Abstract
Background:
The US President’s Emergency Plan for AIDS Relief aims to address the higher risk of cervical cancer among women living with HIV by offering high-quality screening services in the highest burden regions of the world.
Methods:
We analyzed the US President’s Emergency Plan for AIDS Relief Monitoring, Evaluation, and Reporting data from Centers for Disease Control and Prevention–supported sites in 13 countries in sub-Saharan Africa for women living with HIV aged older than 15 years who accessed cervical cancer screening services (mostly visual inspection, with ablative or excisional treatment offered for precancerous lesions), April 2018–March 2022. We calculated the positivity by age, country, and clinical visit type (first lifetime screen or routine rescreening). We fitted negative binomial random coefficient models of log-linear trends in time to estimate the probabilities of testing positive and any temporal trends in positivity.
Results:
Among the 2.8 million completed cancer screens, 5.4% identified precancerous lesions, and 0.8% were positive for suspected invasive cervical cancers (6.1% overall). The positivity rates declined over the study period among those women screening for cervical cancer for the first time and among those women presenting to antiretroviral therapy clinics for routine rescreening.
Conclusions:
These positivity rates are lower than expectations set by the published literature. Further research is needed to determine whether these lower rates are attributable to the high level of consistent antiretroviral therapy use among these populations, and systematic program monitoring and quality assurance activities are essential to ensure women living with HIV have access to the highest possible quality prevention services.
Keywords: cervical cancer, screening, HIV, HPV
INTRODUCTION
Cervical cancer prevention is a critical component of clinical care for women living with HIV (WLHIV). Cervical cancer is an AIDS-defining illness,1 and the risk of developing the disease is six-fold higher for WLHIV compared with those without HIV.2 Cervical cancer is caused by persistent infection with a carcinogenic human papillomavirus (HPV) subtype;3 the risk of acquisition and persistence of oncogenic HPV infection, as well as the incidence of precancerous lesions and cancers, are all increased for WLHIV compared with women without HIV.4 In addition, the incidence of HIV is significantly increased in the presence of prevalent HPV infection.5 Geographic inequalities are evident in the epidemiology of both HPV and HIV; the highest prevalence of HPV infection,3 age-standardized incidence rates of6 and mortality rates from7 invasive cervical cancers, number of people living with HIV, and dying from AIDS-related illness8 are all found in Eastern and Southern Africa compared with all other regions of the world.
The 2021 WHO guidelines recommend that all WLHIV aged 25–49 years should be screened for cervical cancer; screen tests include HPV DNA testing, visual inspection with acetic acid (VIA), and pap smear/cytology.9 HPV tests screen for the necessary but not sufficient viral infection precursor, VIA identifies visible precancerous lesions, and pap smears collect cervical cells to assess for evidence of cytologic abnormalities. A recent systematic review and meta-analysis evaluated the diagnostic accuracy of these screening modalities to identify CIN2+ among thousands of WLHIV, reporting striking variability in the accuracy of VIA and of cytology in the absence of documented quality control procedures and a high sensitivity but low specificity using HPV DNA screening, with some improvement to specificity when a VIA triage follows the HPV screen.10 The same study reported screen positivity ranges for HPV DNA testing of 43.7%–50.6%, for VIA of 5.6%–55.9%, for cytology of 10.0%–20.8%, and for HPV with VIA triage of 22.0%–57.4%.
Global implementation guidelines for cervical cancer screening programs estimate that between 5% and 25% of the general population will screen positive using any available test.11,12 The proportion of women who screen positive with an identified precancerous lesion or a suspected invasive cervical cancer (ICC) will be higher in populations with a higher prevalence of HIV, CIN2+, and oncogenic HPV subtypes.13 Screen-positive rates are higher among WLHIV screened with VIA, cytology, and HPV DNA testing when compared with people without HIV.3 Women younger than 30 years are more likely to screen positive compared with all other age groups because of the higher rates of cervical metaplasia and dysplasia, HPV infection, and low-grade epithelial lesions among young women.3,14 In addition to these patient characteristics, VIA diagnostic accuracy also varies with procedural characteristics, including the light source, the acetic acid concentration, and the training and experience of the test provider, because VIA is inherently subjective and dependent on the judgement of the provider.3
The US President’s Emergency Plan for AIDS Relief (PEPFAR) cocreated the Go Further partnership in 2018 to accelerate progress toward cervical cancer elimination among WLHIV.15,16 As part of this partnership, PEPFAR-supported cervical cancer screen and treat programs have used primarily VIA, as well as some pap smear and HPV DNA testing, to assess candidacy for ablative therapy of precancerous lesions. Both WHO and PEPFAR screening guidance acknowledge the limitations in the performance of visual inspection screening, but cite the benefits of lower cost and same-day treatment feasibility in their recommendations for a realistic, accessible, high-quality screening and treatment approach. Centers for Disease Control and Prevention (CDC) has supported two-thirds of more than 4 million cervical cancer screens in PEPFAR-supported antiretroviral therapy (ART) facilities 2018–2022 through collaboratively developed infrastructure and human resource capacity, provision of ablative or excisional treatment for an increasing proportion of the precancerous lesions identified, and renewed commitment to quality assurance interventions.12
Despite the disproportionate burden of cervical cancer morbidity and mortality in low- and middle-income countries, especially those in Eastern and Southern Africa, global recommendations for cervical cancer prevention are based primarily on data from high-income settings.10 To begin to fill the evidence gaps, we report the screen-positive rates observed in CDC-PEPFAR–supported facilities overall and by country, describe differences by age and clinical screening history, and identify patterns over time.
