Abstract
Background:
Immune checkpoint inhibitors (ICIs) have revolutionized the management of advanced melanoma (AM). However, data on ICI effectiveness have largely been restricted to clinical trials, thereby excluding patients with coexisting malignancies. Chronic lymphocytic leukemia (CLL) is the most prevalent adult leukemia and is associated with increased risk of melanoma. CLL alters systemic immunity and can induce T-cell exhaustion, which may limit the efficacy of ICIs in patients with CLL. We, therefore, sought to examine the efficacy of ICI in patients with these co-occurring diagnoses.
Patients and methods:
In this international multicenter study, a retrospective review of clinical databases identified patients with concomitant diagnoses of CLL and AM treated with ICI (US-MD Anderson Cancer Center, N = 24; US-Mayo Clinic, N = 15; AUS, N = 19). Objective response rates (ORRs), assessed by RECIST v1.1, and survival outcomes [overall survival (OS) and progression-free survival (PFS)] among patients with CLL and AM were assessed. Clinical factors associated with improved ORR and survival were explored. Additionally, ORR and survival outcomes were compared between the Australian CLL/AM cohort and a control cohort of 148 Australian patients with AM alone.
Results:
Between 1997 and 2020, 58 patients with concomitant CLL and AM were treated with ICI. ORRs were comparable between AUS-CLL/AM and AM control cohorts (53% versus 48%, P = 0.81). PFS and OS from ICI initiation were also comparable between cohorts. Among CLL/AM patients, a majority were untreated for their CLL (64%) at the time of ICI. Patients with prior history of chemoimmunotherapy treatment for CLL (19%) had significantly reduced ORRs, PFS, and OS.
Conclusions:
Our case series of patients with concomitant CLL and melanoma demonstrate frequent, durable clinical responses to ICI. However, those with prior chemoimmunotherapy treatment for CLL had significantly worse outcomes. We found that CLL disease course is largely unchanged by treatment with ICI.
Keywords: chronic lymphoid leukemia, melanoma, immune checkpoint inhibitors, anti-PD-1, anti-CTLA-4
INTRODUCTION
Chronic lymphocytic leukemia (CLL) represents the most prevalent adult leukemia in the Western world.1 Defined by monoclonal proliferations of mature B cells in the peripheral blood, CLL is a low-grade lymphoproliferative disease with a heterogeneous clinical course.2 In CLL, malignant B-cell expansion has been shown to impede T-lymphocyte function, leading to impairment of cellular and humoral-mediated immune responses. CLL-associated immunosuppression has been hypothesized to impair tumor recognition which ultimately could lead to immune evasion. Thus, patients with CLL are at increased risk of secondary malignancies, and multiple primary cancer types have been shown to be more aggressive in the context of concomitant CLL.3 Compared to the general population, patients with CLL have a 3.8-fold increased incidence of melanoma.4 Previous reports on concomitant CLL and melanoma have described worse melanoma-related outcomes, including increased risk of recurrence and metastasis, and overall worse prognosis.5–7
In recent years, significant advances have been made in advanced melanoma (AM) management with the advent of immune checkpoint inhibitors (ICIs). However, given the exclusionary nature of clinical trials, results of ICI use among patients with CLL and AM have not been reported. Numerous CLL-related T-cell abnormalities have been described that may diminish ICI efficacy including impaired CD4 signaling, reversed CD4 : CD8 T-cell ratio, and higher cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death-ligand 1 (PD-L1) expression leading to T-cell proliferation and exhaustion.8,9 Lack of clinical data, in combination with these immunologic concerns, has led to clinical uncertainty surrounding the use of ICI and their efficacy for AM and the potential impact on the course of CLL.
In this multi-institutional and international study, retrospective clinical databases of patients with concomitant CLL and AM treated with ICI were utilized to assess ICI response rates and survival outcomes. CLL factors associated with improved AM-related outcomes following ICI treatment were assessed. Furthermore, immune-related adverse events after ICI and the impact of ICI on CLL status were explored.
METHODS
Study design and patients
Electronic medical records at the University of Texas MD Anderson Cancer Center (MDA) and Mayo Clinic were queried for patients diagnosed with concomitant CLL and AM between July 1997 and April 2020 who were treated with ICI for AM. ICI treatments included anti-programmed cell death protein 1 (PD-1), anti-CTLA-4, or a combination of these agents. Audit of prospectively maintained clinical databases from seven tertiary Australian centers resulted in the identification of an Australian cohort (AUS-CLL) of patients with concomitant CLL and AM treated with ICI. Lastly, a control cohort (AUS-CTRL) of patients with AM alone treated with ICI (N = 148) was identified by a retrospective review of records from these Australian centers.
Clinical and pathological data were extracted from medical records and summarized, including: demographic characteristics; clinicopathological features of CLL and melanoma; and treatments received and their outcomes (response, relapse, and progression). Objective radiographic response to ICI was described by an independent dual-physician review using RECIST version 1.1 guidelines.10 Modification of RECIST evaluation was required given concurrent lymphadenopathy from CLL; pathologic lymph nodes were excluded as target or nontarget lesions unless involved by melanoma by biopsy confirmation. Progression-free survival (PFS) was defined as the interval between ICI initiation and date of melanoma progression, or if progression or relapse did not occur, date of death or last follow-up. Overall survival (OS) was defined as the interval between ICI initiation and date of death from any cause or last follow-up.
Data analysis
To assess response to ICI and survival after ICI in patients with CLL and AM compared with AM alone, we compared objective response rate [ORR, defined as complete response (CR) or partial response (PR) by RECIST], as well as OS and PFS between the AUS-CLL and AUS-CTRL cohorts. Next, among patients with concomitant CLL and AM (MDA, Mayo, and AUS-CLL), clinicopathological characteristics, treatment response, and survival outcomes were summarized.
To assess whether prior treatment for CLL had an impact on ICI response and survival, patients with CLL and AM were stratified into two clinical cohorts: prior/concurrent CLL treatment and CLL observation. ORR, OS, and PFS were compared between these clinical cohorts. Sub-analysis by type of CLL treatment (treatment with chemoimmunotherapy versus targeted therapy) was carried out.
