Abstract
Unprotected alicyclic amines undergo α-C–H bond phosphonylation via a two-stage one-pot process involving the oxidation of amine-derived lithium amides with simple ketone oxidants, generating transient imines which are then captured with phosphites or phosphine oxides. Amines with an existing α-substituent undergo regioselective α’-phosphonylation. Amine α-arylation and α’-phosphonylation can be combined, generating a difunctionalized product in a single operation.
Graphical Abstract

α-Aminophosphonic acids and related phosphorous-containing surrogates of α-amino acids have attracted significant interest as synthetic targets, largely due to the biological activities exhibited by these materials.1–3 Of the various strategies devised to synthesize α-aminophosphonates and α-amino phosphine oxides,2 the Kabachnik-Fields reaction4 — a three-component reaction between amines, aldehydes/ketones, and phosphonates — is typically considered to be one of the most useful methods (Scheme 1a). However, due to the difficulty in accessing enolizable cyclic imines, the synthesis of α-aminophosphonates and α-amino phosphine oxides in which the α-P-substituent is placed on the ring of a cyclic amine is difficult to achieve via the traditional Kabachnik-Fields reaction. A highly attractive alternative to the synthesis of these cyclic α-aminophosphonates is based on the α-C–H bond functionalization of cyclic amines.5,6 Such reactions have been achieved via cross-dehydrogenative coupling (CDC),7 photoredox catalysis,8 and electrochemical methods9 (Scheme 1b). Traditionally, these methods have largely been limited to acyclic N,N-dialkylanilines and N-aryl tetrahydroisoquinolines, although recent advances have extended the scope to the synthesis of N-benzoyl protected α-aminophosphonates and α-amino phosphine oxides.8i A mechanistically distinct approach to cyclic N-benzyl α-aminophosphonates and α-amino phosphine oxides was introduced by our group.10 Utilizing the same type of starting materials as in classic Kabachnik-Fields reactions, in combination with catalytic amounts of benzoic acid, this redox-neutral method combines a reductive N-benzylation with an oxidative α-phosphonylation (Scheme 1c). While requiring pre-functionalized substrates, decarboxylative methods with protected α-amino acids have also been developed (Scheme 1 d).11,12 What nearly all methods mentioned thus far have in common is that they generate protected or tertiary aminophos-phonates and are incapable of directly producing unprotected alicyclic products. Unprotected cyclic products have been obtained from nonenolizable imines such as tetrahydroisoquinoline9d, 13 and from imine trimers such as the trimer of 1-piperideine (Scheme 1e).14 Here we report a method for the one-pot installation of phosphorous-containing functionalities onto the α-position of unprotected alicyclic amines (Scheme 1f). A unique feature of our method is that it enables the regioselective α’-functionalization of alicyclic amines with an existing α-substituent.
Scheme 1.

Strategies for amine α-phosphonylation
Based on pioneering work by Wittig and coworkers many decades ago,15 we recently established a new strategy for amine α-C–H bond functionalization where lithium amides 1 engage a ketone oxidant to form a transient imine 2 in addition to a lithium alkoxide byproduct (Scheme 1f).16–18 Imines 2 were found to be valuable intermediates, engaging a broad range of organometallics in addition to nucleophiles such as β-ketoacids and TMSCN to provide α-functionalized products. A unique feature of this approach is that it enables the regioselective α’-functionalization of amines containing an existing α-substituent. To gauge whether a similar strategy would be suitable for α-phosphonylation, 4-benzylpiperidine and diethyl phosphite were evaluated as model substrates. Selected results of this survey are summarized in Table 1. Briefly, the reaction proved to be most sensitive to the amount of diethyl phosphite, with two equivalents being optimal (entry 3). Addition of the Lewis acid boron trifluoride diethyl etherate had no effect on the reaction outcome.
Table 1.
Reaction developmenta

| |||
|---|---|---|---|
| entry | x | additive | yield (%) |
| 1 | 1.2 | - | 45 |
| 2 | 1.5 | - | 64 |
| 3 | 2.0 | - | 65 (63b) |
| 4 | 3.0 | - | 61 |
| 5 | 1.5 | BF3•OEt2 (1.1 equiv) | 65 |
Reactions were performed with 0.5 mmol of 4-benzylpiperidine. Yields correspond to isolated yields of chromatographically purified product.
reaction was performed with 1.0 mmol of 4-benzylpiperidine.