METHODS
Descriptive Analysis
PEPFAR monitors program implementation among people living with HIV using standardized Monitoring, Evaluation, and Reporting (MER) indicators.17 We analyzed the MER cervical cancer data for WLHIV older than 15 years who accessed cervical cancer screening services in April 2018–March 2022, reported semiannually from CDC-supported sites in 13 countries in sub-Saharan Africa. This project was approved as nonresearch according to the agency project determination procedures. The 13 countries are Botswana, Eswatini, Ethiopia, Kenya, Lesotho, Malawi, Mozambique, Namibia, Nigeria, Tanzania, Uganda, Zambia, and Zimbabwe, representing countries with relatively high HIV and cervical cancer prevalence who chose to dedicate a portion of their PEPFAR funding to this initiative in alignment with their national cancer programs. As previously described,16 most PEPFAR ART facilities offer cervical cancer screening using VIA, although some countries have the capacity to screen a small proportion of women using pap or HPV DNA testing, where laboratory systems and infrastructure are in place and as affordable HPV screening tests become more widely available globally. HPV platforms vary according to availability in each country, inclusive of Cepheid GeneXpert, Roche Cobas, Hologics Panther, and Abbott m2000. Screening, quality assurance, and precancerous lesion treatment procedures are conducted by trained health care clinicians (eg, nurses, physicians, and midwives) according to the national cancer guidelines of each country.
Completed screen results were reported as negative, positive for a precancerous cervical lesion, or positive for visual evidence suggestive of ICC. Screen type is not reported in MER; those facilities that used HPV DNA testing or pap triaged all positive screening tests with visual inspection and reported as positive only those positive on both tests. Age groups were reported in 5-year increments: 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, ≥50, and unknown; for this analysis, ages 30–49 years were combined. Screening is reported by clinical visit type, inclusive of women presenting for their first lifetime cervical cancer screening test and those who had a prior negative screen returning for routine rescreening, recommended anywhere from 1 to 5 years depending on the national guidelines (referred to as “rescreen”). Overall screen positivity is defined as the proportion of all completed screens reported as positive for either precancer or suspected ICC among WLHIV. We calculated overall and precancer-specific or suspected ICC-specific positivity rates by country, by age, and by clinical visit type (first time or rescreen). We excluded reported tests with missing information about the age of the woman screened and country-specific semiannual reporting of fewer than 158 tests, which is the sample size required to estimate a positivity of 10% with a 10% absolute margin of error.
Statistical Trend Analysis
We used a multilevel modeling approach to account for clustering of factors by health facility. We used the binom package for R18 (version 4.2.0) to obtain pointwise uncertainty intervals containing the true proportions of women who tested positive for cervical precancers or suspected ICC with 0.95 probability, from their posterior distributions assuming the noninformative Jeffrey’s prior. We fitted negative binomial random coefficient models of log-linear trends in time including and excluding the effects of age groups. The 3 response variables were the total numbers of positive tests across both visit types combined and separately at each of the 2 visit types described above. We used the total numbers of screening tests across all visits combined, and the 2 visit types, as offsets for the 3 response variables so that the models estimated the probabilities of testing positive, and the exponential functions of the model coefficients are risk ratios. For each response variable, we fitted 6 model variations that exhaust the possibilities for country-level random effects on the overall probability of positivity and temporal trend in positivity 2018–2022 with and without overall effects of age and effects of age on trends. We used the rstanarm19 package for R, which implements Hamiltonian Monte Carlo sampling20 from the joint posterior distribution of the model parameters. Posterior inference was based on 3000 samples from each of 4 chains after discarding 3000 warm-up draws from each chain for a total of 12,000 samples. For each of the response variables, the estimated trend risk ratios were obtained from the simpler (fewest parameters) of the model, which produced the largest expected pointwise predictive density of leave-one-out cross-validation21 (elpd-loo), or the simplest model, which was indistinguishable from the model producing the largest elpd-loo based on the Z test.