Statistical analysis
Patient characteristics were summarized using frequency (percentage) for categorical variables and median [with interquartile range (IQR)] for continuous variables. Chi-square and Fisher’s exact tests were used to assess differences in categorical variables. Wilcoxon rank-sum tests were carried out to assess differences in continuous variables. Univariate analyses were carried out to examine differences in clinicopathological variables and ORR. OS and PFS were estimated using the Kaplan–Meier method. Log-rank tests were used to compare OS and PFS among subgroups. The Kaplan–Meier method was used to generate survival curves by clinical characteristics.
RESULTS
In total, 58 patients with concomitant CLL and AM treated with ICI were identified (US-MDA, N = 24; US-Mayo, N = 15; AUS-CLL, N = 19), while 148 patients with melanoma alone treated with ICI were identified (AUS-CTRL) (Figure 1A). Of the 58 patients with concomitant CLL and AM, 15 patients received additional ICI therapies after first-line ICI following disease progression or relapse, amounting to 79 total ICI therapies. A primary analysis of this study focused on first-line therapies, though ORR and survival outcomes were described for all treatments.
Figure 1. AM response rates and survival following ICI, in the absence or presence of concomitant CLL.
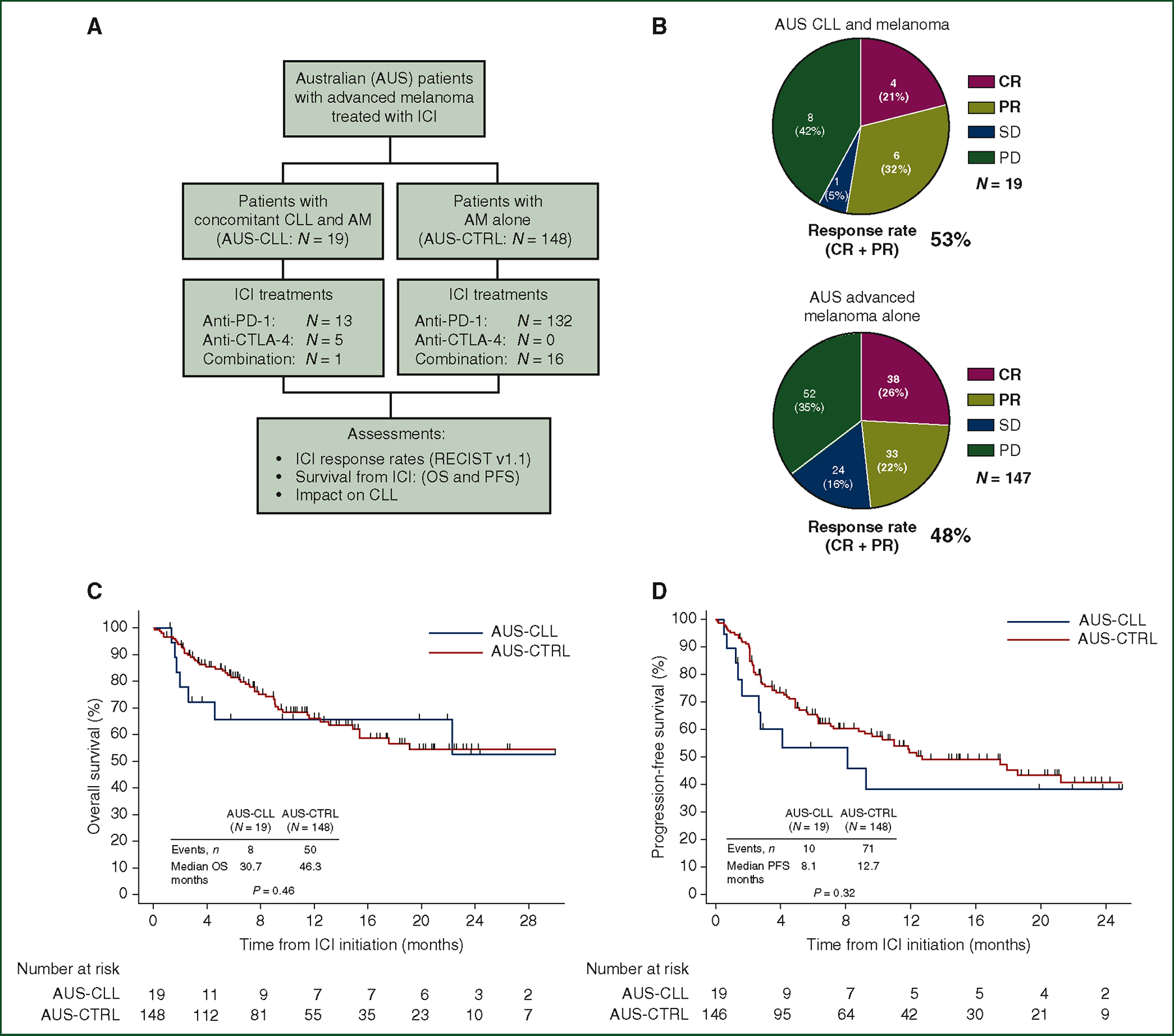
(A) CONSORT diagram describing the comparison of ICI-dependent outcomes between Australian patients with concomitant CLL and AM (N = 19) versus AM alone (N = 148). (B) Pie charts describing radiographic response by RECIST v1.1 assessment in Australian cohorts of patients treated with ICI for AM in the presence of concomitant CLL (top, AUS-CLL, N = 19) or absence of concomitant CLL (bottom, AUS-CTRL, N = 147, *response data available for 147 patients). Objective responses (CR or PR) resulted in 53% of patients with CLL and AM versus 48% in patients with AM alone (P = 0.81). (C) Kaplan–Meier plot comparing OS between patients with concomitant CLL and AM (AUS-CLL, P = 19) and AM alone (AUS-CTRL) (P = 167, P = 0.46 by log-rank test). (D) Kaplan–Meier plot comparing PFS between patients with concomitant CLL and AM (AUS-CLL, N = 19) and AM alone (AUS-CTRL) (N = 167, P = 0.32 by log-rank test).
