Summary
Background
Data on long-term neurodevelopmental outcomes of normocephalic children (born with normal head circumference) exposed to Zika virus in utero are scarce. We aimed to compare neurodevelopmental outcomes in normocephalic children up to age 48 months with and without Zika virus exposure in utero.
Methods
In this prospective cohort study, we included infants from two cohorts of normocephalic children born in León and Managua, Nicaragua during the 2016 Zika epidemic. In León, all women pregnant during the two enrolment periods were eligible. In Managua, mother–child pairs were included from three districts in the municipality of Managua: all women who became pregnant before June 15, 2016, and had a due date of Sept 15, 2016 or later were eligible. Infants were serologically classified as Zika virus-exposed or Zika virus-unexposed in utero and were followed up prospectively until age 48 months. At 36 months and 48 months of age, the Mullen Scales of Early Learning (MSEL) assessment was administered. Primary outcomes were MSEL early learning composite (ELC) scores at 30–48 months in León and 36–48 months in Managua. We used an inverse probability weighting generalised estimating equations model to assess the effect of Zika virus exposure on individual MSEL cognitive domain scores and ELC scores, adjusted for maternal education and age, poverty status, and infant sex.
Findings
The initial enrolment period for the León cohort was between Jan 31 and April 5, 2017 and the second was between Aug 30, 2017, and Feb 22, 2018. The enrolment period for the Managua cohort was between Oct 24, 2019, and May 5, 2020. 478 mothers (482 infants) from the León cohort and 615 mothers (609 infants) from the Managua cohort were enrolled, of whom 622 children (303 from the León cohort; 319 from the Managua cohort) were included in the final analysis; four children had microcephaly at birth and thus were excluded from analyses, two from each cohort. 33 (11%) of 303 children enrolled in León and 219 (69%) of 319 children enrolled in Managua were exposed to Zika virus in utero. In both cohorts, no significant differences were identified in adjusted mean ELC scores between Zika virus-exposed and unexposed infants at 36 months (between-group difference 1·2 points [95% CI –4·2 to 6·5] in the León cohort; 2·8 [–2·4 to 8·1] in the Managua cohort) or at 48 months (–0·9 [–10·8 to 8·8] in the León cohort; 0·1 [–5·1 to 5·2] in the Managua cohort). No differences in ELC scores between Zika virus-exposed and unexposed infants exceeded 6 points at any time between 30 months and 48 months in León or between 36 months and 48 months in Managua, which was considered clinically significant in other settings.
Interpretation
We found no significant differences in neurodevelopmental scores between normocephalic children with in-utero Zika virus exposure and Zika virus-unexposed children at age 36 months or 48 months. These findings are promising, supporting typical neurodevelopment in Zika virus-exposed normocephalic children, although additional follow-up and research is warranted.
Funding
National Institute of Child Health and Development, National Institute of Allergy and Infectious Diseases, and Fogarty International Center.
Introduction
The potential of Zika virus to cause anomalies in the developing fetus was identified during the 2015–17 Zika virus epidemic in the region of the Americas. Although Zika virus transmission in the Americas has declined markedly since that time, low-level transmission of Zika virus continues and new outbreaks have been reported in Cape Verde, Angola, and southeast Asia since 2017,1–3 highlighting the ongoing importance of understanding the impact of congenital Zika virus infections on child health and development. Several studies have reported on the persistent developmental delays in children diagnosed with congenital Zika syndrome.4–6 Other studies have reported on the neurodevelopmental outcomes among children exposed in utero to Zika virus but born without apparent disability or malformations.7–13 These studies have generally, although not exclusively, found that Zika virus-exposed infants had lower scores in various neurodevelopment domains in the first 24 months of life, including motor, social cognition, language, and fine motor skills than did infants without exposure to Zika virus.
In a previous study, we examined neurodevelopmental outcomes up to 24 months of age in a population-based sample of 129 children born to women who were pregnant during the peak of the Zika virus epidemic in León, Nicaragua; 25% of these women had incident Zika virus infection during their pregnancy.10 We found that at 24 months of age, children with in-utero Zika virus exposure had lower neurodevelopment scores than children who were not exposed to Zika virus, although these differences were not deemed clinically significant. In this study, we aimed to include a larger sample of children originating from two population-based cohorts in León and Managua (Nicaragua) and followed up children to age 48 months.
Previous studies, including our previous publication from the León cohort, have generally been restricted to the first 2 years of development.7,9–12,14–16 Identifying delays during this period is important for long-term developmental outcomes in affected children, since previous research has shown that early childhood interventions up to age 5 years can be effective in improving later neurodevelopmental outcomes in children with early delays.17 As children continue to grow and develop, a broader range of development skills can be assessed, and any longer term neurodevelopmental findings in these children would have implications regarding the need for continued therapeutic interventions beyond early infancy for children exposed to Zika virus in utero.18 This study aims to describe these outcomes in normocephalic children up to 48 months of age by comparing children with and without Zika virus exposure in utero.
Methods
Study design and cohorts
This prospective cohort study was done in the most populated cities of Nicaragua: León (approximately 200 000 inhabitants; population density G9·2 people per km2) and Managua (1·58 million inhabitants; 3G4·5 people per km2). No specific sample size restrictions were placed on enrolment, and all eligible women who consented to participate were enrolled.
We utilised an existing prospective cohort in León, Nicaragua,10 which offered enrolment to all pregnant women who attended prenatal care visits during study periods at clinics in the Perla Maria Norori Health Sector (León), which covers 40% of the population of the municipality. The initial enrolment period was between Jan 31 and April 5, 2017, and included women who were pregnant during the Zika virus epidemic that occurred between June and September, 2016.19 Women without an established Zika virus serostatus or who had unknown timing of infection were excluded.
There was a second enrolment period in the same setting between Aug 30, 2017 and Feb 22, 2018. This second enrolment period was undertaken to increase the unexposed sample size for comparison. All children of enrolled mothers without signs of congenital Zika syndrome were eligible for inclusion in the study. Differences in in length-for-age and weight-for-age Z scores based on WHO guidelines were used to measure malnutrition, as per WHO guidelines.19 Congenital Zika syndrome was defined by microcephaly at birth (head circumference <2 SDs the mean for age and sex); children with microcephaly were excluded from final analysis. During both study periods, eligible newborns were enrolled when their mother provided written consent for their child’s participation. Ethical approval was provided by institutional review boards for National Autonomous University of Nicaragua, León, and the University of North Carolina at Chapel Hill (UNAN-Leon IRB Acta No 30, 2017, UNC-CH IRB Study 17–095, 2018).