RESULTS
During April 2018–March 2022, CDC-PEPFAR–supported HIV care facilities completed more than 2.8 million cervical cancer screening tests in 13 African countries (Table 1). These same facilities reported providing ART services for more than 3.4 million WLHIV age 25–49 years during January to March 2022. We excluded 15,208 reported tests (0.5%) with missing information about the age of the woman screened and 68 tests (<0.001%) from countries where the country-specific semiannual reporting was below the sample size requirement described above. Most screens were among WLHIV who had never been screened previously (82.7%, n = 2,323,181/2,807,532); routine rescreening (17.2%, n = 484,351/2,807,532) was less common. The total number of screening tests per country ranged from 41,683 in Lesotho to 634,463 in Mozambique; the absolute numbers of screening tests have increased over time in all countries (not shown) and all age groups in our analysis (Table 1).
TABLE 1.
Numbers of Cervical Cancer Screenings by Visit Type, Age Group, and Country, 2018–2022
| Age | Year | Visit Type |
||
|---|---|---|---|---|
| First | Rescreening | Both | ||
|
| ||||
| 15–24 | 2018 | 4242 | 354 | 4596 |
| 2019 | 20,889 | 879 | 21,768 | |
| 2020 | 43,961 | 2092 | 46,053 | |
| 2021 | 91,971 | 7165 | 99,136 | |
| 2022 | 54,719 | 5147 | 59,866 | |
| All | 215,782 | 15,637 | 231,419 | |
| 25–29 | 2018 | 12,383 | 1282 | 13,665 |
| 2019 | 58,941 | 3082 | 62,023 | |
| 2020 | 103,338 | 6321 | 109,659 | |
| 2021 | 195,032 | 18,871 | 213,903 | |
| 2022 | 116,695 | 14,780 | 131,475 | |
| All | 486,389 | 44,336 | 530,725 | |
| 30–49 | 2018 | 49,811 | 5574 | 55,385 |
| 2019 | 144,295 | 30,162 | 174,457 | |
| 2020 | 292,736 | 59,083 | 351,819 | |
| 2021 | 613,997 | 155,162 | 769,159 | |
| 2022 | 347,275 | 116,047 | 463,322 | |
| All | 1,448,114 | 366,028 | 1,814,142 | |
| 50+ | 2018 | 4421 | 719 | 5140 |
| 2019 | 18,566 | 4332 | 22,898 | |
| 2020 | 43,443 | 9461 | 52,904 | |
| 2021 | 70,581 | 24,467 | 95,048 | |
| 2022 | 35,885 | 19,371 | 55,256 | |
| All | 172,896 | 58,350 | 231,246 | |
|
| ||||
| Country | ||||
|
| ||||
| Botswana | 28,462 | 26,152 | 54,614 | |
| Eswatini | 34,379 | 28,356 | 62,735 | |
| Ethiopia | 92,141 | 4871 | 97,012 | |
| Kenya | 226,818 | 55,766 | 282,584 | |
| Lesotho | 35,779 | 5904 | 41,683 | |
| Malawi | 104,877 | 60,733 | 165,610 | |
| Mozambique | 628,350 | 6113 | 634,463 | |
| Namibia | 36,359 | 20,244 | 56,603 | |
| Nigeria | 54,290 | 2676 | 56,966 | |
| Tanzania | 433,741 | 98,110 | 531,851 | |
| Uganda | 168,361 | 15,519 | 183,880 | |
| Zambia | 302,410 | 58,558 | 360,968 | |
| Zimbabwe | 177,214 | 101,349 | 278,563 | |
| Totals | 2,323,181 | 484,351 | 2,807,532 | |
Reportings from 2018 to 2022 were limited to the second and first halves of those years, respectively.
The overall positivity rate was 6.1% (172,238 positive/ 2,807,532 total tests); this included a rate of 5.4% (n = 150,287) for precancerous lesions and 0.8% (n = 21,951) for suspected ICCs (Table 2). The positivity rate varied by country, ranging from an overall rate of 3.5% in Kenya to 17.8% in Namibia; precancerous lesion positivity ranged from 2.6% in Kenya to 17.2% in Namibia; and suspected ICC positivity ranged from 0.6% in Botswana, Mozambique, and Uganda to 1.2% in both Lesotho and Nigeria.
TABLE 2.