AM, advanced melanoma; CLL, chronic lymphocytic leukemia; CR, complete response; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; OS, overall survival; PD, progressive disease; PD-1, programmed cell death protein 1; PFS, progression-free survival; PR, partial response; SD, stable disease.
Melanoma ICI outcomes in the absence or presence of CLL
To compare response and survival following ICI in patients with AM in the absence or presence of concomitant CLL, we first examined outcomes within the Australian population with concomitant CLL and AM (N = 19) and AM alone (N = 148). AUS-CTRL patients were older compared to the AUS-CLL patients (78% versus 47% were ≥ 65 years, P = 0.009) (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2023.06.007). A review of the staging in the eighth edition of the American Joint Committee on Cancer at ICI initiation revealed more visceral involvement among AUS-CTRL patients compared to AUS-CLL patients (80% versus 42%, P = 0.001). All treatments in the Australian population were first-line ICI, though type of ICI received varied between cohorts (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2023.06.007, P< 0.001). Both cohorts had similar rates of prior non-ICI melanoma treatments (AUS-CLL: 11% versus AUS-CTRL: 29%, P = 0.10). Lactate dehydrogenase (LDH) levels at ICI onset were similar between cohorts [AUS-CLL: 277 U/l (IQR 222–363 U/l) versus AUS-CTRL: 243 U/l (IQR 201–285 U/l), P = 0.15].
ORR to ICI was similar between the AUS-CLL and AUS-CTRL cohorts (Figure 1B: 53% versus 48%, P = 0.81). Among AUS-CLL patients who achieved an objective response (N = 10/19), median duration of response (DOR) was 13 months. Data on DOR were unavailable for the AUS-CTRL cohort. OS and PFS were similar between the AUS-CLL and AUS-CTRL cohorts (Figure 1C: OS, 31 versus 46 months, P = 0.46; Figure 1D: PFS, 8 versus 13 months, P = 0.32).
Characteristics of patients with concomitant CLL and melanoma
Among all US and Australian patients with concomitant CLL and AM (N = 58), 58 first-line and 21 subsequent ICI therapies were administered. First-line therapies included 34 anti-PD-1, 18 anti-CTLA-4, and 6 combination therapies.
Clinicopathologic features of CLL and AM are summarized in Table 1 and Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2023.06.007, respectively. Overall, a majority of patients with available Rai staging11 data had low-risk CLL (56%, N = 25/45) and 64% (N = 37/58) were on observation at ICI onset, with no previous CLL treatment.
Table 1.
CLL characteristics among patients with concomitant CLL and advanced melanoma
| All patients (N = 58) |
No prior CLL treatment (N = 37) |
Prior/concurrent CLL treatment (N = 21) |
P | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
|
| |||||||
| Age at CLL diagnosis, years Median (IQR) | 63 | (57–71) | 63 | (58–71) | 62 | (57–67) | 0.57 |
| CLL Rai stage | N = 45a | N = 28a | N = 17a | 0.34 | |||
| Low risk (0) | 25 | 56 | 17 | 61 | 8 | 47 | |
| Intermediate risk (I/II) | 19 | 42 | 11 | 39 | 8 | 47 | |
| High risk (III/IV) | 1 | 2 | 0 | 0 | 1 | 6 | |
| CLL FISH | N = 39a | N = 22a | N = 17a | ||||
| Del(17p) | 3 | 8 | 1 | 5 | 2 | 12 | 0.57 |
| Trisomy 12 | 7 | 18 | 5 | 23 | 2 | 12 | 0.44 |
| Del(13q) | 16 | 41 | 10 | 46 | 6 | 35 | 0.74 |
| Del(11) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Negative | 15 | 39 | 7 | 32 | 8 | 47 | 0.51 |
| IGHV | N = 22a | N = 12a | N = 10a | 0.004 | |||
| Mutated | 10 | 46 | 9 | 75 | 1 | 10 | |
| Unmutated | 12 | 55 | 3 | 25 | 9 | 90 | |
| N = 29a | N = 18a | N = 11a | |||||
| LDH at CLL diagnosis, median (IQR) | 230 | (179–395) | 338 | (205–427) | 184 | (178–229) | 0.018 |
| CLL mutational analysis | N = 16a | N = 8a | N = 8a | ||||
| TP53 | 5 | 31 | 1 | 13 | 4 | 50 | 0.28 |
| FBXW7 | 1 | 6 | 0 | 0.0 | 1 | 13 | 0.30 |
| SF3B1 | 3 | 18 | 1 | 13 | 2 | 25 | 0.52 |
| MYD88 | 1 | 6 | 1 | 13 | 0 | 0 | 0.30 |
| MUC2 | 1 | 6 | 1 | 13 | 0 | 0 | 0.30 |
| SPEN | 1 | 6 | 1 | 13 | 0 | 0 | 0.30 |
| N = 50c | N = 30c | N = 20c | |||||
| LDH (U/l) at ICI initiation, median (IQR) | 293 | (212–462) | 294 | (214–427) | 272 | (192–470) | 0.69 |
| CLL treatment before ICI | 21 | 36.2 | 0 | 0.0 | 21 | 100.0 | |
| Targeted therapyb | 10 | 17.2 | 0 | 0.0 | 10 | 47.6 | |
| Chemoimmunotherapyc | 11 | 18.9 | 0 | 0.0 | 11 | 52.4 | |
| Months between CLL and AM diagnoses, median (IQR) | 39 | (3–96) | 42 | (2–112) | 36 | (5–78) | 0.70 |
AM, advanced melanoma; CLL, chronic lymphocytic leukemia; ICI, immune checkpoint inhibitor; IGHV, immunoglobulin heavy chain variable region gene; IQR, interquartile range; LDH, lactate dehydrogenase.
N refers to the number of patients with available data for each category.
Targeted therapies included non-T-lymphocyte-depleting CLL therapies.
Chemoimmunotherapies included T-lymphocyte-depleting agents bendamustine, fludarabine, and alemtuzumab.
