress; p = 0.005 between 1 kPa and 20 kPa substrates for 1 h post 10-min stress; p = 3.69×10−4 between 10 kPa and 20 kPa substrates for 1 h post 10-min stress; p = 1.06×10−7 between 0- and 2-min stress; p = 1.41×10−6 between 0 and 10 min stress; p = 0.009 between 0 min and 1 h post 2-min stress; p = 0.005 between 0-min and 1 h post 10-min stress; p = 9.75×10−6 between 2-min and 1 h post 2-min stress; p = 0.002 between 10-min and 1 h post 10-min stress for 1 kPa substrate; p = 3.31×10−7 between 0- and 2-min stress; p = 8.80×10−7 between 0- and 10-min stress; p = 0.008 between 0-min and 1 h post 2-min stress; p = 6.34×10−4 between 0 min and 1 h post 10-min stress; p = 2.61×10−4 between 2-min and 1 h post 2-min stress; p = 2.23×10−4 between 10-min stress and 1 h post 10-min stress for 10 kPa substrate; p = 1.16×10−4 between 0- and 2-min stress; p = 2.94×10−6 between 0 and 10-min stress; p = 2.33×10−5 between 2-min stress and 1 h post 2-min stress; p = 2.62×10−6 between 10-min stress and 1 h post 10-min stress for 20 kPa substrate for anti-H2B coated NP; (C) Chromatin stretching (apparent distance changes between two DHFR GFP spots in the same chromatin domain (chromatin strain)) with anti-H2B-ab coated 200-nm microinjected FMNP applied stress (5 Pa at 0.3 Hz) of CHO cell grown on a 1 kPa, 10 kPa, and 20 kPa substrate stiffness. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for 20 kPa substrates stiffness from three independent experiments for anti-H2B-ab coated FMNP, p= 0.003 between 1 kPa and 10 kPa; p= 0.009 between 10 kPa and 20 kPa; p = 1.14×10−4 between 1 kPa and 20 kPa for anti-H2B-ab coated FMNP; (D) Chromatin complex modulus (ratio of applied stress to measured chromatin strain) of CHO cell grown on a 1 kPa, 10 kPa, and 20 kPa substrate stiffness for microinjected 200-nm FMNP with applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for 20 kPa substrates stiffness from three independent experiments for anti-H2B-ab coated FMNP, p= 0.003 between 1 kPa and 10 kPa; p= 0.009 between 10 kPa and 20 kPa; p = 1.14×10−4 between 1 kPa and 20 kPa for anti-H2B-ab coated FMNP; (E) Chromatin storage modulus (G’ = (σ0/ε0) cos δ) of CHO cell grown on a 1 kPa, 10 kPa, and 20 kPa substrate stiffness for microinjected 200-nm FMNP with applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for 20 kPa substrates stiffness from three independent experiments for anti-H2B-ab coated FMNP, p= 0.003 between 1 kPa and 10 kPa; p= 0.009 between 10 kPa and 20 kPa; p = 1.14×10−4 between 1 kPa and 20 kPa for anti-H2B-ab coated FMNP; (F) Chromatin loss modulus (G” = (σ0/ε0) sin δ) of CHO cell grown on a 1 kPa, 10 kPa, or 20 kPa substrate stiffness for microinjected 200-nm FMNP with applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for 20 kPa substrates stiffness from three independent experiments for anti-H2B-ab coated FMNP, p= 0.003 between 1 kPa and 10 kPa; p= 0.009 between 10 kPa and 20 kPa; p = 1.14×10−4 between 1 kPa and 20 kPa for anti-H2B-ab coated FMNP; (G) Nucleoplasm complex modulus (ratio of applied stress to strain; strain= (Peak NP displacement)/(NP radius)) of CHO cell grown on a 1 kPa, 10 kPa, and 20 kPa substrate stiffness for microinjected 200-nm FMNP with applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for 20 kPa substrates stiffness from three independent experiments for anti-H2B-ab coated FMNP, p= 0.034 between 1 kPa and 10 kPa; p= 0.03 between 10 kPa and 20 kPa; p = 4.35×10−4 between 1 kPa and 20 kPa for anti-H2B-ab coated FMNP. (H) Nucleoplasm storage modulus (G’ = (σ0/ε0) cos δ) of CHO cell grown on a 1 kPa, 10 kPa, and 20 kPa substrate stiffness for microinjected 200-nm FMNP with applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for 20 kPa substrates stiffness from three independent experiments for anti-H2B-ab coated FMNP, p= 0.034 between 1 kPa and 10 kPa; p= 0.031 between 10 kPa and 20 kPa; p = 4.35×10−4 between 1 kPa and 20 kPa for anti-H2B-ab coated FMNP. (I) Nucleoplasm loss modulus (G” = (σ0/ε0) sin δ) of CHO cell grown on a 1 kPa, 10 kPa, and 20 kPa substrate stiffness for microinjected 200-nm FMNP with applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for 20 kPa substrates stiffness from three independent experiments for anti-H2B-ab coated FMNP, p= 0.034 between 1 kPa and 10 kPa; p= 0.03 between 10 kPa and 20 kPa; p = 4.35×10−4 between 1 kPa and 20 kPa for anti-H2B-ab coated FMNP. One-way ANOVA with Tukey and Mann-Whitney test was used.
Supplementary Material
Figure S1. (A) A typical SEM (Scanning Electron Microscopy) image of FMNPs (ferromagnetic nanoparticles). Scale bar, 200 nm. (B) Normal size distribution (202±6.0 nm; mean ± SD) of individual FMNP shown in the histogram. Insets show the boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 108 FMNPs from three independent experiments of SEM. (C) Representative 200-nm FMNP embedded completely in a pure viscous fluid of 1000 poise. An external step-function twisting magnetic field of 50 Gauss was applied for 10 s to calibrate the nanoparticle magnetic moment constant. Scale bar, 5 μm. (D) FMNP displacements in response to the applied step-function twisting magnetic field of 50 Gauss quantified for 10 s to measure strain rate for the nanoparticle magnetic moment constant (c = 0.10±0.0054 Pa/Gauss (mean±SD)). n = 10 FMNPs from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S2. (A) DHFR-GFP spots displaced sinusoidally with a sinusoidal local stress (5 Pa at 0.3 Hz) applied by a 200-nm FMNP coated with anti-H2B antibody via MTC. Individual DHFR-GFP spots displacements of the same four cells as in Figure 1C. (B) Chromatin stretching for anti-H2B antibody coated microinjected 200-nm FMNP with (5 Pa at 0.3 Hz) and without applied stress. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 16 cells for 36 DHFR-GFP spots for anti-H2B-ab coated NP with stress and n = 14 cells for 30 DHFR-GFP spots for anti-H2B-ab coated NP without stress from three independent experiments. P= 3.26×10−8 between with and without stress generated chromatin stretching for anti-H2B coated 200-nm FMNP. (C) Chromatin stretching for Rabbit Anti-Mouse IgG H&L (HRP) coated microinjected 200-nm FMNP with and without applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 12 cells for 30 DHFR-GFP spots for Rabbit Anti-Mouse IgG H&L (HRP) coated NP with stress and n = 10 cells for 27 DHFR-GFP spots for Rabbit Anti-Mouse IgG H&L (HRP) coated NP without stress from three independent experiments. (D) Chromatin stretching for uncoated microinjected 200-nm FMNP with and without applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 11 cells for 25 DHFR-GFP spots for uncoated NP with stress and n = 10 cells for 23 DHFR-GFP spots for uncoated NP without stress from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S3. (A) Chromatin displacements do not depend on DHFR-GFP spots location from anti-H2B-ab coated microinjected FMNP in the absence of stress. Spontaneous (without stress) displacements of individual DHFR-GFP spots at various locations from anti-H2B-ab coated 200-nm FMNP. Each distinct color line represents displacements from a microinjected magnetic FMNP. Each rounded end point on the same line shows the location of individual DHFR-GFP spots from