Positive Cervical Cancer Screening Tests Among Women Living With HIV in CDC-PEPFAR–Supported Programs, April 2018–March 2022
| Total |
Precancers |
Suspected Cancers |
Overall Positivity |
||||
|---|---|---|---|---|---|---|---|
| Tested | N | % | N | % | N | % | |
| All patients | 2,807,532 | 150,287 | 5.4 | 21,951 | 0.8 | 172,238 | 6.1 |
| Country | |||||||
| Botswana | 54,614 | 3315 | 6.1 | 325 | 0.6 | 3640 | 6.7 |
| Eswatini | 62,735 | 2452 | 3.9 | 432 | 0.7 | 2884 | 4.6 |
| Ethiopia | 97,012 | 5740 | 5.9 | 933 | 1.0 | 6673 | 6.9 |
| Kenya | 282,584 | 7479 | 2.6 | 2535 | 0.9 | 10,014 | 3.5 |
| Lesotho | 41,683 | 1355 | 3.3 | 506 | 1.2 | 1861 | 4.5 |
| Malawi | 165,610 | 4420 | 2.7 | 1538 | 0.9 | 5958 | 3.6 |
| Mozambique | 634,463 | 47,606 | 7.5 | 3594 | 0.6 | 51,200 | 8.1 |
| Namibia | 56,603 | 9714 | 17.2 | 373 | 0.7 | 10,087 | 17.8 |
| Nigeria | 56,966 | 3471 | 6.1 | 680 | 1.2 | 4151 | 7.3 |
| Tanzania | 531,851 | 19,637 | 3.7 | 4532 | 0.9 | 24,169 | 4.5 |
| Uganda | 183,880 | 10,273 | 5.6 | 1081 | 0.6 | 11,354 | 6.2 |
| Zambia | 360,968 | 20,499 | 5.7 | 3441 | 1.0 | 23,940 | 6.6 |
| Zimbabwe | 278,563 | 14,326 | 5.1 | 1981 | 0.7 | 16,307 | 5.9 |
| Age group (yrs) | |||||||
| 15–24 | 231,419 | 11,950 | 5.2 | 841 | 0.4 | 12,791 | 5.5 |
| 25–29 | 530,725 | 31,972 | 6.0 | 2766 | 0.5 | 34,738 | 6.5 |
| 30–49 | 1,814,142 | 98,006 | 5.4 | 14,734 | 0.8 | 112,740 | 6.2 |
| 50+ | 231,246 | 8359 | 3.6 | 3610 | 1.6 | 11,969 | 5.2 |
| Visit type | |||||||
| First time | 2,323,181 | 127,489 | 5.5 | 18,518 | 0.8 | 146,007 | 6.3 |
| Rescreened | 484,351 | 22,798 | 4.7 | 3433 | 0.7 | 26,231 | 5.4 |
The positivity rates were highest among WLHIV aged 25–29 years (overall 6.5%, n = 34,738/530,725 and precancers 6.0%, n = 31,972/530,725) and lowest among WLHIV older than 50 years (overall 5.2%, n = 11,969/ 231,246 and precancers 3.6%, n = 8359/231,246); the positivity rates for suspected ICCs was highest in the 50 years or older age band at 1.6% (n = 3610/231,246) and lowest among WLHIV aged 15–24 years (0.4%, n = 841/231,419).