Women were also enrolled from an existing prospective cohort of mother–child pairs from three districts in the municipality of Managua, which included women who became pregnant before June 15, 2016, and had a due date of Sept 15, 2016 or later, with the same eligibility criteria as the León cohort. Women and children were originally enrolled between 9 and 15 months after birth with follow-up visits at 12 and 24 months. Children were enrolled and were followed up with Mullen Scales of Early Learning (MSEL) evaluations at 3G months and 48 months of age, carried out by trained study personnel in consulting rooms within the ministry of health centres. Ethical approval was provided by the Nicaraguan Ministry of Health (NIC-MINSA/CNDR CIRE IRB 00005231).
Procedures
At the time of enrolment, data on household characteristics and maternal health histories were obtained. Trained fieldwork personnel subsequently conducted study visits to the children’s homes, where they obtained child health histories and applied the MSEL assessment every 3 months until 24 months of life and then every G months until 48 months of life. In the León cohorts, the serostatus and timing of incident Zika virus infection were determined using IgM and IgG ELISAs, non-structural protein 1 blockade of binding (NS1 BOB) assays, and foci reduction neutralisation tests. The serological methods for ascertaining the timing of maternal infection in this dengue-endemic region have been described in detail previously.10 Women enrolled between Aug 30, 2017 and Feb 22, 2018 were not considered to be at risk for an incident Zika virus infection during the course of their pregnancy, because the majority of active Zika virus transmission in the region had ceased by the beginning of this period.20 Women were designated as either Zika virus-naive or pre-immune at the start of their pregnancy, and their children were classified as Zika virus-unexposed in the final analysis.
Women enrolled in the Managua cohort were pregnant during the period when more than 80% of PCR-confirmed Zika virus infections in Nicaragua were recorded.20 Considering the low incidence of Zika virus infection during the dry season (March–April), Zika virus seropositivity was considered a reasonable indication of Zika virus infection during pregnancy.21 We used a combination of the Zika virus NS1 BOB assay and a series of custom 5-plex and 10-plex Luminex assays (Luminex, Austin, TX, USA) to determine Zika virus seropositivity.21,22 The capacity of the Zika virus NS1 BOB assay to discriminate Zika virus infection from other flaviviruses is described elsewhere.21
The primary endpoint was MSEL early learning composite score. The secondary endpoints were MSEL subdomain scores, assessed at 30 months in León and at 36 months and 48 months in León and Managua. Neurodevelopmental outcomes among children were assessed by trained psychologists or nurses using MSEL, a comprehensive performance-based test measuring five developmental domains consisting of gross motor, fine motor, expressive language, receptive language, and visual reception.18 The MSEL has excellent correspondence validity with the Bayley Scales of Infant Development and has been used with Spanish-speaking populations in Latin America.23,24 The raw scores for each domain are converted into a standardised t-score (age adjusted), and the t-scores from the four cognitive domains (fine motor, expressive language, receptive language, and visual reception) are summed and converted into a corresponding t-score for the early learning composite (ELC) score. In previous studies, a 6-point difference in MSEL scores has been considered clinically significant.25 The standardised t-scores correspond to percentile rankings, with children scoring in the top and bottom 2% of t-scores categorised as very high and very low for a given measure, respectively, while children scoring in the highest or lowest 15% of t-scores are categorised as above average and below average. Verbal directions for the MSEL assessment were given in Spanish by field staff, who were trained in situ and followed up by monthly virtual teleconferences on MSEL administration by experienced co-authors (IF and MJB). MSEL assessments were conducted at participant home visits for children in the León cohort, and at the health centres for children in the Managua cohort. Timepoints for assessments were within a 3-month range and were not affected by the COVID-19 pandemic.
We adapted a household poverty index developed by the Center for Investigation of Demography and Health for use in Nicaragua.26 The index was calculated as a composite score, with information on parental education, parental employment, housing construction materials, water supply and sanitary conditions in the home. The index is comprised of a score of 0–3 based on these domains; study participants were classified as living in poverty if their household received a score of 2 or greater based on participant survey responses, and that basic needs are met if the household received a score of 0–1.26
Statistical analysis
An inverse probability weighting generalised estimating equations model was used to assess the effect of Zika virus exposure on individual MSEL cognitive domain scores and ELC scores. Stabilised inverse probability weights were constructed by estimating the probability of exposure in each cohort overall and for each individual, accounting for baseline covariates (maternal education and age at the time of birth, poverty status, and infant sex) via logistic regression. An autoregressive correlation structure was specified at the individual level to account for repeated administrations of the MSEL assessment, and child age at follow-up visit was included in the model to allow for non-linear, longitudinal effects of Zika virus exposure on neuro-developmental outcomes. No adjustment was made for multiplicity in this analysis, and interaction terms were not included in the final model. Unadjusted mean MSEL scores and adjusted mean MSEL cognitive domain and ELC scores were calculated and plotted by exposure status and timepoint.
