Abstract
Dermatomyositis (DM) and polymyositis are idiopathic inflammatory myopathies (IIMs), most associated with solid organ malignancies, and less commonly hematological malignancies. We discuss a case of DM associated with diffuse large B-cell lymphoma, followed by a review of literature on the pathogenesis, clinical course, treatment, and prognosis. Various challenges with the diagnosis and management of underlying lymphoproliferative disorders (LPDs) in patients with IIM are discussed. The case demonstrates the importance of being vigilant of the association between IIM and LPD. Cancer screening in patients with IIM is discussed, including the recently published International Guideline for IIM-Associated Cancer Screening. More research is required to address knowledge gaps in cancer screening in IIM.
Keywords: B-LPD, dermatomyositis, diffuse large B-cell lymphoma, diagnostic challenge
Introduction
Dermatomyositis (DM) and polymyositis (PM) are idiopathic inflammatory myopathies (IIMs) known to be associated with solid organ and, less commonly, hematological malignancies. In particular, they are associated with B-cell lymphoproliferative disorders (B-LPDs), more commonly aggressive subtypes. The risk of cancer is highest within the first year of diagnosis of DM/PM. 1
The pathogenesis of LPD in association with IIM is likely multifactorial, including long-term inflammation, epigenetic mechanisms, and environmental factors (e.g., smoking, viral infections). We report a case of DM associated with aggressive B-LPD and discuss various challenges with the diagnosis and management of the condition. The case demonstrates the importance of being vigilant of the association between IIM and LPD.
Case Presentation
A man in his 60s presents with a 7-week history of rash, arthralgia, and malaise without fevers or night sweats. Examination revealed a violaceous heliotrope rash in photosensitive distribution, periorbital edema, and Shawl’s sign. Gottron’s papules were noted, along with proximal myopathy.
Investigations on presentation are summarized in Table 1. Punch biopsy of the back showed mild interface dermatitis with features consistent with DM (Figure 1). Direct immunofluorescence was negative. Intravenous methylprednisolone and immunoglobulin were commenced. Computed tomography of the abdomen performed due to abnormal liver function tests showed pneumobilia and multiple enlarged para-aortic lymph nodes. This prompted an 18F-fludeoxyglucose (FDG) positron emission tomography (PET)/CT showing para-aortic lymphadenopathy (SUVMax 5.5) with extensive muscular and cutaneous activity. Lymph node biopsy showed diffuse infiltrates of large pleomorphic lymphoid cells with prominent nucleoli (Figure 2). Immunohistochemistry was positive for B-cell lymphoma (BCL-6), CD30, MUM-1, and negative for CD10. Ki-67 staining was 80%, and flow cytometry showed a population of kappa light-chain-restricted B cells (31.5%) expressing CD19 and CD20 without CD5 or CD10. Bone marrow aspirate showed normal numbers of lymphocytes with a predominance of small forms without abnormal lymphoid aggregates seen on the trephine. However, flow cytometry reported a small population of kappa light chain-restricted B-lymphocytes (3.7%) expressing CD19, CD20, and CD22 without CD5 or CD10. The final diagnosis was diffuse large B-cell lymphoma (DLBCL) of nongerminal center subtype.
Table 1.
Blood Tests on Presentation.
| Test | Patient | Reference ranges |
|---|---|---|
| Hemoglobin (g/L) | 128 | 135-180 |
| White cell count (×109/L) | 6.2 | 4.0-11.0 |
| Platelets (×109/L) | 220 | 150-400 |
| Mean corpuscular volume (fL) | 86 | 80-96 |
| Creatinine kinase (U/L) | 9664 | 20-200 |
| Erythrocyte sedimentation rate (mm/h) | 41 | 1-20 |
| C-reactive protein (mg/L) | 25.9 | <6.0 |
| Lactate dehydrogenase (U/L) | 795 | 120-250 |
| Serum protein electrophoresis | No monoclonal band | |
| Alanine aminotransferase (U/L) | 168 | <40 |
| Antinuclear antibodies | Not detected | |
| Myositis-specific antibodies | Not detected |
Figure 1.