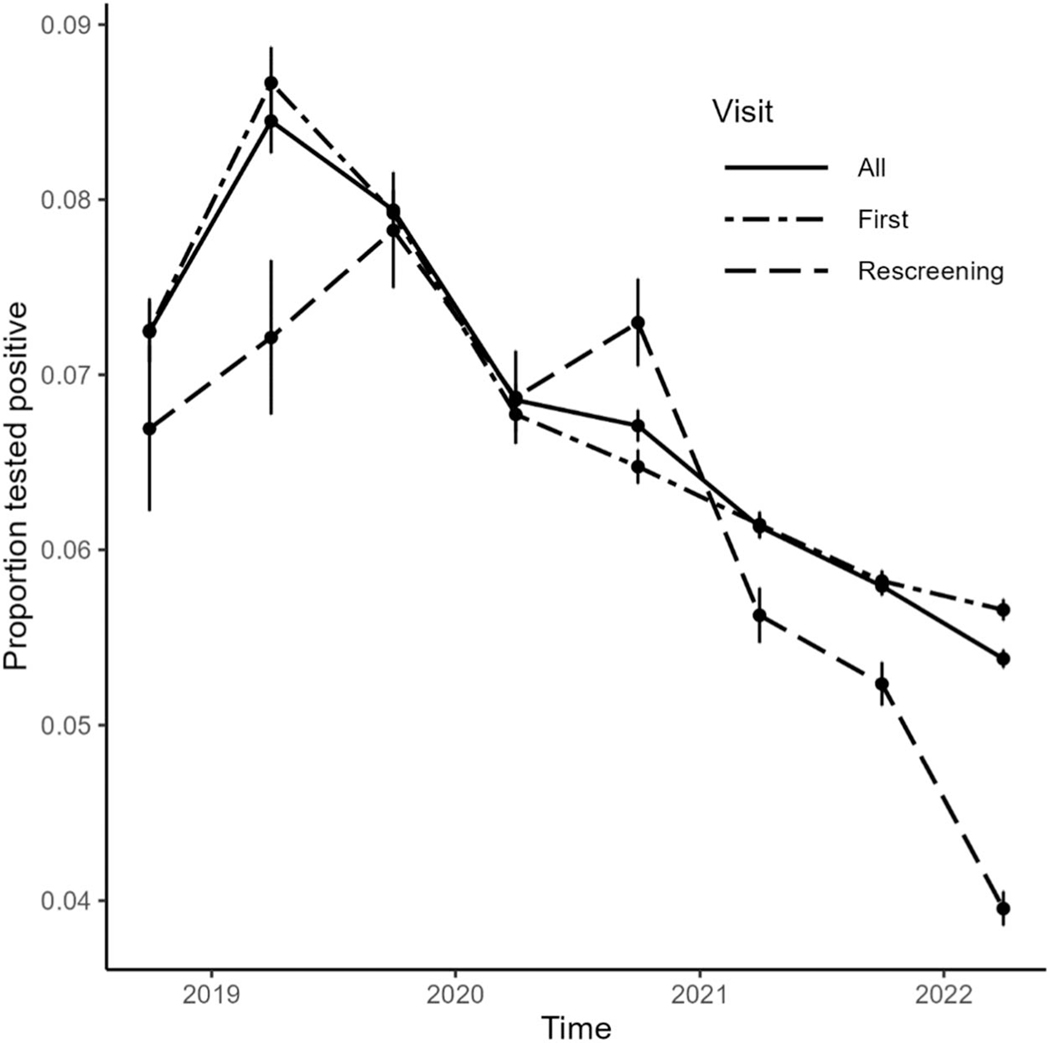
The overall positivity rate was 6.3% (n = 146,007/ 2,323,181) among those screening for the first time and 5.4% (n = 26,231/484,351) among those returning for routine screening. The reported positivity rate at first screen was highest among WLHIV in Namibia (17.2%). The screen positivity rate among WLHIV has declined over time since 2019 in all countries (Fig. 1).
FIGURE 1.
Proportions1 of positive screening tests2 among women living with HIV in CDC-PEPFAR–supported cervical cancer prevention programs by visit type, April 2018–March 2022. 1Vertical bars are pointwise uncertainty intervals containing the true value with 0.95 probability. 2Positive cervical cancer tests include positive for precancerous lesions and for suspected invasive cervical cancers.
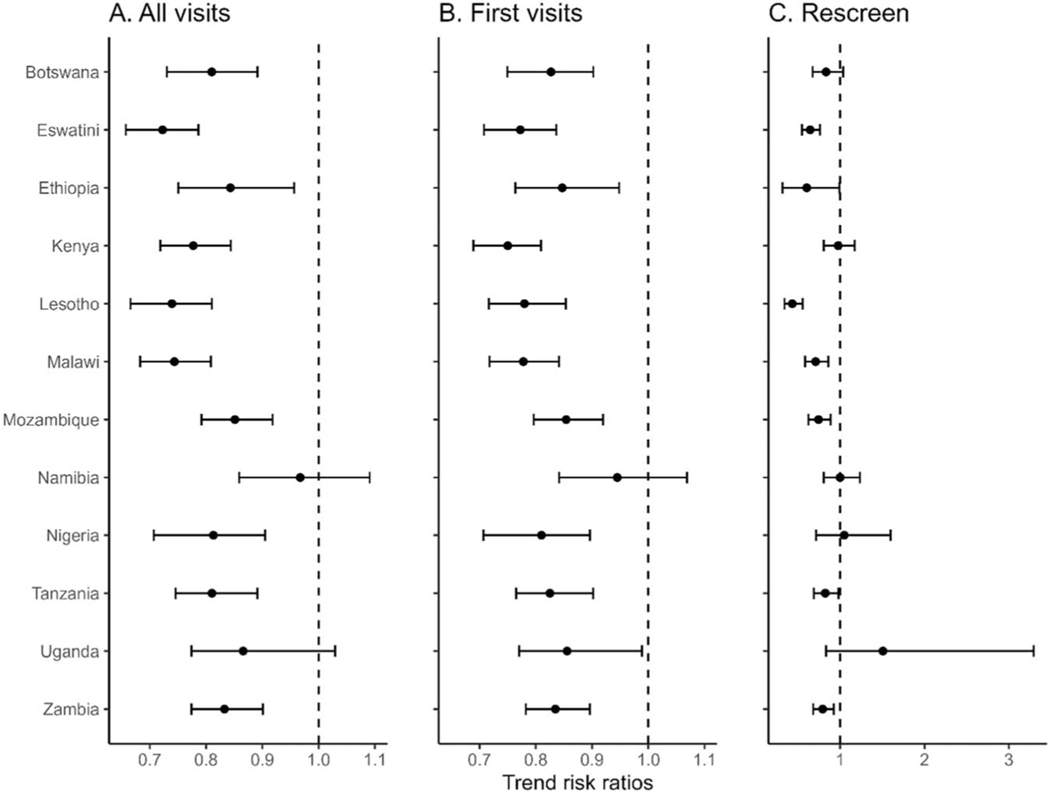
The best fit model included random country-level logscale slopes and intercepts and the main effect of age category; based on this best-fit model, age had no detectable effect on either the overall positivity rate or the temporal trend in the positivity rate. Risk ratios for annual trends in precancers or suspected cervical cancer cases varied by country for both first visits and rescreening visits and all visit-type combined (Fig. 2, see Table, Supplemental Digital Content, http://links.lww.com/QAI/C109). The screen-positive rate at first visit decreased over the observation interval (trend risk ratios < 1) in all countries except Namibia. The screen-positive rate at rescreening visits decreased over time in Eswatini, Lesotho, Malawi, Mozambique, and Zambia. Across all visit types, the positivity rate decreased over time in all countries except Namibia and Uganda.