With the optimized conditions in hand (Table 1, entry 3), the scope of the reaction was evaluated with regard to the amine component (Scheme 2). Simple alicyclic amines of different ring sizes all readily underwent α-phosphonylation (products 3b–3e). In case of pyrrolidine-derived product 3b, addition of trifluoroacetic acid was required to obtain appreciable yields, a strategy that we employed previously in cases where the initially formed lithium alkoxide interferes with the addition step.16d 1-Benzylpiperazine, while also a viable substrate, provided product 3f in low yield. Piperidines containing a substituent in the 4-position provided the corresponding α-phosphonylation products in acceptable yields and with excellent diastereoselectivities (products 3g–3i).
Scheme 2. Scope of aminesa.
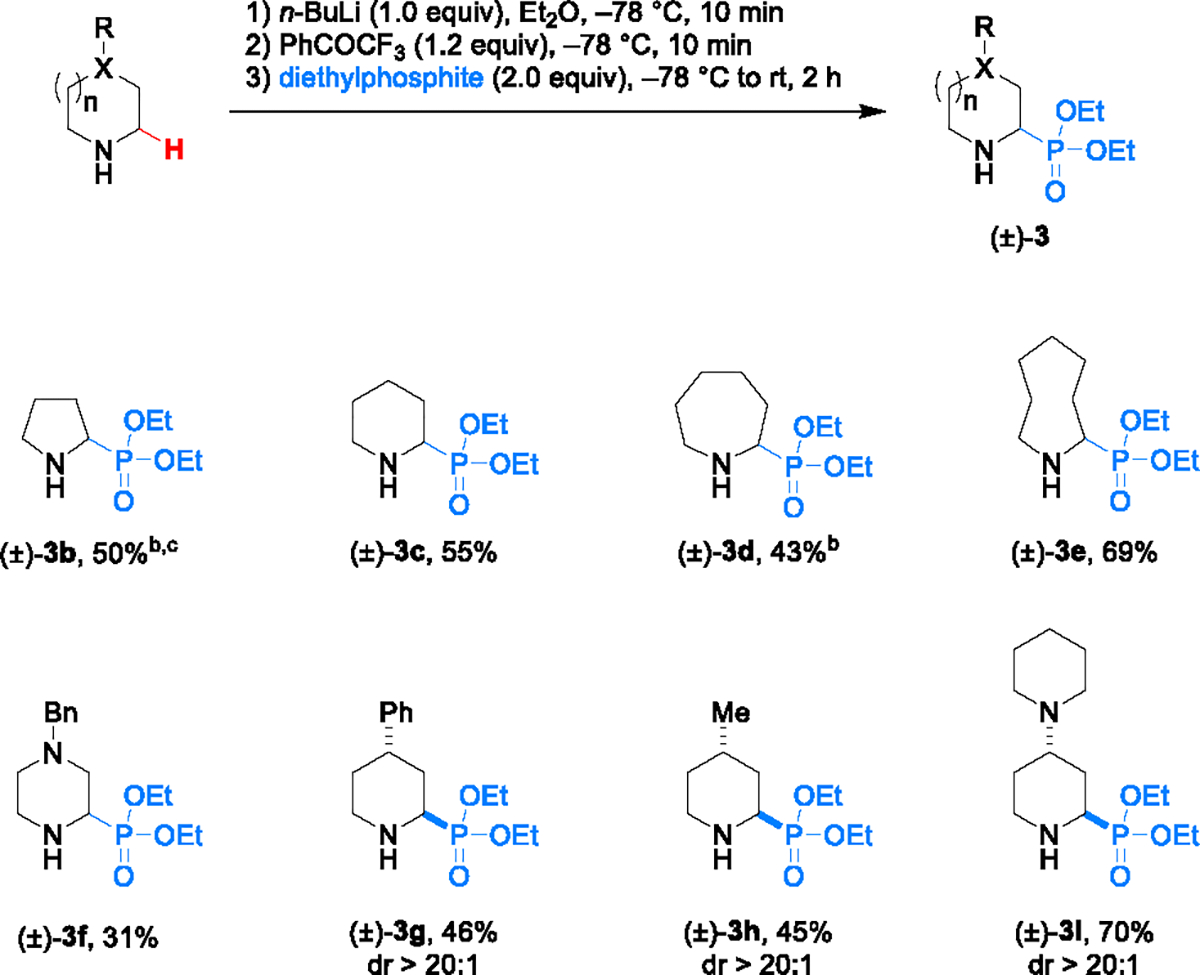
a Reactions were performed with 0.5 mmol of the amine. Yields correspond to isolated yields of chromatographically purified product. b Benzophenone was used as the oxidant. c trifluoroacetic acid (1.05 equiv) was used as an additive (added after step 2).