Response to ICI in patients with concomitant CLL and melanoma
All ICI treatments, including first-line and subsequent therapies, were evaluated for response and survival outcomes. Four patients received ICI in the adjuvant setting, two as first-line and two as subsequent therapies and were excluded from RECIST evaluation. Among all evaluable first-line therapies (N = 56), ORR was 41% (N = 23/56) and median DOR was 18 months (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2023.06.007). ORR by ICI subtype was 39% for anti-PD-1 (N = 13/33), 39% for anti-CTLA-4 (N = 7/18), and 60% for combination ICI (N = 3/5). Subsequent ICI therapies were associated with a lower ORR of 21% (N = 4/19) (anti-PD-1: 17%, N = 2/12; anti-CTLA-4: 33%, N = 1/3; combination: 25%, N = 1/4). Percentage change in the size of metastatic melanoma (sum of longest diameters by RECIST) in response to ICI was available for the US-cohort patients (MDA and Mayo); response to first-line ICI therapies for US patients, categorized by ICI subtype, is illustrated in Figure 2B.
Figure 2. Sub-analysis of ICI-dependent outcomes (ORR, OS, and PFS) among patients with concomitant CLL and AM by ICI subtype and patient history of prior CLL therapy.
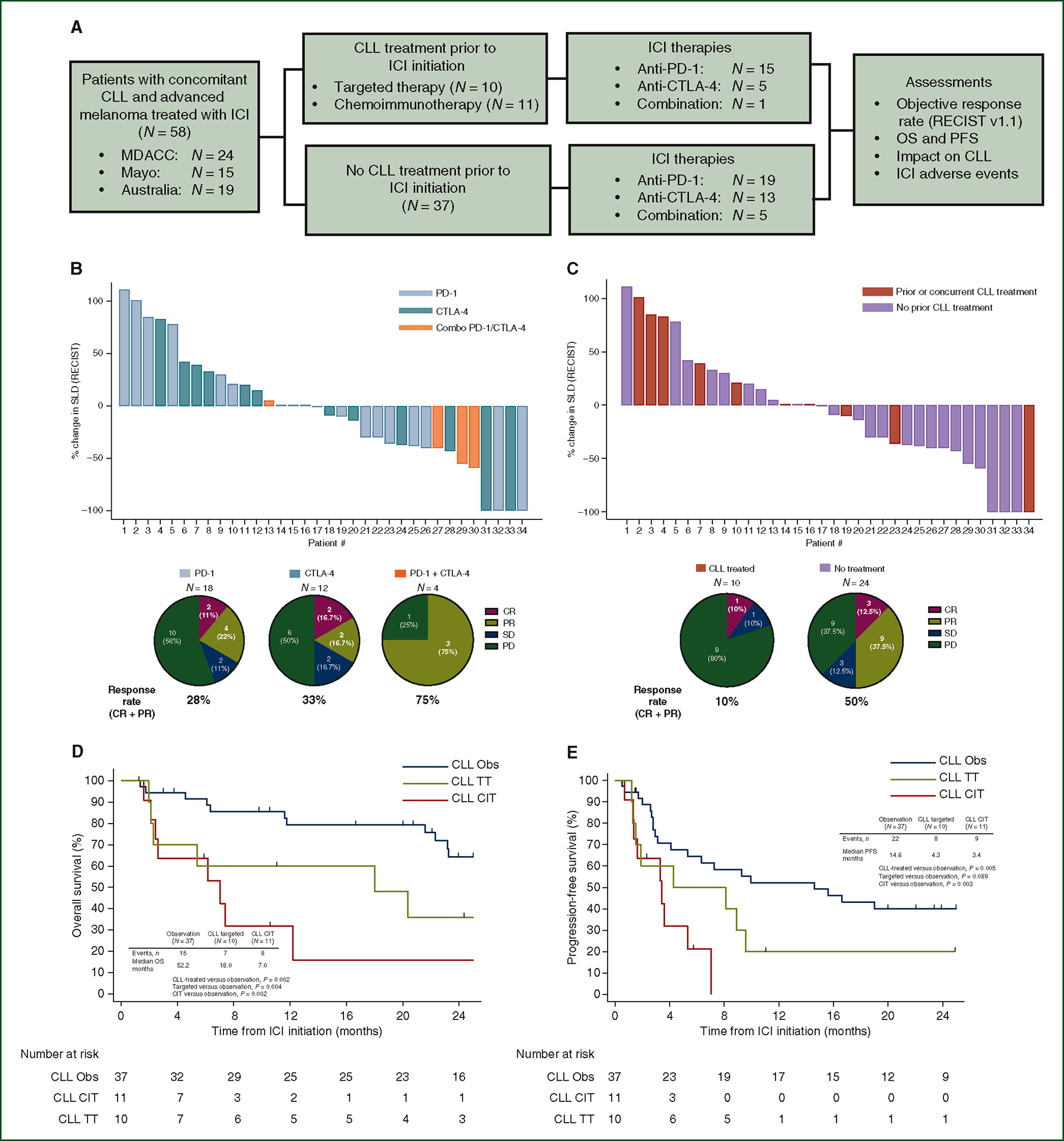
(A) CONSORT diagram describing the comparison of ICI-dependent outcomes among all patients with concomitant CLL and AM, stratified by whether patients had prior treatment for their CLL. (B) Waterfall plot describing response to first-line ICI as percentage change in SLD of metastatic melanoma by RECIST v1.1, stratified by type of ICI subtype. These plots display evaluable radiographic responses (N = 34) from ICI treatments from the US cohorts (N = 39). Pie charts, below, further describe radiographic response in this cohort. (C) Waterfall plots describing radiographic response to first-line ICI as percentage change SLD of metastatic melanoma, stratified by whether patients had received prior CLL treatment. These plots include evaluable radiographic responses (N = 34) from ICI treatments from the US cohorts (N = 39). Pie charts, below, further describe response in this cohort. Kaplan–Meier plots comparing OS (D) and PFS (E) between patients with no prior CLL treatment (CLL observation), prior treatment with targeted therapies, or prior treatment with chemoimmunotherapy.
AM, advanced melanoma; CLL, chronic lymphocytic leukemia; CR, complete response; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; OS, overall survival; PD, progressive disease; PD-1, programmed cell death protein 1; PFS, progression-free survival; PR, partial response; SD, stable disease; SLD, sum of longest diameter.