FIGURE 2.
Risk ratios1 for annual trends in screening test positivity2 for each additional year among women living with HIV in CDC-PEPFAR–supported cervical cancer prevention programs, April 2018–March 2022. Note the expanded scale for trend risk ratios in C. 1Risk ratios of 1.0 imply constant proportions over time, and negative ratios imply exponential declines in positive screens. Horizontal bars are uncertainty intervals containing the true risk ratios with 0.95 probability. 2Positive cervical cancer tests include positive for precancerous lesions and for suspected invasive cervical cancers.
DISCUSSION
Since 2018, cervical cancer screening has been integrated into comprehensive HIV care for women who access services at CDC-PEPFAR–supported facilities. The number of screening tests increased in all countries, age groups, and visit types over time, and these growing programs identified thousands of opportunities to prevent significant morbidity and mortality due to cervical cancer. This integration of cervical cancer care and HIV services enhances the sustainability of both, in alignment with PEPFAR’s strategic plans to end the HIV/AIDS pandemic and WHO’s global strategy for cervical cancer elimination.22,23
Our analysis provides a comparison across programs in 13 African countries and identified variability in positivity rates by country. The factors that drive this variability likely include differences in national guidelines and cancer prevention infrastructure, as well as access to trained human resource capacity and cancer treatment, and the known variability in diagnostic accuracy of available screening tests, particularly VIA. Further cross-country collaboration to explore this variability may provide context for systematic improvements in cervical cancer screening test accuracy globally.
The positivity rate for cervical precancers of 5.4% observed in our study is lower than expected based on the published literature, where reported VIA positivity rates among WLHIV range 5.6%–55.9%, cytology 10.0%–20.8%, HPV DNA test 43.7%–50.6%, and HPV with VIA triage of 22.0%–57.4%.10 Although data disaggregation of positivity by screening modality was not possible in our data, most of these countries exclusively screen using VIA; those that have introduced HPV DNA testing have generally restricted to urban areas more amenable to sample transport and additional clinic visits for follow-up precancer care and with high-quality local laboratory services. The high estimated proportion of VIA-only testing within CDC-PEPFAR–supported programs during the study period offers some explanation for observed rates nearer the lower end of the expected range. In addition, most studies in the published literature that inform expectations include WLHIV screened before 2015, when universal ART was first recommended, and before 2013, when Option B+ began, which allowed for all pregnant WLHIV to initiate ART for life regardless of CD4 results.24 Therefore, in contrast to the consistently high proportion of ART use among our PEPFAR population, which is approaching the UNAIDS target of 95% of the population living with HIV on effective treatment in some countries,8 WLHIV included in the earlier published cervical cancer screening studies may not have been on a consistent prolonged ART regimen, which is protective against HPV acquisition and persistence, progression to CIN2+, and visible precancers.4,25
HPV infection rates are highest overall in young women near the age of first lifetime sexual intercourse, precancer rates peak in young adult women, and cervical cancer rates significantly increase with increasing age.26,27 Age was not statistically significantly associated with positivity in our data, likely because the analysis combined precancers and suspected invasive cervical cancers, which peak in different age groups. We did observe the highest rate for precancers in 25–29-year-old WLHIV and the highest rate for suspected ICC among WLHIV older than 50 years compared with all other age groups, consistent with our understanding of patterns of HPV-associated disease across the lifespan.