Study data were managed using REDCap electronic data capture tools hosted at the University of North Carolina at Chapel Hill (Chapel Hill, NC, USA)27 and analysed using R software (version 4.1). Generalised estimating equations were fit using the geepack package.28
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
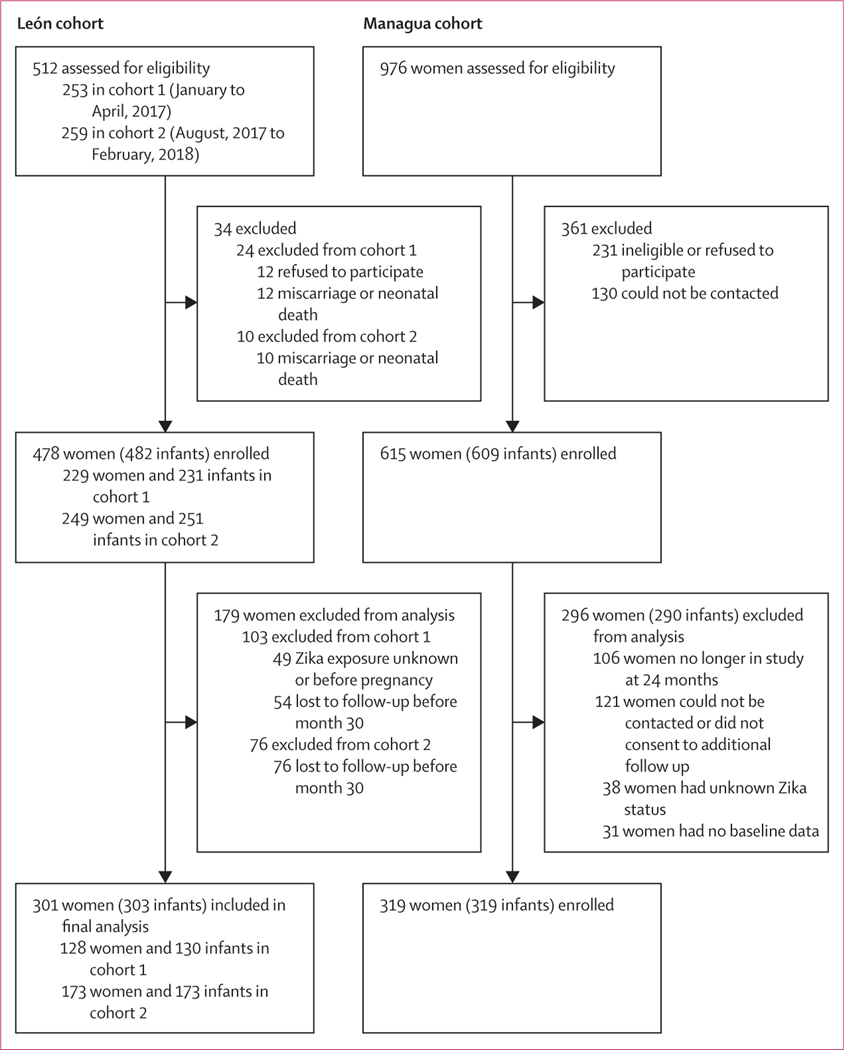
The initial enrolment period for the León cohort was between Jan 31 and April 5, 2017 and the second was between Aug 30, 2017 and Feb 22, 2018. 253 pregnant women were invited to participate during the first enrolment period and 259 women during the second enrolment period. Of these, 12 women declined to participate (first enrolment period), 22 had a miscarriage or neonatal death, and 130 were lost to follow-up before the 30-month MSEL assessment. An additional 32 women who enrolled during this period did not have an established Zika virus serostatus, and 17 had unknown timing of infection, thus 128 women were eligible to have their children included in the final analysis. Thus, 130 children from León born to pregnant women enrolled during the initial enrolment period, including two pairs of twins, and 173 children from León born to pregnant women enrolled during the second enrolment period, including two pairs of twins, were included in the final analysis (figure 1). Of the 303 children included in the analysis from León, 33 (11%) were classified as likely to have been exposed to Zika virus in utero, and 270 (89%) were classified as unexposed to Zika virus.
Figure 1:
Study profile
In the Managua cohort, 615 mothers and their 609 children (three miscarriages, two stillbirths, seven infant deaths, and six sets of twins) were enrolled into the original study. 509 mother–child pairs remained in the study and were approached during the 24-month visit and asked if they would like to participate in the second phase with additional follow-up. During the second phase, 121 mother–child pairs did not sign the informed consent or were lost to follow-up. We included 319 children in the Managua cohort, 219 (69%) of whom were classified as having had in utero exposure to Zika virus, and 100 (31%) of whom were classified as unexposed to Zika virus.
In each of the two cohorts, one child with in-utero exposure to Zika virus and one child who was unexposed to Zika virus (n=4) were born with microcephaly (defined as head circumference ≤2 SD the mean for age and sex); these children were excluded from further analyses of neurodevelopmental outcomes. Thus, 622 children (303 from the León cohort; 319 from the Managua cohort) were included in the final analysis. Mean head circumference, child length for age, and child weight for age were similar between children exposed and unexposed to Zika virus at 36 months and 48 months of age in both the León and Managua cohorts (table 1).
Table 1:
Baseline characteristics of mother–child pairs with and without in-utero exposure to Zika virus in León and Managua, Nicaragua
| León cohort | Managua cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Whole cohort (n=303) | Exposure to Zika virus (n=33) | No exposure to Zika virus (n=270) | Mean difference (95% CI)* | p value | Whole cohort (n=319) | Exposure to Zika virus (n=219) | No exposure to Zika virus (n=100) | Mean difference (95% CI)* | p value | |
| Maternal age, years† | 24.2 (5.7) | 24.8 (6.6) | 24–1 (5.5) | 07 (−1.8 to 31) | 0.59 | 25 9 (6.3) | 25 9 (6.2) | 25 9 (6.8) | 0.0 (−1.6 to 16) | 0.99 |
| Maternal race‡ | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | |
| White | 37 (13%) | 8 (25%) | 29 (12%) | ·· | 0.15 | 8 | 7 (3%) | 1 (1%) | ·· | 034 |
| Black | 13 (5%) | 0 | 13 (5%) | ·· | ·· | 1 (0%) | 0 | 1 (1%) | ·· | ·· |
| Mestizo | 229 (82%) | 24 (75%) | 205 (83%) | ·· | ·· | 303 | 208 (95%) | 95 (96%) | ·· | ·· |
| Other | 1 | 0 | 1 | ·· | ·· | 5 | 3 (1%) | 2 (2%) | ·· | ·· |
| Maternal Hispanic or Latin ethnicity§ | 280 (100%) | 32 (100%) | 248 (100%) | ·· | >0.99 | 304 (100%) | 94 (100%) | 210 (100%) | ·· | >0.99 |
| Maternal education, years¶ | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| <8 | 54 (24%) | 8 (24%) | 46 (24%) | ·· | 014 | 88 (28%) | 58 (27%) | 30 (31%) | ·· | 0.77 |
| 8–12 | 86 (38%) | 8 (24%) | 78 (41%) | ·· | ·· | 148 (47%) | 104 (48%) | 44 (45%) | ·· | ·· |
| >12 | 85 (38%) | 17 (52%) | 68 (35%) | ·· | ·· | 79 (25%) | 55 (25%) | 24 (24%) | ·· | ·· |