anti-H2B-ab coated FMNP in a CHO cell nucleus. n = 14 cells with 30 DHFR-GFP spots for anti-H2B coated 200-nm FMNPs from three independent experiments. (B) Chromatin displacements do not depend on DHFR-GFP spots location from Anti-Mouse IgG H&L (HRP) antibody coated microinjected FMNP. Stress (5 Pa at 0.3 Hz) induced similar peak displacements of individual DHFR-GFP spots at various locations from Rabbit Anti-Mouse IgG H&L (HRP) antibody coated 200-nm FMNP as those NPs without stress in (C). n = 12 cells with 30 DHFR-GFP spots for IgG H&L coated 200-nm FMNPs from three independent experiments. (C) Spontaneous displacements of individual DHFR-GFP spots at various locations from IgG H&L (HRP) antibody coated 200-nm FMNP. n = 10 cells with 27 DHFR-GFP spots for IgG H&L coated 200-nm FMNPs from three independent experiments. (D) Chromatin displacements do not depend on DHFR-GFP spots location from uncoated microinjected FMNP. Stress (5 Pa at 0.3 Hz) induced similar peak displacements of individual DHFR-GFP spots at various locations from uncoated 200-nm FMNP as those NPs in (E). n = 11 cells with 25 DHFR-GFP spots for uncoated 200-nm FMNPs from three independent experiments. (E) Spontaneous displacements of individual DHFR-GFP spots at various locations from uncoated 200-nm FMNP. n = 10 cells with 23 DHFR-GFP spots for uncoated 200-nm FMNPs from three independent experiments. (F) Displacements between two DHFR-GFP spots in the same chromatin domain for anti-H2B, Rabbit Anti-Mouse IgG H&L (HRP) antibody, or uncoated microinjected 200-nm FMNP with applied stress (5 Pa at 0.3 Hz) and anti-H2B-ab coated FMNP without stress. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 16 cells for 36 DHFR-GFP spots for anti-H2B coated NP with stress, n = 12 cells for 30 DHFR-GFP spots for IgG H&L antibody coated NP, n = 11 cells for 25 DHFR-GFP spots for uncoated NP, and n = 14 cells for 30 DHFR-GFP spots for anti-H2B coated NP without stress from three independent experiments. P= 3.03×10−9 between displacements for anti-H2B and Rabbit Anti-Mouse IgG H&L (HRP) antibody coated microinjected NP; P= 2.25×10−8 between displacements for anti-H2B and uncoated microinjected NP; P= 3.75×10−8 between displacements with and without stress for anti-H2B-ab coated microinjected NP. (G) Displacements between two DHFR-GFP spots in the same chromatin domain for Rabbit Anti-Mouse IgG H&L (HRP) antibody coated microinjected 200-nm FMNP with and without applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 12 cells for 30 DHFR-GFP spots for IgG H&L antibody coated NP with stress and n = 10 cells for 27 DHFR-GFP spots for IgG H&L coated NP without stress from three independent experiments. (H) Displacements between two DHFR-GFP spots in the same chromatin domain for uncoated microinjected 200-nm FMNP with and without applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 11 cells for 25 DHFR-GFP spots for uncoated NP with stress and n = 10 cells for 23 DHFR-GFP spots for uncoated NP without stress from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S4. (A) Diffusion coefficient of DHFR-GFP on the chromatin 0 min, 5 min, and 30 min after 2- or 10-min stress (5 Pa at 0.3 Hz) for anti-H2B-ab coated microinjected 200-nm FMNP in CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 23 cells with 27 DHFR-GFP spots for 0 min, n = 14 cells with 17 DHFR-GFP spots for 5 min after 2-min stress, n=13 cells with 20 DHFR-GFP spots for 30 min after 2-min stress, n=14 cells with 16 DHFR-GFP spots for 5 min after 10-min stress, and n=8 cells with 12 DHFR-GFP spots for 30 min after 10-min stress from three independent experiments. P = 0.009 between 0- and 2-min stress for anti-H2B coated NP; P = 3.43×10−4 between 0- and 10- min stress for anti-H2B coated NP; P = 0.016 between 5 min after 2- and 10-min stress; P = 0.025 between 30 min after 2- and 10-min stress for anti-H2B coated NP. (B) Diffusion coefficient of free GFP inside the nucleoplasm (free GFP’s <5 μm and >5 μm distance from location of DHFR-GFP spots) 0 min, and 5 min and 30 min after 2-min stress (5 Pa at 0.3 Hz) for anti-H2B-ab coated microinjected FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 40 cells for 0 min, n = 20 (free GFP’s< 5 μm), n = 20 (free GFP’s> 5 μm) for 5 min after 2-min stress, n = 17 (free GFP’s< 5 μm) and n=17 (free GFP’s> 5 μm) for 30 min after 2-min stress from five independent experiments. P = 7.84×10−8 between 0 and 5 min after 2-min stress for free GFP’s <5 μm distance from DHFR-GFP spots; P = 0.009 between 0 and 5 min after 2 min stress for free GFP’s >5 μm distance from DHFR-GFP spots; P = 0.004 for free GFP’s <5 μm and free GFP’s >5 μm from DHFR-GFP location for 5 min after 2-min stress. (C) Diffusion coefficient of free GFP inside the nucleoplasm (free GFP’s <5 μm and >5 μm distance from location of DHFR-GFP spots) 0 min, and 5 min and 30 min after 10-min stress for anti-H2B-ab coated 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 40 cells for 0-min stress from five independent experiments, n = 24 (free GFP’s< 5 μm), n = 24 (free GFP’s> 5 μm) for 5 min after 10-min stress, n = 22 (free GFP’s< 5 μm) and n=22 (free GFP’s> 5 μm) for 30 min after 10-min stress from six independent experiments. P = 2.67×10−10 between 0-min stress and 5 min after 10-min stress for free GFP’s <5 μm distance from DHFR-GFP spots; P = 3.03×10−4 between 0-min stress and 5 min after 10-min stress for free GFP’s >5 μm distance from DHFR-GFP spots; P = 2.52×10−4 for free GFP’s <5 μm and free GFP’s >5 μm from DHFR-GFP location for 5-min after 2-min stress.; (D) Diffusion coefficient (quantified by the best fit of time vs mean square displacement (MSD) of DHFR-GFP spot movements) of DHFR-GFP 5 min after 0-, 2-, or 10-min stress (5 Pa at 0.3 Hz) or 1 h post 2- and 10-min stress for Rabbit Anti-Mouse IgG H&L (HRP) antibody coated microinjected 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 10 cells with 11 DHFR-GFP spots for 0-min stress, n=11 cells with 11 DHFR-GFP spots for 2-min stress, n=10 cells with 10 DHFR-GFP spots for 10-min stress, n=10 cells with 10 DHFR-GFP spots for 1 h post 2-min stress, n=10 cells with 10 DHFR-GFP spots for 1 h post 10-min stress from three independent experiments. (E) Diffusion coefficient of DHFR-GFP on the chromatin 5 minutes after 0-, 2-, or 10-min stress (5 Pa at 0.3 Hz) or 1 h post 2- and 10-min stress for uncoated microinjected 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 12 cells with 12 DHFR-GFP spots for 0 min, n=11 cells with 11 DHFR-GFP spots for 2-min stress, n=11 cells with 11 DHFR-GFP spots for 10-min stress, n=11 cells with 11 DHFR-GFP spots for 1 h post 2-min stress, n=10 cells with 10 DHFR-GFP spots for 1 h post 10-min stress from three independent experiments. (F) Fluorescence correlation spectroscopy (FCS) based diffusion coefficient of nucleoplasm free GFP inside the nucleus 5 min after 0-, 2-, or 10-min stress and 1 h post 2- or 10-min stress for IgG H&L antibody coated microinjected 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 30 cells for 0-min, n = 12 cells (free GFP’s< 5 μm), n = 12 (free GFP’s> 5 μm) for 2-min, n = 11 cells (free GFP’s< 5 μm) and n=11 cells (free GFP’s> 5 μm) for 10-min stress, n = 24 for 1 h post 2-min stress, n = 22 for 1 h post 10-min stress from three independent experiments for IgG H&L coated NP. (G) Diffusion coefficient of free GFP inside the nucleoplasm 5 min after 0-, 2-, or 10-min stress and 1 h post 2 or 10-min stress for uncoated microinjected 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 28 cells for 0-min stress, n = 15 cells (free GFP’s< 5 μm), n = 15 cells (free GFP’s> 5 μm) for 2-min stress, n = 15 cells (free GFP’s< 5 μm) and n=15 cells (free GFP’s> 5 μm) for the 10-min stress, n = 30 for 1 h post 2-min stress; n = 30 for 1 h post 10-min stress from three independent experiments for uncoated NP. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S5. (A) Diffusion coefficient of RNA Polymerase II spots at 0-min stress, 5 or 30 min after 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at hiPSC surface. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 42 cells with 55 RNA Polymerase II spots for 0 min, n = 20 cells with 20 RNA Polymerase II spots for 5 min after 2-min stress, n = 15 cells with 15 RNA Polymerase II spots for 30 min after 2-min stress, n = 16 cells with 16 RNA Polymerase II spots for 5 min after 10-min stress, n = 15 cells with 15 RNA Polymerase II spots for 30 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP from three independent experiments, n = 22 cells with 32 RNA Polymerase II spots for 5 min after 2-min stress, n = 19 cells with 24 RNA Polymerase II spots for 30 min after 2-min stress, n = 25 cells with 35 RNA Polymerase II spots for 5 min after 10-min stress from four independent experiments, n = 16 cells with 27 RNA Polymerase II spots for 30 min after 10-min stress for a 4-μm RGD-coated bead from three independent experiments. P = 3.03×10−7 between 0-min stress and 5 min after 2-min stress; P = 2.60×10−8 between 0-min stress and 5 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP; P = 2.22×10−8 between 0-min stress and 5 min after 2-min stress; P =7.29×10−8 between 0 and 5 min after the 10-min stress for a 4-μm RGD-coated bead. (B) Fluorescence correlation spectroscopy (FCS) based diffusion coefficient of single Pol IIs in the nucleoplasm in the vicinity (<0.5 μm) of the RNA Polymerase II spot, 0-min stress, 5 and 30 min after 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at hiPSC surface. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 32 cells for 0-min stress, n = 7 cells for 5 min after 2-min stress, n = 7 cells for 30 min after 2-min stress, n = 8 cells for 5 min after 10-min stress, n=8 cells for 30 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP, n=16 cells for 5 min after 2-min stress, n=14 cells for 30 min after 2-min stress, n=15 cells for 5 min after 10-min stress, and n=14 cells for 30 min after 10-min stress for a 4-μm RGD-coated bead from three independent experiments. P = 4.49×10−5 between 0-min stress and 5 min after 2-min stress; P = 1.62×10−5 between 0-min stress and 5 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP; P = 1.01×10−7 between 0-min stress and 5 min after 2-min stress; P = 4.61×10−8 between 0-min stress and 5 min after 10-min stress for a 4-μm RGD-coated bead. (C) Diffusion coefficient of RNA Polymerase II spots (quantified by the best fit of time vs mean square displacement (MSD) of RNA Polymerase II spot movements) 0-min stress, 5 and 60 min after 2- or 10-min stress for uncoated 200-nm microinjected FMNP in hiPSC nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 42 cells with 55 RNA Polymerase II spots for 0 min, n = 17 cells with 17 RNA Polymerase II spots for 5 min after 2-min stress, n = 17 cells with 17 RNA Polymerase II spots for 5 min after 10-min stress, n = 15 cells with 15 RNA Polymerase II spots for 60 min after 2-min stress, and n = 18 cells with 18 RNA Polymerase II spots for 60 min after 10-min stress for uncoated 200-nm microinjected FMNP from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S6. (A) Normalized RNA Polymerase II activity (RNA Pol II Ser 2 phosphorylation fluorescence intensity) 0 min, 5 min, and 30 min after 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at cell surface receptors for Anti-RNA polymerase II (phospho S2) or Anti-Rabbit IgG H&L in hiPSC nuclei. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 78 cells for 0 min, n = 16 cells for 5 min after 2-min stress, n = 16 cells for 30 min after 2-min stress, n=18 cells for 5 min after 10-min stress, and n=14 cells for 30 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP, n = 33 cells for 5 min after 2-min stress, n = 31 cells for 30 min after 2-min stress, n=76 cells for 5 min after 10-min stress, and n=53 cells for 30 min after 10-min stress for a 4-μm RGD-coated bead at cell surface receptors from three independent experiments. P = 4.90×10−4 between 0 and 5 min after 2-min stress; P = 8.52×10−6 between 0-min stress and 30 min after 2 min stress; P = 1.74×10−8 between 0-min stress and 30 min after 10-min stress for anti-H2B coated FMNP; P = 0.002 between 0-min stress and 30 min after 2-min stress; P = 1.74×10−8 between 0-min stress and 30 min after 10-min stress, P = 4.07×10−6 between 5 min and 30 min after 2-min stress, P = 0.005 between 5 min and 30 min after 10-min stress, for a 4-μm RGD-coated bead. P = 2.16×10−4 for 5 min after 2-min stress between Anti-H2B coated FMNP and 4-μm RGD-coated bead; P = 0.0012 for 30 min after 10-min stress between Anti-H2B coated FMNP and 4-μm RGD-coated bead; (B) Normalized RNA Polymerase II activity (RNA Pol II Ser 2 phosphorylation fluorescence intensity) 0-min stress, 5 and 30 min after 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at cell surface receptors for Anti-RNA polymerase II (phospho S2) or Anti-Rabbit IgG H&L in CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 80 cells for 0 min, n = 20 cells for 5 min after 2-min stress, n = 11 cells for 30 min after 2-min stress, n=17 cells for 5 min after 10-min stress, and n=11 cells for 30 min after 10-min stress from three independent experiments for anti-H2B coated microinjected 200-nm FMNP, n = 78 cells for 5 min after 2-min stress from four independent experiments, n = 110 cells for 30 min after 2-min stress, n=79 cells for 5 min after 10-min stress, and n=63 cells for 30 min after 10-min stress from three independent experiments for a 4-μm RGD-coated bead at cell surface receptors. P = 1.38×10−11 between 0-min stress and 5 min after 2-min stress; P = 1.68×10−5 between 0 min and 30 min after 2-min stress; P = 8.76×10−8 between 0-min stress and 30 min after 10-min stress, P = 0.04 between 5 and 30 min after 2-min stress for anti-H2B coated FMNP; P = 4.49×10−8 between 0-min stress and 30 min after 2-min stress; P = 4.05×10−8 between 0-min stress and 30 min after 10-min stress, for a 4-μm RGD-coated bead at cell surface receptors. (C) Representative image of brightfields (BF) and immunofluorescence (used both Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2) and Anti-Rabbit IgG H&L (Alexa Fluor® 555) antibody) of RNA Polymerase II Ser 2 phosphorylation (red) in CHO cell nucleus at 0-min (no stress) or 5 min after, 2- or 10-min stress (5 Pa at 0.3 Hz) for an uncoated 200-nm microinjected FMNP. Scale bars, 5 μm. (D) Normalized RNA Polymerase II Ser 2 phosphorylation fluorescence intensity 0-min stress, 5 and 60 min after 2- or 10-min stress for uncoated 200 nm microinjected FMNP for Anti-RNA polymerase II (phospho S2) or Anti-Rabbit IgG H&L in CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 80 cells for 0 min, n = 28 cells for 5 min after 2-min stress, n = 25 cells for 5 min after 10-min stress, n=24 cells for 60 min after 2-min stress, and n=22 cells for 60 min after 10-min stress from three independent experiments for uncoated 200-nm FMNP. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S7. (A) Displacements between two DHFR-GFP spots in the same chromatin domain of CHO cell nucleus grown on a 1 kPa, 10 kPa, or 20 kPa stiffness Polyacrylamide (PA) gel substrate for anti-H2B-ab coated microinjected 200-nm FMNP with stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for anti-H2B-ab coated NP with stress from three independent experiments. P= 0.002 between chromatin displacements for cells grown on 10-kPa and 20-kPa substrate stiffness; P= 1.39×10−4 between chromatin displacements for cells grown on 1-kPa and 20-kPa substrate stiffness for anti-H2B-ab coated microinjected FMNP. (B) Diffusion coefficient (quantified by the best fit of time vs mean square displacement (MSD) of DHFR-GFP spot movements) of DHFR-GFP at 0 min stress for control (no microinjection), only medium microinjection (microinjection of medium only without 200-nm FMNP), and medium+FMNP microinjection (microinjection of anti-H2B-ab coated 200-nm FMNP). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 33 cells with 33 DHFR-GFP spots for control, n=28 cells with 34 DHFR-GFP spots for medium only microinjection, n=23 cells with 24 DHFR-GFP spots for medium+FMNP microinjection from three independent experiments. (C) Quantified nuclear stiffness (Nucleus Young’s modulus) for control, medium only, and medium+FMNP microinjected CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 11 cells for control, n = 13 cells for only medium microinjection, and n = 18 cells for anti-H2B-ab coated FMNP microinjection from three independent experiments. (D) Representative images of transmission electron microscopy (TEM) from three groups of cell nuclei: control (left, intact cell nuclei with no microinjection), medium (middle, cell nuclei microinjected with medium without any FMNP), or medium+FMNP (right, cell nuclei microinjected with medium plus an anti-H2B-ab coated ferromagnetic nanoparticle (NP) (highlighted by a black arrow and within yellow dashed lines). Each group in a column has four different CHO cells. Note that there is elevated intensity of gray contrast surrounding each NP, suggesting the presence of chromatin aggregation. Scale bar, 2 μm. (E) Diffusion coefficient (quantified by the best fit of time vs mean square displacement (MSD) of DHFR-GFP spot movements) of DHFR-GFP at no-stress conditions for regular CHO cell medium, 20-mM glucose containing medium, 20-mM 2-deoxy-D-glucose containing medium, or 10-μM Antimycin A containing medium. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 23 cells of 27 DHFR-GFP spots for regular medium, n = 25 cells of 37 DHFR-GFP spots for 20-mM glucose medium, n = 23 cells of 28 DHFR-GFP spots for 20-mM 2-deoxy-D-glucose medium, and n = 19 cells of 23 DHFR-GFP spots for 10 μM Antimycin A medium from three independent experiments. P = 4.20×10−4 for DHFR-GFP diffusivity between regular and 20-mM 2-deoxy-D-glucose medium, P = 2.17×10−4 for DHFR-GFP diffusivity between regular and 10 μM Antimycin A medium. Note that the DHFR-GFP spot diffusivity was reduced by 49% for 20-mM 2-deoxy-D-glucose medium and 47.6% in 10-μM Antimycin A medium. (F) Diffusion coefficient of uncoated 200-nm FMNP at no-stress conditions for various control conditions and ATP-depleted conditions. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 22 uncoated FMNP for regular medium, n = 25 uncoated FMNP for 20-mM glucose medium, n = 22 uncoated FMNP for 20-mM 2-deoxy-D-glucose medium, and n = 23 uncoated FMNP for 10 μM Antimycin A medium from three independent experiments. P = 1.13×10−4 for uncoated FMNP diffusivity between regular medium and 20-mM 2-deoxy-D-glucose medium; P = 1.37×10−5 for uncoated FMNP diffusivity between regular and 10-μM Antimycin A medium. Note that the uncoated FMNP diffusivity was reduced by 30.5% for 20-mM 2-deoxy-D-glucose medium and 38.2% in 10-μM Antimycin A medium. One-way ANOVA with Tukey and Mann-Whitney test was used.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Histone H2B antibody | Abcam Incorporated | RRID: AB_52484 |
| Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2) | Abcam Incorporated | RRID: AB_5095 |
| Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 555) preadsorbed | Abcam Incorporated | RRID: AB_150062 |
| Rabbit Anti-Mouse IgG H&L (HRP) | Abcam Incorporated | RRID: AB_6728 |
| Chemicals, peptides, and recombinant proteins | ||
| Fetal Bovine Serum, dialyzed | Gibco | A3382001 |
| TrypLE™ Express Enzyme (1X), no phenol red | Gibco | 12604013 |
| Penicillin-Streptomycin | Gibco | 15070-063 |
| Fibronectin bovine plasma | Sigma-Aldrich | F4759 |
| mTeSR™1 | STEMCELL Technologies | 85850 |
| HEPES | Gibco | 15-630-080 |
| Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix, Phenol Red-free | Corning | 356231 |
| DMEM/F-12, HEPES, no phenol red | Gibco | 11039021 |
| StemPro™ Accutase™ Cell Dissociation Reagent | Thermo Fisher | A1110501 |
| KnockOut™ Serum Replacement | Thermo Fisher | 10828010 |
| ROCK inhibitor | STEMCELL Technologies | Y-27632 |
| Hoechst | Thermo Fisher | 62249 |
| DPBS, without Ca++ or Mg++ | Gibco | 14190-144 |
| PBS | Corning | 21-040-CV |
| N-Hydroxysuccinimide | Sigma-Aldrich | 130672 |
| EDC | Sigma-Aldrich | 341006 |
| Bovine Serum Albumin | Sigma-Aldrich | A9647 |
| Triton™ X-100 | Sigma-Aldrich | A9647 |
| Paraformaldehyde | Santa Cruz | sc-281692 |
| Normal donkey serum | Jackson ImmunoResearch Laboratories Inc.) | 017-000-121 |
| Formamide | Thermo Fisher | AM9344 |
| 2-deoxy-D-glucose | Sigma-Aldrich | D8375 |
| Antimycin A | Sigma-Aldrich | A8674 |
| D-glucose | Thermo Fisher | A2494001 |
| 40% acrylamide | BIO-RAD | 1610140 |
| 2% Bis Solution | BIO-RAD | 1610142 |
| (3-Aminopropyl)trimethoxysilane | Sigma-Aldrich | 281778 |
| Glutaraldehyde solution | Sigma-Aldrich | G6257 |
| Ammonium Persulfate | BIO-RAD | 1610700 |
| TEMED | BIO-RAD | 1610801 |
| Sulfo-SANPAH (sulfosuccinimidyl 6-(4′-azido-2′-nitrophenylamino)hexanoate) | Thermo Fisher | 22589 |
| Trypan Blue solution | Sigma-Aldrich | T8154 |
| Experimental models: Cell lines | ||
| Human induced pluripotent stem cells (hiPSCs) | Coriell Institute | AICS-0096-074 |
| Software and algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| MATLAB | The MathWorks, Inc. | https://www.mathworks.com/products |
| VistaVision | ISS | https://iss.com/software/vistavision |
| Other | ||
| Inverted microscope | Leica | DMIRE2 |
| Olympus confocal scanning system | ISS | Alba |
| DriveAFM | Nanosurf Inc. | https://www.nanosurf.com/en/products |
| Transmission electron microscope | JEOL | JEOL JEM 1010 TEM |
| Scanning Electron Microscope | Hitachi | Hitachi S-4800 FE-SEM |
| FemtoJet 4i and InjectMan 4 | Eppendorf | https://www.eppendorf.com/product |
Highlights.