Amines with an existing α-substituent underwent regioselective α’-phosphonylation, typically with excellent diastereoselectivities (Scheme 3).19 Electronically diverse substituents and other heterocycles (indole, thiophene) were well tolerated. In addition to α-substituted piperidines, α-substituted piperazines, azepanes, and pyrrolidines were also viable substrates. 4-Benzylpiperidine also underwent α-phosphonylation with different phosphites and diphenylphosphine oxide (Scheme 4). The method was further extended to a one-pot double C–H bond functionalization of piperidine (Scheme 5). Following deprotonation, oxidation, and α-arylation, a second oxidation of the intermediate lithium amide was triggered by addition of additional ketone oxidant. Finally, addition of diethyl phosphite facilitated the isolation of product 3m in acceptable overall yield. It should be noted that, with the exception of compounds 3c–3d, all products reported here represent previously unknown materials.20
Scheme 3. Scope of α-functionalized aminesa.

a Reactions were performed with 0.5 mmol of the amine. Yields correspond to isolated yields of chromatographically purified product. b 2,2,2-Trimethylacetophenone was used as the oxidant. c trifluoroacetic acid (1.05 equiv) was used as an additive (added after step 2).
Scheme 4. Scope of nucleophiles.

a Reactions were performed with 0.5 mmol of 4-benzylpiperidine. Yields correspond to isolated yields of chromatographically purified product.
Scheme 5.

One-pot double C–H bond functionalization
In conclusion, we have achieved facile α-phosphonylations of unprotected alicyclic amines. Azacycles with an existing α-substituent underwent regioselective α’-phosphonylation. α-Arylation can also be combined with α’-phosphonylation in a convenient one-pot process.
Supplementary Material
ACKNOWLEDGMENT
Financial support from the NIH–NIGMS (grant no. R35GM149246) is gratefully acknowledged. Mass spectrometry instrumentation was supported by grants from the NIH (S10OD021758–01A1 and S10OD030250–01A1). Fuchao Yu thanks the Program of the China Scholarship Council (201708535014), the National Natural Science Foundation of China (21961018), and the Plan for Funding Outstanding Young Talents of Yunnan Province.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and spectral data (PDF)
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
REFERENCES
- (1).Selected reviews on the bioactivity of α-aminophosphonic acids and related compounds: Kukhar VP; Hudson HR, Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity. John Wiley & Sons: Chichester, 2000;Lejczak B; Kafarski P, Biological Activity of Aminophosphonic Acids and Their Short Peptides. In Phosphorous Heterocycles I, Bansal RK, Ed. Springer Berlin Heidelberg: Berlin, Heidelberg, 2009; pp 31–63;Mucha A; Kafarski P; Berlicki L, Remarkable Potential of the alpha-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980; Abdou MM; O’Neill PM; Amigues E; Matziari M, Phosphinic acids: current status and potential for drug discovery. Drug Discovery Today 2019, 24, 916–929; Talma M; Maślanka M; Mucha A, Recent developments in the synthesis and applications of phosphinic peptide analogs. Bioorg. Med. Chem. Lett. 2019, 29, 1031–1042.