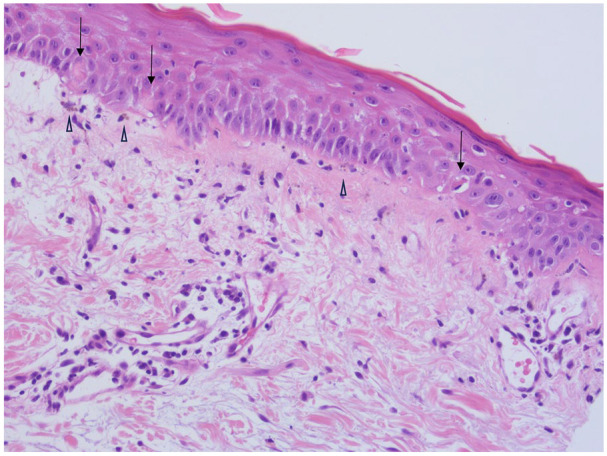
Cutaneous punch biopsy with mild vacuolar interface change along the dermoepidermal junction, occasional Civatte bodies (↓), some pigment incontinence in papillary dermis (△) and perivascular lymphocytic infiltrate in upper dermis.
Figure 2.
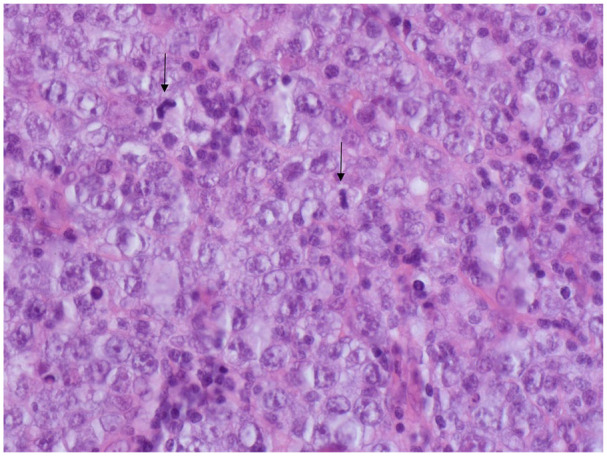
Lymph node excision biopsy showing diffuse infiltrate of large pleomorphic lymphoid cells with prominent nucleoli and plentiful mitotic figures (↓).
He commenced on R-CHOP-14 (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy and received 5 cycles as an inpatient due to significant malnutrition that warranted nasogastric feeding, deconditioning that required rehabilitation, and adjustment disorder. After the sixth cycle, repeat 18F-FDG PET/CT showed complete metabolic response, and he received 2 further doses of rituximab. The DM went into remission in parallel; however, his treatment was complicated by steroid-induced osteoporosis. He remained in remission for more than 5 years postchemotherapy and was discharged from the hematology clinic.
Discussion
Most cases of DM are associated with solid organ malignancies (81%-94%), including tumors of the nasopharynx, ovary, lung, breast, gastrointestinal tract, and bladder.2,3 However, our case emphasizes the importance to be vigilant of the association with hematologic malignancies, particularly aggressive LPD. In a retrospective study of 32 DM and PM patients with hematologic malignancies, B-LPD was most common (62.5%), followed by T-LPD (12.5%), Hodgkin’s lymphoma (6.3%), myelodysplastic syndrome (9.4%), multiple myeloma (3.1%), acute lymphocytic leukemia (3.1%), and hairy cell leukemia (3.1%). 4 Among B-LPD, 13 patients had aggressive subtypes (65%) including 12 DLBCL and 1 Burkitt’s lymphoma. Seven patients (35%) had indolent subtypes including 6 chronic lymphocytic leukemias and 1 follicular lymphoma. There was a female preponderance (59.6%) in the study.