The reasons for the declining screen-positive rate trends we observed over the 4 years of reporting in first-time screens in all countries, and in rescreening in many countries, are unclear. Observed rates are sometimes higher with less-experienced providers; the observed declines may indicate a maturing facility staff proficiency level.13 Conversely, if screening programs expand rapidly without adequate training and supervision, lower rates may indicate a growing proportion of false negatives, missed precancers, and worse outcomes for the women we serve.
Both PEPFAR and WHO stress the importance of quality assurance evaluations and continuous quality improvement activities to ensure that cervical cancer screening services improve and maintain the health and wellness of WLHIV globally. Rigorous training, ongoing retraining, supportive supervision, and ongoing support and guidance for difficult or ambiguous cases are all essential elements of a successful cancer screening program, and global resources are available to assist with implementation.28,29
Our analysis found that screen-positive rates from those rescreening after one or more previous negative screens were lower than first-time screens, likely due to the lower prevalence of HPV in the subpopulation. It will be informative to include relevant details of clinical history (immune status, ART regimen and duration, and HPV vaccination status, as vaccine becomes more available globally) in these systematic monitoring, evaluation, and improvement processes.
An important limitation of this report is that our analysis used PEPFAR MER indicator data, which does not include screening modality (HPV DNA testing, VIA, pap, and other). Similarly, we do not have access to clinical data including HPV vaccination status, ART regimen type and timing, HIV viral load status, and comorbidities. Although countries follow PEPFAR guidance when reporting data, data quality and reporting vary across countries and across facilities.
CONCLUSION
WLHIV are a population at higher risk for cervical disease; integrating cervical cancer prevention and ART services has accelerated progress toward reducing this risk. Continuation of these prevention efforts, and expansion where relevant, can significantly contribute to the global effort to eliminate cervical cancer. This comparison across countries with cervical cancer screening implementation ongoing in CDC-PEPFAR–supported facilities identified a wide range of positivity rates; outcomes at both the low and the high end of this range should prompt further internal review and create opportunities for cross-country collaboration. We found a lower-than-expected screen-positive rate among WLHIV; further investigation is needed to assess whether this is reflective of the positive effects of improved HIV treatment in this population of WLHIV and to ensure that cervical cancer prevention services are consistently of the highest quality.
Supplementary Material
ACKNOWLEDGMENTS
Joseph Kabanda, Evelyn Ngugi, and Mamorapeli Ts’oeu; CDC Uganda, CDC Kenya, and CDC Lesotho.
Supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC).
Footnotes
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
DATA AVAILABILITY
The data underlying this article belong to the 13 host country governments and are not publicly available. Select variables are included in a publicly available data set PEPFAR Panorama Spotlight.
REFERENCES
- 1.World Health Organization (WHO). WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva, Switzerland: World Health Organization (WHO); 2007. Available at: https://apps.who.int/iris/bitstream/handle/10665/43699/9789241595629_eng.pdf?sequence=1&isAllowed=y. Accessed September 12, 2022. [Google Scholar]
- 2.Stelzle D, Tanaka LF, Lee KK, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021;9: e161–e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer (IARC). Cervical Cancer Screening. IARC Handbook of Cancer Prevention. Lyon: International Agency for Research on Cancer (IARC); 2022:Vol 18:1–456. [Google Scholar]
- 4.Liu G, Sharma M, Tan N, et al. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018;32:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Looker KJ, Rönn MM, Brock PM, et al. Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J Int AIDS Soc. 2018;21:e25110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer (IARC); 2020. Available at: https://gco.iarc.fr/today. Accessed September 12, 2022. [Google Scholar]
- 8.The Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV and AIDS Statistics—2021 Fact Sheet. Geneva: Joint United Nations Programme on HIV/AIDS; 2022. Available at: https://aidsinfo.unaids.org/. Accessed November 16, 2022. [Google Scholar]
- 9.World Health Organization (WHO). WHO Guideline for Screening and Treatment of Cervical Pre-cancer Lesions for Cervical Cancer Prevention, Second Edition. Geneva: World Health Organization; 2021. [PubMed] [Google Scholar]