| Maternal medical history|| | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Diabetes | 7 (2%) | 2 (6%) | 5 (2%) | ·· | 0.17 | 9 (3%) | 9 (4%) | 0 | ·· | 0.06 |
| Hypertension | 16 (5%) | 4 (12%) | 12 (5%) | ·· | 0.09 | 24 (8%) | 17 (8%) | 7 (7%) | ·· | >0.99 |
| In poverty | 56 (18%) | 6 (18%) | 50 (19%) | ·· | 0.98 | 64 | 42 (19%) | 22 (22%) | ·· | 0.66 |
| Infant sex** | ||||||||||
| Female | 152 (53%) | 11 (33%) | 141 (56%) | ·· | 0.03 | 147 | 104 (47%) | 43 (43%) | ·· | 0.53 |
| Male | 135 (47%) | 22 (67%) | 113 (44%) | ·· | ·· | ·· | 115 (53%) | 57 (57%) | ·· | ·· |
| Infant birthweight†† | ||||||||||
| Mean (SD) | 3060 (466) | 3179 (512) | 3044(458) | 135 (−54 to 325) | 016 | 3200 (655) | 3197 (734) | 3226 (464) | −29 (−196 to 137) | 073 |
| <2500 g | 20 (7%) | 1 (3%) | 19 (8%) | ·· | 0.48 | 14 | 9 (6%) | 5 (8%) | ·· | 0.77 |
| Gestational age at birth‡‡ | ||||||||||
| Mean (SD) | 38 9 (1.4) | 38.9 (16) | 38 9 (14) | 0 (−0.5 to 07) | 0.85 | 38.4 (1.6) | 38 3 (1.7) | 38. (1.4) | −0.2 (−0.6 to 0.2) | 030 |
| <37 weeks | 24 (9%) | 2 (6%) | 22 (10%) | ·· | 075 | 21 | 15 (8%) | 6 (7%) | ·· | 0.81 |
| Microcephalic§§¶¶ | 2 (1%) | 1 (3%) | 1 (<1%) | ·· | 0.23 | 2 | 1 (<1%) | 1 (1%) | ·· | 0.53 |
| Child head circumference, cm|||| | ||||||||||
| 36 months*** | 49.1 (1.6) | 49.1 (2.4) | 49.1 (1.5) | 0.1 (−0.9 to 1.0) | 0.91 | 49.0 (2.6) | 48.9 (2.9) | 49.1 (1.8) | −0.2 (−0.8 to 0.3) | 0.38 |
| 48 months*** | 50.0 (1.6) | 50.3 (2.0) | 49.9 (1.5) | 04 (−0.4 to 1.2) | 0.34 | 50.0 (1.8) | 49.9 (1.7) | 50.2 (1.8) | −0.3 (−0.6 to 0.2) | 0.32 |
| Child length-for-age WHO Z score††† | ||||||||||
| 36 months*** | −0.6 (1.1) | −0.4 (1.3) | −0.6 (1.1) | 0.2 (−0.3 to 0.7) | 0.39 | −0.5 (1.0) | −0.5 (1.2) | −0.5 (1.1) | 0.0 (−0.3 to 0.3) | 0.98 |
| 48 months*** | −0.5 (1.1) | −0.6 (1.2) | −0.5 (1.1) | −01 (−0.6 to 0.4) | 0.69 | −0.4 (1.2) | −04 (1.3) | −04 (1.1) | 0.0 (−0.3 to 0.3 | 0.87 |
| Child weight-for-age WHO Z scored‡‡‡ | ||||||||||
| 36 months*** | 0.0 (1.2) | 0.2 (1.2) | 0.0 (1.2) | 0.2 (−0.3 to 0.6) | 0.41 | 0.0 (1.2) | 0.0 (1.1) | 0.0 (1.3) | 0.0 (−0.3 to 0.3) | 0.99 |
| 48 months*** | −0.2 (1.2) | 0.0 (1.3) | −0.2 (1.1) | 0.2 (−0.4 to 0.7) | 0.53 | 0.0 (1.2) | 0.0 (1.2) | 0.0 (1.3) | 0.0 (−0.3 to 0.2) | 0.95 |
Data are mean (SD) or n (%), unless otherwise stated.
Exposed vs not exposed.
Data missing for one infant (Zika virus-unexposed) in the León cohort.
Data missing for 25 infants (n=1 Zika-virus exposed; n=22 Zika virus-unexposed) in the León cohort and two infants (n=1 Zika-virus exposed; n=1 Zika-virus unexposed) in the Managua cohort.
Data missing for 23 infants (n=1 Zika-virus exposed; n=22 Zika virus-unexposed) in the León cohort and 15 infants (n=6 Zika-virus exposed; n=9 Zika-virus unexposed) in the Managua cohort.
Data missing for 78 infants (Zika virus-unexposed) in the León cohort and four infants (n=2 Zika-virus exposed; n=2 Zika-virus unexposed) in the Managua cohort.
Data missing for four infants (Zika virus-unexposed) in the León cohort.
Data missing for 16 infants (Zika virus-unexposed) in the León cohort.
Data missing for 32 infants (Zika virus-unexposed) in the León cohort and 113 infants (n=78 Zika-virus exposed; n=35 Zika-virus unexposed) in the Managua cohort.
Data missing for 36 infants (Zika virus-unexposed) in the León cohort and 33 infants (n=8 Zika-virus exposed; n=25 Zika-virus unexposed) in the Managua cohort.
Microcephaly defined as 2 standard deviations below the mean for age and sex at birth.
Data missing for 36 infants (Zika virus-unexposed) in the León cohort.
Data missing for 21 infants (n=4 Zika virus-unexposed; 17 Zika-virus unexposed) in the León cohort and 17 infants (n=8 Zika-virus exposed; n=9 Zika-virus unexposed) in the Managua cohort.
Timepoints within a 3-month range.
Data missing for 47 infants (n=4 Zika virus-unexposed; 43 Zika-virus unexposed) in the León cohort and 39 infants (n=25 Zika-virus exposed; n=14 Zika-virus unexposed) in the Managua cohort.
Data missing for 47 infants (n=4 Zika virus-unexposed; 43 Zika-virus unexposed) in the León cohort and 38 infants (n=24 Zika-virus exposed; n=14 Zika-virus unexposed) in the Managua cohort.
For all timepoints (30, 36, and 48 months of age) and in both cohorts, no differences were identified in the proportion of children with very low or below average MSEL scores and overall ELC scores between Zika virus-exposed and Zika virus-unexposed children in any descriptive category (table 2; appendix 2 p 3).