Chromatin is viscoelastic in response to an anti-H2B-ab nanoparticle force at 0.1–5 Hz
Nucleoplasm is a softer viscoelastic network than chromatin in living cells
Chromatin stretching induces mechanomemory of chromatin diffusivity and RNA Pol II activity
Substrate stiffness regulates chromatin mechanomemory and moduli
ACKNOWLEDGMENTS
This research was funded by the National Institutes of Health grant R01 GM072744 (N.W.) and Northeastern University in Boston (N.W.). We thank Dr. Joseph Irudayaraj of UIUC for the use of his lab for the experiments with the single-molecule fluorescence correlation spectroscopy and Dr. Andrew Belmont for CHO DG44 cells.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2024.114462.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Swift J, Ivanovska IL, Buxboim A, Harada T, Dave PC, Dingal P, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, et al. (2013). Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104. 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain N, Iyer KV, Kumar A, and Shivashankar GV (2013). Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc. Natl. Acad. Sci. USA 110, 11349–11354. 10.1073/pnas.1300801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alisafaei F, Jokhun DS, Shivashankar GV, and Shenoy VB (2019). Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proc. Natl. Acad. Sci. USA 116, 13200–13209. 10.1073/pnas.1902035116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Earle AJ, Kirby TJ, Fedorchak GR, Isermann P, Patel J, Iruvanti S, Moore SA, Bonne G, Wallrath LL, and Lammerding J (2020). Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater 19, 464–473. 10.1038/s41563-019-0563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tajik A, Zhang Y, Wei F, Sun J, Jia Q, Zhou W, Singh R, Khanna N, Belmont AS, and Wang N (2016). Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater 15, 1287–1296. 10.1038/nmat4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Chen J, Mohagheghian E, and Wang N (2020). Force-induced gene up-regulation does not follow the weak power law but depends on H3K9 demethylation. Sci. Adv 6, eaay9095. 10.1126/sciadv.aay9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei F, Xu X, Zhang C, Liao Y, Ji B, and Wang N (2020). Stress fiber anisotropy contributes to force-mode dependent chromatin stretching and gene upregulation in living cells. Nat. Commun 11, 4902. 10.1038/s41467-020-18584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Chen J, Amar K, Wu Y, Jiang M, and Wang N (2023). LAP2β transmits force to upregulate genes via chromatin domain stretching but not compression. Acta Biomater 163, 326–338. 10.1016/j.actbio.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashid F, Liu W, Wang Q, Ji B, Irudayaraj J, and Wang N (2023). Mechanomemory in protein diffusivity of chromatin and nucleoplasm after force cessation. Proc. Natl. Acad. Sci. USA 120, e2221432120. 10.1073/pnas.2221432120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engler AJ, Sen S, Sweeney HL, and Discher DE (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, and Hinz B (2012). The mechanical memory of lung myofibroblasts. Integr. Biol 4, 410–421. 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 12.Yang M, Tibbitt W, Basta L, and Anseth KS (2014). Mechanical memory and dosing influence stem cell fate. Nat. Mater 13, 645–652. 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, Wang L, Chen J, Zhang S, Huang B, Wang N, et al. (2014). Matrix softness regulates plasticity of tumourrepopulating cells via H3K9 demethylation and Sox2 expression. Nat. Commun 5, 4619. 10.1038/ncomms5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, and Hinz B (2017). MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater 16, 379–389. 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Zhu H, Ren X, Gao B, Cheng B, Liu S, Sha B, Li Z, Zhang Z, Lv Y, et al. (2021). Mechanics-driven nuclear localization of YAP can be reversed by N-cadherin ligation in mesenchymal stem cells. Nat. Commun 12, 6229. 10.1038/s41467-021-26454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida JA, Mathur J, Lee YL, Sarker B, and Pathak A (2023). Mechanically primed cells transfer memory to fibrous matrices for invasion across environments of distinct stiffness and dimensionality. Mol. Biol. Cell 34, ar54. 10.1091/mbc.E22-10-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misteli T (2020). The self-organizing genome: principles of genome architecture and function. Cell 183, 28–45. 10.1016/j.cell.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keizer VIP, Grosse-Holz S, Woringer M, Zambon L, Aizel K, Bongaerts M, Delille F, Kolar-Znika L, Scolari VF, Hoffmann S, et al. (2022). Live-cell micromanipulation of a genomic locus reveals interphase chromatin mechanics. Science 377, 489–495. 10.1126/science.abi9810. [DOI] [PubMed] [Google Scholar]
- 19.Strickfaden H, Tolsma TO, Sharma A, Underhill DA, Hansen JC, and Hendzel MJ (2020). Condensed chromatin behaves like a solid on the mesoscale in vitro and in living cells. Cell 183, 1772–1784.e13. 10.1016/j.cell.2020.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, and Burridge K (2014). Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol 16, 376–381. 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guilak F, Tedrow JR, and Burgkart R (2000). Viscoelastic properties of the cell nucleus. Biochem. Bioph. Res. Co 269, 781–786. 10.1006/bbrc.2000.2360. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Kireev I, Plutz M, Ashourian N, and Belmont AS (2009). Large-scale chromatin structure of inducible genes: transcription on a condensed, linear template. J. Cell Biol 185, 87–100. 10.1083/jcb.200809196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhury F, Na S, Collin O, Tay B, Li F, Tanaka T, Leckband DE, and Wang N (2008). Is cell rheology governed by nonequilibrium-to-equilibrium transition of noncovalent bonds? Biophys. J 95, 5719–5727. 10.1529/biophysj.108.139832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alisafaei F, Moheimani H, Elson EL, and Genin GM (2023). A nuclear basis for mechanointelligence in cells. Proc. Natl. Acad. Sci. USA 120, e2303569120. 10.1073/pnas.2303569120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houchmandzadeh B, Marko JF, Chatenay D, and Libchaber A (1997). Elasticity and structure of eukaryote chromosomes studied by micromanipulation and micropipette aspiration. J. Cell Biol 139, 1–12. 10.1083/jcb.139.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirier MG, Eroglu S, and Marko JF (2002). The bending rigidity of mitotic chromosomes. Mol. Biol. Cell 13, 2170–2179. 10.1091/mbc.01-08-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vries AHB, Krenn BE, van Driel R, Subramaniam V, and Kanger JS (2007). Direct observation of nanomechanical properties of chromatin in living cells. Nano Lett 7, 1424–1427. 10.1021/nl070603+. [DOI] [PubMed] [Google Scholar]
- 28.Tseng Y, Lee JSH, Kole TP, Jiang I, and Wirtz D (2004). Micro-organization and visco-elasticity of the interphase nucleus revealed by particle nanotracking. J. Cell Sci 117, 2159–2167. 10.1242/jcs.01073. [DOI] [PubMed] [Google Scholar]
- 29.Solovei I, Cavallo A, Schermelleh L, Jaunin F, Scasselati C, Cmarko D, Cremer C, Fakan S, and Cremer T (2002). Spatial preservation of nuclear chromatin architecture during three-dimensional fluorescence in situ hybridization (3D-FISH). Exp. Cell Res 276, 10–23. 10.1006/excr.2002.5513. [DOI] [PubMed] [Google Scholar]