- (2).Selected reviews on the synthesis of α-aminophosphonic acids and related compounds: Ordóñez M; Rojas-Cabrera H; Cativiela C, An overview of stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron 2009, 65, 17–49; H. Kudzin Z; H. Kudzin M; Drabowicz J; V. Stevens C, Aminophosphonic Acids - Phosphorus Analogues of Natural Amino Acids. Part 1: Syntheses of alpha-Aminophosphonic Acids. Curr. Org. Chem. 2011, 15, 2015–2071;Ordóñez M; Viveros-Ceballos JL; Cativiela C; Sayago FJ, An update on the stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron 2015, 71, 1745–1784;Gazizov AS; Smolobochkin AV; Turmanov RA; Pudovik MA; Burilov AR; Sinyashin OG, Synthesis of Phosphaproline Derivatives: A Short Overview. Synthesis 2019, 51, 3397–3409;Varga PR; Keglevich G, Synthesis of α-Aminophosphonates and Related Derivatives; The Last Decade of the Kabachnik–Fields Reaction. Molecules 2021, 26, 2511; Luo Z; Ding J; Huang D; Wu X; Bi Y, Recent advances in three-component reactions of P(O)-H compounds. Tetrahedron Lett. 2022, 96, 153757;Moiseev DV, Phospha-Mannich reactions of phosphinous acids R2P–OH and their derivatives. Phosphorus, Sulfur, Silicon Relat. Elem. 2023, 198, 867–923;Varga PR; Keglevich G, The Last Decade of Optically Active alpha-Aminophosphonates. Molecules 2023, 28, 6150.
- (3).Selected general reviews on the relevance of azacycles in medicine: Taylor RD; MacCoss M; Lawson ADG, Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859; Vitaku E; Smith DT; Njardarson JT, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274.
- (4).Fields EK, The Synthesis of Esters of Substituted Amino Phosphonic Acids. J. Am. Chem. Soc. 1952, 74, 1528–1531;Kabachnik MI; Medved TY, New method for the synthesis of 1-aminoalkylphosphonic acids Communication 1. Russ. Chem. Bull. 1953, 2, 769–777. For selected reviews, see: Zefirov NS; Matveeva ED, Catalytic Kabachnik-Fields reaction: new horizons for old reaction. ARKIVOC 2008, 1–17;Keglevich G; Bálint E, The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules 2012, 17, 12821–12835.
- (5).Yuanting Huang QC, Recent Advances in C(sp3)–H Phosphorylation Based on Secondary Phosphine Oxides and Phosphite Esters. Chinese J. Org. Chem. 2021, 41, 4138–4153. [Google Scholar]
- (6).For a general overview of amine C–H bond functionalization, see: Dutta S; Li B; Rickertsen DRL; Valles DA; Seidel D, C–H Bond Functionalization of Amines: A Graphical Overview of Diverse Methods. SynOpen 2021, 05, 173–228. Other selected reviews:Campos KR, Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 2007, 36, 1069–1084; Jazzar R; Hitce J; Renaudat A; Sofack-Kreutzer J; Baudoin O, Functionalization of Organic Molecules by Transition-Metal-Catalyzed C(sp3)–H Activation. Chem. Eur. J. 2010, 16, 2654–2672; Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW, Direct alpha-Functionalization of Saturated Cyclic Amines. Chem. Eur. J. 2012, 18, 10092–10142; Peng B; Maulide N, The Redox-Neutral Approach to C–H Functionalization. Chem. Eur. J. 2013, 19, 13274–13287; Girard SA; Knauber T; Li C-J, The Cross-Dehydrogenative Coupling of C sp3-H Bonds: A Versatile Strategy for C–C Bond Formations. Angew. Chem. Int. Ed. 2014, 53, 74–100;Haibach MC; Seidel D, C–H Bond Functionalization through Intramolecular Hydride Transfer. Angew. Chem. Int. Ed. 2014, 53, 5010–5036;Beatty JW; Stephenson CRJ, Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res. 2015, 48, 1474–1484; Cheng M-X; Yang S-D, Recent Advances in the Enantioselective Oxidative α-C–H Functionalization of Amines. Synlett 2017, 28, 159–174;Chu JCK; Rovis T, Complementary Strategies for Directed C(sp3)–H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed. 2018, 57, 62–101;Stateman LM; Nakafuku KM; Nagib DA, Remote C–H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569–1586; Edwards PM; Schafer LL, Early transition metal-catalyzed C–H alkylation: hydroaminoalkylation for C sp3–C sp3 bond formation in the synthesis of selectively substituted amines. Chem. Commun. 