Table 2:
Proportion of enrolled children with very low scores*, by in-utero exposure to Zika virus status, at 30, 36, and 48 months of age
| León cohort | Managua cohort | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| In-utero exposure to Zika virus (n=33) | No in-utero exposure to Zika virus (n=270) | OR (95% CI) | p value | In-utero exposure to Zika virus (n=219) | No in-utero exposure to Zika virus (n=100) | OR (95% CI) | p value | |
| Early learning composite | ||||||||
| 28·5–31·5 months | 6/29 (21%) | 75/241 (31%) | 0·29 (0·18–1·54) | 0·29 | ·· | ·· | ·· | ·· |
| 34·5–37·5 months | 9/33 (27%) | 59/238 (25%) | 1·14 (0·44–2·71) | 0·83 | 35/204 (17%) | 21/90 (23%) | 0·68 (0·36–1·32) | 0·28 |
| 46·5–49·5 months | 12/30 (40%) | 27/86 (31%) | 1·45 (0·55–3·72) | 0·50 | 65/219 (30%) | 34/97 (35%) | 0·78 (0·46–1·35) | 0·41 |
| Gross motor | ||||||||
| 28·5–31·5 months | 2/29 (7%) | 22/240 (9%) | 0·73 (0·08–3·28) | >0·99 | ·· | ·· | ·· | ·· |
| 34·5–37·5 months | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| 46·5–49·5 months | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Fine motor | ||||||||
| 28·5–31·5 months | 3/29 (10%) | 50/241 (21%) | 0·44 (0·08–1·53) | 0·22 | ·· | ·· | ·· | ·· |
| 34·5–37·5 months | 7/33 (21%) | 35/238 (15%) | 1·55 (0·53–4·06) | 0·31 | 58/207 (28%) | 33/91 (36%) | 0·69 (0·39–1·20) | 0·20 |
| 46·5–49·5 months | 7/30 (23%) | 13/86 (15%) | 1·70 (0·51–5·27) | 0·40 | 57/221 (26%) | 29/99 (29%) | 0·84 (0·48–1·48) | 0·61 |
| Visual reception | ||||||||
| 28·5–31·5 months | 4/29 (14%) | 33/241 (14%) | 1·01 (0·24–3·20) | >0·99 | ·· | ·· | ·· | ·· |
| 34·5–37·5 months | 11/33 (33%) | 79/238 (33%) | 1·01 (0·42–2·29) | >0·99 | 38/207 (18%) | 24/90 (27%) | 0·62 (0·33–1·17) | 0·14 |
| 46·5–49·5 months | 17/30 (57%) | 34/86 (39·5%) | 1·99 (0·79–5·08) | 0·16 | 39/221 (18%) | 16/99 (16%) | 1·11 (0·57–2·26) | 0·87 |
| Expressive language | ||||||||
| 28·5–31·5 months | 10/29 (34·5%) | 76/241 (31·5%) | 1·14 (0·45–2·73) | 0·91 | ·· | ·· | ·· | ·· |
| 34·5–37·5 months | 5/33 (15·2%) | 25/238 (10·5%) | 1·52 (0·42–4·50) | 0·38 | 20/207 (10%) | 6/91 (7%) | 1·51 (0·56–4·77) | 0·52 |
| 46·5–49·5 months | 10/30 (33%) | 25/86 (29%) | 1·22 (0·44–3·20) | 0·65 | 54/220 (24·5%) | 23/98 (23%) | 1·06 (0·59–1·95) | 0·95 |
| Receptive language | ||||||||
| 28·5–31·5 months | 2/29 (7%) | 47/241 (19·5%) | 0·31 (0·03–1·30) | 0·13 | ·· | ·· | ·· | ·· |
| 34·5–37·5 months | 9/33 (27%) | 52/238 (22%) | 1·34 (0·52–3·21) | 0·51 | 31/206 (15%) | 16/91 (18%) | 0·83 (0·41–1·73) | 0·72 |
| 46·5–49·5 months | 6/30 (20%) | 17/86 (20%) | 1·01 (0·29–3·11) | >0·99 | 65/219 (30%) | 36/98 (37%) | 0·73 (0·43–1·25) | 0·26 |
Data are n/N (%). OR=odds ratio.
Scores within the lowest 2% of expected scores for children at a given timepoint.
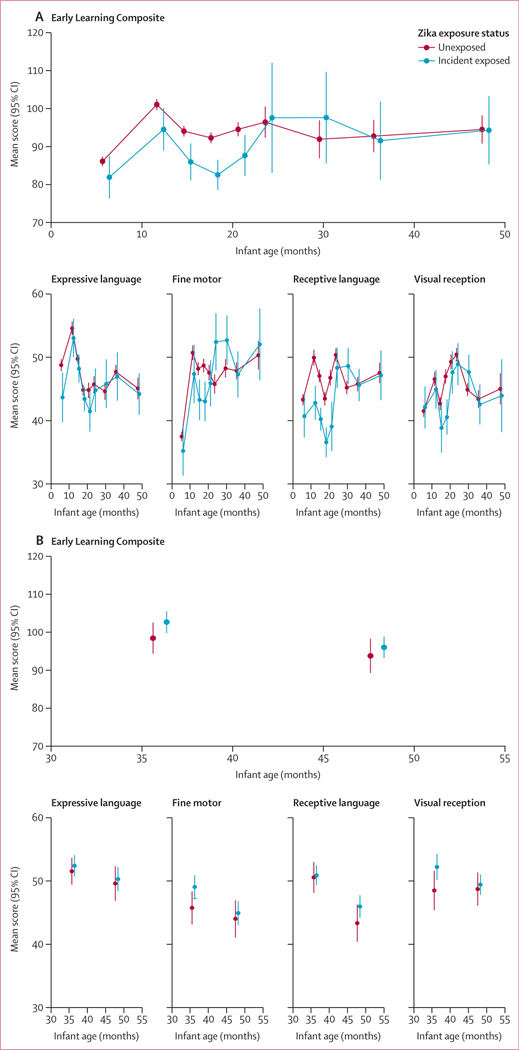
No differences in unadjusted mean ELC scores were identified between infants exposed and unexposed to Zika virus (figure 2) at age 3G months (mean difference –1·2 points [95% CI –6·5 to 4·1]) or age 48 months (–0·2 [–8·7 to 8·3]) in the León cohort or at 36 months (4·2 [–0·7 to 9·2]) or age 48 months (2·2 [–3·1 to 7·5]) in the Managua cohort.