Effect of CLL treatment on ICI response in patients with concomitant CLL and melanoma
Because of the potential long-lasting immunosuppression and myelosuppression associated with certain CLL therapies, response to ICI was compared among patients who received prior or concurrent treatment and patients on observation for their CLL (Figure 2C and Supplementary Figures S1 and S2, available at https://doi.org/10.1016/j.annonc.2023.06.007). Prior or concurrent treatment for CLL was utilized before 30 of the 79 total ICI therapies and 21 of the 58 first-line ICI therapies, respectively. CLL treatments were categorized as targeted therapy (TT), which included non-T-lymphodepleting agents, or chemoimmunotherapy (CIT), which included T-lymphodepleting agents, fludarabine, bendamustine, and alemtuzumab. Demographic, clinicopathological, and treatment variables between CLL-treated and observation patients are described in Table 1 and Supplementary Tables S2 and S3, available at https://doi.org/10.1016/j.annonc.2023.06.007. Neither LDH level nor CNS involvement, two factors thought to impact anti-PD-1 response, were different between cohorts at the start of ICI treatment. Among patients with available immunoglobulin heavy chain variable region gene (IGHV) mutation testing (N = 22), patients with prior CLL treatment were more likely to have unmutated IGHV compared to observed patients (90%, N = 9/10 versus 25%, N = 3/12, P = 0.002). At ICI initiation, laboratory parameters were similar between CLL-treated and observed groups, except for absolute lymphocyte count which was lower for CLL-treated patients [2.8 (1.2–5.4) versus 7.8 (2.7–26.5), P = 0.012].
ORR to first-line ICI treatments was significantly lower for patients with prior/concurrent treatment for CLL compared to observation patients (21%, N = 4/19 versus 51%, N = 19/37, P = 0.044) (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2023.06.007). Among all ICI therapies, ORR was also lower for previously treated patients (19%, N = 5/26 versus 45%, N = 22/49, P = 0.042) (Supplementary Figure S2, available at https://doi.org/10.1016/j.annonc.2023.06.007). Among the patients treated for CLL before first-line ICI, there was a non-significant trend toward lower ORR from assessable CIT-treated compared to TT-treated patients (11%, N = 1/9 versus 33% N = 3/9, P=0.26).
Median DOR was longer for patients on observation compared to CLL-treated patients, although this did not reach statistical significance for all ICI treatments, nor for first-line ICI (all ICI: 19 versus 6 months, P = 0.13; first-line: 18 versus 6 months, P = 0.37).
Survival outcomes in patients with concomitant CLL and AM
Among the 58 patients, 29 were dead at the time of last follow-up (25 melanoma-related deaths). No deaths were related to CLL. Median OS from ICI initiation was 26 months and median PFS for all 79 ICI treatments was 5 months. Median OS was 26 months and PFS was 7 months for first-line ICI treatments. Survival outcomes did not significantly vary by ICI subtype (median OS, PD-1: 26, CTLA-4: 31, Combo: 23 months, P = 0.91; median PFS, PD-1: 7, CTLA-4: 5, Combo: 10 months, P = 0.76). Next, we assessed whether prior/concurrent treatment for CLL impacted survival outcomes. Of the first-line ICI treatments, median OS was 7 months for CLL-treated patients (N = 21) and 52 months for patients on observation (N = 37) (P = 0.002). Median OS among observation patients was longer than CIT-treated patients (52 versus 7 months, P = 0.002) and TT-treated patients (52 versus 18 months, P = 0.009), (Figure 2D). Median OS was shorter in patients treated with CIT compared to TT (7 versus 18 months, P = 0.43); however, the difference did not reach statistical significance. Median PFS was longer for patients on observation than for treated patients (15 versus 4 months, P = 0.005) and CIT-treated patients specifically (15 versus 3 months, P = 0.003) (Figure 2E). A similar trend was seen between observation and TT-treated patients, though this did not reach statistical significance (PFS 15 versus 4 months, P = 0.089).
Effects of ICI on CLL status
CLL status was assessed with the International Workshop on Chronic Lymphocytic Leukaemia criteria (iwCLL) and was carried out before ICI and 3 months following ICI initiation.12 At the 3-month assessment, patients were classified as having disease stability or progression. Data were available for 49 ICI treatments overall. Stable CLL was noted in 94% (N = 46/49) of patients after ICI and no difference was seen between groups with prior/concurrent history of CLL treatment or observation (94%, N = 29/31 versus 94%, N = 17/18, P = 0.90). Three patients developed CLL progression while on anti-PD-1 therapy, though in two of these patients, the AM had an objective, durable response (CR or PR) to ICI.
Immune-related adverse events
Overall, immune-related adverse events (irAEs) occurred following 39% of ICI (Supplementary Table S4, available at https://doi.org/10.1016/j.annonc.2023.06.007). No difference was identified between irAE rates following first-line or subsequent ICI treatments (38% versus 45%, P = 0.61), but a non-significant trend toward lower irAEs for single-agent compared to combination ICI was identified (36% versus 64%, P = 0.10). Rates of irAE were similar by CLL treatment status (33% versus 44%, P = 0.48). Despite the presence of concomitant CLL, few hematologic AEs occurred overall. Among all cohorts, thrombocytopenia occurred in 9% and thrombosis occurred in 9% of patients. Of note, in the AUS-CLL cohort, one patient on anti-PD-1 ICI developed autoimmune hemolytic anemia and another patient on anti-PD-1 therapy developed hemophagocytic lymphohistiocytosis, a severe inflammatory syndrome previously described in ICI literature and known to occur in patients with lymphoproliferative disorders.
DISCUSSION
The optimal management of patients with concomitant CLL and AM has posed significant challenges for oncologists. Because patients with CLL have been excluded from ICI trials for melanoma, formal guidelines are lacking for the treatment of this dual diagnosis. Due to the limited literature on the topic, there is clinical uncertainty about the efficacy of ICI, risk of irAEs, and the impact of ICI on CLL dynamics. To our knowledge, this study reports on the largest cohort of patients managed with ICI with these co-occurring diagnoses and is the first to compare outcomes against patients with melanoma alone.