2018, 54, 12543–12560;Gonnard L; Guérinot A; Cossy J, Transition metal-catalyzed α-alkylation of amines by C(sp3)–H bond activation. Tetrahedron 2019, 75, 145–163;Liu S; Zhao Z; Wang Y, Construction of N-Heterocycles through Cyclization of Tertiary Amines. Chem. Eur. J. 2019, 25, 2423–2441; Antermite D; Bull JA, Transition Metal-Catalyzed Directed C(sp3)–H Functionalization of Saturated Heterocycles. Synthesis 2019, 51, 3171–3204;Zhang T; Wu Y-H; Wang N-X; Xing Y, Advances in C(sp3)–H Bond Functionalization via Radical Processes. Synthesis 2019, 51, 4531–4548;Trowbridge A; Walton SM; Gaunt MJ, New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev. 2020, 120, 2613–2692; Kapoor M; Singh A; Sharma K; Hua Hsu M, Site-Selective C(sp3)–H and C(sp2)–H Functionalization of Amines Using a Directing-Group-Guided Strategy. Adv. Synth. Catal. 2020, 362, 4513–4542;An X-D; Xiao J, Recent advances in hydride transfer-involved C(sp3)–H activation reactions. Org. Chem. Front. 2021, 8, 1364–1383;Basak S; Winfrey L; Kustiana BA; Melen RL; Morrill LC; Pulis AP, Electron deficient borane-mediated hydride abstraction in amines: stoichiometric and catalytic processes. Chem. Soc. Rev. 2021, 50, 3720–3737; Caplin MJ; Foley DJ, Emergent synthetic methods for the modular advancement of sp3-rich fragments. Chem. Sci. 2021, 12, 4646–4660; Ohno S; Miyoshi M; Murai K; Arisawa M, Non-Directed β- or γ-C(sp3)–H Functionalization of Saturated Nitrogen-Containing Heterocycles. Synthesis 2021, 53, 2947–2960;He Y; Zheng Z; Yang J; Zhang X; Fan X, Recent advances in the functionalization of saturated cyclic amines. Org. Chem. Front. 2021, 8, 4582–4606;Chen W; Seidel D, Condensation-Based Methods for the C–H Bond Functionalization of Amines. Synthesis 2021, 53, 3869–3908; Deb ML; Saikia BS; Borpatra PJ; Baruah PK, Progress of Metal-Free Visible-Light-Driven α-C–H Functionalization of Tertiary Amines: A Decade Journey. Asian J. Org. Chem. 2022, 11, e202100706;Chen W; Yang X; Cao X, Transition-Metal-Catalyzed Remote C–H Bond Functionalization of Cyclic Amines. SynOpen 2022, 06, 286–305;Chen W; Cao X; Yang X, Transition-Metal-Free Methods for the Remote C–H Bond Functionalization of Cyclic Amines. Asian J. Org. Chem. 2023, 12, e202200547;Ray N; Jana CK, Iminium and azonium-activated metal and oxidant-free C–H functionalization of aliphatic amines. Chem. Commun. 2023, 59, 8504–8519.
- (7).Selected examples of α-C–H phosphonylations of (protected) amines via CDC: Basle O; Li CJ, Copper-catalyzed aerobic phosphonation of sp3 C-H bonds. Chem. Commun. 2009, 4124–4126;Han W; Ofial AR, Iron-catalyzed dehydrogenative phosphonation of N,N-dimethylanilines. Chem. Commun. 2009, 6023–6025;Han W; Mayer P; Ofial AR, Iron-Catalyzed Oxidative Mono- and Bis-Phosphonation of N,N-Dialkylanilines. Adv. Synth. Catal. 2010, 352, 1667–1676;Xie J; Li H; Xue Q; Cheng Y; Zhu C, A Scalable, Efficient Gold-Catalyzed Oxidative Phosphonation of sp3 C–H Bonds using Air as Sustainable Oxidant. Adv. Synth. Catal. 2012, 354, 1646–1650;Alagiri K; Devadig P; Prabhu KR, CDC Reactions of N-Aryl Tetrahydroisoquinolines Using Catalytic Amounts of DDQ: C–H Activation under Aerobic Conditions. Chem. Eur. J. 2012, 18, 5160–5164; Wang H; Li X; Wu F; Wan B, Direct oxidative phosphonylation of amines under metal-free conditions. Tetrahedron Lett. 2012, 53, 681–683;Alagiri K; Devadig P; Prabhu KR, Molybdenum trioxide catalyzed oxidative cross-dehydrogenative coupling of benzylic sp3 C–H bonds: synthesis of α-aminophosphonates under aerobic conditions. Tetrahedron Lett. 2012, 53, 1456–1459;Dhineshkumar J; Lamani M; Alagiri K; Prabhu KR, A Versatile C–H Functionalization of Tetrahydroisoquinolines Catalyzed by Iodine at Aerobic Conditions. Org. Lett. 2013, 15, 1092–1095; Patil MR; Dedhia NP; Kapdi AR; Kumar AV, Cobalt(II)/N-Hydroxyphthalimide-Catalyzed Cross-Dehydrogenative Coupling Reaction at Room Temperature under Aerobic Condition. J. Org. Chem. 2018, 83, 4477–4490; Lin B; Shi S; Lin R; Cui Y; Fang M; Tang G; Zhao Y, Cobalt-Catalyzed Oxidative C(sp3)–H Phosphonylation for α-Aminophosphonates via C(sp3)–H/P(O)–H Coupling. J. Org. Chem. 2018, 83, 6754–6761.