Figure 2: Unadjusted mean standardised MSEL scores by Zika exposure (unexposed and incident exposed only) in the León cohort (A) and Managua cohort (B).
MSEL=Mullen Scales of Early Learning.
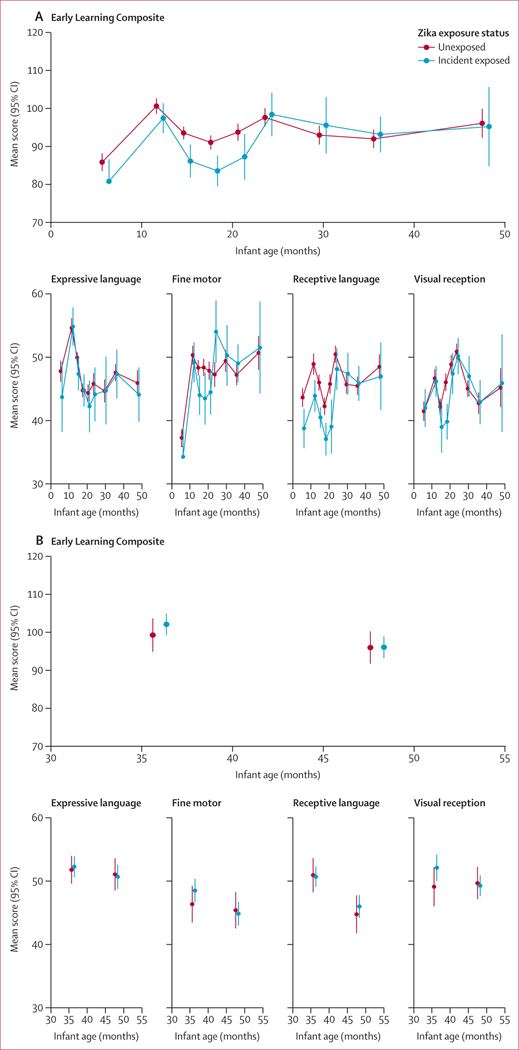
The mean MSEL standardised t-scores after adjustment for maternal education and age, poverty status, and infant sex are shown in figure 3. No differences were identified in adjusted mean ELC scores in the third and fourth years of life between Zika virus-exposed and unexposed infants in the León cohort at age 36 months (between-group difference 1·2 points [95% CI –4·2 to 6·5]) or at age 48 months (–0·9 [–10·8 to 8·8]). In the Managua cohort, no significant differences were identified in mean ELC scores between Zika virus-exposed and unexposed infants at 36 months (between-group difference 2·8 [–2·4 to 8·1]) or at 48 months (0·1 [–5·1 to 5·2]).
Figure 3: Adjusted mean standardised MSEL scores by Zika exposure (unexposed and incident exposed only) in the León cohort (A) and Managua cohort (B).
Inverse probability weighting generalised estimating equations were used to calculate adjusted mean MESL scores. MESL=Mullen Scales of Early Learning.
Discussion
Our analyses of neurodevelopmental outcomes in children without congenital Zika syndrome who were exposed to Zika virus in utero did not indicate neurodevelopmental deficits persisting to preschool age (3–4 years) when compared with children without Zika virus exposure from the same source population. Although early developmental delays in children born with congenital Zika syndrome have been described previously,4–6 findings on the neurodevelopmental outcomes of children exposed to Zika virus in utero but born without congenital Zika syndrome are mixed, and outcomes beyond the first 2 years of life have not been well characterised.7–12,15,16 Our publication reported data from the first 2 years of follow-up of the León cohort and other earlier research have shown that children with Zika virus exposure in utero but without evidence of congenital Zika syndrome at birth might be at risk of neurodevelopmental delays.7,8,10,15 However, the results of this prospective study suggest that delays in neurodevelopment in the first 2 years of life are not detectable in the third or fourth years.
In both the crude and adjusted analyses, the lower mean ELC and MSEL scales scores in the Zika virus-exposed group previously described in the León cohort10 did not persist after 24 months of age. No significant differences in neurodevelopmental outcomes were identified between the Zika virus-exposed and unexposed groups at 3G months or 48 months of age, nor do the point estimates for the ELC score differences exceed the 6-point difference considered clinically significant in other studies that have used the MSEL.25 Additionally, no significant differences were identified in the proportion of children with very low scores or those with very low or below average scores of any MSEL scales or ELC scores, at any timepoints from age 30 months onwards, in either cohort. Although a general increase in the proportion of children with low or very low ELC scores was observed over time in both exposed and unexposed children, we believe this is due to differences between the cultural and socioeconomic context in which the MSEL was developed and the context in which it is being used.
Some strengths of this study include the use of two separate population-based cohorts in different locations in Nicaragua, the inclusion of children born to women asymptomatically infected with Zika virus during their pregnancy, the intensive follow-up and retention of study participants after enrolment, and the training and monitoring of study staff involved in administering neurodevelopmental assessments. Additionally, the use of nuanced serological testing for Zika virus exposure mitigates the risk of misclassification due to cross-reactive antibodies elicited by related endemic flaviviruses such as dengue virus. Another strength of the study is the use of the MSEL, which is a performance-based measure of neurodevelopment that, although originally developed in the USA, has since been adapted and validated for use in diverse settings.24,25,29,30 In each cohort, the inclusion of a control group of children who were born to women from similar environments and with similar characteristics, but without in utero exposure to Zika virus, allows for a comparison that helps ensure that an observed neurodevelopmental delay would have been attributable to Zika virus exposure rather than to the application of the assessment outside of its intended setting.
The compatibility of the results between the León and Managua paediatric cohorts using different methods of Zika virus exposure classification, different study staff, and a different setting for the MSEL assessment administration suggest that the findings are robust to bias that might have been introduced by these particular methodological choices. For example, while the administration of the MSEL assessment at the health clinic in Managua provided a more controlled environment for the assessment than those performed at home visits by study staff in León, it also had the potential to not fully capture the child’s neurodevelopment level due to their discomfort in unfamiliar surroundings. The proportion of children exposed to Zika virus in utero also differed substantially between these cohorts due to differences in the method and timing of enrolment. The consistency of our findings using different methods between cohorts provides some reassurance against these potential biases.