In the Australian retrospective cohorts, we observed an ORR to ICI of 53% among patients with CLL and AM with encouraging OS and PFS, which were not significantly different from the studied cohort without CLL. We were not able to find similar reports comparing ICI-dependent melanoma outcomes among patients with and without concomitant CLL, likely because few centers have treated homogenous populations with these diagnoses with similar standards.
We recognize that the small sample size of patients with CLL and AM (N = 19), as well as the absence of matched cohorts, limits a definitive, formal statement on the efficacy of ICI in the absence or presence of CLL. We also acknowledge that differences in age, stage, and ICI treatments were observed between these two Australian cohorts. However, the comparable results observed in the CLL and AM cohort spurred further interest in evaluating ICI outcomes in a larger cohort and identifying factors that predict favorable outcomes.
Further analysis demonstrated that treatment of AM with ICI in the context of concomitant CLL could result in durable and frequent clinical responses. This finding is consistent with a recent small case series from a tertiary cancer center, in which anti-CTLA-4 treatment resulted in objective responses in 30% of patients (N = 3/10) with concomitant CLL and AM.13 Furthermore, in a national tumor registry study, patients with CLL and AM treated during the era of ICI use (2011 onward) demonstrated a survival benefit compared with prior time periods, supporting a beneficial effect of immunotherapies in patients with these co-occurring diagnosis.14 In our review, AM ORR for first-line ICI among all three cohorts was 41% (CTLA-4: 39%, N = 7/18; PD-1: 39%, N = 13/33; combination: 60%, N = 3/5). Incomparison, historicalresponse rates in melanoma have been reported at 10%–15%15,16 for anti-CTLA-4 therapy, 37%–40% for anti-PD-1,17,18 and 56%–61% for combination anti-PD-1/CTLA-4 therapy.17,19,20 Survival outcomes, OS and PFS, were also similar to historic values, further supporting that patients with CLL can clinically benefit from ICI despite their underlying immune dysfunction.
We aimed to identify clinicopathological factors associated with improved ICI-related outcomes in this population. Previous studies have reported that advanced CLL Rai stage correlates with inferior outcomes for non-basal-cell skin cancers, including melanoma.21 In our analysis, advanced Rai stage and the presence of del(17p)/TP53 aberrations were associated with inferior clinical outcomes, though this did not reach statistical significance on multivariate analysis. Importantly, TP53 aberrations, including those associated with del(17p), are known to be associated with poor prognosis in CLL. Interestingly, TP53 mutation identified in melanoma is also associated with worse outcomes and poor response to anti-CTLA-4 therapy in metastatic melanoma.22 Prior treatment for CLL was the only factor independently associated with worse OS and PFS; ORR was significantly lower among patients who received prior treatment for CLL (21% versus 51%). Furthermore, OS and PFS following ICI treatment were significantly longer for patients without prior CLL treatment. Given the profound and persistent immunosuppression and prolonged lymphopenia associated with certain treatments (fludarabine, bendamustine, and alemtuzumab), a sub-analysis of survival by CLL treatment type demonstrated that patients receiving these therapies had very poor outcomes following ICI treatment. In Figure 3, we highlight two clinical vignettes of patients with concomitant CLL/AM, one with low-risk CLL on observation who achieved a durable melanoma response following anti-CTLA-4 treatment, and another case in which a patient with high-risk CLL, with a history of fludarabine treatment, rapidly progressed on anti-PD-1 ICI.
Figure 3. Clinical vignettes of patients treated with ICI for AM in the context of concomitant CLL.
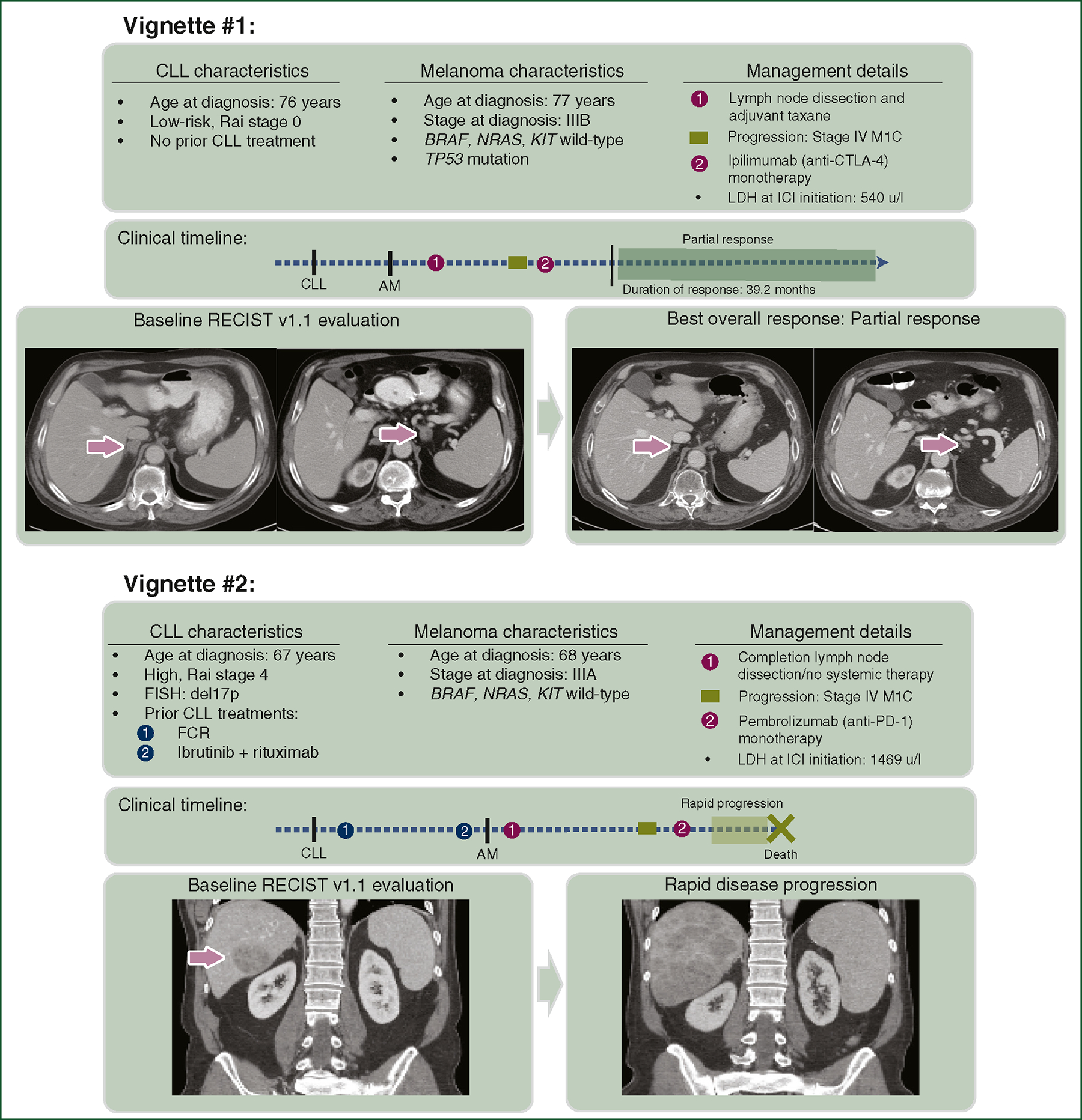
Clinical vignette #1 describes a 76-year-old male who was diagnosed with low-risk CLL and was managed on observation. A year later, he developed a cutaneous melanoma with clinically palpable nodal disease and was managed with wide local excision, lymph node dissection, and adjuvant taxane therapy. Months later, new metastatic disease was identified on surveillance imaging in bilateral adrenal glands. Following treatment with anti-CTLA-4 monotherapy, 3-month post-ICI imaging revealed a near-complete radiographic response. His response has been durable, with no relapse up to the date of this publication. Clinical vignette #2 describes a 67-year-old male who was diagnosed with Rai stage IV CLL with a high-risk 17p deletion by FISH, who was treated, first with combination fludarabine, cyclophosphamide, rituximab, then after CLL progression, with ibrutinib and rituximab. A year later, he developed a cutaneous melanoma treated with wide excision, sentinel lymph node biopsy, and subsequent lymph node dissection due to occult nodal disease. A year later, he developed a large hepatic metastasis and was treated with anti-PD-1 monotherapy. Unfortunately, follow-up imaging demonstrated rapid disease progression.