- (8).Selected examples of α-C–H phosphonylations of (protected) amines via photocatalysis: Rueping M; Zhu SQ; Koenigs RM, Photoredox catalyzed C–P bond forming reactions-visible light mediated oxidative phosphonylations of amines. Chem. Commun. 2011, 47, 8679–8681;Hari DP; Koenig B, Eosin Y Catalyzed Visible Light Oxidative C–C and C–P bond Formation. Org. Lett. 2011, 13, 3852–3855; Xue QC; Xie J; Jin HM; Cheng YX; Zhu CJ, Highly efficient visible-light-induced aerobic oxidative C–C, C–P coupling from C–H bonds catalyzed by a gold(III)-complex. Org. Biomol. Chem. 2013, 11, 1606–1609; Rueping M; Vila C; Bootwicha T, Continuous Flow Organocatalytic C–H Functionalization and Cross-Dehydrogenative Coupling Reactions: Visible Light Organophotocatalysis for Multicomponent Reactions and C–C, C–P Bond Formations. ACS Catal. 2013, 3, 1676–1680;To W-P; Liu Y; Lau T-C; Che C-M, A Robust Palladium(II)–Porphyrin Complex as Catalyst for Visible Light Induced Oxidative C–H Functionalization. Chem. Eur. J. 2013, 19, 5654–5664; Wang X-Z; Meng Q-Y; Zhong J-J; Gao X-W; Lei T; Zhao L-M; Li Z-J; Chen B; Tung C-H; Wu L-Z, The singlet excited state of BODIPY promoted aerobic cross-dehydrogenative-coupling reactions under visible light. Chem. Commun. 2015, 51, 11256–11259;Niu L; Wang S; Liu J; Yi H; Liang X-A; Liu T; Lei A, Visible light-mediated oxidative C(sp3)–H phosphonylation for α-aminophosphonates under oxidant-free conditions. Chem. Commun. 2018, 54, 1659–1662;Shao A; Chen J; Wang L; Yi M; Yang H; Zhang Y; Fan S; Chen S; Wu H; Shi R, Excited-state cobaloxime catalysis enabled scalable oxidant-free dehydrogenative C–H phosphinoylation of undirected heterocycles. Org. Chem. Front. 2022, 9, 4379–4387;Lei Z; Zhang W; Wu J, Photocatalytic Hydrogen Atom Transfer-Induced Arbuzov-Type α-C(sp3)–H Phosphonylation of Aliphatic Amines. ACS Catal. 2023, 13, 16105–16113.
- (9).Selected examples of α-C–H phosphonylations of (protected) amines via electrochemical methods: Bidan G; Genies M, Utilisation en synthese du cation iminium genere in situ par oxydation electrochimique d’amines tertiaires. Tetrahedron 1981, 37, 2297–2301;Baslé O; Borduas N; Dubois P; Chapuzet JM; Chan T-H; Lessard J; Li C-J, Aerobic and Electrochemical Oxidative Cross-Dehydrogenative-Coupling (CDC) Reaction in an Imidazolium-Based Ionic Liquid. Chem. Eur. J. 2010, 16, 8162–8166; Xie W; Liu N; Gong B; Ning S; Che X; Cui L; Xiang J, Electrochemical Cross-Dehydrogenative Coupling of N-Aryl-tetrahydroisoquinolines with Phosphites and Indole. Eur. J. Org. Chem. 2019, 2019, 2498–2501;Huang M; Dai J; Cheng X; Ding M, Electrochemical Approach for Direct C–H Phosphonylation of Unprotected Secondary Amine. Org. Lett. 2019, 21, 7759–7762; Ollivier A; Sengmany S; Rey M; Martens T; Léonel E, Direct Phosphonylation of N-Carbamate-tetrahydroisoquinoline by Convergent Paired Electrolysis. Synlett 2020, 31, 1191–1196;Gao J; Weng X; Ma C; Xu X; Fang P; Mei T, Electrochemical 2,2,6,6-tetramethylpiperidinyl-N-oxyl (TEMPO)-Mediated α-Cyanation and Phosphonylation of Cyclic Amines with Metal-Free Conditions. Chin. J. Org. Chem. 2021, 41, 3223–3234.