The interpretation of these findings requires that limitations also be considered. The short, intense period of transmission was used to establish the Zika virus exposure status for the Managua cohort and second half of the León cohort without testing serum from multiple timepoints, but any potential for exposure misclassification through false positive or negative laboratory results or simplifying assumptions could affect validity. Additionally, the causal factors affecting neuro-development are complex, and as with any observational study, there is a possibility of uncontrolled confounding. A possible example is the poverty index used to characterise and adjust for the household conditions in which children were raised, which is a simplified proxy measure of the factors relevant to neurodevelopment, and further adapted from its original version to accommodate the data available in these populations.
Missing data are another source of potential bias, and some information such as maternal education were not completely available in both cohorts. Although we do not have reason to believe that the participants with missing data differed substantially from those with available data, we are not able to assess this. The behavioural standards used to compute scaled MSEL scores are based on dated American norms for which no alternatives are available. This mismatch between the neurodevelopmental norms used to develop the MSEL score ranges and the norms expected in our study population might be most apparent in the MSEL descriptive categories, which were included to provide meaningful clinical interpretations of scores in addition to comparisons of overall score distributions between the exposure groups. However, it is possible that the higher proportion of children categorised as very low compared with the US-based standard could either reflect a true delay in development due to multiple risk factors in the population or an artifact of using US-based norms and cultural context in the development of the instrument. The computation of a global cognitive performance score using the MSEL requires scaling by age and gender, and previous research in a cohort of younger children in Benin has suggested that if the range of ages at assessment is narrow, then the use of the American behavioural norms in the MSEL can be reasonably applied.30
Taken in combination with the previous study and other research showing neurodevelopmental delays among Zika virus-exposed children in their first 2 years of life,7,8,10,13 the results of this research suggest that early deficits (if they do exist) in neurodevelopment among normocephalic children exposed to Zika virus in utero resolve by the third and fourth years of life when children start preschool in Nicaragua. Further research on the impacts of Zika virus exposure on neurodevelopment in different settings and focusing on different functional outcomes remains important; however, we are cautiously optimistic that these and other recent findings are encouraging for the long-term prognosis of children born with Zika virus exposure.31
Supplementary Material
Research in context.
Evidence before this study
Before undertaking this study, we searched for studies describing the neurodevelopment of children born to women exposed to Zika virus during pregnancy, but without apparent congenital Zika syndrome at birth. We searched PubMed from database inception to Nov 1, 2022, for any articles using the search terms: (“Zika” OR “ZIKV”) AND (“child” OR “children” OR “infant”) AND (“neurodevelopment” OR “neurodevelopmental” OR “developmental”) AND (humans[Filter]). Of the 247 articles returned by the search, 28 relevant full-text articles were reviewed. Some studies found indications of early developmental delay among Zika virus-exposed normocephalic children, but there was substantial heterogeneity in the findings of these articles. These studies were mostly limited to outcome ascertainment during the first 24 months of development, and many called for longer-term follow-up to be done to preschool age (ie, 3–4 years in Nicaragua). Many studies had relatively small sample sizes and did not include a control group of unexposed infants from the study setting to compare neurodevelopmental assessments against. We did not find any large, prospective cohort studies comparing neurodevelopmental indicators in both Zika virus-exposed and Zika virus-unexposed children to preschool age (age 3–5 years).
Added value of this study
To our knowledge, this study represents the largest prospective cohort describing the neurodevelopment of normocephalic children exposed to Zika virus in utero with follow-up to age 4 years. Our findings included women who had asymptomatic Zika virus infections during pregnancy and are strengthened by the comparison of a non-exposed control group of children from the same source population, to contextualise the neurodevelopmental assessment scores in the local setting, and the use of the Mullen Scales of Early Learning (MSEL) assessment, an evaluation of neurological development based on five subscales (gross motor, fine motor, expressive language, receptive language, and visual reception). The MSEL corresponds well with the Bayley Scales of Infant Development, but it is considered more adaptable to different settings and cultural contexts. The approach used and characteristics of this study help to address limitations in existing evidence concerning the sample size of individual studies, and the interpretation of the results of neurodevelopmental assessments compared with standardised values from a different population and setting. The extension of follow-up to preschool age provides valuable information for the understanding of the effect of Zika virus exposure on neurodevelopment, because during the first 4 years of development, expressive and productive language domains develop at a fast pace, during which children’s vocabulary expands rapidly, enabling the production of longer, more complex sentences. 4-year-old children tend to have better gross motor domain, stronger memory, and pro-social skills than 3-year-old children. Collectively, the larger array of neurodevelopmental skills available for evaluation at age 4 years provides a more comprehensive overview of neurodevelopment.
Implications of all the available evidence
Neurodevelopmental delays that are seen in the first 2 years among normocephalic children who are exposed to Zika virus in utero might resolve or they might be mitigated to the extent that they are no longer apparent as children reach their third and fourth years of life.
Acknowledgments
This study is supported by the National Institute of Child Health and Development (grant number 5R01HD094009) and the National Institute of Allergy and Infectious Diseases (NIAID; grant number AI137902). SB-D is supported by an award from the NIAID (5K24AI141744). OZ is supported by an award from the Fogarty International Center (5TD43TW010923). NMB is supported by the AI137902 grant. We would like to thank our study staff in Managua and León, Nicaragua, and express our gratitude to our study participants and their families.
Declaration of interests
EWG is supported by the National Eye Institute, the END Fund, and the Task Force for Global Health. All other authors declare no competing interests.
Footnotes
Equitable partnership declaration
The authors of this paper have submitted an equitable partnership declaration (appendix 3). This statement allows researchers to describe how their work engages with researchers, communities, and environments in the countries of study. This statement is part of The Lancet journals’ broader goal to decolonise global health.
Data sharing
The study team will provide a de-identified version of the database used for this analysis will be available in the University of North Carolina at Chapel Hill dataverse by G months after publication to enhance reproducibility and encourage future research. This database will include the key derived variables used to conduct this analysis along with a data dictionary, but it might not contain the original underlying data used to derive these variables in the interest of privacy and in concordance with data sharing policies as written in the informed consent for this study. Additional documents will not be available.