AM, advanced melanoma; CLL, chronic lymphocytic leukemia; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; PD-1, programmed cell death protein 1.
In previous studies, prior CLL treatment with fludarabine was associated with increased risk of developing melanoma and other malignancies.23 However, to our knowledge, no study to date has reported melanoma survival and outcomes following treatment with fludarabine or other immunosuppressant agents. A recent single-institution retrospective study reported that prior treatment of CLL was not associated with melanoma-related mortality among 56 patients with the dual diagnosis.24 That series included any-stage melanoma and only 25% of that population received immunotherapy, potentially contributing to the differences in outcomes seen in our ICI-focused study. Furthermore, authors of this review contend that immunosuppression from prior CLL treatment is unlikely to affect melanoma treatment if given years later. However, CLL treatment with chemoimmunotherapies can have long-term T-lymphocyte depletion, and in our analysis, were particularly associated with poor outcomes following ICI. The immunosuppressive mechanisms by which lymphodepleting agents interfere with ICI response are not completely understood. The landscape of treatment of CLL has changed recently and, particularly in the United States, chemoimmunotherapy has been replaced by targeted agents. Future studies are needed to investigate how patients with prior CLL treatment with targeted therapies respond to both ICI and other melanoma therapies.
Importantly, our experience did not identify an increased risk of CLL progression following ICI, regardless of CLL treatment history. In one case report of a patient with TP53-mutated CLL, a discordant clinical course was noted in which melanoma responded to anti-PD-1 ICI but developed rapidly progressive CLL.25 Conversely, other small series have not reported accelerated CLL disease progression in patients treated for AM with ICI. Additionally, in our experience, any-grade irAE occurred following 39% (N = 31/79) of all ICI treatments and 26% (N = 20/79) required ICI discontinuation. In contrast, two previously referenced reviews reported grade 3 or 4 toxicity in 40% of patients (N = 6/15)13 and AEs requiring cessation in 43% (N = 6/14).24 While AE rates vary significantly across studies, rates of grade 3 or 4 toxicity in the general melanoma population have been reported as high as 42% for ICI monotherapy and 59% for combination ICI.26,27 Overall, this review supports the relative safety of ICI in patients with CLL and AM, though selection of therapies should always be done cautiously with appropriate patient counseling.
The biological effects of ICI on CLL physiology need further evaluation. In patients with CLL, T cells have up-regulated PD-1 expression, and in preclinical models, PD-1 inhibition resulted in correction of leukemia-induced CD8 dysfunction and protected mice from CLL development.28 The PD-1/PD-L1 axis has been shown to be functionally intact in patients with CLL and, further, is hypothesized to contribute to T-cell function restoration in CLL. Due to these observations, trials have investigated PD-1 blockade for CLL and Richter’s transformations and observed ORRs of up to 44% for Richter’s transformation component whereas no changes were observed in the CLL component.29,30 T-cell CTLA-4 expression has also been shown to be significantly elevated in CLL and is associated with T-cell anergy.9 Whether CTLA-4 inhibition can revitalize immune responses among leukemic cells is in question, and clinical trials evaluating management of hematological malignancies with anti-CTLA-4 therapy are underway.
In addition to the limitations of the retrospective review, clinicopathological characteristics of patients, including treatment history, varied among our study populations. Prospective studies focused on ICI response, survival, and irAE rates in patients with CLL and AM would be needed to confirm our findings and to further explore CLL-related factors that either support or inhibit ICI response.
Conclusion
Treatment with ICI resulted in frequent durable clinical responses of AM without exacerbation of CLL or clearly increased irAEs, and thus should be considered a valuable therapeutic option in patients with concomitant CLL and melanoma. Our results also suggest that prior treatment for CLL may impact ICI efficacy and AM outcomes, and should be considered in patient management. Given the increasing use of ICI for many solid organ malignancies, confirmation of ICI effectiveness for treating other cancer types with concurrent CLL is necessary.