- (10).Das D; Seidel D, Redox-Neutral α-C–H Bond Functionalization of Secondary Amines with Concurrent C–P Bond Formation/N-Alkylation. Org. Lett. 2013, 15, 4358–4361. See also: Hu G; Chen W; Ma D; Zhang Y; Xu P; Gao Y; Zhao Y, Silver-Catalyzed, Aldehyde-Induced α-C–H Functionalization of Tetrahydroisoquinolines with Concurrent C–P Bond Formation/N-Alkylation. J. Org. Chem. 2016, 81, 1704–1711; Li X; Xie Y; Yin K; Shen R; Zhu D, p-Quinol Ethers and p-Quinone Monoacetals as Arylation and Oxidation Reagents: Tandem N-Arylation and α-Functionalization of Pyrrolidine via Redox-Neutral Three-Component Reaction. Synthesis 2022, 54, 2574–2584. For an overview of other reactions of this type, see reference 6x.
- (11).Selected examples of related decarboxylative α-phosphonylations: Boto A; Gallardo JA; Hernández R; Saavedra CJ, One-pot synthesis of α-amino phosphonates from α-amino acids and β-amino alcohols. Tetrahedron Lett. 2005, 46, 7807–7811;Hu J; Zhao N; Yang B; Wang G; Guo L-N; Liang Y-M; Yang S-D, Copper-Catalyzed C–P Coupling through Decarboxylation. Chem. Eur. J. 2011, 17, 5516–5521; Reich D; Noble A; Aggarwal VK, Facile Conversion of α-Amino Acids into α-Amino Phosphonates by Decarboxylative Phosphorylation using Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2022, 61, e202207063.
- (12).For a three-component decarboxylative approach, see: Yang D; Zhao D; Mao L; Wang L; Wang R, Copper/DIPEA-Catalyzed, Aldehyde-Induced Tandem Decarboxylation-Coupling of Natural α-Amino Acids and Phosphites or Secondary Phosphine Oxides. J. Org. Chem. 2011, 76, 6426–6431; Firouzabadi H; Iranpoor N; Ghaderi A; Ghavami M, Cerium(IV) oxide as a neutral catalyst for aldehyde-induced decarboxylative coupling of l-proline with triethyl phosphite and nitromethane. Tetrahedron Lett. 2012, 53, 5515–5518;Kaboudin B; Karami L; Kato JY; Aoyama H; Yokomatsu T, A catalyst-free, three-component decarboxylative coupling of amino acids with aldehydes and H-dialkylphosphites for the synthesis of α- aminophosphonates. Tetrahedron Lett. 2013, 54, 4872–4875;Wang X; Zhang C; Shen R; Han L-B, Three-Component Reactions of α-Amino Acids, p-Quinone Monoacetals, and Diarylphosphine Oxides to Selectively Afford 3-(Diarylphosphinyl)anilides and N-Aryl-2-diarylphosphinylpyrrolidines. J. Org. Chem. 2020, 85, 14753–14762.