For the Spanish translation of the abstract see Online for appendix 1
See Online for appendix 2
References
- 1.Chu DT, Ngoc VTN, Tao Y. Zika virus infection in Vietnam: current epidemic, strain origin, spreading risk, and perspective. Eur J Clin Microbiol Infect Dis 2017; 36: 2041–42. [DOI] [PubMed] [Google Scholar]
- 2.Hill SC, Vasconcelos J, Neto Z, et al. Emergence of the Asian lineage of Zika virus in Angola: an outbreak investigation. Lancet Infect Dis 2019; 19: 1138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lourenço J, de Lourdes Monteiro M, Valdez T, Monteiro Rodrigues J, Pybus O, Rodrigues Faria N. Epidemiology of the Zika virus outbreak in the Cabo Verde Islands, West Africa. PLoS Curr 2018; 10: ecurrents.outbreaks.19433b1e4d007451c691f138e1e67e8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalcante TB, Ribeiro MRC, Sousa P da S, et al. Congenital Zika syndrome: growth, clinical, and motor development outcomes up to 3G months of age and differences according to microcephaly at birth. J Infect Dis 2021; 105: 399–408. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler AC, Toth D, Ridenour T, et al. Developmental outcomes among young children with congenital Zika syndrome in Brazil. JAMA Netw Open 2020; 3: e204096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hcini N, Kugbe Y, Rafalimanana ZHL, et al. Association between confirmed congenital Zika infection at birth and outcomes up to 3 years of life. Nat Commun 2021; 12: 3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulkey SB, Arroyave-Wessel M, Peyton C, et al. Neurodevelopmental abnormalities in children with in utero Zika virus exposure without congenital Zika syndrome. JAMA Pediatr 2020; 174: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Familiar I, Boivin M, Magen J, et al. Neurodevelopment outcomes in infants born to women with Zika virus infection during pregnancy in Mexico. Child Care Health Dev 2021; 47: 311–18. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar Ticona JP, Nery N Jr, Ladines-Lim JB, et al. Developmental outcomes in children exposed to Zika virus in utero from a Brazilian urban slum cohort study. PLoS Negl Trop Dis 2021; 15: e0009162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringer EM, Martinez E, Blette B, et al. Neurodevelopmental outcomes of children following in utero exposure to Zika in Nicaragua. Clin Infect Dis 2021; 72: e146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerzson LR, de Almeida CS, Silva JHD, Feitosa MMA, de Oliveira LN, Schuler-Faccini L. Neurodevelopment of nonmicrocephalic children, after 18 months of life, exposed prenatally to Zika virus. J Child Neurol 2020; 35: 278–82. [DOI] [PubMed] [Google Scholar]
- 12.Díaz-Martínez LA, Rojas MA, Pinilla-García LS, et al. Neurodevelopmental outcome of infants without central nervous system anomalies born to symptomatic RT-PCR ZIKV positive women. PLoS Negl Trop Dis 2022; 16: e0009854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marbán-Castro E, Vazquez Guillamet LJ, Pantoja PE, et al. Neurodevelopment in normocephalic children exposed to Zika virus in utero with no observable defects at birth: a systematic review with meta-analysis. Int J Environ Res Public Health 2022; 19: 7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peçanha PM, Junior SCG, Pone SM, et al. Neurodevelopment of children exposed intra-uterus by Zika virus: a case series. PLoS One 2020; 15: e0229434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen-Saines K, Brasil P, Kerin T, et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med 2019; 25: 1213–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faiçal AV, de Oliveira JC, Oliveira JVV, et al. Neurodevelopmental delay in normocephalic children with in utero exposure to Zika virus. BMJ Paediatr Open 2019; 3: e000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prado EL, Larson LM, Cox K, Bettencourt K, Kubes JN, Shankar AH. Do effects of early life interventions on linear growth correspond to effects on neurobehavioural development? A systematic review and meta-analysis. Lancet Glob Health 2019; 7: e1398–413. [DOI] [PubMed] [Google Scholar]
- 18.Mullen EM. Mullen scales of early learning. Circle Pines, MN: AGS, 1995. [Google Scholar]
- 19.WHO. WHO guideline on the prevention and management of wasting and nutritional oedema (acute malnutrition) in infants and children under 5 years. https://www.who.int/publications/i/item/9789240082830 (accessed May 21, 2024). [PubMed]
- 20.Pan American Health Organization. Zika epidemiological report–Nicaragua. https://www.paho.org/hq/dmdocuments/2017/2017-phezika-situation-report-nic.pdf (accessed Dec 8, 2022).
- 21.Balmaseda A, Stettler K, Medialdea-Carrera R, et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci USA 2017; 114: 8384–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balmaseda A, Zambrana JV, Collado D, et al. Comparison of four serological methods and two reverse transcription-pcr assays for diagnosis and surveillance of Zika virus infection. J Clin Microbiol 2018; 56: e01785–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley-Johnson S. Cognitive assessment for the youngest children: a critical review of tests. J Psychoeduc Assess 2001; 19: 19–44. [Google Scholar]
- 24.Connery AK, Colbert AM, Lamb MM, et al. Receptive language skills among young children in rural Guatemala: the relationship between the Test de Vocabulario en Imagenes Peabody and a translated and adapted version of the Mullen Scales of Early Learning. Child Care Health Dev 2019; 45: 702–08. [DOI] [PubMed] [Google Scholar]
- 25.Boivin MJ, Maliwichi-Senganimalunje L, Ogwang LW, et al. Developmental effects of antepartum and postpartum antiretroviral exposure for HIV-exposed versus HIV-unexposed uninfected children in Uganda and Malawi: a prospective cohort study.Lancet HIV 2019; 6: e518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peña R, Pérez W, Meléndez M, Källestål C, Persson LÅ. The Nicaraguan Health and Demographic Surveillance Site, HDSS-Leon: a platform for public health research. Scand J Public Health 2008; 36: 318–25. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw 2006; 15: 1–11. [Google Scholar]
- 29.Swineford LB, Guthrie W, Thurm A. Convergent and divergent validity of the Mullen Scales of Early Learning in young children with and without autism spectrum disorder. Psychol Assess 2015; 27: 1364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodeau-Livinec F, Davidson LL, Zoumenou R, Massougbodji A, Cot M, Boivin MJ. Neurocognitive testing in West African children 3–6 years of age: challenges and implications for data analyses. Brain Res Bull 2019; 145: 129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulkey SB, Peyton C, Ansusinha E, et al. Preschool neurodevelopment in Zika virus-exposed children without congenital Zika syndrome. Pediatr Res 2023; 94: 178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.