- 10.Kelly HJI, Chung M, Arbyn M, et al. Evidence reviews of interventions and accuracy of tests for women living with HIV. WHO Guidelines For Screening and Treatment of Cervical Precancer Lesions for Cervical Cancer Prevention, Second Edition. Geneva, Switzerland: World Health Organization; 2021:17–34. Available at: https://apps.who.int/iris/bitstream/handle/10665/342366/9789240030886-eng.pdf. Accessed September 12, 2022. [Google Scholar]
- 11.Alliance for Cervical Cancer Prevention (ACCP). Planning and Implementing Cervical Cancer Prevention and Control Programs. Lyon: Alliance for Cervical Cancer Prevention (ACCP); 2004. Available at: https://screening.iarc.fr/doc/ACCP_screen.pdf. Accessed September 12, 2022. [Google Scholar]
- 12.US President’s Emergency Plan for AIDS Relief (PEPFAR). The PEPFAR 2022 Country Operational Plan Guidance. Washington, DC: US Department of State; 2022. Available at: https://www.state.gov/2022-country-operational-plan-guidance/#:;:text=COP%2FROP%202022%20guidance%20for%20program%20implementation%20in%20FY,health%20systems%20and%20capabilities%2C%20and%20establishing%20lasting%20collaborations. Accessed September 12, 2022. [Google Scholar]
- 13.World Health Organization (WHO). Monitoring National Cervical Cancer Prevention and Control Programmes: Quality Control and Quality Assurance for Visual Inspection with Acetic Acid (VIA)-based Programmes. Geneva: World Health Organization (WHO); 2013. Available at: https://apps.who.int/iris/handle/10665/79316. Accessed September 12, 2022. [Google Scholar]
- 14.Castle PE, Qiao YL, Zhao FH, et al. Clinical determinants of a positive visual inspection after treatment with acetic acid for cervical cancer screening. BJOG. 2014;121:739–746. [DOI] [PubMed] [Google Scholar]
- 15.George W. Bush Institute. Go Further Fact Sheets. Dallas: George W. Bush Institute; 2021. Available at: https://www.bushcenter.org/publications/resources-reports/reports/go-further-fact-sheets.html. Accessed September 12, 2022. [Google Scholar]
- 16.Godfrey C, Prainito A, Lapidos-Salaiz I, et al. Reducing cervical cancer deaths in women living with HIV: PEPFAR and the Go Further partnership. Prev Med. 2021;144:106295. [DOI] [PubMed] [Google Scholar]
- 17.US Department of State. Monitoring, Evaluation, and Reporting Indicator Reference Guide Version 2.6.1. Washington, DC: US Department of State; 2022. [Google Scholar]
- 18.RFoundation. R: A Language and Environment for Statistical Computing. Vienna: R Core Team; 2022. Available at: https://www.R-project.org. Accessed September 15, 2022. [Google Scholar]
- 19.Goodrich B, Gabry J, Ali I, et al. rstanarm: Bayesian Applied Regression Modeling via Stan. New York: rstanarm; 2022. Available at: https://mc-stan.org/rstanarm/. Accessed September 15, 2022. [Google Scholar]
- 20.Carpenter B, Gelman A, Hoffman MD, et al. Stan: a probabilistic programming language. J Stat Softw. 2017;76:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vehtari A, Gelman A, Gabry J, et al. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput. 2017;27: 1413–1432. [Google Scholar]
- 22.US Department of State. Fulfilling America’s Promise to End the HIV/ AIDS Pandemic by 2030: PEPFAR’s Five-Year Strategy. Washington, DC: United States Department of State; 2022. Available at: https://www.state.gov/wp-content/uploads/2022/11/PEPFARs-5-Year-Strategy_WAD2022_FINAL_COMPLIANT_3.0.pdf. Accessed June 30, 2023. [Google Scholar]
- 23.World Health Organization (WHO). Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneva, Switzerland: World Health Organization (WHO); 2020. Available at: https://www.who.int/publications/i/item/9789240014107. Accessed February 6, 2023. [Google Scholar]
- 24.Gopalappa C, Stover J, Shaffer N, et al. The costs and benefits of Option B+ for the prevention of mother-to-child transmission of HIV. AIDS. 2014;28(suppl 1):S5–S14. [DOI] [PubMed] [Google Scholar]
- 25.Kelly HA, Sawadogo B, Chikandiwa A, et al. Epidemiology of high-riskhuman papillomavirus and cervical lesions in African women living with HIV/AIDS: effect of anti-retroviral therapy. AIDS. 2017;31:273–285. [DOI] [PubMed] [Google Scholar]
- 26.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus andcervical cancer. Lancet. 2007;370:890–907. [DOI] [PubMed] [Google Scholar]
- 27.Schiffman M, Castle PE. The promise of global cervical-cancer prevention. N Engl J Med. 2005;353:2101–2104. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO). Improving Data for Decision Making: A Toolkit for Cervical Cancer Prevention and Control Programmes. Geneva: World Health Organization (WHO); https://www.who.int/publications/i/item/9789241514255 (2018, February 6, 2023). [Google Scholar]
- 29.World Health Organization (WHO). Improving Data for Decision Making: A Toolkit for Cervical Cancer Prevention and Control Programmes. Geneva, Switzerland: World Health Organization (WHO); 2018. Available at: https://www.who.int/publications/i/item/9789241514255. Accessed February 6, 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article belong to the 13 host country governments and are not publicly available. Select variables are included in a publicly available data set PEPFAR Panorama Spotlight.