- (13).(a) Gross H; Ozegowski S, α-Substituierte Phosphonate. 43. Synthese und Reaktivität von 1,2,3,4-Tetrahydroisochinolin-1-phosphonaten. J. Prakt. Chem. 1983, 325, 437–445; [Google Scholar]; (b) Hernández-Moreno JT; Romero-Estudillo I; Cativiela C; Ordóñez M, Practical Synthesis of 1,2,3,4-Tetrahydroisoquinoline-1-phosphonic and 1-phosphinic Acids through Kabachnik–Fields and Aza-Pudovik Reaction. Synthesis 2020, 52, 769–774. [Google Scholar]
- (14).Couture A; Deniau E; Grandclaudon P; Lebrun S, Dramatically different photochemical behaviour of 1-aroyl-2-methylene piperidine and pyrrolidine derivatives. An expeditious synthesis of ruspolinone. Tetrahedron Lett. 1996, 37, 7749–7752. [Google Scholar]
- (15).For an early review, see: Majewski M; Gleave DM, Reduction with lithium dialkylamides. J. Organomet. Chem. 1994, 470, 1–16. Selected key contributions:Wittig G; Schmidt HJ; Renner H, Über Lithium-diäthylamid als Hydrid-Donator. Chem. Ber. 1962, 95, 2377–2383;Wittig G; Hesse A, Zur Reaktionsweise N-metallierter acyclischer und cyclischer sekundärer Amine. Liebigs Ann. Chem. 1971, 746, 149–173;Wittig G; Hesse A, Hydrid-Übertragung von Lithium-pyrrolidid auf Azomethine. Liebigs Ann. Chem. 1971, 746, 174–184;Wittig G; Häusler G, Über die Reaktivität von metallierten Aminen als Hydrid-Donatoren. Liebigs Ann. Chem. 1971, 746, 185–199.
- (16).(a) Chen W; Ma L; Paul A; Seidel D, Direct α-C–H bond functionalization of unprotected cyclic amines. Nat. Chem. 2018, 10, 165; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Paul A; Seidel D, α-Functionalization of Cyclic Secondary Amines: Lewis Acid Promoted Addition of Organometallics to Transient Imines. J. Am. Chem. Soc. 2019, 141, 8778–8782; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen W; Paul A; Abboud KA; Seidel D, Rapid functionalization of multiple C–H bonds in unprotected alicyclic amines. Nat. Chem. 2020, 12, 545–550; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Paul A; Kim JH; Daniel SD; Seidel D, Diversification of Unprotected Alicyclic Amines by C–H Bond Functionalization: Decarboxylative Alkylation of Transient Imines. Angew. Chem. Int. Ed. 2021, 60, 1625–1628; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kim JH; Paul A; Ghiviriga I; Seidel D, α-C–H Bond Functionalization of Unprotected Alicyclic Amines: Lewis-Acid-Promoted Addition of Enolates to Transient Imines. Org. Lett. 2021, 23, 797–801; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen W; Seidel D, α-C–H/N–H Annulation of Alicyclic Amines via Transient Imines: Preparation of Polycyclic Lactams. Org. Lett. 2021, 23, 3729–3734; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Valles DA; Dutta S; Paul A; Abboud KA; Ghiviriga I; Seidel D, α,α′-C–H Bond Difunctionalization of Unprotected Alicyclic Amines. Org. Lett. 2021, 23, 6367–6371; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Paul A; Vasseur C; Daniel SD; Seidel D, Synthesis of Polycyclic Isoindolines via α-C–H/N–H Annulation of Alicyclic Amines. Org. Lett. 2022, 24, 1224–1227; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yu F; Valles DA; Chen W; Daniel SD; Ghiviriga I; Seidel D, Regioselective α-Cyanation of Unprotected Alicyclic Amines. Org. Lett. 2022, 24, 6364–6368; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Dutta S; Bhatt K; Cuffel F; Seidel D, Synthesis of Polycyclic Imidazoles via α-C–H/N–H Annulation of Alicyclic Amines. Synthesis 2023, 55, 2343–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).For an excellent recent review on organic oxidants serving as hydride acceptors, see: Miller JL; Lawrence J-MIA; Rodriguez del Rey FO; Floreancig PE, Synthetic applications of hydride abstraction reactions by organic oxidants. Chem. Soc. Rev. 2022, 51, 5660–5690.
- (18).For other selected approaches to the direct α-C–H bond functionalization of unprotected alicyclic amines, see: Payne PR; Garcia P; Eisenberger P; Yim JCH; Schafer LL, Tantalum Catalyzed Hydroaminoalkylation for the Synthesis of α- and β-Substituted N-Heterocycles. Org. Lett. 2013, 15, 2182–2185; Lennox AJJ; Goes SL; Webster MP; Koolman HF; Djuric SW; Stahl SS, Electrochemical Aminoxyl-Mediated α-Cyanation of Secondary Piperidines for Pharmaceutical Building Block Diversification. J. Am. Chem. Soc. 2018, 140, 11227–11231.
- (19).The complete loss of diastereoselectivity observed for product 3k may in part be due to a directing group effect of the silyl ether substituent.
- (20).This statement is based on a Reaxys search conducted on May 8, 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