The risk of cancer is highest within the first year of diagnosis of DM/PM. 1 In the aforementioned study, DM/PM was diagnosed before the hematological malignancy in half of the cases (n = 16). 4 In this group, 14 patients had a ≤2-year interval between the diagnoses. Six patients (18.8%) had a concurrent diagnosis of DM/PM with a hematological malignancy, and 10 (31.2%) had DM/PM that was diagnosed later. In the latter group, 6 patients had a ≤2-year interval between the diagnoses. In terms of prognosis, half of the patients (n = 16) died, and the cause was hematological malignancy (56.3%), followed by infection (31.2%), and cardiac failure (12.5%). 4
The clinical course of DM associated with LPD may be protean. In the retrospective study, DM/PM paralleled the clinical course of the underlying hematological malignancy in only 21/32 of the cases (65.6%). 4 The nonparallel cases may pose a management challenge and highlight the incomplete understanding of the underlying pathogenesis.
The pathogenesis of LPD in autoimmune conditions is multifactorial. Similar to many autoimmune conditions, many non-Hodgkin lymphomas (NHL) involve B-lymphocytes as the origin. This is thought to be due to the role of B-lymphocytes at a cellular level performing important functions such as presenting antigen to T-cells, producing antibodies, and secreting inflammatory cytokines. 5 Epigenetic mechanisms may also play a role by causing T-helper 17 and T regulatory dysfunction which may lead to autoimmune conditions and hematological malignancies. 6 Other factors proposed to contribute to the pathogenesis include high inflammatory activity and long-term immunosuppressant use. The degree of inflammation with chronic B-lymphocyte activation and antigen stimulation are thought to be important risk predictors for lymphoma.5,7 Environmental factors such as cigarette smoking and viral infections including Epstein-Barr virus, Hepatitis C, and human immunodeficiency virus have also been implicated.5,8 Both NHL and autoimmune conditions are influenced by genetic variations in the major histocompatibility complex. It is hypothesized that there is a shared genetic mechanism between NHL and autoimmune conditions due to the presence of common genetic variants. 9
There are various challenges in diagnosing LPD associated with IIM which are partly illustrated by this case. First, constitutional symptoms associated with lymphomas are not always present. Extensive muscular and cutaneous avidity may make the interpretation of 18F-FDG PET/CT difficult, a pitfall that has been highlighted by previous studies. 10 Although elevated lactate dehydrogenase (LDH) can be seen in LPD, the marker is nonspecific and is also raised in inflammatory states. Another consideration is that corticosteroids, a cornerstone therapy for IIM, may affect or delay the histological diagnosis of LPD hence a timely approach is required. 11 Masson et al 12 described 2 cases of seronegative DM associated with mycosis fungoides, illustrating the diagnostic challenge due to the overlap in clinical and histologic findings between the conditions.
More importantly, this highlights the need to be vigilant of the cancer risk in IIM. The International Guideline for IIM-Associated Cancer Screening was recently published with 18 recommendations made. 13 The cancer risk in patients with adult-onset IIM can be categorized according to autoantibody status, clinical features, and subtype of IIM to high, moderate, or standard (strong recommendation, level of evidence: B). High-risk factors are DM, anti-TIF1-γ, or anti-NXP2 autoantibodies, older than 40 years of age at onset of IIM, moderate to severe dysphagia, cutaneous necrosis, and persistently high disease activity despite immunosuppression. Our patient had 2 or more high-risk factors, indicating a high cancer risk. In addition, male gender is also an intermediate-risk factor. In high-risk patients, both basic and enhanced screening panels are recommended, with the latter incorporating the use of cross-sectional imaging and tumor markers.
However, there is a need for further research into cancer screening in IIM as emphasized in recent literature including the aforementioned guideline. This includes the optimal interval between repeated screenings, as well as investigating the potential harm and long-term outcomes from screening. Further research is also required to determine the accuracy of the risk stratification method proposed above.13,14
In conclusion, although less common, the association between DM and LPD should not be overlooked. We discussed a case of DM associated with DLBCL and the various challenges in the diagnosis and management of IIM in LPDs. The case demonstrates the importance of being vigilant of the association between IIM and LPD. Future research is required to address knowledge gaps in cancer screening in IIM is required.