Supplementary Material
FUNDING
JAW is supported by NIH [grant number 1 R01 CA219896-01A1], Melanoma Research Alliance [grant number 4022024], American Association for Cancer Research Stand Up To Cancer [grant number SU2C-AACR-IRG-19-17], and MD Anderson Cancer Center’s Melanoma Moon Shots Program. JLM is supported by the Melanoma Research Alliance, an American Society of Clinical Oncology and Conquer Cancer Foundation Career Development Award, the Elkins Foundation, Seerave Foundation, Rising Tide Foundation, the Mark Foundation, an MD Anderson Cancer Center (MDACC) Melanoma SPORE (1 P50 CA221703-01A1) Developmental Research Program Award, the MD Anderson Cancer Center Melanoma Moon Shots Program, and the MD Anderson Physician Scientist Program and acknowledges the Transdisciplinary Research in Energetics and Cancer Research Training Workshop R25CA203650 and the MDACC Center for Energy Balance in Cancer Prevention and Survivorship. Alessandra Ferrajoli is supported by the MD Anderson’s Cancer Center CLL Moon Shots Program.
ROLE OF THE FUNDER/SPONSOR
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
DISCLOSURE
JAW is an inventor on a US patent application (PCT/US17/53.717); reports compensation for speaker’s bureau and honoraria from Imedex, Dava Oncology, Omniprex, Illumina, Gilead, PeerView, MedImmune, and Bristol-Myers Squibb (BMS); serves as a consultant/advisory board member for Roche/Genentech, Novartis, AstraZeneca, GlaxoSmithKline (GSK), BMS, Merck, Biothera Pharmaceuticals, and Micronoma. JAW holds stock options from Micronoma. JLM serves as a consultant for Merck and in advisory board for Bristol-Myers Squibb. GVL is a consultant advisor for Agenus, Amgen, Array Biopharma, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Evaxion, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA, Merck Sharpe & Dohme, Novartis, OncoSec, PHMR Ltd., Pierre Fabre, Provectus, Qbiotics, Regeneron. LW is an advisory board consultant/advisor for Novartis, Merck Sharp and Dohme, and Bristol-Myers Squibb. All other authors have declared no conflicts of interest.
DATA SHARING
The data that support the findings of this study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:23–33. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391:1524–1537. [DOI] [PubMed] [Google Scholar]
- 3.Solomon BM, Rabe KG, Slager SL, et al. Overall and cancer-specific survival of patients with breast, colon, kidney, and lung cancers with and without chronic lymphocytic leukemia: a SEER population-based study. J Clin Oncol. 2013;31:930–937. [DOI] [PubMed] [Google Scholar]
- 4.Olsen CM, Lane SW, Green AC. Increased risk of melanoma in patients with chronic lymphocytic leukaemia: systematic review and meta-analysis of cohort studies. Melanoma Res. 2016;26:188–194. [DOI] [PubMed] [Google Scholar]
- 5.Brewer JD, Christenson LJ, Weenig RH, Weaver AL. Effects of chronic lymphocytic leukemia on the development and progression of malignant melanoma. Dermatol Surg. 2010;36:368–376. [DOI] [PubMed] [Google Scholar]
- 6.Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignant melanoma and merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30:843–849. [DOI] [PubMed] [Google Scholar]
- 7.Royle JA, Baade PD, Joske D, et al. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105:1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motta M, Rassenti L, Shelvin BJ, et al. Increased expression of CD152 (CTLA-4) by normal T lymphocytes in untreated patients with B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1788–1793. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 11.Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234.1139039 [Google Scholar]
- 12.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. [DOI] [PubMed] [Google Scholar]
- 13.Smithy JW, Pianko MJ, Maher C, et al. Checkpoint blockade in melanoma patients with underlying chronic lymphocytic leukemia. J Immunother. 2021;44:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi R, Bordeaux JS, Rothermel L, Mangla A. Impact of immunotherapy on survival differences in patients with melanoma and chronic lymphocytic leukemia. J Clin Oncol. 2021;39:e21550. [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 17.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–1862. [DOI] [PubMed] [Google Scholar]
- 19.Postow MA,Chesney J,Pavlick AC,et al.Nivolumabandipilimumabversus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlino MS, Atkinson V, Cebon JS, et al. KEYNOTE-029: efficacy and safety of pembrolizumab (pembro) plus ipilimumab (ipi) for advanced melanoma. J Clin Oncol. 2017;35:9545. [Google Scholar]
- 21.Velez NF, Karia PS, Vartanov AR, et al. Association of advanced leukemic stage and skin cancer tumor stage with poor skin cancer outcomes in patients with chronic lymphocytic leukemia. JAMA Dermatol. 2014;150:280–287. [DOI] [PubMed] [Google Scholar]
- 22.Xiao W, Du N, Huang T, et al. TP53 mutation as potential negative predictor for response of anti-CTLA-4 therapy in metastatic melanoma. EBioMedicine. 2018;32:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Cunha-Bang C, Rostgaard K, Andersen MA, et al. Risk of new malignancies among patients with CLL treated with chemotherapy: results of a Danish population-based study. Br J Haematol. 2021;193: 339–345. [DOI] [PubMed] [Google Scholar]
- 24.Jobson D, McCormack CJ, Mar V, et al. Impact of chronic lymphocytic leukaemia on melanoma outcomes: a retrospective case-control study. Br J Haematol. 2022;197:320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess M, Keane C, Tobin JW, et al. Resolution of melanoma to PD-1 blockade but simultaneous rapid progression of concomitant chronic lymphocytic leukemia. Acta Haematol. 2023;146:166–171. [DOI] [PubMed] [Google Scholar]
- 26.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 27.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClanahan F, Hanna B, Miller S, et al. PD-L1 checkpoint blockade prevents immune dysfunction and leukemia development in a mouse model of chronic lymphocytic leukemia. Blood. 2015;126: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129: 3419–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain N, Basu S, Thompson PA, et al. Nivolumab combined with ibrutinib for CLL and Richter transformation: a phase II trial. Blood. 2016;128:59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