- 30.Brangwynne CP, Mitchison TJ, and Hyman AA (2011). Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes oocytes. Proc. Natl. Acad. Sci. USA 108, 4334–4339. 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zidovska A, Weitz DA, and Mitchison TJ (2013). Micron-scale coherence in interphase chromatin dynamics. Proc. Natl. Acad. Sci. USA 110, 15555–15560. 10.1073/pnas.1220313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, et al. (2017). Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 171, 1397–1410.e14. 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Wu PH, Aroush DRB, Asnacios A, Chen WC, Dokukin ME, Doss BL, Durand-Smet P, Ekpenyong A, Guck J, Guz NV, et al. (2018). A comparison of methods to assess cell mechanical properties. Nat. Methods 15, 491–498. 10.1038/s41592-018-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Aguilar ZP, Yang L, Kuang M, Duan H, Xiong Y, Wei H, and Wang A (2011). Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials 32, 9758–9765. 10.1016/j.biomaterials.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Na S, and Wang N (2008). Application of fluorescence resonance energy transfer and magnetic twisting cytometry to quantify mechanochemical signaling activities in a living cell. Sci. Signal 1, pl1. 10.1126/scisignal.134pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaine M, Imamura H, and Yoshida S (2022). High and stable ATP levels prevent aberrant intracellular protein aggregation in yeast. Elife 11, e67659. 10.7554/eLife.67659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandel LJ, Doctor RB, and Bacallao R (1994). ATP depletion: a novel method to study junctional properties in epithelial tissues. II. Internalization of Na+, K (+)-ATPase and E-cadherin. J. Cell Sci 107, 3315–3324. 10.1242/jcs.107.12.3315. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, and Irudayaraj J (2020). Perfluorooctanoic acid (PFOA) exposure inhibits DNA methyltransferase activities and alters constitutive heterochromatin organization. Food Chem. Toxicol 141, 111358–111359. 10.1016/j.fct.2020.111358. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, and Irudayaraj J (2009). Quantitative investigation of compartmentalized dynamics of ErbB2 targeting gold nanorods in live cells by single molecule spectroscopy. ACS Nano 3, 4071–4079. 10.1021/nn900743v. [DOI] [PubMed] [Google Scholar]
- 40.Tse JR, and Engler AJ (2010). Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol 47, 10–16. 10.1016/j.micron.2022.103228. [DOI] [PubMed] [Google Scholar]
- 41.Kontomaris SV, Malamou A, and Stylianou A (2022). The Hertzian theory in AFM nanoindentation experiments regarding biological samples: Overcoming limitations in data processing. Micron 155, 103228. 10.1016/j.micron.2022.103228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A) A typical SEM (Scanning Electron Microscopy) image of FMNPs (ferromagnetic nanoparticles). Scale bar, 200 nm. (B) Normal size distribution (202±6.0 nm; mean ± SD) of individual FMNP shown in the histogram. Insets show the boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 108 FMNPs from three independent experiments of SEM. (C) Representative 200-nm FMNP embedded completely in a pure viscous fluid of 1000 poise. An external step-function twisting magnetic field of 50 Gauss was applied for 10 s to calibrate the nanoparticle magnetic moment constant. Scale bar, 5 μm. (D) FMNP displacements in response to the applied step-function twisting magnetic field of 50 Gauss quantified for 10 s to measure strain rate for the nanoparticle magnetic moment constant (c = 0.10±0.0054 Pa/Gauss (mean±SD)). n = 10 FMNPs from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S2. (A) DHFR-GFP spots displaced sinusoidally with a sinusoidal local stress (5 Pa at 0.3 Hz) applied by a 200-nm FMNP coated with anti-H2B antibody via MTC. Individual DHFR-GFP spots displacements of the same four cells as in Figure 1C. (B) Chromatin stretching for anti-H2B antibody coated microinjected 200-nm FMNP with (5 Pa at 0.3 Hz) and without applied stress. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 16 cells for 36 DHFR-GFP spots for anti-H2B-ab coated NP with stress and n = 14 cells for 30 DHFR-GFP spots for anti-H2B-ab coated NP without stress from three independent experiments. P= 3.26×10−8 between with and without stress generated chromatin stretching for anti-H2B coated 200-nm FMNP. (C) Chromatin stretching for Rabbit Anti-Mouse IgG H&L (HRP) coated microinjected 200-nm FMNP with and without applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 12 cells for 30 DHFR-GFP spots for Rabbit Anti-Mouse IgG H&L (HRP) coated NP with stress and n = 10 cells for 27 DHFR-GFP spots for Rabbit Anti-Mouse IgG H&L (HRP) coated NP without stress from three independent experiments. (D) Chromatin stretching for uncoated microinjected 200-nm FMNP with and without applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 11 cells for 25 DHFR-GFP spots for uncoated NP with stress and n = 10 cells for 23 DHFR-GFP spots for uncoated NP without stress from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S3. (A) Chromatin displacements do not depend on DHFR-GFP spots location from anti-H2B-ab coated microinjected FMNP in the absence of stress. Spontaneous (without stress) displacements of individual DHFR-GFP spots at various locations from anti-H2B-ab coated 200-nm FMNP. Each distinct color line represents displacements from a microinjected magnetic FMNP. Each rounded end point on the same line shows the location of individual DHFR-GFP spots from anti-H2B-ab coated FMNP in a CHO cell nucleus. n = 14 cells with 30 DHFR-GFP spots for anti-H2B coated 200-nm FMNPs from three independent experiments. (B) Chromatin displacements do not depend on DHFR-GFP spots location from Anti-Mouse IgG H&L (HRP) antibody coated microinjected FMNP. Stress (5 Pa at 0.3 Hz) induced similar peak displacements of individual DHFR-GFP spots at various locations from Rabbit Anti-Mouse IgG H&L (HRP) antibody coated 200-nm FMNP as those NPs without stress in (C). n = 12 cells with 30 DHFR-GFP spots for IgG H&L coated 200-nm FMNPs from three independent experiments. (C) Spontaneous displacements of individual DHFR-GFP spots at various locations from IgG H&L (HRP) antibody coated 200-nm FMNP. n = 10 cells with 27 DHFR-GFP spots for IgG H&L coated 200-nm FMNPs from three independent experiments. (D) Chromatin displacements do not depend on DHFR-GFP spots location from uncoated microinjected FMNP. Stress (5 Pa at 0.3 Hz) induced similar peak displacements of individual DHFR-GFP spots at various locations from uncoated 200-nm FMNP as those NPs in (E). n = 11 cells with 25 DHFR-GFP spots for uncoated 200-nm FMNPs from three independent experiments. (E) Spontaneous displacements of individual DHFR-GFP spots at various locations from uncoated 200-nm FMNP. n = 10 cells with 23 DHFR-GFP spots for uncoated 200-nm FMNPs from three independent experiments. (F) Displacements between two DHFR-GFP spots in the same chromatin domain for anti-H2B, Rabbit Anti-Mouse IgG H&L (HRP) antibody, or uncoated microinjected 200-nm FMNP with applied stress (5 Pa at 0.3 Hz) and anti-H2B-ab coated FMNP without stress. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 16 cells for 36 DHFR-GFP spots for anti-H2B coated NP with stress, n = 12 cells for 30 DHFR-GFP spots for IgG H&L antibody coated NP, n = 11 cells for 25 DHFR-GFP spots for uncoated NP, and n = 14 cells for 30 DHFR-GFP spots for anti-H2B coated NP without stress from three independent experiments. P= 3.03×10−9 between displacements for anti-H2B and Rabbit Anti-Mouse IgG H&L (HRP) antibody coated microinjected NP; P= 2.25×10−8 between displacements for anti-H2B and uncoated microinjected NP; P= 3.75×10−8 between displacements with and without stress for anti-H2B-ab coated microinjected NP. (G) Displacements between two DHFR-GFP spots in the same chromatin domain for Rabbit Anti-Mouse IgG H&L (HRP) antibody coated microinjected 200-nm FMNP with and without applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 12 cells for 30 DHFR-GFP spots for IgG H&L antibody coated NP with stress and n = 10 cells for 27 DHFR-GFP spots for IgG H&L coated NP without stress from three independent experiments. (H) Displacements between two DHFR-GFP spots in the same chromatin domain for uncoated microinjected 200-nm FMNP with and without applied stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 11 cells for 25 DHFR-GFP spots for uncoated NP with stress and n = 10 cells for 23 DHFR-GFP spots for uncoated NP without stress from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S4. (A) Diffusion coefficient of DHFR-GFP on the chromatin 0 min, 5 min, and 30 min after 2- or 10-min stress (5 Pa at 0.3 Hz) for anti-H2B-ab coated microinjected 200-nm FMNP in CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 23 cells with 27 DHFR-GFP spots for 0 min, n = 14 cells with 17 DHFR-GFP spots for 5 min after 2-min stress, n=13 cells with 20 DHFR-GFP spots for 30 min after 2-min stress, n=14 cells with 16 DHFR-GFP spots for 5 min after 10-min stress, and n=8 cells with 12 DHFR-GFP spots for 30 min after 10-min stress from three independent experiments. P = 0.009 between 0- and 2-min stress for anti-H2B coated NP; P = 3.43×10−4 between 0- and 10- min stress for anti-H2B coated NP; P = 0.016 between 5 min after 2- and 10-min stress; P = 0.025 between 30 min after 2- and 10-min stress for anti-H2B coated NP. (B) Diffusion coefficient of free GFP inside the nucleoplasm (free GFP’s <5 μm and >5 μm distance from location of DHFR-GFP spots) 0 min, and 5 min and 30 min after 2-min stress (5 Pa at 0.3 Hz) for anti-H2B-ab coated microinjected FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 40 cells for 0 min, n = 20 (free GFP’s< 5 μm), n = 20 (free GFP’s> 5 μm) for 5 min after 2-min stress, n = 17 (free GFP’s< 5 μm) and n=17 (free GFP’s> 5 μm) for 30 min after 2-min stress from five independent experiments. P = 7.84×10−8 between 0 and 5 min after 2-min stress for free GFP’s <5 μm distance from DHFR-GFP spots; P = 0.009 between 0 and 5 min after 2 min stress for free GFP’s >5 μm distance from DHFR-GFP spots; P = 0.004 for free GFP’s <5 μm and free GFP’s >5 μm from DHFR-GFP location for 5 min after 2-min stress. (C) Diffusion coefficient of free GFP inside the nucleoplasm (free GFP’s <5 μm and >5 μm distance from location of DHFR-GFP spots) 0 min, and 5 min and 30 min after 10-min stress for anti-H2B-ab coated 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 40 cells for 0-min stress from five independent experiments, n = 24 (free GFP’s< 5 μm), n = 24 (free GFP’s> 5 μm) for 5 min after 10-min stress, n = 22 (free GFP’s< 5 μm) and n=22 (free GFP’s> 5 μm) for 30 min after 10-min stress from six independent experiments. P = 2.67×10−10 between 0-min stress and 5 min after 10-min stress for free GFP’s <5 μm distance from DHFR-GFP spots; P = 3.03×10−4 between 0-min stress and 5 min after 10-min stress for free GFP’s >5 μm distance from DHFR-GFP spots; P = 2.52×10−4 for free GFP’s <5 μm and free GFP’s >5 μm from DHFR-GFP location for 5-min after 2-min stress.; (D) Diffusion coefficient (quantified by the best fit of time vs mean square displacement (MSD) of DHFR-GFP spot movements) of DHFR-GFP 5 min after 0-, 2-, or 10-min stress (5 Pa at 0.3 Hz) or 1 h post 2- and 10-min stress for Rabbit Anti-Mouse IgG H&L (HRP) antibody coated microinjected 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 10 cells with 11 DHFR-GFP spots for 0-min stress, n=11 cells with 11 DHFR-GFP spots for 2-min stress, n=10 cells with 10 DHFR-GFP spots for 10-min stress, n=10 cells with 10 DHFR-GFP spots for 1 h post 2-min stress, n=10 cells with 10 DHFR-GFP spots for 1 h post 10-min stress from three independent experiments. (E) Diffusion coefficient of DHFR-GFP on the chromatin 5 minutes after 0-, 2-, or 10-min stress (5 Pa at 0.3 Hz) or 1 h post 2- and 10-min stress for uncoated microinjected 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 12 cells with 12 DHFR-GFP spots for 0 min, n=11 cells with 11 DHFR-GFP spots for 2-min stress, n=11 cells with 11 DHFR-GFP spots for 10-min stress, n=11 cells with 11 DHFR-GFP spots for 1 h post 2-min stress, n=10 cells with 10 DHFR-GFP spots for 1 h post 10-min stress from three independent experiments. (F) Fluorescence correlation spectroscopy (FCS) based diffusion coefficient of nucleoplasm free GFP inside the nucleus 5 min after 0-, 2-, or 10-min stress and 1 h post 2- or 10-min stress for IgG H&L antibody coated microinjected 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 30 cells for 0-min, n = 12 cells (free GFP’s< 5 μm), n = 12 (free GFP’s> 5 μm) for 2-min, n = 11 cells (free GFP’s< 5 μm) and n=11 cells (free GFP’s> 5 μm) for 10-min stress, n = 24 for 1 h post 2-min stress, n = 22 for 1 h post 10-min stress from three independent experiments for IgG H&L coated NP. (G) Diffusion coefficient of free GFP inside the nucleoplasm 5 min after 0-, 2-, or 10-min stress and 1 h post 2 or 10-min stress for uncoated microinjected 200-nm FMNP. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 28 cells for 0-min stress, n = 15 cells (free GFP’s< 5 μm), n = 15 cells (free GFP’s> 5 μm) for 2-min stress, n = 15 cells (free GFP’s< 5 μm) and n=15 cells (free GFP’s> 5 μm) for the 10-min stress, n = 30 for 1 h post 2-min stress; n = 30 for 1 h post 10-min stress from three independent experiments for uncoated NP. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S5. (A) Diffusion coefficient of RNA Polymerase II spots at 0-min stress, 5 or 30 min after 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at hiPSC surface. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 42 cells with 55 RNA Polymerase II spots for 0 min, n = 20 cells with 20 RNA Polymerase II spots for 5 min after 2-min stress, n = 15 cells with 15 RNA Polymerase II spots for 30 min after 2-min stress, n = 16 cells with 16 RNA Polymerase II spots for 5 min after 10-min stress, n = 15 cells with 15 RNA Polymerase II spots for 30 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP from three independent experiments, n = 22 cells with 32 RNA Polymerase II spots for 5 min after 2-min stress, n = 19 cells with 24 RNA Polymerase II spots for 30 min after 2-min stress, n = 25 cells with 35 RNA Polymerase II spots for 5 min after 10-min stress from four independent experiments, n = 16 cells with 27 RNA Polymerase II spots for 30 min after 10-min stress for a 4-μm RGD-coated bead from three independent experiments. P = 3.03×10−7 between 0-min stress and 5 min after 2-min stress; P = 2.60×10−8 between 0-min stress and 5 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP; P = 2.22×10−8 between 0-min stress and 5 min after 2-min stress; P =7.29×10−8 between 0 and 5 min after the 10-min stress for a 4-μm RGD-coated bead. (B) Fluorescence correlation spectroscopy (FCS) based diffusion coefficient of single Pol IIs in the nucleoplasm in the vicinity (<0.5 μm) of the RNA Polymerase II spot, 0-min stress, 5 and 30 min after 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at hiPSC surface. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 32 cells for 0-min stress, n = 7 cells for 5 min after 2-min stress, n = 7 cells for 30 min after 2-min stress, n = 8 cells for 5 min after 10-min stress, n=8 cells for 30 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP, n=16 cells for 5 min after 2-min stress, n=14 cells for 30 min after 2-min stress, n=15 cells for 5 min after 10-min stress, and n=14 cells for 30 min after 10-min stress for a 4-μm RGD-coated bead from three independent experiments. P = 4.49×10−5 between 0-min stress and 5 min after 2-min stress; P = 1.62×10−5 between 0-min stress and 5 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP; P = 1.01×10−7 between 0-min stress and 5 min after 2-min stress; P = 4.61×10−8 between 0-min stress and 5 min after 10-min stress for a 4-μm RGD-coated bead. (C) Diffusion coefficient of RNA Polymerase II spots (quantified by the best fit of time vs mean square displacement (MSD) of RNA Polymerase II spot movements) 0-min stress, 5 and 60 min after 2- or 10-min stress for uncoated 200-nm microinjected FMNP in hiPSC nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 42 cells with 55 RNA Polymerase II spots for 0 min, n = 17 cells with 17 RNA Polymerase II spots for 5 min after 2-min stress, n = 17 cells with 17 RNA Polymerase II spots for 5 min after 10-min stress, n = 15 cells with 15 RNA Polymerase II spots for 60 min after 2-min stress, and n = 18 cells with 18 RNA Polymerase II spots for 60 min after 10-min stress for uncoated 200-nm microinjected FMNP from three independent experiments. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S6. (A) Normalized RNA Polymerase II activity (RNA Pol II Ser 2 phosphorylation fluorescence intensity) 0 min, 5 min, and 30 min after 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at cell surface receptors for Anti-RNA polymerase II (phospho S2) or Anti-Rabbit IgG H&L in hiPSC nuclei. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 78 cells for 0 min, n = 16 cells for 5 min after 2-min stress, n = 16 cells for 30 min after 2-min stress, n=18 cells for 5 min after 10-min stress, and n=14 cells for 30 min after 10-min stress for anti-H2B coated microinjected 200-nm FMNP, n = 33 cells for 5 min after 2-min stress, n = 31 cells for 30 min after 2-min stress, n=76 cells for 5 min after 10-min stress, and n=53 cells for 30 min after 10-min stress for a 4-μm RGD-coated bead at cell surface receptors from three independent experiments. P = 4.90×10−4 between 0 and 5 min after 2-min stress; P = 8.52×10−6 between 0-min stress and 30 min after 2 min stress; P = 1.74×10−8 between 0-min stress and 30 min after 10-min stress for anti-H2B coated FMNP; P = 0.002 between 0-min stress and 30 min after 2-min stress; P = 1.74×10−8 between 0-min stress and 30 min after 10-min stress, P = 4.07×10−6 between 5 min and 30 min after 2-min stress, P = 0.005 between 5 min and 30 min after 10-min stress, for a 4-μm RGD-coated bead. P = 2.16×10−4 for 5 min after 2-min stress between Anti-H2B coated FMNP and 4-μm RGD-coated bead; P = 0.0012 for 30 min after 10-min stress between Anti-H2B coated FMNP and 4-μm RGD-coated bead; (B) Normalized RNA Polymerase II activity (RNA Pol II Ser 2 phosphorylation fluorescence intensity) 0-min stress, 5 and 30 min after 2- or 10-min stress for anti-H2B-ab coated microinjected 200-nm FMNP and a 4-μm RGD-coated bead at cell surface receptors for Anti-RNA polymerase II (phospho S2) or Anti-Rabbit IgG H&L in CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 80 cells for 0 min, n = 20 cells for 5 min after 2-min stress, n = 11 cells for 30 min after 2-min stress, n=17 cells for 5 min after 10-min stress, and n=11 cells for 30 min after 10-min stress from three independent experiments for anti-H2B coated microinjected 200-nm FMNP, n = 78 cells for 5 min after 2-min stress from four independent experiments, n = 110 cells for 30 min after 2-min stress, n=79 cells for 5 min after 10-min stress, and n=63 cells for 30 min after 10-min stress from three independent experiments for a 4-μm RGD-coated bead at cell surface receptors. P = 1.38×10−11 between 0-min stress and 5 min after 2-min stress; P = 1.68×10−5 between 0 min and 30 min after 2-min stress; P = 8.76×10−8 between 0-min stress and 30 min after 10-min stress, P = 0.04 between 5 and 30 min after 2-min stress for anti-H2B coated FMNP; P = 4.49×10−8 between 0-min stress and 30 min after 2-min stress; P = 4.05×10−8 between 0-min stress and 30 min after 10-min stress, for a 4-μm RGD-coated bead at cell surface receptors. (C) Representative image of brightfields (BF) and immunofluorescence (used both Anti-RNA polymerase II CTD repeat YSPTSPS (phospho S2) and Anti-Rabbit IgG H&L (Alexa Fluor® 555) antibody) of RNA Polymerase II Ser 2 phosphorylation (red) in CHO cell nucleus at 0-min (no stress) or 5 min after, 2- or 10-min stress (5 Pa at 0.3 Hz) for an uncoated 200-nm microinjected FMNP. Scale bars, 5 μm. (D) Normalized RNA Polymerase II Ser 2 phosphorylation fluorescence intensity 0-min stress, 5 and 60 min after 2- or 10-min stress for uncoated 200 nm microinjected FMNP for Anti-RNA polymerase II (phospho S2) or Anti-Rabbit IgG H&L in CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 80 cells for 0 min, n = 28 cells for 5 min after 2-min stress, n = 25 cells for 5 min after 10-min stress, n=24 cells for 60 min after 2-min stress, and n=22 cells for 60 min after 10-min stress from three independent experiments for uncoated 200-nm FMNP. One-way ANOVA with Tukey and Mann-Whitney test was used.
Figure S7. (A) Displacements between two DHFR-GFP spots in the same chromatin domain of CHO cell nucleus grown on a 1 kPa, 10 kPa, or 20 kPa stiffness Polyacrylamide (PA) gel substrate for anti-H2B-ab coated microinjected 200-nm FMNP with stress (5 Pa at 0.3 Hz). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 13 cells for 26 DHFR-GFP spots for 1 kPa, n = 15 cells for 30 DHFR-GFP spots for 10 kPa, n = 14 cells for 28 DHFR-GFP spots for anti-H2B-ab coated NP with stress from three independent experiments. P= 0.002 between chromatin displacements for cells grown on 10-kPa and 20-kPa substrate stiffness; P= 1.39×10−4 between chromatin displacements for cells grown on 1-kPa and 20-kPa substrate stiffness for anti-H2B-ab coated microinjected FMNP. (B) Diffusion coefficient (quantified by the best fit of time vs mean square displacement (MSD) of DHFR-GFP spot movements) of DHFR-GFP at 0 min stress for control (no microinjection), only medium microinjection (microinjection of medium only without 200-nm FMNP), and medium+FMNP microinjection (microinjection of anti-H2B-ab coated 200-nm FMNP). Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 33 cells with 33 DHFR-GFP spots for control, n=28 cells with 34 DHFR-GFP spots for medium only microinjection, n=23 cells with 24 DHFR-GFP spots for medium+FMNP microinjection from three independent experiments. (C) Quantified nuclear stiffness (Nucleus Young’s modulus) for control, medium only, and medium+FMNP microinjected CHO cell nucleus. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 11 cells for control, n = 13 cells for only medium microinjection, and n = 18 cells for anti-H2B-ab coated FMNP microinjection from three independent experiments. (D) Representative images of transmission electron microscopy (TEM) from three groups of cell nuclei: control (left, intact cell nuclei with no microinjection), medium (middle, cell nuclei microinjected with medium without any FMNP), or medium+FMNP (right, cell nuclei microinjected with medium plus an anti-H2B-ab coated ferromagnetic nanoparticle (NP) (highlighted by a black arrow and within yellow dashed lines). Each group in a column has four different CHO cells. Note that there is elevated intensity of gray contrast surrounding each NP, suggesting the presence of chromatin aggregation. Scale bar, 2 μm. (E) Diffusion coefficient (quantified by the best fit of time vs mean square displacement (MSD) of DHFR-GFP spot movements) of DHFR-GFP at no-stress conditions for regular CHO cell medium, 20-mM glucose containing medium, 20-mM 2-deoxy-D-glucose containing medium, or 10-μM Antimycin A containing medium. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 23 cells of 27 DHFR-GFP spots for regular medium, n = 25 cells of 37 DHFR-GFP spots for 20-mM glucose medium, n = 23 cells of 28 DHFR-GFP spots for 20-mM 2-deoxy-D-glucose medium, and n = 19 cells of 23 DHFR-GFP spots for 10 μM Antimycin A medium from three independent experiments. P = 4.20×10−4 for DHFR-GFP diffusivity between regular and 20-mM 2-deoxy-D-glucose medium, P = 2.17×10−4 for DHFR-GFP diffusivity between regular and 10 μM Antimycin A medium. Note that the DHFR-GFP spot diffusivity was reduced by 49% for 20-mM 2-deoxy-D-glucose medium and 47.6% in 10-μM Antimycin A medium. (F) Diffusion coefficient of uncoated 200-nm FMNP at no-stress conditions for various control conditions and ATP-depleted conditions. Data shown as boxplot with values of minimum, 5% percentile, 25% percentile, median, 75% percentile, maximum, and mean. n = 22 uncoated FMNP for regular medium, n = 25 uncoated FMNP for 20-mM glucose medium, n = 22 uncoated FMNP for 20-mM 2-deoxy-D-glucose medium, and n = 23 uncoated FMNP for 10 μM Antimycin A medium from three independent experiments. P = 1.13×10−4 for uncoated FMNP diffusivity between regular medium and 20-mM 2-deoxy-D-glucose medium; P = 1.37×10−5 for uncoated FMNP diffusivity between regular and 10-μM Antimycin A medium. Note that the uncoated FMNP diffusivity was reduced by 30.5% for 20-mM 2-deoxy-D-glucose medium and 38.2% in 10-μM Antimycin A medium. One-way ANOVA with Tukey and Mann-Whitney test was used.
Data Availability Statement
All data that are needed to evaluate the conclusions in the current study are shown in the paper and the supplemental information.
The MATLAB codes used for this research are available on GitHub (https://github.com/fazlurr2/Mechanomemory_FMNP_GFP_Pol-II.git).
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.