Footnotes
Author Contributions: NP conceived of the presented idea and revised it critically for important intellectual content. JN, MM, HK, and AM drafted the work and revised it critically for important intellectual content. All authors reviewed and contributed to the final approval of the version to be published.
Data Availability Statement: All data analyzed during this study are included in this article. Enquiries about data access should be made to the corresponding author.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethical approval is not required for case reports in accordance with local guidelines
Informed Consent: Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Prior Presentation of Abstract Statement: This abstract was previously presented at Blood conference in Australia, as a virtual poster. The meeting dates were 20 to 24 September 2021.
ORCID iD: Jun Yen Ng  https://orcid.org/0000-0002-7995-4696
https://orcid.org/0000-0002-7995-4696
References
- 1. Aggarwal R, Oddis CV. Paraneoplastic myalgias and myositis. Rheum Dis Clin North Am. 2011;37(4):607-621. doi: 10.1016/j.rdc.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 2. Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi: 10.1016/S0140-6736(00)03540-6 [DOI] [PubMed] [Google Scholar]
- 3. Hsu J-L, Liao M-F, Chu C-C, et al. Reappraisal of the incidence, various types and risk factors of malignancies in patients with dermatomyositis and polymyositis in Taiwan. Sci Rep. 2021;11:4545. doi: 10.1038/s41598-021-83729-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marie I, Guillevin L, Menard JF, et al. Hematological malignancy associated with polymyositis and dermatomyositis. Autoimmun Rev. 2012;11(9):615-620. doi: 10.1016/j.autrev.2011.10.024 [DOI] [PubMed] [Google Scholar]
- 5. Yadlapati S, Efthimiou P. Autoimmune/inflammatory arthritis associated lymphomas: who is at risk. Biomed Res Int. 2016;2016:8631061. doi: 10.1155/2016/8631061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ngalamika O, Zhang Y, Yin H, Zhao M, Gershwin ME, Lu Q. Epigenetics, autoimmunity and hematologic malignancies: a comprehensive review. J Autoimmun. 2012;39(4):451-465. doi: 10.1016/j.jaut.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 7. Stübgen J-P. Inflammatory myopathies and lymphoma. J Neurol Sci. 2016;369:377-389. doi: 10.1016/j.jns.2016.08.060 [DOI] [PubMed] [Google Scholar]
- 8. Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25(10):2025-2030. doi: 10.1093/annonc/mdu365 [DOI] [PubMed] [Google Scholar]
- 9. Conde L, Bracci PM, Halperin E, Skibola CF. A search for overlapping genetic susceptibility loci between non-Hodgkin lymphoma and autoimmune diseases. Genomics. 2011;98(1):9-14. doi: 10.1016/j.ygeno.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahman WT, Wale DJ, Viglianti BL, et al. The impact of infection and inflammation in oncologic 18F-FDG PET/CT imaging. Biomed Pharmacother. 2019;117:109168. doi: 10.1016/j.biopha.2019.109168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kan E, Levi I, Benharroch D. Alterations in the primary diagnosis of lymphomas pretreated with corticosteroid agents. Leuk Lymphoma. 2011;52(3):425-428. doi: 10.3109/10428194.2010.544049 [DOI] [PubMed] [Google Scholar]
- 12. Masson A, Ram-Wolff C, Bouaziz JD, et al. Dermatomyositis versus mycosis fungoides: challenges in the diagnosis of erythroderma with associated myositis. Ann Dermatol Venereol. 2023;150(2):129-133. doi: 10.1016/j.annder.2021.04.011 [DOI] [PubMed] [Google Scholar]
- 13. Oldroyd AGS, Callen JP, Chinoy H, et al. International Guideline for Idiopathic Inflammatory Myopathy-Associated Cancer Screening: an International Myositis Assessment and Clinical Studies Group (IMACS) initiative. Nat Rev Rheumatol. 2023;19:805-817. doi: 10.1038/s41584-023-01045-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi: 10.1001/jamadermatol.2021.5841 [DOI] [PubMed] [Google Scholar]


